Abstract
Background:
Young, premenopausal women with breast cancer often experience more aggressive disease biology and poorer survival than older women. Diagnostic and therapeutic advances, including human epidermal growth factor receptor-2 (HER2)-directed therapy, may lessen treatment burden and improve survival for these young women, but contemporary incidence and survival data by HER2 status are limited.
Patients and Methods:
We identified women aged 20–49 years (n=68,530) diagnosed with stage I-III breast cancer during 2010–2016 from the United States Surveillance, Epidemiology and End Results 18 registries database. Age-adjusted, average annual percentage changes in incidence (diagnosis 2010–2016) and five-year Kaplan-Meier survival curves (diagnosis 2010–2015) were estimated by HER2 and hormone receptor (HR) status and stratified independently by cancer stage and race/ethnicity.
Results:
With increasing age decade, proportions of HER2−/HR+ cancer increased, whereas proportions of HER2+/HR+, HER2+/HR−, and HER2−/HR− decreased. The greatest increases in incidence during 2010–2016 were observed for HER2+ among women 20–49 and HER2−/HR− among women 20–29. Incidence decreased for HER2−/HR− among women 40–49. Five-year survival was lowest for HER2−/HR− status compared to other receptor-based subtypes among women 20–49. HER2+ status was more beneficial for five-year survival than HR+ status among women 20–29, with the opposite observed among women 30–49, particularly those 40–49.
Conclusions:
HER2+ breast cancer increased among premenopausal women and was also associated with higher early survival within each HR status. HER2−/HR− cancer also increased among women 20–29 and was associated with lower early survival. Our contemporary data provide important insights to help inform preventive and therapeutic strategies for premenopausal women.
Keywords: Breast Neoplasms, Genes, HER2, Incidence, Neoplasm Staging, Survival
MicroAbstract
Contemporary data of breast cancer incidence and survival by HER2 status among premenopausal women are limited. In our analysis of 68,530 women in the SEER database aged 20–49 years, HER2+ cancer increased in incidence and was also associated with higher early survival within each HR status. Our findings provide insights to help inform preventive and therapeutic strategies for premenopausal women.
Introduction
Premenopausal women with breast cancer experience a high risk of disease recurrence and cancer-related death.1 Gene-expression profiles of the tumors in these women suggest that these tumors tend to have more aggressive molecular characteristics, including more frequent basal-like and human epidermal growth factor receptor-2 (HER2)-enriched tumors, than those that occur in older women.2–5 Reports combining tumor receptor status and other histologic features also suggest women under age 35 years tend to have more aggressive cancer disease biology than older women of premenopausal age.6, 7 These findings, together with advances in diagnostics and treatment – including the availability of HER2-directed therapy in the United States (US) – help underscore the need for improved understanding of contemporary breast cancer incidence and survival data among US premenopausal women by HER2 receptor status; to date, available data are limited.
We identified five recent US studies that reported frequencies of HER2 receptor-based subtypes in women of premenopausal age.1, 4, 8–10 These studies included women <50 years of age of differing sample sizes, ranging from 399 to 6,570, and time periods of breast cancer diagnoses, ranging from 1996–2013. Most studies grouped women either under age 40 or age 50 years into a single age category,1, 4, 8, 10 some studies were limited to women seen at tertiary care centers,1, 8 had limited racial/ethnic diversity,8 or had a sizable proportion of tumors that could not be classified9; no study reported changes in incidence estimates over time by receptor-based subtypes. We identified two US studies that examined survival by HER2 status among premenopausal women.1, 10 One study analyzed 2010–2013 breast cancer diagnoses ascertained by the population-based cancer registries in the US National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program and reported on clinical characteristics and survival across all disease stages, but classified premenopausal women into a single group <50 years of age.10 Another study examined outcomes among women ≤40 years of age presenting to eight National Comprehensive Cancer Network (NCCN) centers from 2000–2007, largely in the pre-trastuzumab era.1
In 2010, the US SEER Program began reporting breast cancer HER2 status. Analysis by age decade of these non-trial, population-based data can add to our understanding of incidence patterns and survival for premenopausal women. Using data in the SEER 18 registries database for 2010–2016, we report on breast cancer incidence and early survival by receptor-based subtype, including HER2, for women aged 20–49 years diagnosed with stage I-III breast cancer. We excluded stage IV cancer as its treatment is generally delivered with palliative rather than curative intent. Our findings can inform preventive and therapeutic strategies aimed at reducing treatment burden and improving survival for these women.
Patients and Methods
Study population
We obtained data for our retrospective cohort study from the SEER 18 registries database (November 2018 submission, 2010–2016); access to these data can be obtained at www.seer.cancer.gov. Jurisdictions covered by these 18 population-based registries comprise approximately 28% of the total US population.11 Our study was approved by the University of Iowa Institutional Review Board.
We identified 79,000 women aged 20–49 years whose initial breast cancer diagnosis occurred during 2010–2016. Of these, we excluded 271 whose diagnoses were not microscopically confirmed and 18 whose diagnosis was reported only from a nursing or convalescent home, hospice, or autopsy. Of the remaining 78,711 women, we excluded 6,349 whose cancer was not confirmed stage I-III (Supplementary Table S1); stage was assigned using the American Joint Committee on Cancer adjusted 7th edition (2010–2015)12 and SEER combined stage (2016).13 Of the 72,362 women with stage I-III cancer, we excluded women with borderline HER2 status (n=1,354), as well as women with unknown HER2 status (n=1,271), hormone receptor (HR) status (n=102), or both (n=1,105) (Supplementary Table S2). In the SEER 18 registries database, HER2+ status was defined as a positive test result (borderline excluded). HR+ status was defined as having either positive or borderline estrogen receptor (ER) or progesterone receptor (PR) status, and HR− status was defined as having both ER− and PR− status. Women with borderline ER and PR status were grouped with ER+ and PR+, respectively, due to changes in assay interpretation guidelines that no longer allow for a borderline result and indicate a cutoff of 1% positive tumor cell nuclei be used, compared to historical cutoffs of up to 10%.14 After all exclusions, our analytic sample comprised 68,530 women.
Statistical analysis
We grouped age at breast cancer diagnosis into three decades, 20–29, 30–39, and 40–49 years, and classified receptor-based subtypes as HER2+/HR+, HER2+/HR−, HER2−/HR+ and HER2−/HR−. We stratified breast cancer stage as I, II, or III and applied SEER definitions for race/ethnicity (non-Hispanic white, black, American Indian/Alaska Native, Asian/Pacific Islander; Hispanic; and non-Hispanic [unknown race]). Due to sparse totals for non-Hispanic American Indian/Alaska Native women and non-Hispanic women of unknown race, we did not present results for these strata.
Incidence was estimated for each age decade as the number of annual breast cancer diagnoses per 100,000 women, age-adjusted to the 2000 US standard population by five-year age groups using SEER*Stat Version 8.3.5 software.15 In order to provide a summary measure of incidence change over the study time period, the average annual percent change (AAPC) in incidence and corresponding 95% confidence interval (CI) for 2010–2016 were estimated for each age decade and receptor-based subtype using Joinpoint Regression Program software, version 4.6.0.0,16 applying least-squares regression models with the natural logarithm of the age-adjusted rates as the outcome and diagnosis year as the predictor. The errors were assumed to be normally distributed. Model assumptions were evaluated by examining the residuals, and no violations were observed. Five-year survival was estimated for women diagnosed in 2010–2015 (n=58,618); those diagnosed in 2016 (n=9,880) were excluded due to lack of follow-up time, as were those diagnosed in 2010–2015 with no follow-up time (n=32). We generated Kaplan-Meier estimates and 95% CIs for five-year survival using SEER*Stat; five-year Kaplan-Meier curves were plotted using R Version 3.5.1.17 Because previous data suggest that young women may have a relatively high prevalence of germ-line mutations,18 we assessed whether a second primary malignancy confounded our survival estimates by conducting a sub-analysis excluding women who were later diagnosed with a second primary malignancy.
Results
Of the 68,530 women with stage I-III breast cancer in our analytic sample, there were 1,818 women 20–29 years, 14,550 women 30–39 years, and 52,162 women 40–49 years (Table 1). HER2−/HR+ was the most frequent receptor-based subtype for each age decade, whereas HER2+/HR− was the least frequent. The proportion of cancers that were HER2−/HR+ subtype increased with increasing age; the opposite pattern was observed for HER2+/HR+, HER2+/HR−, and HER2−/HR− subtypes. These observed patterns tended to persist for each cancer stage and racial/ethnic group.
Table 1.
Frequencies of HER2/HR Status among women aged 20–49 years diagnosed with stage I-III breast cancer, 2010–2016 SEER 18 registries
| 20–29 Years | 30–39 Years | 40–49 Years | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Analytic sample | 1,818 | 14,550 | 52,162 | |||
| HER2+/HR+ | 385 | 21.2 | 2,677 | 18.4 | 6,935 | 13.3 |
| HER2+/HR− | 133 | 7.3 | 1,041 | 7.2 | 2,607 | 5.0 |
| HER2−/HR+ | 879 | 48.4 | 8,008 | 55.0 | 36,021 | 69.1 |
| HER2−/HR− | 421 | 23.2 | 2,824 | 19.4 | 6,599 | 12.7 |
| Stage I | 476 | 4,191 | |23,093 | |||
| HER2+/HR+ | 106 | 22.3 | 708 | 16.9 | 2,549 | 11.0 |
| HER2+/HR− | 34 | 7.1 | 243 | 5.8 | 837 | 3.6 |
| HER2−/HR+ | 265 | 55.7 | 2,657 | 63.4 | 17,791 | 77.0 |
| HER2−/HR− | 71 | 14.9 | 583 | 13.9 | 1,916 | 8.3 |
| Stage II | 930 | 7,334 | 21,268 | |||
| HER2+/HR+ | 199 | 21.4 | 1,397 | 19.0 | 3,136 | 14.7 |
| HER2+/HR− | 62 | 6.7 | 494 | 6.7 | 1,148 | 5.4 |
| HER2−/HR+ | 423 | 45.5 | 3,770 | 51.4 | 13,510 | 63.5 |
| HER2−/HR− | 246 | 26.5 | 1,673 | 22.8 | 3,474 | 16.3 |
| Stage III | 412 | 3,025 | 7,801 | |||
| HER2+/HR+ | 80 | 19.4 | 572 | 18.9 | 1,250 | 16.0 |
| HER2+/HR− | 37 | 9.0 | 304 | 10.1 | 622 | 8.0 |
| HER2−/HR+ | 191 | 46.4 | 1,581 | 52.3 | 4,720 | 60.5 |
| HER2−/HR− | 104 | 25.2 | 568 | 18.8 | 1,209 | 15.5 |
| Non-Hispanic white | 903 | 7,503 | 30,282 | |||
| HER2+/HR+ | 207 | 22.9 | 1,455 | 19.4 | 3,863 | 12.8 |
| HER2+/HR− | 70 | 7.8 | 525 | 7.0 | 1,278 | 4.2 |
| HER2−/HR+ | 438 | 48.5 | 4,190 | 55.8 | 21,761 | 71.9 |
| HER2−/HR− | 188 | 20.8 | 1,333 | 17.8 | 3,380 | 11.2 |
| Non-Hispanic black | 308 | 2,090 | 6,253 | |||
| HER2+/HR+ | 52 | 16.9 | 365 | 17.5 | 817 | 13.1 |
| HER2+/HR− | 21 | 6.8 | 136 | 6.5 | 422 | 6.7 |
| HER2−/HR+ | 143 | 46.4 | 1,032 | 49.4 | 3,598 | 57.5 |
| HER2−/HR− | 92 | 29.9 | 557 | 26.7 | 1,416 | 22.6 |
| Non-Hispanic Asian or Pacific Islander | 184 | 1,824 | 6,353 | |||
| HER2+/HR+ | 44 | 23.9 | 326 | 17.9 | 909 | 14.3 |
| HER2+/HR− | 13 | 7.1 | 134 | 7.3 | 387 | 6.1 |
| HER2−/HR+ | 93 | 50.5 | 1,117 | 61.2 | 4,532 | 71.3 |
| HER2−/HR− | 34 | 18.5 | 247 | 13.5 | 525 | 8.3 |
| Hispanic (all races) | 398 | 2,937 | 8,701 | |||
| HER2+/HR+ | 74 | 18.6 | 494 | 16.8 | 1,262 | 14.5 |
| HER2+/HR− | 27 | 6.8 | 224 | 7.6 | 491 | 5.6 |
| HER2−/HR+ | 195 | 49.0 | 1,572 | 53.5 | 5,741 | 66.0 |
| HER2−/HR− | 102 | 25.6 | 647 | 22.0 | 1,207 | 13.9 |
Abbreviations: HER2, human epidermal growth factor receptor-2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results.
Results for non-Hispanic American Indian/Alaska Native women aged 20–49 years (n=435) and non-Hispanic women of unknown race aged 20–49 years (n=359) were not presented due to sparse totals for these strata.
Because of rounding, percentages might not total 100.
Incidence
For the years 2010–2016, we observed increased incidence estimates for each receptor-based subtype among women 20–29 years except HER2−/HR+, for which incidence remained rather stable (Table 2, Figure 1). Estimates among women 30–49 years increased most for the HER2+ subtypes with more modest increases for HER2−/HR+; estimates for HER2−/HR− remained rather stable among women 30–39 years and decreased among women 40–49 years. Estimated AAPCs stratified by cancer stage or race/ethnicity tended to be imprecise. The difference in AAPCs for HER2-/HR- between women 30–39 years and 40–49 years in the total analytic sample was largest for stage III cancer. Hispanic women aged 30–49 years experienced larger increases of HER2+/HR+ cancer compared to the total analytic sample for these respective age decades. The modest improvement of classification of cancer stage, HER2 status, and HR status over time throughout the study period (Supplementary Tables S1 and S2) may have positively biased AAPC estimates.
Table 2.
Average annual percentage changes in incidence by HER2/HR status among women aged 20–49 years diagnosed with stage I-III breast cancer, 2010–2016, SEER 18 registries
| 20–29 Years | 30–39 Years | 40–49 Years | |
|---|---|---|---|
| AAPC (95% CI) | AAPC (95% CI) | AAPC (95% CI) | |
| Analytic sample | |||
| HER2+/HR+ | 3.58 (−1.56, 9.00) | 4.43 (2.75, 6.14) | 3.70 (1.26, 6.20) |
| HER2+/HR− | 5.80 (−3.45, 15.95) | 3.04 (0.49, 5.66) | 2.63 (−0.54, 5.90) |
| HER2−/HR+ | 0.49 (−4.43, 5.65) | 1.20 (−1.21, 3.67) | 1.43 (0.32, 2.55) |
| HER2−/HR− | 3.64 (−1.82, 9.40) | 0.44 (−0.97, 1.87) | −1.73 (−4.28, 0.89) |
| Stage I | |||
| HER2+/HR+ | −0.90 (−18.11, 19.91) | 3.68 (0.28, 7.21) | 2.37 (−1.33, 6.21) |
| HER2+/HR− | −2.68 (−28.89, 33.18) | 1.91 (−7.37, 12.10) | −0.34 (−5.48, 5.08) |
| HER2−/HR+ | −1.77 (−8.63, 5.61) | 1.35 (−1.34, 4.10) | 2.02 (0.48, 3.59) |
| HER2−/HR− | −4.34 (−11.76, 3.70) | −1.63 (−5.67, 2.58) | −2.13 (−5.35, 1.20) |
| Stage II | |||
| HER2+/HR+ | 4.33 (−0.79, 9.72) | 6.04 (2.65, 9.55) | 5.52 (1.60, 9.59) |
| HER2+/HR− | 13.51 (−1.07, 30.24) | 6.68 (0.66, 13.06) | 7.82 (3.15, 12.70) |
| HER2−/HR+ | 1.22 (−5.71, 8.66) | 2.04 (−0.53, 4.67) | 1.56 (0.48, 2.65) |
| HER2−/HR− | 5.31 (−5.28, 17.08) | 0.67 (−2.20, 3.62) | −0.67 (−3.17, 1.91) |
| Stage III | |||
| HER2+/HR+ | 3.53 (−5.36, 13.26) | 1.36 (−4.49, 7.58) | 2.05 (−3.14, 7.52) |
| HER2+/HR− | 2.28 (−14.96, 23.02) | −1.92 (−6.00, 2.33) | −2.42 (−5.35, 0.61) |
| HER2−/HR+ | 2.12 (−3.90, 8.52) | −1.00 (−4.54, 2.67) | −1.10 (−2.56, 0.37) |
| HER2−/HR− | 3.09 (−11.86, 20.57) | 1.99 (−0.78, 4.84) | −4.07 (−6.94, −1.12) |
| Non-Hispanic white | |||
| HER2+/HR+ | 6.13 (−1.99, 14.92) | 3.77 (0.87, 6.76) | 3.09 (−0.81, 7.15) |
| HER2+/HR− | 10.48 (−1.28, 23.64) | 3.60 (1.83, 5.40) | 2.78 (−2.58, 8.43) |
| HER2−/HR+ | 0.99 (−3.92, 6.15) | 0.76 (−1.98, 3.58) | 1.39 (0.17, 2.64) |
| HER2−/HR− | 5.34 (−3.25, 14.69) | 0.49 (−2.96, 4.06) | −2.07 (−4.03, −0.08) |
| Non-Hispanic black | |||
| HER2+/HR+ | −7.27 (−19.76, 7.17) | 2.41 (−2.01, 7.04) | 2.42 (−1.21, 6.19) |
| HER2+/HR− | −8.19 (−24.68, 11.92) | 1.64 (−2.95, 6.44) | 4.71 (−0.53, 10.24) |
| HER2−/HR+ | −5.83 (−15.65, 5.14) | 2.48 (−2.41, 7.63) | 0.57 (−1.30, 2.47) |
| HER2−/HR− | −2.04 (−10.20, 6.87) | 0.25 (−5.07, 5.87) | −0.70 (−5.35, 4.19) |
| Non-Hispanic Asian or Pacific Islander | |||
| HER2+/HR+ | 7.81 (−8.52, 27.05) | 3.09 (1.45, 4.76) | 3.13 (−2.77, 9.40) |
| HER2+/HR− | −4.81 (−22.48, 16.87) | 2.48 (−8.86, 15.25) | 1.24 (−3.58, 6.30) |
| HER2−/HR+ | 1.64 (−10.29, 15.16) | 0.20 (−3.49, 4.02) | 2.85 (0.20, 5.57) |
| HER2−/HR− | −5.25 (−28.29, 25.19) | −2.06 (−7.11, 3.28) | −2.23 (−10.23, 6.49) |
| Hispanic (all races)a | |||
| HER2+/HR+ | 3.58 (−7.16, 15.56) | 9.42 (5.11, 13.91) | 7.46 (4.48, 10.53) |
| HER2+/HR− | 2.67 (−22.83, 36.60)b | 1.86 (−5.43, 9.72) | 0.30 (−5.93, 6.93) |
| HER2−/HR+ | 1.63 (−4.25, 7.88) | 2.42 (−0.56, 5.49) | 2.48 (−0.78, 5.85) |
| HER2−/HR− | 6.55 (3.32, 9.88) | 1.24 (−1.70, 4.27) | −1.16 (−7.26, 5.35) |
Abbreviations: AAPC, average annual percent change; CI, confidence interval; HER2, human epidermal growth factor receptor-2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results.
Women identified by the Alaska Native Registry were excluded from incidence estimations for the Hispanic group, as this registry only collects cases from the Native American and Alaska Native populations within the state.
No cases were observed in 2012 for this group. A case count of 0.25 was used for 2012 in order to estimate the AAPC.
Results for non-Hispanic American Indian/Alaska Native women were not presented due to sparse case counts for these strata.
Figure 1.
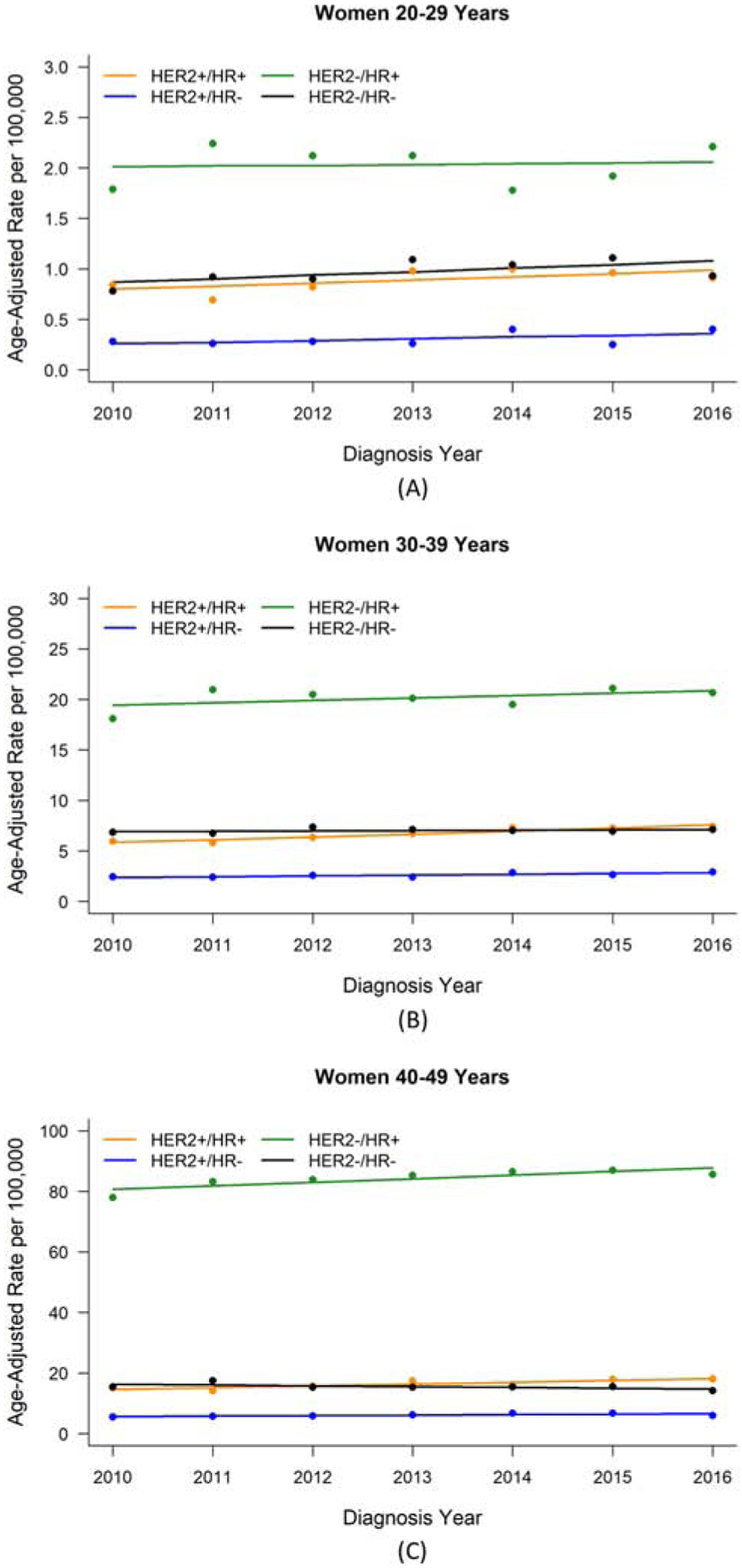
Breast cancer incidence rate by HER2/HR status for women aged 20–49 years diagnosed with stage I-III breast cancer, 2010–2016, SEER 18 registries. (A) Women aged 20–29 years. (B) Women aged 30–39 years. (C) Women aged 40–49 years.
Abbreviations: HER2, human epidermal growth factor receptor-2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results.
Five-year survival
Women 20–49 years with HER2−/HR− cancer experienced the lowest five-year survival compared to the other receptor-based subtypes (Table 3, Figure 2). The highest five-year survival was observed for women with HER2+ cancers among those 20–29 years, for women with HER2+/HR+ cancer among those 30–39 years, and for women with HR+ cancers among women 40–49 years. These patterns tended to persist for each cancer stage and racial/ethnic group.
Table 3.
Five-year survival by HER2/HR status among women aged 20–49 years diagnosed with stage I-III breast cancer, 2010–2015, SEER 18 registries
| 20–29 Years | 30–39 Years | 40–49 Years | ||||
|---|---|---|---|---|---|---|
| N | Survival (95% CI) | N | Survival (95% CI) | N | Survival (95% CI) | |
| Analytic sample | ||||||
| HER2+/HR+ | 326 | 91.9 (86.1, 95.3) | 2,238 | 94.6 (93.2, 95.8) | 5,872 | 94.8 (94.0, 95.5) |
| HER2+/HR− | 107 | 91.8 (78.3, 97.0) | 867 | 88.6 (85.4, 91.2) | 2,251 | 89.2 (87.4, 90.8) |
| HER2−/HR+ | 735 | 89.0 (85.5, 91.7) | 6,793 | 90.9 (89.9, 91.8) | 30,912 | 95.3 (95.0, 95.6) |
| HER2−/HR− | 361 | 79.3 (73.3, 84.0) | 2,399 | 78.3 (76.2, 80.3) | 5,757 | 80.1 (78.8, 81.3) |
| Stage I | ||||||
| HER2+/HR+ | 89 | 98.5 (89.9, 99.8) | 586 | 99.4 (98.1, 99.8) | 2,169 | 98.2 (97.3, 98.8) |
| HER2+/HR− | 32 | 100 | 200 | 95.1 (89.4, 97.8) | 734 | 95.6 (92.8, 97.3) |
| HER2−/HR+ | 219 | 97.0 (92.1, 98.9) | 2,259 | 96.9 (95.6, 97.8) | 15,247 | 98.5 (98.2, 98.8) |
| HER2−/HR− | 62 | 90.2 (75.2, 96.3) | 494 | 92.8 (89.5, 95.1) | 1,682 | 93.0 (91.2, 94.4) |
| Stage II | ||||||
| HER2+/HR+ | 168 | 95.6 (85.8, 98.7) | >1,171 | 95.7 (93.7, 97.1) | 2,660 | 95.2 (93.9, 96.2) |
| HER2+/HR− | 46 | 95.5 (71.9, 99.3) | 406 | 92.5 (88.0, 95.3) | 969 | 92.4 (89.9, 94.3) |
| HER2−/HR+ | 354 | 90.4 (85.0, 93.9) | 3,185 | 92.1 (90.7, 93.3) | 11,549 | 95.0 (94.4, 95.5) |
| HER2−/HR− | 209 | 83.0 (74.4, 88.9) | 1,430 | 83.4 (80.7, 85.7) | 3,005 | 82.4 (80.6, 84.0) |
| Stage III | ||||||
| HER2+/HR+ | 69 | 74.9 (57.0, 86.2) | 481 | 86.5 (81.8, 90.0) | 1,043 | 87.1 (84.2, 89.5) |
| HER2+/HR− | 29 | 77.7 (42.7, 92.8) | 261 | 78.4 (70.7, 84.3) | 548 | 75.7 (70.7, 80.0) |
| HER2−/HR+ | 162 | 73.8 (63.1, 81.8) | 1,349 | 78.4 (75.3, 81.2) | 4,116 | 84.4 (82.9, 85.8) |
| HER2−/HR− | 90 | 62.6 (50.0, 72.9) | 475 | 48.4 (42.9, 53.7) | 1,070 | 54.2 (50.5, 57.6) |
| Non-Hispanic white | ||||||
| HER2+/HR+ | 175 | 92.9 (84.2, 96.9) | 1,218 | 95.3 (93.4, 96.7) | 3,322 | 95.4 (94.3, 96.3) |
| HER2+/HR− | 54 | 89.8 (64.8, 97.4) | 438 | 91.0 (86.7, 93.9) | 1,125 | 90.3 (87.7, 92.4) |
| HER2−/HR+ | 366 | 88.9 (83.7, 92.4) | 3,574 | 91.4 (90.1, 92.6) | 18,883 | 95.9 (95.6, 96.3) |
| HER2−/HR− | 162 | 84.0 (75.9, 89.5) | 1,139 | 80.4 (77.4, 83.1) | 2,970 | 82.2 (80.5, 83.8) |
| Non-Hispanic black | ||||||
| HER2+/HR+ | 47 | 87.7 (64.8, 96.1) | 310 | 90.1 (84.8, 93.6) | 700 | 89.7 (86.2, 92.4) |
| HER2+/HR− | 18 | 90.9 (50.8, 98.7) | 113 | 86.4 (76.3, 92.4) | 356 | 80.4 (74.3, 85.1) |
| HER2−/HR+ | 124 | 89.4 (79.2, 94.8) | 866 | 84.5 (81.0, 87.4) | 3,083 | 89.7 (88.2, 91.0) |
| HER2−/HR− | 79 | 72.6 (56.6, 83.5) | 464 | 72.3 (67.2, 76.7) | 1,226 | 74.1 (71.0, 76.9) |
| Non-Hispanic Asian or Pacific Islander | ||||||
| HER2+/HR+ | 38 | 100 | 272 | 97.3 (93.3, 98.9) | 744 | 97.4 (95.4, 98.6) |
| HER2+/HR− | 12 | 100 | 117 | 88.7 (75.9, 94.9) | 325 | 92.9 (88.5, 95.6) |
| HER2−/HR+ | 75 | 97.9 (85.8, 99.7) | 938 | 94.0 (91.3, 95.9) | 3,809 | 97.2 (96.3, 97.8) |
| HER2−/HR− | 30 | 76.8 (49.4, 90.6) | 214 | 81.2 (73.7, 86.7) | 470 | 84.2 (79.5, 88.0) |
| Hispanic (all races) | ||||||
| HER2+/HR+ | 59 | 87.9 (72.0, 95.1) | 405 | 94.3 (89.7, 96.9) | 1,036 | 94.4 (92.0, 96.1) |
| HER2+/HR− | 21 | 94.1 (65.0, 99.2) | 183 | 84.6 (75.3, 90.7) | 420 | 91.2 (87.1, 94.0) |
| HER2−/HR+ | 163 | 84.6 (74.9, 90.8) | 1,336 | 91.4 (89.1, 93.2) | 4,807 | 95.0 (94.1, 95.8) |
| HER2−/HR− | 85 | 79.1 (66.8, 87.2) | 547 | 77.8 (72.8, 81.9) | 1,033 | 78.7 (75.2, 81.8) |
Abbreviations: CI, confidence interval; HER2, human epidermal growth factor receptor-2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results.
Results for non-Hispanic American Indian/Alaska Native women aged 20–49 years (n=366) and non-Hispanic women of unknown race aged 20–49 years (n=301) were not presented due to sparse totals for these strata.
Figure 2.
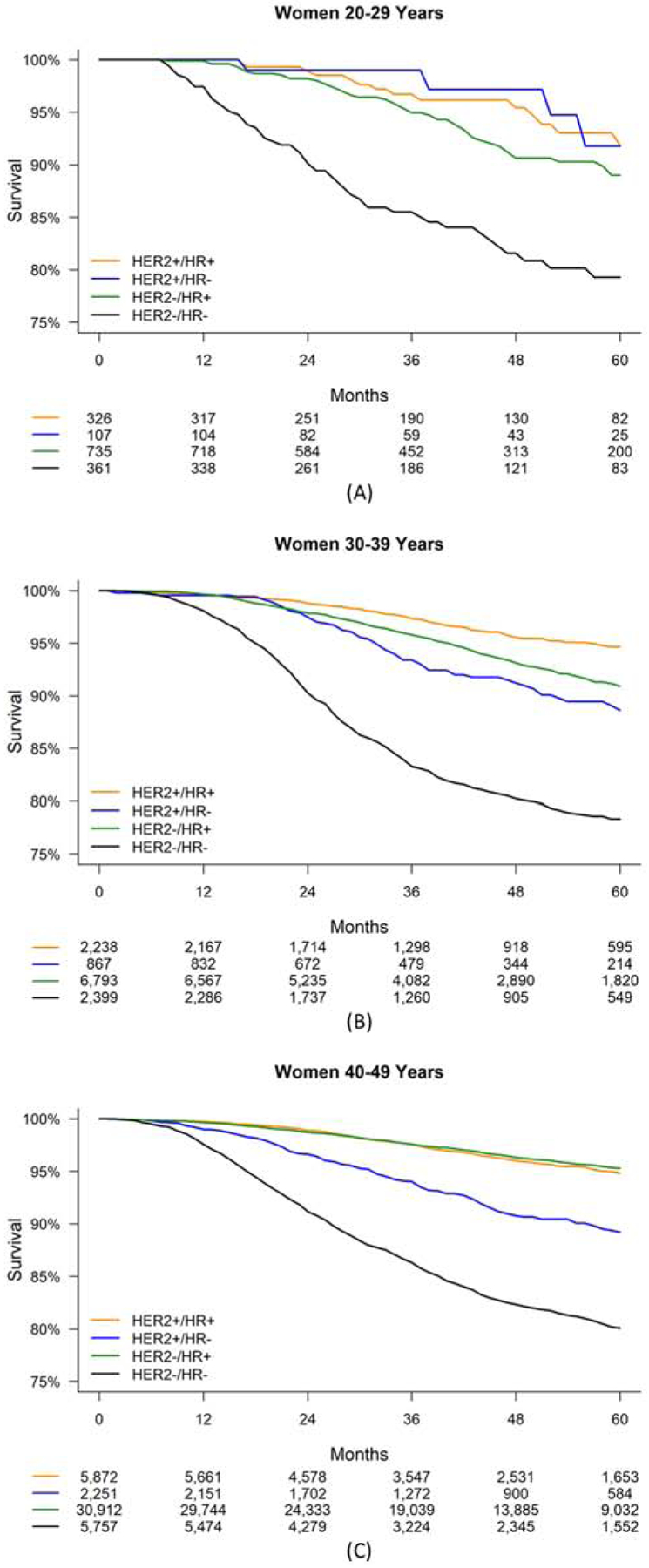
Five-year survival by HER2/HR status for women aged 20–49 years diagnosed with stage I-III breast cancer, 2010–2015, SEER 18 registries. (A) Women aged 20–29 years. (B) Women aged 30–39 years. (C) Women aged 40–49 years.
Abbreviations: HER2, human epidermal growth factor receptor-2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results.
Numbers of patients at risk are given below the x-axis.
The greater positive impact of HER2+ status compared to HR+ status among women 20–29 years was observed for each cancer stage and primarily among Hispanic women, although estimates for this age group were imprecise (Table 3, Supplementary Figure S1). Among women 30–39 years, survival was modestly better with HER2−/HR+ cancer compared to HER2+/HR− cancer; this was primarily observed for stage I cancer and among non-Hispanic Asian/Pacific Islander women and Hispanic women. The greater positive impact of HR+ status compared to HER2+ status among women 40–49 years persisted for each cancer stage and racial/ethnic group.
Comparing survival estimates across age decades, estimates for women with HR+ cancers, particularly HER2−/HR+ cancer, decreased with decreasing age decade (Table 3). Stratifying by cancer stage revealed that this pattern was primarily observed for stage III cancer, although the increase for HER2−/HR+ was also observed for stage II cancer. A similar comparison across age decades stratified by race/ethnicity revealed that the largest increase in survival with increasing age decade for HR+ cancers was among Hispanic women, although this pattern was also observed for non-Hispanic white women (Table 3, Supplementary Figure S1). The median follow-up time for our sample was 42 months. Removing women diagnosed in 2010–2015 with a second primary malignancy from the analytic sample (n=4,079, 7.0%) did not substantively alter findings from our survival analyses (data not shown).
Discussion
Our large, population-based cohort provides insights into contemporary incidence patterns and early survival for premenopausal women with breast cancer by receptor-based subtypes. The incidence of breast cancer increased over the study period for most subtypes in each age decade, remaining stable for HER2−/HR+ among women 20–29 years and for HER2−/HR− among women 30–39 years, and decreasing for HER2−/HR− among women 40–49 years. In particular, HER2+ cancers increased in each age decade, and HER2−/HR− cancer increased among women 20–29 years. Lastly, in this study of women diagnosed from 2010–2016, a period when HER2-directed therapy was available for curative intent, we observed a greater five-year survival benefit for HER2+ receptor status than HR+ receptor status among women 20–29 years, with the opposite pattern observed among women 30–49 years, particularly those 40–49 years.
Our incidence estimates are not directly comparable to previous US studies, as most of these studies examined women either under age 40 or age 50 years as a single age category, and no study reported changes in incidence estimates over time by breast cancer receptor-based subtype.1, 4, 8−10 The overall proportions of HER2+ cancer diagnoses that we observed for women 20–29 and 30–39, however, were intermediate between the proportion (31%) reported for women aged 18–40 years enrolled in the Young Women’s Health Study,8 and the proportion (24%) among patients ≤40 years of age diagnosed at eight NCCN centers.1 These proportions reported were based on patients seen at tertiary care centers1, 8 or those with limited racial/ethnic diversity8 and may not fully reflect the broader population of young women with breast cancer.
Among women with HER2+ cancer, we observed similar proportions of HER2+/HR+ cancer among women 20–29 and 30–39 years to that (17.9%) previously reported among women 15–39 years enumerated by the California Cancer Registry9; however, a sizeable proportion (16.0%) of the total cases in this California cohort had unclassifiable tumors, which well exceeded that observed in our study. Recent data from a study outside the US, the Prospective Outcomes in Sporadic and Hereditary Breast Cancer (POSH) study, reported a somewhat smaller proportion (15.6%) of HER2+/ER+ diagnoses among women <40 years.19 Among women with HER2− cancer, our finding of a higher proportion of HER2−/HR− cancer among women 20–29 and 30–39 years compared to those 40–49 years has been previously reported.1 Also, the previous study that analyzed SEER Program data from 2010–2013 reported a higher proportion of HER2−/HR− cancer in women <50 years compared to those ≥50 years.10
Our findings for incidence cover the full and most contemporary study period for which SEER 18 data include HER2 status. Of note, during this time period, new consensus guidelines on HER2 testing were published,20 which minimally broadened the group of tumors considered to be HER2+.21, 22 This change in HER2 testing interpretation is unlikely to fully account for the observed increased incidence of HER2+ cancer, as these guidelines were only available for the most recent two of the six years we studied and a review of the Herceptin Adjuvant (HERA) trial screening data demonstrated that applying the 2013 guidelines, instead of the 2007 guidelines, led to a 0.7% increase in tumors considered to be HER2+.21
Our use of SEER 18 data also provided an initial look at survival outcomes for women 20–49 years with breast cancer fully in the era of HER2-directed therapy. Our findings are not directly comparable to two recent US registry-based studies, one which examined patients treated at tertiary care centers,1 and the other which classified premenopausal women into a single group <50 years of age.10 Our findings extend understanding of contemporary survival of young women with HER2+ breast cancer beyond that from the HERA trial, which had a two-year median follow-up period due to post-trial patient crossover to trastuzumab.23 Our observation that five-year survival was similar or higher for women with HER2+ cancer compared to HER2− cancer within each strata of HR status contrasted with findings from the POSH study, which suggested poorer distant disease-free and overall survival for women ≤40 years of age with HER2+ cancer compared to those with HER2− cancer for women diagnosed largely in the pre-trastuzumab era.19 Also, a previous study reported that women of all ages with HER2+ cancer treated in the pre-trastuzumab era experienced higher rates of cancer recurrence compared to those with HER2− cancer.24
Several Phase III trials for operable HER2+ and HER2−/HR− breast cancer – subtypes considered to have higher risk of rapid recurrence – have recently reported early overall survival estimates for five-years or longer. For HER2+ breast cancer, the APHINITY trial (n=4,805) reported 6-year overall survival of 93.9% (trastuzumab and placebo) to 94.8% (trastuzumab and pertuzumab).25 For HER2−/HR− breast cancer, the BEATRICE trial (n=2,591) reported five-year overall survival of 88% in both treatment arms (chemotherapy with and without bevacizumab),26 and the CREATE-X trial (n=286), which was conducted in Asia, reported five-year overall survival of 70.3% (chemotherapy without capecitabine) to 78.8% (chemotherapy with capecitabine).27 Comparisons of overall survival between population-based and clinical trial reports are challenging due to differences in cohort make-up and other methodologic differences. Also, these trials did not report overall Kaplan-Meier survival estimates by patient age, therefore our survival estimates are not directly comparable.
Our finding that survival for women with HR+ breast cancer decreased with decreasing age was observed previously.1, 7, 28 Our observation of a greater decrease for those with HER2−/HR+ cancer compared to HER2+/HR+ cancer suggests that either HER2-directed therapy or disease biology may overcome the increased risk associated with young age for HR+ cancers. This finding was suggested clinically by the recent report from the Suppression of Ovarian Function Trial and the Tamoxifen and Exemestane Trial that patients with HER2+/HR+ breast cancer experienced less benefit from ovarian function suppression and exemestane than women with HER2−/HR+ breast cancer.29 These findings may become even more pronounced given the newer HER2-directed therapies being used in the adjuvant treatment setting.30–32 Also, age <45 years has been associated with non-adherence to anti-estrogen therapy,33, 34 which in turn, has been associated with increased mortality.35
A strength of our work is that it examines a large, population-based sample, which represents over one-quarter of the US population. Another strength is that our data are contemporary, including women diagnosed from 2010–2016, well within the period of HER2-directed therapy and widespread use of taxanes. Conversely, our study was limited due to the unavailability of data for neo-adjuvant or adjuvant treatment, in particular for receipt of HER2-directed therapy. Also, due to modest improvements in the rates of classification of cancer stage, HER2 status, and HR status throughout the study period, observed incidence estimates may have been subject to positive classification bias. Additionally, because the SEER 18 registries database did not report HER2 status prior to 2010, our follow-up time was limited to five years, and future study of these data will be needed to fully describe longer-term outcomes in premenopausal women. Lastly, we were unable to report how parity or reproductive health status, which have demonstrated associations with breast cancer in premenopausal women,36–39 might have influenced our incidence and survival results.
Conclusions
In summary, we describe frequencies, incidence patterns, and early survival for a large cohort of premenopausal-aged women diagnosed with stage I-III breast cancer from 2010–2016 by receptor-based subtypes, including HER2. We observed a greater proportion of cancer subtypes were HER2+/HR+ and HER2−/HR− with decreasing age. HER2+ cancer increased in incidence over the study period in each age decade, as did HER2−/HR− among women 20–29 years. HER2+ status was a better predictor than HR+ status of superior early survival among women 20–29 years; HR+ status was the better predictor for women 30–49 years. These contemporary incidence and survival data can be used to help guide preventive and therapeutic strategies aimed at lowering incidence and treatment burden and improving survival for premenopausal women with breast cancer.
Supplementary Material
Clinical Practice Points.
Premenopausal women with breast cancer experience a high risk of disease recurrence and cancer-related death.
Contemporary data on incidence and survival for premenopausal women by human epidermal growth factor receptor-2 (HER2) receptor status are limited.
Understanding contemporary incidence and survival trends is of particular importance given advances in HER2-directed therapy.
Increases in incidence of HER2+ breast cancer were observed among women aged 20–49 years, including both HER2+/hormone receptor (HR)+ and HER2+/HR− subtypes.
Incidence of HER2−/HR− breast cancer also increased among women aged 20–29 years.
Five-year survival among women aged 20–49 years varied by disease subtype, being lowest among those with HER2−/HR− cancer.
Among women aged 20–29 years, HER2+ disease status, rather than HR+ disease status, was more beneficial for five-year survival.
Among women aged 30–49 years, HR+ disease status was more beneficial for five-year survival than HER2+ disease status.
These findings provide important information to counsel young women on early disease outcomes.
Highlights:
Breast cancer incidence and survival were analyzed for 68,530 women aged 20–49.
Incidence of human epidermal growth factor receptor-2 (HER2)+ cancer increased.
Among women 20–29, incidence of HER2−/hormone receptor (HR)− cancer also increased.
Survival was lowest among women with HER2−/HR− cancer.
Women 20–29 benefitted more from HER2+ status, and women 30–49 from HR+ status.
Acknowledgements
This work was supported by grants from the Centers for Disease Control and Prevention (grant numbers U01DD001035, U01DD001223); National Cancer Institute (grant number HHSN2612013000201); and the Nealie Belk Stevens Fund for Breast Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no potential conflicts of interest to disclose. This work was supported by grants from the Centers for Disease Control and Prevention (U01DD001035, U01DD001223), National Cancer Institute (HHSN2612013000201) and the Nealie Belk Stevens Fund for Breast Cancer Research.
References
- 1.Partridge AH, Hughes ME, Warner ET, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol. 2016;34:3308–3314. 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 2.Azim HA Jr., Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–1351. 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 3.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney C, Bernard PS, Factor RE, et al. Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23:714–724. 10.1158/1055-9965.EPI-13-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azim HA Jr., Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16:427 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13:273–279. 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 7.Han W, Kang SY, Korean Breast Cancer S. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010;119:193–200. 10.1007/s10549-009-0388-z. [DOI] [PubMed] [Google Scholar]
- 8.Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–1066. 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 9.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14:R55 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:619–626. 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. [Google Scholar]
- 12.Edge SB, American Joint Committee on Cancer AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl J, Adamo M, Dickie L. (February 2016). SEER Program Coding and Staging Manual 2016: Section V. National Cancer Institute, Bethesda, MD: 20850–9765. [Google Scholar]
- 14.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 8.3.5. [Google Scholar]
- 16.Joinpoint Regression Program, Version 4.6.0.0 - April 2018; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. [Google Scholar]
- 17.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 18.Sun J, Meng H, Yao L, et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin Cancer Res. 2017;23:6113–6119. 10.1158/1078-0432.CCR-16-3227. [DOI] [PubMed] [Google Scholar]
- 19.Copson E, Eccles B, Maishman T, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18–40 years at diagnosis: the POSH study. J Natl Cancer Inst. 2013;105:978–988. 10.1093/jnci/djt134. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 21.Stoss OC, Scheel A, Nagelmeier I, et al. Impact of updated HER2 testing guidelines in breast cancer--re-evaluation of HERA trial fluorescence in situ hybridization data. Mod Pathol. 2015;28:1528–1534. 10.1038/modpathol.2015.112. [DOI] [PubMed] [Google Scholar]
- 22.Press MF, Villalobos I, Santiago A, et al. Assessing the New American Society of Clinical Oncology/College of American Pathologists Guidelines for HER2 Testing by Fluorescence In Situ Hybridization: Experience of an Academic Consultation Practice. Arch Pathol Lab Med. 2016. 10.5858/arpa.2016-0009-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Partridge AH, Gelber S, Piccart-Gebhart MJ, et al. Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol. 2013;31:2692–2698. 10.1200/JCO.2012.44.1956. [DOI] [PubMed] [Google Scholar]
- 24.Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2015;33:65–73. 10.1200/JCO.2014.57.2461. [DOI] [PubMed] [Google Scholar]
- 25.Piccart M, Procter M, Fumagalli D, et al. Interim overall survival analysis of APHINITY (BIG 4–11): A randomized multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer Presentation at: 2019 San Antonio Breast Cancer Symposium. December 10-14, 2019; San Antonio, TX: Abstract GS1–04. [Google Scholar]
- 26.Bell R, Brown J, Parmar M, et al. Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann Oncol. 2017;28:754–760. 10.1093/annonc/mdw665. [DOI] [PubMed] [Google Scholar]
- 27.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376:2147–2159. 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 28.Lian W, Fu F, Lin Y, et al. The Impact of Young Age for Prognosis by Subtype in Women with Early Breast Cancer. Sci Rep. 2017;7:11625 10.1038/s41598-017-10414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis PA, Pagani O, Fleming GF, et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med. 2018;379:122–137. 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377:122–131. 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380:617–628. 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 32.Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 33.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 34.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azim HA Jr., Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 2012;38:834–842. 10.1016/j.ctrv.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Hartman EK, Eslick GD. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat. 2016;160:347–360. 10.1007/s10549-016-3989-3. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen B, Venet D, Azim HA Jr., et al. Breast cancer diagnosed during pregnancy is associated with enrichment of non-silent mutations, mismatch repair deficiency signature and mucin mutations. NPJ Breast Cancer. 2018;4:23 10.1038/s41523-018-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols HB, Schoemaker MJ, Cai J, et al. Breast Cancer Risk After Recent Childbirth: A Pooled Analysis of 15 Prospective Studies. Ann Intern Med. 2019;170:22–30. 10.7326/m18-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


