Abstract
Key characteristics of chronic obstructive pulmonary disease (COPD) that significantly affect health-related quality of life (HRQoL) include chest symptoms, dyspnea, cough, sputum production, and exacerbations. Additional areas of impact are sleep, fatigue, emotional well-being, social functioning, and coping. Patient-reported outcomes (PROs) are essential to evaluate symptoms, impact of symptoms on activities of daily living, and treatment response. This review summarizes COPD-specific PRO endpoints from randomized controlled trials of approved and commonly used COPD drugs. A search conducted in “ClinicalTrials.gov” to identify COPD clinical trials (only completed Phase III and IV) incorporating PRO endpoints yielded a total of 104 clinical trials for inclusion in this analysis. Both symptom-based and HRQoL-specific PRO measures were reported. Several COPD-specific PRO measures are available; however, the St. George’s Respiratory Questionnaire (SGRQ) and the Baseline and Transition Dyspnea Indexes (BDI/TDI) were reported in the majority of the studies. Results reflected a gap in terms of full coverage of key impacted areas from a patient’s perspective. Methodological issues identified in this review related to scoring of instruments require careful consideration, as these challenges may limit the complete assessment of drug benefits. Selection of PRO measures aligned with the expected treatment benefit of a drug in a clinical trial should reflect patients’ perspective holistically.
Keywords: chronic obstructive pulmonary disease, patient-reported outcomes, clinical trials, endpoints, Patient-Focused Drug Development, randomized controlled trials
Introduction
Chronic obstructive pulmonary disease (COPD) is a global health issue, with progressive morbidity and high mortality incurring huge societal costs.1–3 It is characterized by persistent symptoms such as dyspnea, wheeze, chest tightness, cough, and sputum production.4 A recent “social media listening” study indicated that from a patient’s perspective, relief from cough, mucus production, and shortness of breath are the most sought-after symptoms to be treated.5 Exacerbations (worsening of condition) are important predictors of disease progression.6 Besides pulmonary impact, COPD affects multiple organs, ultimately leading to systemic effects.7 Sleep gets impacted substantially due to nighttime symptoms; fatigue and progressive dyspnea limit daily activities of patients with COPD to a large extent.8–10 Urinary incontinence is also a prevalent bothersome symptom in these patients.11 While COPD symptoms negatively influence the quality of life (QoL) of patients, pharmacological control of symptoms has an overall positive impact on their health status. Nevertheless, despite the availability of a range of therapies, an unmet treatment need persists. Several patients (including even patients on dual or triple therapy) experience a huge symptomatic burden and report low levels of medication adherence.12,13 Both direct (eg, cost of drugs) and indirect (eg, work productivity losses) costs contribute toward the economic burden on patients and their families.3,14
Health-related quality of life (HRQoL) is significantly impaired in patients with COPD across all severities.15,16 An increased number of emergency hospital admissions, mainly during exacerbation of the disease, are associated with poor HRQoL.17 COPD symptoms have a substantial detrimental impact on emotional and social functioning and present challenges with coping due to the progressive nature of HRQoL deterioration.4,18 Comorbid depressive symptoms further lead to reduced physical health and low survival rates, forming a vicious cycle.19 Patient-reported outcomes (PROs)—reports directly provided by patients on their perceived health status—are essential to determine disease severity and its impact on health conditions in patients with COPD.4,20 Traditionally, both generic (eg, the 36-Item Short Form Health Survey [SF-36]) and disease-specific (eg, the St. George’s Respiratory Questionnaire [SGRQ]) PRO measures have been used in COPD studies.21,22 PRO measures such as the Exacerbation of Chronic Pulmonary Disease Tool (EXACT) have also been used for detection, quantification, and evaluation of COPD exacerbations.6
COPD clinical trials aimed at proving the efficacy of drugs focus on clinical parameters and biomarkers as primary endpoints. Decline in lung function over time is a fundamental measure of disease progression; hence, spirometry (forced expiratory volume in 1 second [FEV1]) decline has been widely used as a central outcome measure in randomized controlled trials (RCTs).23 However, FEV1 alone might not capture all the beneficial effects of therapy on clinically relevant patient outcomes and disease modification.24,25 In addition, only a small proportion of patients show clinically relevant improvements in PROs along with improvements in lung function.25 Previous studies have shown low to moderate correlations between lung function and patients’ perception of disease impairment.25–27 Furthermore, most of these correlations were observed in large populations,28 and individual-level data are lacking.
To demonstrate additional efficacy benefits of drugs by improvements in HRQoL, sponsors include PROs as key secondary, exploratory, or other pre-specified endpoints. For patient-centered drug development, it becomes crucial to include PRO endpoints in addition to clinical endpoints. However, there is limited information in the literature on overall trends (usual practices over the years) for PRO inclusion in COPD clinical studies. The present review is a trend analysis of PRO endpoints in RCTs, encompassing an overview of disease-specific PRO endpoints in completed RCTs of commonly used and approved COPD drug therapies. This review highlights the current challenges in the selection of PRO measures and also touches upon methodological issues related to PRO scoring that can impact their selection. This work will support the development of PRO strategies aligned with the Food and Drug Administration (FDA) Patient-Focused Drug Development (PFDD) initiative, providing insights on incorporating patients’ perspectives into drug development. Overall, this review can be considered and referred to as a groundwork on the selection of PRO endpoints for future COPD clinical trials.
Methods
Study Design and Data Source
This cross-sectional (at a single time point) descriptive review was conducted by including completed phase III and IV clinical trials posted on the “ClinicalTrials.gov” database as of April 2020. RCTs that assessed approved and commonly used COPD therapies such as aclidinium, budesonide, beclomethasone, fluticasone, formoterol, glycopyrronium, indacaterol, olodaterol, roflumilast, salmeterol, tiotropium, umeclidinium, and vilanterol, either as monotherapy or in combinations, were included. It is noteworthy that some of the recent trials included in the analysis comprise drugs that are still in the pre-approval phase. The search was restricted to completed phase III and IV trials, and only articles published in English were included. There was no restriction on the time frame of the search and no limitations on the inclusion of studies based on the age group or gender of COPD patients. Terminated, recruiting, or withdrawn trials were excluded from the search. Keywords such as “chronic obstructive pulmonary disease,” “COPD,” “patient-reported outcomes,” “PRO,” and “health-related quality of life” were used to initiate the search in the selected database “ClinicalTrials.gov.” Other region-specific databases such as “EudraCT” were excluded in this review to avoid duplication in terms of retrieval of the same studies registered under different trial numbers or names.
Data Collection and Retrieval
From each of the selected RCT, data were summarized in an Excel sheet, providing basic study details such as registration number, intervention and comparator arms, and reported COPD-specific PROs from each RCT. This information is available as supplementary file (S1). Published literature of corresponding RCTs was referenced (wherever available) to cross-check for details related to PRO endpoints. Furthermore, PRO endpoints from each trial were reported as primary, secondary, or exploratory (other/additional), and this information was also collected under “classification of endpoints.” Wherever there was a discrepancy in reporting of endpoints, the corresponding publication of the respective clinical trial was referred for a final decision.
Outcomes Included in the Review
The primary outcomes include data and descriptions related to disease-specific PRO endpoints. A trend in terms of reported PRO endpoints was summarized from the included RCTs. All types of endpoints (primary, secondary, exploratory, and non-specified) reported across the trials were captured. Both validated (developed and psychometrically evaluated in accordance with FDA guidance, 200920) and unvalidated PRO measures (without evidence of formal validation) were integrated into the analysis. However, owing to the specific focus of the review on disease-specific PRO endpoints, no generic and health utility measures were included in the analysis. Results presented are mutually inclusive, ie, while counting the number of PRO endpoints, there were trials wherein more than one PRO measure was used. In such cases, results consisted of the frequency of usage (total count) of that individual PRO measure from all the trials. Furthermore, wherever available (ie, published), full-text articles were retrieved to capture additional details of PRO endpoints. PRO measures identified were further analyzed for their respective domains and sub-domains.
Gaps in terms of how adequately a PRO measure covers key impacted areas were explored by looking into the domains of each PRO measure. To support the assessment of such gaps and inform on key impacted domains, a conceptual framework was constructed based on published literature on the impact of COPD on HRQoL,1–29 including the Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD guidelines, 2020,4 and the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines on outcomes for COPD pharmacological trials 2008.29
Presentation of Results
Based on domains covered, each PRO measure was classified either as symptom-based or HRQoL-specific. Results presented embrace the frequency of inclusion of PRO endpoints and the domains covered by each PRO measure. Descriptive tables comprising the PRO endpoints are provided as results after analysis in Table 1, while domain-level analysis for each PRO measure is presented in Table 2.
Table 1.
Frequency of PRO Measures Reported in the Included Trials (n=104)
| Category | PRO Measures | No. of Trials (n) |
|---|---|---|
| Symptom-based | Baseline/Transition Dyspnea Indexes (BDI/TDI)a | 60 |
| Unvalidated symptom-based instrumentsb | 32 | |
| Evaluating Respiratory Symptoms in COPD (E-RS™: COPD)c | 9 | |
| Exacerbations of Chronic Pulmonary Disease Tool (EXACT) | 4 | |
| Shortness of Breath with Daily Activities (SOBDA) | 4 | |
| Chronic Respiratory Questionnaire Self-Administered Standardized (CRQ-SAS) dyspnea domaind | 4 | |
| Early Morning and Nighttime Symptoms of COPD Instrument (EMSCI and NiSCI) | 4 | |
| Borg Dyspnea Scale | 3 | |
| Shortness of Breath Questionnaire (SOBQ) | 2 | |
| Global Chest Symptoms Questionnaire (GCSQ) | 1 | |
| Cough Symptom Score (CSS) | 1 | |
| HRQoL-specific | St. George’s Respiratory Questionnaire (SGRQ) | 64 |
| St. George’s Respiratory Questionnaire COPD-specific (SGRQ-C) | 12 | |
| COPD Assessment Test (CAT) | 21 | |
| Cough and Sputum Assessment Questionnaire (CASA-Q) | 2 | |
| Capacity of Daily Living during the Morning (CDLM) | 2 | |
| Clinical COPD Questionnaire (CCQ) | 2 | |
| Leicester Cough Questionnaire (LCQ) | 1 |
Notes: Results presented are mutually inclusive, which means that while counting the number of PRO endpoints, there may have been cases wherein more than one PRO was used in the same trial. In such cases, results reported are based on the frequency of usage of that individual PRO from the total trials. aBDI/TDI are interview-based instruments (except the self-administered computerized [SAC] version); bUnvalidated instruments are instruments that do not have published evidence for their validation; cE-RSTM: COPD is subscale of EXACT; dCRQ-SAS is an HRQoL instrument, but in clinical trials, only the dyspnea domain was used; hence, it has been classified under symptom.
Abbreviations: COPD, chronic obstructive pulmonary disease; HRQoL, health-related quality of life; PRO, patient-reported outcome.
Table 2.
Domain-Level Analysis of PRO Measures Used in Included Clinical Trials (n=104)
| Domains and Sub-Domains (Number of Items) |
SGRQ (50) |
SGRQ-Ca (40) |
CDLM (8) |
LCQ (19) |
CAT (8) |
CCQ (10) |
CASA-Q (20) |
EXACT (14) |
CSS (2) |
SOBQ (24) |
SOBDA (13) |
CRQ-SASb (20) |
GCSQ (2) |
EMSCI/ NiSCIc (6) |
Borg Scale (1) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dyspnea | General/at rest/severity | + | + | - | - | - | + | + | + | - | + | + | - | + | + | + |
| During activities | + | + | - | - | + | + | - | + | - | + | + | + | - | - | + | |
| Impact | + | + | + | - | - | - | - | - | - | + | + | + | - | - | - | |
| Chest symptoms | Frequency/severity | + | + | - | - | + | - | - | + | - | - | - | - | + | + | - |
| Impact | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | |
| Wheeze | Frequency/severity | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Impact | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Cough | Frequency/severity | + | + | - | + | + | + | + | + | + | - | - | - | - | + | - |
| Impact | + | + | - | + | - | - | + | - | - | - | - | - | - | - | - | |
| Sputum | Frequency/severity | + | + | - | - | + | + | + | + | - | - | - | - | - | + | - |
| Impact | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | |
| Other symptom related | Disease-free days | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Morning symptoms | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | |
| Sleep | Sleep quality | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - |
| Sleep disturbance | + | + | - | + | - | - | + | + | - | - | - | - | - | - | - | |
| Nighttime awakening | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | |
| Energy | Fatigue/tiredness/activeness | + | + | + | + | + | - | - | + | - | - | - | - | - | - | - |
| Activities of daily living | Daily activities limitations | + | + | + | + | + | + | + | + | - | + | + | - | - | + | - |
| Leisure/fun activities/sports | + | + | - | + | - | - | + | - | - | - | - | - | - | - | - | |
| Impact on mobility | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | |
| Psychosocial | Depression/anxiety | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - |
| Embarrassment | + | + | - | + | - | - | + | - | - | - | - | - | - | - | - | |
| Fear/panic/worry/concern | + | + | - | + | - | + | - | + | - | + | - | - | - | - | - | |
| Hopelessness | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Happiness/sadness | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Annoyance/frustration | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | |
| Family/personal relationship | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | |
| Social life | - | - | - | + | - | + | + | - | - | - | - | - | - | - | - | |
| Treatment impact | Disease control | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - |
| Treatment satisfaction | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Medication/side effects | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Work | Work productivity/ job |
+ | - | - | + | - | - | - | - | - | - | - | - | - | - | - |
| Other | Environmental exposured | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - |
Notes: (+) indicates the presence and (-) indicates the absence of a domain or sub-domain. aAn item on overall health is included in SGRQ-C but is not included in the total score; bCRQ-SAS is an HRQoL instrument, but in clinical trials, only the dyspnea domain was used; hence, it has been classified under symptom; cEMSCI/NISCI items are included based on available literature as these instruments were not available in the public domain at the time of execution of the study; unvalidated instruments are not included in this table; dPrecipitation/increase in symptoms due to environmental exposure.
Abbreviations: CASA-Q, Cough and Sputum Assessment Questionnaire; CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire; CDLM, Capacity of Daily Living during the Morning; COPD, chronic obstructive pulmonary disease; CRQ-SAS, Chronic Respiratory Questionnaire Self-Administered Standardized; CSS, Cough Symptom Score; EMSCI/NiSCI, Early Morning/Nighttime Symptoms of COPD Instrument; EXACT, Exacerbations of Chronic Pulmonary Disease Tool; GCSQ, Global Chest Symptoms Questionnaire; HRQoL, health-related quality of life; LCQ, Leicester Cough Questionnaire; PRO, patient-reported outcome; SGRQ, St. George’s Respiratory Questionnaire; SGRQ-C, St. George’s Respiratory Questionnaire COPD-specific; SOBDA, Shortness of Breath with Daily Activities; SOBQ, Shortness of Breath Questionnaire.
Results
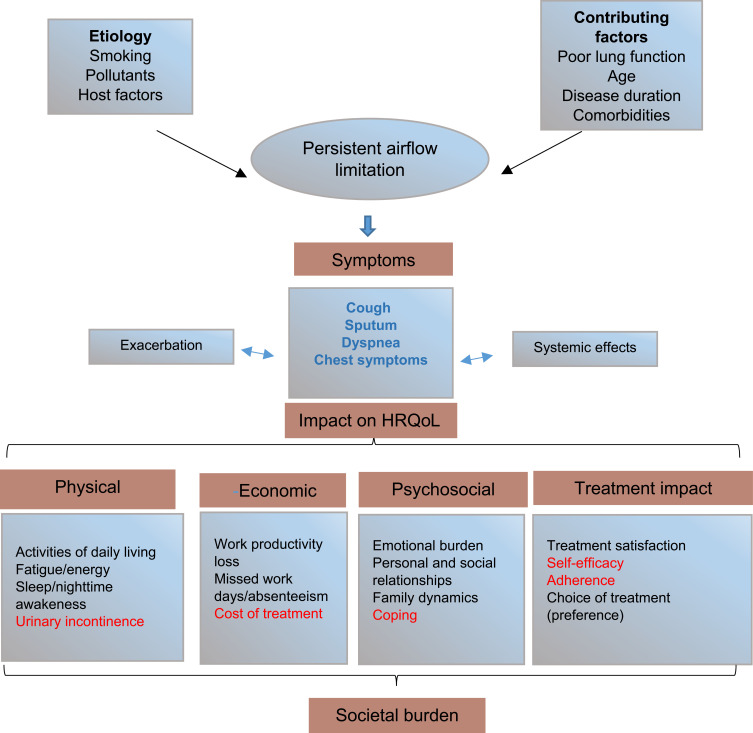
The conceptual framework presented in Figure 1 gives an overview of the impacted domains in COPD, along with the etiology and contributing factors of the disease. HRQoL, which consists of physical, psychosocial, and economic domains, was largely impacted by COPD symptoms, as depicted in Figure 1. Additionally, treatment impact, which is an important aspect of overall HRQoL, was also included in the framework. The physical domain included sleep, fatigue, and activities of daily living, while the psychosocial domain concentrates on emotional, family dynamics, personal and social relationships. The economic domain included work productivity losses as well as direct and indirect costs of COPD treatment. Furthermore, impacted areas within each domain were classified into two categories based on their coverage in the included RCTs by use of PROs. Symptoms highlighted in blue font in Figure 1 were the most widely covered and were captured by both validated and unvalidated PROs. On the other hand, areas of impact highlighted in red font were not covered by PROs in any of the included trials.
Figure 1.
Overview of impact of COPD symptoms on HRQoL.
Notes: Data from these studies.1–29 Clinical endpoints, symptom diaries, and EXACT were used to capture exacerbations. Cost of treatment includes both direct and indirect costs, but this aspect was not captured in this review.
Abbreviations: EXACT, Exacerbations of Chronic Pulmonary Disease ToolHRQoL, health-related quality of life.
Features of the 104 Trials
Trends in the selection and reporting of PRO endpoints from the included studies are summarized in this section. In total, 104 clinical trials were included, and the results presented are mutually inclusive. Results comprise both phase III (n=84) and IV RCTs (n=20). Table 1 summarizes the frequency of usage of each PRO measure from all included studies. To assess the health status of patients, both symptom-based and HRQoL PROs were reported in the RCTs. The reported symptoms mainly comprise dyspnea, wheeze, chest symptoms, cough, and sputum production. The Baseline and Transition Dyspnea Indexes (BDI/TDI) were frequently used (60/104 studies) for measurement of dyspnea based on activities of daily living in symptomatic individuals. Unvalidated symptom-based instruments (electronic daily diaries) were reported in nearly one-third (n=32) of the included clinical trials; these instruments usually capture common COPD symptoms such as cough, sputum production, dyspnea, and wheeze. Hence, in addition to symptom-based PRO measures, symptoms were captured by clinical endpoints (not reported in this review), interview/clinician-based (BDI/TDI), or unvalidated PRO measures.
HRQoL was measured using the SGRQ in a majority of the trials (n=64), along with the SGRQ COPD-specific version (SGRQ-C) in 12 trials, adding up to 76 trials (73.0%) in which the SGRQ was used (Table 1). Four trials used SGRQ as a primary endpoint, while the remaining 72 used it as a key secondary, exploratory, additional, or other pre-specified endpoints. Table 1 also lists the number of trials that utilized other HRQoL measures such as the COPD Assessment Test (CAT), the Cough and Sputum Assessment Questionnaire (CASA-Q), the Capacity of Daily Living during the Morning (CDLM), the Leicester Cough Questionnaire (LCQ), and the Clinical COPD Questionnaire (CCQ).
Additional PRO endpoints used in the included RCTs but not depicted in Table 1 include the “impact on activities of daily living” captured by “percentage of days able to perform usual activities” in nine trials. Furthermore, patients’ global rating (or evaluation) of their health (n=5) and patient preference, such as inhaler preference and device satisfaction (n=5) were also reported. One study used a validated single-item component of the Breathlessness Cough and Sputum Scale (BCSS) to measure dyspnea. Details are available in the supplementary file (S1).
Domain-Level Analyses of PRO Measures in Included Studies
Table 2 summarizes the domain-level analysis of PRO measures in the included trials. Domains were categorized into “symptom and its impact,” “sleep,” “energy,” “activities of daily living,” “psychosocial,” “treatment impact,” “work,” and “other.” Among the symptoms, dyspnea and its impact was the most elaborately reported domain (Table 2). Among the HRQoL-specific PROs, the SGRQ and the LCQ covered a wide spectrum of impacted domains. These PRO measures also covered areas such as fatigue, daily activities, and emotional concerns (Table 2). While the scoring algorithm of these instruments can provide both domain and total scores, only total score was reported in most of the studies.
Symptom-Based Instruments
Validated Symptom-Based Instruments
EXACT, a 14-item daily diary, is used to quantify and measure exacerbations of COPD; its subscale, the Evaluating Respiratory Symptoms in COPD (E-RSTM: COPD) has 11 items that quantify the severity of respiratory symptoms.30 The Shortness of Breath with Daily Activities (SOBDA) and the Shortness of Breath Questionnaire (SOBQ) are13-item and 24-item PRO measures, respectively, which determine the impact of dyspnea on daily activities.31,32 The Chronic Respiratory Questionnaire Self-Administered Standardized (CRQ-SAS) is a self-administered format with standardized dyspnea-related questions.33 The Global Chest Symptoms Questionnaire (GCSQ) includes two items related to dyspnea and chest tightness, and the Early Morning and Nighttime Symptoms of COPD Instruments (EMSCI and NiSCI) also have individual items on COPD symptoms.34–36 The BDI and TDI are three-item, interview-based measures (except the self-administered computerized [SAC] version) that capture breathlessness related to daily activities.37 The Cough Symptom Score (CSS) is a two-part questionnaire referring to daytime and nighttime symptoms to evaluate cough frequency.38 The Borg dyspnea scale is a single-item rating scale of 0 to 10 for perceived dyspnea.39
Unvalidated Symptom-Based Instruments
Several unvalidated scales and scores (daily diaries) were used to assess the severity and frequency of clinical symptoms. One study used an electronic diary to assess COPD symptoms over 24 hours (breathlessness on a scale of 0 to 4; cough, sputum production, and wheeze on a scale of 0 to 3) and use of daily rescue medication and concomitant medication.40
HRQoL Instruments
The SGRQ is the most widely used and US Food and Drug Administration (FDA)–qualified COPD-specific instrument that has 50 items comprising three domains: symptoms, activity, and impact (psychosocial), while the SGRQ-C has 40 items and the same domains.41,42 Both domain and total scores can be calculated for the SGRQ. The CAT has a total of eight items, including COPD symptoms (such as cough, sputum, chest tightness, and breathlessness), activity limitation, sleep, and energy.43 The LCQ is a 19-item measure that has 17 items assessing the impact of chronic cough on HRQoL and one item each on sputum and energy.44 The CDLM, an eight-item measure, evaluates the ability to perform basic morning activities.34
Measurement of Direct Impact of Symptoms on HRQoL
This section provides a description of PRO measures that focus on the measurement of the impact of symptoms on HRQoL. The CASA-Q evaluates the impact of cough and sputum on a patient’s QoL. This instrument includes four domains, namely, cough symptoms (3 items), cough impact (8 items), sputum symptoms (3 items), and sputum impact (6 items).45 The SGRQ also captures the impact of symptoms on HRQoL and activities of daily living, with few missing domains such as the impact of sputum production on overall HRQoL (Table 2). The CDLM and LCQ focus on the impact of chest symptoms and cough on HRQoL. The BDI and TDI capture the impact of dyspnea along with the impact on activities of daily living. Similarly, the dyspnea domain of the SOBQ, SOBDA, and CRQ-SAS focuses on the impact of dyspnea.
Discussion
Inclusion of PROs in clinical trials is gaining importance for a better understanding of disease impact on HRQoL. PRO data provide important supporting evidence of the treatment benefit of drugs. Hence, sponsors highly value patient views of product efficacy in addition to safety and efficacy parameters. In order to meet their commitment to Patient-Focused Drug Development (PFDD), the US FDA give high importance to patients’ input.20,46 The European Medicines Agency (EMA) also considers patients’ perspective as an important aspect of drug approval.47 The analysis presented in this review focuses on PRO endpoints and reveals a trend on coverage of impacted HRQoL domains in COPD clinical studies. It informs on usual practices and trends of PRO inclusion in RCTs and foregrounds the need for using a battery of instruments to capture HRQoL comprehensively. Important issues and gaps related to current practices on the selection of PRO measures in the context of COPD clinical studies are also brought to light.
From a clinical perspective, the most prominent and bothersome symptoms include dyspnea, wheeze, cough, sputum, chest troubles or attacks, and exacerbations. The sponsors also reflect this focus on the selection of symptom-based PROs. COPD symptoms such as breathlessness, chest symptoms, cough, and sputum are comprehensively captured (Tables 1 and 2) by the use of PRO measures. Domain-level analysis of PRO measures showed that dyspnea and its impact on patients were the most elaborately reported domains. However, BDI and TDI, which were used most frequently in trials to measure dyspnea, are interview-based measures (except the SAC version) and not direct reports from patients; hence, there is a likelihood of potential interviewer bias during their administration.48 Despite the availability of various validated COPD-specific symptom-based PRO measures, a large number of trials utilized trial-specific, custom-based unvalidated PRO measures. Although sensitive to changes in symptom burden, such PRO measures lack evidence of formal validation and content generation. Widespread use of unvalidated symptom-based PRO measures reflects a gap in terms of a standard format for symptom assessment from a patients’ perspective. As regulatory scrutiny of PRO measures on content validity and documentation of psychometric evidence have increased in the recent past, clinical studies should implement validated PRO measures developed following regulatory guidance. Another challenge observed through domain-level analyses is the direct measurement of the impact of symptoms on HRQoL. As shown in the domain-level analyses (Table 2), the impact of symptoms is often included in the total score or a domain score (which are combinations of various items). These total scores have often been referred to as “black box,” providing little or no insights on the specific nature of disease progression or drug benefit.27 Total scores do not necessarily reflect improvements (or deterioration) in the discrete impact of a particular symptom. In a nutshell, from a patient perspective, a standardized approach for the measurement of symptoms (and their impact on HRQoL) is still lacking.
To measure HRQoL, various COPD-specific PRO measures with proven validity and reliability are available. However, owing to various instances of acceptance by regulatory bodies (FDA and EMA) and the availability of an extensive historical database on use, the SGRQ is the most preferred PRO measure by both regulators and sponsors. The results of this review also reflect the preponderance of the SGRQ in terms of usage and reporting (in published evidence). The SGRQ was used in 73.0% of clinical studies included in this analysis, mainly as a secondary endpoint. Given that the latest FDA guidance (2018) on the use of the SGRQ prefers a total score over domain scores,49 assessment of all the impacted domains by total scores of SGRQ alone can be challenging. For an instance, although the SGRQ includes one item for sleep, estimation of impacted sleep is not possible owing to the scoring algorithm of the SGRQ, which reports only the combined scores (total and domain scores) of different items. The use of other HRQoL instruments (eg, CAT and LCQ) also poses similar limitations and challenges. Depicted in the conceptual framework (Figure 1), COPD affects multiple areas (beyond symptoms) including sleep, activities of daily living, fatigue, emotional wellbeing (eg, depression or anxiety), and social functioning, resulting in severely impacted HRQoL. Work productivity and treatment impact are also important determinants of QoL of patients with COPD. The analyzed trials lacked the use of dedicated validated PRO measures on key affected areas such as fatigue, sleep, and activities of daily living. Patient preference (in the form of inhaler preference or device satisfaction) was captured in five trials. Essentially, a gap exists in coverage of key impacted HRQoL domains, mainly due to a lack of use of PRO measures despite their availability. To overcome such challenges, incorporation of additional PRO endpoints providing a holistic overview of HRQoL becomes indispensable to improve clinical study designs.
A previous study reported only poor to moderate correlations between physiological, functional, and psychosocial consequences of COPD.50 Hence, improvement in one domain does not guarantee improvement in another. Interestingly, the correlations between improvements in lung function (eg, FEV1) and PROs and the associations between different PRO scores are also modest or weak.25 Moreover, the identification of responders based on the achievement of minimal clinically important differences shows a small overlap between FEV1 and different PRO measures.25 Such findings suggest that different PRO measures should be used in a specific and targeted way and that similar PRO measures cannot be used interchangeably.
Nevertheless, incorporation of PRO endpoints in a clinical study should be based on meticulous planning of trial endpoints to avoid unnecessary administrative burden on patients and study personnel. A meta-analysis conducted to determine the relationship between response rates and questionnaire length revealed that it is preferable to base decisions on the content rather than on the length of PROs.51 On the other hand, the use of numerous PRO measures results in an increased time of administration, which is perceived as burdensome by patients.52 Concisely, there should be an adequate balance between conceptual coverage and administrative burden to patients in RCTs. This can be achieved only by customized planning for PRO inclusion aligned with the aim of a study.
For future clinical trials, the PRO strategy should be grounded on the most critical needs of COPD patients, and the most commonly used PRO measures do not necessarily cover these needs. Capturing all the key impacted areas (as observed in the conceptual framework) may contribute towards a comprehensive patient-centered assessment. With the upcoming COPD therapies, particularly non-bronchodilators, the inclusion of PRO endpoints in clinical trials can play an important role in the development and characterization of newer treatment options.
This review summarizes the analysis of 104 COPD RCTs from ClinicalTrials.gov, ie, from a single database only. Hence, this is not an overview of all the completed COPD trials. By reporting the results from RCTs of selected commonly used approved drugs, we attempted to perform a trend analysis. Future research is required to capture ongoing trials to provide a complete understanding of such trends.
Conclusion
Overall, HRQoL in COPD clinical studies was measured using disease-specific PRO measures, majorly the SGRQ. A significant proportion of RCTs utilized the interviewer-administered BDI/TDI or unvalidated symptom-based PRO measures. Hence, a standardized approach for assessment of COPD symptoms and their impact on HRQoL from a patient’s perspective are still lacking. Moreover, validated PROs covering key affected areas such as sleep, fatigue, activities of daily living, and treatment impact were missing, and such PRO measures should be integrated into clinical trials. With growing demands of including patient centricity (aligned with FDA’s PFDD) into drug development, a PRO strategy grounded on the most critical needs of patients can ensure a comprehensive assessment of drug benefits. HRQoL assessment in clinical trials also presents methodological challenges related to total scores of instruments, which require careful context-specific considerations.
Funding Statement
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Abbreviations
BCSS, Breathlessness Cough and Sputum Scale; BDI, Baseline Dyspnea Index; CASA-Q, Cough and Sputum Assessment Questionnaire; CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire; CDLM, Capacity of Daily Living during the Morning; COA, Clinical Outcomes Assessment; COPD, chronic obstructive pulmonary disease; CRQ-SAS, Chronic Respiratory Questionnaire Self-Administered Standardized; CSS, Cough Symptom Score; EMA, European Medicines Agency; EMSCI, Early Morning Symptoms of COPD Instruments; E-RSTM: COPD, Evaluating Respiratory Symptoms in COPD; EXACT, Exacerbation of Chronic Pulmonary Disease Tool; FDA, Food and Drug Administration; FEV1, forced expiratory volume in 1 second; GCSQ, Global Chest Symptoms Questionnaire; HRQoL, Health-related quality of life; LCQ, Leicester Cough Questionnaire; NiSCI, Nighttime Symptoms of COPD Instruments; PFDD, Patient-Focused Drug Development; PRO, patient-reported outcome; QoL, quality of life; RCT, randomized controlled trial; SAC, self-administered computerized; SF-36, 36-Item Short Form Health Survey; SGRQ, St. George’s Respiratory Questionnaire; SGRQ-C, SGRQ COPD-specific version; SOBDA, Shortness of Breath with Daily Activities; TDI, Transition Dyspnea Index.
Data Sharing Statement
Data supporting the results are available as supplementary file (S1).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Terms Used in the Review
HRQoL: A multi-domain concept that represents the patient’s general perception of the effect of illness and treatment on various aspects of life, such as physical, psychological, and social.20
PRO domain: A sub-concept represented by a score of an instrument that measures a larger concept comprising multiple domains (eg, depression).20
Validated PRO measures: Instruments with formal developmental and validation evidence meeting regulatory guidance such as the FDA guidance, 2009, in the form of published literature were classified as “validated” in this review.
Unvalidated PRO measures: Instruments with no published evidence of developmental and validation steps following regulatory guidance (or are not available in the public domain) were classified as "unvalidated" in this review. These are custom-based, trial-specific, and usually capture symptoms using a Likert scale.
Qualified instruments: Clinical Outcomes Assessment (COA) qualification by the FDA is based on a review of evidence to support that COA is a well-defined and reliable assessment of a specified concept of interest for use in adequate and well-controlled studies in a specified context of use, eg, SGRQ and EXACT.53
Disclosure
Nuzhat Afroz is an employee of Novartis Healthcare Pvt. Ltd., Hyderabad, India. Florian S. Gutzwiller, Francesco Patalano, and Christel Naujoks are employees and shareholders of Novartis Pharma AG, Basel, Switzerland. Konstantinos Kostikas was an employee of Novartis Pharma AG, Basel, Switzerland, at the time of execution of this analysis, and reports grants and personal fees from AstraZeneca, Chiesi, Boehringer-Ingelheim, ELPEN, Novartis, and GSK, outside the submitted work; Alex J. Mackay was a European Respiratory Society fellow with Industry working with Novartis Pharma AG, Basel, Switzerland, at the time of execution of this analysis, and is currently an employee of AstraZeneca; they also report personal fees from Pfizer, outside the submitted work. The authors report no other possible conflicts of interest in this work.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) COPD key facts; 2018. Available from: http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Accessed April16, 2020..
- 3.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi: 10.1378/chest.14-0972 [DOI] [PubMed] [Google Scholar]
- 4.Global Strategy for Diagnosis, Management and Prevention of COPD. The Global Initiative for Chronic Obstructive Lung Diseases (GOLD). 2020 report. Available from: https://goldcopd.org/gold-reports/. Accessed April16, 2020.
- 5.Cook NS, Kostikas J, Gruenberger J-B, et al. Patients’ perspectives on COPD: findings from a social media listening study. ERJ Open Res. 2019;5(1):00128–2018. doi: 10.1183/23120541.00128-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay AJ, Kostikas K, Murray L, et al. Patient-reported outcomes for the detection, quantification, and evaluation of chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2018;198(6):730–738. doi: 10.1164/rccm.201712-2482CI [DOI] [PubMed] [Google Scholar]
- 7.Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703 [DOI] [PubMed] [Google Scholar]
- 8.Garrow AP, Yorke J, Khan N, Vestbo J, Singh D, Tyson S. Systematic literature review of patient-reported outcome measures used in assessment and measurement of sleep disorders in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:293–307. doi: 10.2147/COPD.S68093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spruit MA, Vercoulen JH, Sprangers MAG, Wouters EFM. FAntasTIGUE consortium. Fatigue in COPD: an important yet ignored symptom. Lancet Respir Med. 2017;5(7):542–544. doi: 10.1016/S2213-2600(17)30158-3 [DOI] [PubMed] [Google Scholar]
- 10.Janaudis-Ferreira T, Beauchamp MK, Robles PG, Goldstein RS, Brooks D. Measurement of activities of daily living in patients with COPD: a systematic review. Chest. 2014;145(2):253–271. doi: 10.1378/chest.13-0016 [DOI] [PubMed] [Google Scholar]
- 11.Burge AT, Lee AL, Kein C, et al. Prevalence and impact of urinary incontinence in men with chronic obstructive pulmonary disease: a questionnaire survey. Physiotherapy. 2017;103(1):53–58. doi: 10.1016/j.physio.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Small M, Lindner L, Xu X. Symptomatic burden of COPD for patients receiving dual or triple therapy. Int J Chron Obstruct Pulmon Dis. 2018;13:1365–1376. doi: 10.2147/COPD.S163717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanduzzi A, Balbo P, Candoli P, et al. COPD: adherence to therapy. Multidiscip Respir Med. 2014;9(1):60. doi: 10.1186/2049-6958-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sin DD, Stafinski T, Ng YC, Bell NR, Jacobs P. The impact of chronic obstructive pulmonary disease on work loss in the United States. Am J Respir Crit Care Med. 2002;165(5):704–707. doi: 10.1164/ajrccm.165.5.2104055 [DOI] [PubMed] [Google Scholar]
- 15.Jones PW, Brusselle G, Dal Negro RW, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med. 2011;105(1):57–66. doi: 10.1016/j.rmed.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 17.Garrido PC, de Miguel Díez J, Gutiérrez JR, et al. Negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes. 2006;4:31. doi: 10.1186/1477-7525-4-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ Prim Care Respir Med. 2016;26:16051. doi: 10.1038/npjpcrm.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60–67. doi: 10.1001/archinte.167.1.60 [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims; 2009. Available from: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed April 21, 2020.
- 21.Ekström M, Sundh J, Larsson K. Patient reported outcome measures in chronic obstructive pulmonary disease: which to use? Expert Rev Respir Med. 2016;10(3):351–362. doi: 10.1586/17476348.2016.1146595 [DOI] [PubMed] [Google Scholar]
- 22.Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD–revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725–738. doi: 10.2147/COPD.S77368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suissa S. Lung function decline in COPD trials: bias from regression to the mean. Eur Respir J. 2008;32(4):829–831. doi: 10.1183/09031936.00103008 [DOI] [PubMed] [Google Scholar]
- 24.ZuWallack RL, Nici L. Modifying the course of chronic obstructive pulmonary disease: looking beyond the FEV1. COPD. 2012;9(6):637–648. doi: 10.3109/15412555.2012.710668 [DOI] [PubMed] [Google Scholar]
- 25.Kostikas K, Greulich T, Mackay AJ, et al. Treatment response in COPD: does FEV1 say it all? A post hoc analysis of the CRYSTAL study. ERJ Open Res. 2019;5(1):00243–2018. doi: 10.1183/23120541.00243-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880–887. doi: 10.1136/thorax.56.11.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV1 in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. doi: 10.2147/COPD.S32675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donohue JF, Jones PW, Bartels C, et al. Correlations between FEV1 and patient-reported outcomes: a pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018;49:11–19. doi: 10.1016/j.pupt.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 29.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306 [DOI] [PubMed] [Google Scholar]
- 30.Leidy NK, Wilcox TK, Jones PW, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient-reported outcome (PRO) measure. Value Health. 2010;13(8):965–975. doi: 10.1111/j.1524-4733.2010.00772.x [DOI] [PubMed] [Google Scholar]
- 31.Howard K, Berry P, Petrillo J, et al. Development of the Shortness of Breath with Daily Activities questionnaire (SOBDA). Value Health. 2012;15(8):1042–1050. doi: 10.1016/j.jval.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 32.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. Chest. 1998;113(3):619–624. doi: 10.1378/chest.113.3.619 [DOI] [PubMed] [Google Scholar]
- 33.Schünemann HJ, Griffith L, Jaeschke R, et al. A comparison of the original chronic respiratory questionnaire with a standardized version. Chest. 2003;124(4):1421–1429. doi: 10.1378/chest.124.4.1421 [DOI] [PubMed] [Google Scholar]
- 34.Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T. Development and validation of the capacity of daily living during the morning questionnaire and the global chest symptoms questionnaire in COPD. Eur Respir J. 2010;36(1):96–104. doi: 10.1183/09031936.00123709 [DOI] [PubMed] [Google Scholar]
- 35.Mocarski M, Zaiser E, Trundell D, Make BJ, Hareendran A. Evaluation of the psychometric properties of the Nighttime symptoms of COPD instrument. Int J Chron Obstruct Pulmon Dis. 2015;10:475–487. doi: 10.2147/COPD.S75776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia Gil E, Zaiser E, Hareendran A, Make BJ. A summary of the development and validation of the Early Morning Symptoms of COPD Instrument (EMSCI). Am J Respir Crit Care Med. 2017;195:A3661. doi: 10.1164/rccm.201701-0150WS [DOI] [Google Scholar]
- 37.Mahler DA, Brunel V, White T, et al. Development and validation of an innovative self-administered computerized (SAC) version of baseline/transition dyspnea index (BDI/TDI) for evaluating breathlessness in patients with COPD: the BLAZE study. Eur Respir J. 2013;42(Suppl 57):P718. [Google Scholar]
- 38.Hsu JY, Stone RA, Logan-Sinclair RB, Worsdell M, Busst CM, Chung KF. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J. 1994;7(7):1246–1253. doi: 10.1183/09031936.94.07071246 [DOI] [PubMed] [Google Scholar]
- 39.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 40.Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res. 2011;12(1):55. doi: 10.1186/1465-9921-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85(SupplB):25–31. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 42.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest. 2007;132(2):456–463. doi: 10.1378/chest.06-0702 [DOI] [PubMed] [Google Scholar]
- 43.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 44.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford B, Monz B, Hohlfeld J, et al. Development and validation of a cough and sputum assessment questionnaire. Respir Med. 2008;102(11):1545–1555. doi: 10.1016/j.rmed.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 46.Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522–531. doi: 10.1046/j.1524-4733.2003.65309.x [DOI] [PubMed] [Google Scholar]
- 47.Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. European Medicines Agency; 2005. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf. Accessed April21, 2020.
- 48.Jones P, Lareau S, Mahler DA. Measuring the effects of COPD on the patient. Respir Med. 2005;99(Suppl):B:S11–S18. doi: 10.1016/j.rmed.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research. Chronic Obstructive Pulmonary Disease: use of the St. George’s Respiratory Questionnaire as a PRO assessment tool guidance for industry; 2018. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm071575.pdf. Accessed April 21, 2020.
- 50.Engström CP, Persson LO, Larsson S, Sullivan M. Health-related quality of life in COPD: why both disease-specific and generic measures should be used. Eur Respir J. 2001;18(1):69–76. doi: 10.1183/09031936.01.00044901 [DOI] [PubMed] [Google Scholar]
- 51.Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011;14(8):1101–1108. doi: 10.1016/j.jval.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 52.Bodart S, Byrom B, Crescioni M, Eremenco S, Flood E. Perceived burden of completion of patient-reported outcome measures in clinical trials: results of a preliminary study. Ther Innov Regul Sci. 2019;53(3):318–323. doi: 10.1177/2168479018788053 [DOI] [PubMed] [Google Scholar]
- 53.US Department of Health and Human Services Food and Drug Administration. Clinical Outcome Assessment (COA) Qualification Program. Available from: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/clinical-outcome-assessment-coa-qualification-program. Accessed April23, 2020..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization (WHO) COPD key facts; 2018. Available from: http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Accessed April16, 2020..
- Global Strategy for Diagnosis, Management and Prevention of COPD. The Global Initiative for Chronic Obstructive Lung Diseases (GOLD). 2020 report. Available from: https://goldcopd.org/gold-reports/. Accessed April16, 2020.
- US Department of Health and Human Services Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims; 2009. Available from: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed April 21, 2020.
- Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. European Medicines Agency; 2005. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf. Accessed April21, 2020.
- U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research. Chronic Obstructive Pulmonary Disease: use of the St. George’s Respiratory Questionnaire as a PRO assessment tool guidance for industry; 2018. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm071575.pdf. Accessed April 21, 2020.
- US Department of Health and Human Services Food and Drug Administration. Clinical Outcome Assessment (COA) Qualification Program. Available from: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/clinical-outcome-assessment-coa-qualification-program. Accessed April23, 2020..