Abstract
Introduction:
Recently, a combined reflectance confocal microscopy (RCM)-optical coherence tomography (OCT) has been tested for the diagnosis of basal cell carcinoma (BCC). Evaluating the role of RCM-OCT in management of complex BCCs has not been studied. The objective of the study was to investigate the utility of a new combined RCM-OCT device in the evaluation and management of complex BCCs in a descriptive study.
Methods:
Prospective study of consecutive cases (July 2018- June 2019) of biopsy proven ‘complex’ BCC defined as BCC in the head and neck area with multiple high risk criteria such as large size in the mask area, multiple recurrences, and infiltrative subtype. All cases were evaluated with a combined RCM-OCT device that provided simultaneous image viewing on a screen. Lesions were evaluated bedside with RCM-OCT according to previously described criteria.
Results:
Ten patients with complex head and neck BCCs had mean age of 73.1±13.0 years. Six (60%) patients were males. Mean BCC clinical size was 1.9±1.2 cm (range 0.6–4.0 cm). RCM detected residual BCC in 8 out of 10 cases (80%) and OCT detected residual BCC in all 10 cases (100%). Six BCCs (60%) had a depth estimate of >1000 μm under OCT. In 5 cases, (50%) RCM-OCT imaging results led to a change/modification in BCC management.
Conclusion:
The use of a combined RCM-OCT device may help in the evaluation of complex head and neck BCCs by guiding treatment selection and defining the extent of surgery.
Keywords: basal cell carcinoma, residual, reflectance confocal microscopy, biopsy, surgery, Mohs, dermoscopy
Introduction:
Basal cell carcinoma (BCC) is the most common skin tumor in Caucasians with more than 2 million cases per year in the United States alone.1,2 Diagnosis of BCC has been traditionally made clinically with the aid of a biopsy, but in the last 30 years, dermoscopy3 and reflectance confocal microscopy4 have been used as an aid to improve diagnostic accuracy. Despite the increasing number of cases, presurgical evaluation has remained unchanged with up to 15% of BCCs displaying a more aggressive histologic subtype at the time of the surgery when compared to a biopsy.5,6
Multiple non-invasive imaging modalities have emerged as an aid for the diagnosis and management of BCC. Although dermoscopy has demonstrated a high diagnostic accuracy,3,7 its use in BCC margin mapping prior to Mohs surgery is not reliable.8,9 Reflectance confocal microscopy (RCM) is a non-invasive, quasi-histological device that has improved the diagnosis of both melanocytic and non-melanocytic tumors.10 It has greatly improved the diagnosis of BCC.11-14 However, the main limitation of RCM is that it is limited to a depth of 200 – 250 μm; similar to a shave biopsy ‘transecting’ the tumor base.10 Many aggressive, large, or recurrent BCCs have a deeper component not evaluable by RCM alone, limiting the role of RCM in the evaluation of BCC ‘deep’ component.12
Optical coherence tomography (OCT), on the other hand, is a non-invasive imaging modality that lacks the ‘quasi-histological’ resolution of RCM, but provides deeper tumor information due to its higher tissue penetration.5,15 The combination of contrasting non-invasive imaging modalities or ‘multimodal imaging’ enables the synergistic exploitation of each individual technology advantages.5,15,16 Recently, a new combined multimodal RCM-OCT device17 has shown excellent diagnostic accuracy and depth estimation for the diagnosis of BCCs.5,18 However, the studies included only small BCCs and was designed primarily to measure its diagnostic role.5,18 Herein, we sought to perform a descriptive study in the utility of a combined RCM-OCT device to guide evaluation and management of complex head and neck BCCs.
Patients and methods:
This was a prospective IRB-approved (No 99-099) study conducted between July 2018 to June 2019 at a tertiary care cancer center. All patients gave written informed consent prior to imaging. We included consecutive patients with biopsy-proven head and neck BCC referred for evaluation and management. The definition of ‘complex’ BCC was made based on: (1) recurrent BCC in a high-risk location (H-zone) according to the National Comprehensive Cancer Network (NCCN) guidelines;19 (2) multiple recurrent BCC on the head and neck (≥2 recurrences); (3) histological high-risk subtype (infiltrative, morpheaform, basosquamous) in an H-zone; (4) BCCs larger than 2 cm in an H-zone; and (5) BCCs where excision would likely require a complex reconstruction (e.g. forehead flap). Patients were excluded when the RCM-OCT probe could not access the anatomical site, such as the conchal bowl.
Demographic and clinical characteristics:
Demographic characteristics (age, sex), BCC location, clinical size, and predominant histologic subtype were recorded. All biopsy specimens were evaluated by an expert dermatopathologist who confirmed the diagnosis and subtype of BCC. Clinical images of the lesions were captured using a digital camera (VeosDS3, Canfield Scientific, NJ, USA).
Reflectance confocal microscopy-optical coherence tomography imaging protocol:
RCM-OCT imaging was performed using the hand-held probe following the technical details previously described.20 Briefly, RCM imaging has a lateral resolution of ~1μm and 3-5μm axial resolution; with an en face field of view (FOV) of 750x750μm. OCT imaging has a lateral resolution of ~5μm, axial resolution of 10μm, and orthogonal FOV of 1000μm deep x2000μm wide. The en-face RCM images are centered on the OCT FOV and both images are co-registered and viewed simultaneously in real-time on a single monitor. We used adhesive paper rings (3M, Flemington, NJ) or paper tape to delineate the lesion and margin demarcation, as described.21,22
RCM-OCT evaluations were performed in a systematic fashion. First, the center of the lesion was evaluated to assess for residual status with the intent to scout a 100% of the area. Then, margins were assessed; each quadrant was circumferentially imaged from 12-3, 3-6, 6-9, and 9-12 o’clock, respectively. If suspicious areas were found in any of the quadrants, we explored by maneuvering the handheld RCM-OCT probe in those areas. Upon encountering suspicious foci, we acquired RCM stacks (every 3 μm to 150 μm). OCT rasters were recorded simultaneously and OCT estimated-depth was measured.18 Peri-lesional skin was also imaged in all patients to evaluate for subclinical disease/margins.
Image evaluation:
The combined (co-registered) RCM-OCT images were valuated and recorded bedside in a database in ‘real-time’ for consensus by 3 investigators with more than 2 years’ experience in RCM-OCT (C.N-D., S.A., M.C.). The RCM features used for BCC detection were based on a recent systematic review and Longo et al: streaming, atypical honeycomb, dendritic cells in the epidermis, cord-like structures, tumor nests, clefting, palisading, dark silhouettes, horizontal vessels, vertical vessels, inflammation, plump cells, and collagen type (reticular or bundled).23,24 OCT features used for BCC detection were those previously described by Sahu et al. and others: hyporeflective/gray structures attached to the dermal-epidermal junction (DEJ), thickened epidermis, hyporeflective/gray ovoid structures in the dermis, and hyper-reflective peritumoral stroma (‘white lines’).25-27 OCT-estimated depth for BCC was measured (in microns) from the first hyperreflective layer of the epidermis (corneal layer) to the deepest area with tumor. BCC lesions extending beyond maximum measured depth (1000 μm) were recorded as >1000μm.
Outcomes and statistical analysis:
The primary outcome was to describe the features of complex BCC seen under RCM-OCT. The secondary outcome was the clinical relevance of RCM-OCT imaging by describing any changes/modification in clinical decision making. Unless otherwise noted, all values are expressed as mean ± standard deviation (SD). Descriptive and relative frequencies were used to describe the survey respondents and the results of the consensus. Data was analyzed using SPSS 23.0 (SPSS, Armonk, NY, USA).
Results:
Ten patients with complex head and neck BCCs met the inclusion criteria for the study. Reasons for inclusion were: 4 (40%) multiple recurrent BCC, 3 (30%) larger than 2 cm BCC, and 3 (30%) had a complex reconstruction predicted. Details are available in Table 1.
Table 1:
Demographics, tumor characteristics, reflectance confocal microscopy, and optical coherence tomography features of complex head and neck basal cell carcinomas. *Palisading and clefting was recorded for both tumor nodules and cord-like structures. SD = standard deviation; BCC = basal cell carcinoma (n=10).
| Variable | Value |
|---|---|
| Age, y, mean (SD) | 73.1 (13.0) |
| Gender, male (%) | 6 (60%) |
| Size, cm (SD) | 1.9 (1.2) |
| BCC subtype | |
| - Superficial, n (%) | 3 (30%) |
| - Nodular, n (%) | 4 (40%) |
| - Infiltrative, n (%) | 2 (20%) |
| - Micronodular, n (%) | 1 (10%) |
| Location | |
| - Tip of the nose | 3 (30%) |
| - Nasal ala | 2 (20%) |
| - Forehead | 2 (20%) |
| - Nasal dorsum | 1 (10%) |
| - Medial canthus | 1 (10%) |
| - Eyebrow | 1 (10%) |
| Reflectance confocal microscopy-optical coherence tomography | |
| Reflectance confocal microscopy | N (%) |
| - Streaming | 4 (40%) |
| - Atypical honeycomb | 2 (20%) |
| - Dendritic cells in the epidermis | 1 (10%) |
| - Cord-like structures | 5 (50%) |
| - Tumor nodules | 6 (60%) |
| - Palisading* | 8 (80%) |
| - Clefting* | 7 (70%) |
| - Dark silhouettes | 2 (20%) |
| - Inflammation | 5 (50%) |
| - Plump cells | 1 (10%) |
| - Reticular collagen | 1 (10%) |
| - Horizontal vessels | 4 (40%) |
| - Vertical vessels | 1 (10%) |
| Optical coherence tomography features | |
| - Hyporeflective nodules connected to the epidermis | 5 (50%) |
| - Tumor nodules in the dermis | 7 (70%) |
| - White lines | 5 (50%) |
| - Thickened epidermis | 0 (0%) |
| - Depth >1000μm | 6 (60%) |
Mean age at diagnosis was 73.1±13.0 years (range 46–87 years). Six (60%) patients were males. All BCC cases were located on the head and neck. Mean BCC clinical size was 1.9±1.2 cm (range 0.6–4.0 cm). Regarding BCC main histopathological subtype 4 BCCs were nodular (40%), 3 (30%) were superficial, 2 (20%) were infiltrative, and 1 (10%) was micronodular subtype.
RCM-OCT features and histopathological correlation:
RCM and OCT imaging details are found in Table 1. RCM detected residual BCC features in 8 out of 10 cases (80%). Most common BCC features seen with RCM were tumor nodules (6/10; 80%) and cord-like structures (5/10; 50%). Dark silhouettes were seen in 2 out of 10 cases (20%) (Figures 1-3). Two cases did not detect BCC with RCM; both cases corresponded to infiltrative histologic subtype with a deep component.
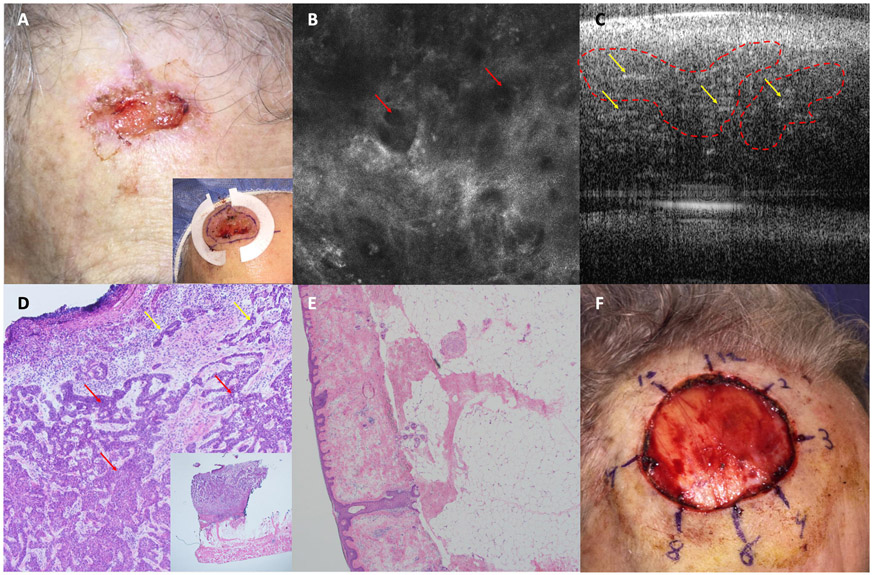
Figure 1: Complex Basal cell Carcinoma (BCC):
A. Biopsy proven BCC on right forehead with multiple high risk features (3 cm, H zone, infiltrative histology) . Inset shows paper rings to estimate margins. B. Reflectance confocal microscopy (RCM) image of lesion center showing dark silhouettes (red arrows) (750 x 750 μm). C. Optical coherence tomography (OCT) image showing hyporeflective tumor nodules in the dermis (red dashed lines) and white lines (yellow arrows). D. Photomicrograph of lesion center showing deep infiltrative tumor strands (red arrows) (H&E, 20X). Inset shows tumor extending to the adipose tissue but sparing muscle (H&E 2X). E. Photomicrograph of a Mohs micrographic surgery margin at the RCM-OCT estimated negative margin showing no residual BCC (H&E, 4X). F. Image of the surgical defect.
Figure 3: Complex Basal cell Carcinoma (BCC):
A. Biopsy proven superficial BCC on nasal tip, clinically measuring 2 cm; surgical resection will likely require reconstruction with a forehead flap. B. Reflectance confocal microscopy (RCM) image showing cord-like structures (red arrows), with palisading (green arrows) and horizontal vessels (blue arrows) (750 x 750 μm). C. Optical coherence tomography (OCT) image showing hyporeflective tumor nodules attached to the epidermis. D. Clinical appearance 12 months after ALA-photodynamic therapy. No clinical or RCM signs of recurrence were seen.
OCT detected BCC in all 10 cases (100%) (Figures 1-3). The most common features seen with OCT were tumor nodules in the dermis (7/10; 70%) and hyporeflective nodules connected to the epidermis (5/5; 50%). Six cases (60%) had a BCC depth estimate of >1000 μm (Figure 1).
RCM-OCT imaging impact on BCC management:
In 5 cases (50%), RCM-OCT imaging results lead to a change in BCC management. In 3 cases (30%), there was a change in the extent of BCC surgical planning as greater margins were necessary (Figure 2). In 2 cases, there was a change in therapy from surgery to non-surgical treatments. One patient chose off-label topical imiquimod and 1 patient chose off-label photodynamic therapy [Figure 3]) due to presence of superficial BCC only (to a depth of <400 μm based on OCT estimates) and desire to avoid extensive surgery requiring complex reconstruction in setting of multiple co-morbidities. In the other 5 cases (50%), RCM-OCT correctly determined presence of residual BCC, subtype, and extent of disease but there was no change in the initial surgical plan.
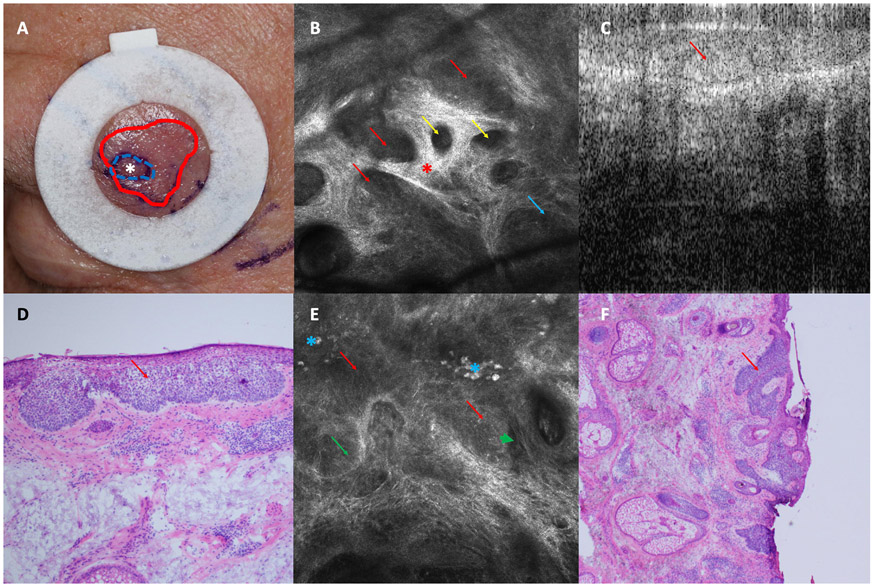
Figure 2: Complex Basal cell Carcinoma (BCC):
A. Biopsy proven superficial BCC on left eyebrow clinically measuring 0.8-cm. After reflectance confocal microscopy (RCM)-optical coherence tomography (OCT) evaluation, actual BCC extent was estimated 2.1 cm(White asterisk represents biopsy site. Blue-dashed line represents initial clinically estimated surgical margins. Red line represents RCM-OCT estimated margins). B. Reflectance confocal microscopy (RCM) image showing tumor nodules (red arrows) with palisading (blue arrows), dark silhouettes (yellow arrows), and bundled collagen (red asterisk) (750 x 750 μm). C. Optical Coherence Tomography (OCT) image showing hyporeflective tumor attached to the epidermis. D. Photomicrograph of the blue dashed area in panel A confirming superficial BCC (H&E, 20X). E. RCM image at the margins showing cord-like structures (red arrows) with palisading (blue arrow) and clefting (green arrow). Plump cells were also seen (blue asterisk) (750 x 750 μm). F. Photomicrograph of a Mohs layer showing superficial BCC at the margins (red arrows) (H&E, 4X).
Discussion:
In this descriptive, prospective case-series of 10 patients with complex head and neck BCC, we found that the use of a combined, co-registered, RCM-OCT was able to detect residual BCC and extent of disease in all cases. Two cases of infiltrative BCC were missed with RCM imaging alone; but both cases were successfully detected by the simultaneous RCM-OCT evaluation. Since RCM has the potential to miss deep BCC,5,12 these results highlight the relevance and potential benefits of having a multimodal co-registered approach. Moreover, in half of the cases, there was a change or modification in management of BCC after RCM-OCT evaluation. These results are particularly applicable to complex BCCs arising in the head and neck area with multiple functional and cosmetic considerations. Pre-surgical evaluation of these patients may potentially help select the best treatment modality and plan reconstruction accordingly. This may also lead to increased patient satisfaction by eliminating the “unknown’ factor and be included in shared decision-making tools.28-31
Multimodal imaging has the advantage of combining 2 or more imaging modalities that have specific characteristics and inherent strengths and limitations which synergistically enhance overall imaging capabilities. Sahu et al. showed that the combined RCM-OCT device evaluated in this study had a sensitivity of a 100% and a specificity of 23.1% for the diagnosis of BCC in previously-biopsied lesions. In non-biopsied suspicious lesions, sensitivity was 100% and specificity 75%.18 Recently, Aleissa et al. showed a sensitivity of 82.6% and a specificity of 93.8% for the diagnosis of residual BCC and margin evaluation in 35 patients prior to Mohs surgery.5 In the present study, we were able to visualize BCC-specific features imaged with a combined RCM-OCT device. Additionally, Sahu et al. and Aleissa et al. showed a good-to-excellent correlation between RCM-OCT calculated BCC depth and histopathology-measured BCC depth of (r2=0.75-0.9).5,18 This is relevant since studies have shown that imiquimod treatment is associated with no recurrences when used in BCCs <400 μm depth vs 58% for tumors>400 μm.32 Based on this, we advised 2 patients presenting with large superficial BCCs to undergo non-surgical treatments given tumor was limited to a maximum depth <300μm. This approach could be invaluable when selecting non-surgical treatment modalities for large superficial BCC as the handheld RCM-OCT device can theoretically scout the entirety of the lesion.
In the future, the information provided by non-invasive imaging devices such as RCM-OCT can potentially help address patients’ and physicians’ concerns pre surgically. The use of RCM-OCT and other non-invasive imaging modalities can estimate margin extent, predicted defect size, and potential surgical reconstruction type. This can help select the best treatment modality (surgery vs other therapies such as imiquimod), potentially reduce costs (by reducing number of stages), as well as improve patient satisfaction by providing with more and better pre-surgical information.5,12,13
Limitations:
The main limitation of our study was the small sample size, this makes it challenging to compare the diagnostic accuracy with other imaging techniques (i.e. RCM alone). However, this was a descriptive study to evaluate the feasibility of the new RCM-OCT device in this specific subgroup of patients with complex BCCs on the head and neck. This was conducted at a tertiary cancer center with experience in RCM and OCT imaging; new users of this imaging modality would need to learn both RCM and OCT to use the device. Specific anatomical locations are difficult to evaluate with the current probe size (e.g. conchal area, retroauricular area). Also, the cost of the device and that it is not yet commercially available are additional limitations to dissemination of this technology. Ideally, all Mohs surgeons and other skin cancer experts could use it or incorporate it for complex cases.
Conclusions:
The use of a combined, co-registered RCM-OCT non-invasive imaging device may help in the evaluation of complex BCCs arising on the head and neck by guiding treatment selection and defining the extent of surgery ultimately improving patient management and experience.
Acknowledgement:
Development of the RCM-OCT microscope was supported by NIH grants (NCI SBIR R44CA162561, NIBIB R01EB020029 and NCI MSK Cancer Center P30CA008748). Further technical support (provision, troubleshooting, software modifications, image analysis) was provided by Drs. Nicusor Iftimia and Gopi Maguluri, Physical Sciences Inc. (Andover, MA).
Funding source: This research was funded in part by a grant from the National Cancer Institute/National Institutes of Health (P30-CA008748) made to Memorial Sloan Kettering Cancer Center.
Footnotes
Conflicts of interest:
Anthony Rossi: Dr. Rossi has no relevant conflicts of interest related to this manuscript but served on advisory board, as a consultant, or given educational presentations: for Allergan, Inc; Galderma Inc; Evolus Inc; Elekta; Biofrontera, Quantia; Merz Inc; Dynamed; Skinuvia, Perf-Action.
Nehal KS: received royalties from publishing companies for books and book chapters.
IRB approval status: MSKCC #99-099
Prior presentation: None.
References:
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. [DOI] [PubMed] [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. [DOI] [PubMed] [Google Scholar]
- 3.Navarrete-Dechent C, Bajaj S, Marchetti MA, Rabinovitz H, Dusza SW, Marghoob AA. Association of Shiny White Blotches and Strands With Nonpigmented Basal Cell Carcinoma: Evaluation of an Additional Dermoscopic Diagnostic Criterion. JAMA Dermatol. 2016;152(5):546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nori S, Rius-Diaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51(6):923–930. [DOI] [PubMed] [Google Scholar]
- 5.Aleissa S, Navarrete-Dechent C, Cordova M, et al. Presurgical evaluation of basal cell carcinoma using combined reflectance confocal microscopy-optical coherence tomography: A prospective study: Combined Reflectance Confocal Microscopy-Optical Coherence Tomography for Basal Cell Carcinoma. J Am Acad Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyllo RL, Staser KW, Rosman I, Council ML, Hurst EA. Histopathologic upgrading of nonmelanoma skin cancer at the time of Mohs micrographic surgery: A prospective review. J Am Acad Dermatol. 2019;81(2):541–547. [DOI] [PubMed] [Google Scholar]
- 7.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136(8):1012–1016. [DOI] [PubMed] [Google Scholar]
- 8.Que SKT. Research Techniques Made Simple: Noninvasive Imaging Technologies for the Delineation of Basal Cell Carcinomas. J Invest Dermatol. 2016;136(4):e33–e38. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med. 2017;49(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarrete-Dechent C, Mori S, Cordova M, Nehal KS. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80(1):e7–e8. [DOI] [PubMed] [Google Scholar]
- 12.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J Am Acad Dermatol. 2019;81(2):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarrete-Dechent C, Cordova M, Liopyris K, et al. Reflectance confocal microscopy-guided carbon dioxide laser ablation of low-risk basal cell carcinomas: A prospective study. J Am Acad Dermatol. 2019;81(4):984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liopyris K, Navarrete-Dechent C, Yelamos O, Marchetti MA, Rabinovitz H, Marghoob AA. Clinical, dermoscopic and reflectance confocal microscopy characterization of facial basal cell carcinomas presenting as small white lesions on sun-damaged skin. Br J Dermatol. 2019;180(1):229–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarrete-Dechent C, Rajadhyaksha M, Nehal KS. Can optical coherence tomography improve the management of basal cell carcinoma? Br J Dermatol. 2019;180(3):448–449. [DOI] [PubMed] [Google Scholar]
- 16.Rossi AM, Navarrete-Dechent C, Nehal KS. Beyond skin deep: taking bedside dermatology to the next level with noninvasive technologies. Br J Dermatol. 2018;178(5):994–996. [DOI] [PubMed] [Google Scholar]
- 17.Iftimia N, Yelamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22(7):76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahu A, Yelamos O, Iftimia N, et al. Evaluation of a Combined Reflectance Confocal Microscopy-Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network N. Basal Cell Skin Cancer (Version 1.2018). 2018.
- 20.Iftimia N, Yélamos O, Chen C-SJ, et al. Handheld optical coherence tomography–reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22(7):076006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marino M, Rogers T, Sierra Gil H, Rajadhyaksha M, Cordova M, Marghoob A. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol. 2016;22(4):519–520. [DOI] [PubMed] [Google Scholar]
- 22.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Use of paper tape to guide reflectance confocal microscopy navigation of large skin lesions. J Am Acad Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarrete-Dechent C, DeRosa AP, Longo C, et al. Reflectance confocal microscopy terminology glossary for nonmelanocytic skin lesions: A systematic review. J Am Acad Dermatol. 2019;80(5): 1414–1427 e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longo C, Lallas A, Kyrgidis A, et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J Am Acad Dermatol. 2014;71(4):716–724 e711. [DOI] [PubMed] [Google Scholar]
- 25.Maier T, Braun-Falco M, Hinz T, Schmid-Wendtner MH, Ruzicka T, Berking C. Morphology of basal cell carcinoma in high definition optical coherence tomography: en-face and slice imaging mode, and comparison with histology. J Eur Acad Dermatol Venereol. 2013;27(1):e97–104. [DOI] [PubMed] [Google Scholar]
- 26.Hinz T, Ehler LK, Hornung T, et al. Preoperative characterization of basal cell carcinoma comparing tumour thickness measurement by optical coherence tomography, 20-MHz ultrasound and histopathology. Acta Derm Venereol. 2012;92(2):132–137. [DOI] [PubMed] [Google Scholar]
- 27.Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173(6):1371–1380. [DOI] [PubMed] [Google Scholar]
- 28.Anstey A, Edwards A. Shared decision making in dermatology: asking patients, 'What is important to you?'. Br J Dermatol. 2014;170(4):759–760. [DOI] [PubMed] [Google Scholar]
- 29.Giordano CN, Mori S, Navarrete-Dechent C, et al. Patient Concerns in the Immediate Postoperative Period After Mohs Micrographic Surgery. Dermatol Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee EH, Brewer JD, MacFarlane DF. Optimizing Informed Decision Making for Basal Cell Carcinoma in Patients 85 Years or Older. JAMA Dermatol. 2015;151(8):817–818. [DOI] [PubMed] [Google Scholar]
- 31.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Lentigo maligna melanoma mapping using reflectance confocal microscopy correlates with staged excision: A prospective study. J Am Acad Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay KM, Sambrano BL, Fox PS, Bassett RL, Chon S, Prieto VG. Thickness of superficial basal cell carcinoma (sBCC) predicts imiquimod efficacy: a proposal for a thickness-based definition of sBCC. Br J Dermatol. 2013;169(3):549–554. [DOI] [PubMed] [Google Scholar]