Abstract
The healthy and diverse microbes living in our gut provide numerous benefits to our health. It is increasingly recognized that the gut microbiome affects the host’s neurobehavioral state through production of metabolites, modulation of intestinal immunity (e.g., cytokines) and other mechanisms (e.g., gut neuropeptides). By sending the sensed information (e.g., metabolic and immunologic mediators) about the state of the inner organs to the brain via afferent fibers, the vagus nerve maintains one of the connections between the brain and GI tract, and oversees many critical bodily functions (e.g., mood, immune response, digestion and heart rate). The microbiome-gut-brain axis is a bidirectional communication between the gut, its microbiome, and the nervous system. In the present review, the roles of microbiome in neuroendocrine and neuroimmune interactions have been discussed using naturally occurring isoflavones, particularly the phytoestrogen genistein, as there are sex differences in the interactions among the microbiome, hormones, immunity and disease susceptibility. A deep understanding of the mechanisms underlying the interactions among the endocrine modulators, brain, endocrine glands, gut immune cells, vagus nerve, enteric nervous system and gut microbiome will provide important knowledges that may ultimately lead to treatment and prevention of debilitating disorders characterized by deficits of microbiome-neuroendocrine-neuroimmune relationships.
Keywords: human/animal microbiome, gut-brain axis, genistein
1. Introduction
It is well known that the gastrointestinal (GI) tract and central nervous system (CNS) interact, and mechanisms underlying the bidirectional gut–brain interactions have gradually been revealed. The microbiome-gut-brain axis is a dynamic interaction among various tissues and organs, including the brain, endocrine glands, gut immune cells, vagus nerve, enteric nervous system and gut microbiome (GMB) that communicate in a multidirectional manner to maintain organism homeostasis. This interaction is now recognized as a regulator of mood, fear, cognition, pain, sleep and behaviors (Chu et al., 2019). The GMB is a dynamic ecosystem formed by thousands of distinct bacterial species in the gut. The first evidence associating GMB disturbances (dysbiosis) and neurobehavioral disorders originated from germ-free mice. These mice exhibited abnormalities in the GI tract as well as the hypothalamic-pituitary-adrenal gland axis by showing more anxieties and fear-associated behaviors, and less exploratory, cognitive and social behaviors (Chu et al., 2019). These deficits could be reversed with bacterial reconstitution or fecal transplantation, suggesting a critical role for GMB in postnatal development of the enteric nervous system. In addition, other experimental paradigms including treatment with antibiotics or pre-/probiotics have demonstrated that GMB influence many facets of CNS physiology e.g., neurotransmitter signaling, synaptic plasticity, myelination and neurogenesis.
Gut houses 70% of the body’s immune system along with 80% of plasma cells (Vighi, Marcucci, Sensi, Di Cara, & Frati, 2008), and intestinal inflammation and imbalance of GMB (dysbiosis) and associated metabolic activities are linked to many diseases, including neurological symptoms (e.g., depression), diabetes and obesity (metabolic), malnutrition and inflammatory bowel disease (IBD; immune) (Day, 2018; Lazar et al., 2019; Valles-Colomer et al., 2019). The objective of this manuscript was to review the roles of GMB in neuroendocrine and neuroimmune interactions by focusing on naturally occurring isoflavones, particularly the phytoestrogen genistein. There are sex differences in the interactions among the GMB, hormones, immunity and disease susceptibility. In addition, there are pathophysiological differences between the microbiome of humans and animals. For example, all rodent guts contain equol-producing bacteria, while only 30–50% of humans harbor such bacteria (Cross et al., 2017). To this end, we have discussed human and lab animal microbiome separately for the phytoestrogen genistein.
2. Possible Pathways in the Microbiome-Gut-Brain Axis
It has been realized a long time ago that intestinal dysfunctions, such as IBD, and psychiatric disease (e.g., depression) might share the same pathogenesis and biological mechanisms, including alterations in the hypothalamic-pituitary-adrenal gland axis mediated by corticotropin releasing factor in response to stress, cytokine secretion and immunological modulations. The microbiome-gut-brain axis is a bidirectional communication between the gut, its microbiome, and the nervous system. The efferent brain-gut signaling includes neuroendocrine, neuroimmune and autonomic regulations (Mayer, Tillisch, & Gupta, 2015). The afferent gut–brain signaling involves the enteroendocrine system, cytokines, sensory epithelial cells, and GMB (Figure 1). Both the vagus nerve and palatine nerve can relay cytokine-induced signals to the brain, and neurotransmitters, such as serotonin, play a key role in the activation of immune cells to produce proinflammatory cytokines (Banks, 2008; Herr, Bode, & Duerschmied, 2017). In addition, cytokines alter the concentrations of several neurotransmitters that regulate the communications in brain, including serotonin, dopamine, and glutamate. Cytokines, together with neurotransmitters and hormones, are critical in the maintenance of neuro-immune-endocrine system homeostasis. Crosstalk among the intestinal epithelium, intestinal immune system and GMB can modulate systemic immunity and affect the interaction between GMB and CNS-restricted immune cells, the microglia (Chu et al., 2019).
Figure 1.
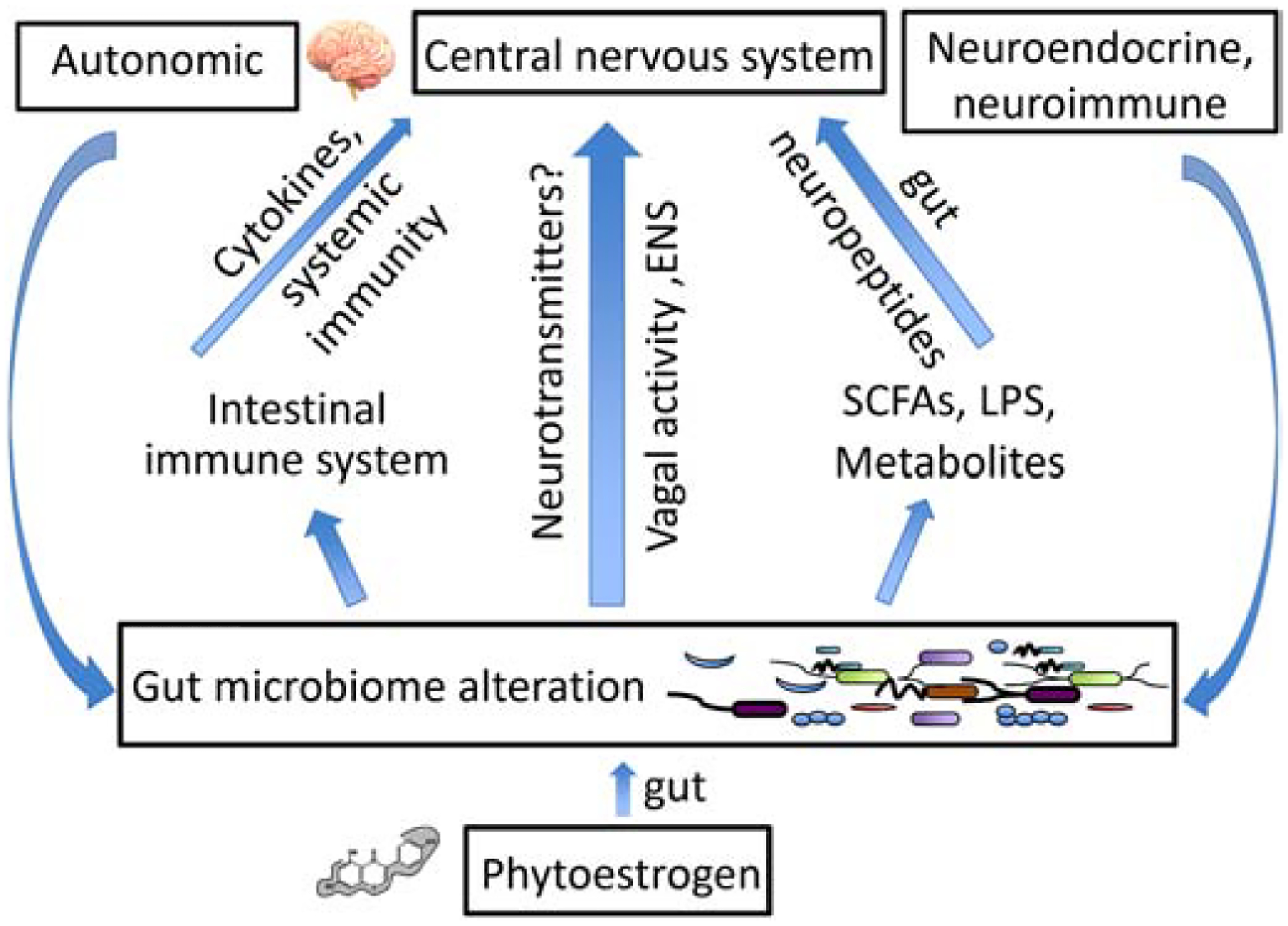
Illustrated are three major ways for gut microbiome to send signals to the brain. (1) Gut microbes may signal the brain through the vagus nerve using neurotransmitters. (2) Gut microbes stimulate gut immune cells to secrete cytokines for brain signaling. (3) The gut-brain communication occurs through metabolites produced by gut microbes. SCFAs = short chain fatty acids, LPS = lipopolysaccharides, ENS = enteric nervous system.
The CNS can alter GMB composition and behavior via the autonomic nervous system, and neuroendocrine and neuroimmune pathways (Wasilewska & Klukowski, 2015). For example, reducing hypothalamic inflammation improves leptin sensitivity (Milanski et al., 2012), which is under GMB control (Cani & Knauf, 2016). The present review focuses more on the enteric-afferent pathways than on the complicated neuro-efferent events. There exist at least three major ways for GMB to send signals to the brain (Figure 1). Firstly, GMB may signal the brain through the vagus nerve, which connects networks of nerves in the gut to various brain regions (e.g., hypothalamus) using neurotransmitters. It is also possible for GMB to stimulate gut immune cells to secrete cytokines that travel to the brain via the bloodstream. The third possible way for gut-brain communication to occur is through metabolites produced by GMB, e.g., short chain fatty acids (SCFAs), which may stimulate enteric neurons and enteroendocrine cells to produce gut neuropeptides. Metabolites such as SCFAs can also travel to the brain through the bloodstream and directly modulate microglia density, morphology and maturity (Erny et al., 2015). However, these three pathways are not mutually exclusive. There exist interactions and overlaps among them that allow for these processes to amplify each other. For example, the vagus nerve has immunomodulatory properties (Breit, Kupferberg, Rogler, & Hasler, 2018), and SCFAs can stimulate free fatty acid receptors in enterochromaffin cells to trigger serotonin biosynthesis (Reigstad et al., 2015).
2.1. Vagus nerve and neurotransmitters in the microbiome-gut-brain axis
The vagus nerve bridges the direct communication between GMB and the brain (Figure 1). In the gut, the sensed information, such as metabolic mediators from GMB and immunologic mediators, is integrated at the vagal nuclei and then transmitted to different brain regions to alter behavioral responses. Furthermore, GMB can alter the neurochemical levels in the vagus nerve. GMB produces a range of neurotransmitters through the metabolism of indigestible fibers (i.e., cellulose, hemicellulose, lignin, pectin and beta-glucans) (Lattimer & Haub, 2010). These include dopamine and noradrenaline by members of the Bacillus family, GABA (γ-aminobutyric acid) by the Bifidobacteria family, serotonin (5-hydroxytryptamine) by the Enterococcus and Streptococcus families, noradrenaline and serotonin by the Escherichia family, and GABA and acetylcholine by the Lactobacilli family (Sarkar et al., 2016). These neurotransmitters stimulate the vagus nerve, and it may in turn alter the activities in hypothalamus and other brain regions. It is also possible that some of these neurotransmitters reach the brain via blood and circumventricular organs. However, more studies are required to determine what are the physiological implications of GMB-mediated alterations of these neurotransmitters, although it has been proposed that some of these neurotransmitters may reach the brain through the vagus nerve (Bonaz, Bazin, & Pellissier, 2018; Klarer et al., 2014).
The expressions of neurotransmitters, such as GABA and serotonin, can be regulated by GMB (Martin et al., 2019; Strandwitz et al., 2019). GABAergic transmission plays a key role in controlling emotional state and participates in the regulation of various psychophysiological phenomena. There are GABA-producing bacteria found in the stool samples from healthy people, e.g., Bacteroides, Parabacteroides and Escherichia species (Strandwitz et al., 2019), and more have been identified from various dietary sources. In patients with depressive disorders, the relative abundance of fecal GABA-producing Bacteroides is decreased and negatively correlates with the depressive signatures in the brain (Strandwitz et al., 2019). In adult male BALB/c mice, administration of the potential psychobiotic Lactobacillus rhamnosus (JB-1) over 28 days lowered the level of stress-induced corticosterone, and decreased the anxiety and depression-like behaviors in the forced swim test (Bravo et al., 2011). At the same time, JB-1-treated mice exhibited region-dependent alterations in the expression of GABAB1b and GABAAα2 mRNAs, which are related to the modulations of memory and anti-depression, respectively (Bravo et al., 2011). Further studies in vagotomized mice did not show either behavioral or neurochemical changes in the same tests, suggesting an indispensable role of the vagus nerve in the communication between JB-1 and brain via regulating inhibitory neurotransmitter GABA (Bravo et al., 2011).
Serotonin has been used in the form of drugs and nutraceuticals for vagus nerve stimulation and for sleep and feelings of well-being. However, about 95% of the body’s serotonin locates in the gut but not in the brain (Fung et al., 2019). Germ-free mice display lower serotonin levels in cecum and colon, and lower percentage of unconjugated serotonin (bioactive form) than the germ-free mice recolonized with specific pathogen-free fecal flora (Hata et al., 2017). One possible explanation is that some bacterial species, such as lactic acid bacteria (e.g., Streptoccocus thermophilus) and E. coli, produce serotonin. In addition, indigenous spore-forming bacteria from GMB promote the serotonin biosynthesis in enterochromaffin cells through secreting metabolites, e.g., α-tocopherol, butyrate, cholate, deoxycholate, p-aminobenzoate, propionate and tyramine (Yano et al., 2015). In spite of a plethora of information showing that serotonin is vital for emotional and basic physiological functions, and that GMB can regulate the serotonin levels, comprehensive evidence is missing to directly link GMB-regulated serotonin level to emotion and behavior. It is also unclear whether the vagus nerve is mediating this communication.
2.2. Cytokines and gut neuropeptides in the microbiome-gut-brain axis
Although the interactions between GMB and intestinal immune system (Figure 2) have been well studied (Guo et al., 2018), the interplays among cytokine production, neuroendocrine regulation and GMB have not been investigated extensively. In a study to determine the effect of probiotics on chronic stress induced by maternal separation during perinatal stages in C57BL/6 mice, the introduction of the potential probiotic Bifidobacterium pseudocatenulatum CECT 7765 downregulated maternal separation-induced intestinal inflammation by reducing IFN-γ and intestinal hypercatecholaminergic activity, e.g., dopamine and adrenaline, at postnatal day 21 (Moya-Perez, Perez-Villalba, Benitez-Paez, Campillo, & Sanz, 2017). In a rat study, modulation of GMB by a multi-species probiotic treatment significantly reduced depressive-like behavior by 34% in the forced swim test, and altered the cytokine production by the stimulated blood mononuclear cells towards IFN-γ, IL-2 and IL-4 at the expense of TNF-α and IL-6 (Abildgaard, Elfving, Hokland, Wegener, & Lund, 2017). Interestingly, the probiotic use lowered the transcript levels of factors involved in the regulation of hypothalamic-pituitary-adrenal gland axis, including corticotropin releasing hormone receptor-1, −2 and mineralocorticoid in hippocampus (Abildgaard et al., 2017). In a mechanistic study, it was demonstrated that depression was associated with a decreased GMB richness (Kelly et al., 2016): Fecal microbiota transplantation from depressed patients to microbiota-depleted rats induced behavioral and physiological features related to depression in the recipient animals, including anhedonia and anxiety-like behaviors, as well as alterations in tryptophan metabolism. In addition, the depressed rats showed an elevated IL-8 and TNF-α (Kelly et al., 2016).
Figure 2.
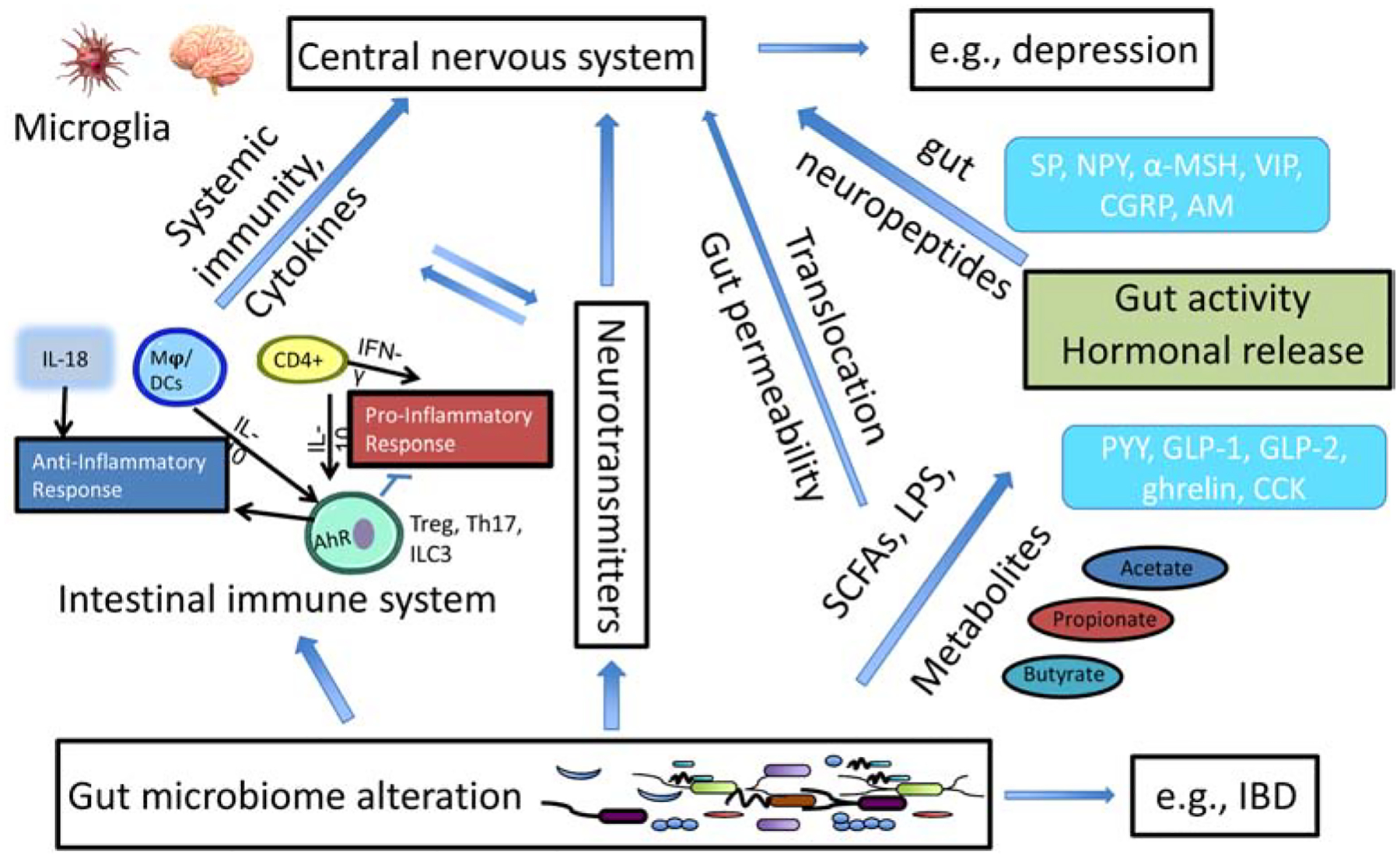
Interactions and overlaps among the three pathways described in Figure 1. AM = adrenomedullin, α-MSH = α-melanocyte stimulating hormone, CCK = cholecystokinin, CGRP = calcitonin gene-related peptide, GLP = glucagon-like peptide, ILC3 = type 3 innate lymphoid cells, NPY = neuropeptide Y, PYY = peptide YY, SP = substance P, Treg = regulatory T cells, VIP = vasoactive intestinal peptide, Mφ = macrophage, DCs = dendritic cells, IBD = inflammatory bowel disease.
In addition to immune mediators, gut neuropeptides originated from enteric neurons and enteroendocrine cells can serve as a mediator between GMB and host (Figure 2). Common gut neuropeptides include substance P, neuropeptide Y, α-melanocyte stimulating hormone, vasoactive intestinal peptide, calcitonin gene-related peptide and adrenomedullin, and they are likely to play important roles in the bidirectional gut-brain communication. The function of gut neuropeptide-releasing enteroendocrine cells is directly influenced by metabolites (e.g., SCFAs) generated by GMB from indigestible fiber, and gut neuropeptides may control the impact of GMB on inflammatory processes, pain, brain function and behavior. The effects of gut neuropeptides on GMB can be direct or indirect when the stimuli are sensed. Gut neuropeptides can cross the epithelial barrier and exert antimicrobial activity in the gut lumen by different mechanisms (direct) or induce immune responses (innate or adaptive), and subsequently result in microbial imbalance (indirect) (Aresti Sanz & El Aidy, 2019).
In the CNS, the microglia are the innate sentinel immune cells that can detect subtle changes in molecules in their locality. The proper functioning of microglia in brain regions (e.g., the hypothalamus) is critical for maintaining brain health and regulating metabolism (Figure 2). When activated, they perform functions such as removing damaged cells at a site of injury. A critical role for GMB in microglia maturation, morphology and immunological function has been shown (Erny et al., 2015), and a healthy and diverse GMB is essential for the continuous preservation of healthy microglia and proper brain function throughout host lifespans. Furthermore, it has also become clear that microglia have a crucial role in synaptic connectivity. By engulfing and degrading unwanted synapses, microglia can ensure that neuronal connections are pruned or maintained as needed, which have been shown to be critical for fear extinction (Chu et al., 2019). In addition, there exist a neuroimmune circuit involving microglia activation and an altered sympathetic neural tone to the peripheral immune system to recruit inflammatory monocytes to the brain (Wohleb, Mckim, Sheridan, & Godbout, 2015). Taken together, GMB closely interact with the body’s major neuroendocrine and neuroimmune systems that control various physiological processes in response to stress, metabolic dysfunction and infections (Figure 2).
2.3. Bacterial-derived metabolites in the microbiome-gut-brain axis
The microbial metabolism is seen as a complement to the host metabolism. Dietary metabolites derived from GMB play a critical role in the regulation of multiple neural behaviors (e.g., anxiety, depression) through the microbiome-gut-brain axis, and gut dysbiosis favoring pro-inflammatory microbial communities precedes depression development (Macedo et al., 2017). GMB can metabolize dietary compounds into metabolites (e.g., phenolic acids) with important biological activities. These small, gut-derived metabolites may be responsible for the health benefits of diets high in fruits and vegetables. Nuclear magnetic resonance and liquid chromatography-mass spectrometry-based metabolomic studies have shown that microbial metabolites are often the compounds most markedly altered in the disease state when compared to healthy individuals (L. S. Zhang & Davies, 2016). Importantly, many studies suggest that these metabolites may be effective anxiolytic, antidepressant, and/or anti-inflammatory agents. Furthermore, these metabolites may exert their biological effects using various pathways simultaneously rather than acting through a single mechanism (e.g., biological signature; Figure 2). Although GMB is highly variable, the summation of genomes composing it tends to be quite conserved when considering the microbial metabolic pathways.
SCFAs such as acetate (C2), propionate (C3), butyrate (C4) and pentanoate (valerate, C5) are mainly produced by bacterial fermentation of dietary fiber or glycosylated host proteins such as mucins in the colon. Bacteroidetes (gram-negative) and Firmicutes (gram-positive) are the most abundant phyla in the intestine, with members of the Bacteroidetes mainly producing acetate and propionate, while Firmicutes mostly produce butyrate in the human gut (Parada Venegas et al., 2019). SCFAs are not only able to protect host from mucosal inflammation and colorectal tumorigenesis, but may also act in a systemic manner to ameliorate T cell-driven autoimmunity in the brain (Luu et al., 2019). Systemic sodium butyrate injections in rats produce antidepressant effects, and increase central serotonin neurotransmission and brain-derived neurotrophic factor expression (Sun et al., 2016). Dysbiosis in patients with multiple sclerosis, an autoimmune disease affecting the CNS, is characterized by a reduction of species belonging to Clostridia XIVa and IV clusters (Miyake et al., 2018). These species produce SCFAs by the fermentation of soluble fiber contained in the diet. However, unfavorable health effects of SCFAs have also been described. For example, butyrate has been shown to act on the locus of enterocyte effacement pathogenicity island of enterohemorrhagic E. coli, which enables this pathogen to efficiently colonize the host epithelium (Luu et al., 2019).
Children, especially newborns and fetuses, are more sensitive to environmental toxicants compared to adults (Kamai, McElrath, & Ferguson, 2019). There is a clear association between alterations of GMB and metabolites in children and the risk of developing depression in adulthood (Frye et al., 2015; Petra et al., 2015). In the fetus, early-life gut bacterial colonization plays an important role in metabolic tissue development and in influencing the risk of immune related diseases because intestinal immune system development starts as early as 11 weeks of gestation in humans (Romano-Keeler & Weitkamp, 2015; Younge et al., 2019). Modulation of GMB by probiotics (Lactobacillus rhamnosus or Bifidobacterium lactis) during pregnancy alters infant immune responses (Prescott et al., 2008). The mode of delivery, antibiotic use after birth and infant formula consumption could all help shape the infant GMB and further modulate the immune system.
3. Phytoestrogens and the Gut-Brain Axis
The complex symbiotic interaction between GMB species can be perturbed by endocrine modulators. Furthermore, GMB can interact with the endocrine modulators by altering their processes of absorption, disposition, metabolism and excretion (Lai et al., 2018). Dietary isoflavones, especially the phytoestrogen genistein (GEN, Formula: C15H10O5, CAS ID: 446-72-0), have been proposed as possible preventive or complementary medicines for depression, and they might improve the overall quality of life and decrease self-rating depression scores (Atteritano et al., 2014). The widely used dietary supplement GEN has been explored for its potential effects in cognitive function, cancer therapy, and bone and cardiovascular health. GEN presents as glycosides (genistin; Formula : C21H20O10, CAS Number : 529-59-9; Figure 3) in intact soybeans. Orally administered glycoside form is hydrolyzed by β-glucosidase to aglycones in the GI tract. The aglycone form is either absorbed intact or further metabolized by GMB. Only a small fraction of dietary GEN is absorbed in the small intestine, and large proportions of that reach the colon where they undergo modifications by GMB. It has been estimated that at least 30% of metabolites have a bacterial origin. GEN and gut microbe-derived GEN metabolites (MGMs) can interact with estrogen receptors (ERs), and function as either antagonist or agonist depending on the estrogen level (Hwang et al., 2006). GEN is first converted by GMB to dihydrogenistein (Formula : C15H12O5, CAS Number 21554-71-2; Figure 3), which can bind to ERs and exert biological effects (e.g., antioxidative). Dihydrogenistein has been detected in human urine and plasma at high concentrations, which may act as bioactive component of GEN (Kobayashi, Shinohara, Nagai, & Konishi, 2013). Dihydrogenistein is further metabolized to 6’-hydroxy-O-desmethylangolensin (C15H14O5), with unknown health effects, through absorption and enterohepatic circulation (Kobayashi et al., 2013). A peak with molecular weight (257.0819 g/mol) identical to 5-hydroxy-equol was also found, suggesting that the production of this compound could be more common than equol. Interestingly, 5-hydroxy-equol showed an antioxidant activity superior to that of GEN (Gaya, Medina, Sanchez-Jimenez, & Landete, 2016). The 5-hydroxy-equol is also expected to bind to ERs, preferably to ER-β. Complete cleavage of the C-ring can also produce 2,4,6-trihydroxybenzoic acid and p-ethyl phenol. C-ring fission may also generate 2-(4-hydroxyphenyl)-propionic acid and trihydroxybenzene (Figure 3).
Figure 3.
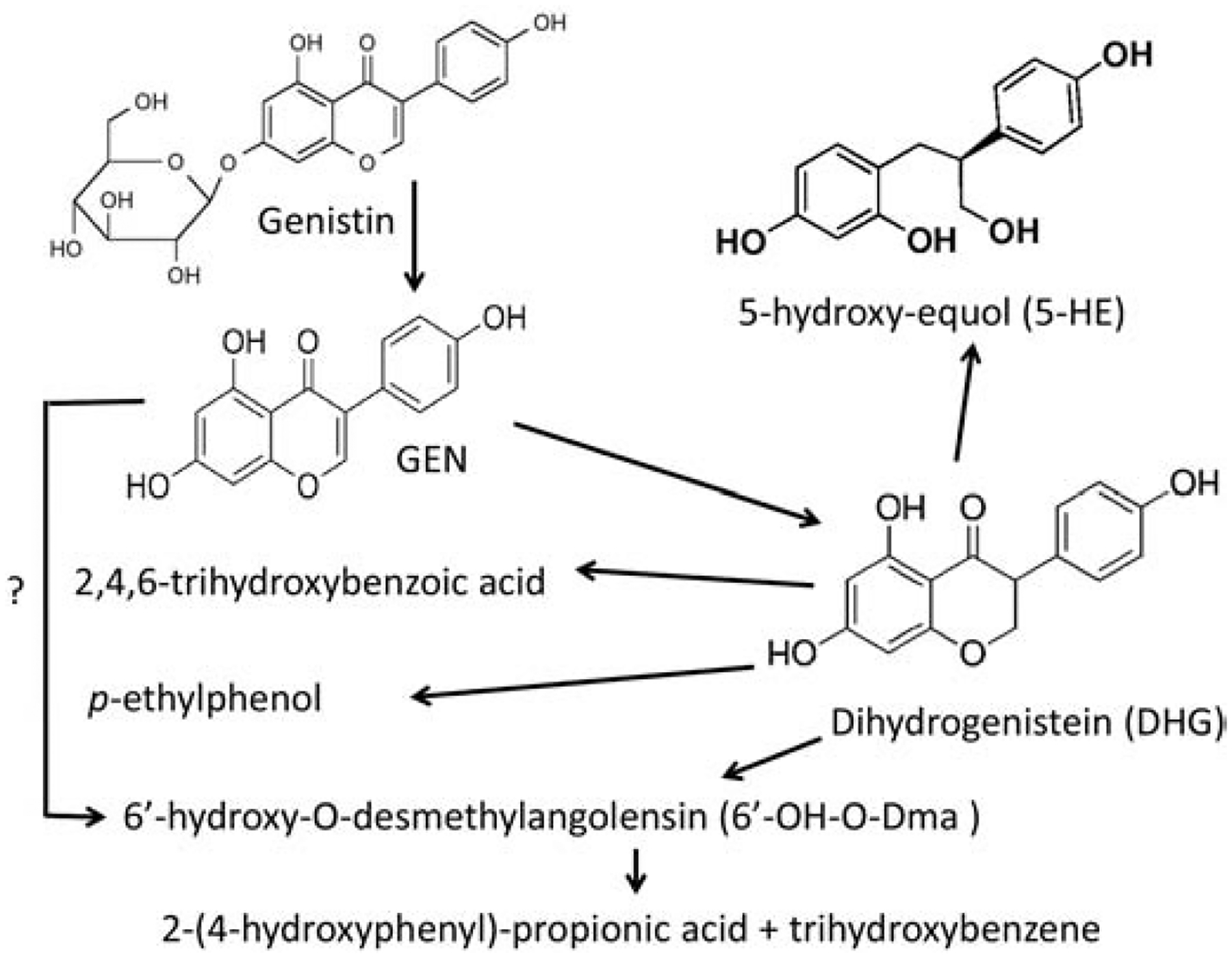
Possible gut microbe-derived GEN metabolites.
The actions of GEN have been studied for more than 20 years, and a great deal has been learned, but the research up to date has not led to the significant clinical successes. More than 30 clinical trials of GEN with various disease indications have been conducted to evaluate its clinical efficacy, and ambiguous therapeutic effects and large interindividual variations have been observed (Yang, Kulkarni, Zhu, & Hu, 2012). The discrepancy between clinical studies of GEN could be attributed to a failure to distinguish between MGM producers and nonproducers in the metabolism of GEN and sex difference in GMB (Vemuri et al., 2019). The solubility of GEN in water and most aqueous buffers is low, e.g., 0.9 μg GEN/ml in water, and the oral availability of GEN is only 23.4% for the dose administered (Yang et al., 2012). In contrast, various MGMs have been detected in plasma and urine. These MGMs are more bioavailable than GEN per se, and they have increased biological activities, e.g., estrogenic or antiestrogenic, antioxidant, anti-inflammatory, antiproliferative and apoptosis-inducing (Gaya et al., 2016). The clinical effectiveness of GEN in depression may be attributed to MGMs. On one hand, GMB that is altered through GEN intake modulates active estrogen in the serum by secreting β-glucuronidase that deconjugates estrogen (Plottel & Blaser, 2011). On the other hand, the metabolites of isoflavones and estrogen from GMB can modulate the immune responses. They may be transmitted through the vagal nerve or systemic circulation to affect neural function. As the gut-associated lymphoid tissue represented 70% of entire immune system, the mechanism of GEN affecting GMB also needs to be studied further from the perspective of metabolome. In addition, as an endocrine disrupting chemical, GEN has been linked to some detrimental health effects, especially during developmental exposure (discussed later). For example, mice treated neonatally with GEN developed cancer of the uterus later in life (Newbold, Banks, Bullock, & Jefferson, 2001). Therefore, understanding the mechanisms underlying GEN’s beneficial and detrimental actions (e.g., depending on the dose and windows of exposure) will help form a more targeted therapy that have fewer side effects.
Human microbiome studies - Adult exposure.
Because limited human studies are available specifically for GEN, this section has considered both in vivo and in vitro gut microbial profile changes following either GEN exposure or soy consumption (Table S1). Soy intake can modulate GMB, estrogen metabolism and immunity. Isoflavone administration in the human GMB-associated mice led to a significant increase in fecal Clostridia (Tamura, 2004), and modified numbers of key bacterial species in the gut in vitro (Vazquez, Florez, Guadamuro, & Mayo, 2017). By culturing human feces in reactor vessels and introducing soy powder upon stabilization, an increase of several bacterial strains (Lactobacillus sp.) together with a 30% increase of SCFAs were found (De Boever, Deplancke, & Verstraete, 2000). In postmenopausal women, supplementation of isoflavones aglycon stimulated dominant microorganisms of the Clostridium coccoides-Eubacterium rectale cluster, Lactobacillus-Enterococcus group, Faecalibacterium prausnitzii subgroup and Bifidobacterium genus (Bolca et al., 2007; Clavel et al., 2005). Similarly, a week of diet supplementation with soy bars containing isoflavones (160 mg soy isoflavones/day) significantly increased Bifidobacterium (Nakatsu et al., 2014). In overweight and obese men, consuming soymilk altered the microbiome including a potentially beneficial alteration of the Firmicutes to Bacteroidetes ratio (Fernandez-Raudales et al., 2012). In athymic nude mice transplanted with human microbiome, GEN at the dose of 0.25 g/kg modulates the microbiome and contributes to its effects on increasing the latency of breast tumor and reducing tumor growth (Paul et al., 2017). Thus, adult exposure to GEN seemed to produce an overall beneficial effect.
Human microbiome studies - Developmental exposure (Table S1).
In humans, a critical period influencing lifelong health is the period from conception to 24 months (the first 1000 days) when GMB composition and eating patterns are established (Schwarzenberg, Georgieff, & Committee On, 2018). Infants who had their cow’s milk-based formula replaced with soymilk were associated with a decrease in the intestinal bifidobacterial population (Piacentini, Peroni, Bessi, & Morelli, 2010). A study in Australian children of 2 to 3 years old found soy intake was positively associated with the relative abundance of bacteria related to Bacteroides xylanisolvens (Smith-Brown, Morrison, Krause, & Davies, 2016). A cross-sectional study found that the urinary concentration of soy isoflavone GEN in infants consuming soy-based formula was 500 times higher than in those consuming cow’s milk-based formula (Cao et al., 2009). However, human studies have shown that twice as many children with type 1 diabetes consumed soy-based formula in infancy as compared to controls (Fort et al., 1986; Strotmeyer et al., 2004). In addition, soy milk formula consumption during infancy was associated with a significant increase in the use of asthma or allergy drugs in young women (Strom et al., 2001), and possible increases in autistic behaviors (Westmark, 2013). These conditions are associated with an overactive immune system, suggesting that GEN might have some adverse effects on children, especially newborns and fetuses.
Lab animal microbiome studies - Adult exposure (Table S2).
It was reported that soy milk could rescue cholesterol-disturbed GMB in male Sprague–Dawley rats (S. M. Lee, Han, & Yim, 2015), which was supported by additional four studies: (1) Soy protein isolate modulated the effects of prebiotic oligosaccharides on gut fermentation and microbiota in female Wistar rats (Bai, Ni, Tsuruta, & Nishino, 2016), (2) Dietary soy exerts a beneficial shift in gut microbial communities in ovariectomized rats with low-running capacity (Cross et al., 2017), (3) Soy exposure resulted in a lower Firmicutes:Bacteroidetes ratio in ovariectomized rats with low-running capacity (Vieira-Potter et al., 2018), and (4) In male BALB/c mice, Odoribacter (Bacteroidales family), Lactobacillus (Lactobacillales order), and Alistipes (Rikenellaceae family) were enriched in soymilk while bacterial taxa from Bacteroides and Lactobacillus were enriched in L. rhamnosus-fermented soymilk (Dai et al., 2019). For phytoestrogen GEN, it was shown that GMB alteration by ovariectomy may affect GEN bioavailability in C57BL/6 mice (D. H. Lee et al., 2017). Our in vivo studies showed that GEN could modulate GMB and immune homeostasis in adult non-obese diabetic (NOD) mice (Huang et al., 2017). In NOD male mice, it was found that GEN treatment during adulthood induced decreases in GM-CSF (75.6%), IFN-γ (22.9%), IL-5 (36.3%), IL-10 (45.3%), and MCP-1 (72.2%), suggesting an anti-inflammatory effect (Huang et al., 2017). These cytokines/chemokines have a strong association with depressive responses by interacting with GMB. When the composition of GMB at the genus level was compared, GEN treatment induced an increased Prevotella, and a decreased Alistipes and Blautia in terms of relative abundance, suggesting an anti-inflammatory response (Huang et al., 2017). Prevotella can also help maximize energy harvest from a plant-based diet (Y. J. Zhang et al., 2015), while Alistipes show a significant association with depressive symptoms as they are overly represented in patients with depression (Jiang et al., 2015; Naseribafrouei et al., 2014). Overall, consistent with human studies, adult exposure to GEN produced beneficial effects in lab animals (López et al., 2018).
Lab animal microbiome studies - Developmental exposure (Table S2).
In 21-day old DF508 mice, exposure to GEN at the dose of 600 mg/kg resulted in a lower within-sample diversity and significant differences in beta diversity when compared to control (Corrie Whisner, 2019). In California mice (Peromyscus californicus), early GEN exposure disrupted normal socio-communicative behaviors, which might be due to GEN-induced microbiota shifts and resultant changes in gut metabolites but might also be attributed to GEN disruptions on neural programming (Marshall et al., 2019). However, early-life GEN intake could also attenuate the harmful effects of maternal high fat diet in adult offspring, and the protective effects were associated with the alterations in GMB (Zhou, Xiao, Zhang, Zheng, & Deng, 2019). Our studies have also shown significant alterations of immune responses in mouse offspring exposed to GEN during in utero and lactation (Guo, Auttachoat, & Chi, 2005; Guo, Chi, Germolec, & White, 2005; Huang et al., 2018). In NOD mice, we have found that the effect of GEN in type 1 diabetes depended on sex and windows of exposure: perinatal exposure produced an exacerbation of type 1 diabetes in females and an anti-inflammatory effect in males (Huang et al., 2018), while adult exposure exerted a protection of type 1 diabetes in both sexes (Guo et al., 2015; Huang et al., 2017). RNA-seq analysis of gene expression in the ileum tissues in perinatal GEN-treated female offspring showed that intestinal α-defensin expression was decreased by 70% (Huang et al., 2018). Importantly, a case-control study using whole-genome copy number analysis showed that decreased dosage of defensin was a predisposing factor to idiopathic autism spectrum disorder (Cho et al., 2009).
Defensins, whose expression is modulated by estrogen, are 2–6 kDa, cationic, antibacterial peptides active against many Gram-negative and Gram-positive bacteria. We conducted GMB analysis in NOD offspring exposed to GEN during in utero and lactation and found that GMB from postnatal day (PND) 90 female offspring was significantly altered following perinatal GEN exposure with an increased level of Enterobacteriales (Genus), suggesting a pro-inflammatory response. Some members of the Enterobacteriaceae (e.g., E. coli) produce endotoxins that are an etiopathogenic agent of type 1 diabetes. In the NOD ileum, E. coli was the sole bacterium correlating with the insulitis score (Sane et al., 2018). Significantly, higher levels of Enterobacteriaceae genera and species were found in children with autism than healthy children (De Angelis et al., 2013). Moreover, compared with healthy subjects, serum levels of endotoxin were significantly higher in autistic patients, and inversely and independently correlated with socialization scores on the Vineland Adaptive Behavior Scales (VABS) and ADI-R Domain A score (social) (Emanuele et al., 2010). In addition, animal studies have shown that early exposure to GEN can lead to altered brain development and behavioral abnormalities (Ponti et al., 2017), and girls exposed during infancy to soy formula show reduced female-typical play behavior (Adgent, Daniels, Edwards, Siega-Riz, & Rogan, 2011). Taken together, developmental exposure to GEN might be harmful, which is dependent on sex.
4. Conclusions and Future Directions
The fundamental question driving the GMB field is - how do differences in microbial composition among individuals (i.e., interindividual diversity) affect human health and disease? It has been suggested that there are likely four major patterns of interindividual variability in GMB functions (Rosen & Palm, 2017). Type I functions show very low interindividual variability among humans. Type II functions are normally distributed among the population but show a wider range of variability, and the host can tolerate a broader range of activity. Type III functions are present in the majority of humans but absent in a small population. Type IV functions are present in a minority of individuals and absent in the majority. Future studies should focus on categorizing GMB and metabolite functions by type, which may not only help delineate the roles of specific microbial activities, or even microbes themselves, in health and diseases, but also act as a guide for which functions should be targeted therapeutically. A better understanding of the roles of GMB in neuroendocrine and neuroimmune interactions following exposure to endocrine modulating chemicals will permit individuals seeking dietary supplements to make better-educated choices. This is especially true for IBD, a chronic recurrent inflammatory disease in which sex hormones play an important role in its prevalence (Shah et al., 2018). If GMB contributes to neuroendocrine and neuroimmune interactions, therapeutics including prebiotics, probiotics, fecal transplants, isoflavone supplements or remediation strategies may be designed to induce/prevent such GMB alterations and thereby improve neurobehavioral outcomes.
Supplementary Material
Acknowledgments
This study was supported in part by NIH R21ES24487, NIH R41AT009523, USDA National Institute of Food and Agriculture [grant no. 2016-67021-24994/project accession no. 1009090] and R41DK121553.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests:
There are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abildgaard A, Elfving B, Hokland M, Wegener G, & Lund S (2017). Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology, 79, 40–48. doi: 10.1016/j.psyneuen.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Adgent MA, Daniels JL, Edwards LJ, Siega-Riz AM, & Rogan WJ (2011). Early-life soy exposure and gender-role play behavior in children. Environ Health Perspect, 119(12), 1811–1816. doi: 10.1289/ehp.1103579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aresti Sanz J, & El Aidy S (2019). Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Psychopharmacology (Berl), 236(5), 1597–1609. doi: 10.1007/s00213-019-05224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atteritano M, Mazzaferro S, Bitto A, Cannata ML, D’Anna R, Squadrito F, … Bagnato G (2014). Genistein effects on quality of life and depression symptoms in osteopenic postmenopausal women: a 2-year randomized, double-blind, controlled study. Osteoporos Int, 25(3), 1123–1129. doi: 10.1007/s00198-013-2512-5 [DOI] [PubMed] [Google Scholar]
- Bai G, Ni K, Tsuruta T, & Nishino N (2016). Dietary Casein and Soy Protein Isolate Modulate the Effects of Raffinose and Fructooligosaccharides on the Composition and Fermentation of Gut Microbiota in Rats. J Food Sci, 81(8), H2093–2098. doi: 10.1111/1750-3841.13391 [DOI] [PubMed] [Google Scholar]
- Banks WA (2008). The blood-brain barrier: connecting the gut and the brain. Regul Pept, 149(1–3), 11–14. doi: 10.1016/j.regpep.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, … Van de Wiele T (2007). Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr, 137(10), 2242–2246. doi: 10.1093/jn/137.10.2242 [DOI] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, & Pellissier S (2018). The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Frontiers in Neuroscience, 12 10.3389/fnins.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, … Cryan JF (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A, 108(38), 16050–16055. doi: 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, & Hasler G (2018). Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Frontiers in Psychiatry, 9, 44. doi: 10.3389/fpsyt.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, & Knauf C (2016). How gut microbes talk to organs: The role of endocrine and nervous routes. Mol Metab, 5(9), 743–752. doi: 10.1016/j.molmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, … Rogan WJ (2009). Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol, 19(2), 223–234. doi: 10.1038/jes.2008.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SC, Yim SH, Yoo HK, Kim MY, Jung GY, Shin GW, …. Chung YJ (2009). Copy number variations associated with idiopathic autism identified by whole-genome microarray-based comparative genomic hybridization. Psychiatr Genet, 19(4), 177–185. doi: 10.1097/YPG.0b013e32832bdafa [DOI] [PubMed] [Google Scholar]
- Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, … Artis D (2019). The microbiota regulate neuronal function and fear extinction learning. Nature, 574(7779), 543–548. doi: 10.1038/s41586-019-1644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel T, Fallani M, Lepage P, Levenez F, Mathey J, Rochet V, … Coxam V (2005). Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J Nutr, 135(12), 2786–2792. doi: 10.1093/jn/135.12.2786 [DOI] [PubMed] [Google Scholar]
- Corrie Whisner KA, Karen Sweazea, and Layla Al-Nakkash. (2019). Impacts of Genistein on the Gut Microbiota of a Mouse Model of Cystic Fibrosis. Current Developments in Nutrition, 3, P06-070-019. [Google Scholar]
- Cross TL, Zidon TM, Welly RJ, Park YM, Britton SL, Koch LG, … Vieira-Potter VJ (2017). Soy Improves Cardiometabolic Health and Cecal Microbiota in Female Low-Fit Rats. Sci Rep, 7(1), 9261. doi: 10.1038/s41598-017-08965-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Pan M, El-Nezami HS, Wan JMF, Wang MF, Habimana O, … Shah NP (2019). Effects of Lactic Acid Bacteria-Fermented Soymilk on Isoflavone Metabolites and Short-Chain Fatty Acids Excretion and Their Modulating Effects on Gut Microbiota. J Food Sci, 84(7), 1854–1863. doi: 10.1111/1750-3841.14661 [DOI] [PubMed] [Google Scholar]
- Day AS (2018). The impact of exclusive enteral nutrition on the intestinal microbiota in inflammatory bowel disease. AIMS Microbiol, 4(4), 584–593. doi: 10.3934/microbiol.2018.4.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, … Francavilla R (2013). Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS One, 8(10). 10.1371/journal.pone.0076993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever P, Deplancke B, & Verstraete W (2000). Fermentation by gut microbiota cultured in a simulator of the human intestinal microbial ecosystem is improved by supplementing a soygerm powder. Journal of Nutrition, 130(10), 2599–2606. Retrieved from <Go to ISI>://WOS:000089728200032 [DOI] [PubMed] [Google Scholar]
- Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, … Politi P (2010). Low-grade endotoxemia in patients with severe autism. Neurosci Lett, 471(3), 162–165. doi: 10.1016/j.neulet.2010.01.033 [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, … Prinz M (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci, 18(7), 965–977. doi: 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Raudales D, Hoeflinger JL, Bringe NA, Cox SB, Dowd SE, Miller MJ, & Gonzalez de Mejia E (2012). Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microbes, 3(6), 490–500. doi: 10.4161/gmic.21578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P, Lanes R, Dahlem S, Recker B, Weyman-Daum M, Pugliese M, & Lifshitz F (1986). Breast feeding and insulin-dependent diabetes mellitus in children. J Am Coll Nutr, 5(5), 439–441. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3155358 [DOI] [PubMed] [Google Scholar]
- Frye RE, Slattery J, MacFabe DF, Allen-Vercoe E, Parker W, Rodakis J, … Midtvedt T (2015). Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis, 26, 26878. doi: 10.3402/mehd.v26.26878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, … Hsiao EY (2019). Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol, 4(12), 2064–2073. doi: 10.1038/s41564-019-0540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaya P, Medina M, Sanchez-Jimenez A, & Landete JM (2016). Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules, 21(8). doi: 10.3390/molecules21081034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Auttachoat W, & Chi RP (2005). Genistein enhancement of respiratory allergen trimellitic anhydride-induced IgE production by adult B6C3F1 mice following in utero and postnatal exposure. Toxicol Sci, 87(2), 399–408. doi: 10.1093/toxsci/kfi268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Chi RP, Germolec DR, & White KL Jr. (2005). Stimulation of the immune response in B6C3F1 mice by genistein is affected by exposure duration, gender, and litter order. Journal of Nutrition, 135(10), 2449–2456. doi: 10.1093/jn/135.10.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Germolec DR, Zheng JF, Kooistra L, Auttachoat W, Smith MJ, … Elmore SA (2015). Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol Pathol, 43(3), 435–448. doi: 10.1177/0192623314526318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Xu J, Chen Y, Lefever D, Huang G, & Lawrence DA (2018). Molecular Mechanisms of Immunotoxicity In Robert C Smart EH (Ed.), Molecular and biochemical toxicology (Fifth ed. ed., pp. 773): John Wiley & Sons, Inc. [Google Scholar]
- Hata T, Asano Y, Yoshihara K, Kimura-Todani T, Miyata N, Zhang XT, … Sudo N (2017). Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One, 12(7), e0180745. doi: 10.1371/journal.pone.0180745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr N, Bode C, & Duerschmied D (2017). The Effects of Serotonin in Immune Cells. Front Cardiovasc Med, 4, 48. doi: 10.3389/fcvm.2017.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Xu J, Cai D, Chen SY, Nagy T, & Guo TL (2018). Exacerbation of Type 1 Diabetes in Perinatally Genistein Exposed Female Non-Obese Diabetic (NOD) Mouse Is Associated With Alterations of Gut Microbiota and Immune Homeostasis. Toxicol Sci, 165(2), 291–301. doi: 10.1093/toxsci/kfy162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, & Guo TL (2017). Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol Appl Pharmacol, 332, 138–148. doi: 10.1016/j.taap.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, … Han KO (2006). Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. Journal of Steroid Biochemistry and Molecular Biology, 101(4–5), 246–253. doi: 10.1016/j.jsbmb.2006.06.020 [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, … Ruan B (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun, 48, 186–194. doi: 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Kamai EM, McElrath TF, & Ferguson KK (2019). Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health, 18(1), 43. doi: 10.1186/s12940-019-0480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, … Dinan TG (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res, 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Klarer M, Arnold M, Gunther L, Winter C, Langhans W, & Meyer U (2014). Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci, 34(21), 7067–7076. doi: 10.1523/JNEUROSCI.0252-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Shinohara M, Nagai T, & Konishi Y (2013). Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci Biotechnol Biochem, 77(11), 2210–2217. doi: 10.1271/bbb.130404 [DOI] [PubMed] [Google Scholar]
- Lai KP, Ng AH, Wan HT, Wong AY, Leung CC, Li R, & Wong CK (2018). Dietary Exposure to the Environmental Chemical, PFOS on the Diversity of Gut Microbiota, Associated With the Development of Metabolic Syndrome. Front Microbiol, 9, 2552. doi: 10.3389/fmicb.2018.02552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimer JM, & Haub MD (2010). Effects of dietary fiber and its components on metabolic health. Nutrients, 2(12), 1266–1289. doi: 10.3390/nu2121266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar V, Ditu L-M, Pircalabioru GG, Picu A, Petcu L, Cucu N, & Chifiriuc MC (2019). Gut Microbiota, Host Organism, and Diet Trialogue in Diabetes and Obesity. Frontiers in Nutrition, 6(21). doi: 10.3389/fnut.2019.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Kim MJ, Song EJ, Kim JH, Ahn J, Nam YD, … Jung CH (2017). Nutrikinetic study of genistein metabolites in ovariectomized mice. PLoS One, 12(10), e0186320. doi: 10.1371/journal.pone.0186320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Han HW, & Yim SY (2015). Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats. Food Funct, 6(2), 492–500. doi: 10.1039/c4fo00731j [DOI] [PubMed] [Google Scholar]
- López P, Sánchez M, Perez-Cruz C, Velázquez-Villegas LA, Syeda T, Aguilar-López M, … Tovar AR (2018). Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxemia, and Cognitive Function in Mice Fed a High-Fat Diet. Mol Nutr Food Res, 62(16), e1800313. doi: 10.1002/mnfr.201800313 [DOI] [PubMed] [Google Scholar]
- Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, … Visekruna A (2019). The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun, 10(1), 760. doi: 10.1038/s41467-019-08711-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo D, Chaves AJM, de Sousa CNS, Quevedo J, Barichello T, Nobre HV, & de Lucena DF (2017). Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. Journal of Affective Disorders, 208, 22–32. doi: 10.1016/j.jad.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Marshall BL, Liu Y, Farrington MJ, Mao J, Helferich WG, Schenk AK, … Rosenfeld CS (2019). Early genistein exposure of California mice and effects on the gut microbiota-brain axis. J Endocrinol, 242(2), 139–157. doi: 10.1530/JOE-19-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Yabut JM, Choo JM, Page AJ, Sun EW, Jessup CF, … Keating DJ (2019). The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci U S A, 116(40), 19802–19804. doi: 10.1073/pnas.1909311116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, & Gupta A (2015). Gut/brain axis and the microbiota. Journal of Clinical Investigation, 125(3), 926–938. doi: 10.1172/jci76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, … Velloso LA (2012). Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes, 61(6), 1455–1462. doi: 10.2337/db11-0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Tanaka K, Okubo H, Sasaki S, Furukawa S, & Arakawa M (2018). Soy isoflavone intake and prevalence of depressive symptoms during pregnancy in Japan: baseline data from the Kyushu Okinawa Maternal and Child Health Study. Eur J Nutr, 57(2), 441–450. doi: 10.1007/s00394-016-1327-5 [DOI] [PubMed] [Google Scholar]
- Moya-Perez A, Perez-Villalba A, Benitez-Paez A, Campillo I, & Sanz Y (2017). Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun, 65, 43–56. doi: 10.1016/j.bbi.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Nakatsu CH, Armstrong A, Clavijo AP, Martin BR, Barnes S, & Weaver CM (2014). Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS One, 9(10), e108924. doi: 10.1371/journal.pone.0108924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, & Rudi K (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterology and Motility, 26(8), 1155–1162. doi: 10.1111/nmo.12378 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Banks EP, Bullock B, & Jefferson WN (2001). Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res, 61(11), 4325–4328. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11389053 [PubMed] [Google Scholar]
- Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, … Hermoso MA (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology, 10(277). doi: 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS, … Tollefsbol TO (2017). Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PLoS One, 12(12), e0189756. doi: 10.1371/journal.pone.0189756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, & Theoharides TC (2015). Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin Ther, 37(5), 984–995. doi: 10.1016/j.clinthera.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini G, Peroni D, Bessi E, & Morelli L (2010). Molecular characterization of intestinal microbiota in infants fed with soymilk. J Pediatr Gastroenterol Nutr, 51(1), 71–76. doi: 10.1097/MPG.0b013e3181dc8b02 [DOI] [PubMed] [Google Scholar]
- Plottel CS, & Blaser MJ (2011). Microbiome and malignancy. Cell Host Microbe, 10(4), 324–335. doi: 10.1016/j.chom.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Rodriguez-Gomez A, Farinetti A, Marraudino M, Filice F, Foglio B, … Gotti S (2017). Early postnatal genistein administration permanently affects nitrergic and vasopressinergic systems in a sex-specific way. Neuroscience, 346, 203–215. doi: 10.1016/j.neuroscience.2017.01.024 [DOI] [PubMed] [Google Scholar]
- Prescott SL, Wickens K, Westcott L, Jung W, Currie H, Black PN, … Probiotic Study G (2008). Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin A detection. Clin Exp Allergy, 38(10), 1606–1614. doi: 10.1111/j.1365-2222.2008.03061.x [DOI] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, … Kashyap PC (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J, 29(4), 1395–1403. doi: 10.1096/fj.14-259598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Keeler J, & Weitkamp JH (2015). Maternal influences on fetal microbial colonization and immune development. Pediatr Res, 77(1–2), 189–195. doi: 10.1038/pr.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CE, & Palm NW (2017). Functional Classification of the Gut Microbiota: The Key to Cracking the Microbiota Composition Code: Functional classifications of the gut microbiota reveal previously hidden contributions of indigenous gut bacteria to human health and disease. Bioessays, 39(12). doi: 10.1002/bies.201700032 [DOI] [PubMed] [Google Scholar]
- Sane F, Scuotto A, Pierrat V, Kacet N, Hober D, & Romond MB (2018). Diabetes progression and alterations in gut bacterial translocation: prevention by diet supplementation with human milk in NOD mice. J Nutr Biochem, 62, 108–122. doi: 10.1016/j.jnutbio.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, & Burnet PWJ (2016). Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci, 39(11), 763–781. doi: 10.1016/j.tins.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberg SJ, Georgieff MK, & Committee On N (2018). Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics, 141(2). doi: 10.1542/peds.2017-3716 [DOI] [PubMed] [Google Scholar]
- Shah SC, Khalili H, Gower-Rousseau C, Olen O, Benchimol EI, Lynge E, … Colombel JF (2018). Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology, 155(4), 1079–1089.e1073. doi: 10.1053/j.gastro.2018.06.043 [DOI] [PubMed] [Google Scholar]
- Smith-Brown P, Morrison M, Krause L, & Davies PS (2016). Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Sci Rep, 6, 32385. doi: 10.1038/srep32385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, … Lewis K (2019). GABA-modulating bacteria of the human gut microbiota. Nat Microbiol, 4(3), 396–403. doi: 10.1038/s41564-018-0307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, … Hanson SA (2001). Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA, 286(7), 807–814. doi: 10.1001/jama.286.7.807 [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Yang Z, LaPorte RE, Chang YF, Steenkiste AR, Pietropaolo M, … Dorman JS (2004). Infant diet and type 1 diabetes in China. Diabetes Res Clin Pract, 65(3), 283–292. doi: 10.1016/j.diabres.2004.02.007 [DOI] [PubMed] [Google Scholar]
- Sun J, Wang F, Hong G, Pang M, Xu H, Li H, … Liu J (2016). Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett, 618, 159–166. doi: 10.1016/j.neulet.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Tamura M, Hirayama K, Itoh K, Shinohara K. (2004). Effects of human intestinal flora on plasma and caecal isoflavones, and effects of isoflavones on the composition and metabolism of flora in human flora-associated (HFA) mice. Microb Ecol Health Dis, 16, 18–22. [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, … Raes J (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 4(4), 623–632. doi: 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- Vazquez L, Florez AB, Guadamuro L, & Mayo B (2017). Effect of Soy Isoflavones on Growth of Representative Bacterial Species from the Human Gut. Nutrients, 9(7). doi: 10.3390/nu9070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, & Flanagan KL (2019). The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol, 41(2), 265–275. doi: 10.1007/s00281-018-0716-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Potter VJ, Cross TL, Swanson KS, Sarma SJ, Lei Z, Sumner LW, & Rosenfeld CS (2018). Soy-Induced Fecal Metabolome Changes in Ovariectomized and Intact Female Rats: Relationship with Cardiometabolic Health. Sci Rep, 8(1), 16896. doi: 10.1038/s41598-018-35171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vighi G, Marcucci F, Sensi L, Di Cara G, & Frati F (2008). Allergy and the gastrointestinal system. Clin Exp Immunol, 153 Suppl 1, 3–6. doi: 10.1111/j.1365-2249.2008.03713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska J, & Klukowski M (2015). Gastrointestinal symptoms and autism spectrum disorder: links and risks - a possible new overlap syndrome. Pediatric Health Med Ther, 6, 153–166. doi: 10.2147/phmt.S85717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ (2013). Soy Infant Formula may be Associated with Autistic Behaviors. Autism Open Access, 2013, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Mckim DB, Sheridan JF, & Godbout JP (2015). Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Frontiers in Neuroscience, 8. doi: 10.3389/fnins.2014.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Kulkarni K, Zhu W, & Hu M (2012). Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anticancer Agents Med Chem, 12(10), 1264–1280. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22583407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, … Hsiao EY (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. doi: 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younge N, McCann JR, Ballard J, Plunkett C, Akhtar S, Araujo-Perez F, … Seed PC (2019). Fetal exposure to the maternal microbiota in humans and mice. JCI Insight, 4(19). doi: 10.1172/jci.insight.127806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LS, & Davies SS (2016). Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med, 8(1), 46. doi: 10.1186/s13073-016-0296-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, & Li HB (2015). Impacts of gut bacteria on human health and diseases. Int J Mol Sci, 16(4), 7493–7519. doi: 10.3390/ijms16047493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Xiao X, Zhang Q, Zheng J, & Deng M (2019). Maternal Genistein Intake Mitigates the Deleterious Effects of High-Fat Diet on Glucose and Lipid Metabolism and Modulates Gut Microbiota in Adult Life of Male Mice. Front Physiol, 10, 985. doi: 10.3389/fphys.2019.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


