Abstract
Background
Candida albicans is associated with high mortality among immunocompromised patients. Resistance to and toxic side effects of antifungal drugs require the development of alternative antifungal agents. AMP-17 is a novel antimicrobial peptide derived from Musca domestica that exerts excellent antifungal effects against the Candida species. In this article, we discuss the potential mechanism of AMP-17 against C. albicans from the perspective of affecting the latter’s cell external structure.
Methods
Recombinant AMP-17 was prepared by prokaryotic expression system, and its anti-C. albicans activity was detected by microdilution method. Microscopy and scanning electron microscopy were used to examine morphological changes in C. albicans. Cell wall-specific staining method was used to detect the change of cell wall integrity of C. albicans after AMP-17 treatment. AMP-17-induced damage to the C. albicans cell membrane was analyzed by fluorescent probes and glycerol assay kit. The expression of genes related to fungal cell wall and cell-membrane synthesis was detected by qRT-PCR.
Results
Morphological observations showed that the growth of C. albicans was significantly inhibited in AMP-17-treated cells; the cells appeared aggregated and dissolved, with severe irregularities in shape. Furthermore, AMP-17 damaged the integrity of C. albicans cell walls. The cell wall integrity rate of AMP-17-treated cells was only 21.7% compared to untreated cells. Moreover, the change of membrane dynamics and permeability suggested that the cell membrane was disrupted by AMP-17 treatment. Genetic analysis showed that after AMP-17 treatment, the cell wall synthesis-related gene FKS2 of C. albicans was up-regulated 3.46-fold, while the cell membrane ergosterol synthesis-related genes ERG1, ERG5, ERG6, and MET6 were down-regulated 5.88-, 17.54-, 13.33-, and 7.14-fold, respectively.
Conclusion
AMP-17 treatment disrupted the cell wall integrity and membrane structure of C. albicans and is likely a novel therapeutic option for prevention and control of C. albicans infections.
Keywords: AMP-17, C. albicans, cell wall, membrane dynamics and permeability
Introduction
In recent times, the incidence of life-threatening fungal infections has become more widespread owing to the increasing number of critical and immunodeficient patients such as those with HIV infection or undergoing extensive antibacterial treatment organ transplantation, or receiving frequent invasive treatments.1,2 Candida albicans is a known opportunistic fungal pathogen. When humans are immunocompromised, C. albicans can invade the skin, mucous membranes, and internal organs to cause acute or chronic fungal infections.3,4 In recent years, drug resistance has been an increasingly widespread issue that seriously affects the efficacy of antifungal drugs and increases the cost of treatment.5 In addition, conventional antifungal drugs are limited and the side effects are obvious.6 Therefore, there is an urgent need for novel antifungal agents to treat C. albicans infections.
Antimicrobial peptides (AMPs) are an important part of the innate immune defense system of insects. When the body is infected or immunostimulated, insects can produce AMPs to protect against pathogenic invasion against several bacteria, fungi, viruses, and parasites.7,8 Since the discovery of the insect AMP—cecropin—by Swedish scientist Boman9 in the mid-1970s, insect AMPs have become a research hotspot in life sciences.
The AMP produced by Musca domestica has some unique characteristics. Houseflies usually gather and breed in human and animal waste, garbage dumps, and other decaying substances, thereby carrying a large number of pathogenic bacteria, which are transmitted to humans or animals during the contact process. However, the housefly itself stays uninfected, mainly because of its powerful congenital immune system.10–12 Reportedly, house flies can produce attacin, cecropin, defensin, diptericin, and other AMP molecules to resist the invasion of pathogens.13 These biologically active proteins and peptides are considered potential alternatives to the conventional antibiotics. In recent years, with extensive research on the AMP-related functions of M. domestica, increasingly new classes of AMP molecules have been discovered.
AMP-17 (M. domestica antimicrobial peptide-17) is encoded by a specific high-expression gene selected from M. domestica transcriptome database constructed 12 hours after microbial infection. In the previous study, our research team successfully produced the recombinant protein AMP-17 in a prokaryotic expression system and purified it by a nickel ion metal chelator affinity chromatography. The purified recombinant protein AMP-17 showed excellent antifungal activity in vitro.14–16 However, the mechanism by which AMP-17 exerts antifungal effects is still unclear. To answer this question, we conducted an in-depth study on the potential anti-Candida mechanism of AMP-17 from the perspective of its influence on the cell wall integrity and cell membrane structure of C. albicans.
The cell wall and cell membrane act as barriers to fungal cells and are the potential targets of antifungal drugs and AMPs. Intracellular target organelles such as the mitochondrial membrane, nucleotides (RNA and DNA), and protein synthesis can be regulated once the AMP destroys the cell wall and cell membrane.17,18 Mannoprotein, β-glucan, and chitin are the main components of the fungal cell wall and are potential targets for AMPs to recognize fungal cells.19 Human antibacterial peptide LL-37 is reported to inhibit C. albicans adhesion to host cells by preferentially binding to mannan, a major component of the C. albicans cell wall.20 Antifungal activity of the ethanol extract from Salvia miltiorrhiza against C. albicans is associated with an increase in the membrane permeability and the reduction of (1,3)-β-D-glucan synthase activity.21 Once the drug and AMP destroy the cell wall barrier, the next potential and highly sensitive target is the fungal cell membrane. It is well known that most AMPs have direct membrane activity, which is essential for effective antimicrobial activities of these natural peptides.22 Jelleine-I isolated from the royal jelly of honeybees (Apis mellifera) could potently inhibit the growth of Candida cells both in vitro and in vivo. Scanning electron microscopy (SEM) showed that membrane surfaces of C. albicans and C. glabrata cells were inflated and rough after treatment with Jelleine-I. Further studies have found that Jelleine-I can increase the production of cellular reactive oxygen species (ROS) and bind to genomic DNA, which may contribute to its antifungal activity.23
In this study, the effects of AMP-17 on the morphological structure of C. albicans were determined by microscopy and SEM. To further clarify the mode of antifungal action, changes in cell wall integrity of C. albicans after AMP-17 treatment were assessed by cell wall staining, and cell membrane damage caused by AMP-17 was detected by fluorescent probes and glycerol assay kit. Furthermore, at the molecular level, we investigated the expression levels of the cell wall and cell membrane synthesis related-genes of C. albicans by using real-time PCR.
Materials and Methods
Chemicals
Ni-NTA beads (Novagen, Germany); Yeast RNAiso Kit, PrimeScript RT reagent Kit with gDNA Eraser, DEPC (Takara Bio, Japan); 1.6-diphenyl-1,3,5-hexatriene (DPH), and propidium iodide (PI) were purchased from Sigma Chemicals (St Louis, MO, USA). Sabouraud dextrose agar (SDA) and Sabouraud dextrose broth (SDB) were purchased from Solarbio (Beijing, China).
Strains and Culture Conditions
C. albicans (ATCC10231) was stored in a tube containing 30% glycerol at −80°C and sub-cultured twice on an SDA plate. Before each experiment, cells were cultured in SDB for 18 h on a shaker incubator (200 rpm) at 37°C.
Preparation of M. domestica AMP-17
The recombinant expression plasmid pet-28a (+) - (amp-17) prepared by our research group. A positive single colony of E. coli BL21 (DE3) harboring the plasmid pET-28a (+) - (AMP-17) was inoculated in 5 mL of liquid Luria broth (LB) medium containing kanamycin under shaking at 37°C incubation for 8 hours. The culture was transferred to 500 mL LB medium containing kanamycin. The transferred culture was allowed to grow on a shaker at 37°C until the OD600 reached 0.5. Then, a final concentration of 0.05 mmol/L IPTG was added and the culture was incubated for another 24 h at 32°C. Bacterial cells were collected by centrifugation (5000 g for 10 min) at 4°C; re-suspended in lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 100 mM NaCl); and disrupted by sonication (160 W, for 1 s and pause for 2 s, 3 min in total). The lysate was dissolved in urea. Solubilized target protein was purified by Ni-NTA beads (Novagen, USA) according to the manufacturer’s manual.
The Minimum Inhibitory Concentration (MIC) Value Determination
The turbidity of the C. albicans solution in the logarithmic growth phase was adjusted to 0.5–2.5×103 colony forming units (CFU)/mL using a blood cell counting plate. An aliquot of 100 μL of the final suspension was added into each well on a sterile 96-well plate containing 100 μL of medium containing AMP-17 at double-diluted concentrations. Blank medium was used as a negative control. The plate was incubated at 37°C for 48 h. MIC value was determined as the minimal concentration at which no growth of microbes can be observed by naked eyes according to the standard criteria from the US Clinical Laboratory and Standards Institute.
Analysis of Cell Morphology
Upright Microscope
C. albicans cells at an initial density of 1.0–5.0×106 CFU/mL were treated with 40 μg/mL AMP-17 at 37°C for 8 h and 16 h. Cells without drug treatment served as the control. Then, cells were collected by centrifugation (5000 rpm for 10 min) and washed twice in PBS buffer. The samples were pelleted on a slide and observed under a microscope (Nikon, Japan).
Scanning Electron Microscopy (SEM)
C. albicans cells at a concentration of 1.0–5.0×106 CFU/mL were cultured in SDB containing 40 μg/mL AMP-17 at 37°C for 8 h and 16 h, and then collected by centrifugation (5000 rpm for 10 min). Cells without drug treatment served as the control. The samples of each group were washed with PBS buffer twice and fixed with 1 mL 2.5% glutaraldehyde at 4°C overnight. Then, the fixed samples were washed twice in PBS and dehydrated in a graded sequence of ethanol (50%, 75%, and 100%). Finally, they were observed under a Hitachi H-7650 SEM (Tokyo, Japan).
Cell Wall Integrity Test
C. albicans cells at a concentration of 1.0–5.0×106 CFU/mL were cultured in SDB containing 40 μg/mL AMP-17 at 37°C for 12 h. Sterile water was used as the negative control, and caspofungin (CS) at a final concentration of 20 μg/mL was the positive control. The cells were centrifuged for 10 min at 5000 rpm and washed twice in PBS. The fungal precipitate was transferred to a slide, and the cells were treated with 10% citric acid for 10 min and then stained with 5% crystal violet for 1 min. After washing, the cells were observed under a microscope (Nikon, Japan); 100 cells were randomly counted to determine the cell wall integrity.
Plasma Membrane Dynamics Detection
The fluorescent dye diphenylhexatriene (DPH) is a hydrophobic molecule that can be embedded in the lipid bilayer without causing membrane perturbation. The fluorescence intensity reflects the fluidity and order of the cell membrane. C. albicans cells with an initial density of 1.0–5.0×106 CFU/mL were treated with 0 (control), 20, 40, and 80 μg/mL AMP-17 at 37°C for 12 h. Amphotericin B (AMB) was used as a positive control. Cells were collected by centrifugation (5000 rpm for 10 min) and fixed in 0.37% formaldehyde for 30 min. After fixation, the samples were washed twice in PBS, rapidly frozen in liquid nitrogen, and re-suspended in PBS after thawing. Then, the cells were stained with DPH dye at a final concentration of 0.6 mM and incubated at 30°C for 30 min. Stained cells were measured by spectrophotometry (Berthold Biotechnologies, Bad Wildbad, Germany) at 350 nm and 425 nm excitation and emission wavelengths, respectively.
Cell Membrane Permeability Test
The integrity of the cell membranes of AMP-17 treated C. albicans was determined by confocal laser scanning microscopy (CLSM). Freshly collected logarithmic fungal inoculum at a concentration of approximately 1.0–5.0×106 CFU/mL was incubated with 0 (control), 20, 40, and 80 μg/mL of AMP-17 at 37°C for 12 h. After incubation, the fungal suspension was stained with propidium iodide (PI) at the final concentration of 20 μg/mL for 15 min in the dark. Then, the stained cells were washed twice in PBS and re-suspended in PBS. Microscopic analysis was conducted using CLSM (Olympus FV1000, Japan).
Detecting Changes in Intracellular Osmotic Pressure
C. albicans cells at the logarithmic growth stage were diluted to 1.0–5.0×106 CFU/mL in SDB medium. AMP-17 at final concentrations of 20, 40, and 80 μg/mL were taken as the experimental groups and sterile water was considered the control group. Each group was placed at 37°C and cultured for 12 h. C. albicans cells were collected from each group. According to the manufacturer’s protocol, we used the Glycerol Assay Kit (Jiancheng, Nanjing, China) to measure the glycerin content to determine the changes in intracellular osmotic pressure. Protein concentrations were measured using the Bradford reagent (Beyotime, Shanghai, China) with bovine serum albumin as the standard. Glycerol concentrations were calculated as the values of the content of glycerol divided by the content of protein. Results are expressed as mean±SD. All experiments were conducted in triplicate.
Measurement of Gene Expression
We performed qPCR to quantify the expression of genes, namely the high-osmolarity glycerol (HOG) pathway genes (HOG1 and RHR2) and genes involved in biosynthesis of cell membrane and cell wall assembly. C. albicans cells were diluted to a concentration of 1.0–5.0×106 CFU/mL in SDB medium. After incubation with AMP-17 at a final concentration of 40 μg/mL at 37°C for 12 h, the cells were collected and washed by centrifugation at 5000 rpm for 10 min at 4°C. Sterile water was used as the negative control and fluconazole (FLC, 20 μg/mL) was used as the positive control group. The total RNA was extracted by Yeast RNAiso Kit (TaKaRa, Japan), and cDNA was synthesized using the PrimeScript RT (PrimeScript™ RT reagent Kit with gDNA Eraser, Takara Bio, Japan) according to the manufacturer’s instructions. The qPCR was performed using the SYBR Premix (SYBR Premix Ex Taq™ II, Takara Bio, Japan), and reactions were set up using the real-time PCR system (ABI PRISM 7300, Applied Biosystems, USA). Reaction mixtures were incubated for 30 s at 95°C, followed by 40 cycles of 5 s at 95°C and 34 s at 60°C. The primer sequences are listed in Table 1. The housekeeping gene 18S rRNA served as the internal reference gene, and relative gene expression was calculated based on the formula 2−ΔΔCT.
Table 1.
Gene-Specific Primers Used for Relative Quantification of Genes Expressions by RT-PCR
| Primers | Sequence | |
|---|---|---|
| 18S | FORWARD | 5ʹ-AATTACCCAATCCCGACAC −3’ |
| REVERSE | 5ʹ-TGCAACAACTTTAATATACGC-3’ | |
| FKS2 | FORWARD | 5ʹ-GATCACGAGTCTGTGATTG-3’ |
| REVERSE | 5ʹ-AATACATGAGACCAGCCTC-3’ | |
| ERG1 | FORWARD | 5ʹ-GCAACCGGCTGGTATCAAGGC-3’ |
| REVERSE | 5ʹ-ACACCATCAACGGCATCAGGAAC-3’ | |
| ERG5 | FORWARD | 5ʹ-GATACCGTCCACCAGTCTTGA-3’ |
| REVERSE | 5ʹ-TTTAGGAGCAGTGTAGGATTCAG-3’ | |
| ERG6 | FORWARD | 5ʹ-TGGTTGGGGTTCTTCATTCC −3’ |
| REVERSE | 5ʹ-CCAGGACCACCTACACCACA −3’ | |
| ERG11 | FORWARD | 5ʹ-CATTTGGTGGTGGTAGACATAGA-3’ |
| REVERSE | 5ʹ-AATCAGGGTCAGGCACTTTA-3’ | |
| MET6 | FORWARD | 5ʹ-ATGAAGGGTATGTTGACTG-3’ |
| REVERSE | 5ʹ-AAGTGGCAACTCTAAATGA-3’ | |
| HOG1 | FORWARD | 5ʹ-GTCTGTGGGTTGTATCTTAG-3’ |
| REVERSE | 5ʹ-TCACTAAATGGGATAGGGTC-3’ | |
| RHR2 | FORWARD | 5ʹ-GCCGTACATTTGATGTCATT-3’ |
| REVERSE | 5ʹ-AAAGTACCAGAAGTGACAAC-3’ | |
Results
Preparation of AMP-17 Recombinant Protein with Effective Antifungal Activity
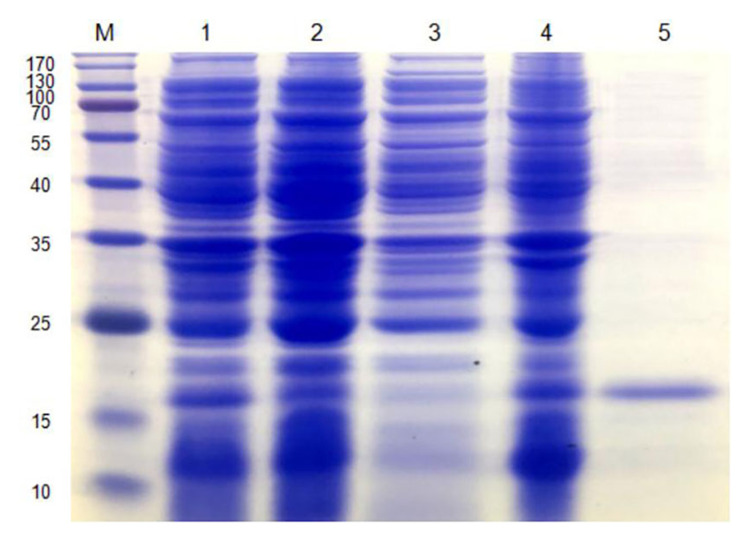
The pet-28a (+) - (AMP-17) recombinant plasmid was introduced in E. coli for expression and AMP-17 recombinant protein was purified by Ni-NTA beads. A clear protein band was observed between 15 kD and 25 kD (the sum of the molecular weights of AMP-17 peptide and His-tag), with respect to the standard molecular weight marker (Figure 1). Antifungal activity of purified AMP-17 recombinant protein against C. albicans was detected by the micro-liquid dilution method. The minimal inhibitory concentration (MIC) of recombinant AMP-17 on C. albicans was 20 μg/mL, and the minimal fungicidal concentration (MFC) was 40 μg/mL.
Figure 1.
Preparation of AMP-17 recombinant protein. SDS-PAGE analysis of AMP-17 recombinant protein from the E. coli expression (lane 1, expression plasmid pET-AMP17 without IPTG; lane 2, expression plasmid pET-AMP17 with IPTG; lane 3, supernatant after disruption of cells; lane 4, sediment after disruption of cells; lane 5, purified protein; M: protein marker).
Effect of AMP-17 on the Morphological Structure of C. albicans
Optical Microscopy
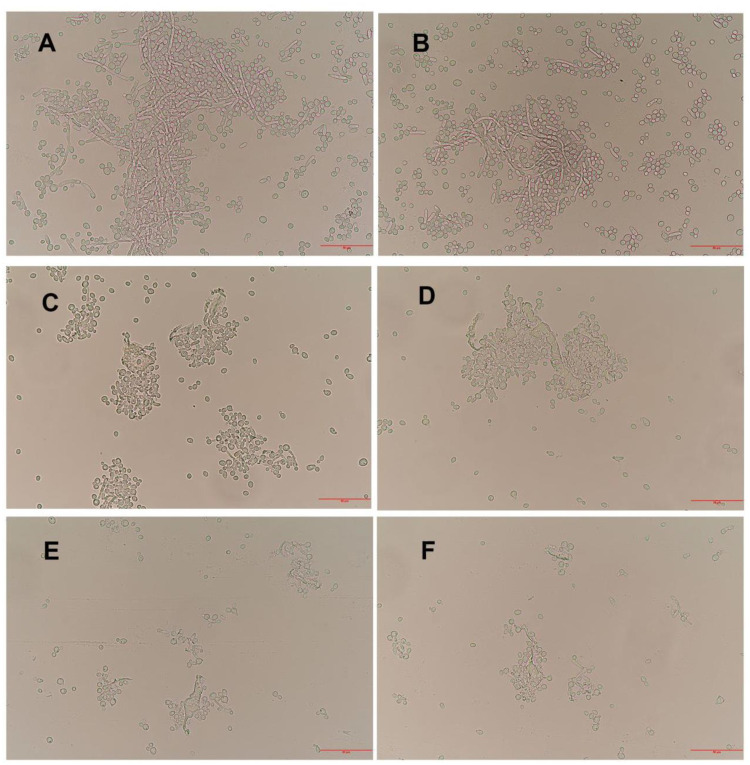
Untreated C. albicans cells were healthy and showed a round or ovoid shape, and the cells produced blastospores and formed a large number of filamentous pseudohyphae. The internal material distribution was uniform, and the refractive index was consistent (Figure 2A and B). The budding cells and filamentous pseudohyphae of C. albicans in AMP-17 treated cells were significantly reduced, and the cells were aggregated and dissolved (Figure 2C–F).
Figure 2.
Observation of the morphological changes of C. albicans by optical microscopy (400×). (A and B) Untreated C. albicans cells are healthy, with the budding cells and pseudohyphae. (C and D) and (E and F) C. albicans treated with AMP-17 (40 μg/mL) at 8 h and 16 h, respectively, showed significantly reduced budding cells and filamentous pseudohyphae, and the cells aggregated and lysed. The bars indicate 50 μm.
SEM
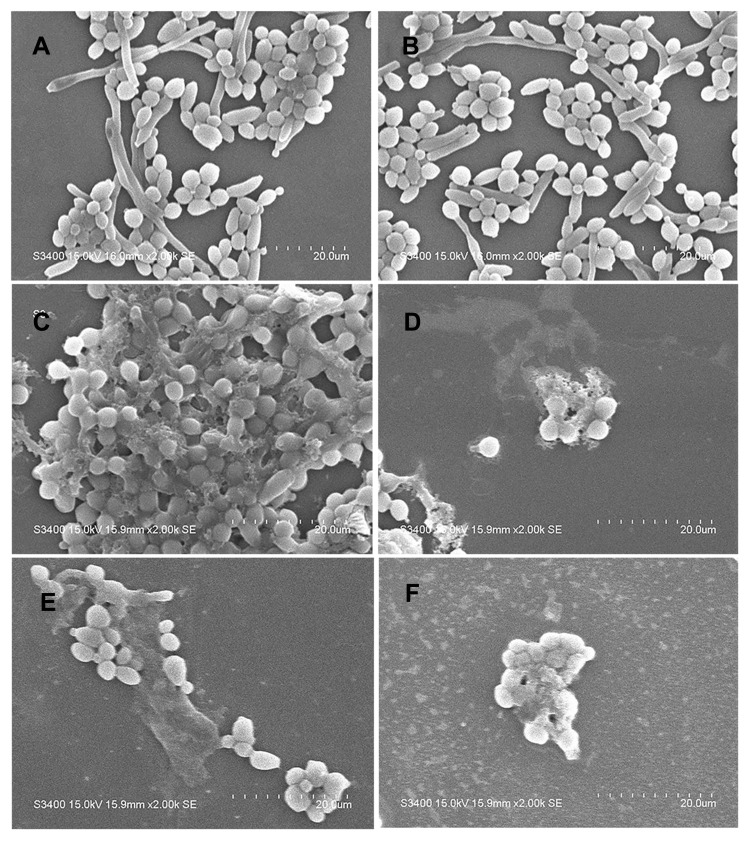
Untreated C. albicans cells showed normal and complete cellular morphology with plump appearance, smooth surface, and clear cell boundaries. Cells were uniform in size and filamentous pseudohyphae were seen on untreated cells (Figure 3A and B). After treatment with 40 μg/mL AMP-17 for 8 h and 16 h, C. albicans showed severe irregularities in shape, and cells adhered tightly to each other so that the cells had ambiguous cell boundaries. Some diseased cells were broken, and thus substances that could be the contents of the cells were seen around the cells. At two time points, filamentous pseudohyphae could not be observed and some cell debris appeared in the visual field (Figure 3C–F).
Figure 3.
SEM images of the effect of AMP-17 on the morphological structure of C. albicans. (A and B) Untreated C. albicans cells are intact, with plump appearance, smooth surface, and clear cell boundaries. (C and D) C. albicans treated with AMP-17 (40 μg/mL) at 8 h showed severe irregularities in shape, and cells adhered tightly to each other; Some diseased cells ruptured and leaked out their contents (red arrowheads). (E and F) C. albicans treated with AMP-17 (40 μg/mL) at 16 h showed lysed cells and some cell debris (red arrowheads). At two time points, pseudohyphae could not be observed. The bars indicate 20 μm.
Effect of AMP-17 on the C. albicans Cell Wall
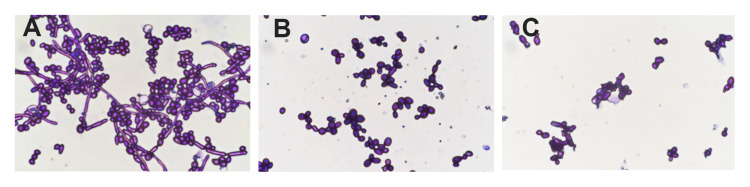
After staining, the complete cell wall structure of C. albicans cells was seen as a dark purple cell wall enclosing a light purple cytoplasm. Treatment with drugs, injury, or other factors destroys the C. albicans cell wall, and the dye enters the cell making it appear dark purple. As seen in Figure 4A, untreated C. albicans cells appeared ovoid with dark purple cell walls and light purple cytoplasm. After treatment with 40 μg/mL AMP-17 for 12 h, the cell walls and cytoplasm appeared dark purple (Figure 4B). The cell wall integrity rate of AMP-17-treated cells and untreated cells was 21.7% and 93.7%, respectively (P<0.05) (Table 2). Most of the cells in the caspofungin group displayed irregular morphology, with dark purple-stained cells (Figure 4C).
Figure 4.
The effect of AMP-17 on the cell wall of C. albicans observed by light microscopy after cell wall staining (400×). (A) Untreated C. albicans cells. (B) C. albicans treated with 40 μg/mL AMP-17 at 37°C for 12 h. (C) C. albicans treated with 20 μg/mL of caspofungin at 37°C for 12 h.
Table 2.
Effect of AMP-17 on the Cell Wall Integrity of Candida albicans
| Groups | Control | AMP-17 | Caspofungin |
|---|---|---|---|
| Cell wall integrity (%) | 93.7±2.1▲ | 21.7±3.0*▲ | 8.7±2.1* |
Notes: *Compared with negative control P<0.05; ▲Compared with CS control P<0.05.
Effect of AMP-17 on Cell Membrane
The cell membrane is an essential barrier for fungal cells, but its location and functional importance makes them particularly vulnerable to chemical toxicity. To determine the effect of AMP-17 on cell membranes, we detected plasma membrane dynamics, cell membrane permeability, changes in intracellular osmotic pressure, and expression of genes involved in cell membrane biosynthesis.
Plasma Membrane Dynamics
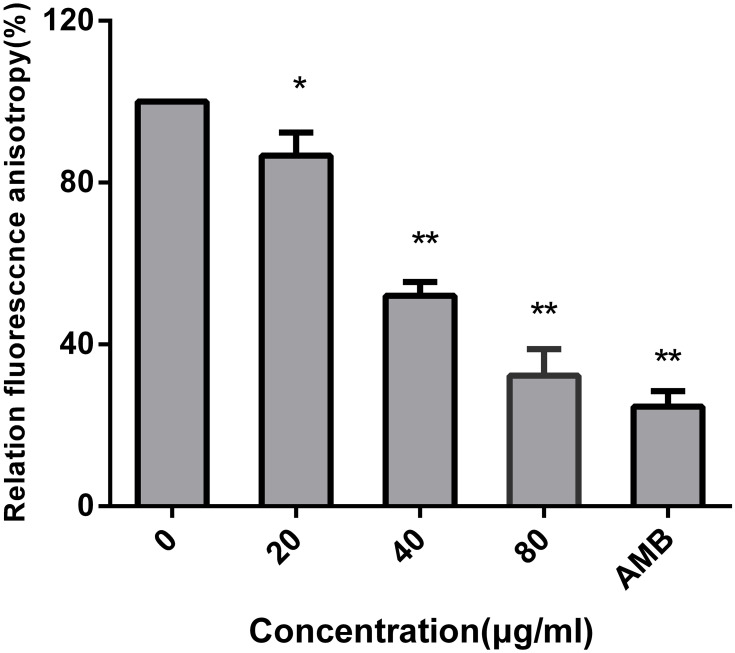
The effect of AMP-17 on the membrane dynamics of C. albicans was detected by using DPH as an indicator. Our results showed that AMP-17 decreased the relative fluorescence intensity of DPH in a dose-dependent manner. Compared with the relative fluorescence intensity of DPH in the control cells (100%), that in the 20, 40, and 80 μg/mL AMP-17 treated cells was 86.6%, 52.0%, and 32.3%, respectively. AMB (8 μg/mL) as a positive control showed a reduction in the relative fluorescence intensity of DPH of 24.7% (Figure 5).
Figure 5.
Effect of AMP-17 on the cell membrane structure of C. albicans. Cells were treated with various concentrations of AMP-17 or 8 μg/mL of AMB (positive control) for 12 h following with DPH staining for spectrofluorophotometric detection (*P<0.05, **P<0.01). Bars indicate the standard deviations.
Cell Membrane Permeability
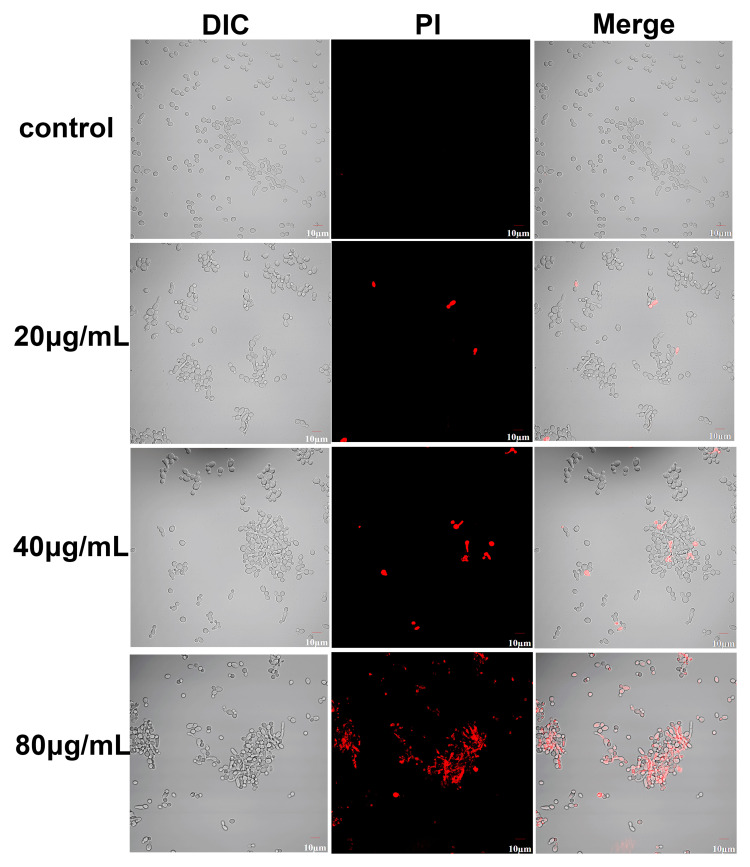
PI is a cell membrane-selective permeable dye that can only pass through damaged cell membranes. CLSM revealed that the red fluorescence seen in 12-h, AMP-17-treated C. albicans cells showed a gradual increase with the increasing concentration of AMP-17; the control group showed no fluorescence (Figure 6). This result showed that PI could penetrate the cell membrane after AMP-17 treatment and bind with DNA to show the characteristic red fluorescence.
Figure 6.
Effect of AMP-17 on permeability of C. albicans cell membrane (600×). C. albicans cells were incubated with 0 (control), 20, 40, and 80 μg/mL AMP-17 at 37°C for 12 h. After staining with 20 μg/mL propidium iodide, the red fluorescence in samples was detected by confocal laser scanning microscopy at 488 nm and 525 nm of excitation and emission, respectively. The bars indicate 10 μm.
Intracellular Glycerol Contents
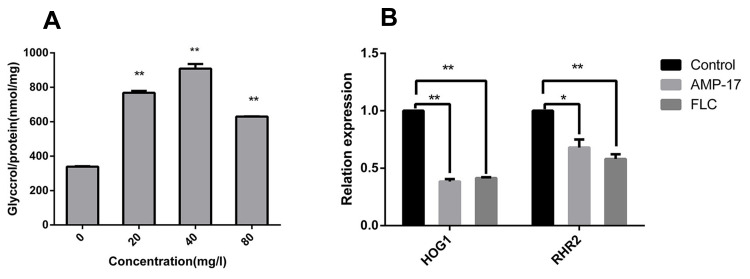
Destruction of the cell membrane may cause changes to intracellular osmotic pressure, and high-osmotic stress can activate the intracellular HOG pathway to synthesize more glycerol to balance the osmotic pressure changes. Therefore, the contents of intracellular glycerol were examined, and the results showed an increase from 338.5±3.5 nmol/mg in the control group to 768.0±11.3 and 909.0±26.9 nmol/mg under 20 μg/mL and 40 μg/mL AMP-17 treatment. However, when the concentration of AMP-17 was 80 μg/mL, the intracellular glycerol concentrations of C. albicans decreased to 630.0±1.4, which may be attributed to leakage of intracellular substances (Figure 7A). In addition, the expression of HOG pathway genes (HOG1 and RHR2) was determined by qPCR. HOG1 plays a general role in regulating stress response in C. albicans, and RHR2 is a glycerol 3-phosphatase gene involved in glycerol biosynthesis. Compared with the untreated control group, the expression levels of HOG1 and RHR2 were reduced by 2.6- and 1.5-fold, respectively, after treatment with AMP-17 (P<0.01) (Figure 7B). The results showed that the osmotic pressure of C. albicans cells was changed after AMP-17 treatment, suggesting damage to the cell membrane.
Figure 7.
Effect of AMP-17 on intracellular glycerol concentration of C. albicans. (A) The concentration of intracellular glycerol was measured using the Glycerol Assay Kit after treatment with various concentrations of AMP-17 for 12 h. (B) The total RNA was extracted with the Yeast RNAiso Kit and the expressions of HOG1 and RHR2 were measured using qPCR. Bars indicate standard deviations (*P<0.05, **P<0.01).
Expression of Cell Wall and Cell Membrane Genes
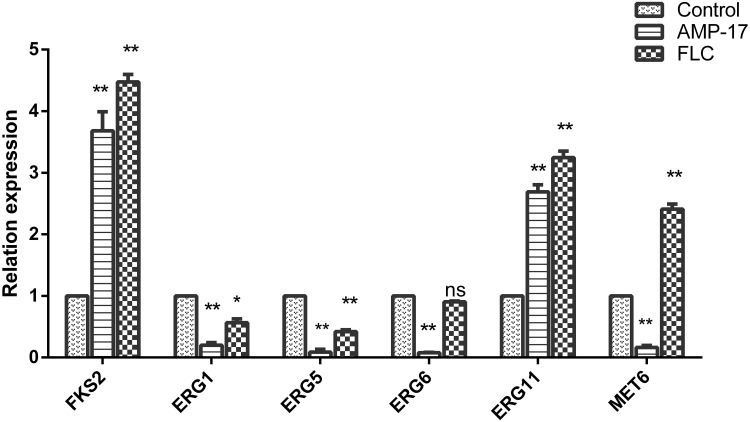
We detected the expression of genes related to cell wall and cell membrane of C. albicans by qPCR. The experimental results are shown in Figure 8. When treated with 40 μg/mL AMP-17, the C. albicans cell wall synthesis-related gene FKS2 was up-regulated by 3.46-fold, compared with the control group. The expression of some genes (ERG1, ERG5, ERG6, ERG11, and MET6) is involved in the biosynthesis of ergosterol, which plays an important role in the structure and function of the C. albicans cell membrane. As shown in Figure 8, ERG1, ERG5, ERG6, and MET6 were down-regulated by 5.88-, 17.54-, 13.33-, and 7.14-fold, respectively, while ERG11 was up-regulated by 2.69-fold when compared with the control group.
Figure 8.
Expression of AMP-17 on C. albicans cell membrane and cell wall associated genes. After treatment with 40 μg/mL of AMP-17 for 12 h, the total RNA was extracted with the Yeast RNAiso Kit and the expressions of genes related with cell membrane synthesis and cell wall assembly were assessed using qPCR (*P<0.05, **P<0.01). Bars indicate standard deviations.
Discussion
C. albicans is a common opportunistic fungal pathogen in humans, particularly among the immunodeficiency population.24–26 Owing to increasing challenges caused by toxicity, high treatment cost, and drug resistance of traditional antifungal agents in recent times, there is an urgent need to develop effective antifungal agents and therapies.27 Antibacterial peptides are a class of biologically active proteins that are generally considered to have multiple targets and modes of action. Therefore, AMPs are considered as potential alternatives to traditional antimicrobial agents.28 Many researchers have proven that genetically engineering the expression of AMPs provides an effective method for large-scale AMP production.29 In the present study, the recombinant protein AMP-17 with obvious anti-C. albicans activity was successfully produced by prokaryotic expression method. Further, the antifungal activity of purified AMP-17 recombinant protein against C. albicans was detected by micro-liquid dilution method. The MIC of recombinant AMP-17 on C. albicans was 20 μg/mL, and the MFC was 40 μg/mL.
To better understand how AMP-17 affects C. albicans cells, we used optical microscopy and SEM to observe changes to cell morphology after AMP-17 treatment for different durations. Under light microscopy, the number of cells, blastospores and filamentous pseudohyphae were significantly reduced after treatment with AMP-17. The treated cells showed aggregation and dissolution. The results of SEM showed that after AMP-17 treatment, C. albicans showed obvious pathological changes, with an abnormal and irregular cell structure. The cells were deformed and the cell boundaries were ambiguous. In addition, substances that could be cell contents and cell debris were observed around the cells. These cytopathic changes suggest that the potential targets of AMP-17 may be the cell wall and cell membrane.
The fungal cell wall surrounds the cell membrane, provides cells with rigidity and strength, and maintains osmotic support via the turgor pressure of the protoplast.21,30 The cell wall are potential targets for AMPs to recognize fungal cells. Upon cell wall staining, the dye penetrates into the cells in the event of cell wall damage; this makes the cell wall and cytoplasm of C. albicans in the AMP-17 treatment group appear dark purple. Real-time PCR showed that expression of FKS2, a gene related to fungal cell wall synthesis, was up-regulated after treatment with AMP-17. This is likely because C. albicans can increase the stability of the cell wall by up-regulating the expression of this gene involved in cell wall synthesis to cope with the AMP-17-induced damage to the fungal cell wall.
The cell membrane is the second barrier of fungal cells, and most cationic AMPs exert antifungal activity by acting on cell membranes.31 The fluorescent dyes DPH and PI are indicators of cell membrane kinetics and permeabilization. DPH can easily associate with the hydrocarbon tail region of phospholipids in the cytoplasmic membrane without disrupting the intactness of the cell membrane. This study showed that AMP-17 treatment decreased the fluorescence intensity of DPH in a dose-dependent manner. Decrease in fluorescence intensity indicates low structural order or high fluidity of the cell membrane.32 Therefore, the decrease of DPH fluorescence intensity revealed the perturbation of the cell membrane by AMP-17 treatment. PI is a nucleic acid dye that cannot penetrate viable cells with intact cell membranes, rather only damaged or permeabilized cell membranes to make stained cells emit a red fluorescence.33 Therefore, the increase in the number of stained cells revealed that high-dose AMP-17 treatment induced more cell membrane permeabilization. In our study, AMP-17-treated C. albicans cells showed a marked increase in the number of PI-stained cells, implying that AMP-17 treatment could damage the integrity of the fungal cell membrane. Damage to the cell membrane causes imbalance in the osmotic pressure of C. albicans cells. High osmotic pressure is known to activate the intracellular HOG pathway to synthesize more glycerol to balance the change in osmotic pressure.34,35 Our results showed that the intracellular glycerol concentration of C. albicans increased in a dose-dependent manner upon AMP-17 treatment. However, at an AMP-17 concentration of 80 μg/mL, the intracellular glycerol concentration of C. albicans decreased, which may be related to leakage of intracellular substance. In addition, at the genetic level, the expression levels of genes related to osmotic pressure, such as HOG1 and RHR2, were down-regulated compared with the control group. These results suggested that AMP-17 treatment disrupted the cell membrane and induced changes to the intracellular osmotic pressure of C. albicans.
Ergosterol is an important component of fungal cell membrane structure and plays an important role in ensuring cell viability, retaining plasma membrane integrity, and maintaining cellular material transport.36 Biosynthesis of the fungal cell membrane ergosterol is an extremely complex process catalyzed by various enzymes. Previous studies have shown that at least 21 gene products are involved in the biosynthesis pathway of ergosterol in yeast; thus, ergosterol regulation is a multi-level process.37 ERG and MET6 are related genes of ergosterol synthesis. The results showed that most tested genes were down-regulated except ERG11. In other words, AMP-17 can inhibit the biosynthesis of ergosterol by affecting the expression of these genes, thereby altering the integrity of the C. albicans cell membrane. ERG11 also encodes lanosterol 14α-demethylase; therefore, when the ERG11 gene is mutated, the spatial configuration of 14α-demethylase changes, which may reduce the affinity between C. albicans and azole drug molecules, resulting in drug resistance.38 Changes in gene expression provide a molecular basis for C. albicans cell wall and cell membrane damage.
Conclusion
In this study, we confirmed that AMP-17 inhibits the growth of C. albicans and affects its morphological structure. Further research shows that AMP-17 can reduce the cell wall integrity of C. albicans, destroy the cell membrane structure and increase cell membrane permeability. In addition, AMP-17 also can change the level of cell wall and membrane-associated synthetic gene expression. Multiple targets for AMP-17 in the cell membrane and cell wall of C. albicans illustrate that it could be a novel therapeutic option for prevention and control of C. albicans infections.
Acknowledgments
This study was supported by National Natural Science Foundation of China [grant numbers: 81760647]. Funders had no role in study design, data collection or analysis, preparation of the manuscript or the decision to publish it. We are grateful for the support of scientific research equipment from the School of Basic Medicine of Guizhou Medical University.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Guo Guo reports a patent ZL201610428119.8 licensed to National Intellectual Property Administration, PRC. The authors report no other possible conflicts of interest in this work.
References
- 1.Schelenz S, Barnes RA, Barton RC, et al. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect Dis. 2015;15:461–474. doi: 10.1016/S1473-3099(15)70006-X [DOI] [PubMed] [Google Scholar]
- 2.Seth R, Xess I, Jana M. Diagnosis of invasive fungal infections in children. Indian Pediatr. 2019;56(3):229–236. doi: 10.1007/s13312-019-1505-7 [DOI] [PubMed] [Google Scholar]
- 3.Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11(2):142–151. doi: 10.1016/S1473-3099(10)70218-8 [DOI] [PubMed] [Google Scholar]
- 4.Spitzer M, Robbins N, Wright GD. Combinatorial strategies for combating invasive fungal infections. Virulence. 2017;8(2):169–185. doi: 10.1080/21505594.2016.1196300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Res. 2017;10:249–259. doi: 10.2147/IDR.S124918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45(6):737–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonk M, Vilcinskas A. The medical potential of antimicrobial peptides from insects. Curr Top Med Chem. 2017;17(5):554–575. doi: 10.2174/1568026616666160713123654 [DOI] [PubMed] [Google Scholar]
- 8.Sheehan G, Garvey A, Croke M, et al. Innate humoral immune defences in mammals and insects: the same, with differences? Virulence. 2018;9(1):1625–1639. doi: 10.1080/21505594.2018.1526531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boman HG, Nilsson-Faye I, Paul K, Rasmuson T. Insect immunity I. Characteristics of an inducible cell-free antibacterial reaction in hemolymph of Samia cynthia pupae. Infect Immun. 1974;10(1):136–145. doi: 10.1128/IAI.10.1.136-145.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang T, Li X, Yang X, et al. Transcriptional response of Musca domestica larvae to bacterial infection. PLoS One. 2014;9(8):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra S, Kumar P, Malik A. Evaluation of Beauveria bassiana infection in the hemolymph serum proteins of the housefly, Musca domestica L. (Diptera: muscidae). Environ Sci Pollut Res. 2017;24(31):1–11. doi: 10.1007/s11356-017-0193-x [DOI] [PubMed] [Google Scholar]
- 12.Ai H, Wang F, Zhang N, Zhang L, Lei C. Antiviral, immunomodulatory, and free radical scavenging activities of a protein-enriched fraction from the larvae of the housefly, Musca domestica. J Insect Sci. 2013;13(112):1–16. doi: 10.1673/031.013.11201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai H, Wang F, Xia Y, Chen X, Lei C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012;132(1):493–498. doi: 10.1016/j.foodchem.2011.11.033 [DOI] [PubMed] [Google Scholar]
- 14.Tao W, Jiangfan X, Yingchun Z, et al. Transcriptional responses of Candida albicans to antimicrobial peptide MAF-1A. Front Microbiol. 2017;8:894–904. doi: 10.3389/fmicb.2017.00894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiu JF, Wang T, Wang Y, et al. Histological observation and expression patterns of antimicrobial peptides during fungal infection in Musca domestica (Diptera: muscidae) larvae. Braz Arch Biol Technol. 2016;59(6):1–13. [Google Scholar]
- 16.Guo G, Tao R, Li Y, et al. Identification and characterization of a novel antimicrobial protein from the housefly Musca domestica. Biochem Biophys Res Commun. 2017;490(3):746–752. doi: 10.1016/j.bbrc.2017.06.112 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Chang W, Zhang M, et al. Diorcinol D exerts fungicidal action against Candida albicans through cytoplasm membrane destruction and ROS accumulation. PLoS One. 2015;10(6):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weerden NLVD, Bleackley MR, Anderson MA. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol Life Sci. 2013;70(19):3545–3570. doi: 10.1007/s00018-013-1260-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabib E, Arroyo J. How carbohydrates sculpt cells: chemical control of morphogenesis in the yeast cell wall. Nat Rev Microbiol. 2013;11(9):648–655. doi: 10.1038/nrmicro3090 [DOI] [PubMed] [Google Scholar]
- 20.Tsai PW, Yang CY, Chang HT, et al. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One. 2011;6(3):e17755. doi: 10.1371/journal.pone.0017755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HS, Kim Y. Antifungal activity of Salvia miltiorrhiza against Candida albicans is associated with the alteration of membrane permeability and (1,3)-β-D-glucan synthase activity. J Microbiol Biotechnol. 2015;26(3):610–617. doi: 10.4014/jmb.1511.11009 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29(9):464–472. doi: 10.1016/j.tibtech.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 23.Jia F, Wang J, Peng J, et al. The in vitro, in vivo antifungal activity and the action mode of Jelleine-I against Candida species. Amino Acids. 2018;50(2):229–239. doi: 10.1007/s00726-017-2507-1 [DOI] [PubMed] [Google Scholar]
- 24.Richardson JP, Moyes DL. Adaptive immune responses to Candida albicans infection. Virulence. 2015;6(4):327–337. doi: 10.1080/21505594.2015.1004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SC, Joosten LA, Kullberg BJ, Netea MG, Maurelli AT. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun. 2012;80(4):1304–1313. doi: 10.1128/IAI.06146-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–128. doi: 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford CB, Funt JM, Abbey D, et al. The evolution of drug resistance in clinical isolates of Candida albicans. Elife. 2015;4:1–27. doi: 10.7554/eLife.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prashant K, Jayachandran K, Suzana S. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecular. 2018;8(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu DF, Hu JH, Liu XY. Research progress in genetic engineering expression of antimicrobial peptides. China Anim Husbandry Vet Med. 2010;37(9):124–126. [Google Scholar]
- 30.Onishi J, Meinz M, Thompson J, et al. Discovery of novel antifungal (1,3)-β-D-glucan synthase inhibitors. Antimicrob Agents Chemother. 2000;44(2):368–377. doi: 10.1128/AAC.44.2.368-377.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rautenbach M, Troskie AM, Vosloo JA. Antifungal peptides: to be or not to be membrane active. Biochimie. 2016;130:132–145. doi: 10.1016/j.biochi.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 32.Jr KK, Kataoka R, Kimura Y, et al. Dynamic structure of biological membranes as probed by 1,6-diphenyl-1,3,5-hexatriene: a nanosecond fluorescence depolarization study. Biochem. 1981;20(15):4270–4277. doi: 10.1021/bi00518a006 [DOI] [PubMed] [Google Scholar]
- 33.Choi H, Cho J, Jin Q, et al. Antifungal property of dihydrodehydrodiconiferyl alcohol 9-O-β-d-glucoside and its pore-forming action in plasma membrane of Candida albicans. Biochim Biophys Acta Biomembr. 2012;1818(7):1648–1655. doi: 10.1016/j.bbamem.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 34.Brewster J, De Valoir T, Dwyer N, et al. An osmosensing signal transduction pathway in yeast. Science. 1993;259(5102):1760–1763. doi: 10.1126/science.7681220 [DOI] [PubMed] [Google Scholar]
- 35.Gregori C, Schuller C, Roetzer A, et al. The high-osmolarity glycerol response pathway in the human fungal pathogen Candida glabrata strain ATCC 2001 lacks a signaling branch that operates in Baker’s yeast. Eukaryot Cell. 2007;6(9):1635–1645. doi: 10.1128/EC.00106-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HS, Kim Y. Paeonia lactiflora inhibits cell wall synthesis and triggers membrane depolarization in Candida albicans. J Microbiol Biotech. 2017;27(2):395–404. doi: 10.4014/jmb.1611.11064 [DOI] [PubMed] [Google Scholar]
- 37.Mercer EI. The biosynthesis of ergosterol. Pest Manag Sci. 1984;15(2):133–155. doi: 10.1002/ps.2780150206 [DOI] [Google Scholar]
- 38.Dagan A, Efron L, Gaidukov L, et al. In vitro antiplasmodium effects of dermaseptin S4 derivatives. Antimicrob Agents Chemother. 2002;46(4):1059–1066. doi: 10.1128/AAC.46.4.1059-1066.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]