Abstract
The investigation of diverse reactivity of β,γ-unsaturated carbonyl compounds is of great value in asymmetric catalytic synthesis. Numerous enantioselective transformations have been well developed with β,γ-unsaturated carbonyl compounds as nucleophiles, however, few example were realized by utilizing them as not only nucleophiles but also electrophiles under a same catalytic system. Here we report a regioselective catalytic asymmetric tandem isomerization/α-Michael addition of β,γ-unsaturated 2-acyl imidazoles in the presence of chiral N,N′-dioxide metal complexes, delivering a broad range of optically pure 1,5-dicarbonyl compounds with two vicinal tertiary carbon stereocenters in up to >99% ee under mild conditions. Meanwhile, stereodivergent synthesis is disclosed to yield all four stereoisomers of products. Control experiments suggest an isomerization process involved in the reaction and give an insight into the role of NEt3. In addition, Mannich reaction and sulfur-Michael addition of β,γ-unsaturated 2-acyl imidazoles proceed smoothly as well under the same catalytic system.
Subject terms: Asymmetric catalysis, Homogeneous catalysis, Stereochemistry, Synthetic chemistry methodology
The investigation of reactivity of β,γ-unsaturated carbonyl compounds is of great synthetic value, especially in asymmetric transformations. Here, the authors report a catalytic asymmetric tandem isomerization/α-Michael addition of β,γ-unsaturated 2-acyl imidazoles in presence of chiral N,N′-dioxide metal catalysts.
Introduction
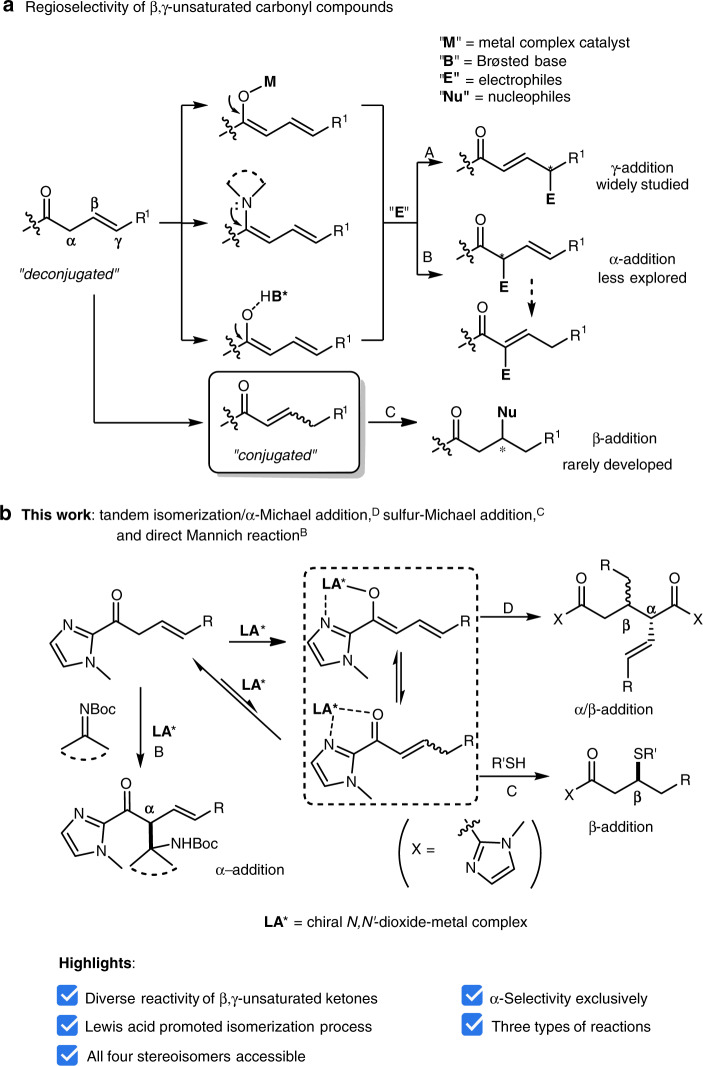
The exploration of reaction diversity from β,γ-unsaturated carbonyl compounds is interesting and of great synthetic value. These compounds and their analogs bearing one potential enolization have been demonstrated as highly active nucleophiles in a number of catalytic asymmetric reactions for the synthesis of natural products and bioactive compounds1–16. Especially, γ-addition as dienolate pronucleophiles with either metal catalysis17–28 or organocatalysis29–36 has been widely documented during the past several years, and the maintained π-conjugation of γ-addition process leading to thermodynamically stable conjugated products (Fig. 1a, A). The regioselectivity changing from γ-addition to α-addition seems to be plaguing37,38, and α-addition of specific substrates, such as γ,γ-disubstituted ones, has been reported39–43. Notably, in some cases, C=C isomerization occurred after α-addition which further expanded the reaction diversity (Fig. 1a, B)44–46.
Fig. 1. Strategies for γ- and α-addition of β,γ-unsaturated carbonyl compounds.
a Regioselectivity of decojugated carbonyl compounds. b Our strategies for diverse reactivity of β,γ-unsaturated 2-acyl imidazoles.
Although versatile catalytic asymmetric reactions have been demonstrated by utilizing β,γ-unsaturated carbonyl compounds as mentioned above, however, few examples were investigated by employing them as electrophiles upon isomerization to conjugated α,β-unsaturated carbonyl compounds (Fig. 1a, C)47,48. We envision that, by careful design of β,γ-unsaturated carbonyl compounds, these could serve not only as nucleophiles but also electrophiles. Based on this assumption, here we report the synthesis of a series of β,γ-unsaturated 2-acyl imidazoles by introducing an imidazole moiety which would address the following two points: (1) bidentate coordination with a Lewis acid of acyl imidazole exhibits good stereocontrol49–55 and (2) the strong coordination facilitates isomerization of the β,γ-unsaturated ketone to an α,β-unsaturated ketone. Chiral N,N′-dioxide-metal56–59 complexes catalyze diverse reactions of β,γ-unsaturated 2-acyl imidazoles, including tandem isomerization/α-Michael addition (Fig. 1b, D), Mannich reaction (Fig. 1b, B), and sulfur-Michael addition (Fig. 1b, C) with high efficiency and stereoinduction. In addition, stereodivergent catalysis60–63 is also disclosed and provides a unified and predictable route for the access to all four stereoisomers of 1,5-dicorbonyl compounds by matching the configuration between the Lewis acid catalysts and substrates.
Results
Optimization of the reaction conditions
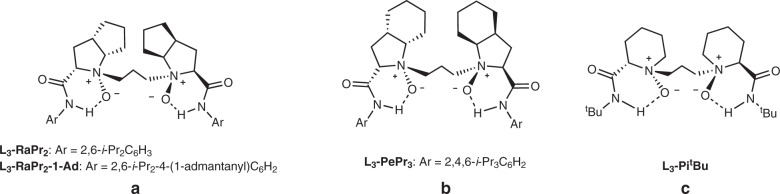
We began our study by employing β,γ-unsaturated 2-acyl imidazole E-1a as the model substrate to optimize the reaction conditions. Several metal salts coordinated with the N,Nʹ-dioxide ligand L3-RaPr2 (Fig. 2) were evaluated, such as Sc(OTf)3, Ni(OTf)2, and Mg(OTf)2; however, only trace amount of the self-α/β-addition product 2a was observed, which was generated from α-addition of E-1a with the corresponding α,β-unsaturated 2-acyl imidazole upon C=C isomerization (Table 1, entry 1). Pleasingly, the Y(OTf)3/L3-RaPr2 complex was efficient to promote the tandem isomerization/α-Michael addition and provided the corresponding product 2a with 60% yield, 2.2:1 anti:syn ratio, and 96% ee in CH2ClCH2Cl (entry 2). Lanthanide metal salts La(OTf)3 and Yb(OTf)3 could also mediate the reaction but gave lower yields and ee values (entries 3 and 4). The screening of chiral backbones and steric hindrance of the amide moiety on the N,Nʹ-dioxide ligands afforded no better results (for details, see Supplementary Table 1). When toluene was used as solvent instead, the isolated yield of anti-2a was increased to 73% with 5.2:1 dr and 97% ee (entry 5). To our delight, the diastereoselectivity could be improved to 10:1 with addition of NEt3 (entry 6). Other common chiral ligands such as Box, Pybox, and BINAP were also explored, and 32% yield, 5:1 dr with 60% ee were observed as the best results (for details, see Supplementary Table 3).
Fig. 2. Representative chiral N,Nʹ-dioxide ligands used in the study.
a l-Ramipril-derived ligand L3-RaPr2 and L3-RaPr2–1-Ad. b l-Perindopril-derived ligand L3-PePr3. c S-pipecolic acid-derived ligand L3-PitBu.
Table 1.
Optimization of the reaction conditions.
| Entry | metal salt | Yield (%)a | anti:synb | ee (%)c |
|---|---|---|---|---|
| 1 | Sc(OTf)3/Ni(OTf)2/Mg(OTf)2 | Trace | — | — |
| 2 | Y(OTf)3 | 60 | 2.2:1 | 96/−34 |
| 3 | La(OTf)3 | 58 | 2.7:1 | 92/63 |
| 4 | Yb(OTf)3 | 46 | 2.2:1 | 84/13 |
| 5d | Y(OTf)3 | 73 | 5.2:1 | 97/0 |
| 6d,e | Y(OTf)3 | 74 | 10:1 | 98/N.D. |
Unless otherwise noted, all reactions were performed with metal salt/ligand (1:1, 2.5 mol%), E-1a (0.20 mmol) in CH2ClCH2Cl (1.0 mL) at 25 °C under N2 atmosphere for 24 h. aIsolated yield of anti-isomer. bDetermined by 1H NMR analysis of crude products. cDetermined by HPLC analysis on a chiral stationary phases. dToluene was used as solvent. eAddition of NEt3 (10 mol%) and for 12 h.
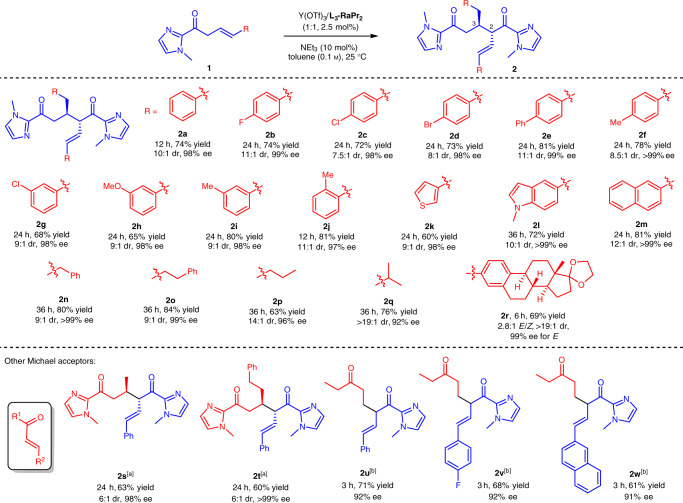
Substrate scope in isomerization/α-Michael addition reaction
The generality of the tandem isomerization/α-Michael addition reaction was investigated under the optimized conditions (Fig. 3). An array of β,γ-unsaturated 2-acyl imidazoles bearing different substituents on the γ-phenyl group (both electron-withdrawing and electron-donating groups at the para-, meta-, or ortho-positions) were converted into the corresponding dimerization products 2a–2j in good yields (65–81%), high diastereoselectivities (7.5:1 to 11:1), and excellent ee values (97–>99%). Furthermore, β,γ-unsaturated carbonyl compounds containing 3-thienyl, N-methyl-5-indolyl and 2-naphthyl moieties were also proven to be suitable substrates, affording 2k–2m with good results (60–81% yields, 9:1 to 12:1 dr, and 98–>99% ee). Moreover, aliphatic-substituted β,γ-unsaturated 2-acyl imidazoles exhibited high tolerance as well, generating the desired products 2n–2q with a high level of yields (63–84%) and stereoselectivities (9:1 to >19:1 dr; 92–>99% ee). Estrone-derived 1r could be transformed into 2r smoothly in 69% yield, 2.8:1 E/Z, >19:1 dr, and 99% ee for E-isomer. Other Michael acceptors such as α,β-unsaturated 2-acyl imidazole and ethyl vinyl ketone were aslo suitable in this reaction, delivering 2s–2w with good yields (60–71%) and stereoselectivities (6:1 dr, 91–>99% ee). The absolute configuration of 2j was determined to be (2S, 3R) by X-ray crystallography analysis.
Fig. 3. Substrate scope in isomerization/α-Michael addition reaction.
Unless otherwise noted, all reactions were performed with Y(OTf)3/L3-RaPr2 (1:1, 2.5 mol%), 1 (0.20 mmol), NEt3 (10 mol%) in toluene (1.0 mL) at 25 °C under N2 atmosphere. The yield was based on isolated anti-isomer. The dr value was determined by 1H NMR of crude products. The ee value was determined by HPLC analysis on chiral stationary phases. The substrates 1l and 1n–1r were used as Z/E mixutres. [a] 5 mol% catalyst was used for 2s and 2t. [b] With 5 mol% Y(OTf)3/L3-RaPr2-1-Ad as a catalyst and CH2Cl2 as a solvent in the absence of NEt3 for 2u–2w.
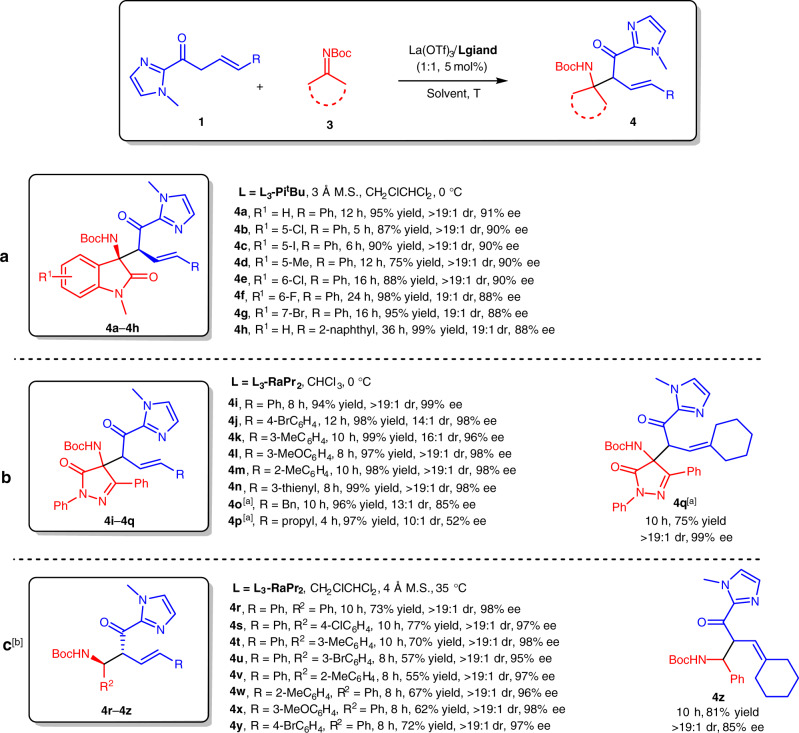
Substrate scope in α-Mannich reaction of β,γ-unsaturated 2-acyl imidazoles and imines
The reaction described above indicated that β,γ-unsaturated 2-acyl imidazoles performed both α-addition reaction and β-addition upon isomerization under proper Lewis acid catalysts. Next, to extend the scope of α-addition of β,γ-unsaturated 2-acyl imidazoles, several types of imines 3 were explored as the electrophiles. By switching the catalyst to La(OTf)3/L3-PitBu complex (for detailed screening of the conditions, see Supplementary Table 4), the Mannich reaction between E-1 and isatin-derived ketimines 3a–3h was successfully realized to deliver the desired β-amino 2-acyl imidazoles 4a–4h as single isomers in 75–99% yields and 88–91% ee (Fig. 4a). Moreover, pyrazolinone-derived ketimine was also suitable in this α-addition reaction, no matter β-aryl-substituted or β-alkyl-substituted β,γ-unsaturated 2-acyl imidazoles could react with it smoothly, producing the corresponding products 4i–4o and 4q with good results (75–99% yields, 13:1–>19:1 dr, 85–99% ee) except for 4p with 52% ee (Fig. 4b). Aldimines were used as the Mannich acceptors, and were transformed into the β-amino 2-acyl imidazoles 4r–4z with good yields (55–81%) and high enantioselectivities (85–98% ee) as single isomers (Fig. 4c). The absolute configuration of 4r was determined to be (1S, 2R) by X-ray crystallography analysis.
Fig. 4. Substrate scope in α-Mannich reaction of β,γ-unsaturated 2-acyl imidazoles and imines.
a Substrate scope with isatin-derived ketimins. b Substrate scope with pyrazolinone-derived ketimins. c Substrate scope with aldimines. Unless otherwise noted, all the reactions were performed with La(OTf)3/Ligand (1:1, 5 mol%), 1 (0.10 mmol), 3 (0.10 mmol for 4a–4q, 0.15 mmol for 4r–4z) in the indicated solvent. The dr value was determined by 1H NMR of crude products. The ee value was determined by HPLC analysis on chiral stationary phases. [a] At 20 °C. [b] With 10 mol% of catalyst.
Substrate scope in isomerization/sulfur-Michael reaction
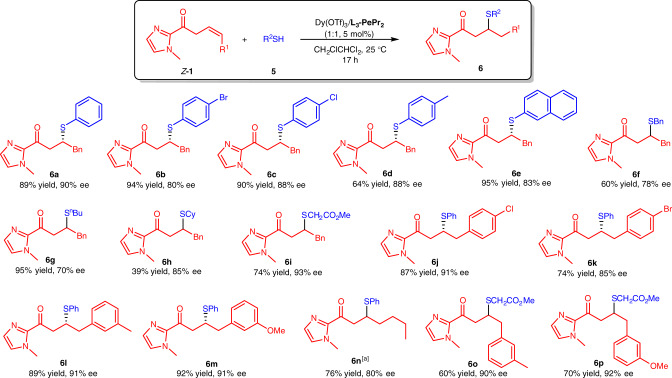
Inspired by the isomerization process of β,γ-unsaturated 2-acyl imidazoles into α,β-unsaturated 2-acyl imidazoles, we next enlarged the diverse reactivity of β,γ-unsaturated compounds as the electrophiles under the current catalytic system. However, only a trace amount of desired tandem isomerization/sulfur-Michael addition product 6a was achieved if E-1a reacted with thiophenol 5a. After examination of the reaction conditions (for details, see Supplementary Table 5), Z-1a was used instead, and 6a could be obtained in 89% yield with 90% ee (Fig. 5). The scope of isomerization/sulfur-Michael reaction was investigated next. Thiolphenols and alkyl-substituted thiols could be converted into the final products (6a–6i) in 39–95% yields with 70–93% ee values. For the Michael acceptors, aryl- and alkyl-substituted β,γ-unsaturated 2-acyl imidazoles were also tolerated in this reaction, giving 6j–6p in 60–92% yields with 80–92% ee.
Fig. 5. Substrate scope in isomerization/sulfur-Michael reaction.
Unless otherwise noted, all reactions were performed with Dy(OTf)3/L3-PePr3 (1:1, 5 mol%), 1 (0.25 mmol), 5 (0.10 mmol) in CH2ClCHCl2 (1.0 mL) at 25 °C for 17 h. [a] Z/E mixture of β,γ-unsaturated 2-acyl imidazole was used for 6n. The reaction time was 5 days.
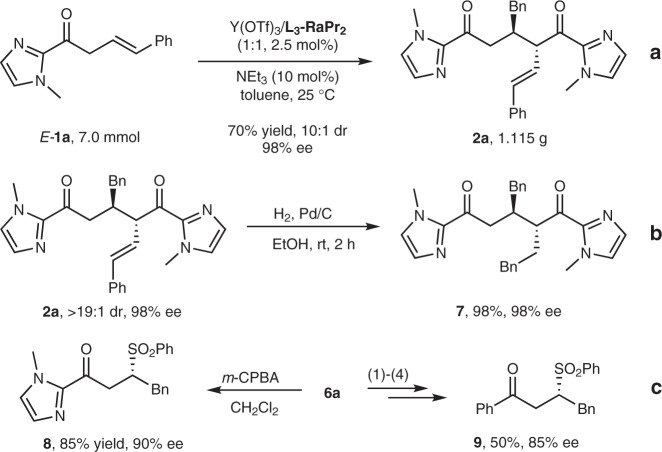
Gram-scale synthesis and derivatization of products
To evaluate the synthetic utility of this methodology, a gram-scale synthesis of 2a was conducted. The current reaction could be carried out at 7.0 mmol scale without loss of yield (70%), diastereoselectivity (10:1 dr), and ee value (98%) (Fig. 6a). Furthermore, hydrogenation of 2a in the presence of Pd/C and H2 afforded derivative 7 in 98% yield with 98% ee (Fig. 6b). Chiral sulfone motif is found in numerous biological compounds64–67 as well as drug candidates68. Upon treatment of 6a with m-CPBA, the oxidized sulfone product 8 was obtained in 85% yield with 90% ee. Moreover, 6a went through further transformations to afford sulfone 9 in 50% yield with 85% ee (Fig. 6c)69.
Fig. 6. Gram-scale synthesis and derivatization of products.
a Gram-scale synthesis of 2a. b Hydrogenation of 2a. c The derivatization of 6a, (1) PhMgBr, THF; (2) MeOTf, MeCN; (3) K2CO3 (aq); (4) m-CPBA, CH2Cl2.
Mechanistic studies
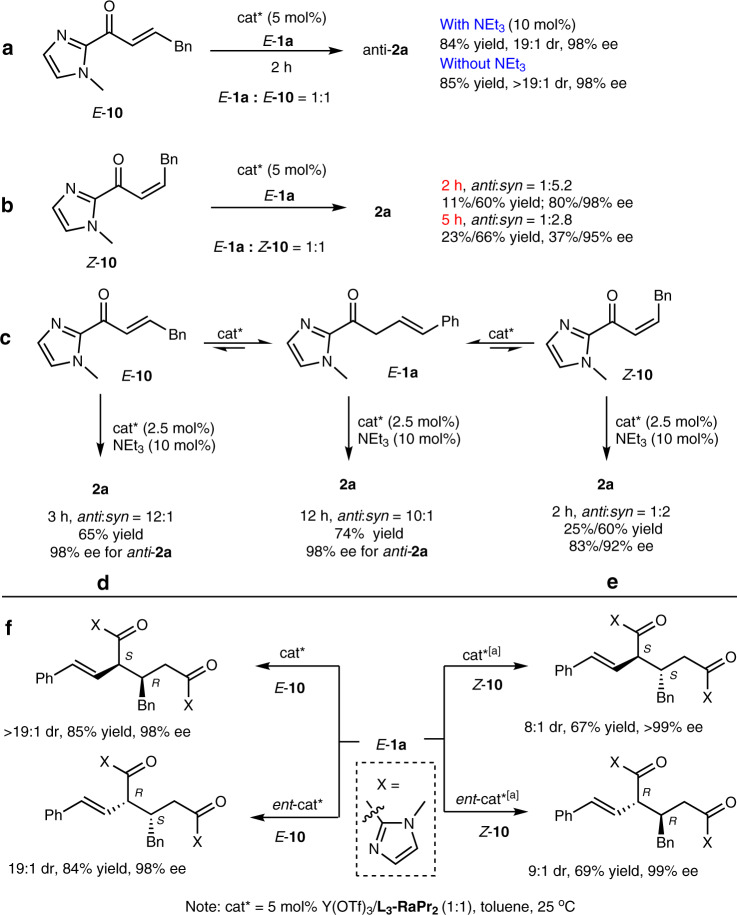
To gain insight into the mechanism of tandem isomerization/α-Michael addition, some control experiments were carried out. Firstly, we wondered why the addition of NEt3 led to an increase in diastereoselectivity (Table 1, entry 6). Treating the product 2a (2.9:1 dr, 85%/12% ee) under the standard conditions for 12 h (for details, see Supplementary Note 5), no change of enantioselectivity and diastereoselectivity was observed, which ruled out the possibility that the diastereoselectivity increased via epimerization of syn-2a in the presence of NEt3. Subsequently, E-α,β-unsaturated 2-acyl imidazole E-10 was synthesized to react with E-1a, affording anti-2a in good yields (84–85%), excellent diastereoselectivities (19:1 to >19:1), and 98% ee within 2 h no matter with or without addition of NEt3 (Fig. 7a). Moreover, when Z-α,β-unsaturated 2-acyl imidazole Z-10 was used to react with E-1a, the product 2a was obtained in 1:5.2 anti:syn after 2 h, and decreased to 1:2.8 anti:syn after 5 h (Fig. 7b). These experiments confirmed the isomerization of β,γ-unsaturated C=C bond into α,β-unsaturated C=C bond in the presence of N,N′-dioxide-metal complexes, and this process was likely to be the rate-determining step. It also suggests the diastereoselectivity was mainly controlled by the E/Z-configuration of the α,β-unsaturated 2-acyl imidazole intermediate, and the addition of NEt3 might improve the E/Z ratio during the isomerization process. As a result of equilibrium between E-1a, E-10, and Z-10 (Fig. 7c), the use of E-10 as the starting substrate alone, albeit unstable yielded the corresponding anti-2a as the major product in 98% ee after 3 h (Fig. 7d), while the reaction from only Z-10 gave the syn-2a product in 60% isolated yield and 92% ee (Fig. 7e). In addition, operando IR experiments were also performed to interpret the reaction process (for details, see Supplementary Note 7). Furthermore, we set out to establish the availability of stereodivergent access to 2a. All four stereoisomers of 2a could be readily obtained in good yields (67–85%) and diastereoselectivities (8:1–>19:1) with excellent ee values by matching the E/Z-configurated 10 and the chiral ligand (Fig. 7f).
Fig. 7. Mechanistic studies.
a Reaction of E-10 with E-1a. b Reaction of Z-10 with E-1a. c Isomerization of E-1a with E-10 and Z-10. d Reaction of single E-10. e Reaction of single Z-10. f Stereodivergent synthesis of 2a. [a] m-Xylene was used instead of toluene.
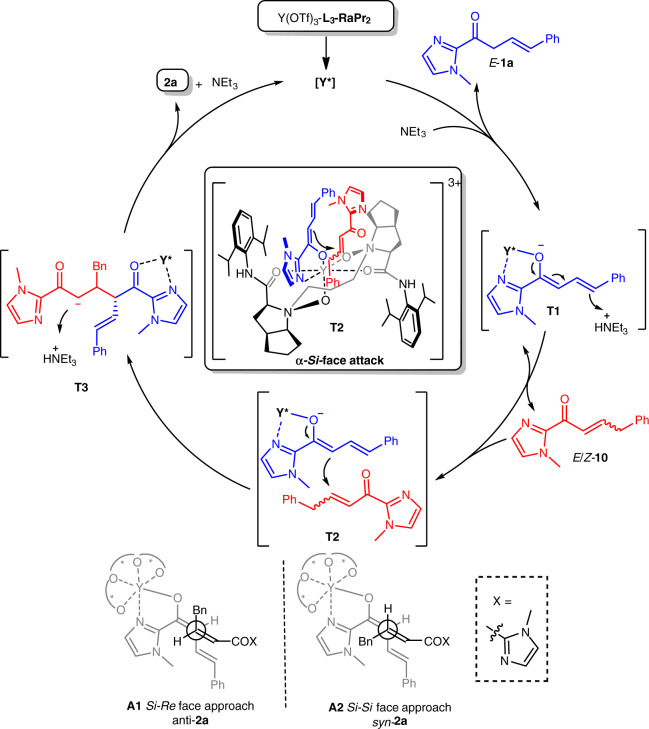
Proposed catalytic cycle
Based on the absolute configuration of the product 2j, control experiments and our previous studies56–59, a possible catalytic cycle with a transition-state model was proposed (Fig. 8). First, the coordination of chiral N,N′-dioxide L3-RaPr2 and metal salt in situ to form chiral metal complex (Y*). Then, the β,γ-unsaturated ketone E-1a attaches to Y* as a dienolate in the presence of NEt3 to give the intermediate T1, and which partly transforms into the α,β-unsaturated ketone E/Z-10 upon 1,5-proton shift. Next, the catalyst-bonded dienolate will react with the newly formed Michael acceptors. The α-Re-face of β,γ-unsaturated 2-acyl imidazole E-1a is strongly shielded by the nearby aryl ring of the ligand. Therefore, the dienolate prefers to attack E/Z-10 from its α-Si-face (T2). Finally, the desired product 2a dissociates after a protonation of the intermediate T3, and the catalyst is regenerated to accomplish one catalytic cycle.
Fig. 8. Proposed catalytic cycle.
The in situ formed chiral catalyst [Y*] catalyzes isomerization of E-1a into E/Z-10 in the presence of NEt3, followed by nucleophilic addition of E-1a and protonation to deliver the final product 2a.
Discussion
In summary, we have disclosed the diverse transformation of β,γ-unsaturated 2-acyl imidazoles in the presence of chiral Lewis acid catalysts, involving catalytic asymmetric tandem isomerization/α-Michael addition, sulfur-Michael addition, and direct Mannich reaction. A wide range of chiral 1,5-dicarbonyl and functionalized carbonyl compounds was afforded with good to excellent levels yields, diastereoselectivities, and enantioselectivities. The β,γ-unsaturated 2-acyl imidazoles features various reactivities, acting as both α-nucleophile and β-electrophile upon isomerization, which provides a route for conjugate addition of unstable α,β-unsaturated carbonyl compounds. Meanwhile, all four stereoisomers with two vicinal tertiary stereocenters could be prepared by matching the configuration between substrates and chiral ligand. Besides, the desired products could be easily transformed into useful compounds with good results under mild conditions. Further studies on this methodology are ongoing.
Methods
Tandem isomerization/α-Michael addition
Y(OTf)3 (0.005 mmol), L3-RaPr2 (0.005 mmol), β,γ-unsaturated 2-acyl imidazole E-1a (0.20 mmol), and NEt3 (0.02 mmol) were dissolved in 1.0 mL of toluene under N2 atmosphere. The mixture was stirred at 25 °C for 12 h and subjected to column chromatography on silica to afford the product 2a (Pet/EtOAc = 1:1 as eluent) as a colorless foam.
Mannich reaction with isatin-derived ketimines
A dry reaction tube was charged with L3-PitBu (2.2 mg, 5 mol%), La(OTf)3 (2.9 mg, 5 mol%), 3 Å M.S. (30 mg), and E-1a (27.1 mg, 0.12 mmol) in CH2ClCHCl2 (1.0 mL). The mixture was stirred at 30 °C for 30 min, and then 3a (0.10 mmol, 26.0 mg) was added at 0 °C. After 3a was consumed (detected by thin-layer chromatography (TLC)), the residue was purified by column chromatography on silica gel to afford the product 4a (Pet/EtOAc = 1:1 as eluent) as a colorless foam.
Mannich reaction with pyrazolinone-derived ketimines
A dry reaction tube was charged with L3-RaPr2 (3.5 mg, 5 mol%), La(OTf)3 (2.9 mg, 5 mol%), E-1a (24.9 mg, 0.11 mmol), and pyrazolinone-derived ketimine (34.9 mg, 0.10 mmol) in CHCl3 (1.0 mL). After ketimine was consumed (detected by TLC), the residue was purified by column chromatography on silica gel to afford the product 4i (Pet/EtOAc = 2:1 as eluent) as a colorless foam.
Mannich reaction with aldimines
A dry reaction tube was charged with L3-RaPr2 (7.0 mg, 10 mol%), La(OTf)3 (5.9 mg, 10 mol%), E-1a (24.9 mg, 0.10 mmol), 4 Å M.S. (20 mg), and benzaldehyde-dervived aldimine (30.8 mg, 0.15 mmol) in CH2ClCHCl2 (1.0 mL). After E-1a was consumed (detected by TLC), the residue was purified by column chromatography on silica gel to afford the product 4r (Pet/EtOAc = 2:1 as eluent) as a colorless oil.
Isomerization/sulfur-Michael reaction
A dry reaction tube was charged with L3-PePr3 (4.2 mg, 5 mol%), Dy(OTf)3 (3.0 mg, 5 mol%), and Z-1a (56.5 mg, 0.25 mmol) in CH2ClCHCl2 (1.0 mL). PhSH (0.10 mmol) was added and the mixture was stirred at 25 °C for 17 h. After PhSH was consumed (detected by TLC), the residue was purified by column chromatography on silica gel to afford the product 6a (Pet/EtOAc = 3:1 as eluent) as a pale yellow oil.
Supplementary information
Acknowledgements
We appreciate the National Natural Science Foundation of China (Nos. 21890723 and 21921002) for financial support. Thanks Dr. Yuqiao Zhou for the assistance in X-ray analysis.
Author contributions
T.K. performed experiments and prepared the Supplementary Information and paper. L.H. took part in the reaction development and synthesized several substrates. S.R. repeated some experiments. W.C. and X.L. helped with modifying the paper and Supplementary Information. X.F. conceived and directed the project.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1972987 (2j), 2001513 (4r), and 1972937 (11). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Zhihui Shao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaohua Liu, Email: liuxh@scu.edu.cn.
Xiaoming Feng, Email: xmfeng@scu.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-17681-9.
References
- 1.Denmark SE, Heemstra JR, Jr, Beutner GL. Catalytic, enantioselective, vinylogous aldol reactions. Angew. Chem. Int. Ed. 2005;44:4682–4698. doi: 10.1002/anie.200462338. [DOI] [PubMed] [Google Scholar]
- 2.Casiraghi G, Battistini L, Curti C, Rassu G, Zanardi F. The vinylogous aldol and related addition reactions: ten years of progress. Chem. Rev. 2011;111:3076–3154. doi: 10.1021/cr100304n. [DOI] [PubMed] [Google Scholar]
- 3.Pansare SV, Paul EK. The organocatalytic vinylogous aldol reaction: recent advances. Chem. Eur. J. 2011;17:8770–8779. doi: 10.1002/chem.201101269. [DOI] [PubMed] [Google Scholar]
- 4.Schneider C, Abels F. Catalytic, enantioselective vinylogous Michael reactions. Org. Biomol. Chem. 2014;12:3531–3543. doi: 10.1039/c4ob00332b. [DOI] [PubMed] [Google Scholar]
- 5.Kalesse M, Cordes M, Symkenberg G, Lu H-H. The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis. Nat. Prod. Rep. 2014;31:563–594. doi: 10.1039/c3np70102f. [DOI] [PubMed] [Google Scholar]
- 6.Yin YL, Jiang ZY. Organocatalytic asymmetric vinylogous Michael reactions. ChemCatChem. 2017;9:4306–4318. [Google Scholar]
- 7.Li H, Yin L. Recent progress on direct catalytic asymmetric vinylogous reactions. Tetrahedron Lett. 2018;59:4121–4135. [Google Scholar]
- 8.Hosokawa S. Remote asymmetric induction reactions using a E,E-vinylketene silyl N,O-acetal and the wide range stereocontrol strategy for the synthesis of polypropionates. Acc. Chem. Res. 2018;51:1301–1314. doi: 10.1021/acs.accounts.8b00125. [DOI] [PubMed] [Google Scholar]
- 9.Tong GH, et al. Highly enantio- and diastereoselective allylic alkylation of Morita–Baylis–Hillman carbonates with allyl ketones. J. Org. Chem. 2013;78:5067–5072. doi: 10.1021/jo400496z. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B, et al. Direct asymmetric vinylogous aldol reaction of allyl ketones with isatins: divergent synthesis of 3-hydroxy-2-oxindole derivatives. Angew. Chem. Int. Ed. 2013;52:6666–6670. doi: 10.1002/anie.201302274. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Wang Y, Yu T-Y, Liang Y-M, Xu P-F. Rationally designed multifunctional supramolecular iminium catalysis: direct vinylogous Michael addition of unmodified linear dienol substrates. Angew. Chem. Int. Ed. 2014;53:14128–14131. doi: 10.1002/anie.201406786. [DOI] [PubMed] [Google Scholar]
- 12.Jing ZZ, et al. Organocatalytic enantioselective vinylogous aldol reaction of allyl aryl ketones to activated acyclic ketones. Org. Lett. 2016;18:260–263. doi: 10.1021/acs.orglett.5b03412. [DOI] [PubMed] [Google Scholar]
- 13.Shi M-L, Zhan G, Zhou S-L, Du W, Chen Y-C. Asymmetric inverse-electron-demand oxa-Diels–Alder reaction of allylic ketones through dienamine catalysis. Org. Lett. 2016;18:6480–6483. doi: 10.1021/acs.orglett.6b03384. [DOI] [PubMed] [Google Scholar]
- 14.Akula PS, Hong B-C, Lee G-H. Catalyst- and substituent-controlled switching of chemoselectivity for the enantioselective synthesis of fully substituted cyclobutane derivatives via 2 + 2 annulation of vinylogous ketone enolates and nitroalkene. Org. Lett. 2018;20:7835–7839. doi: 10.1021/acs.orglett.8b03335. [DOI] [PubMed] [Google Scholar]
- 15.Qin JL, et al. Asymmetric inverse-electron-demand Diels–Alder reaction of β,γ-unsaturated amides through dienolate catalysis. Org. Lett. 2019;21:7337–7341. doi: 10.1021/acs.orglett.9b02629. [DOI] [PubMed] [Google Scholar]
- 16.Ran G-Y, Yang X-X, Yue J-F, Du W, Chen Y-C. Asymmetric allylic alkylation with deconjugated carbonyl compounds: direct vinylogous umpolung strategy. Angew. Chem. Int. Ed. 2019;58:9210–9214. doi: 10.1002/anie.201903478. [DOI] [PubMed] [Google Scholar]
- 17.Jusseau X, Chabaud L, Guillou C. Synthesis of γ-butenolides and α,β-unsaturated γ-butyrolactams by addition of vinylogous nucleophiles to Michael acceptors. Tetrahedron. 2014;70:2595–2615. [Google Scholar]
- 18.Zhang Q, Liu XH, Feng XM. Recent advances in enantioselective synthesis of γ-substituted butenolides via the catalytic asymmetric vinylogous reactions. Curr. Org. Synth. 2013;10:764–785. [Google Scholar]
- 19.Yamaguchi A, Matsunaga S, Shibasaki M. Direct catalytic asymmetric Mannich-type reactions of γ-butenolides: effectiveness of Brønsted acid in chiral metal catalysis. Org. Lett. 2008;10:2319–2322. doi: 10.1021/ol800756r. [DOI] [PubMed] [Google Scholar]
- 20.Trost BM, Hitce J. Direct asymmetric Michael addition to nitroalkenes: vinylogous nucleophilicity under dinuclear zinc catalysis. J. Am. Chem. Soc. 2009;131:4572–4573. doi: 10.1021/ja809723u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd NE, Tanabe H, Xu YJ, Matsunaga S, Shibasaki M. Direct catalytic asymmetric vinylogous Mannich-type and Michael reactions of an α,β-unsaturated γ-butyrolactam under dinuclear nickel catalysis. J. Am. Chem. Soc. 2010;132:3666–3667. doi: 10.1021/ja1002636. [DOI] [PubMed] [Google Scholar]
- 22.Yin L, Takada H, Kumagai N, Shibasaki M. Direct catalytic asymmetric vinylogous Mannich-type reaction of γ-butenolides with ketimines. Angew. Chem. Int. Ed. 2013;52:7310–7313. doi: 10.1002/anie.201303119. [DOI] [PubMed] [Google Scholar]
- 23.Yang DX, et al. Direct site-specific and highly enantioselective γ-functionalization of linear α,β-unsaturated ketones: bifunctional catalytic strategy. Angew. Chem. Int. Ed. 2013;52:6739–6742. doi: 10.1002/anie.201301146. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H-J, Shi C-Y, Zhong F, Yin L. Direct asymmetric vinylogous and bisvinylogous Mannich-type reaction catalyzed by a copper(I) complex. J. Am. Chem. Soc. 2017;139:2196–2199. doi: 10.1021/jacs.6b13042. [DOI] [PubMed] [Google Scholar]
- 25.Trost BM, Gnanamani E, Tracy JS, Kalnmals CA. Zn-ProPhenol catalyzed enantio- and diastereoselective direct vinylogous Mannich reactions between α,β- and β,γ-butenolides and aldimines. J. Am. Chem. Soc. 2017;139:18198–18201. doi: 10.1021/jacs.7b11361. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H-J, Yin L. Asymmetric synthesis of α,β-unsaturated δ-lactones through copper(I)-catalyzed direct vinylogous aldol reaction. J. Am. Chem. Soc. 2018;140:12270–12279. doi: 10.1021/jacs.8b07929. [DOI] [PubMed] [Google Scholar]
- 27.Zhong F, Yue W-J, Zhang H-J, Zhang C-Y, Yin L. Catalytic asymmetric construction of halogenated stereogenic carbon centers by direct vinylogous Mannich-type reaction. J. Am. Chem. Soc. 2018;140:15170–15175. doi: 10.1021/jacs.8b09484. [DOI] [PubMed] [Google Scholar]
- 28.Trost BM, Gnanamani E, Kalnmals CA, Hung C-I, Tracy JS. Direct enantio- and diastereoselective vinylogous addition of butenolides to chromones catalyzed by Zn-prophenol. J. Am. Chem. Soc. 2019;141:1489–1493. doi: 10.1021/jacs.8b13367. [DOI] [PubMed] [Google Scholar]
- 29.Jurberg ID, Chatterjee I, Tannert R, Melchiorre P. When asymmetric aminocatalysis meets the vinylogy principle. Chem. Commun. 2013;49:4869–4883. doi: 10.1039/c3cc41270a. [DOI] [PubMed] [Google Scholar]
- 30.Marcos V, Alemán J. Old tricks, new dogs: organocatalytic dienamine activation of α,β-unsaturated aldehydes. Chem. Soc. Rev. 2016;45:6812–6832. doi: 10.1039/c6cs00438e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bencivenni G, Galzerano P, Mazzanti A, Bartoli G, Melchiorre P. Direct asymmetric vinylogous Michael addition of cyclic enones to nitroalkenes via dienamine catalysis. Proc. Natl Acad. Sci. USA. 2010;107:20642–20647. doi: 10.1073/pnas.1001150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan G, He Q, Yuan X, Chen Y-C. Asymmetric direct vinylogous Michael additions of allyl alkyl ketones to maleimides through dienamine catalysis. Org. Lett. 2014;16:6000–6003. doi: 10.1021/ol503017h. [DOI] [PubMed] [Google Scholar]
- 33.Dell’Amico L, et al. Exploring the vinylogous reactivity of cyclohexenylidene malononitriles: switchable regioselectivity in the organocatalytic asymmetric addition to enals giving highly enantioenriched carbabicyclic structures. J. Am. Chem. Soc. 2014;136:11107–11114. doi: 10.1021/ja5054576. [DOI] [PubMed] [Google Scholar]
- 34.Guo QS, Fraboni AJ, Brenner-Moyer SE. Direct diastereo- and enantioselective vinylogous Michael additions of linear enones. Org. Lett. 2016;18:2628–2631. doi: 10.1021/acs.orglett.6b01050. [DOI] [PubMed] [Google Scholar]
- 35.Curti C, et al. Bifunctional cinchona alkaloid/thiourea catalyzes direct and enantioselective vinylogous Michael addition of 3-alkylidene oxindoles to nitroolefins. Angew. Chem. Int. Ed. 2012;51:6200–6204. doi: 10.1002/anie.201202027. [DOI] [PubMed] [Google Scholar]
- 36.Wang JM, Chen J, Kee CW, Tan C-H. Enantiodivergent and γ-selective asymmetric allylic amination. Angew. Chem. Int. Ed. 2012;51:2382–2386. doi: 10.1002/anie.201107317. [DOI] [PubMed] [Google Scholar]
- 37.Iriarte I, et al. Controlling the α/γ-reactivity of vinylogous ketone enolates in organocatalytic enantioselective Michael reactions. Angew. Chem. Int. Ed. 2017;56:8860–8864. doi: 10.1002/anie.201703764. [DOI] [PubMed] [Google Scholar]
- 38.Urruzuno I, et al. α-Branched ketone dienolates: base-catalysed generation and regio- and enantioselective addition reactions. Chem. Eur. J. 2019;25:9701–9709. doi: 10.1002/chem.201901694. [DOI] [PubMed] [Google Scholar]
- 39.Marqués-López E, et al. Crossed intramolecular Rauhut−Currier-type reactions via dienamine activation. Org. Lett. 2009;11:4116–4119. doi: 10.1021/ol901614t. [DOI] [PubMed] [Google Scholar]
- 40.Han B, et al. Organocatalytic regio- and stereoselective inverse-electron-demand aza-Diels–Alder reaction of α,β-unsaturated aldehydes and N-tosyl-1-aza-1,3-butadienes. Angew. Chem. Int. Ed. 2009;48:5474–5477. doi: 10.1002/anie.200902216. [DOI] [PubMed] [Google Scholar]
- 41.Han B, Xiao Y-C, He Z-Q, Chen Y-C. Asymmetric Michael addition of γ,γ-disubstituted α,β-unsaturated aldehydes to nitroolefins via dienamine catalysis. Org. Lett. 2009;11:4660–4663. doi: 10.1021/ol901939b. [DOI] [PubMed] [Google Scholar]
- 42.Stiller J, et al. Enantioselective α- and γ-alkylation of α,β-unsaturated aldehydes using dienamine activation. Org. Lett. 2011;13:70–73. doi: 10.1021/ol102559f. [DOI] [PubMed] [Google Scholar]
- 43.Enders D, Yang XN, Wang C, Raabe G, Runsik J. Dienamine activation in the organocatalytic asymmetric synthesis of cis-3,4-difunctionalized chromans and dihydrocoumarins. Chem. Asian J. 2011;6:2255–2259. doi: 10.1002/asia.201100320. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi A, Aoyama N, Matsunaga S, Shibasaki M. Ba-catalyzed direct Mannich-type reactions of a β,γ-unsaturated ester providing β-methyl aza-Morita−Baylis−Hillman-type products. Org. Lett. 2007;9:3387–3390. doi: 10.1021/ol071380x. [DOI] [PubMed] [Google Scholar]
- 45.Yazaki R, Nitabaru T, Kumagai N, Shibasaki M. Direct catalytic asymmetric addition of allylic cyanides to ketoimines. J. Am. Chem. Soc. 2008;130:14477–14479. doi: 10.1021/ja806572b. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi A, Matsunaga S, Shibasaki M. Catalytic asymmetric synthesis of α-alkylidene-β-hydroxy esters via dynamic kinetic asymmetric transformation involving Ba-catalyzed direct aldol reaction. J. Am. Chem. Soc. 2009;131:10842–10843. doi: 10.1021/ja904575e. [DOI] [PubMed] [Google Scholar]
- 47.Mitsudo T, Suzuki N, Kondo T, Watanabe Y. Ruthenium complex-catalyzed carbonylation of allylic compounds. J. Org. Chem. 1994;59:7759–7765. [Google Scholar]
- 48.Olaizola O, et al. Brønsted base catalyzed one-pot synthesis of stereodefined six-member carbocycles featuring transient trienolates and a key intramolecular 1,6-addition. Angew. Chem. Int. Ed. 2019;58:14250–14254. doi: 10.1002/anie.201908693. [DOI] [PubMed] [Google Scholar]
- 49.Shen XD, et al. Octahedral chiral-at-metal iridium catalysts: versatile chiral Lewis acids for asymmetric conjugate additions. Chem. Eur. J. 2015;21:9720–9726. doi: 10.1002/chem.201500922. [DOI] [PubMed] [Google Scholar]
- 50.Huang XQ, Meggers E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes. Acc. Chem. Res. 2019;52:833–847. doi: 10.1021/acs.accounts.9b00028. [DOI] [PubMed] [Google Scholar]
- 51.Mansot J, Vasseur J-J, Arseniyadis S, Smietana M. α,β-Unsaturated 2-acyl-imidazoles in asymmetric biohybrid catalysis. ChemCatChem. 2019;11:5686–5704. [Google Scholar]
- 52.Evans DA, Fandrick KR, Song H-J. Enantioselective Friedel−Crafts alkylations of α,β-unsaturated 2-acyl imidazoles catalyzed by bis(oxazolinyl)pyridine−scandium(III) triflate complexes. J. Am. Chem. Soc. 2005;127:8942–8943. doi: 10.1021/ja052433d. [DOI] [PubMed] [Google Scholar]
- 53.Evans DA, Song H-J, Fandrick K. Enantioselective nitrone cycloadditions of α,β-unsaturated 2-acyl imidazoles catalyzed by bis(oxazolinyl)pyridine−cerium(IV) triflate complexes. Org. Lett. 2006;8:3351–3354. doi: 10.1021/ol061223i. [DOI] [PubMed] [Google Scholar]
- 54.Drissi-Amraoui S, et al. Copper-catalyzed asymmetric conjugate addition of dimethylzinc to acyl-N-methylimidazole Michael acceptors: a powerful synthetic platform. Angew. Chem. Int. Ed. 2015;54:11830–11834. doi: 10.1002/anie.201506189. [DOI] [PubMed] [Google Scholar]
- 55.Rout S, Das A, Singh VK. An asymmetric vinylogous Mukaiyama–Michael reaction of α,β-unsaturated 2-acyl imidazoles catalyzed by chiral Sc(III)– or Er(III)–pybox complexes. Chem. Commun. 2017;53:5143–5146. doi: 10.1039/c7cc01763d. [DOI] [PubMed] [Google Scholar]
- 56.Liu XH, Lin LL, Feng XM. Chiral N,Nʹ-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 2011;44:574–587. doi: 10.1021/ar200015s. [DOI] [PubMed] [Google Scholar]
- 57.Liu XH, Lin LL, Feng XM. Chiral N,Nʹ-dioxide ligands: synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014;1:298–302. [Google Scholar]
- 58.Liu XH, Zheng HF, Xia Y, Lin LL, Feng XM. Asymmetric cycloaddition and cyclization reactions catalyzed by chiral N,Nʹ-dioxide-metal complexes. Acc. Chem. Res. 2017;50:2621–2631. doi: 10.1021/acs.accounts.7b00377. [DOI] [PubMed] [Google Scholar]
- 59.Liu XH, Dong SX, Lin LL, Feng XM. Chiral amino acids−derived catalysts and ligands. Chin. J. Chem. 2018;36:791–797. [Google Scholar]
- 60.Lin LL, Feng XM. Catalytic strategies for diastereodivergent synthesis. Chem. Eur. J. 2017;23:6464–6482. doi: 10.1002/chem.201604617. [DOI] [PubMed] [Google Scholar]
- 61.Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 62.Zhan G, Du W, Chen Y-C. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 2017;46:1675–1692. doi: 10.1039/c6cs00247a. [DOI] [PubMed] [Google Scholar]
- 63.Beletskaya IP, Nájera C, Yus M. Stereodivergent catalysis. Chem. Rev. 2018;118:5080–5200. doi: 10.1021/acs.chemrev.7b00561. [DOI] [PubMed] [Google Scholar]
- 64.Doswald S, et al. Large scale preparation of chiral building blocks for the P3 site of renin inhibitors. Bioorg. Med. Chem. 1994;2:403–410. doi: 10.1016/0968-0896(94)80007-3. [DOI] [PubMed] [Google Scholar]
- 65.Skarżewski J, Siedlecka R, Wojaczyńska E, Zielińska-Błajet M. A new and efficient route to homochiral γ-hydroxysulfoxides and γ-hydroxysulfones. Tetrahedron Asymmetry. 2002;13:2105–2111. [Google Scholar]
- 66.Reck F, et al. Identification of 4-substituted 1,2,3-triazoles as novel oxazolidinone antibacterial agents with reduced activity against monoamine oxidase A. J. Med. Chem. 2005;48:499–506. doi: 10.1021/jm0400810. [DOI] [PubMed] [Google Scholar]
- 67.Scott JP, et al. A practical synthesis of a γ-secretase inhibitor. J. Org. Chem. 2007;72:4149–4155. doi: 10.1021/jo070407n. [DOI] [PubMed] [Google Scholar]
- 68.Wolf WM. The fungicidal activity of β-keto sulfones. Molecular conformation of α-phenylhydrazono-β-ketosulfones as determined by an X-ray analysis. Mol. Struct. 1999;474:113–124. [Google Scholar]
- 69.The absolute configuration of 9 was assigned to be R compared with the literature data. Li, L., Liu, Y. D., Peng, Y., Yu, L., Wu, X. Y. & Yan, H. L. Kinetic resolution of β-sulfonyl ketones through enantioselective β-elimination using a cation-binding polyether catalyst. Angew. Chem. Int. Ed. 55, 331–335 (2016). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1972987 (2j), 2001513 (4r), and 1972937 (11). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the corresponding author upon reasonable request.