Abstract
The Radiation and Nuclear Countermeasures Program at the National Institute of Allergy and Infectious Diseases (NIAID) mandated that medical countermeasure for treating the Acute Radiation Syndrome (ARS) must have efficacy when administered at least 24 hours after radiation exposure. At this time point, many cells within key target tissues, such as the hematopoietic system and the gastrointestinal (GI) tract will already be dead. Therefore, drugs that promote the regeneration of surviving cells may improve outcomes. The serine/threonine kinase glycogen synthase kinase-3 (GSK-3) regulates stem and progenitor cell self-renewal and regeneration in the hematopoietic and GI compartments. We tested inhibition of GSK-3β by SB216763 24 hours after total body irradiation (TBI) and sub-total body Irradiation (SBI). Here, we show that subcutaneous administration of SB216763 promotes the regeneration of surviving hematopoietic stem/progenitor cells (HSPCs) including myeloid progenitor cells and improves survival of C57Bl/6 male mice when administered 24 hours after TBI. However, these results were not recapitulated in female C57Bl/6 animals suggesting a sex difference in GSK-3β signaling in HSPCs. Subcutaneous administration of SB216763 in male mice stimulated activation of Sox2 transcription, but failed to induce Sox2 transcription in female C57Bl/6 mice. Using TCF/lef-GFP reporter mice we examined Wnt signaling in HSPCs of irradiated male and female mice treated with SB216763. GSK-3 inhibition elevated Wnt reporter activity in HSPCs isolated from male but not female mice. SB216763 did not mitigate hematopoietic ARS in males or females of a second strain of wild type mice, C3H. In addition, administration of SB216763 did not mitigate hematopoietic ARS beyond the currently available standard approved therapy of ciprofloxacin and granulocyte-colony stimulating factor (G-CSF) in male C57Bl/6 mice. Further, SB216763 did not mitigate GI-ARS after SBI in C57Bl/6 male mice. The lack of efficacy in both sexes and multiple strains of mice indicate that SB216763 is not suitable for further drug development as a mitigator of ARS. Our studies demonstrate that activation of Wnt signaling in HSPC promotes hematopoietic regeneration following radiation exposure and targeting this pathway downstream of GSK-3β may mitigate ARS in a sex and strain independent manner.
Introduction
High dose radiation exposure in a nuclear disaster or attack leads to Acute Radiation Syndrome (ARS) (Dainiak, 2018). ARS is a potentially lethal condition that primarily effects tissues with a high rate of cellular turnover including the hematopoietic system and gastrointestinal (GI) tract (Mettler and Voelz, 2002). Rapidly cycling stem cells are vulnerable to radiation damage and death which leads to loss of tissue integrity (Williams et al., 2010). Total body irradiation (TBI) of doses between 4 and 8 Gy results in extensive hematopoietic cell loss and life-threatening neutropenia and thrombocytopenia. In circumstances where the abdomen is exposed to high dose radiation (>10 Gy) and shielding preserves some bone marrow to prevent hematopoietic ARS, GI ARS occurs (Mettler and Voelz, 2002). Radiation-induced GI injury is mediated by crypt stem cell death and impaired regeneration of the intestinal epithelial cell (IEC) layer, which causes a loss of the mucosal barrier, and sepsis (Kim et al., 2017).
The current therapeutic strategy for treating ARS patients is to provide supportive care such as fluids and treatment of burns and injuries, to give antibiotics such as ciprofloxacin to prevent infection, and to administer granulocyte-colony stimulating factor (G-CSF) to stimulate bone marrow recovery (Williams et al., 2016). The development of pharmaceuticals to mitigate the ARS beyond supportive care, antibiotics, and G-CSF remains an unmet challenge. Logistically, in a mass casualty radiation disaster it may take up to 24 hours to administer potential mitigator therapeutics to victims. Therefore, in order for a mitigator to be included in the national countermeasure stockpile it must be efficacious when delivered 24 hours after radiation exposure. At this timepoint, many of the stem cells in the hematopoietic and GI systems have died (Williams et al., 2010) and thus stimulating regeneration of the remaining stem cells is a major therapeutic goal.
Activation of Wnt/β-Catenin signaling stimulates hematopoietic stem and progenitor cells (HSPCs) self-renewal and proliferation (Reya et al., 2003). Indeed, β-catenin knockout mice exhibit impaired whole bone marrow cellular recovery and reduced HSPC levels after TBI compared to wild type mice (Lento et al., 2014). GSK-3β is a kinase that phosphorylates β-catenin and targets it for degradation; thus inhibition of GSK-3β stabilizes β-Catenin for translocation to the nucleus to activate TCF/lef transcription factors. We previously showed that inhibiting GSK-3β promotes renewal of HSPC populations in mice following TBI (Lee et al., 2014). We found that the GSK-3β inhibitor SB216763, a small molecule derivative of malemide, mitigates hematopoietic ARS when mice are treated at 1 or 24 hours after TBI (Lee et al., 2014). Here, we tested whether inhibition of GSK-3β by SB216763 is a viable countermeasure strategy by determining if SB216763 mitigates ARS in males and females of multiple strains of mice. Our data revealed a sex dependent variability in GSK-3β signaling in HSPCs that impacts the mitigation potential of SB216763 for hematopoietic ARS. Our results suggest that activation of wnt signaling in HSPCs is a promising strategy for mitigating hematopoietic ARS and targeting this pathway downstream of GSK-3β may be a more fruitful approach.
Results
Impact of SB216763 on radiation injury to the GI tract
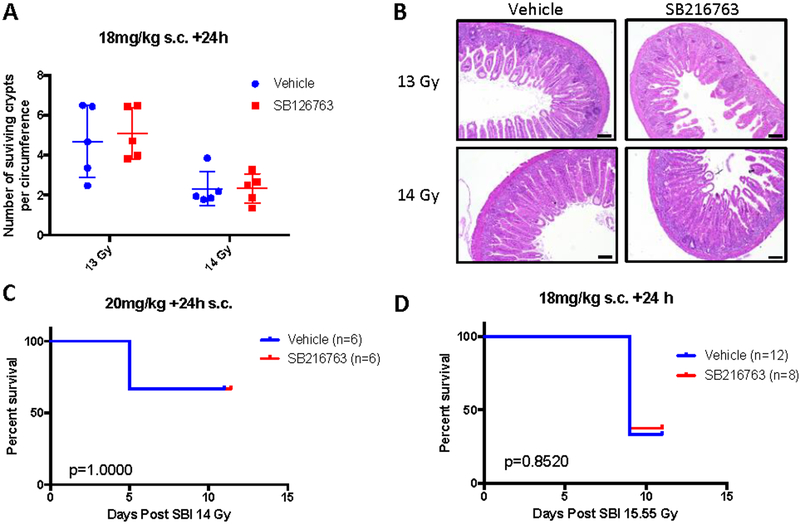
The GI ARS usually occurs at higher doses of radiation and more quickly (less than 10 days in mice) than the hematopoietic ARS (Williams et al., 2010). Because some civilians exposed to a radiological disaster would be expected to have some bone marrow shielding, for example if they are inside of a building at the time of exposure, we utilize a sub-total body irradiation (SBI) model that preserves some bone marrow function to study the GI ARS (Kirsch et al., 2010) and drugs that protect (Lee et al., 2018) against the GI ARS. Previous studies demonstrated that inhibition of GSK-3β by CHIR99021 or SB415286 before irradiation protects the GI tract from radiation-induced injury (Thotala et al., 2010; Wang et al., 2015). Therefore, we examined whether administration of SB216763 after SBI would mitigate the GI syndrome. C57Bl/6 male mice were irradiated with 13 or 14 Gy and 24 hours later treated with vehicle or SB216763 (18mg/kg) by subcutaneous injection. The number of surviving proliferative crypts was not altered with SB216763 treatment (Figure 1A and B). Additional cohorts of male and female mice were irradiated with 14 Gy (LD40/10, Lethal Dose for 40 percent of the mice in 10 days) or 15.55 Gy (LD60/10), treated with vehicle or SB216763 24 hours later and followed for the GI syndrome (Figure 1C and D). No difference was observed in GI ARS of mice treated with SB216763 compared to vehicle control.
Figure 1. SB216763 does not mitigate GI-ARS in C57Bl/6 male mice.
A, B. SB216763 or vehicle control was administered to male C57Bl/6 mice in a single subcutaneous injection 24 hours after a 13 or 14 Gy dose of SBI using X rays. Small intestine of animals was harvested 96 hours post-IR, H&E slides prepared, and surviving crypts per circumference were counted. Each dot represents the average of 7 circumferences from the duodenum and jejunum of one mouse. Scale bars, 100μm. C, D. SB216763 or vehicle control delivered to mice 24 hours after 14 or 15.55 Gy SBI. Mice were followed for the GI-ARS. P value was calculated by log-rank test.
Influence of mouse strain, sex, and supportive care on SB216763 mitigation after TBI
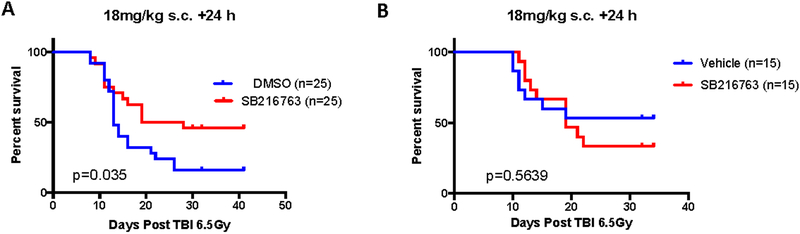
We previously reported that SB216763 mitigates the hematopoietic syndrome in C57Bl/6 mice when delivered in a single subcutaneous injection 24 hours after irradiation (Lee et al., 2014). Male C57Bl/6 mice were exposed to 6.5 Gy TBI and 24 hours later treated with SB216763 (18mg/kg) or vehicle control. Similar to our previous report (Lee et al., 2014), SB216763 treatment improved the 30 day mouse survival from hematopoietic ARS (Figure 2A). Currently approved treatments for hematopoietic ARS include granulocyte-colony stimulating factor (G-CSF) and ciprofloxacin, improve the survival of mice when administered after TBI (Plett et al., 2012). We therefore sought to determine if treatment with SB216763 provided additional mitigation benefit in the setting of these FDA approved treatments. Male C57Bl/6 animals were irradiated with 6.5 Gy TBI. Then, starting 24 hours after TBI, G-CSF (125ug/kg) was administered by subcutaneous injection daily for 16 days, and ciprofloxacin was provided in the drinking water and wet food (0.67mg/ml) beginning day 4 post-irradiation (Plett et al., 2012). Vehicle or SB216763 (18mg/kg) were delivered by a single subcutaneous injection 24 hours after TBI. SB216763 did not further mitigate the hematopoietic ARS over conventional supportive care alone (Figure 2B).
Figure 2. SB216763 alone, but not in combination with approved standard of care, mitigates hematopoietic ARS in C57Bl/6 male mice.
A. SB216763 or vehicle control was administered to male C57Bl/6 mice in a single subcutaneous injection 24 hours after a 6.5 Gy dose of TBI using X rays. B. SB216763 or vehicle was administered to male C57Bl/6 mice 24 hours after a 6.5 Gy dose of TBI using X rays. In this experiment all males received: G-CSF, 125ug/kg subcutaneous injection daily starting 24 hours after TBI for 16 days, and Ciprofloxacin, 0.67mg/ml in the drinking water and wet food beginning at day 4 post-irradiation according to Plett et al. Health Physics, 2012 (Plett et al., 2012). Mice were followed for the hematopoietic ARS. P value was calculated by log-rank test.
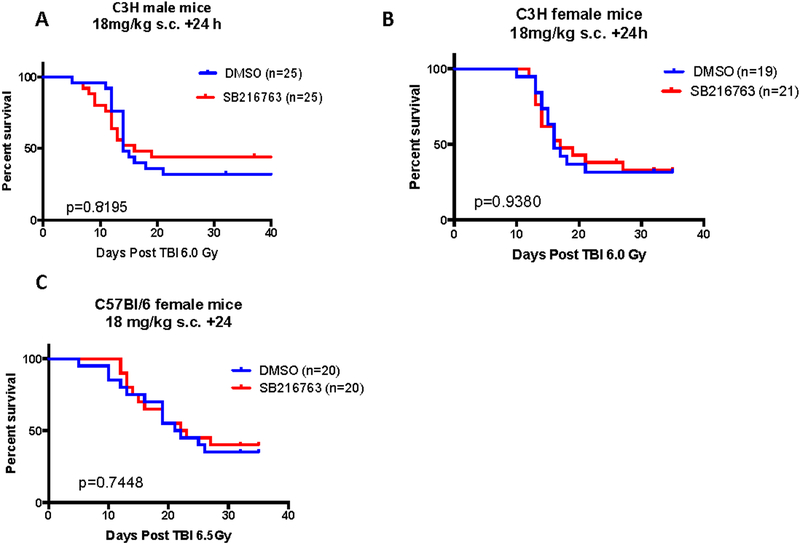
To further characterize SB216763 as a mitigator of hematopoietic ARS we performed experiments in a second strain of mice, C3H. Male C3H mice received 6 Gy TBI and 24 hours later one dose of SB216763 (18mg/kg) or vehicle was administered by subcutaneous injection. Mice were followed for hematopoietic ARS. While a slightly higher portion of SB216763 mice survived compared to vehicle treated animals, no significant difference was observed (Figure 3A). Similar experiments were performed in female C3H and C57Bl/6 strains of mice. SB216763 administration did not mitigate hematopoietic ARS in female mice of either strain (Figure 3B and C). These results indicate that SB216763 mitigates hematopoietic ARS in a strain and sex dependent manner.
Figure 3. SB216763 failed to mitigate hematopoietic ARS in C3H mice or in C57Bl/6 female mice.
A. SB216763 or vehicle control was administered to male C3H mice in a single subcutaneous injection 24 hours after a 6.0 Gy dose of TBI using X rays. B. SB216763 or vehicle control delivered to female C3H mice 24 hours after 6.0 Gy TBI. C. SB216763 or vehicle control delivered to female C57Bl/6 mice 24 hours after 6.5 Gy TBI. Mice were followed for the hematopoietic ARS. P value was calculated by log-rank test.
Comparison of SB216763 inhibition of GSK-3β in the bone marrow of male and female mice
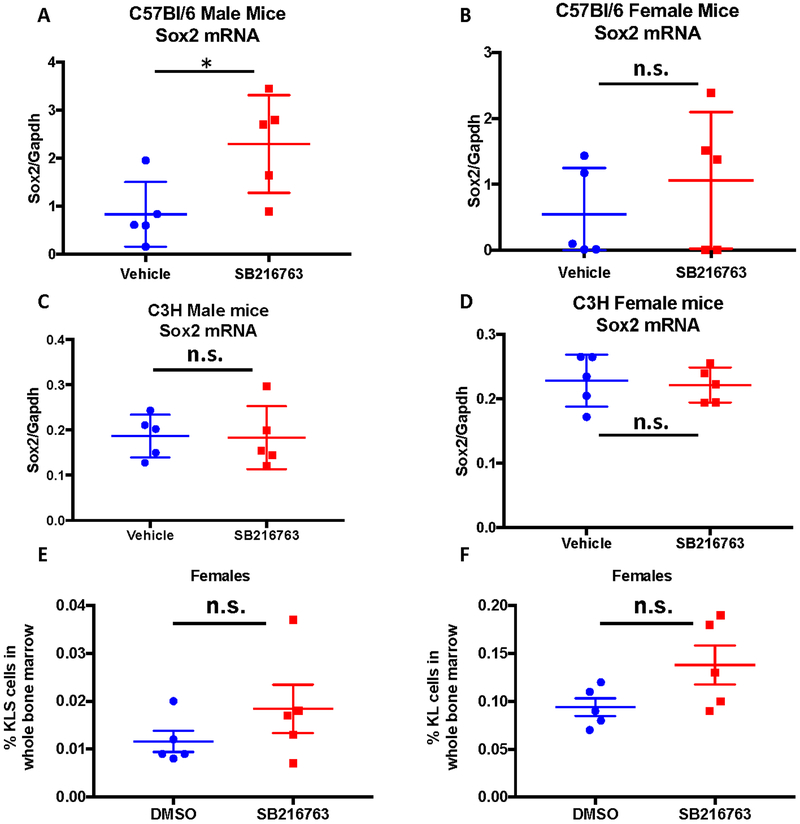
Recently published data provide evidence of a sex difference in GSK-3β activation in normal human hematopoietic cells, namely adhesion induced GSK-3β activation occurred in cells from male patients, but not from female patients (Bertrand et al., 2012). These data suggest that GSK-3β inhibition may impact hematopoietic ARS in males because the pathway may be more responsive to activation following TBI. To explore this potential explanation for the sex difference of SB216763 as a mitigator of the hematopoietic ARS, we examined a downstream target of GSK-3β inhibition in bone marrow cells. GSK-3β inhibition has been shown to induce gene expression of the stem cell marker Sox2 (Ke et al., 2017; Kirby et al., 2012). To investigate sex and strain differences in the bone marrow following inhibition of GSK-3β, male or female C57Bl/6 and C3H mice were treated with a single dose of SB216763 (18mg/kg) or vehicle and 3 hours later whole bone marrow was harvested for Sox2 gene expression analysis by Real-time PCR. SB216763 treatment of C57Bl/6 male mice induced a robust increase in Sox2 expression in whole bone marrow preparations (Figure 4A). However, Sox2 mRNA levels were not significantly elevated in SB216763 treated C57Bl/6 female nor C3H male and female mice compared to vehicle treated animals (Figure 4B–D).
Figure 4. SB216763 does not stimulate increased HSPC recovery in irradiated C57Bl/6 female mice.
A, B SB216763 or vehicle control was administered to male (left) and female (right) C57Bl/6 mice in a single subcutaneous injection and bone marrow was harvested 3 hours later for RNA isolation and Sox2 analysis. P value was calculated by Student T-test. C, D SB216763 or vehicle control was administered to male (left) and female (right) C3H mice in a single subcutaneous injection and bone marrow was harvested 3 hours later for RNA isolation and Sox2 analysis. P value was calculated by Student T-test. E, F C57Bl/6 female mice were irradiated with 4 Gy TBI and 24 hours later one dose of SB216763 or vehicle control was administered subcutaneously. Bone marrow was harvested 15 days after irradiation and hematopoietic cell populations were analyzed using flow cytometry. The percent KLS (left) and KL (right) cells are plotted. P value was calculated by Student T-test.
We previously published that treatment with SB216763 following TBI mitigates hematopoietic ARS by improving the recovery of the hematopoietic stem and progenitor cell (HSPC) population in male C57Bl/6 mice (Lee et al., 2014). We therefore examined HSPC recovery following TBI in female C57Bl/6 mice treated with SB216763 or vehicle. Mice were irradiated with 4 Gy TBI and 24 hours later treated with a single subcutaneous dose of SB216763 (18mg/kg) or vehicle. Whole bone marrow was harvested 15 days after irradiation and HSPCs were evaluated by flow cytometry using previously published markers (Lee et al., 2014). In contrast to male C57Bl/6 mice (Lee et al., 2014), the percent of multipotent HSPCs as defined by c-Kit+ lineage− Sca-1+ (KLS) cells (Figure 4C), or oligopotent myeloid progenitor cells as defined by c-Kit+ lineage− (KL) cells (Figure 4D), were not significantly increased in female mice that received SB216763 24 hours after TBI.
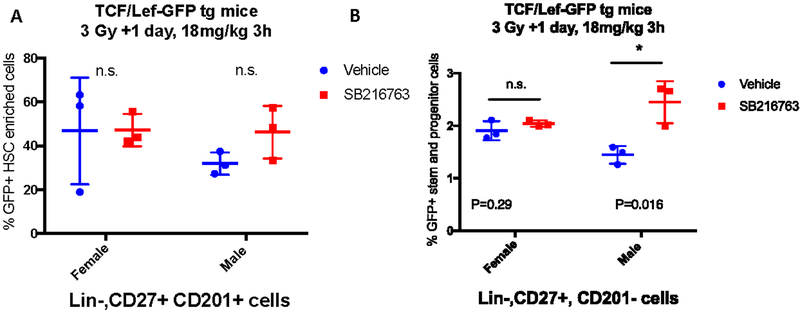
To directly assess the activation of Wnt signaling in HSPC cells downstream of GSK-3β inhibition by SB216763 we used TCF/Lef1-HIST1H2BB/EGFP mice on a C57Bl/6 background (Ferrer-Vaquer et al., 2010). In these reporter mice, Wnt/β-catenin signaling activates the TCF/Lef promoter and drives expression of EGFP. We examined GFP expression in bone marrow cells in vivo in male and female mice after TBI. Reporter mice were irradiated with 3 Gy TBI, 24 hours later mice they were treated with vehicle or SB216763 (18mg/kg), and 3 hours later whole bone marrow was harvested. Flow cytometry was performed using markers identified to efficiently define the cell populations enriched for HSPCs in irradiated mice including Lineage-, CD27+, CD201+ cells and lineage-, CD27+, CD201- cells (Vazquez et al., 2015). Quantification of GFP positive cells within these populations showed that SB216763 significantly induced Wnt activation and therefore GFP expression in Lineage- CD27+ CD201- cells from male mice, but not female mice (Figure 5, A and B). Collectively, our results suggest that SB216763 inhibits GSK-3β activity in HSPCs of C57Bl/6 male mice, but not female mice, to mitigate hematopoietic ARS when administered 24 hours after TBI. Because GSK-3β pathway inhibition is sex dependent, the potential utility of SB216763 as a countermeasure is limited.
Figure 5. SB216763 increases Wnt signaling in stem and progenitor enriched populations of bone marrow cells in male mice, but not in female mice, following TBI.
TCF/Lef-GFP reporter male and female mice were irradiated and 24 hours later treated with vehicle or SB216763 (18mg/kg). Three hours later whole bone marrow was harvested and flow cytometry was performed to determine the percent of Lin-CD27+CD201+ cells (A) or Lin-CD27+CD201- cells (B) that express GFP. P value by ANOVA test.
Discussion
Our data reveal that although SB216763 is an effective mitigator of hematopoietic ARS in male C57Bl/6 mice, SB216763 does not provide additional benefit when combined with standard antibiotic and G-CSF therapy. This finding underscores the importance of comparing mitigators of the ARS with drugs that are already in the national stockpile for radiation disaster scenarios. Studying mitigators of the hematopoietic ARS in the context of G-CSF therapy is particularly important for drugs whose mechanism of action stimulate the regeneration of surviving HSPCs, which can also be efficiently accomplished with G-CSF (Plett et al., 2012). In addition, our data show that SB216763 treatment 24 hours after SBI does not mitigate GI ARS. Interestingly, in C3H and female C57Bl/6 mice, SB216763 failed to mitigate hematopoietic ARS. We showed that in C3H and female C57Bl/6 mice Sox2 activation in the bone marrow and in female C57Bl/6 mice Wnt signaling in HSPCs can be uncoupled from GSK-3β inhibition. These data demonstrate that in mice, similar to humans (Bertrand et al., 2012), GSK-3β activity in bone marrow cells is sex dependent and therefore may not be a relevant therapeutic target in females. These results underscore the importance of testing potential mitigators in both sexes and more than one strain of mice.
Inhibition of GSK-3β and subsequent activation of Wnt signaling has been shown to improve bone marrow regeneration from irradiation and chemotherapy (Congdon et al., 2008; Lee et al., 2014; Trowbridge et al., 2006). In bone marrow transplant studies, treatment of mice with SB216763 to activate Notch, Wnt and Hedgehog signaling in HSPCs stimulated improved bone marrow progenitor cell recovery compared to vehicle controls (Trowbridge et al., 2006). Interestingly, while these studies were performed in female recipient mice it is unclear if the human and mouse HSPCs were from male or female donors. We previously demonstrated that SB216763 administration mitigates hematopoietic ARS (Lee et al., 2014). Further examination of these data reveal that the mitigation phenotype at high dose TBI was driven by the improved survival of male mice in the cohorts. These results suggest that GSK-3β may not be as active in female bone marrow cells and therefore inhibiting GSK-3β is not sufficient to elicit downstream signaling effects to promote regeneration of HSPCs. This notion is supported by data in human HSCs from healthy donors where GSK-3β activity is markedly increased in male cells plated on plastic compared to suspension cells, however GSK-3β in female HSCs remains inactive under suspension and adhesion conditions (Bertrand et al., 2012). Further, SB216763 treatment in female HSCs did not affect activity readouts indicating low basal activity.
Importantly, our data indicate that the downstream effects of blocking active GSK-3β in C57Bl/6 male HSPCs leads to mitigation of hematopoietic ARS and these results may be useful for identifying more effective therapeutic targets. Indeed, SB216763 administration results in increased expression of the target gene Sox2 in bone marrow cells and activates Wnt signaling in HSPCs of male mice leading to increased survival from high dose TBI. Therefore, these results suggest that other downstream signaling nodes that bypass the sex dependent signaling of GSK-3β and function in both male and female mice might improve hematopoietic regeneration when targeted following radiation injury. Therefore, even though our results do not support further development of SB216763 as a mitigator for the ARS, they suggest that activation of downstream effectors, such as Wnts, remain promising targets for medical countermeasures against radiation.
Methods
Animals and SB216763 treatment
All animal procedures for this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Duke University. Experiments were carried out with mice that were between 8 to 12 weeks old and contemporaneous vehicle controls were used. C57Bl/6 mice were purchased from Jackson Laboratories (stock number 000664) and C3H mice were purchased from Charles River (catalog number 25). The TCF/Lef:H2B-GFP transgenic mice were purchased from Jackson laboratories (stock number 013752) and bred in-house. SB216763 was administered in 100% DMSO at the indicated dose by subcutaneous injection.
Mouse Irradiation
An X-RAD 320 biological irradiator (Precision X-ray Inc., North Branford, CT) was used to irradiate the mice. Irradiators are maintained and dosimetry performed by the Duke University Radiation Safety Division staff using an ion chamber (Belley et al., 2014). For TBI, unanesthetized mice were placed in a pie cage without restraints and with no shielding. For SBI, unanesthetized animals were restrained in jigs and placed under lead shielding to protect the head and front limbs from exposure, as previously described (Kirsch et al., 2010). All mice were placed 50 cm from the source and irradiated with 320 kVp, 12.5 mA X rays. A 2.5-mm aluminum and 0.1-mm copper filter was used. Mouse radiation exposures were performed at a consistent time of day, to minimize the effect of circadian rhythm on radiation response across experiments. No supportive care was given to mice after irradiation except for the study when ciprofloxacin and G-CSF was administered (Figure 2B). The health status of irradiated mice was monitored by trained laboratory personnel daily. The criteria for euthanasia (Plett et al., 2012) was based on two parameters: decreased activity and squinted/closed eyes on a scale of 1–3. A mouse receiving a score of 3 in either category was considered moribund and was euthanized by carbon dioxide asphyxiation.
Quantitative Real-Time PCR
Whole bone marrow cells were isolated and resuspended in 1 ml of TRI Reagent (Invitrogen™, Grand Island, NY). Total RNA was extracted and quantified. cDNA was generated from 1 μg of RNA using iScript™ cDNA Synthesis Kit (Bio-Rad® Laboratories Inc., Hercules, CA). Relative expression levels were determined using qPCR assays performed on the QuantStudio™ 6 Flex Real-Time PCR System with TaqMan™ Fast Advanced Master Mix and specific Taqman probes (Thermo Fisher Scientific): Sox2 Mm03053810_s1 and Gapdh Mm99999915_g1. Target gene quantification levels were normalized to the housekeeper gene, Gapdh.
Crypt Survival Assay
A 12-cm segment of the duodenum and jejunum was harvested from mice 96 h after 13 or 14 Gy SBI (n = 5 mice per treatment group per dose). BrdU (Sigma-Aldrich) was administered by intraperitoneal injection (200 ul of 10 mg/ml in PBS) 2 hours prior to sacrificing the animal. Immunohistochemistry was performed on tissue sections from the duodenum and jejunum using anti-BrdU (1:100; Abcam). The number of surviving crypts per cross section was scored in approximately 7 circumferences per mouse. To correct for the increased likelihood of counting a larger crypt, we multiplied the surviving number of crypts by a correction factor as previously described (Potten et al., 1981).
Analyzing Hematopoietic Stem/Progenitor Cells (HSPCs) in the Bone Marrow
Whole bone marrow cells were isolated from femurs and tibias by grinding the bones in hematopoietic stem cell (HSC) buffer [Hanks’ balanced salt solution (HBSS) with Ca2+ and Mg2+, 5% fetal bovine serum, 2 mM EDTA]. Red blood cells (RBCs) were lysed using ACK lysing buffer (Lonza, Basel, Switzerland). Cells were blocked with a rat anti-mouse CD16/32 antibody (BD Pharmingen) and stained with PE-Cy5 conjugated lineage cocktail containing anti-mouse CD3, CD4, CD8, B220, CD11b, Gr-1 and Ter-119 antibodies (eBioscience). Cells were stained with either PE conjugated anti-mouse Sca1 and APC conjugated anti-mouse c-Kit (eBioscience) or with CD27 conjugated to APC (Thermo Fisher) and CD201 conjugated to PE (eBioscience). Dead cells were excluded by staining with 7-AAD (BD Pharmingen). Data were collected from 1 million single cells by FACSCanto (BD Biosciences) and analyzed by FlowJo software.
Acknowledgements:
This work was supported by National Institute of Health grants 1R35 CA197616 (DGK) and 2U19 AI067798 (DGK). C-LL is supported by the Whitehead Scholar Award from the Duke University School of Medicine.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicting financial interests with this manuscript. DGK is a cofounder of and stockholder in XRAD Therapeutics, which is developing radiosensitizers. DGK is a member of the scientific advisory board for and owns stock in Lumicell Inc, a company commercializing intraoperative imaging technology. He is an inventor of a handheld imaging device under U.S. patent 20140301950-A1 and is a co-inventor on a submitted patent on radiosensitizers. XRAD Therapeutics, Merck, Bristol Myers Squibb, and Eli Lilly provide research support to DGK.
References
- Belley MD, Wang C, Nguyen G, Gunasingha R, Chao NJ, Chen BJ, Dewhirst MW, and Yoshizumi TT (2014). Toward an organ based dose prescription method for the improved accuracy of murine dose in orthovoltage x-ray irradiators. Med Phys 41, 034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Despeaux M, Joly S, Bourogaa E, Gallay N, Demur C, Bonnevialle P, Louache F, Maguer-Satta V, Vergnolle N, et al. (2012). Sex differences in the GSK3beta-mediated survival of adherent leukemic progenitors. Oncogene 31, 694–705. [DOI] [PubMed] [Google Scholar]
- Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, and Reya T (2008). Activation of Wnt signaling in hematopoietic regeneration. Stem Cells 26, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Dainiak N (2018). Medical management of acute radiation syndrome and associated infections in a high-casualty incident. J Radiat Res 59, ii54–ii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, and Hadjantonakis AK (2010). A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Yuan Y, Guo C, Yang Y, Pu Q, Hu X, Tang K, Luo X, Jiang Q, Su X, et al. (2017). MiR-410 induces stemness by inhibiting Gsk3beta but upregulating beta-catenin in non-small cells lung cancer. Oncotarget 8, 11356–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Yang VW, and Bialkowska AB (2017). The Role of Intestinal Stem Cells in Epithelial Regeneration Following Radiation-Induced Gut Injury. Curr Stem Cell Rep 3, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LA, Schott JT, Noble BL, Mendez DC, Caseley PS, Peterson SC, Routledge TJ, and Patel NV (2012). Glycogen synthase kinase 3 (GSK3) inhibitor, SB-216763, promotes pluripotency in mouse embryonic stem cells. PLoS One 7, e39329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch DG, Santiago PM, di Tomaso E, Sullivan JM, Hou WS, Dayton T, Jeffords LB, Sodha P, Mercer KL, Cohen R, et al. (2010). p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327, 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CL, Lento WE, Castle KD, Chao NJ, and Kirsch DG (2014). Inhibiting glycogen synthase kinase-3 mitigates the hematopoietic acute radiation syndrome in mice. Radiat Res 181, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CL, Oh P, Xu ES, Ma Y, Kim Y, Daniel AR, and Kirsch DG (2018). Blocking Cyclin-Dependent Kinase 4/6 During Single Dose Versus Fractionated Radiation Therapy Leads to Opposite Effects on Acute Gastrointestinal Toxicity in Mice. Int J Radiat Oncol Biol Phys 102, 1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lento W, Ito T, Zhao C, Harris JR, Huang W, Jiang C, Owzar K, Piryani S, Racioppi L, Chao N, et al. (2014). Loss of beta-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev 28, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler FA Jr., and Voelz GL (2002). Major radiation exposure--what to expect and how to respond. N Engl J Med 346, 1554–1561. [DOI] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, et al. (2012). Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys 103, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Rezvani M, Hendry JH, Moore JV, and Major D (1981). The correction of intestinal microcolony counts for variation in size. Int J Radiat Biol Relat Stud Phys Chem Med 40, 321–326. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, and Weissman IL (2003). A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414. [DOI] [PubMed] [Google Scholar]
- Thotala DK, Geng L, Dickey AK, Hallahan DE, and Yazlovitskaya EM (2010). A new class of molecular targeted radioprotectors: GSK-3beta inhibitors. Int J Radiat Oncol Biol Phys 76, 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge JJ, Xenocostas A, Moon RT, and Bhatia M (2006). Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med 12, 89–98. [DOI] [PubMed] [Google Scholar]
- Vazquez SE, Inlay MA, and Serwold T (2015). CD201 and CD27 identify hematopoietic stem and progenitor cells across multiple murine strains independently of Kit and Sca-1. Exp Hematol 43, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wei L, Cramer JM, Leibowitz BJ, Judge C, Epperly M, Greenberger J, Wang F, Li L, Stelzner MG, et al. (2015). Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci Rep 5, 8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, et al. (2010). Animal models for medical countermeasures to radiation exposure. Radiat Res 173, 557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Calvi L, Chakkalakal JV, Finkelstein JN, O’Banion MK, and Puzas E (2016). Addressing the Symptoms or Fixing the Problem? Developing Countermeasures against Normal Tissue Radiation Injury. Radiat Res 186, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]