Abstract
Newly diagnosed HIV positive children may be unique index cases to identify undiagnosed parents. Data was used from the Pediatric Urgent Start of HAART ( NCT02063880) trial, which enrolled hospitalized, ART-naïve, HIV positive children ages 0-12 years in Kenya. Exact McNemar’s tests were used to compare proportions of mothers and fathers tested for HIV, linked to care, and on ART at baseline and 6 months. This analysis included 87 newly diagnosed children with HIV who completed 6 months of follow-up. Among 83 children with living mothers, there were improvements in maternal linkage to care and treatment comparing baseline to 6 months (36% vs. 78%; p<0.0001 and 22% vs. 52%; p<0.0001). Among 80 children with living fathers, there were increases from baseline to 6 months in the number of fathers who knew the child’s HIV status (34% vs. 78%; p<0.0001), fathers ever tested for HIV (43% vs. 65%; p<0.0001), fathers ever tested HIV positive (21% vs. 43%; p<0.0001), fathers ever linked to care (15% vs. 35%; p<0.0001), and fathers ever initiated on ART (11% vs. 23%; p=0.0039). Newly diagnosed HIV positive children can be important index cases to identify parents with undiagnosed HIV or poor engagement in care.
Keywords: HIV, HIV testing, family testing, index case testing, case identification, HIV care cascade
Introduction
Antiretrovirals have substantially reduced HIV transmission and AIDS-related mortality (Cohen et al., 2011; Palella et al., 1998; Tanser, Baernighausen, Graspa, Zaidi, & Newell, 2013; Vittinghoff et al., 1999). However, in Eastern and Southern Africa, an estimated 6.7 million people living with HIV remain untreated (UNAIDS, 2018a) either because they are undiagnosed or face challenges that hinder treatment initiation (Patel et al., 2016; UNAIDS, 2018b). Of those living with HIV in Eastern and Southern Africa, UNAIDS estimates that 81% know their HIV status, 81% of those who know their HIV status are on ART, and 79% of those on ART are virally suppressed (UNAIDS, 2018b). While free HIV testing is widely available, it relies on adults seeking HIV testing, and uptake is poor among men and non-pregnant women (Snow, Madalane, & Poulsen, 2010). Individual barriers to testing among adults include fear of stigma or discrimination by community or partners, and accessibility to HTS services (Meehan, Draper, Burger, & Beyers, 2017; Patel et al., 2016; Rankin-Williams, Geoffroy, Schell, & Mguntha, 2017), while barriers to antiretroviral therapy (ART) initiation include denial, stigma, fear of disclosure, perceived opposition from community or religious groups, perceived side effects, and logistical and health system issues (Patel et al., 2016).
Index case testing (ICT), which refers to testing untested contacts of an HIV positive person, has been an effective and efficient strategy to increase yield of testing of children with HIV positive parents and sexual partners of HIV positive adults (Ahmed et al., 2017; Cherutich et al., 2017; Govindasamy et al., 2015; Simon, 2018; Wagner et al., 2016). Family-based testing and treatment has been found to significantly improve testing and treatment outcomes for children and adolescents (Lewis Kulzer et al., 2012; Njuguna et al., 2016). While children are usually tested through early infant diagnosis (EID) programs or through ICT as a result of a positive parent, there are still children newly diagnosed in inpatient wards and outpatient departments (Govindasamy et al., 2015). These children are likely part of families with suboptimal historical engagement in HIV testing and prevention services, like prevention of mother-to-child transmission programs, EID, and voluntary HIV counseling and testing services (Njuguna et al., 2016). Additionally, parental uptake of HIV testing and treatment services may be poor due to complex relationship dynamics, non-disclosure and the emotional burden of a new pediatric HIV diagnosis.
This analysis aims to determine parental engagement in HIV testing and treatment among parents of newly diagnosed children in inpatient pediatric wards and understand whether these children can serve as index cases to identify families in need of testing services.
Methods
Study design and population
This secondary analysis was nested in the Pediatric Urgent Start of HAART (PUSH; NCT02063880) study, a randomized clinical trial of urgent versus post-stabilization ART among hospitalized HIV positive children newly initiating ART. The study’s methodology and inclusion criteria have been reported elsewhere (Njuguna et al., 2018). Briefly, HIV positive, ART-naïve children ages 0-12 years enrolled in the PUSH study and their parents were included in this analysis. Participants were recruited from four hospitals in Kenya (two in Nairobi and two in western Kenya). Children with suspected or confirmed central nervous system infection prior to enrollment were excluded. For this analysis, we restricted to parents of newly diagnosed HIV positive children who survived and completed 6 months of follow-up.
Data collection
Participants were enrolled from April 2013 and follow-up of all participants was completed in November 2015. At baseline and 6-month follow-up, information was collected about the caregiver’s relationship to the child, the child’s status as an orphan or vulnerable child, whether each biological parent was fulfilling his/her parental duties (i.e. parent was living in the same household as the child), who knew about the child’s HIV status, and parental HIV testing, care, and treatment.
Statistical analysis
Parental outcomes were dichotomized as follows: tested for HIV (yes vs. no/don’t know), linked to care (yes vs. no/don’t know), and on ART (yes vs. no/don’t know). Parental outcomes were summarized using counts and proportions. We used exact McNemar’s tests to compare differences between baseline and 6 months post-enrollment in the proportions of mothers and fathers tested for HIV, linked to care, and on HIV treatment. Analyses were conducted using Stata 14 (StataCorp, College Station, TX); all tests were 2-sided with alpha of 0.05.
Ethical considerations
This study was approved by the Kenyatta National Hospital (KNH) Ethics Research Committee (ERC) and the University of Washington Institutional Review Board (IRB). The primary study is registered in ClinicalTrials.gov ( NCT02063880).
Results
Of the 181 children enrolled in the PUSH trial, 87 (48%) were newly diagnosed with HIV during the enrollment hospitalization and completed 6 months of follow-up. Of these 87, median child age was 2.0 years (IQR 0.8-6.1) and 39 (45%) were female. Sixty (69%) were born in hospital and the majority of mothers (95%) had tested HIV positive.
Among the 87 children included in this analysis, 78 (90%) had two living parents, 5 (6%) had only a living mother, 2 (2%) had only a living father, and 2 (2%) did not have living parents at 6 months. Most caregiver respondents were the child’s biological mother (82 [94%] at baseline and 77 [89%] at 6-month follow-up). Median age of caregiver respondents was 28 years (IQR 24-33); most caregivers were married (66 [76%]) and had completed primary school (50 [57%]) (Table 1).
Table 1:
Child and Caregiver Demographics
| n (%) or Median (IQR) N=87 | |
|---|---|
|
Child demographics | |
| Age (years) | 2.0 (0.8, 6.1) |
| Female | 39 (45) |
| Mother primary caregiver | 82 (94) |
| Number of siblings | 2 (1, 2) |
| Place of birth | |
| Home | 25 (29) |
| Hospital | 60 (69) |
| Unknown | 2 (2) |
|
Caregiver demographics | |
| Age (years) | 28 (24, 33) |
| Female | 86 (99) |
| Marital status | |
| Married/steady partner | 66 (76) |
| Separated/widow | 15 (17) |
| Never married | 6 (7) |
| Highest level of education completed | |
| None | 1 (1) |
| Primary | 50 (57) |
| Secondary | 29 (33) |
| College | 7 (8) |
| Number of years of education | 8 (8, 10) |
Among 83 children with living mothers, 25 (30%) mothers reported that they knew their HIV positive status during pregnancy and 13 (16%) received antiretroviral medication during pregnancy. At baseline, 30 (36%) were already linked to HIV care compared to 65 (78%) after 6 months (p<0.0001). Eighteen (22%) mothers were on ART at baseline compared to 43 (52%) after 6 months (p<0.0001) (Figure 1 and Table 2).
Figure 1:
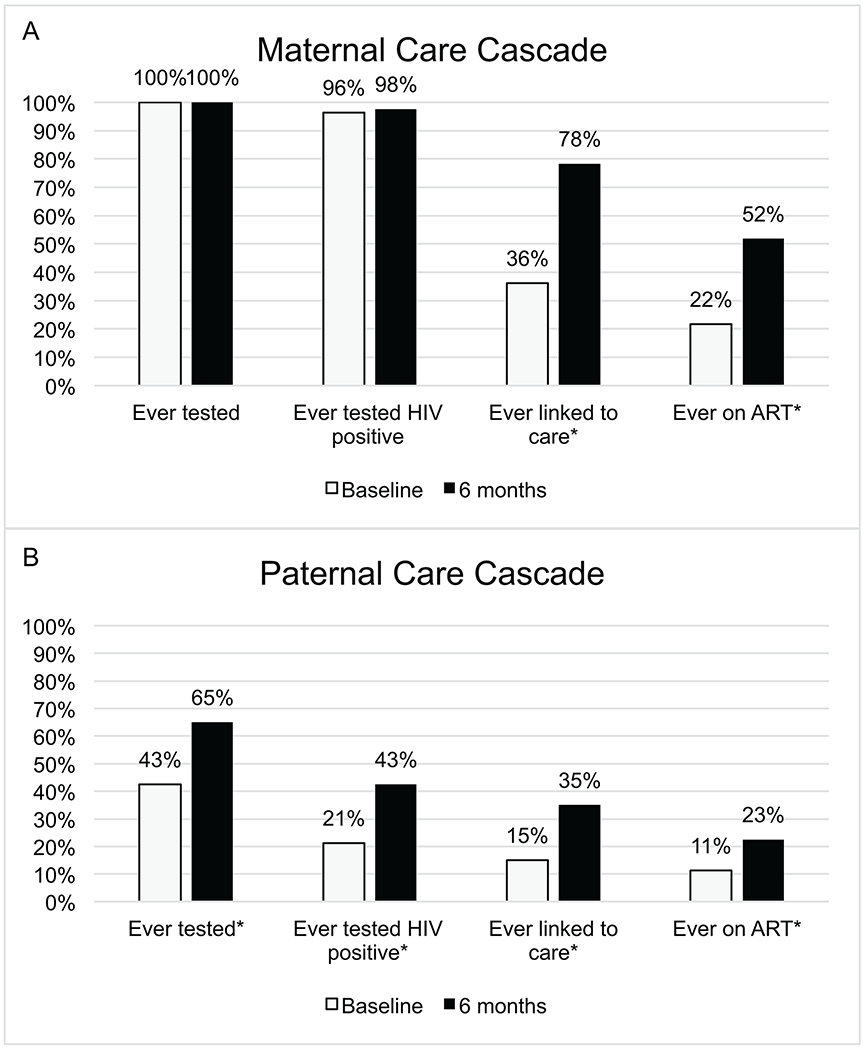
HIV Care Cascade for parents of newly diagnosed HIV positive children at baseline and 6 months
*p<0.05
Table 2:
Paternal Knowledge of child’s status and care cascade among parents of newly diagnosed HIV positive children at baseline and 6 months
| Father N=80 n(%) |
Mother N=83 n(%) |
|||||
|---|---|---|---|---|---|---|
| At/prior to baseline | 6 months | p-value* | At/prior to baseline | 6 months | p-value* | |
| Knows child’s HIV status | 27 (33.8) | 62 (77.5) | <0.0001 | 82 (98.8) | 83 (100.0) | >0.9999 |
| Ever tested | 34 (42.5) | 52 (65.0) | <0.0001 | 83 (100.0) | 83 (100.0) | >0.9999 |
| Ever tested HIV positive | 17 (21.3) | 34 (42.5) | <0.0001 | 80 (96.4) | 81 (97.6) | >0.9999 |
| Ever linked to care | 12 (15.0) | 28 (35.0) | <0.0001 | 30 (36.1) | 65 (78.3) | <0.0001 |
| Ever initiated ARVs | 9 (11.3) | 18 (22.5) | 0.0039 | 18 (21.7) | 43 (51.8) | <0.0001 |
Exact McNemar’s test was used to calculate p-values
Among 80 children with living fathers, the number of fathers who knew the child’s HIV positive status increased from 27 (34%) at baseline to 62 (78%) after 6 months (p<0.0001). The reported proportion of fathers ever tested for HIV increased from 34 (43%) at baseline to 52 (65%) after 6 months (p<0.0001), with 17 (21%) of fathers ever testing HIV positive at baseline compared to 34 (43%) after 6 months (p<0.0001). There was an increase in the number of fathers ever linked to care from baseline to 6 months (12 [15%] vs. 28 [35%]; p<0.0001), and 9 (11%) fathers had ever initiated ART at baseline compared to 18 (23%) after 6 months (p=0.0039) (Figure 1 and Table 2).
Among children with living parents, 22 (27%) of fathers and 1 (1%) of mothers were not fulfilling parental duties, defined as not residing in the same household as the child, at some point during the 6-month study period, which has implications for accessing parents for HIV testing or HIV care engagement.
Discussion
Among a cohort of newly diagnosed, HIV positive children identified in inpatient settings, there was low overall parental engagement in HIV care, despite high prevalence of HIV. Six months after a child’s diagnosis, there were increases in parental engagement across the HIV care cascade, indicating that a child’s diagnosis can prompt parental HIV testing, care, and treatment. Testing fathers of newly diagnosed children revealed a high prevalence of previously undiagnosed HIV among men, a historically challenging population to reach.
Despite these increases, important gaps existed 6 months after child diagnosis; a fifth of mothers remained not linked to care, nearly half of mothers were not on ART, and over a third of fathers had never tested for HIV. Among known HIV positive fathers, nearly a fifth were not linked to care and almost half were not on ART.
UNAIDS has set 90-90-90 goals, aiming to have 90% of people living with HIV diagnosed, 90% of those individuals on sustained ART, and 90% of those individuals virally suppressed (UNAIDS, 2014). To achieve these goals, populations with historically low uptake and engagement in care must be reached. In sub-Saharan Africa, men are less likely to test, link to care, and initiate ART (Ackers et al., 2014; Sherr & Croome, 2012; Snow et al., 2010; UNAIDS, 2018a) compared to women. Consistent with the literature, male uptake of testing in this analysis was low; less than half of fathers with HIV positive sexual partners had ever been tested. Despite a significant increase in uptake of HIV testing from baseline to 6 months, over a third of fathers with HIV positive children remained untested after 6 months.
Men face common barriers to HIV testing and linkage to care including fear of stigma (Meehan et al., 2017; Rankin-Williams et al., 2017) or discrimination by the community or a partner (Rankin-Williams et al., 2017), and lack of transport or availability during clinic hours (Camlin et al., 2016). While fear of stigma and discrimination are typical barriers to HIV testing and care among all adults (Musheke et al., 2013), women who have ever been pregnant are more likely to have contact with the healthcare system, whereas men are required to be more proactive in seeking healthcare (Dovel, Yeatman, Watkins, & Poulin, 2015; Sherr & Croome, 2012) and may perceive HIV services as female-oriented (Rankin-Williams et al., 2017). Consequently, HIV positive men tend to be tested at older ages after being medically referred or symptomatic (Schatz & Knight, 2018). Complex relationship dynamics also likely contributed to fathers’ low uptake of HIV testing. Our study found that a quarter of fathers were not residing in the same household as the child. Disclosure of the mother’s or child’s status to the father can be difficult in any circumstance; in cases where mothers and fathers live separately, there are added logistical and psychological challenges to disclosure. Additionally, supportive partners can influence male uptake of HIV testing (Rankin-Williams et al., 2017), which fathers may be lacking if they live separately from their partner. Knowledge of the child’s HIV positive status is likely an important motivator to test for HIV; after 6 months, nearly a quarter of fathers were still unaware of their child’s status.
In this analysis, only a third of mothers knew their HIV positive status during pregnancy; however, an earlier analysis found that 77% of these mothers were HIV tested during pregnancy, of whom 60% reported HIV-negative results (Njuguna et al., 2016). Programs to identify incident infections, such as repeat maternal HIV testing, are necessary, as mothers remain at high risk of HIV acquisition during pregnancy and postpartum (Drake, Wagner, Richardson, & John-Stewart, 2014). Early diagnosis of incident maternal infections is also important for maternal initiation of ART, which could substantially reduce mother-to-child transmission of HIV (John & Kreiss, 1996).
It is critical to find innovative strategies to engage those who may not have regular contact with the healthcare system and identify undiagnosed people living with HIV. ICT is an efficient strategy for increasing yield of HIV testing by identifying undiagnosed family members of HIV positive index cases. Several studies have determined that using parents as index cases is an effective and high-yield strategy to identify undiagnosed children(Ahmed et al., 2017; Govindasamy et al., 2015; Simon, 2018; Wagner et al., 2016). Similarly, assisted partner services, in which HIV positive adolescents or adults serve as the index case for sexual partners, has been an effective approach to increase testing of individuals at high risk for HIV (Cherutich et al., 2017; Henley et al., 2013). ICT approaches have focused on improving HIV care cascade of children, adolescents and sexual partners (Simon, 2018). Our study suggests that hospitalized, HIV positive children could be important index cases for testing hard-to-reach adults. Currently, uptake of testing and care is generally low in passive HIV testing service models, such as voluntary counseling and testing (Snow et al., 2010). Leveraging ICT to identify parents and family members of HIV positive children in outpatient and inpatient settings could be a feasible, effective and high-yield intervention.
This analysis was nested within the PUSH RCT, which allowed us to focus on a unique cohort of recently diagnosed, hospitalized children enrolled at 4 hospitals in Kenya. We were limited to responses from the available caregiver at the time of baseline or follow-up. One parent’s reports may have inaccurately captured HIV care engagement and testing for the other parent. There were also cases in which the caregiver answering questions at baseline differed from the caregiver at 6 months; however, these cases were few.
This analysis supports a new ICT model, using HIV positive children as index cases to identify HIV positive parents who are untested or unengaged in HIV care. There were significant increases in parental HIV testing, linkage to care, and ART uptake from baseline to 6 months. Despite these increases, important gaps in HIV care still remain. Innovative strategies to engage hard-to-reach adults are needed to achieve UNAIDS 90-90-90 targets.
Acknowledgements
We thank the PUSH study team, caregivers, and children who participated in the trial, the Kenyan Ministry of Health, and our funders for their dedication and support. The authors have no conflicts of interest to disclose.
Sources of Funding and Conflicts of Interest:
INN, LMC, VOO, CM, HMO, SBN, BAR, JS, EMO, DCW, and GJS were supported by National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH) (R01 HD023412 and K24 HD054314-06 to GJS, K12 HD000850 to LMC). JN was supported by the National Institutes of Health (New Investigator Award supported by P30 AI027757). This publication was supported in part by Fogarty International Center (FIC) D43TW009783 to IN. SBN was supported by National Institute of Neurological Disorders and Stroke (K01 NS080637). ADW was supported by NICHD (F32 HD088204-01). Research reported in this publication was supported by the University of Washington / Fred Hutch Center for AIDS Research, an NIH-funded program under award number AI027757 which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK.
Footnotes
Abstract presented at 10th Workshop on HIV Pediatrics; 2018 July; Amsterdam, Netherlands
References
- Ackers ML, Hightower A, Obor D, Ofware P, Ngere L, Kubaje A, & Laserson KF (2014). Health care utilization and access to human immunodeficiency virus (HIV) testing and care and treatment services in a rural area with high HIV prevalence, Nyanza Province, Kenya, 2007. American Journal of Tropical Medicine and Hygiene, 90(2), 224–233. 10.4269/ajtmh.13-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Sabelli RA, Simon K, Rosenberg NE, Kavuta E, Harawa M, … Kim MH (2017). Index case finding facilitates identification and linkage to care of children and young persons living with HIV/AIDS in Malawi. Tropical Medicine and International Health, 22(8), 1021–1029. 10.1111/tmi.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camlin CS, Ssemmondo E, Chamie G, El Ayadi AM, Kwarisiima D, Sang N, … Havlir D (2016). Men “missing” from population-based HIV testing: insights from qualitative research. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV, 28, 67–73. 10.1080/09540121.2016.1164806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherutich P, Golden MR, Wamuti B, Richardson BA, Ásbjörnsdóttir KH, Otieno FA, … Farquhar C (2017). Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. The Lancet HIV, 4(2), e74–e82. 10.1016/S2352-3018(16)30214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Study H (2011). Prevention of HIV-1 Infection with Early Antiretroviral Therapy. The New England Journal of Medicine, 365(6), 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovel K, Yeatman S, Watkins S, & Poulin M (2015). Men’s heightened risk of AIDS-related death: The legacy of gendered HIV testing and treatment strategies. Aids, 29(10), 1123–1125. 10.1097/QAD.0000000000000655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AL, Wagner A, Richardson B, & John-Stewart G (2014). Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis. PLoS Medicine, 11(2). 10.1371/journal.pmed.1001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy D, Ferrand RA, Wilmore SMS, Ford N, Ahmed S, Afnan-Holmes H, & Kranzer K (2015). Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: A systematic review. Journal of the International AIDS Society, 18(1), 1–9. 10.7448/IAS.18.1.20182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, & Muffih PT (2013). Scale-Up and Case-Finding Effectiveness of an HIV Partner Services Program in Cameroon. Sexually Transmitted Diseases, 40(12), 909–914. 10.1097/olq.0000000000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GC, & Kreiss J (1996). Mother-to-child transmission of human immunodeficiency virus type 1. Epidemiol Rev, 18(2), 149–157. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9021309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Kulzer J, Penner JA, Marima R, Oyaro P, Oyanga AO, Shade SB, … Cohen CR (2012). Family model of HIV care and treatment: A retrospective study in Kenya. Journal of the International AIDS Society, 15(1), 1–6. 10.1186/1758-2652-15-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan S, Draper H, Burger R, & Beyers N (2017). What drives “first-time testers” to test for HIV at community-based HIV testing services? Public Health Action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musheke M, Ntalasha H, Gari S, McKenzie O, Bond V, Martin-Hilber A, & Merten S (2013). A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Saharan Africa. BMC Public Health, 13, 220 Retrieved from http://queens.ezp1.qub.ac.uk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medl&AN=23497196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuguna IN, Cranmer LM, Otieno VO, Mugo C, Okinyi HM, Benki-Nugent S, … John-Stewart GC (2018). Urgent versus post-stabilisation antiretroviral treatment in hospitalised HIV-infected children in Kenya (PUSH): a randomised controlled trial. The Lancet HIV, 5(1), e12–e22. 10.1016/S2352-3018(17)30167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuguna IN, Wagner AD, Cranmer LM, Otieno VO, Onyango JA, Chebet DJ, … Wamalwa DC (2016). Hospitalized Children Reveal Health Systems Gaps in the Mother–Child HIV Care Cascade in Kenya. AIDS Patient Care and STDs, 30(3), 119–124. 10.1089/apc.2015.0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, … Investigators HOS (1998). Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. The New England Journal of Medicine, 853–860. [DOI] [PubMed] [Google Scholar]
- Patel RC, Odoyo J, Anand K, Stanford-Moore G, Wakhungu I, Bukusi EA, … Brown JM (2016). Facilitators and barriers of antiretroviral therapy initiation among HIV discordant couples in Kenya: Qualitative insights from a pre-exposure prophylaxis implementation study. PLoS ONE, 11(12), 1–15. 10.1371/journal.pone.0168057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin-Williams AC, Geoffroy EM, Schell ES, & Mguntha AM (2017). How can male rates of HIV testing be increased? Recommendations from a mixed methods study in southern Malawi. International Health, 9(6), 367–373. 10.1093/inthealth/ihx042 [DOI] [PubMed] [Google Scholar]
- Schatz E, & Knight L (2018). “I was referred from the other side”: Gender and HIV testing among older South Africans living with HIV. PLoS ONE, 13(4), 1–14. 10.1371/journal.pone.0196158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, & Croome N (2012). Involving fathers in prevention of mother to child transmission initiatives - What the evidence suggests. Journal of the International AIDS Society, 15(Suppl 2). 10.7448/IAS.15.4.17378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon KR et al. (2018). Family Testing: An Index Case Finding Strategy to Close the Gaps in Pediatric HIV Diagnosis. JAIDS Journal of Acquired Immune Deficiency Syndromes, 78, S88–S97. 10.1097/QAI.0000000000001731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RC, Madalane M, & Poulsen M (2010). Are men testing? Sex differentials in HIV testing in Mpumalanga province, South Africa. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV, 22(9), 1060–1065. 10.1080/09540120903193641 [DOI] [PubMed] [Google Scholar]
- Tanser F, Baernighausen T, Graspa E, Zaidi J, & Newell M-L (2013). High Coverage of ART Associated with Decline in Risk of HIV Acquisition in Rural KwaZulu-Natal, South Africa. Science, 339(966), 966–972. 10.1177/1045389X05047210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2014). 90-90-90: An ambitious treatment target to help end the AIDS epidemic.
- UNAIDS. (2018a). AIDSInfo: UNAIDS 2018 estimates. Retrieved from https://aidsinfo.unaids.org/
- UNAIDS. (2018b). AIDSInfo: UNAIDS special analysis, 2018. Retrieved from https://aidsinfo.unaids.org/
- Vittinghoff E, Scheer S, O’Malley P, Colfax G, Homberg SD, & Buchbinder SP (1999). Combination Antiretroviral Therapy and Recent Declines in AIDS Incidence and Mortality. 94105, 717–720. [DOI] [PubMed] [Google Scholar]
- Wagner ADAD, Mugo C, Njuguna ININN, Maleche-Obimbo E, Sherr K, Inwani IWIW, … Slyker JAAJA (2016). Active referral of children of HIV-positive adults reveals high prevalence of undiagnosed HIV. J Acquir Immune Defic Syndr, 73(5), E89 10.1097/QAI.0000000000001184 [DOI] [PMC free article] [PubMed] [Google Scholar]


