Abstract
The current opioid epidemic is one of the most severe public health crisis in US history. Responding to it has been difficult due to its rapidly changing nature and the severity of its associated outcomes. This review examines the origin and evolution of the crisis, the pharmacological properties of opioids, the neurobiology of opioid use and opioid use disorder (OUD), medications for opioid use disorder (MOUD), and existing and promising approaches to prevention. The results of the review indicate that the opioid epidemic is a complex, evolving phenomenon that involves neurobiological vulnerabilities and social determinants of health. Successfully addressing the epidemic will require advances in basic science, development of more acceptable and effective treatments, and implementation of public health approaches, including prevention. The advances achieved in addressing the current crisis should also serve to advance the science and treatment of other substance use disorders.
Origins and Evolution of the Crisis
The current opioid epidemic is one of the most severe public health crisis in US history. Providing an effective response has been difficult because of its changing nature, geographic and demographic diversity, multiplicity of its causes, and the severity of adverse outcomes associated with opioid use and opioid use disorder (OUD). Furthermore, opioid analgesics, which fueled the origins of the opioid epidemic, are therapeutically beneficial when used properly. This, compounds the difficulties of regulating their availability because they cannot be banned, in contrast with illicit drugs. While earlier phases (i.e., first wave) of the crisis were predominantly driven by non-medical use and addiction to prescription opioid analgesics, heroin (second wave) and subsequently illicit synthetic opioids (third wave) have become progressively important as the crisis progressed and more recently there is emerging evidence of increasing fatalities associated with the combination of psychostimulant drugs with opioids (fourth wave). These changes explain why despite the decreases in the number of opioid prescription dispensed, the number of opioid fatalities has continued to rise unabated.1 Although the opioid crisis is often seen through the prism of fatal overdoses, its most dramatic manifestation, the misuse of opioids and OUD also lead to death, disease and suffering from many other causes with devastating medical, social and economic consequences.2-5
Like most complex problems, the opioid crisis has multiple roots,5 including changing social and economic conditions, limited availability of safe and effective analgesics, insufficient treatment capacity for OUD and legal approaches that criminalize OUD rather than fostering treatment. The two major driving factors of the crisis, however, were the steady increase in the rate of opioid prescriptions, particularly in the early stages of the crisis, and the decrease in price and the increase in availability of heroin and synthetic opioids.
Increases in the rate of opioid prescribing followed the identification of undertreatment of pain in the 1990s as an important clinical problem6 and the mistaken belief, based on anecdotal evidence, that patients in pain were not at risk for OUD.7 Although less than 10% of individuals to whom opioids are prescribed develop OUD,8 large increases in the rates of opioid prescriptions inevitably led to more individuals being exposed to opioids and subsequent increases in OUD prevalence. More importantly, increased availability of opioids generated an enormous surplus of medication that was diverted for non-medical use.9 From 1991 to 2013, the prevalence of non-medical use of prescription opioids in the US more than doubled, from 1.5% to 4.1%, and the prevalence of prescription OUD tripled, from 0.3% to 0.9%.10, 11 At the same time, the severity of nonmedical use, as measured by the frequency of use, also increased among nonmedical users.12
A second major factor in the crisis was the increased accessibility and purity of heroin coupled with reduced price partly due to increases in the efficiency of its distribution channels, which led to increases in heroin use and heroin use disorder. From 2001-2002 and 2012-2013 the prevalence of lifetime heroin use in the US increased from 0.33% to 1.6% and the lifetime prevalence of heroin use disorder increased from 0.21% to 0.69%.13, 14 There is controversy regarding whether efforts to decrease use of prescription opioids led to increases in heroin use and use disorder. Although individuals who use prescription opioids are more likely than those who don’t to use heroin, only 3%-5% of individuals who used prescription drugs non-medically in the previous year also reported using heroin during the same year.15 Furthermore, increases in heroin use among nonmedical prescription opioids users preceded the development of policies to address misuse of prescription opioids. Thus, it may be the case that efforts to improve prescription of opioids have played a more limited role in the increases in heroin use than that ascribed to them.16
A more recent entrant has been fentanyl and other very potent synthetic fentanyl analogues. From 2010 to 2017, deaths from fentanyl and other synthetic opioids increased nearly ten -fold, from around 3,000 (14.3% of opioid-related deaths) to over 28,466 (59.8%).17, 18 Synthetic opioids are now almost twice as commonly involved in overdose deaths as prescription opioids or heroin.17 The low production costs of fentanyl and its potency (50- fold compared to heroin) make it an attractive option to mix (“lace”) with heroin and illicit manufactured prescription opioids.19 At present, it is not known how many users actively seek fentanyl, but regardless of intent, heroin users are being exposed to fentanyl or other analogues without realizing it, increasing their risk of overdosing. Overdoses from fentanyl by itself or combined with heroin appear to be harder to reverse with naloxone than overdoses due to prescription opioids or pure heroin, contributing to the lethality of fentanyl or drugs laced with it. The reasons for the decreased efficacy of naloxone for reversing fentanyl overdoses are unclear and might reflect, its very high potency at the mu opioid receptor (MOR), its very fast pharmacokinetics (entering the brain very rapidly minimizing time for intervention), longer duration of its respiratory depressing effects (that might explain re-narcotization after a temporary reversal of an overdose by naloxone) and/or an additive effects when fentanyl is combined with other drugs including heroin.20
Pharmacological properties of opioids
In addition to their effect on MOR, opioid drugs also bind to kappa- (KOR) and delta-opioid (DOR) receptors, although their affinity, intrinsic efficacy, pharmacokinetics and bioavailability vary by drug. In particular opioid drugs with fast uptake into the brain and full agonist effects at MOR such as heroin and fentanyl are particularly rewarding.20 A strategy for developing opioid medications with lower abuse liability entails opioids formulations with slower entry into the brain and/or formulations that cannot be injected, since this is the route of administration that results in the faster rate of drug uptake in brain. The intrinsic efficacy of full agonist drugs such as heroin and fentanyl leads to greater rewarding effects than for partial agonists such as buprenorphine. Additionally, the rate of clearance of opioid drugs from the brain determines their duration of action and the severity of withdrawal symptoms upon their discontinuation. For that reason, heroin is associated with a much more severe withdrawal than a drug such as buprenorphine, which clears the brain more slowly. Opioids drugs with longer half-lives, slower clearance rates and slower brain uptake are favored for the treatment of OUD. By binding to MOR they decrease craving and prevent the emergence of withdrawal symptoms. Methadone enters the brain rapidly, which is why it is given orally when used for OUD treatment, for this will slow its entry into the brain. Also while it is a full MOR agonist, it has agonist effects at galanin receptors, which are co-expressed with MOR in brain reward regions antagonizing them, and thus reducing methadone’s rewarding effects.31
The positive (increase reward) and negative (avoid pain) reinforcing effects of opioids, trigger learned associations between the receipt of the drug and these experiences resulting in conditioning. In parallel, the repeated administration of opioids triggers physiological adaptations that result in tolerance and in physical dependence. Tolerance necessitates increasing opioid doses in order to achieve the same levels of analgesia and when misusing them increasing the doses or shifting to more potent opioids such as fentanyl in order to experience their rewarding effects. The development of tolerance does not occur at the same rate for the various pharmacological effects of opioids. In general, tolerance to the analgesic and hedonic effects develops faster than to respiratory depression, whereas tolerance to constipation might never develop.32 This explains why increases in dose to maintain analgesia (or reward) or doses injected to get high can markedly increase the risk of overdose.
Physical dependence to opioids also occurs rapidly and is responsible for the emergence of withdrawal symptoms when opioids are abruptly discontinued, which creates a negative reinforcement mechanism that can contribute to the maintenance of opioid use. The severity of withdrawal symptoms varies as a function of the opioid drug used; greater for higher potency short acting opioids than for longer lasting opioids as well as the chronicity of exposure. The symptoms from acute withdrawal usually resolve within days and are rarely lethal, but are extremely uncomfortable and are a powerful trigger for relapse in those with an OUD and for continued opioid use among those being treated with opioid analgesics. The risk of withdrawal is minimized or prevented by tapering opioids gradually.
Repeated use of opioids often results in physical dependence as a result of multiple neuroadaptations including desensitization and internalization of the MOR, impaired MOR signaling with intracellular effectors, and adaptations in glial signaling and in neuropeptide systems that interact with MOR-sensitive neurons, among others33, 34. In contrast to physical dependence, OUD develops only in a minority of individuals exposed to opioids. It is characterized by a pattern of maladaptive opioid use that leads to clinically significant impairment or distress and is manifested as intense craving for opioids, erosion of inhibitory control over efforts to refrain from using them, persistent thinking about procuring the drug, and impaired control over opioid use. Because of their repeated opioid use, those with OUD also suffer from physical dependence and tolerance, unless they have undergone supervised medical withdrawal (formerly known as detoxification) in which case they can have OUD without having physical dependence or tolerance. This is clinically relevant in that patients undergoing medically supervised withdrawal without subsequent treatment for OUD are at an extremely high risk of relapse and of overdosing since they crave the drug as intensely as prior to their withdrawal but have lost their tolerance to the opioids. The behavioral manifestations of OUD are associated with structural and functional changes in the brain’s reward, executive control, emotion and interoceptive circuits. 58,59
Biological factors contributing to OUD
In addition to the environmental contributions to the crisis, multiple biological factors modulate an individuals’ vulnerability for opioid use, non-medical use and OUD, including genetic predisposition, brain development, mental illness and social factors.
Genetics
Studies of genetic epidemiology indicate that genes contribute about 50% of the vulnerability to SUD, including OUD. Yet identifying specific genetic variants for increased OUD risk has been difficult, which is likely to reflect in part the fact that OUD, like other psychiatric disorders is a polygenic disease. Genes influence brain development and function of brain circuits and neurotransmitter systems that mediate the reactivity to the environment including drug responses. Furthermore, genes can intervene at different stages of OUD development, including propensity to use (i.e. genes that modulate personality traits) risk of transition from use to OUD (i.e. gene involved in conditioning and neuroplasticity), and vulnerability to relapse (i.e. genes that modulate severity of withdrawal symptoms, sensitivity to stress or other potential triggers). Because OUD often co-occurs with other psychiatric disorders, genes that increase the risk for those co-occurring disorders can also indirectly increase OUD risk. The effects of genes are also moderated by environmental influences.
Despite these complexities, studies have been able to identify genes that appear to contribute to OUD risk (Table 1). For example, OPRM1 the gene that encodes for MOR has been implicated in increased vulnerability to OUD.35 Similarly, converging evidence of genome-wide association, neuroimaging and rodent studies support a role in OUD for CNIH3, a gene that encodes for the Cornichon Family AMPA Receptor Auxiliary Protein that regulates trafficking and gating properties of AMPA receptors.36 Preliminary results also suggest that the BDNF Val(66) Met genotype, which has been associated with neurobehavioral deficits, may promote drug-seeking in individuals with OUD.37
Table 1.
Genes associated with increased vulnerability to opioid use disorder
| Gene | Molecular Product or Target |
Hypothesized mechanism |
|---|---|---|
| OPRM135 | Mu-opioid receptor | Changes in Mu-receptor expression |
| CNIH336 | AMPA receptor auxiliary protein | Influences control over opioid use |
| BDNF37 | Brain-derived neurotrophic factor | Moderates propensity to drug-seeking |
| MAOA38, 39 | Monoamine oxidase A | Changes in propensity to externalizing behaviors |
| COMT40, 41 | Catecholamine-O-methyltransferase | Modulation of prefrontal cortex, amygdalar activity, and reward circuitry |
| FKBP550 | FK506 binding protein 5 | Moderates responsivity to stress |
| Homer 1-347 | Homer proteins | Influences response to rewards |
| MMP948 | Matrix metalloproteinase 9 | Moderates propensity to drug-seeking |
Other genes, though not directly linked to OUD, relate to risk factors, such as personality traits, or brain regions implicated in the circuitry of addiction. For example, polymorphisms associated with low activity in MAOA, the gene for monoamine oxidase A, have been linked to a predisposition to externalizing behaviors and disorders that is moderated by environmental exposures.38, 39 Similarly, the catecholamine-O-methyltransferase (COMT) gene variant V(108/158)M, leads to greater dopamine degradation and impaired modulation of prefrontal cortex40, and has been associated with increased amygdalar reactivity41 and with disrupted modulation of cortical and striatal activation during anticipation or receipt of a reward.42, 43 Numerous animal and human studies have also demonstrated the role of dopamine receptors in reward related behaviors,44-46 but to date, no study has directly linked any dopamine-related gene to the risk of OUD.
Genes whose product influence synaptic plasticity can also contribute to OUD risk, such as Homer proteins, which regulate the level and activity of glutamate receptors,47 and matrix metalloproteinase 9 (MMP-9), which in animal models increases motivation for drug-seeking.48, 49 Genes that influence response to stress by modulating the glucocorticoid receptor’s affinity for cortisol such as the FKBP5 chaperone protein50 may also increase risk of OUD.
Brain Development
Drug experimentation is commonly initiated during adolescence and the risk to addiction is increased with early drug use. The greater vulnerability of adolescents to drug use and experimentation is driven by multiple factors including genetics that are associated with the developmental trajectories of the human brain. At the same time the social environments during childhood will influence brain development in ways that can increase its vulnerability to drugs or provide with resilience. Notably, the development of the human brain has a greater sensitivity to environmental factor during the first than second decades of life whereas genes influence brain development throughout the first and second decades.51 This explain why social stressors are particularly harmful during early childhood. However while it is heuristically useful to distinguish environmental from genetic factors, it is likely that it is their interactions that ultimately determine how the brain will develop.
A better characterization of human neurodevelopment has allowed us to start to understand the role of the environment at critical moments of brain development and how differences in the rate of development of neuronal circuits influence vulnerability for drug use. For example, during adolescence reward and emotion circuits develop faster than those related to executive function, creating an imbalance between systems that favor experimentation, reward-seeking and drug use and systems underpinning self-regulation. Early exposure to drugs of abuse may further impair the development of the prefrontal cortex, decreasing self-regulating capabilities and increasing the long-term risk for SUD.52
Several brain imaging studies have started or will soon start to generate data to inform the development of the brain and the influence of substance use in this development. The Adolescent Brain Cognitive Development Study (ABCD) is a longitudinal study of children 9-10 years old who will be assessed with brain imaging, genotyping, and deep phenotyping and followed for 10 years. It recently completed the baseline assessment of 11,875 children and has started to provide valuable data on normal variability in brain development and its influence by environmental factors.53 The Baby Connectome Project is a four-year study of children from birth through five years of age, intended to provide a better understanding of how the brain develops from infancy through early childhood and the factors that contribute to healthy brain development.54 It will help characterize human brain connectivity and map patterns of structural and functional connectivity to important behavioral skills. Additional biological (e.g., genetic markers) and environmental measures (e.g., family demographics) will be collected and examined to provide a more comprehensive picture of the factors that affect brain development. Finally, the planned HEALthy Brain and Child Development (HBCD) study aims to prospectively follow 7,500 infants through childhood (e.g., age 9-10) to assess structural and functional brain development as well as cognitive, behavioral, social, and emotional development and the long-term impacts of pre/postnatal drug (expected oversampling for opioid prenatal exposures) and adverse environmental exposures on brain and behavioral health and risk for substance use and mental disorders.
Social determinants
Epigenetics are implicated in the persistent neuroplastic changes associated with exposure to environmental factors that increase vulnerability to addiction such as social stressors or drug exposures.55 For example, the environment’s ability to shape the circuits of emotion, particularly those impacted during critical periods of prenatal, postnatal, and adolescent brain development,56 taps heavily on epigenetic mechanisms.46, 57 Studies have also started to assess the effects of social stressors on the development of the human brain, and these studies are relevant for understanding why social stressors increase the risk for SUD and other psychiatric disorders. Studies evaluating the effects of social deprivation during infancy and early childhood have reported delayed maturation that results in impaired brain connectivity, which could underlie increased impulsivity in these children.58 Fortunately, preliminary studies suggest that interventions that provide social support may help reverse some of these impairments.59 Nevertheless, stressful events at any age can increase vulnerability to opioid use and OUD.
Though the developing brain is the most sensitive to adverse social environments, these can also negatively influence the adult brain in ways that increase the vulnerability to drugs use and addiction. This is apparent in the current opioid epidemic and the other “deaths of despair” (overdoses, suicide and alcohol-driven cirrhosis) that have prominently affected adult white Americans from economically impoverished environments. There is a scarcity of research on the effects of adverse social environments on adult brain function.60, 61
Neurocircuitry of addiction
Reward Circuitry
Although much remains to be learned, our growing knowledge of the brain’s reward circuitry (originating in the dopamine neurons in the ventral tegmental area projecting to the nucleus accumbens, ventral prefrontal cortex and amygdala) and its changes have been fundamental for understanding drug taking and addiction. Reward (more precisely, reinforcement) can be defined as any event that increases the probability of repeating the action. Animal62,63 and human studies consistently indicate that drugs release dopamine (DA) in the nucleus accumbens (NAc), which is fundamental to their rewarding effects. Additionally, increases in endogenous opioids and cannabinoids are also associated with the rewarding effects of various drugs.64, 65 It is thought that the rapid release of DA and its binding to D1 receptors (D1R) in the ventral (location of NAc) and dorsal striatum, which stimulates cyclic AMP (cAMP) signaling is associated with euphoria and pleasure (so-called “high”) and triggers conditioning (learned association between the drug effects and situation where it occurred). By contrast, stimulation of D2 receptors (D2R), which inhibit cAMP signaling, does not appear to be associated with rewarding effects per se but blocks the aversive effects when D2R-expressing medium spiny neurons (D2R-MSN) are not inhibited by DA.66 Individuals with SUD often have decreased baseline DA release in the striatum (including in nucleus accumbens) and experience an attenuated DA increase and less intense reward from drug use (i.e., tolerance), which is interpreted to reflect a hypofunction of their reward circuit. It is unknown to what extent this hypofunction reflects a predisposition of the individual versus consequences from chronic drug exposures.
With repeated drug use, conditioning strengthens and drives the motivation to procure the drug reinforcers. Exposure to conditioned stimuli (referred to as cues) by themselves triggers firing of DA neurons and DA release, energizing the motivation to obtain the drug. When previously neutral stimuli become conditioned to the drug they acquire incentive salience, becoming desirable67. Conditioning and the associated DA increases in the striatum are hypothesized to underlie the intense desire for drugs that addicted individuals experience upon exposure to environments or situations where they have taken drugs that frequently leads them to relapse.
The drug-induced stimulation of D1R signaling involved with conditioning triggers synaptic changes in N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that enhance glutamatergic signaling in the affected synapses.68, 69 At the circuit levels these neuroplastic changes strengthen striatal- pallidal-thalamo-cortical loops that include the ventral and the dorsal striatum, resulting in habit formation70 and compulsive response for drugs. As SUD progresses, a prominent hypothesis is that these circuits become increasingly sensitized to drug-cues, environmental stressors or negative emotional states, which can then more readily trigger compulsive drug consumption. In more advanced stages, substance use behaviors are more driven by a growing importance of dorsal striatum and habits and a decreasing role of positive drug reward. In parallel, as drug-procuring and taking becomes increasingly salient in the addicted individual, non-drug related activities become less motivating and rewarding.71, 72
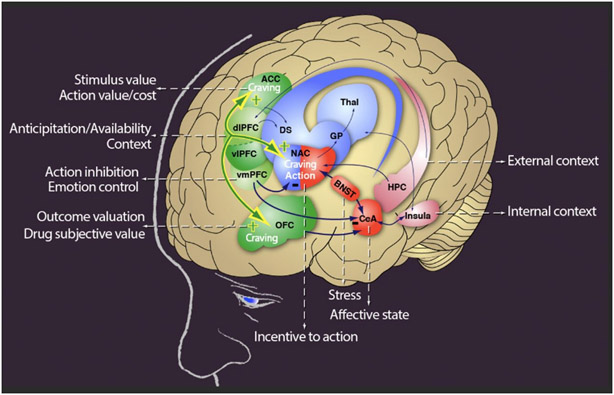
Although DA and glutamate remain central to our understanding of the reward circuits, these circuits are also modulated by γ-aminobutyric acid (GABA), serotonin, acetylcholine, and the endogenous opioid and cannabinoid systems. Similarly, it is important to realize that, like any other, the reward circuit (as well as the executive control circuit) interact and overlap with circuitry involved in the perception of internal bodily states or interoception (including primary sensory cortex, insula, anterior cingulate cortex and precuneus), homeostasis (hypothalamus), stress (amygdala, hypothalamus, and habenula), salience attribution (orbitofrontal cortex) and learning and memory (amygdala, hippocampus and dorsal striatum) and that the interplay with these circuits modulates the responses to rewards and to conditioned cues (Figure 1).73
Figure 1.
The circuitry of opioid use disorder (reprinted with permission from: George O, Koob GF. Control of craving by the prefrontal cortex. Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):4165-4166
Emotion Circuitry
Although positive reinforcers play a major role in the initial phases of drug taking and the development of OUD, in more advance stages negative emotional experiences such as withdrawal symptoms, craving and enhanced sensitivity to stress become increasingly important and drive drug taking. The increased role of negative reinforcement in drug taking (as a means to escape the negative emotional state) is a considerable barrier to abstinence and a formidable obstacle to successful treatment.
The negative emotional state can be understood as enhancement of processes that are the inverse of those involved in reward (i.e., “anti-reward processes”) 74 that are derived from neuroadaptions to drug-induced stimulation.62 In the case of opioids most of the physical manifestations of withdrawal, which emerge with drug discontinuation are caused by decreased MOR-triggered intracellular signaling and by enhanced autonomic reactivity.75 Early signs of withdrawal, appearing generally in the first 8-36 hours of heroin discontinuation include mydriasis, piloerection, muscle twitching, lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, myalgia, abdominal pain, nausea/vomiting, tremor and insomnia. Individuals with fully developed opioid withdrawal can also experience tachycardia, tachypnea, hypertension or hypotension, dehydration, hyperglycemia, fever, anorexia and diarrhea.
In addition to these physiological manifestations, opioid withdrawal is often associated with neuropsychological symptoms that include, irritability, emotional pain, dysphoria, insomnia and subjective distress that can last several months. The subjective aspects of the negative emotional state are associated with disrupted dopaminergic, serotonergic, noradrenergic, glutamatergic, GABAergic neurotransmission in reward and emotion networks (including NAc).67 Stress-related neurotransmitters, such as CRF, norepinephrine, and dynorphin, are also recruited in the extended amygdala and contribute to the distress and increased irritability often present in withdrawal and protracted abstinence.76 The lateral habenula, which decreases DA neuronal firing in VTA when expected rewards do not materialize77-79 and is a target of serotonin neurons from the dorsal raphe that modulate mood, also plays a crucial role in triggering and maintaining negative emotional states.80 Similarly, the insula, which is reciprocally connected to several limbic regions and to the default mode network (DMN) has an interoceptive function that integrates autonomic and visceral information with emotion and motivation81, plays a key role in the self-awareness of negative emotional states and of craving.82-84
Executive Control Circuitry
Impairments in executive function (including self-regulation), which can precede as a vulnerability factor for SUD or develop secondary to chronic drug exposures, are important contributors to impulsive and compulsive drug taking.67
During withdrawal the emergence of the preoccupation/anticipation phase along with mounting craving for the drug results from the interplay of two opposing systems: a Go and a Stop system. The Go system includes most of the circuitry described in the two previous sections, which underlies the hedonic, habitual and emotional aversive aspects that leads to continued drug taking.85, 86 The Stop system seeks to control the incentive value of choices, regulate habitual behavior, maintain goal-directed behavior and suppress affective responses to negative emotional signals.87-89 In this framework, the Stop system seeks to inhibit signals generated by the Go system.
The Stop system involves a widely distributed and complex prefrontal cortex–subcortical circuitry. It is mediated through glutamatergic projections from prefrontal cortex to the caudate and ventral striatum to modulate the striatal-pallidal-thalamocortical direct (D1 receptor-mediated) and indirect (D2 receptor-mediated) pathways, and to modulate the mesocortical dopamine neurons in the ventral tegmental area, which exert control over DA release in the prefrontal cortex.73 Deficits in prefrontal cortex in individuals with SUD are associated in impairments in executive function that can interfere with decision making, self-regulation, inhibitory control, and working memory.90 Reduced activity in the prefrontal cortex (including dorsolateral prefrontal cortex, anterior cingulate gyrus, and medial orbitofrontal cortex) is also associated with downregulation of D2R in the striatum,91-93 and disrupted GABAergic activity in striatum and prefrontal cortex.94, 95
The orbital frontal cortex, anterior cingulate cortex and dorsolateral prefrontal cortex are involved with incentive salience, emotional regulation, and decision making, respectively. Deficits in striatal D2R-mediated DA signaling, which modulates them, may underlie the enhanced motivational value of drugs and the loss of control over drug intake in SUD.96 Furthermore, because dysfunction of the orbital frontal cortex and the anterior cingulate cortex are associated with compulsive behaviors and impulsivity,97 DA’s impaired modulation of these regions may contribute to compulsive and impulsive drug taking in SUD.96
Several pathways can encode signals that increase the risk of relapse with exposure to cues, which can activate the ventral prefrontal cortex, including ventral anterior cingulate, and medial and lateral orbitofrontal cortices.98-101 Cue-induced reinstatement involves glutamatergic projections from the prefrontal cortex, basolateral amygdala, and ventral subiculum to the NAc, and DA projections to the basolateral amygdala and dorsal striatum.102-104
In contrast to cue-induced relapse, stress-induced reinstatement depends on the activation of both CRF and norepinephrine in parts of the extended amygdala (i.e., central nucleus of the amygdala and bed nucleus of the stria terminalis) and the VTA.105 Protracted abstinence, mostly described for alcohol, appears to involve overactive glutamatergic and CRF systems.106, 107 A third pathway to relapse is through interoceptive stimuli related to the insula82-84 and its activation during craving has been associated with relapse.108
Pharmacological treatment of opioids
A range of pharmacological treatments exists to treat different components of OUD. Information on these medications was recently reviewed by the U.S. Substance Abuse and Mental Health Administration109 and by the National Academy of Medicine.110
Withdrawal
Medically supervised withdrawal (formerly referred to as detoxification) is the gradual taper of opioid agonist medications (methadone or buprenorphine) guided by a clinician to alleviate withdrawal symptoms. The use of α2-agonists such as lofexidine (recently approved by the FDA for the treatment of opioid withdrawal) or clonidine can also help attenuate symptoms of withdrawal,111 though subsequent initiation of medications for OUD (MOUD) is required to prevent relapse into drug taking. Indeed, medically supervised withdrawal is not recommended as an isolated strategy, as most patients without subsequent MOUD initiation relapse shortly thereafter112-114 and are at increased risk for overdosing due to the loss of tolerance.115 Medically supervised withdrawal is required, though, for patients starting naltrexone, as described in the next section.
Maintenance
Ongoing outpatient treatment with MOUD leads to better retention and outcomes for OUD116 and reduced risk of HIV and HCV infection and overdose death.117-121 At present, MOUD constitutes the standard of care for most patients with OUD. There are three FDA-approved MOUD: methadone, buprenorphine and naltrexone. In deciding on a specific medication the clinician should evaluate the patient’s responses to prior MOUD treatment, tolerance to opioids and patient preferences. Patients, in turn, should be informed of the efficacy, risks, benefits and relative advantages of each of these medications.
Methadone has been available the longest and has the largest evidence of efficacy.119, 121 Higher doses of methadone are associated with better retention in treatment, less heroin use during treatment and lower withdrawal symptoms, until around 100 mg/day, after which the reliability of evidence is lower. 122 Because methadone is a full MOR agonist, it has no ceiling effect. It can lead to overdoses if it is used at doses above the patient’s tolerance or combined with other central nervous depressants such as alcohol, benzodiazepines or other opioids. To minimize the risk of intoxication or overdose, methadone should be started at low doses and increased gradually with daily monitoring over several weeks. An important barrier to the use of methadone, particularly in rural settings is that, with a few exceptions, in the US methadone has to be administered in a licensed opioid treatment programs (methadone clinics) and cannot be prescribed by office-based clinicians.
The use of buprenorphine123, 124 has certain advantages over methadone, which explains its growing use for OUD treatment. First, although it can still be lethal when combined with other central nervous depressant substances, as a partial agonist, buprenorphine has lower lethality than methadone.124 Second, its antagonist effects at the KOR may contribute to its efficacy for OUD.125, 126 Its KOR antagonist properties may also have antidepressant effects, which could help improve the depressive symptoms that are common in OUD. Buprenorphine is also an agonist at the nociceptin receptor, which is also implicated on its therapeutic benefits. To deter the injection of buprenorphine, which would enhance its rewarding effects, it is often prescribed in a formulation that includes naloxone, a short-acting opioid antagonist that has poor bioavailability when sublingually administered, but blocks buprenorphine effects if injected. Currently, in the US, prescribers of buprenorphine for the treatment of OUD need to obtain a waiver from the Drug Enforcement Administration (DEA) after completing an 8-hour training.
Extended-release (XR) formulations of buprenorphine have been developed to improve patient adherence. Although, 6-months buprenorphine implants were shown to be as effective as low-dose sublingual buprenorphine,127 its benefits are restricted to patients who respond to low doses of buprenorphine (8mg). In 2017, the FDA approved a 1-month XR buprenorphine injection for patients with OUD who have been treated with sublingual buprenorphine for at least one week. Another 1-month and a 1-week XR buprenorphine are currently under FDA review. It would be important to know whether those new formulations can increase treatment retention.
The third FDA-approved MOUD is naltrexone, which is a MOR antagonist that can be prescribed by clinicians in outpatient practice. The efficacy of its immediate release formulation was limited by poor adherence, but the development of a monthly extended release formulation (XR-NTX) significantly improved outcomes.128-131 It has been particularly useful in justice system settings reluctant to use agonist therapies,130 although there is need to assess whether it would be superior to XR-buprenorphine in those settings. Naltrexone is also a KOR antagonist, and while its affinity is lower than that for MOR, a recent brain imaging study reported KOR occupancies > 80% in patients with alcohol use disorder treated with naltrexone.132 Antagonism of KOR by naltrexone could contribute to the mood improvements reported in OUD patients treated with naltrexone.133 A potential barrier for the use of XR-NTX is the need for medically supervised withdrawal. Patients cannot use short-acting opioids for at least 7 days and long-acting opioids for 10-14 days preceding induction onto naltrexone to avoid triggering a withdrawal syndrome. A rapid taper consisting of a single day of buprenorphine followed by ascending doses of oral naltrexone along with clonidine and other adjunctive medications (e.g., clonazepam and prochlorperazine) may allow for faster induction protocols for XR-NTX,131 but requires further study before it can be recommended as standard strategy.
At present, limited data are available regarding the comparative effectiveness of MOUD or about which patients will respond better to each medication.109 A Cochrane review concluded that flexible-dose methadone leads to greater retention than sublingual buprenorphine,119 but whether the same results would hold when compared with XR-buprenorphine is unknown. There are currently no published Cochrane reviews of XR-NTX versus buprenorphine, but two studies134, 135 have suggested that patients who can be inducted onto XR-NTX have similar outcomes to those treated with sublingual buprenorphine. However, in one of those studies134 a substantial proportion of patients were unable to complete XR-NTX induction, mostly due to early relapse, leading to superior outcomes for the buprenorphine group in the intent-to-treat analysis.
As knowledge about MOUDs continues to grow, three priority areas need to be addressed. First, studies suggest that longer time in treatment is associated with better outcomes and that the risk of relapse greatly increases after medication discontinuation, yet rates of MOUD discontinuation in the first 6 months of treatment remain very high. Thus, there is a need to improve retention in MOUD treatment. It is also important to know which patients, when and under what circumstances can safely discontinue MOUD.
Second, current evidence indicates that counseling or psychotherapy do not increase retention in buprenorphine treatment or improve abstinence rates116, 136-139 and that methadone treatment140 and buprenorphine without concomitant counseling is vastly superior to no treatment.141 It is important to determine whether the potential benefits of concurrent psychotherapy outweigh the barrier to treatment created by requiring provision of psychotherapy when delivering buprenorphine treatment (such counseling is at present not required for XR-NTX). This was highlighted in the recent report of the National Academy of Medicine , which emphasized that lack of behavioral treatment support is not a reason for withholding MOUD.110 Nevertheless, evidence-based behavioral interventions can be useful in engaging some patients with OUD in treatment, retaining them in treatment, improving outcomes, and helping them achieve recovery .142 More research is needed to determine which behavioral interventions provided in conjunction with MOUD are most helpful for which patients, including evidence on the effectiveness peer support. There is also a need to develop models of care that better attract and retain patients in care and can overcome the barriers posed by the limited availability of well-trained clinicians. The use of technology-based approaches (i.e Telehealth, mHealth) may be a promising avenue to achieve these goals.
Third, research is needed to evaluate whether residential or inpatient treatment is superior to outpatient treatment for initiating MOUD. This question is important to evaluate the optimal allocation of resources for the treatment of OUD.
Preventing Opioid-Related Overdoses
Despite national and local efforts, the number of opioid-related overdoses continue to increase, highlighting the need of clinicians to educate patients and their families about the risk of overdose and how to respond to it. Several medication- and patient-related factors increase the risk of overdose.143 Patient-related factors include: 1) Age above 65 years; 2) respiratory problems; 3) long-term opioid use; 4) substance use disorder (including alcohol use disorder); 5) comorbid mood disorders and/or suicidality; 6) use after a period of abstinence (e.g. following medically supervised withdrawal or incarceration); 7) prior overdose. Medication-related factors include: 1) Daily dose above 100 morphine milligram equivalents; 2) use of higher doses than prescribed (or than usually consumed, in the case of illicit opioids); 3) combination with fentanyl, other high-potency opioids or other substances, such as alcohol or benzodiazepines.
The acute treatment of overdose is administration of naloxone. For many years naloxone, which required injection, could only be administered by health care providers, but the availability of an auto-injectable naloxone device and a naloxone spray now allow laypersons to administer naloxone to revert overdoses.144, 145 Nevertheless, increasing the availability of naloxone to ensure that it can reach those who need it on short notice remains a challenge, as there is considerable variability in the availability of naloxone by locality.146 A 2019 analysis concluded that making naloxone available without prescription (“over the counter”) would substantially increase its availability.147
In most cases a single dose of naloxone is sufficient to reverse the overdose. However, when high doses or high potency opioids such as fentanyl are used more than one dose is necessary to restore or maintain spontaneous breathing.148 Furthermore, the fast pharmacokinetics of fentanyl can result in abrupt respiratory depression, which in some instances does not provide sufficient time to administer naloxone. It is believed that the duration of the respiratory depression from fentanyl or other analogues is longer than the duration of its reversal from naloxone. Moreover, thoracic rigidity associated with serotonergic effects from some of the opioids drugs might also interfere with overdose reversals. Naloxone can also fail to reverse overdoses in which opioids are combined with other CNS depressants, such as alcohol or benzodiazepines. First-responders to an overdose should stay with patients until emergency medical services arrive and transport them to an emergency room for a more systematic evaluation. Reversal of the overdose generally triggers an acute withdrawal syndrome,111 which can lead patients to leave medical supervision to seek opioids for relieving withdrawal. Upon reversal of an overdose OUD patients should be linked to OUD treatment otherwise their risk of overdosing again is very high. The emergency room offers unique opportunities to start patients on MOUD if they can be linked with ongoing services.149 Nevertheless, more research is needed to improve the management of patients immediately after reversal of an overdose, to retain them under care and to initiate effective MOUD.
Prevention
Because of the urgency of the crisis, most efforts to date have emphasized treatment approaches. There is growing recognition, however, of the need to develop effective preventive interventions for OUD.1, 5, 150 Initial preventive approaches in the US focused on improving prescription practices for opioid analgesics and increasing the availability of naloxone to prevent overdoses. The growing role of heroin, fentanyl and other synthetic opioids in the opioid crisis,1, 16, 151 has required broadening the scope of preventive interventions. There are no evidence-based primary and secondary prevention for OUD for adults or for youth transitioning into adulthood, but several approaches appear as promising directions.
One approach would be to adapt existing interventions that have been successful for youth. Evidence-supported prevention interventions delivered in community or school settings have shown effectiveness at reducing substance use and other related problem behaviors, including middle-school interventions that have specifically demonstrated an impact on reducing prescription opioid misuse.152 However, whether those interventions would work in adults is unknown. An obvious difficulty in adapting these interventions is the lack of a setting similar to school where adults could be easily reached.
A second direction would be the development of conceptual frameworks that articulate the relationship among risk factors for OUD to help guide which interventions might be most effective given the prevalence of the risk factors, how they relate to other relevant risk factors and how modifiable the risk factors are. These models could help examine how risk factors present at birth (e.g., family history of substance use disorders) or childhood (e.g., adverse childhood events) can increase the likelihood of risk factors in adolescence (e.g., early onset of psychiatric disorders, low educational achievement), which in turn increase the risk of OUD in adulthood.153 Simulations could help identify which interventions might be most promising at the full population level or for subgroups with specific constellations of risk factors, as well as to identify potential unintended consequences of those interventions.
A third approach would be to increase the focus on populations at risk that can be accessed for screening and treatment. Those include, for example, individuals who receive regular health care and those in the justice system. Improved management of opioid prescriptions and of treatment of OUD for pregnant women is also a high priority, as it could benefit the mother and simultaneously decrease the risk of neonatal abstinence syndrome (NAS) in newborns by decreasing their in-utero exposure to opioids.
Even with wider use of non-opioid analgesics or use of non-pharmacological approaches, opioids will continue to be necessary for the treatment of many patients with severe pain. Efforts to prevent the development of opioid misuse and OUD can be started in clinical settings. This includes assessment of risk of OUD before opioids are prescribed, periodic assessment of the need for opioid use, and use of urine testing to rule out illicit use of other substances. There is evidence that prescribing lower doses/fewer pills in the emergency room/post-surgery is associated with lower rates of long-term use and possibly OUD. There is also a need to assess each patient for licit and illicit use of other substances, particularly benzodiazepines and alcohol, which can increase the lethality of opioids by potentiating their depressing respiratory effects. Individuals who misuse opioids or develop OUD should be treated by their primary physicians if they have the necessary expertise and support or otherwise be referred to an addiction specialist.
Prevention approaches should also consider supply approaches. The most common sources of diverted opioid analgesics are friends or relatives who were legitimately prescribed opioids.154 As with any other medication, it is important to educate patients who receive legitimate prescriptions about the health hazards they create for others when they give them their medications. Similarly, it is important to educate patients about the health risks they incur when they take medications (including opioids) that were not prescribed to them. Use of Prescription Drug Monitoring Programs (PDMPs) can help reduce doctor shopping and overdoses, but their use to date is inconsistent. This may be due in part to the voluntary nature of the programs in many states, delays in updating the information, restrictions in data sharing across jurisdictions and the need to access them through a separate computer from the one used to access electronic health records.150 Approaches that could help decreased the availability of heroin and fentanyl, traditionally in the purview of law enforcement primarily, would also advance prevention, but their development and implementation are challenging in the face of the evolving nature of the epidemic.
Research Gaps and Translational Opportunities
Basic Science
In addition to making progress in clinical and public health approaches, there is a need to advance fundamental science that can provide the foundations of more effective interventions for the current crisis and provide the basis to combat future ones. For example, there is a need to better understand the genes implicated in individual differences in vulnerability to the stages and sequelae along the OUD trajectory, including the development of tolerance, physical dependence, addiction, hyperalgesia, and respiratory depression for they could help identify new medication targets and in the future could serve as biomarkers to predict risk for side effects. Animal models could be used to identify the functions of the relevant genetic variants, epigenetic modifications, and gene networks.
Similarly, technological advances, such as those emerging from the BRAIN initiative could be leveraged to gain a deeper knowledge of endogenous opioids and to identify the consequences of exogenous opioid administration on the endogenous opioid systems and the cells and circuits they modulate across a trajectory of opioid use, ranging from acute administration, tolerance, physical dependence, addiction, and recovery to relapse. This could include studying profile changes in the transcriptome and methylome of opioid-synthesizing neurons, imaging the intracellular signaling cascades following opioid receptor activation (and how those cascades change across the trajectory of opioid use) and using quantitative brain-wide optical imaging combined with computational pipelines for cellular registration to provide unbiased documentation of the stereotyped distributions of cellular activity and cell-types engaged by opioids under different conditions. Post-mortem brains could be used to identify human-specific genes and their proteins, as has been done in the study of other psychiatric disorders. Use of post-mortem brains would enable the discovery and integration of genomic, epigenomic, proteomic, metabolomic, and non-coding RNA features across many brain regions and cell types that distinguish individuals with vulnerabilities to OUD and opioid-induced respiratory depression. This knowledge could be combined to validate findings in animal models (i.e., does the animal model recapitulate the human brain biology?) and to reverse translate from human to test a hypothesis in rodents.
A systematic investigation of developmental trajectories of the human brain is relevant for our understanding of the mechanism through which adverse child experiences as well as drug exposures during fetal development increase risk for drug use and other mental illnesses. To address this question, the HEALthy Brain and Child Development (HBCD) Study, which is part of the HEAL Initiative from the National Institutes of Health,155 will establish a large cohort of pregnant women from regions of the country significantly affected by the opioid crisis, and follow them and their children for at least 10 years. The study will help better understand typical brain development, beginning in the prenatal period through early childhood, including variability in development and how it contributes to cognitive, behavioral, social, and emotional function.
New Medications
Despite the existence of effective medications for the treatment of OUD, current rates of engagement and retention in treatment suggest that new medications and devices are needed. The introduction of more acceptable and effective medications is crucial to improve the outcomes of individuals with OUD. In turn, improved outcomes could lead to changes in societal attitudes towards OUD, with broader acceptance of OUD as a treatable brain disorder and lower stigmatization of those suffering from it. Because the circuitry and neurotransmitters of the different phases of OUD only partially overlap, several complementary approaches may be necessary to treat the whole spectrum of severity from sporadic to chronic use, withdrawal and relapse.
To help speed the development of novel pharmacotherapies (i.e., excluding already known mechanisms) NIDA recently created a list of medication development priorities based on data from published literature and internal studies with the most direct relevance to OUD.156 This list, which is not exclusive did not prioritize any of the 10 proposed mechanisms and included: 1) orexin-1 or 1/2 antagonists; 2) Kappa opioid antagonists; 3) GABA-B agonists; 4) Muscarinic M5 antagonists; 5) AMPA antagonists; 6) nociception opioid peptide receptors/opioid receptor like agonists or antagonists; 7) mGluR2/3 agonists; 8) Ghrelin antagonists; 9) Dopamine D3 partial agonists; and, 10) Cannabinoid CB-1 antagonists.
In addition to medications that target specific receptors, the development of allosteric modulators of those receptors is also a high priority. Negative allosteric modulators (NAMs) and positive allosteric modulators (PAMs) may provide more physiologically relevant effects compared with agonists and antagonists acting on the same receptor, which may ultimately result in improved clinical outcomes.157 The development of opioids that selectively activate MOR G-protein intracellular pathways (“biased agonists”) may lead to medication with potent analgesic properties but with lower risk of respiratory depression and addictive liability, which would contribute to decrease the incidence and prevalence of OUD.158 Other potential directions could include epigenetic, micro RNA and neuroimmune targets.
Better training of health professionals
Despite the severity of the dual crises of chronic pain and OUD, very few medical schools offer adequate training in pain management, and still fewer offer even one course in addiction. Furthermore, surprisingly little is known about how best to train physicians and other health professionals on the management of OUD including the use of medications.159 There is also evidence that many individuals trained to provide MOUD do not provide that treatment. Some national organizations offer a combination of didactics, supervision and mentoring to provide training beyond residency, 159 and the new subspecialty of addiction medicine is likely to further increase the availability of well-trained professionals that can treat individuals with OUD. However, broader changes, including combating stigma, enhancing institutional support and increasing reimbursement rates may be necessary to increase rates of treatment among those trained to provide it. Increasing the number of addiction treatment and prevention programs, particularly in low-resource communities is also essential.
Conclusion
The opioid crisis is a complex, evolving phenomenon. It involves neurobiological vulnerabilities and social determinants of health. Successfully addressing the crisis will require advances in basic science, development of more effective treatments, and public health approaches to implement current and emerging knowledge. We hope that this review will alert clinicians and researchers about the current approaches to the crisis and suggest opportunities for future research and for practice improvement. The advances achieved in addressing the current crisis should also serve to expand the science and treatment of other substance use disorders.
Acknowledgments:
The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies or the US government.
Footnotes
Conflict of Interest: The authors have no competing financial interests in relation to the work described in this manuscript.
References
- 1.Compton WM JC, Baldwin G, Harding F, Blanco C, Wargo EM. Using Prevention Science Principles to Address the U.S. Opioid Crisis. AJPH, in press 2016. [Google Scholar]
- 2.Han B, Compton WM, Blanco C, Jones CM. Correlates of Prescription Opioid Use, Misuse, Use Disorders, and Motivations for Misuse Among US Adults. The Journal of clinical psychiatry 2018; 79(5). [DOI] [PubMed] [Google Scholar]
- 3.Olfson M, Crystal S, Wall M, Wang S, Liu S, Blanco C. Causes of death after nonfatal opioid overdose. JAMA Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olfson M, Wall M, Wang S, Crystal S, Blanco C. Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug and alcohol dependence 2018; 190: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pincus HA BC. The Opioid Crisis in America: An Overview In: Confronting Our Nation’s Opioid Crisis, A Report of the Aspen Health Strategy Group. The Aspen Institute; 2017: 23–46. [Google Scholar]
- 6.Medicine Io. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 7.Addiction Rare in Patients Treated with Narcotics. New England Journal of Medicine 1980; 302(2): 123–123. [DOI] [PubMed] [Google Scholar]
- 8.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4): 569–576. [DOI] [PubMed] [Google Scholar]
- 9.Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychological medicine 2012; 42(6): 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco C, Alderson D, Ogburn E, Grant BF, Nunes EV, Hatzenbuehler ML et al. Changes in the prevalence of non-medical prescription drug use and drug use disorders in the United States: 1991-1992 and 2001-2002. Drug and alcohol dependence 2007; 90(2-3): 252–260. [DOI] [PubMed] [Google Scholar]
- 11.Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J et al. Nonmedical Prescription Opioid Use and DSM-5 Nonmedical Prescription Opioid Use Disorder in the United States. The Journal of clinical psychiatry 2016; 77(6): 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han B, Compton WM, Jones CM, Cai R. Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003-2013. Jama 2015; 314(14): 1468–1478. [DOI] [PubMed] [Google Scholar]
- 13.Mars SG, Fessel JN, Bourgois P, Montero F, Karandinos G, Ciccarone D. Heroin-related overdose: The unexplored influences of markets, marketing and source-types in the United States. Social science & medicine (1982) 2015; 140: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unick G, Rosenblum D, Mars S, Ciccarone D. The relationship between US heroin market dynamics and heroin-related overdose, 1992-2008. Addiction (Abingdon, England) 2014; 109(11): 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Associations of nonmedical pain reliever use and initiation of heroin use in the United States. http://archive.samhsa.gov/data/2k13/DataReview/DR006/nonmedical-pain-reliever-use-2013.pdf, 2013, Accessed Date Accessed 2013 Accessed.
- 16.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. The New England Journal of Medicine 2016; 374(2): 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drug overdose deaths in the United States, 1999–2017. 2018, Accessed Date Accessed 2018 Accessed.
- 18.Jones CM, Einstein EB, Compton WM. Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010-2016Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010-2016Letters. Jama 2018; 319(17): 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank RG, Pollack HA. Addressing the Fentanyl Threat to Public Health. The New England Journal of Medicine 2017; 376(7): 605–607. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki J, El-Haddad S. A review: Fentanyl and non-pharmaceutical fentanyls. Drug and alcohol dependence 2017; 171: 107–116. [DOI] [PubMed] [Google Scholar]
- 21.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annual review of neuroscience 1984; 7: 223–255. [DOI] [PubMed] [Google Scholar]
- 22.Pattinson KT. Opioids and the control of respiration. British journal of anaesthesia 2008; 100(6): 747–758. [DOI] [PubMed] [Google Scholar]
- 23.Elliott JA SH. Handbook of Acute Pain Management. CRC Press: 73–109. [Google Scholar]
- 24.Foley KM. The treatment of cancer pain. The New England Journal of Medicine 1985; 313(2): 84–95. [DOI] [PubMed] [Google Scholar]
- 25.Foley KM, Inturrisi CE. Analgesic drug therapy in cancer pain: principles and practice. The Medical clinics of North America 1987; 71(2): 207–232. [DOI] [PubMed] [Google Scholar]
- 26.Freye E LJ. Opioids in medicine. A comprehensive review on the mode of action and the use of analgesics in different clinical pain studies. Springer; 2008; Dordrecht, The Netherlands. [Google Scholar]
- 27.Pharmacists TASoH-S. Fentanyl, Fentanyl Citrate, Fentanyl Hydrochloride. 2018.
- 28.Reisine T PG. Opioid analgesics and antagonists. In: Hardman JG, Limbird LE. Goodman & Gilman’s The pharmacological basis of therapeutics. 1996: 521–556. [Google Scholar]
- 29.Smith H PS. Pain and Chemical Dependency. Oxford University Press, New York, NY: 183–202. [Google Scholar]
- 30.Vallejo R, Barkin RL, Wang VC. Pharmacology of opioids in the treatment of chronic pain syndromes. Pain physician 2011; 14(4): E343–360. [PubMed] [Google Scholar]
- 31.Cai NS, Quiroz C, Bonaventura J, Bonifazi A, Cole TO, Purks J et al. Opioid-galanin receptor heteromers mediate the dopaminergic effects of opioids. The Journal of clinical investigation 2019; 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life sciences 1989; 45(18): 1627–1636. [DOI] [PubMed] [Google Scholar]
- 33.Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 2008; 154(2): 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends in neurosciences 2007; 30(3): 119–125. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Lu Z, Xu M, Pan L, Deng Y, Xie X et al. A heroin addiction severity-associated intronic single nucleotide polymorphism modulates alternative pre-mRNA splicing of the mu opioid receptor gene OPRM1 via hnRNPH interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014; 34(33): 11048–11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson EC, Agrawal A, Heath AC, Bogdan R, Sherva R, Zhang B et al. Evidence of CNIH3 involvement in opioid dependence. Molecular psychiatry 2016; 21(5): 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwald MK, Steinmiller CL, Sliwerska E, Lundahl L, Burmeister M. BDNF Val(66)Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addiction biology 2013; 18(5): 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children, vol. 851e854. Science 297 (5582)2002. [DOI] [PubMed] [Google Scholar]
- 39.Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J et al. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biological psychiatry 2009; 65(5): 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature neuroscience 2005; 8(5): 594–596. [DOI] [PubMed] [Google Scholar]
- 41.Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Archives of general psychiatry 2006; 63(12): 1396–1406. [DOI] [PubMed] [Google Scholar]
- 42.Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Therapeutic drug monitoring 2002; 24(1): 163–171. [DOI] [PubMed] [Google Scholar]
- 43.Tunbridge EM, Huber A, Farrell SM, Stumpenhorst K, Harrison PJ, Walton ME The role of catechol-O-methyltransferase in reward processing and addiction. CNS Neurol Disord Drug Targets 2012; 11(3): 306e323. [DOI] [PubMed] [Google Scholar]
- 44.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Frontiers in neuroanatomy 2011; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samochowiec A, Grzywacz A, Kaczmarek L, Bienkowski P, Samochowiec J, Mierzejewski P et al. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain research 2010; 1327: 103–106. [DOI] [PubMed] [Google Scholar]
- 46.Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proceedings of the National Academy of Sciences of the United States of America 2012; 109(43): 17675–17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Current opinion in neurobiology 2006; 16(3): 251–257. [DOI] [PubMed] [Google Scholar]
- 48.Stefaniuk M, Beroun A, Lebitko T, Markina O, Leski S, Meyza K et al. Matrix Metalloproteinase-9 and Synaptic Plasticity in the Central Amygdala in Control of Alcohol-Seeking Behavior. Biological psychiatry 2017; 81(11): 907–917. [DOI] [PubMed] [Google Scholar]
- 49.Samochowiec J, Ladehoff M, Pelz J, Smolka M, Schmidt LG, Rommelspacher H et al. Predominant influence of the 3’-region of dopamine D2 receptor gene (DRD2) on the clinical phenotype in German alcoholics. Pharmacogenetics 2000; 10(5): 471–475. [DOI] [PubMed] [Google Scholar]
- 50.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama 2008; 299(11): 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitt JE, Neale MC, Fassassi B, Perez J, Lenroot RK, Wells EM et al. The dynamic role of genetics on cortical patterning during childhood and adolescence. Proceedings of the National Academy of Sciences of the United States of America 2014; 111(18): 6774–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology 2014; 76 Pt B: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.https://abcdstudy.org. , 2019, Accessed Date Accessed 2019 Accessed.
- 54.Baby Connectome Project. https://fnih.org/what-we-do/programs/baby-connectome, 2019, Accessed Date Accessed 2019 Accessed.
- 55.Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cellular and molecular life sciences : CMLS 2006; 63(9): 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Champagne FA, Curley JP. How social experiences influence the brain. Current opinion in neurobiology 2005; 15(6): 704–709. [DOI] [PubMed] [Google Scholar]
- 57.Sun H, Maze I, Dietz DM, Scobie KN, Kennedy PJ, Damez-Werno D et al. Morphine epigenomically regulates behavior through alterations in histone H3 lysine 9 dimethylation in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012; 32(48): 17454–17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS). Cerebral cortex (New York, NY : 1991) 2010; 20(3): 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA 3rd. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America 2012; 109(32): 12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedman EM, Karlamangla AS, Almeida DM, Seeman TE. Social strain and cortisol regulation in midlife in the US. Social science & medicine (1982) 2012; 74(4): 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karatsoreos IN, McEwen BS. Annual Research Review: The neurobiology and physiology of resilience and adaptation across the life course. Journal of Child Psychology and Psychiatry 2013; 54(4): 337–347. [DOI] [PubMed] [Google Scholar]
- 62.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science (New York, NY) 1997; 278(5335): 52–58. [DOI] [PubMed] [Google Scholar]
- 63.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends in neurosciences 2009; 32(10): 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gianoulakis C Endogenous opioids and addiction to alcohol and other drugs of abuse. Current topics in medicinal chemistry 2004; 4(1): 39–50. [DOI] [PubMed] [Google Scholar]
- 65.Panlilio LV, Justinova Z. Preclinical Studies of Cannabinoid Reward, Treatments for Cannabis Use Disorder, and Addiction-Related Effects of Cannabinoid Exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2018; 43(1): 116–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology 2014; 76 Pt B: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 2016; 3(8): 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 2011; 69(4): 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proceedings of the National Academy of Sciences of the United States of America 2009; 106(18): 7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behavioural brain research 2009; 199(1): 89–102. [DOI] [PubMed] [Google Scholar]
- 71.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry 2005; 162(8): 1403–1413. [DOI] [PubMed] [Google Scholar]
- 72.Perez de los Cobos J, Batlle F, Casas M. [Proposal for improving the integration of drug dependencies in psychiatric nosology]. Actas luso-espanolas de neurologia, psiquiatria y ciencias afines 1996; 24(2): 63–65. [PubMed] [Google Scholar]
- 73.Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Reviews in the neurosciences 2008; 19(4-5): 227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koob GF, Le Moal M. Addiction and the brain antireward system. Annual review of psychology 2008; 59: 29–53. [DOI] [PubMed] [Google Scholar]
- 75.Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience 1990; 37(3): 767–773. [DOI] [PubMed] [Google Scholar]
- 76.Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE et al. Addiction as a stress surfeit disorder. Neuropharmacology 2014; 76 Pt B: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimura M, Satoh T, Matsumoto N. What does the habenula tell dopamine neurons? Nature neuroscience 2007; 10(6): 677–678. [DOI] [PubMed] [Google Scholar]
- 78.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nature reviews Neuroscience 2010; 11(7): 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007; 447(7148): 1111–1115. [DOI] [PubMed] [Google Scholar]
- 80.Metzger M, Bueno D, Lima LB. The lateral habenula and the serotonergic system. Pharmacology, biochemistry, and behavior 2017; 162: 22–28. [DOI] [PubMed] [Google Scholar]
- 81.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in neurosciences 2009; 32(1): 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2002; 26(3): 376–386. [DOI] [PubMed] [Google Scholar]
- 83.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage 2004; 23(4): 1486–1493. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007; 27(51): 14035–14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and biobehavioral reviews 2014; 38: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, affective & behavioral neuroscience 2012; 12(2): 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience 1999; 19(13): 5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical transactions of the Royal Society of London Series B, Biological sciences 1996; 351(1346): 1413–1420. [DOI] [PubMed] [Google Scholar]
- 89.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007; 27(33): 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America 2011; 108(37): 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. The American journal of psychiatry 2001; 158(12): 2015–2021. [DOI] [PubMed] [Google Scholar]
- 92.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse (New York, NY) 1993; 14(2): 169–177. [DOI] [PubMed] [Google Scholar]
- 93.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007; 27(46): 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences 2012; 109(44): 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim HJ, Lee JH, Yun K, Kim JH. Alterations in Striatal Circuits Underlying Addiction-Like Behaviors. Molecules and cells 2017; 40(6): 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral cortex (New York, NY : 1991) 2000; 10(3): 318–325. [DOI] [PubMed] [Google Scholar]
- 97.Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2010; 35(3): 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN. Brain Activity During Cocaine Craving and Gambling Urges: An fMRI Study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2016; 41(2): 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Applied psychophysiology and biofeedback 2005; 30(3): 195–204. [DOI] [PubMed] [Google Scholar]
- 100.Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage 2005; 26(4): 1097–1108. [DOI] [PubMed] [Google Scholar]
- 101.Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience 2005; 25(15): 3932–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khaled MA, Pushparaj A, Di Ciano P, Diaz J, Le Foll B. Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2014; 39(13): 3049–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016; 36(33): 8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yager LM, Garcia AF, Donckels EA, Ferguson SM. Chemogenetic inhibition of direct pathway striatal neurons normalizes pathological, cue-induced reinstatement of drug-seeking in rats. Addiction biology 2019; 24(2): 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annual review of pharmacology and toxicology 1996; 36: 359–378. [DOI] [PubMed] [Google Scholar]
- 106.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS drugs 2005; 19(6): 517–537. [DOI] [PubMed] [Google Scholar]
- 107.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism, clinical and experimental research 2002; 26(10): 1494–1501. [DOI] [PubMed] [Google Scholar]
- 108.Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological psychiatry 2010; 67(8): 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Administration SAaMHS. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63, Full Document 2018; HHS Publication No. (SMA) 18-5063FULLDOC. .
- 110.National Academies of Sciences E, and Medicine Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. [PubMed] [Google Scholar]
- 111.Gowing L, Ali R, White JM. Opioid antagonists with minimal sedation for opioid withdrawal. The Cochrane Database of Systematic Reviews 2017; 5: Cd002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. The Cochrane Database of Systematic Reviews 2011; (9): Cd005031. [DOI] [PubMed] [Google Scholar]
- 113.Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D et al. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction (Abingdon, England) 2005; 100(8): 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Irish medical journal 2010; 103(6): 176–179. [PubMed] [Google Scholar]
- 115.Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction (Abingdon, England) 2010; 105(9): 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of addictive diseases 2016; 35(1): 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. Journal of substance abuse treatment 2005; 28(4): 321–329. [DOI] [PubMed] [Google Scholar]
- 118.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug and alcohol dependence 2009; 105(1-2): 9–15. [DOI] [PubMed] [Google Scholar]
- 119.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. The Cochrane Database of Systematic Reviews 2014; (2): Cd002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McDermott KA, Griffin ML, Connery HS, Hilario EY, Fiellin DA, Fitzmaurice GM et al. Initial response as a predictor of 12-week buprenorphine-naloxone treatment response in a prescription opioid-dependent population. The Journal of clinical psychiatry 2015; 76(2): 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. The Cochrane Database of Systematic Reviews 2016; (5): Cd011117. [DOI] [PubMed] [Google Scholar]
- 122.Faggiano F V-TF, Versino E, Lemma P. . Methadone maintenance at different dosages for opioid dependence. . The Cochrane Database of Systematic Reviews 2003; 3(Cd002208). [DOI] [PubMed] [Google Scholar]
- 123.Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2019; 393(10173): 778–790. [DOI] [PubMed] [Google Scholar]
- 124.Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. Journal of addiction medicine 2014; 8(5): 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG et al. Antidepressant-like Effects of Buprenorphine are Mediated by Kappa Opioid Receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2016; 41(9): 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khanna IK, Pillarisetti S. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. Journal of pain research 2015; 8: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenthal RN, Lofwall MR, Kim S, Chen M, Beebe KL, Vocci FJ. Effect of Buprenorphine Implants on Illicit Opioid Use Among Abstinent Adults With Opioid Dependence Treated With Sublingual Buprenorphine: A Randomized Clinical Trial. Jama 2016; 316(3): 282–290. [DOI] [PubMed] [Google Scholar]
- 128.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Archives of general psychiatry 2006; 63(2): 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet (London, England) 2011; 377(9776): 1506–1513. [DOI] [PubMed] [Google Scholar]
- 130.Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA Jr. et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. The New England Journal of Medicine 2016; 374(13): 1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sullivan M, Bisaga A, Pavlicova M, Choi CJ, Mishlen K, Carpenter KM et al. Long-Acting Injectable Naltrexone Induction: A Randomized Trial of Outpatient Opioid Detoxification With Naltrexone Versus Buprenorphine. The American journal of psychiatry 2017; 174(5): 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vijay A, Morris E, Goldberg A, Petrulli J, Liu H, Huang Y, and Krishnan-Sarin S. Naltrexone occupancy at kappa opioid receptors investigated in alcoholics by PET occupancy at kappa opioid receptors investigated in alcoholics by PET J Nucl Med 2017; 58(1297). [Google Scholar]
- 133.Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ et al. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2008; 33(3): 653–665. [DOI] [PubMed] [Google Scholar]
- 134.Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet (London, England) 2018; 391(10118): 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma-Haase K et al. Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA Psychiatry 2017; 74(12): 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cushman P. Abstinence following detoxification and methadone maintenance treatment. The American Journal of Medicine 1978; 65(1): 46–52. [DOI] [PubMed] [Google Scholar]
- 137.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of substance abuse treatment 2018; 85: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nosyk B, Sun H, Evans E, Marsh DC, Anglin MD, Hser YI et al. Defining dosing pattern characteristics of successful tapers following methadone maintenance treatment: results from a population-based retrospective cohort study. Addiction (Abingdon, England) 2012; 107(9): 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ (Clinical research ed) 2017; 357: j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwartz RP, Highfield DA, Jaffe JH, Brady JV, Butler CB, Rouse CO et al. A randomized controlled trial of interim methadone maintenance. Archives of general psychiatry 2006; 63(1): 102–109. [DOI] [PubMed] [Google Scholar]
- 141.Sigmon SC, Schwartz RP, Higgins ST. Buprenorphine for persons on waiting lists for treatment for opioid dependence. The New England Journal of Medicine 2017; 376(10): 1000–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carroll KM, Weiss RD. The Role of Behavioral Interventions in Buprenorphine Maintenance Treatment: A Review. The American journal of psychiatry 2017; 174(8): 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Volkow ND, McLellan AT. Mitigation Strategies for Opioid Abuse. The New England Journal of Medicine 2016; 375(1): 96. [DOI] [PubMed] [Google Scholar]
- 144.Albert S, Brason FW 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain medicine (Malden, Mass) 2011; 12 Suppl 2: S77–85. [DOI] [PubMed] [Google Scholar]
- 145.Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ (Clinical research ed) 2013; 346: f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Freeman PR, Hankosky ER, Lofwall MR, Talbert JC. The changing landscape of naloxone availability in the United States, 2011 - 2017. Drug and alcohol dependence 2018; 191: 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murphy SM, Morgan JR, Jeng PJ, Schackman BR. Will converting naloxone to over-the-counter status increase pharmacy sales? Health services research 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Substance Abuse and Mental Health Services Administration SAaMHS. SAMHSA opioid overdose prevention toolkit. . HHS Publication (2016); (No. (SMA) 16-4742).
- 149.D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. Jama 2015; 313(16): 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA Psychiatry 2019; 76(2): 208–216. [DOI] [PubMed] [Google Scholar]
- 151.Cicero TJ, Ellis MS, Kasper ZA. Increased use of heroin as an initiating opioid of abuse. Addictive Behaviors 2017; 74: 63–66. [DOI] [PubMed] [Google Scholar]
- 152.Spoth R, Trudeau L, Shin C, Ralston E, Redmond C, Greenberg M et al. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. American journal of public health 2013; 103(4): 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blanco C WM, Liu SM, Olfson M. . Towards a comprehensive developmental model of prescription opioid use disorder. . J Clin Psychiatry, in press. [DOI] [PubMed] [Google Scholar]
- 154.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Annals of Internal Medicine 2017; 167(5): 293–301. [DOI] [PubMed] [Google Scholar]
- 155.Collins FS, Koroshetz WJ, Volkow ND. Helping to End Addiction Over the Long-term: The Research Plan for the NIH HEAL Initiative. Jama 2018; 320(2): 129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rasmussen K, White DA, Acri JB. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2019; 44(4): 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nature reviews Drug discovery 2009; 8(1): 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016; 537(7619): 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Levin FR, Bisaga A, Sullivan MA, Williams AR, Cates-Wessel K. A review of a national training initiative to increase provider use of MAT to address the opioid epidemic. The American journal on addictions 2016; 25(8): 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]