Abstract
The clinical manifestations of atherosclerosis are nowadays the main cause of death in industrialized countries, but atherosclerotic disease was found in humans who lived thousands of years ago, before the spread of current risk factors. Atherosclerotic lesions were identified on a 5300-year-old mummy, as well as in Egyptian mummies and other ancient civilizations. For many decades of the twentieth century, atherosclerosis was considered a degenerative disease, mainly determined by a passive lipid storage, while the most recent theory of atherogenesis is based on endothelial dysfunction. The importance of inflammation and immunity in atherosclerosis’s pathophysiology was realized around the turn of the millennium, when in 1999 the famous pathologist Russell Ross published in the New England Journal of Medicine an article entitled “Atherosclerosis – an inflammatory disease”. In the following decades, inflammation has been a topic of intense basic research in atherosclerosis, albeit its importance has ancient scientific roots. In fact, in 1856 Rudolph Virchow was the first proponent of this hypothesis, but evidence of the key role of inflammation in atherogenesis occurred only in 2017. It seemed interesting to retrace the key steps of atherosclerosis in a historical context: from the teachings of the physicians of the Roman Empire to the response-to-injury hypothesis, up to the key role of inflammation and immunity at various stages of disease. Finally, we briefly discussed current knowledge and future trajectories of atherosclerosis research and its therapeutic implications.
Keywords: atherosclerosis, history of medicine, cardiovascular disease, inflammation
Introduction
Although coronary heart disease mortality peaked in the 1960s and then reduced drastically, the clinical manifestations of atherosclerosis represent the most frequent cause of death and are one of the most important sources of morbidity, disability and hospitalization.1
Atherosclerosis is a chronic inflammatory process that affects large- and medium-sized arteries, characterized by the presence of diffuse medium-intimal fibrofatty lesions.
This disease related to industrialization, urbanization and the lifestyle changes is present all over the world, regardless of races, ethnic groups and cultures.
Nevertheless, atherosclerosis is a disease found since ancient times with a venerable history, according to indications of paleopathology.
Convinced that the disease is best understood when examined in the context of history, we have reviewed the key steps of atherosclerosis over the centuries, with a rapid overview of the current research and future prospects.
Historical Survey
Atherosclerosis in Ancient Populations: Pioneering Work
Atherosclerosis was found in humans who lived thousands of years ago and is not just a disease typical of contemporary civilizations, as shown by autopsy findings and tomographic scans performed on mummies.
The description of the earliest evidence of atherosclerosis was made in 2003 by William A. Murphy et al on a 5300-year-old mummy named Ötzi, the Iceman. The researchers identified, by CT scan, calcific atheromatous lesions in the aorta, and in the coronary, carotid and iliac arteries.2
However, atherosclerosis in ancient populations was identified for the first time in 1852 by Austrian paleopathologist Johann Nepomuk Czermak (1828–1873). He found in the well-preserved mummy of an elderly woman “multiple considerably large and calcified plaques” in the descending thoracic aorta.3
In 1907, anatomist Grafton Elliot Smith (1871–1937) reported that the Pharaoh Menephtah, who ruled Egypt from 1213 to 1203 BC., “affected with severe atheromatous disease, large calcified patches being distinctly visible”,4 adding that “the aorta was in an extreme stage of calcareous degeneration,5 large bone-like patches standing out prominently from the walls of the vessel”.
In those same years, the paleopathologist Marc Armand Ruffer (1859–1917)6 identified atherosclerotic plaques in the aorta as well as in several large arteries of numerous Egyptian mummies, noting that atherosclerosis was a widespread disease in antiquity as in his time.7 He commented as follows:
When we consider that few of the arteries examined were quite healthy, it would appear that such lesions were as frequent three thousand years ago as they are today.8
The first description of coronary atherosclerosis of an Egyptian mummy dates back to 1931 by Allen R. Long, who performed the autopsy of Lady Teye, who lived during the Twenty-first Dynasty (about 1000 BC). He found myocardial fibrosis suggestive of previous myocardial infarction and vascular calcifications also in aorta, coronary and renal arteries.9
Lessons Learned from Mummies
About one hundred years after Ruffer’s investigations, the Horus study researchers applied the current diagnostic imaging to examine atherosclerotic disease in ancient Egyptian mummies and others from different geographical areas.
The HORUS study – named after the famous Egyptian god – was intended to determine whether the ancient Egyptians and non-Egyptian ancient populations (people from ancient Peru, the Ancestral Puebloans, and the Unangans of the Aleutian Islands) had atherosclerosis, and to study similarities and differences between the atherosclerotic disease of ancient and contemporary populations. 10−13
Researchers performed whole-body CT scans of 137 mummies, examining a period of approximately 4000 years: arterial calcifications identified by CT scan were used as a marker of atherosclerosis.13
They discovered atheromatous plaques in more than a third of the mummies examined, confirming that atherosclerosis was a disease spread across four ancient different populations, with similar percentages to those found of modern societies.12 Moreover, there was a clear relationship between the severity of atherosclerotic disease and aging, with evidence of multiple atherosclerotic lesions in the older age groups. Atherosclerosis was prevalent in the aorta, followed by the peripheral vessels, carotids, iliacs and coronaries, with no frequency difference between sexes. Atherosclerotic lesions in both modern and ancient populations were observed in the aortoiliac beds almost ten years earlier than the coronary and carotid beds.10−13
Of course, the prevalence of risk factors (such as hypertension, dyslipidaemia, diabetes) in ancient Egypt is not known. Anyway, the Egyptian mummies – expression of the wealthiest classes such as royalty and clergy – certainly led a sedentary lifestyle and practiced an “atherogenic” diet, rich in saturated fats and sugars. These factors probably had a significant impact on atherogenesis.14,15 However, this explanation does not hold for the other peoples examined: indeed, the Peruvians were farmers and shepherds, the Unangans were mainly fishermen, the Ancestral Puebloans were farmers and gatherers. All three groups practiced intense physical activity and a simple diet.
A possible explanation of the Horus study findings is based on the pro-atherogenic role played by chronic inflammation caused by frequent microbial and parasitic infections, common in the populations of those times, and favored by poor hygiene conditions and the total absence of antimicrobial treatments. This inflammatory burden of chronically infected ancient individuals may have accelerated the progression of atherosclerosis, as well as other diseases of aging, both conditions related to inflammation. Another possible pro-inflammatory cause of the time would be linked to domestic smoke inhalation caused by the fire used for cooking and lighting, as documented by frequent findings of pulmonary anthracosis in mummies. At the same time, other unknown pro-atherogenic causes cannot be excluded.15,16
The complex of these data suggests that atherosclerosis is an aging-related disease with a strong interaction between genetic and environmental factors.
The Surprising Modernity of Ancient Hypotheses
Also in ancient times, scientific studies were carried out on inflammation and atherosclerosis.17
Already in the Ebers Papyrus (1534 BC) some clinical signs of inflammation as well as the symptoms of angina pectoris are described. Writings on inflammation are already found in the first-century AD, when Cornelius Celsus (c. 25 BC – c. 50 AD) first identified the four cardinal signs of inflammation, namely: rubor or redness, tumor or swelling, calor or heat, and dolor or pain.17
In the following century, Galen (129–216 AD) considered inflammation as a reaction of the organism against injuries, adding the disturbance of normal function (functio laesa) as the fifth cardinal sign.18 The first attempts to correlate the pathological changes of the coronary arteries to clinical symptoms, in particular angina pectoris, date back to Leonardo da Vinci (1452–1519), who observed the modifications induced by arteriosclerosis in olden men, stating: “vessels in the elderly, through the thickenings of the tunics, restrict the transit of the blood”.19
However, the first detailed and accurate description of arteriosclerosis is due to the Italian surgeon and academic Antonio Scarpa (1752–1832), who rejected the hypothesis that an aortic dilatation was the real cause of an aneurysm tending to rupture. In his book, Sull’ Aneurisma, Riflessioni ed Osservazioni Anatomico-chirurgiche he describes the cause of aneurysm as “a slow, morbid ulcerated, steatomatous, fungus, squamous degeneration of the internal coat of the artery”. Scarpa also emphasized that
… especially the internal coat is subject, from slow internal cause, to an ulcerated and steatomatous disorganization, as well as to a squamous and earthy rigidity and brittleness,
introducing modern concepts of lipid infiltration and intimal involvement in the process causing atherosclerosis.20
In 1755, the term atheroma (from the Greek athere “gruel” or “porridge”) was first used with reference to the human arteries used by the Swiss physiologist Albrecht von Haller (1708–1777).20
In 1833, Jean-Fréderic Lobstein (1777–1835) in his “Traité d’anatomie pathologique” first coined the term “arteriosclerosis”21 to indicate generally all forms of arterial hardening, while in 1903 the German pathologist Johann Georg Mönckeberg (1877–1925) identified calcifications only localized in the tunica media of medium arteries, highlighting that it was a specific pathological entity unrelated to other forms of arteriosclerosis (Mönckeberg’s sclerosis and Mönckeberg’s medial calcinosis).22
The word “atherosclerosis” was introduced in 1904 by the German pathologist Felix Marchand (1846–1928) to emphasize the presence of lipid material in some of the lesions, causing vascular stiffening and obstructions.23
In the middle of the 19th century, several scholars extensively investigated the histopathological aspects of atherosclerosis, proposing several pathogenetic theories. In particular, two opposing theories on atherogenesis emerged: that of the German pathologist Rudolf Virchow (1821–1902) (Figure 1) and that of the Austrian baron Carl von Rokitansky (1804–1878) (Figure 2). Both pathologists emphasized the presence of histological signs of inflammation in atherosclerotic plaques, but gave an opposite interpretation.
Figure 1.

Rudolf Virchow (1821–1902).
Note: Photo courtesy of Carl Günther (public domain).105
Figure 2.

Carl von Rokitansky (1804–1878).
Note: Photo courtesy of Adolf Dauthage (public domain).106
Virchow supported the primary role of inflammation in atherogenesis (“endoarteritis chronica deformans”), considering it an initiating factor.24 Rokitansky, on the contrary, believed that the cellular inflammatory changes in atherosclerotic vessel walls were secondary in nature, stating that
the deposit cannot be regarded as the product (exudation) of an inflammation of the arteries. The chronic inflammation of the cellular sheath of the diseased vessel is almost always a secondary consecutive appearance which associates itself with the already established deposit.25
The Virchow’s inflammatory theory of atherosclerosis is surprisingly similar to the current view, since it considered atherosclerosis as a chronic inflammation determined by an active tissue reaction process, and not a simple thrombus incrustation or passive lipid deposition. He asserted as follows:
the frequency with which cells in a state of fatty degeneration are found in inflamed parts, affords sufficient proof, that in the course of inflammatory processes, which it is impossible we should ever regard as simply passive processes, such transformations must take place.26
Substantially, Virchow believed that atherosclerotic lesions resulted from injury to the artery wall, with an association of inflammatory and proliferative responses, preceding the morphological changes observable in advanced lesions.27
The conceptual evolution of Virchow’s theory has contributed significantly to the current knowledge of the cellular and molecular mechanisms of atherosclerosis, laying the basis of the famous Response-to -Injury Hypothesis of Russel Ross (1929–1999). However, it took more than a century before these mechanisms were elucidated, so his hypothesis was forgotten, since the theory that considered atherosclerotic plaque as a passive fat accumulation prevailed.
Virchow’s concept was then deepened and reworked over time by Duguid,28 French,29 Mustard and Packham,30 Wissler,31 Thomas et al.32 Above all, Ross and Glomset, at first emphasized the intimal proliferation of smooth muscle cells as a key event and one of the main early alterations in atherosclerosis.33 In essence, Ross’s response-to-injury hypothesis asserted that atherosclerosis was caused by certain forms of endothelial injury, resulting in endothelial desquamation and subsequent platelet aggregation in the damaged site; platelets then elaborated factors stimulating migration and proliferation of smooth muscle cells.
The “injury response” hypothesis – now widely accepted as a unifying pathogenetic mechanism operating in atherosclerosis – has contributed to study and deepen the inflammatory response in atherosclerosis, leading to a more comprehensive understanding of its immunological and molecular aspects.
Albeit injury was initially supposed to cause endothelial denudation, following research demonstrated that the lesion can be very slight, emphasizing endothelial cell dysfunction rather than denudation. In 1993, Russell Ross perfected the response-to-injury hypothesis, largely coding the current view of atherogenesis stating as follows:
… the lesions of atherosclerosis represent a specialized form of a protective, inflammatory-proliferative response to various forms of insult to the artery wall. Depending upon the nature and duration of the insult, the protective response may become excessive and over many years in its excess become a disease state.34
Finally, in 1999 Ross published a paper in the New England Journal of Medicine, in which he definitively stated that atherosclerosis was an inflammatory disease and did not result simply from lipid accumulation.35
Cholesterol and Atherosclerosis: More Than a Century of Studies
During the twentieth century, intense studies and research on lipoprotein metabolism helped to clarify the pro-atherogenic effect of LDL-C, as well as the pathophysiological and molecular mechanisms of atherosclerosis. Furthermore, several clinical and epidemiological works showed a good correlation between plasma cholesterol levels and the risk of cardiovascular clinical events.
Therefore, the importance of the role of cholesterol in atherogenesis is considered one of the most relevant scientific acquisitions of the twentieth century.
In the early 1900s, several scholars focused on the relationship between high-fat diet and atherosclerosis.
In 1910, Adolf Windaus (1876–1959) – Nobel Prize in Chemistry 1928 – showed that atheromatous lesions contained 25-fold more cholesterol than the normal arterial wall.36
A few years later, Russian researchers tried to induce experimental atherosclerosis in an animal model, feeding laboratory animals with pure cholesterol. They demonstrated that cholesterol alone caused atherosclerotic lesions in the artery wall.
In particular, in 1913 the pathologist Nikolai N. (1885–1964) Anitschkow first showed that rabbits fed high amounts of purified cholesterol developed – in association with very high blood cholesterol – vascular lesions similar to human atherosclerosis.37,38 He accurately described the various cell types found in atherosclerotic lesions, including macrophages, lymphocytes and smooth muscle cells, partly anticipating a unifying view of the atherosclerosis’s pathophysiology which links dyslipidaemia with inflammation.
In addition, Anitschkow first identified the “cholesterinesterphagozyten”, deriving from macrophages and currently known as foam cells.
However, Anitschkow’s work aroused little interest in the American and European medical world of the time, and several decades passed before the originality of his research was scientifically recognized. Only in 1958, William Dock highlighted the importance of Anitschkow’s theory of atherosclerosis, assimilating it to the discovery of the tubercle bacillus by Robert Koch.
In 1964, whit a paper on coronary atherosclerosis published in Circulation entitled “Compensatory adjustments in the structure of coronary arteries of the heart with stenotic atherosclerosis”, Anitschkow opened an innovative research line in this field.39
Numerous subsequent epidemiological and clinical research confirmed the central role of cholesterol in atherosclerosis, showing that its reduction prevented cardiovascular events.
The 20th century brought many other contributions to the study of atherosclerosis, the most important of which focused on LDL as the primary cause of this disease. Among these we remember above all the discovery, in 1973, of the LDL receptor by Michael S. Brown and Joseph L. Goldstein (jointly winners of the Nobel Prize in Physiology or Medicine 1985);40 the identification of the first HMG CoA reductase inhibitor (called compactin) which ushered in the class of cholesterol-lowering drugs known as statins;41,42 finally, the recent introduction of a new class of effective lipid-lowering drugs, the anti-PCSK9 (proprotein convertase subtilisin/kexin type 9) antibodies. PCSK9 is an enzyme secreted by the endoplasmic reticulum in liver cells that promotes the degradation of LDL receptors. Today it is known that adding an anti-PCSK9 antibody to standard therapy drastically reduces plasma LDL –C, also significantly decreasing cardiovascular event incidence.43,44
A Brief Overview of the Current Atherosclerosis’s Pathophysiology
Atherosclerosis is a chronic inflammatory disease of large- and medium-sized arteries, characterized by subendothelial accumulation and subsequent oxidative modification of lipoproteins, immune cells, and extracellular matrix.45–47 Endothelial dysfunction,48,49 underlying the disease, is predisposed by numerous risk factors (such as modified or oxidized LDL, diabetes mellitus, hypertension, cigarette smoking, infectious agents, age, and association of these or other factors) and involves disorders of the anti-thrombotic, pro-fibrinolytic and anti-inflammatory and anti-oxidant properties of the endothelium.50
Several lines of evidence support the importance of inflammation and immunity in the pathogenesis of atherosclerosis: inflammatory and immune mechanisms link traditional and emerging risk factors to altered biology of arterial wall cells, and open new pathways for therapeutic approaches.51,52
Immunity operates in atherogenesis through a complex network of interactions between its components, balancing pro-atherogenic inflammatory and atheroprotective anti-inflammatory responses and thus affecting plaque progression or stability.52–55
Innate immunity is a non-specific immunity, able to give rapid and non-selective responses, as a first line of defense, mediated by NK (Natural killer) lymphocytes, monocytes, macrophages, neutrophils, eosinophils, mast cells, and dendritic cells.52–55 Innate immune cells detect both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs),56 through the expression of host sensor proteins, called pattern recognition receptors (PRRs), including toll-like receptors (TLRs).57–59 The effector mechanisms of innate immunity consist of inflammation, phagocytosis, activation of the complement cascade, cytotoxicity mediated by NK cells, the production of interferon, apoptosis and autophagy.
The innate response is followed by an antigen-specific adaptive immune responses triggered and mediated by T and B cells.52–55
Initiation and Progression of Atherosclerosis: Role of Innate Immunity
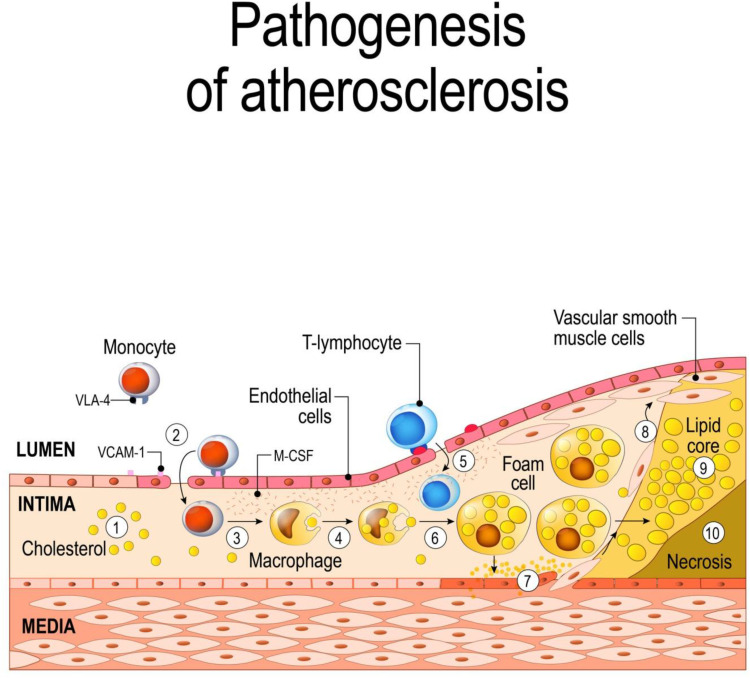
Atherosclerosis starts with the subendothelial buildup of cholesterol-carrying LDL, which stimulates both the innate and adaptive immune responses. LDL, particularly oxidized-LDL and subclass B of LDL, exhibit properties of damage-associated molecular patterns and induce the activation of endothelial cells, thereby triggering an inflammatory response.60–64 Endothelial activation, by local pro-inflammatory cytokines elaboration, triggers expression of leukocyte adhesion molecules on endothelial cells (such as E and P-selectins, intercellular adhesion molecule 1 called ICAM-1 and vascular cell adhesion molecule 1, VCAM-1), and consequent monocyte adhesion to the endothelium65,66 (Figure 3).
Figure 3.
Schematic of atherogenesis.
Notes: The oxidized-LDLs induce the activation of endothelial cells and the expression of various leukocyte adhesion molecules (such as VCAM1) and consequent monocyte adhesion to the endothelium (1,2); subsequent transmigration of monocytes into the intima, where they differentiate into macrophages (3,4); T lymphocytes join macrophages in the intima during plaque evolution (5); macrophages, incorporating modified lipoproteins, become lipid-rich foam cells (6); the inflammatory response stimulates migration and replication of vascular smooth muscle cells, which accumulate in the plaque to form a fibroproliferative lesion (7,8); macrophages in the plaque show abnormal lipid metabolism with a reduction of the cholesterol efflux, (9) which leads to accumulation of apoptotic bodies and necrotic debris, forming a necrotic core (10). ©[designua]/123RF.COM
This is followed by the secretion of chemokines, such as monocyte chemoattractant protein-1 (MCP-1), which recruits circulating monocytes to transmigrate into the intima, where they differentiate into macrophages in response to macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Recruited monocytes and other immune cells also produce proinflammatory cytokines and chemokines that further promote a positive feedback loop of increased cell transmigration into the developing lesion.67–69
In particular, a pro-inflammatory monocyte subset, that has high Ly6C expression in mice differentiates into macrophages which – incorporating modified lipoproteins via an array of scavenger receptors – become lipid-rich foam cells, found in the early stages of atherosclerosis.70
Continued inflammation induces migration and proliferation of vascular smooth muscle cells, that accumulate in the areas of inflammation to form a fibroproliferative lesion with a progressive narrowing of the vessel lumen.71
Macrophages in the plaque acquire altered lipid metabolism with a reduction of the cholesterol efflux, which leads to the accumulation of apoptotic bodies and necrotic debris, forming a necrotic core covered by thin fibrous caps at risk of rupture. Increased cell death, reduced efferocytosis and extensive matrix remodelling may thus contribute to the growth and progression of atheromatous plaques. Moreover, resident macrophages proliferate and release several growth factors and cytokines, including tumour necrosis factor (TNF)-α, interleukin (IL)-1β or IL-6, as well as CD40 ligand (CD154) and interstitial collagenases (matrix metalloproteinase: MMP-1, MMP-8, MMP-13), perpetuating local inflammation and contributing to plaque rupture.72–75
B, T lymphocytes join macrophages in the intima during plaque evolution.76
The recruitment and activation of neutrophils increase endothelial adhesion and the consequent infiltration of monocytes and macrophages inside the vessel wall, through the release of ROS, which intensifies even more ROS production in resident macrophages and subsequent foam cell formation. Neutrophils release pro-inflammatory molecules, such as TNF-α, IL-1, IL-6, IL-8 and GM-CSF, as well as the pro-oxidant enzyme myeloperoxidase and MMP-8 and MMP-9, further increasing inflammation and setting the stage for plaque disruption, acute thrombosis with subsequent vascular occlusion.77,78
Role of Adaptive Immunity
The innate immune response is quickly followed by an antigen-specific adaptive immune response to a series of antigens presented to effector T lymphocytes by antigen-presenting cells, such as dendritic cells.72
Several studies suggest that plasma levels of LDL and its core protein ApoB are a major self-antigen within the plaque which elicits an autoimmune response against self-proteins in the atherosclerotic plaque, as well as HSPs (heat shock proteins) and microbial peptides such as Cytomegalovirus hepatitis C virus, HIV, human papillomavirus.
Adaptive immunity is mediated by T and B cells, which recognize modified auto-antigens presented by antigen-presenting cells, such as macrophages or dendritic cells. T cells are among the first cells to be recruited into atherosclerotic lesion.79–82
The adaptive response is based on CD4 + and CD8 + T cells activation and antibody secretion by B cells. Once activated, CD4+ T cells multiply and differentiate into specialized effector T helper (Th) lymphocytes, while activated CD8+ T cells multiply and differentiate into cytotoxic CD8+ T lymphocytes. In response to the local milieu of cytokines, CD4+ T cells can differentiate into various lymphocyte subgroups, including helper T cells (Th1, Th2, and Th17) and Treg cells.
Th1 pathway exhibits a potent pro-atherogenic effect, by producing high levels of IFN-γ (interferon-gamma), promoting the inflammatory response. The role of Th2 cells in atherosclerosis is still controversial: they can dampen inflammatory responses promoting lesion healing through the production of IL-10 and IL-4, although some Th2-slanted responses appear to trigger aneurysm formation. Th17 cells produce IL-17 which has different effects on lesions, emphasizing endothelial inflammation but also increasing fibrosis and promoting plaque stability.79–82 Treg cells are immunoregulatory cells with atheroprotective properties: they secrete the immunosuppressive cytokines such as transforming growth factor-beta (TGF-β), IL-10 and IL-35, which exert anti-inflammatory actions.83
As can be seen, T cells not only promote inflammatory responses but can also mitigate them.
B lymphocytes also emerged as important immune cells in atherosclerosis and are considered important modulators of both pro and anti-inflammatory effects in atherosclerosis: these can be subclassified as B1 and B2 cells.
B1 cells are heterogeneous and exhibit atheroprotective effects, mainly via the production of IgM antibodies against oxidation-specific epitopes and antigens derived from apoptotic cells.
B2 cells of the adaptive system – that must be activated by T-follicular helper cells to differentiate into plasma cells producing IgG antibodies – mediate pro-atherogenic responses, via secretion of inflammatory cytokines or via the production of IgG autoantibodies.84,85
In sum, the inflammatory and immune-mediated response can act either by promoting atherosclerosis or by facilitating lesions healing; moreover, they influence coagulation and fibrinolysis, thus being able to modulate the thrombotic complications of the disease.
Role of the NLRP3 Inflammasome
In the 1990s, Polly Matzinger developed an innovative concept of immunity models, called the “danger theory”. According to this view, the main function of the immune system was not to discriminate between self and non-self, but rather “the need to detect and protect against danger”.86 The immune system is able to recognize not only pathogen-associated molecular patterns, but also damage-associated molecular patterns from cellular or tissue lesions (such as in atherosclerosis).
Endogenous molecules released or activated during tissue stress or damage can be detected by pattern recognition receptors (PRRs), triggering immune and inflammatory responses similar to those induced by pathogens.
In fact, PRRs – expressed in the innate immune system cells, such as macrophages, dendritic cells and neutrophil – are proteins that mediate the recognition of both molecules found in pathogens (pathogen-associated molecular patterns-PAMPs) and molecules released by damaged cells (Damage-Associated Molecular Pattern -DAMPs).57,58
Among the different families of PRRs, a member of the NLR family called NLR pyrin domain containing 3 (NLRP3) is particularly important, as it detects several DAMPs to form an NLRP3 inflammasome.
The NLRP3 inflammasome is the most widely studied of the known inflammasomes: it is an intracellular multi-protein consisting of NLRP3, the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) and caspase-1. The NLRP3 inflammasome can be triggered by pathogen-associated molecular patterns or damage-associated molecular patterns; after these stimuli, NLRP3 oligomerizes and interacts with the ASC, promoting the autocatalytic activation of procaspase-1. Once enzymatically active, caspase-1 convert proIL-1β and proIL-18 to mature forms, markedly pro-inflammatory cytokines that initiate the inflammatory cascade. In addition, inflammasome-mediated caspase-1 activity induces a specific inflammatory form of programmed cell death, called pyroptosis.87,88
Growing evidence indicated the crucial role in driving atherogenesis of this inflammasome.
NLRP3 inflammasome is considered as a link between lipid metabolism and inflammation, since it is activated in macrophages by various danger signals (mainly crystalline cholesterol, oxidized low-density lipoprotein, but also reactive oxygen species, mitochondrial dysfunction, endoplasmic reticulum stress, and lysosome rupture) which trigger the inflammatory response.
NLRP3 inflammasome activation leads to the maturation of IL-1β and IL-18, which are both considered the main responsible for atherogenesis, highly expressed in human atherosclerotic plaques in proportion to the severity of the disease.
IL-1ß enhances the inflammatory response by amplifying the production of IL-6, TNF-α, IL-8, adhesion molecules such as ICAM 1 and VCAM1, chemokines such as MCP-1 thus promoting recruitment and following infiltration of neutrophils and monocytes. IL-1ß further causes the production of platelet-derived growth factor, which stimulates the proliferation of smooth muscle cells, facilitating the formation of foam cells, and increases MMPS production and following degradation of the extracellular matrix.89,90
Conclusive Remarks and Future Perspectives
Our knowledge of the pathophysiology of atherosclerosis has essentially evolved in the last century.
Experimental and clinical studies focused on the link between lipoproteins and atherogenesis until the 1970s, while vascular biology research investigated the growth factors and proliferation of smooth muscle cells in the 1970s and 1980s.
In the recent decades, the modulatory role of inflammation and immunity has gained increasing importance in experimental atherosclerosis research.
Vascular inflammation is now considered a key event of atherosclerosis which contributes to plaque instability and major adverse cardiovascular events, as brilliantly proposed by Virchow more than 160 years ago, laying the foundation of Russel Ross’s Response-to -Injury Theory. As often in science, current acquisitions have ancient roots.
After many years of electron microscopy and immunohistochemical research, the current framework of atherosclerosis is not fully clarified. Above all, molecular and immune mechanisms underlying atherosclerosis remain widely unsolved; of course, this has an impact on the therapeutic approach.
Due to their anti-inflammatory and metabolic benefits, effective lifestyle interventions – such as Mediterranean diet, regular exercise, weight control and smoking cessation – are prescribed as a non-pharmacological treatment for atherosclerosis, preceding or accompanying drug therapy for reducing cardiovascular risk. In this regard, it should be noted that Tsimane – a Bolivian population conducting a subsistence lifestyle with few coronary risk factors – have the lowest prevalence of ischemic heart disease in the world, notwithstanding a high inflammatory burden.91
Many anti-inflammatory drugs, such as statins, antithrombotics and antihypertensives are widely used, but at most, they only delay the progression of atherosclerosis; therefore, new anti-atherosclerotic therapies that address the residual inflammatory risk are warranted.
The CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) trial demonstrated the effectiveness of targeting inflammation in atherosclerosis. Canakinumab - a therapeutic monoclonal antibody targeting interleukin-1β - led to a significantly lower rate of recurrent cardiovascular events than placebo in 10,061 patients who have sustained a previous myocardial infarction, with no significant difference in all-cause mortality.92,93
Interestingly, CANTOS was the first trial to prove the inflammatory hypothesis of atherothrombosis.
In fact, the treatment significantly reduces plasma inflammatory markers (such as C‐reactive protein and interleukin‐6) without changing the levels of LDL cholesterol or HDL cholesterol; individuals with the highest levels of hs-CRP benefited most from the IL-1ß block.94
The data from the CANTOS strongly indicate that other potential pathways targeting inflammation – such as those that directly inhibit NLRP3 function or that alter downstream IL-6 signaling – may constitute a breeding ground for future research. For example, arglabin – a natural product with anti-inflammatory properties – limits atherosclerotic development in atherosclerotic risk mice, by impeding the activation of the NLRP3 inflammasome in macrophages.95 Similarly, colchicine96 and atorvastatin97 were tested to contain atherosclerosis by targeting the NLRP3 inflammasome.
However, specific inhibition of cytokines, rather than broad-spectrum anti-inflammatory therapy would be essential for atheroprotection, since low-dose methotrexate did not reduce biomarkers of inflammation, nor lowered cardiovascular events in the CIRT (Cardiovascular Inflammation Reduction Trial).98,99 Furthermore, canakinumab only moderately (≈15%) reduced CV events in individuals after myocardial infarction leaving a high residual CV risk.
A new field of research is focused on the study of dysregulated immune cells within atherosclerotic lesions. The use of innovative single-cell proteomic and transcriptomic analyses allows to detect the immune diversity and to identify the molecular alterations of immune cells in the atherosclerotic plaques, promoting the design of new and more precise cardiovascular immunotherapies, tailored to immune molecular and cellular defects.100 High-parameter technologies, such as mass cytometry (CyTOF) and single-cell RNA sequencing (scRNAseq), showed an unexpected diversity among murine aortic leukocytes; in particular, CyTOF has proven more specific in distinguishing the phenotypic diversity of the leukocyte subsets, while scRNAseq provides more information on their likely functions. The definition of leukocyte diversity may be useful to establish a functional relevance for lesional leukocytes in human atherosclerosis.101
Recently, in-depth analysis of immune cells using cytometry by time of flight and single‐cell RNA sequencing led to the identification of three main macrophage subsets residing in the atherosclerotic arterial wall of mice and men: resident‐like, pro‐inflammatory and anti‐inflammatory foamy TREM2hi macrophages.102 The identification and mapping of immune cells clusters and their functions in the atherosclerotic plaque will greatly expand our pathophysiological knowledge of atherosclerosis, enabling a more accurate risk stratification, as well as innovative and personalized therapeutic approaches.
Finally, vaccination against atherosclerosis is a promising and perhaps inexpensive approach to induce effective adaptive immunity; several candidate antigens – including OxLDL, apoB100, CETP (Cholesteryl ester transfer protein), PCSK9, HSP60, MHC-II-derived peptides, and interleukins – have been tested and shown to reduce disease in animal models, while very few studies have been conducted in humans.103,104
The coming years should prove useful in understanding the unexplored mechanisms of atherogenesis and in translating new acquisitions into clinical practice.
Abbreviations
CETP, Cholesteryl ester transfer protein; CVD, cardiovascular disease; CyTOF, mass cytometry; DAMPs, damage-associated molecular patterns; GM-CSF, granulocyte-macrophage colony-stimulating factor; HMG CoA, 3-hydroxy-3-methyl-glutaryl-CoA; HSPs, heat shock proteins; ICAM-1, intercellular adhesion molecule 1; IFN-γ, interferon-γ; IL, interleukin; LDL, low-density lipoprotein; MCP-1, monocyte chemo-attractant protein-1; M-CSF, macrophage colony-stimulating factor; MHC, major Histocompatibility Complex; MMPs, matrix metalloproteinase; NLRP3, NOD-like receptor family, pyrin domain-containing protein 3; NK, natural killer; PAMPs, pathogen-associated molecular patterns; PCSK9, proprotein convertase subtilisin/kexin type 9; PRRs, pattern recognition receptors; ROS, reactive oxygen species; single-cell RNA sequencing (scRNAseq); TGF-β, transforming growth factor beta; Th, T helper; TLRs, Toll-like receptors; TNF, tumor necrosis factor; Treg, regulatory T cells; VCAM-1, vascular cell adhesion molecule 1.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association [published correction appears in Circulation. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Murphy WA Jr, Zur Nedden D, Gostner P, Knapp R, Recheis W, Seidler H. The Iceman: discovery and imaging. Radiology. 2003;226(3):614–629. [DOI] [PubMed] [Google Scholar]
- 3.Czermak J. Description and microscopic findings of two Egyptian mummies. Meet Acad Sci. 1852;9:27–69. [Google Scholar]
- 4.Smith GE. 61079: the mummy of Menephtah In: Smith GE, editor. The Royal Mummies. Cairo, Egypt: Imprimerie de l’Institut Francais d’Archeologic Orientale; 1912:65–70. [Google Scholar]
- 5.Shattock SG. A report upon the pathological condition of the Aorta of King Menephtah, traditionally regarded as the Pharaoh of the Exodus. Proc R Soc Med. 1909;2(PatholSect):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandison AT. Sir Marc Armand Ruffer (1859-1917) pioneer of palaeopathology. Med Hist. 1967;11(2):150‐156. doi: 10.1017/s002572730001200x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruffer MA. Remarks on the histology and pathological anatomy of Egyptian mummies. Cairo Sci J. 1910;4:40. [Google Scholar]
- 8.Ruffer MA. On arterial lesions found in Egyptian mummies (1580 B.C.—525 A.D.). J Pathol Bacteriol. 1911;16:453–462. doi: 10.1002/path.1700150403 [DOI] [Google Scholar]
- 9.Long AR Cardiovascular renal disease: a report of a case three thousand years ago. Arch Pathol (Chic) 1931; 12:92–94 [Google Scholar]
- 10.Allam AH, Thompson RC, Wann LS, et al. Atherosclerosis in ancient Egyptian mummies: the Horus study. JACC Cardio-Vasc Imaging. 2011;4(4):315–327. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RC, Allam AH, Lombardi GP, et al. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet. 2013;381(9873):1211–1222. doi: 10.1016/S0140-6736(13)60598-X [DOI] [PubMed] [Google Scholar]
- 12.Allam AH, Mandour Ali MA, Wann LS, et al. Atherosclerosis in ancient and modern Egyptians: the Horus study. Glob Heart. 2014;9(2):197–202. doi: 10.1016/j.gheart.2014.03.2454 [DOI] [PubMed] [Google Scholar]
- 13.Thompson RC, Allam AH, Zink AR, et al. Computed tomographic evidence of atherosclerosis in the mummified remains of humans from around the world. Glob Heart. 2014;9(2):187–196. doi: 10.1016/j.gheart.2014.03.2455 [DOI] [PubMed] [Google Scholar]
- 14.David AR, Kershaw A, Heagerty A. Atherosclerosis and diet in ancient Egypt. The Lancet. 2010;375(9716):718–719. doi: 10.1016/S0140-6736(10)60294-2 [DOI] [PubMed] [Google Scholar]
- 15.Minelli P, Montinari MR. The mediterranean diet and cardioprotection: historical overview and current research. J Multidiscip Healthc. 2019;12:805–815. doi: 10.2147/JMDH.S219875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas GS, Wann LS, Allam AH, et al. Why did ancient people have atherosclerosis? From autopsies to computed tomography to potential causes. Glob Heart. 2014;9(2):229–237. doi: 10.1016/j.gheart.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 17.Rocha e Silva M. A brief survey of the history of inflammation. Agents Actions. 1978;8(1–2):45–49. doi: 10.1007/bf01972401 [DOI] [PubMed] [Google Scholar]
- 18.Rather LJ. Disturbance of function (functio laesa): the legendary fifth cardinal sign of inflammation, added by Galen to the four cardinal signs of Celsus. Bull N Y Acad Med. 1971;47(3):303–322. [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MK, Eollman A. Leonardo da Vinci (1452–1519). Heart. 1996;76(6):464. doi: 10.1136/hrt.76.6.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibowitz J. The History of Coronary Disease. London: Wellcome Institute of the History of Medicine; 1970:107. [Google Scholar]
- 21.André E Jean-Frédéric Lobstein: “Artériosclérose” et “ostéoporose”, Historie des Sciences Medicales - Tome LII - N° 2, 197-208, 2018.
- 22.Mönckeberg JG, Die Reine Ü. Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virchows Arch Path Anat. 1903;171:141–167. doi: 10.1007/BF01926946 [DOI] [Google Scholar]
- 23.Marchand F. Ueber Atherosclerosis. Vol. 21 Kongresse: Verhandlungen der Kongresse fuer Innere Medizin; 1904. [Google Scholar]
- 24.Virchow R. Cellular Pathology. London, United Kingdom: John Churchill; 1858. [Google Scholar]
- 25.Rokitansky K, Day EG, Moore HC, Sieveking EH, Swaine EW. A Manual of Pathological Anatomy. Philadelphia: Blanchard & Lea; 1855:201–205. [Google Scholar]
- 26.Virchow R. Phlogose und Thrombose im Gefasssystem. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. Frankfurt: Meidinger Sohn and Co.; 1856:p 458. [Google Scholar]
- 27.Virchow R. Der Ateromatose Prozess der Arterien. Wien Med Wochenschr; 1856:825–827. [Google Scholar]
- 28.Duguid JB. Pathogenesis of arteriosclerosis. Lancet. 1949;2:925–927. doi: 10.1016/S0140-6736(49)91503-2 [DOI] [PubMed] [Google Scholar]
- 29.French JE. Atherosclerosis in relation to the structure and function of the arterial intima, with special reference to the endothelium. Int Rev Exp Pathol. 1966;5:253–353. [PubMed] [Google Scholar]
- 30.Mustard JF, Packham MA. The role of blood and platelets in atherosclerosis and the complications of atherosclerosis. Thromb Diath Haemor. 1975;33:444–456. doi: 10.1055/s-0038-1647838 [DOI] [PubMed] [Google Scholar]
- 31.Wissler RW. Development of the atherosclerosis plaque In: Braunwald E, editor. The Myocardium: Failure and Infarction. New York: H.P. Publishing Co; 1974:155–166. [Google Scholar]
- 32.Thomas WA, Jones R, Scott RF, Morrison E, Goodale F, Imai H. Production of early atherosclerotic lesions in rats characterized by proliferation of “modified smooth muscle cells. Exp Mol Pathol. 1963;2(suppl 1):40–61. doi: 10.1016/0014-4800(63)90005-4 [DOI] [PubMed] [Google Scholar]
- 33.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332 [DOI] [PubMed] [Google Scholar]
- 34.Ross R. Rous-Whipple Award Lecture. Am J Pathol. 1993;143(4):987–1002. [PMC free article] [PubMed] [Google Scholar]
- 35.Ross R. Atherosclerosis-an inflammatory disease. Atherosclerosis: a defense mechanism gone awry. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- 36.Windaus A. Ueber der Gehalt normaler und ateromatoser Aorten an Colesterolo und colesterinester. Zeitschrift Physiol Chemie. 1910;67:174. doi: 10.1515/bchm2.1910.67.2.174 [DOI] [Google Scholar]
- 37.Anichkov NN. Experimental arteriosclerosis in animals In: Cowdry EV, editor. Arteriosclerosis: A Survey of the Problem. New York: MacMillan Publishing; 1933:271–322. [Google Scholar]
- 38.Anichkov NN. A history of experimentation on arterial atherosclerosis in animals In: Blumenthal HT, editor. Cowdry’s Arteriosclerosis: A Survey of the Problem. 2nd ed. Springfield, (IL): Charles C Thomas Publishing; 1967:21–46. [Google Scholar]
- 39.Dock W. Research in arteriosclerosis; the first fifty years. Ann Intern Med. 1958;49:699–707. doi: 10.7326/0003-4819-49-3-699 [DOI] [PubMed] [Google Scholar]
- 40.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9 [DOI] [PubMed] [Google Scholar]
- 42.Brown MS, Faust JR, Goldstein JL, Kaneko I, Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978;253(4):1121–1128. [PubMed] [Google Scholar]
- 43.Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–156. doi: 10.1038/ng1161 [DOI] [PubMed] [Google Scholar]
- 44.Rosenson RS, Hegele RA, Fazio S, Cannon CP. The evolving future of PCSK9 inhibitors. J Am Coll Cardiol. 2018;72(3):314–329. doi: 10.1016/j.jacc.2018.04.054 [DOI] [PubMed] [Google Scholar]
- 45.Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91(3A):3A–6A. doi: 10.1016/s0002-9149(02)03143-0 [DOI] [PubMed] [Google Scholar]
- 46.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 47.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 48.Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 2000;902:230–240. doi: 10.1111/j.1749-6632.2000.tb06318.x [DOI] [PubMed] [Google Scholar]
- 49.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8 [DOI] [PubMed] [Google Scholar]
- 50.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23Suppl 1):III15–III19. doi: 10.1161/01.CIR.0000131513.33892.5b [DOI] [PubMed] [Google Scholar]
- 51.Libby P, Hansson GK. From focal lipid storage to systemic inflammation: JACC review topic of the week. J Am Coll Cardiol. 2019;74(12):1594–1607. doi: 10.1016/j.jacc.2019.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307–311. doi: 10.1161/CIRCRESAHA.116.301313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ketelhuth DFJ, Lutgens E, Bäck M, et al. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res. 2019;115(9):1385–1392. doi: 10.1093/cvr/cvz166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 55.Zhao TX, Mallat Z. Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(13):1691–1706. doi: 10.1016/j.jacc.2018.12.083 [DOI] [PubMed] [Google Scholar]
- 56.Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev. 2015;24:29–39. doi: 10.1016/j.arr.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 57.Sellge G, Kufer TA. PRR-signaling pathways: learning from microbial tactics. Semin Immunol. 2015;27:75–84. doi: 10.1016/j.smim.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805‐820. doi: 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 59.Lin J, Kakkar V, Lu X. Essential roles of toll-like receptors in atherosclerosis. Curr Med Chem. 2016;23(5):431‐454. doi: 10.2174/0929867323666151207111408 [DOI] [PubMed] [Google Scholar]
- 60.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22. doi: 10.1083/jcb.201412052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. doi: 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 62.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51(Suppl 1):33S–37S. doi: 10.2967/jnumed.109.069633 [DOI] [PubMed] [Google Scholar]
- 64.Hua J, Malinski T. Variable effects of LDL subclasses of cholesterol on endothelial nitric oxide/peroxynitrite balance - the risks and clinical implications for cardiovascular disease. Int J Nanomedicine. 2019;14:8973–8987. doi: 10.2147/IJN.S223524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(11):2292–2301. doi: 10.1161/ATVBAHA.107.149179 [DOI] [PubMed] [Google Scholar]
- 66.Habas K, Shang L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell. 2018;54:139–143. doi: 10.1016/j.tice.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 67.Libby P, Ridker PM, Hansson GK. Leducq transatlantic network on atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramji DP, Davies TS. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26(6):673–685. doi: 10.1016/j.cytogfr.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trus E, Basta S, Gee K. Who’s in charge here? Macrophage colony stimulating factor and granulocyte macrophage colony stimulating factor: competing factors in macrophage polarization. Cytokine. 2020;127:154939. doi: 10.1016/j.cyto.2019.154939 [DOI] [PubMed] [Google Scholar]
- 70.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basatemur GL, Jørgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–744. doi: 10.1038/s41569-019-0227-9 [DOI] [PubMed] [Google Scholar]
- 72.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans [published correction appears in Immunity. Immunity. 2013;38(6):1092–1104. doi: 10.1016/j.immuni.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdolmaleki F, Gheibi Hayat SM, Bianconi V, Johnston TP, Sahebkar A. Atherosclerosis and immunity: a perspective. Trends Cardiovasc Med. 2019;29(6):363–371. doi: 10.1016/j.tcm.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 75.Swirski FK, Robbins CS, Nahrendorf M. Development and function of arterial and cardiac macrophages. Trends Immunol. 2016;37(1):32–40. doi: 10.1016/j.it.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ammirati E, Moroni F, Magnoni M, Camici PG. The Role of T and B cells in human atherosclerosis and atherothrombosis. Clin Exp Immunol. 2015;179:173–187. doi: 10.1111/cei.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 78.Silvestre-Roig C, Braster Q, Ortega-Gomez A, et al. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020. doi: 10.1038/s41569-019-0326-7 [DOI] [PubMed] [Google Scholar]
- 79.Ilatovskaya DV, Halade GV, DeLeon-Pennell KY. Adaptive immunity-driven inflammation and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2019;317(6):H1254–H1257. doi: 10.1152/ajpheart.00642.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ketelhuth DF, Hansson GK. Adaptive response of T and B cells in atherosclerosis. Circ Res. 2016;118(4):668–678. doi: 10.1161/CIRCRESAHA.115.306427 [DOI] [PubMed] [Google Scholar]
- 81.Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47(4):621–634. doi: 10.1016/j.immuni.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bartlett B, Ludewick HP, Misra A, Lee S, Dwivedi G. Macrophages and T cells in atherosclerosis: a translational perspective. Am J Physiol Heart Circ Physiol. 2019;317(2):H375–H386. doi: 10.1152/ajpheart.00206.2019 [DOI] [PubMed] [Google Scholar]
- 83.Ou HX, Guo BB, Liu Q, et al. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol Sin. 2018;39(8):1249–1258. doi: 10.1038/aps.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srikakulapu P, McNamara CA. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol. 2017;312(5):H1060–H1067. doi: 10.1152/ajpheart.00859.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sage AP, Tsiantoulas D, Binder CJ, Mallat Z. The role of B cells in atherosclerosis. Nat Rev Cardiol. 2019;16(3):180–196. doi: 10.1038/s41569-018-0106-9 [DOI] [PubMed] [Google Scholar]
- 86.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991‐1045. doi: 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- 87.Liu D, Zeng X, Li X, Mehta JL, Wang X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 2018;113:5. doi: 10.1007/s00395-017-0663-9 [DOI] [PubMed] [Google Scholar]
- 88.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang X, Wang F, Wang Y, et al. Inflammasome-Driven Interleukin-1α and Interleukin-1β production in atherosclerotic plaques relates to hyperlipidemia and plaque complexity. JACC Basic Transl Sci. 2019;4(3):304–317. doi: 10.1016/j.jacbts.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satish M, Agrawal DK. Atherothrombosis and the NLRP3 inflammasome - endogenous mechanisms of inhibition. Transl Res. 2020;215:75–85. doi: 10.1016/j.trsl.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaplan H, Thompson RC, Trumble BC, et al. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet. 2017;389(10080):1730–1739. doi: 10.1016/S0140-6736(17)30752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162(4):597–605. doi: 10.1016/j.ahj.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 93.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 94.Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. doi: 10.1016/S0140-6736(17)32814-3 [DOI] [PubMed] [Google Scholar]
- 95.Abderrazak A, Couchie D, Mahmood DF, et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation. 2015;131(12):1061–1070. doi: 10.1161/CIRCULATIONAHA.114.013730 [DOI] [PubMed] [Google Scholar]
- 96.Nidorf SM, Fiolet ATL, Eikelboom JW, et al. The effect of low-dose colchicine in patients with stable coronary artery disease: the LoDoCo2 trial rationale, design, and baseline characteristics. Am Heart J. 2019;218:46–56. doi: 10.1016/j.ahj.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 97.Wu LM, Wu SG, Chen F, et al. Atorvastatin inhibits pyroptosis through the lncRNA NEXN-AS1/NEXN pathway in human vascular endothelial cells. Atherosclerosis. 2020;293:26–34. doi: 10.1016/j.atherosclerosis.2019.11.033 [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762. doi: 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. 2019;124(3):437–450. doi: 10.1161/CIRCRESAHA.118.313129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernandez DM, Rahman AH, Fernandez NF, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25(10):1576‐1588. doi: 10.1038/s41591-019-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winkels H, Ehinger E, Vassallo M, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122(12):1675‐1688. doi: 10.1161/CIRCRESAHA.117.312513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Willemsen L, de Winther MP. Macrophage subsets in atherosclerosis as defined by single-cell technologies. J Pathol. 2020;250(5):705‐714. doi: 10.1002/path.5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roy P, Ali AJ, Kobiyama K, Ghosheh Y, Ley K. Opportunities for an atherosclerosis vaccine: from mice to humans [published online ahead of print, 2020 Jan 18]. Vaccine. 2020;S0264-410X(19):31681. doi: 10.1016/j.vaccine.2019.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kobiyama K, Saigusa R, Ley K. Vaccination against atherosclerosis. Curr Opin Immunol. 2019;59:15–24. doi: 10.1016/j.coi.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wikimedia Commons [webpage on the Internet]. Germany: Rudolf Virchow; 1902. Available from: https://commons.wikimedia.org/wiki/File:Rudolf_Virchow_(Carl_G%C3%BCnther).png. Accessed July01, 2020.. [Google Scholar]
- 106.Wikimedia Commons [webpage on the Internet]. Germany: Carl vo Rokitanskay Litho; 1853. Available from: https://commons.wikimedia.org/wiki/File:Carl_von_Rokitansky_Litho.jpg. Accessed July01, 2020. [Google Scholar]