Abstract
This study examined a potential age-dependency of both posture and stability (balance) control in children and adolescents in a healthy population. Body posture with open and closed eyes was examined for a total of 456 test persons (age 6.7–17.6 years. Posture parameters (posture index, upper body tilt, trunk tilt) were assessed in the sagittal plane. Additionally, the oscillation of the center of pressure with open and closed eyes was additionally analyzed in a sub-sample of 318 subjects.
Absolute values of stability control parameters changed significantly during childhood and adolescence for both boys (p = 0.005) and girls (p = 0.01). Relative changes of stability and posture parameters when closing the eyes did not change (p > 0.05) and were independent of age, gender or sports activity in healthy children and adolescents.
The shifting of the body segments towards each other, as a result of the loss of visual information, does not seem to be primarily responsible for the increase in COP fluctuation. This is a further indication that stability control and posture control are complex interdependent mechanisms whose interaction is not yet fully understood.
Keywords: Health sciences, Pediatrics, Evidence-based medicine, Clinical research, Diagnostics, Posturography, Posture orientation, Posture measurement, Postural stability, Equilibrium, Neuromuscular control, Athletic activity, Stability, Balance control
Health sciences; Pediatrics; Evidence-based medicine; Clinical research; Diagnostics; Posturography; Posture orientation; Posture measurement; Postural stability; Equilibrium; Neuromuscular control; Athletic activity; Stability; Balance control
1. Introduction
Posture and stability control are fundamental motor skills and represent the basis for daily routine tasks (Punakallio, 2003) and athletic activity (De Kegel et al., 2016; Mickle et al., 2011). For example, a good stability control is essential for a stable gait (Ganz et al., 2007). Good posture regulation is thus the basis for almost every movement. On the other hand, poor posture can lead to strain in tendon and joint structures, e.g., when picking up heavy items. In 25–60% of children and adolescents, posture weakness is already present in the form of hollow back, hanging shoulders, or an anteverted pelvis (Dolphens et al., 2012; Kratenova et al., 2007; Gh Maghsoud et al., 2012; Wirth et al., 2013; Lee, 2016).
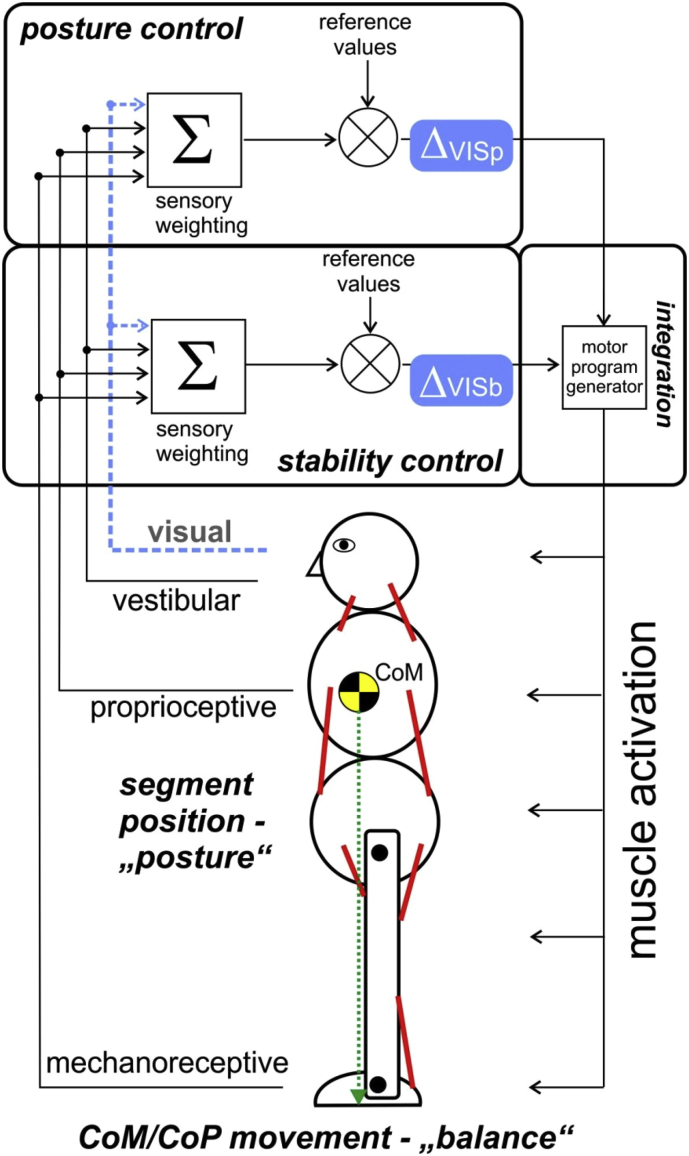
According to Massion (1992), the body is composed of superimposed segments (‘modules’) whose position can be regulated specifically and independently by the central nervous system (CNS). He was able to show that anticipatory postural adjustments are related to both balance control and postural stabilization. Postural stability (in the sense of balance control) can be defined as an adequate response to disturbances of the center of mass (COM) caused by body sway, motor activity or conscious interaction with the environment (Błaszczyk et al., 2020). It is known that both balance control and postural control are interlinked dynamic processes (Massion, 1992) that use signals of the same receptor systems (Chiba et al., 2016; Danis et al., 1998) and the same actuators - both regulate the activity of the postural musculature (Figure 1). Therefore, controlling posture while ensuring its stability is a fundamental task of the CNS (Błaszczyk et al., 2020; Sousa et al., 2012). By systematically activating the muscles, the central nervous system (CNS) is able to stabilize the biomechanically unstable body segments and at the same time keep the stabilized system in equilibrium (Assaiante et al., 2005; Sousa et al., 2012; Massion, 1992).
Figure 1.
A conceptual schematic diagram of the feedback control processes that regulate the upright posture (compare Massion, 1992 and Sousa et al., 2012). Sensory information is weighted in the CNS (Σ) and compared with target values (⊗) and in case of deviations, actuators (muscles) are activated to adjust the posture. Changes in visual information (blue) generate different changes in posture orientation and balance control. Σ = summation of afferent input for the posture and balance (stability) control circuit. Δ VIS is the control deviation between the eyes open- (feedback-) and eyes closed- (feed-forward-) condition. Comparison between the control deviation of the posture control circuit (Δ VISp) and the balance control circuit (Δ VISb) may give information on the interdependence of both systems. CoM = center of Mass, CoP = center of pressure.
In the following, we will distinguish between stability control (control of the center of pressure (COP) in relation to the base of support = ‘postural stability’) and posture control (control of body alignment and tonus = ‘postural orientation’; Sousa et al., 2012).
During somatosensory maturation and growth-related changes of biomechanical parameters, stability and posture control need to be continuously adapted (Yamamoto et al., 2015). The central nervous system learns and optimizes both functions in the course of child maturation (Peterka, 2002; Assaiante et al., 2005), with the somatosensory integration playing an important role (Machado et al., 2010). Perception and control of the position of the trunk are therefore key factors (Assaiante et al., 2005). Literature describes an age dependency pertaining to stability control, measurable for example by a reduction of the sway of the center of pressure (COP) in the course of maturation (Gouleme et al., 2014; Verbecque et al., 2016). The discussion about whether this development is linear or shows slumps due to anthropometric changes during growth periods is rather controversial (Kirshenbaum et al., 2001; Mallau et al., 2010; Schwesig et al., 2013; Verbecque et al., 2016).
Posture control involves adopting a stable and active posture in which the body segments are aligned perpendicular to each other and is an important basis for the prevention of postural deficiencies. The question therefore arises as to whether changes in posture stability, or more precisely in the oscillation of the COP, become apparent when adopting an active posture or when this posture deteriorates. Only a few studies examined the development of posture and stability control in children and adolescents, and they especially focused on pathologies, mostly without considering sports activities (balance: Mickle et al., 2011, overview in Verbecque et al., 2016; posture: Calvo-Muñoz et al., 2012, Latalski et al., 2013). Children with a weak posture do not necessarily seem to have a worse stability control (Nagymáté et al., 2018). Therefore, many questions remain open regarding stability and posture control in children and adolescents, their interrelationship and their possible correlations with athletic performance (Schwesig et al., 2013). As some studies found differences in posture regulation between girls and boys (Błaszczyk et al., 2014; Mickle et al., 2011; Smith et al., 2012; Steindl et al., 2006), we examined both age and sex as possible influencing factors. An age-related development can also be assumed (Assaiante et al., 2005).
Therefore, the aim of our study was to examine the interrelationships between posture and stability control with closed eyes, depending on age, sex, and sports activity.
2. Methods
The tests were conducted within the framework of an interdisciplinary research project (Kid-Check) over 14 examination days between 2015 and 2017. All 456 participants and their parents or guardians were informed prior to the tests about the tests' objective and test procedure, in accordance with the requirements of the Helsinki Declaration. Participants and parents gave their written informed consent. The ethics commission of the university had approved the study (Ref.-No. 15-6-08). Exclusion criteria were acute orthopedic or neurological problems (e.g. imbalance, vertigo) and known ADHD (attention deficit hyperactivity disorder).
Anthropometric data were collected on site and athletic activity was surveyed by means of a questionnaire filled in by parents and children together. The questionnaire contained questions about the types of sport performed, number of years of athletic practice, and average time spent within a week with the respective type of sport. Participants were requested to provide this information as plain text. When the participants or parents were unsure when answering the questions, a team member helped them by interviewing.
Afterwards, the test persons were assigned to four chronologically identical age groups (6.0–8.9 years, 9.0–11.9 years, 12.0–14.9 years, 15.0–17.9 years; in the following this will be abbreviated by integers). It is known that both the type of sport and the level of activity may have an effect on postural and/or balance control (Schwesig et al., 2009, Pion et al., 2015). Therefore, athletic activity (AA) was grouped based on sports with a high share of coordinative and balance skills and an intense control of body tension (AA = 3, pertaining to sports like gymnastics, rhythmic gymnastics, martial arts), with a lower share of these skills (AA = 2, pertaining to sports like soccer, handball, running sports), and persons who were not active in sports (AA = 1). This is a simplified subdivision, reduced to three categories, but based on common assumptions (Pion et al., 2015; Opstoel et al., 2015). Sports motor skills depend not only on the type of sport practised, but also on the number of hours spent doing it (Fransen et al., 2012). Therefore, to estimate athletic activity, an athletic activity index (AAI) was established based on AA ∗ hours of athletic activity per week ∗ years of athletic practice divided by age. Every subject was assigned an index value AAI according to their reported athletic activity. The participants were divided into three equally sized groups (level 1 to level 3) based on the AAI for each gender (boys: Ntotal = 284, AAI level 1: N = 95, level 2: N = 95, level 3: N = 94; girls: Ntotal = 172, AAI level 1: N = 57, level 2: N = 57, level 3: N = 58).
During the posture and balance assessment, all subtests were performed only once in order to avoid decrease of attention. This experiment setup was also applied in other studies (Nolan et al., 2005). The whole experiment, including 3 posture photos and 2 balance measurements, only took about 3 min. Therefore, according to pilot tests a loss of concentration could not be expected.
2.1. Posture measurement
For this study, posture regulation was measured, which means the ability to transfer one's own body posture from a relaxed, passive state (habitual posture) into an active, upright state. The concept is based on the idea that humans are naturally allowed to adopt relaxed body postures during their daily routine lives without suffering any ill effects, but that they must be able to consciously straighten their posture, for example, to carry loads or perform athletic activities. Therefore, we did not evaluate only the current posture status, but also the regulatory ability of the CNS relating to change body posture by analyzing the changes in postural parameters during the transition between postural situations, especially when closing the eyes. Figure 2 describes the experimental setup.
Figure 2.
Experimental setup. Δ POS = changes in postural parameters, CoP = center of pressure, Δ SPL = changes in sway path length.
In our analysis we compared balance and posture parameters in two situations: habitual posture with open eyes and activated posture with closed eyes. Differences between the posture and balance values were calculated for these situations. Activation of posture is a means to stimulate the neural posture regulation circuits, and habitual posture is relevant because it represents the most common posture during the day (Ludwig et al., 2016b).
456 test persons aged 6.7–17.6 years (see Table 1 for anthropometric data) participated in the posture measurement. First, digital photographs of the habitual posture in the sagittal plane were taken of the participants who were wearing swimwear or underwear (Ludwig et al., 2016b) in front of a calibration wall. Afterwards, the child was instructed to actively straighten up its posture. The investigator helped the child by means of verbal instructions to adopt an active body posture, so that malleolus lateralis, trochanter major, acromion, and tragus were positioned vertically one above the other as much as possible. As an additional, optical aid, a perpendicular laser beam was projected onto the body starting at malleolus lateralis, as it is known that verbal instructions alone sometimes do not result in targeted straightening up of the posture (Czaprowski et al., 2013; D'Amico et al., 2017). The participant was now to retain the best possible posture for 30 s, and then a second posture photo was taken. The child was then requested to keep the straight-up posture and close the eyes. After 60 s, a third posture photo was taken. The following posture parameters were determined by means of the Corpus Concepts posture analysis software (AFG, Idar-Oberstein, Germany) for each posture state: posture index, upper body tilt, trunk tilt (Krawczky et al., 2014; Ludwig et al., 2016a; Stolinski et al., 2017). Figure 3 explains these posture parameters. The photo-based, two-dimensional posture analysis and the posture parameters examined have been established as valid and reliable test methods (Hazar et al., 2015; Ruivo et al., 2015; Ludwig et al., 2016a).
Table 1.
Anthropometric data for the whole group (‘total’, N = 456) and for the balance measurement subgroup (‘SPL’, N = 318); mean value ± standard deviation; SPL = Sway path length, BMI = body mass index.
| Sex |
Age group |
Entire group |
Subgroup balance measurement |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (total) | Age (total) [years] | Mass (total) [kg] | Height (total) [cm] | BMI (total) [kg/m2] | N (SPL) | Age (SPL) [years] | Mass (SPL) [kg] | Height (SPL) [cm] | BMI (SPL) [kg/m2] | ||
| Girls | 6.0–8.9 yrs. | 23 | 8.07 ± 0.62 | 28.20 ± 5.26 | 129.65 ± 6.85 | 16.72 ± 2.43 | 17 | 8.01 ± 0.61 | 27.69 ± 5.35 | 130.59 ± 7.27 | 16.14 ± 2.14 |
| 9.0–11.9 yrs. | 57 | 10.51 ± 0.79 | 37.18 ± 8.31 | 146.15 ± 8.19 | 17.24 ± 2.48 | 47 | 10.55 ± 0.79 | 37.50 ± 8.51 | 146.90 ± 8.15 | 17.21 ± 2.52 | |
| 12.0–14.9 yrs. | 57 | 13.35 ± 0.94 | 50.93 ± 9.99 | 162.19 ± 9.32 | 19.26 ± 2.94 | 37 | 13.35 ± 0.94 | 52.78 ± 8.80 | 165.19 ± 7.95 | 19.28 ± 2.50 | |
| 15.0–17.9 yrs. | 35 | 15.84 ± 0.59 | 58.29 ± 9.40 | 169.34 ± 7.54 | 20.25 ± 2.54 | 26 | 15.79 ± 0.59 | 56.65 ± 8.80 | 169.65 ± 8.46 | 19.59 ± 1.88 | |
| Boys | 6.0–8.9 yrs. | 24 | 8.12 ± 0.43 | 30.08 ± 7.72 | 132.08 ± 8.33 | 17.14 ± 3.23 | 15 | 8.16 ± 0.42 | 31.14 ± 8.84 | 132.36 ± 7.73 | 17.55 ± 3.29 |
| 9.0–11.9 yrs. | 104 | 10.31 ± 0.77 | 37.51 ± 10.24 | 145.52 ± 9.67 | 17.45 ± 2.68 | 73 | 10.36 ± 0.80 | 38.95 ± 11.41 | 146.08 ± 10.45 | 17.91 ± 2.83 | |
| 12.0–14.9 yrs. | 105 | 13.40 ± 0.80 | 52.74 ± 12.13 | 165.54 ± 10.52 | 19.05 ± 3.05 | 70 | 13.39 ± 0.78 | 52.89 ± 11.82 | 165.93 ± 10.84 | 19.02 ± 2.96 | |
| 15.0–17.9 yrs. | 51 | 15.89 ± 0.70 | 66.13 ± 12.02 | 177.98 ± 6.88 | 20.83 ± 3.33 | 33 | 15.80 ± 0.70 | 67.35 ± 12.95 | 178.79 ± 7.20 | 15.80 ± 0.70 | |
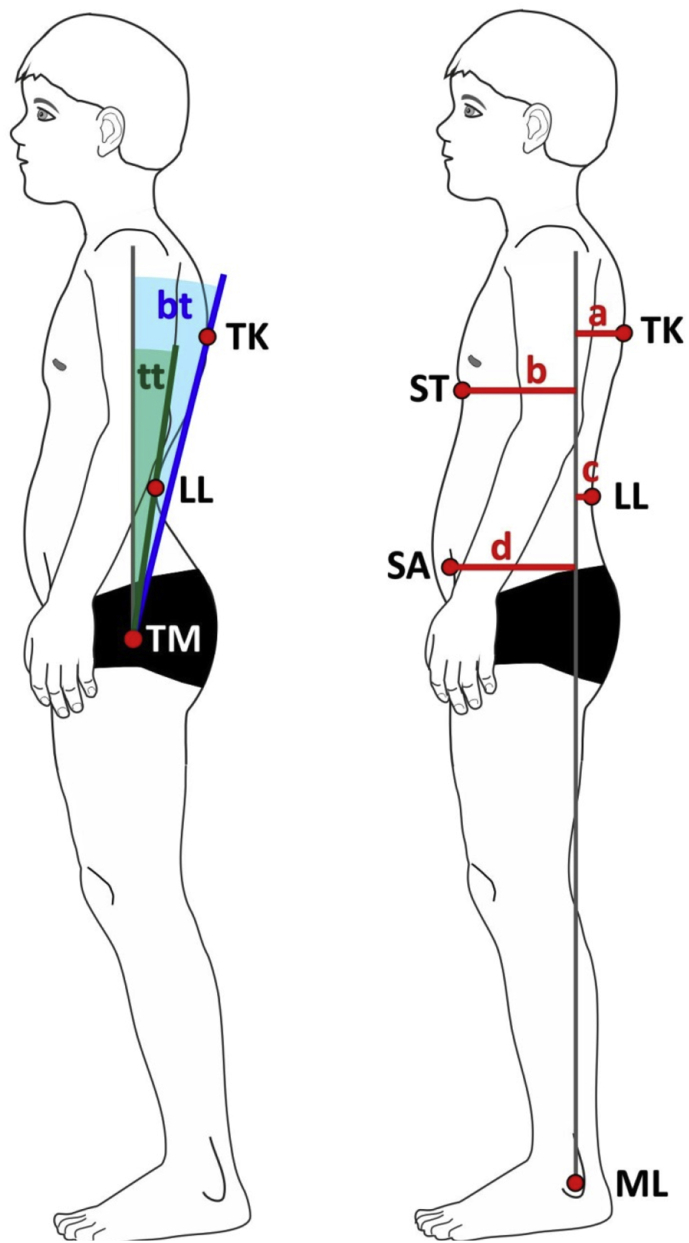
Figure 3.
Posture parameters: tt – trunk tilt, bt – upper body tilt, TK - maximal thoracic kyphosis, LL – maximal lumbar lordosis, TM – trochanter major, ST – distal sternum, SA – spina iliaca anterior superior, ML – malleolus lateralis, a, b, c, d – horizontal distances to plumb line. Posture index PI = (a+d)/(b + c).
The change in posture parameters during the transition from habitual to active posture describes the ability of the CNS to purposefully regulate posture (Ludwig et al., 2016b). Therefore, the differences of the posture parameters between the eyes open and eyes closed conditions were calculated and included in the following evaluation.
2.2. Balance measurement
Posturography is a valid tool for measuring the extent of body sway during a defined period by registering the movement of the center of pressure (Riemann et al., 2002; Ruhe et al., 2010). Instrumental measurement using force plates allows an easy and reproducible assessment of balance control under different sensory conditions (Verbecque et al., 2016). During posture measurement, the children and adolescents were standing barefoot and as still as possible on a force plate (Zebris PDM, Zebris Medical, Isny, Germany, 2560 calibrated sensors, sampling frequency 100 Hz) with their feet at hip width, their arms hanging naturally, and their head up straight. The outside rotation of the feet was chosen in such a way that the knees were aligned straight ahead. The same investigator supervised foot placement and gave the instructions for all participants. Two balance measurements were performed for 30 s while the posture photos of the habitual posture with open eyes and the active posture with closed eyes were taken (see 2.1) As the balance measurement device was only available on ten of 14 examination days, only 318 of the test persons (127 girls, 191 boys) participated in the balance measurement.
Based on the pressure shift during 30 s, the oscillation of the center of pressure (CoP) was calculated. This parameter (also known as sway path length – SPL) has been proven as a measurand for posture balance (Riemann et al., 2002; Ruhe et al., 2010; Cretual, 2015). The differences of the SPL values between the open and closed eyes conditions were calculated (delta SPL) and included in the statistical evaluation.
2.3. Statistical evaluation
For statistical testing, six sets of multi-factorial (4 × 3) ANOVAs based on the factors of age group (4 levels) and athletic activity index (3 levels) were calculated for girls and boys, respectively. Each ANOVA was conducted for the balance variables SPL (eyes open), SPL (eyes closed), and delta SPL, and for the posture variables delta posture index (delta PI), delta upper body tilt, and delta trunk tilt. The homogeneity of the variances was verified by means of the Levene test. A post-hoc pairwise analysis was performed based on the Tukey's HSD test.
After verification of the normal distribution, the Spearman rank correlation was applied to check for interrelationships between changes in the balance variable (delta SPL) and changes in the posture variables (delta posture index, delta upper body tilt, delta trunk tilt) after closing the eyes.
If data were missing, the corresponding data sets were not included in the calculations. Tables 2, 3, and 4 show the number of participants included in each calculation.
Table 2.
Descriptive statistics of the four age groups for girls and boys.
| Sex/age group | N (SPL) | SPL habitual (cm) | SPL eyes closed (cm) | N (total) | Delta SPL (cm) | Delta PI (-) | Delta Trunk Tilt (°) | Delta Upper Body Tilt (°) |
|---|---|---|---|---|---|---|---|---|
| Girls 6–8 yrs. | 17 | 165.86 ± 47.42 [141.48; 190.24] | 207.98 ± 63.07 [175.55; 240.41] | 23 | 42.12 ± 45.96 [18.49; 65.75] | 0.20 ± 0.30 [0.07; 0.33] | -4.13 ± 4.55 [-6.10; -2.16] | -3.04 ± 5.14 [-5.27; -0.82] |
| Girls 9–11 yrs. | 47 | 140.19 ± 42.51 [127.71; 152.68] | 164.44 ± 49.56 [149.89; 178.99] | 57 | 24.25 ± 30.74 [15.22; 33.28] | 0.09 ± 0.18 [0.05; 0.14] | -1.68 ± 4.61 [-2.91; - 0.46] | -1.68 ± 4.17 [-2.79; -0.58] |
| Girls 12–14 yrs. | 37 | 127.49 ± 44.46 [112.66; 142.31] | 157.16 ± 54.69 [138.92; 175.39] | 57 | 29.67 ± 30.29 [19.58; 39.77] | 0.15 ± 0.29 [0.07; 0.23] | -1.72 ± 5.88 [-3.28; -0.16] | -2.37 ± 4.27 [-3.50; -1.24] |
| Girls 15–17 yrs. | 26 | 123.03 ± 36.53 [108.27; 137.78] | 156.82 ± 56.45 [134.02; 179,62] | 35 | 33.80 ± 37.73 [18.56; 49.03] | 0.14 ± 0.23 [0.06; 0.22] | -1.77 ± 3.90 [-3.11; -0.43] | -2.49 ± 3.65 [-3.74; -1,23] |
| Boys 6–8 yrs. | 15 | 175.57 ± 51.08 [146.08; 205.07] | 215.98 ± 52.93 [185.42; 246.54] | 24 | 40.41 ± 36.99 [19.05; 61.76] | 0.06 ± 0.27 [-0.06; 0.17] | 0.00 ± 4.98 [-2.10; 2.10] | -0.79 ± 4.00 [-2.48; 0.90] |
| Boys 9–11 yrs. | 73 | 158.25 ± 52.85 [145.92; 170.58] | 193.13 ± 67.65 [177.34; 208.91] | 104 | 34.87 ± 47.45 [23.80; 45.94] | 0.09 ± 0.20 [0.05; 0.13] | -1.37 ± 5.13 [-2.36; -0.37] | -1.61 ± 3.62 [-2.31; -0.90] |
| Boys 12–14 yrs. | 70 | 146.90 ± 52.87 [134.30; 159.51] | 178.58 ± 64.69 [163.15; 194.00] | 105 | 31.67 ± 33.26 [23.74; 39.60] | 0.13 ± 0.27 [0.08; 0.18] | -1.55 ± 4.80 [-2.48; -0.62] | -2.41 ± 4.57 [-3.29; -1.52] |
| Boys 15–17 yrs. | 33 | 118.50 ± 34.39 [106.50; 130.50] | 141.33 ± 42.01 [126.00; 155.32] | 51 | 22.83 ± 29.65 [12.32; 33.34] | 0.10 ± 0.17 [0.05; 0.14] | -0.86 ± 3,49 [-1.85; 0.12] | -1.43 ± 3.52 [-2.42; -0.44] |
Data are reported as mean ± standard deviation. Values in brackets specify the 95%-confidence interval of the mean value. SPL = Sway Path Length, PI = Posture Index. Data with the prefix ‘Delta’ are differences between the eyes open and eyes closed measurements.
Table 3.
Results of the ANOVAs: interrelationships between athletic activity index (AAI) and balance/posture parameters and for girls and boys.
| AAI versus | N | F | p | |
|---|---|---|---|---|
| Girls | Balance: Delta Sway Path Length | 127 | 0.95 | 0.45 |
| Posture: Delta Posture Index | 170 | 0.75 | 0.59 | |
| Posture: Delta Trunk Tilt | 172 | 1.06 | 0.39 | |
| Posture: Delta Upper Body Tilt | 172 | 0.54 | 0.74 | |
| Boys | Balance: Delta Sway Path Length | 190 | 1.81 | 0.11 |
| Posture: Delta Posture Index | 277 | 0.68 | 0.64 | |
| Posture: Delta Trunk Tilt | 284 | 0.57 | 0.72 | |
| Posture: Delta Upper Body Tilt | 284 | 1.04 | 0.39 | |
Table 4.
Interrelationship between changes of balance parameters (Delta SPL) and changes of posture parameters when closing the eyes.
| Delta SPL [cm] versus | Sex | N | Mean ± standard deviation | Rho | p |
|---|---|---|---|---|---|
| Delta Upper Body Tilt [°] | Girls | 127 | -2.26 ± 4.23 | 0.13 | 0.08 |
| Boys | 190 | -1.83 ± 4.00 | 0.01 | 0.24 | |
| Delta Trunk Tilt [°] | Girls | 127 | -2.04 ± 4.96 | 0.01 | 0.28 |
| Boys | 190 | -1.25 ± 4.72 | 0.01 | 0.26 | |
| Delta Posture Index [-] | Girls | 127 | 0.19 ± 0.23 | 0.02 | 0.12 |
| Boys | 190 | 0.10 ± 0.23 | 0.01 | 0.29 |
Rho = Spearman's rank correlation coefficient, SPL = sway path length.
The significance level was set to 5%.
3. Results
The results are presented in Tables 2, 3, and 4.
3.1. Sway of the center of pressure with open eyes
We found a significant main effect on the sway path length with open eyes for girls (F(5,126) = 2.72, p < 0.02) and for boys (F(5,190) = 4.28, p = 0.001). The age group had a significant effect (girls: F(3) = 4.20, p = 0.01; boys: F(3) = 6.19, p = 0.0005), but for athletic activity no significant effect was identified (girls: F(3) = 0.51, p = 0.60; boys: F(3) = 1.08, p = 0.34). Post-hoc pairwise comparisons showed that 6 - 8 year-old girls had a significantly greater sway path length than 12–14 and 15 - 17 year-old girls (p < 0.01). For boys we found a significant difference between the oldest group (15–17 years) and all younger age groups (p < 0.05, see Figure 4). With increasing age, the sway path length of the CoP decreased.
Figure 4.
SPL (sway path length) with open eyes for girls (grey bars) and boys (black bars) in the different age groups. ∗p < 0.05, ∗∗p < 0.01.
3.2. Sway of the center of pressure with closed eyes
With closed eyes, the ANOVA showed a significant main effect for both girls (F(5,126) = 2.79, p = 0.02) and boys (F(5,190) = 4.99, p = 0.0003). The age group showed a significant effect on the sway path length for girls (F(3) = 4.14, p = 0.008) and for boys (F(3) = 7.29, p = 0.0001). Athletic activity did not show any significant influence (girls: F(3) = 1.04, p = 0.36; boys: F(3) = 1.54, p = 0.22). Post-hoc pairwise comparisons showed that 6–8 year old girls had a significantly greater sway path length than all older girls (all p < 0.02). For boys we found a significant difference between the oldest group (15–17 years) and all younger age groups (all p < 0.02, details see Figure 5).
Figure 5.
SPL (sway path length) with closed eyes for girls (grey bars) and boys (black bars) in the different age groups. ∗p < 0.05, ∗∗p < 0.01.
3.3. Delta SPL when closing the eyes
The degree of change of the CoP sway (delta SPL) during the transition from open to closed eyes was not significant in terms of age group and athletic activity neither for girls (F(5,126) = 0.95, p = 0.45) nor for boys (F(5,189) = 1.81, p = 0.11).
3.4. Changes in body posture
The body posture assumed with the eyes closed differed significantly from the habitual posture, even after Bonferroni correction, for the parameters examined: posture index (t = 1.96, p < 0.0001), upper body tilt (t = 6.44, p < 0.0001), and trunk tilt (t = 4.25, p < 0.0001).
For the changes in posture parameters (delta values), the calculated ANOVAs did not show any significant effects. We did not find any interrelationship between athletic activity, age group, and posture parameter changes during the transition from open to closed eyes (Table 3).
3.5. Interrelationships between posture and balance variables
No significant interrelationship was identified in any of the cases between the changes of the posture variables when closing the eyes and the changes of the balance variables (see Table 4).
3.6. Summary of the results
-
•
The absolute values of balance control parameters changed significantly with age.
-
•
The difference values of balance and posture parameters between open- and closed-eyes- conditions did not change with age.
-
•
Athletic activity, as far as representable by an athletic activity index, did not influence the absolute and relative balance and posture control parameters, independent of age and sex.
-
•
The difference values of balance and posture parameters that represent the reaction of control processes to the loss of vision did not show any interrelationship, independent of age and sex.
4. Discussion
The objective of this study was to examine a potential interrelationship and age-dependency of the neuromuscular regulation processes of both posture control and stability control in childhood and adolescence in a healthy population. Stability control was operationalized by the differences in sway path length between postures with open eyes and closed eyes. Posture control was operationalized by the differences in the posture parameters posture index (PI), upper body tilt (BT), and trunk tilt (TT) between the above mentioned postures. Compared to other studies, this was a new approach because we used the difference values of balance and posture parameters instead of the absolute values to quantify the response strength to the disturbance when the optical analyzer was turned off.
4.1. Posture control, stability control and athletic activity
We did not find any significant correlation between athletic activity, age, and sex and stability control or posture control parameters. Athletic activity develops sport-specific skills, such as balance or the execution of specialized motor patterns that generate sport-specific movements (Wrotniak et al., 2006). We thought it reasonable to expect that the development of such motor skills should have had a clearer positive effect on posture regulation and - in a second step - on effective stability control. To be able to maintain balance by adequate efferent muscle activity, afferent sensory information must be processed and a controlling system must exist when taking a cybernetic point of view (compare Figure 1; Sousa et al., 2012; Latash et al., 2010; Baratto et al., 2002). In a neurophysiological context, this is based on a representation of the body and its segments in the cortex, the so called 'body schema' (Maravita et al., 2003). The formation of a body schema is seen to be taking place in early childhood (Schmitz et al., 2002; Maravita et al., 2003). Assuming that body posture on this basis is controlled in a combination of open-loop (feedforward) and closed-loop (feedback) mechanisms (Collins and De Luca, 1995; Schmitz et al., 2002), it is safe to suppose that the quality of posture control depends on the formation of a valid body schema and also on the optimization of control loops (Figure 1). For example, with a well-developed internal representation, the CNS can compensate for the effects of changes in body position by anticipatory movements, thereby keeping the COG stable above the supporting surface of the feet (Błaszczyk et al., 2020). Although we were not able to find any clear dependency on athletic activity (i.e., participants with long-term praxis in demanding types of sport with a high share of coordinative and balance skills and an intense control of body tension did not exhibit improved posture control ability), it must be remembered that the control system worked successfully in all cases, which means the test persons did not lose their balance even with closed eyes. Although an increase in the COP fluctuation indicates that the system is approaching its stability limits (Baratto et al., 2002), it can also be argued that a well-trained system can afford to use this range. Our results correspond to the findings of current studies that concluded that the ability to correct posture depends on body perception rather than on visual information (D'Amico et al., 2017).
Pertaining to stability control, other working groups did identify interrelationships with athletic activity in as far as more athletically active persons exhibited a lower degree of COP sway (Keller et al., 2014; Kiers et al., 2013; Muehlbauer et al., 2013). Schwesig et al. (2009), for example, found improved stability control in adult athletes, especially in shooting competitors. However, these studies only examined adult subgroups or various types of professional sports (Kiers et al., 2013; Schwesig et al., 2009). We assume that these results are not directly comparable to our study because sensory systems and body schema are still maturing in children, and we examined subjects who performed sports at a non-professional level. Nevertheless, we had expected a better postural and stability control in the sense of a smaller posture deviation or smaller increase in the COP fluctuation with closed eyes in children active in sports, as we had assumed that athletic experience would have trained their sensorimotor system, leading to improved sensory information processing. Smith et al. (2012) did not find any consistent correlations between physical activity and postural stability, either. The results of our study do not corroborate the assumption that leisure sports 'automatically' is associated with a better stability and balance control (as far as we can measure the effects of these control processes). At the same time, it must be remembered that the operationalization of stability control via the sway path length of the COP is only one scientific approach among several. Other studies use for example the COP velocity or the sway ratio for analysis and come to different results, e.g. they did not find differences for all parameters between the eyes open and eyes closed conditions (Błaszczyk et al., 2014; Błaszczyk, 2008). Here, other analysis methods (COP frequency, speed) would probably be more suitable evaluation parameters.
The heterogeneity of our test group may have disguised positive effects of particular types of sport, though. We also need to point out that the definition of an 'athletic activity index' is a somewhat artificial theoretical construct. We tried to incorporate the most important factors that might have influenced the motor skills of our subjects, but we are aware that this predefinition of influencing factors that we used for calculation may have influenced the result.
4.2. Posture and stability control depending on age and gender
We analyzed the interrelationship between posture and stability control with closed eyes pertaining to age and gender. The CNS applies visual, vestibular, mechanoreceptive, and proprioceptive information to regulate balance and posture (Chiba et al., 2016; Peterka and Loughlin, 2004; Maurer et al., 2006; Massion, 1992). The process of adjusting sensory information to stability control is called sensory integration (Asslander and Peterka, 2014). Stability control, in our study measured in absolute SPL values, showed a significant change over age for both girls and boys, with the SPL with open and closed eyes diminishing with increasing age. For girls, we identified significant differences to the youngest age group. From age 9, significant differences were no longer detectable. This is in accordance with other studies that found stability control in 11 years old girls to be as stable as in young adults (Błaszczyk et al., 2020; Nolan et al., 2005). For boys, there were significant differences between the oldest age group (15–17 years) and the younger ones. These results apply to both open and closed eyes conditions. Nolan et al. (2005) found sex specific differences in COP oscillation in children, with boys showing greater oscillations with closed eyes than girls in the age group of 9–10 years. They interpreted this as a time-lag in development of posture control.
The elimination of sensory information (the visual one) leads to an increase in COP sway, which can be interpreted as a reduction in quality of the control process (Chiba et al., 2016), respectively an increase of the noise in the control system (Błaszczyk, 2008; Baratto et al., 2002). A reduction of COP sway and thus an improvement of postural stability in adolescence indicate a refinement of regulatory processes in the course of maturation. This finding corresponds to the results of other studies (Alonso et al., 2015; Paniccia et al., 2018). Kirshenbaum et al. (2001) proposed that sensory integration that begins between the age of 7 and 9 years leads to an integrated open- and closed-loop strategy to control COP oscillations. As closed-loop strategies (control processes with a sensory feedback) use visual sense, Nolan et al. (2005) suggest that increased COP oscillations with closed eyes may primarily indicate a closed-loop strategy in the above-mentioned age group.
Nolan et al. (2005) and Smith et al. (2012) supposed that girls use a combined strategy to control stability from an early age on. This would explain a better balance regulation with closed eyes. Nevertheless, we found a statistically significant improvement in stability control already in the group of 9–11 year olds, while Nolan and colleagues described this for girls older than 11 years. On the other side, in our study a significant improvement of body sway occurred in the group of 15–17 year-old boys, which is in accordance with Steindl et al. (2006), who described maturation of the visual component of posture control at the age of 15–16. Summarizing the above, it can be said that literature shows general agreement on maturation-dependent development of COP sway in childhood and adolescence, but not on its linearity (Foudriat et al., 1993; Schwesig et al., 2013; Steindl et al., 2006; Verbecque et al., 2016). Some authors describe age ranges in which COP sway decreases more strongly (Kirshenbaum et al., 2001), others found uniform improvement (Mallau et al., 2010). In adulthood, COP control is described to deteriorate only in seniors (Duarte and Sternad, 2008).
The youngest of the children we examined were six years old. While the process of somatosensory maturation progresses in early childhood, both sensory processing and posture control strategies develop (Woollacott and Shumway-Cook, 1990; Foudriat et al., 1993; Polastri and Barela, 2013). From the age of seven years, the maturation period during which somatosensory integration matures sets in. Steindl et al. state some proprioceptive maturation even in three- to four-year-olds (Steindl et al., 2006). This leads to a problem that may have influenced the results of all previous studies, including ours: chronological age is not always parallel to biological age. Effects of physique influence stability control in children and adolescents (Lipowicz et al., 2019). Therefore, the degree of comparability of studies may be reduced as the degree of the subjects' maturation was not considered in the different age groups.
We were also not able to find any dependencies on age or sex for posture and balance control with closed eyes. In our opinion, this is an interesting result because it shows that the changes in posture and balance parameters when the visual analyzer was turned off were not age- or gender-specific, regardless of the individual initial value. In other words: it did not matter whether a girl or boy exhibited good or poor posture control or stability control. Whenever they closed their eyes, their values deteriorated to the same extent, and regardless of age, too. This means that the differences in the control quality of the COP, caused by the elimination of visual input, did not depend on the age of the test persons. Ultimately, this parameter describes the influence of the visual system within the complex interaction of different receptor systems (Collins and De Luca, 1995). Both older and younger children were able to compensate to the same extent for the elimination of visual information.
Steindl et al. assume that visual and vestibular afferent systems reach an adult level only at the age of 15–16 (Steindl et al., 2006). The reduction of body sway could therefore rather be based on the improved processing of proprioreceptive receptor information. This can improve in the course of daily life routine. Athletic activity, insofar as this could be sufficiently described by our activity index, does not seem to play a primary role in this, though.
According to our results, sports training or therapeutic approaches with the aim of improving balance and posture control in children or adolescents (Behm et al., 2015, Bruhn et al., 2004; Page, 2006; Barczyk-Pawelec et al., 2015; Karaleić et al., 2014) should include both facets simultaneously. We would suggest that improving balance skills through a therapeutic exercise program or a sports exercise program will not automatically improve posture regulation (e.g. correcting a hollow back) and vice versa.
4.3. Interrelationship of posture and balance
Current models of stability control regard the human body in a simplified way as an inverted pendulum, in which the mass is concentrated in one point (Maurer and Peterka, 2005, Winter et al., 1990). Several strategies have been described with which the CNS balances the center of mass over the support surface. The ankle joint angle, respectively the torque generated by the calf muscles, plays a decisive role in the control in anterior-posterior (AP) direction. A second strategy which controls stability in the medio-lateral (ML) direction was described by Winter (Winter et al., 1990). Although the inverted pendulum model provides insights into the regulatory principles of the CNS, it simplifies reality considerably. The trunk is a very flexible multi-joint system, and through muscle activation the CNS can change the position of individual trunk segments in relation to each other which automatically shifts the position of the COM and as a result displaces the COP (Massion, 1992; Reeves et al., 2007). On the other hand, postural deficiencies are characterized, for example, by a hollow back, which also leads to a shifting of the pelvic, lumbar and thoracic segment and, as a consequence, to a relocation of the COM (Reeves et al., 2007). In order to keep the COM above the support area by the feet, COM control is thus necessary and according to current knowledge realized by bottom-up (e.g. through the ankle joint) and top-down strategies by repositioning the body segments via anticipatory movements.
A conscious straightening of the posture initially causes a reorientation especially of the segments pelvis, lumbar and thoracic spine and head to each other. This involves activating the corresponding muscles to bring the respective joints (hip joint, shoulder joint, upper cervical joint) closer to a common plumb line. Conversely, deterioration in posture is characterized by the fact that these joints move away from each other and the trunk is stabilized rather passively via the ligaments and joint capsules. Figure 6 shows a typical example of postural deterioration when the eyes are closed.
Figure 6.
Typical example of postural deterioration, from the state with open eyes (left, body segments lie on a plumb line) to the state with closed eyes (right).
Studies of human posture have shown that a previously upright posture often worsens again when the eyes are closed, which means the body segments move away from a common plumb line (Ludwig et al., 2016b). As this can be observed within 30 s, muscular fatigue can be excluded as the cause. Rather, the causes seem to lie in the neuronal control of the posture stabilizing muscles. One interpretive approach is that due to the suppression of optical information, an important sensory input into the control system is missing and, in case of poor proprioceptive perception, the quality of control decreases (Baratto et al., 2002).
Most studies show an increase in the amplitude of COP sway in the AP direction when closing the eyes (Cornilleau-Pérès et al., 2005). This is explained by the intrinsic noise within the control system with the motor controller increasing the safety margin of the COP shifts and the precision of the COM estimation deteriorating (Baratto et al., 2002). The basic question is therefore whether the increase in COP sway in the AP direction is accompanied (and possibly caused) by an analogous change in body posture in the AP direction.
As the objective of our study was to examine the reaction of the posture and balance control system to an omission of the visual information, we did not only compare the absolute values, but also the differences in the posture and balance variables when the subjects closed their eyes. The transition from 'eyes open' to 'eyes closed' is a disturbance that changes the input into the control systems (it ‘turns off’ the visual analyzer). We could find both an increase in COP oscillation (as found in other studies, e.g. Aoki et al., 2018; Błaszczyk, 2008) and a deterioration of the posture parameters. Interestingly, we did not find any direct interrelationship between these two sets of variables (posture and balance). Subjects who showed a great increase in COP sway when they closed their eyes did not automatically show a comparable decrease in their posture parameters, and vice versa.
Nevertheless, stability and posture regulation are interlinked processes because the CNS has to control COP oscillation in order to maintain stability when body segments shift (Feldman et al., 2014; Sousa et al., 2012; Massion, 1992). A hierarchical structure of the control paths is proposed by Sousa et al. and seems logical because changes in body posture often result in COP shifts that, in extreme cases, can also affect stability (Sousa et al., 2012).
Literature that describes interrelationships between posture (anomalies) and changes in stability is sparse. For adults, Drzal-Grabiec et al. (2014), Lopes et al. (2014) and Willigenburg et al. (2013) identified relationships between posture and balance parameters, but this applied particularly to patients. For adolescents with idiopathic scoliosis, Nault et al. (2002) and Stylianides et al. (2013) found that standing imbalance was related to altered body posture parameters. Other authors, such as Danis et al. (1998) did not find any relationships at all. Nagymáté et al. (2018) did no find a significant degradation of postural control in children with weak posture.
The changes in COP sway (in our study changes in sway path length) and the changes in macroscopically measurable body position in AP direction when closing the eyes are not statistically related according to our results. This is interesting because a change in body position in the AP direction also changes the position of the body's COM and as a consequence also the position of the COP. It would therefore have been reasonable to assume that the sway of the COP in the AP direction increases to the same extent.
Massion (1992) has stated that anticipatory postural adjustments are both related to balance control and to the stabilization of given posture positions. We found that the increase in body sway is not directly related to the deterioration in posture. The shifting of the body segments towards each other does not seem to be primarily responsible for the increase in COP fluctuation. Rather, this is a further indication that stability control and posture control are interdependent mechanisms whose complex interaction is not yet fully understood.
4.4. Limitations
Our tests are subject to some limitations which we have for the most part already listed in the corresponding paragraphs. In addition, a point to be viewed critically is that the categorization into only four age groups does not allow for differentiated observation of individual somatic development. The grouping was, however, selected in line with typical stages of maturation. When evaluating athletic activity, creation of an index value always leads to weighting of individual components. Nevertheless, this did seem to us an appropriate option of combining the lifetime span of practicing a sport (i.e., the overall volume of physical conditioning) with the motor requirements of that same sport. At the same time, we are aware that the quality of practicing a sport is a parameter that is difficult to measure, but that certainly has an influence on the motor performance of the test persons. Leisure habits (such as sedentary behavior) were not included in the evaluation. Future studies could deliver additional findings in this respect.
To determine the quality of the stability control, the sway path length (SPL) is not the only, and probably not the most meaningful parameter. Other studies describe sway velocity, stability indices or oscillation frequencies as suitable parameters. Nevertheless, we consider SPL as a simple parameter to measure that is logically related to the postural changes in the AP direction. SPL has also been described as a suitable parameter in other studies (Kitabayashi et al., 2018; Low et al., 2017).
4.5. Outlook
In further studies the transition of the COP position between habitual and active posture and/ or eyes-open eyes-closed condition should be investigated more closely. By analyzing the reaction to disturbing stimuli, the control properties of a system can be understood. E.g., Błaszczyk et al. (2020) have investigated the motor response to toe stand upright and have shown that the trajectory of toe stand position depends on individual characteristics of the children studied, such as muscle strength, anthropometry and neuromuscular development. This indicates perspectives in the analysis of active versus passive postural states and possibly also postural deficiencies, with the speed of the COP oscillation obviously being a crucial parameter (Błaszczyk et al., 2020).
5. Conclusion
The increase in body sway is not directly related to the deterioration in posture when the eyes are closed. Therefore, we conclude that the shifting of the body segments towards each other, as a result of the loss of visual information, does not seem to be primarily responsible for the increase in COP fluctuation, nor does it depend on age, gender, or sports activity in healthy children and adolescents.
Declarations
Author contribution statement
O. Ludwig: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Hammes: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
E. Schmitt: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
J. Kelm: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
M. Fröhlich: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank their students for their help with data acquisition, Martin Lindemann for organising the examination days and Monika Schutz for translating the manuscript.
References
- Alonso A.C., Mochizuki L., Silva Luna N.M., Ayama S., Canonica A.C., Greve J.M. Relation between the sensory and anthropometric variables in the quiet standing postural control: is the inverted pendulum important for the static balance control? BioMed Res. Int. 2015;2015:985312. doi: 10.1155/2015/985312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H., Demura S., Hirai H. Age-related changes in body sway when standing with eyes closed or open and on stable and unstable surfaces. Am. J. Sports Sci. Med. 2018;6(1):33–38. [Google Scholar]
- Assaiante C., Mallau S., Viel S., Jover M., Schmitz C. Development of postural control in healthy children: a functional approach. Neural Plast. 2005;12:109–118. doi: 10.1155/NP.2005.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asslander L., Peterka R.J. Sensory reweighting dynamics in human postural control. J. Neurophysiol. 2014;111:1852–1864. doi: 10.1152/jn.00669.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratto L., Morasso P.G., Re C., Spada G. A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Mot. Contr. 2002;6(3):246–270. doi: 10.1123/mcj.6.3.246. [DOI] [PubMed] [Google Scholar]
- Barczyk-Pawelec K., Piechura J.R., Dziubek W., Rozek K. Evaluation of isokinetic trunk muscle strength in adolescents with normal and abnormal postures. J. Manip. Physiol. Ther. 2015;38(7):484–492. doi: 10.1016/j.jmpt.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Muehlbauer T., Kibele A., Granacher U. Effects of strength training using unstable surfaces on strength, power and balance performance across the lifespan: a systematic review and meta-analysis. Sports Med. 2015;45(12):1645–1669. doi: 10.1007/s40279-015-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Błaszczyk J.W., Fredyk A.F., Błaszczyk P.M., Ashtiani M. Step response of human motor system as a measure of postural stability in children. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28(4):895–903. doi: 10.1109/TNSRE.2020.2974784. [DOI] [PubMed] [Google Scholar]
- Błaszczyk J.W., Beck M., Sadowska D. Assessment of postural stability in young healthy subjects based on directional features of posturographic data: vision and gender effects. Acta Neurobiol. Exp. 2014;74:433–442. doi: 10.55782/ane-2014-2006. [DOI] [PubMed] [Google Scholar]
- Błaszczyk J.W. Sway ratio – a new measure for quantifying postural stability. Acta Neurobiol. Exp. 2008;68:51–57. doi: 10.55782/ane-2008-1672. [DOI] [PubMed] [Google Scholar]
- Bruhn S., Kullmann N., Gollhofer A. The effects of a sensorimotor training and a strength training on postural stabilisation, maximum isometric contraction and jump performance. Int. J. Sports Med. 2004;25(1):56–60. doi: 10.1055/s-2003-45228. [DOI] [PubMed] [Google Scholar]
- Calvo-Muñoz I., Gómez-Conesa A., Sánchez-Meca J. Preventive physiotherapy interventions for back care in children and adolescents: a meta-analysis. BMC Muscoskel. Disord. 2012;13 doi: 10.1186/1471-2474-13-152. 152-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba R., Takakusaki K., Ota J., Yozu A., Haga N. Human upright posture control models based on multisensory inputs; in fast and slow dynamics. Neurosci. Res. 2016;104:96–104. doi: 10.1016/j.neures.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Collins J.J., De Luca C.J. The effects of visual input on open-loop and closed-loop postural control mechanisms. Exp. Brain Res. 1995;103:151–163. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- Cornilleau-Pérès V., Shabana N., Droulez J., Goh J., Lee G., Chew P. Measurement of the visual contribution to postural steadiness from the COP movement: methodology and reliability. Gait Posture. 2005;22(2):96–106. doi: 10.1016/j.gaitpost.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Cretual A. Which biomechanical models are currently used in standing posture analysis? Neurophysiol. Clin. 2015;45:285–295. doi: 10.1016/j.neucli.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Czaprowski D., Pawłowska P., Stoliński Ł., Kotwicki T. Active self-correction of back posture in children instructed with ‘straighten your back’ command. Man. Ther. 2013;19(5):392–398. doi: 10.1016/j.math.2013.10.005. [DOI] [PubMed] [Google Scholar]
- D’Amico M., Kinel E., Roncoletta P. 3D quantitative evaluation of spine proprioceptive perception/motor control through instinctive self-correction manoeuvre in healthy young subjects’ posture: an observational study. Eur. J. Phys. Rehabil. Med. 2017;54(3):428–439. doi: 10.23736/S1973-9087.17.04738-4. [DOI] [PubMed] [Google Scholar]
- Danis C.G., Krebs D.E., Gill-Body K.M., Sahrmann S. Relationship between standing posture and stability. Phys. Ther. 1998;78:502–517. doi: 10.1093/ptj/78.5.502. [DOI] [PubMed] [Google Scholar]
- De Kegel A., Dhooge I., Peersman W., Rijckaert J., Baetens T., Cambier D., Van Waelvelde H. Construct validity of the assessment of balance in children who are developing typically and in children with hearing impairments. Phys. Ther. 2016;90:1783–1794. doi: 10.2522/ptj.20100080. [DOI] [PubMed] [Google Scholar]
- Dolphens M., Cagnie B., Coorevits P. Sagittal standing posture and its association with spinal pain: a school-based epidemiological study of 1196 Flemish adolescents before age at peak height velocity. Spine. 2012;37:1657–1666. doi: 10.1097/BRS.0b013e3182408053. [DOI] [PubMed] [Google Scholar]
- Drzal-Grabiec J., Rachwal M., Podgorska-Bednarz J., Rykala J., Snela S., Truszczynska A., Trzaskoma Z. The effect of spinal curvature on the photogrammetric assessment on static balance in elderly women. BMC Muscoskel. Disord. 2014;15:186. doi: 10.1186/1471-2474-15-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M., Sternad D. Complexity of human postural control in young and older adults during prolonged standing. Exp. Brain Res. 2008;191:265–276. doi: 10.1007/s00221-008-1521-7. [DOI] [PubMed] [Google Scholar]
- Feldman A.G., Ilmane N., Sangani S., Raptis H. Motor control and position sense: action-perception coupling. Adv. Exp. Med. Biol. 2014;826:17–31. doi: 10.1007/978-1-4939-1338-1_2. [DOI] [PubMed] [Google Scholar]
- Foudriat B.A., Di Fabio R.P., Anderson J.H. Sensory organization of balance responses in children 3-6 years of age: a normative study with diagnostic implications. Int. J. Pediatr. Otorhinolaryngol. 1993;27:255–271. doi: 10.1016/0165-5876(93)90231-q. [DOI] [PubMed] [Google Scholar]
- Fransen J., Pion J., Vandendriessche J., Vandorpe B., Vaeyens R., Lenoir M., Philippaerts M.R. Differences in physical fitness and gross motor coordination in boys aged 6–12 years specializing in one versus sampling more than one sport. J. Sports Sci. 2012;30(4):379–398. doi: 10.1080/02640414.2011.642808. [DOI] [PubMed] [Google Scholar]
- Ganz D.A., Bao Y., Shekelle P.G., Rubenstein L.Z. Will my patient fall? JAMA. 2007;297:77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- Gh Maghsoud E., Alilou A., Ghafurinia S., Fereydounnia S. Biomedical Human Kinetics. 2012. Prevalence of faulty posture in children and youth from a rural region in Iran. [Google Scholar]
- Gouleme N., Ezane M.D., Wiener-Vacher S., Bucci M.P. Spatial and temporal postural analysis: a developmental study in healthy children. Int. J. Dev. Neurosci. 2014;38:169–177. doi: 10.1016/j.ijdevneu.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Hazar Z., Karabicak G.O., Tiftikci U. Reliability of photographic posture analysis of adolescents. J. Phys. Ther. Sci. 2015;27:3123–3126. doi: 10.1589/jpts.27.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaleić S., Milenković V., Savić Z. The influence of Physical Education curriculum on the correction of bad body posture and changes of motor status in adolescence period of schoolgirls. Activit. Phys. Educ. Sport. 2014;4(1):92–94. [Google Scholar]
- Keller M., Rottger K., Taube W. Ice skating promotes postural control in children. Scand. J. Med. Sci. Sports. 2014;24(6):e456–461. doi: 10.1111/sms.12230. [DOI] [PubMed] [Google Scholar]
- Kiers H., Van Dieen J., Dekkers H., Wittink H., Vanhees L. A systematic review of the relationship between physical activities in sports or daily life and postural sway in upright stance. Sports Med. 2013;43:1171–1189. doi: 10.1007/s40279-013-0082-5. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum N., Riach C.L., Starkes J.L. Non-linear development of postural control and strategy use in young children: a longitudinal study. Exp. Brain Res. 2001;140(4):420–431. doi: 10.1007/s002210100835. [DOI] [PubMed] [Google Scholar]
- Kitabayashi T., Demura S., Aoki H. Examination of effective body sway parameters for healthy elderly. Am. J. Sports Sci. Med. 2018;6(1):22–27. [Google Scholar]
- Kratenova J., Zejglicova K., Maly M., Filipova V. Prevalence and risk factors of poor posture in school children in the Czech Republic. J. Sch. Health. 2007;77:131–137. doi: 10.1111/j.1746-1561.2007.00182.x. [DOI] [PubMed] [Google Scholar]
- Krawczky B., Pacheco A.G., Mainenti M.R. A systematic review of the angular values obtained by computerized photogrammetry in sagittal plane: a proposal for reference values. J. Manip. Physiol. Ther. 2014;37:269–275. doi: 10.1016/j.jmpt.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Latalski M., Bylina J., Fatyga M., Repko M., Filipovic M., Jarosz M.J., Trzpis T. Risk factors of postural defects in children at school age. Ann. Agric. Environ. Med. 2013;20(3):583–587. [PubMed] [Google Scholar]
- Latash M.L., Levin M.F., Scholz J.P., Schöner G. Motor control theories and their applications. Medicina (Kaunas, Lithuania) 2010;46:382–392. [PMC free article] [PubMed] [Google Scholar]
- Lee J.H. Effects of forward head posture on static and dynamic balance control. J. Phys. Ther. Sci. 2016;28:274–277. doi: 10.1589/jpts.28.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowicz A., Szurmik T., Bugdol M.N., Graja K., Kurzeja P., Mitas A.W. Paper Presented at the International Conference on Information Technologies in Biomedicine. 2019. Relationship between body sway and body building in girls and boys in developmental age. [Google Scholar]
- Lopes A., Silva D., Kasuki L., Roberto Gadelha M., Camilo G., Guimaraes F. Posture and balance control in patients with acromegaly: results of a cross-sectional study. Gait & Posture. 2014;40:154–159. doi: 10.1016/j.gaitpost.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Low C.D., Walsh S.G., Arkesteijn M. Effectiveness of exercise interventions to improve postural control in older adults: a systematic review and meta-analyses of centre of pressure measurements. Sports Med. 2017;47(1):101–112. doi: 10.1007/s40279-016-0559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig O., Hammes A., Kelm J., Schmitt E. Assessment of the posture of adolescents in everyday clinical practice: intra-rater and inter-rater reliability and validity of a posture index. J. Bodyw. Mov. Ther. 2016;20(4):761–766. doi: 10.1016/j.jbmt.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Ludwig O., Mazet C., Mazet D., Hammes A., Schmitt E. Changes in habitual and active sagittal posture in children and adolescents with and without visual input – implications for diagnostic analysis of posture. J. Clin. Diagn. Res. 2016;10:SC14–17. doi: 10.7860/JCDR/2016/16647.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado S., Cunha M., Velasques B., Minc D., Teixeira S., Domingues C.A., Silva J.G., Bastos V.H., Budde H., Cagy M. Sensorimotor integration: basic concepts, abnormalities related to movement disorders and sensorimotor training-induced cortical reorganization. Rev. Neurol. 2010;51:427–436. [PubMed] [Google Scholar]
- Mallau S., Vaugoyeau M., Assaiante C. Postural strategies and sensory integration: no turning point between childhood and adolescence. PloS One. 2010;5(9):13078. doi: 10.1371/journal.pone.0013078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A., Spence C., Driver J. Multisensory integration and the body schema: close to hand and within reach. Curr. Biol. 2003;13:R531–R539. doi: 10.1016/s0960-9822(03)00449-4. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog. Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Maurer C., Peterka R.J. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J. Neurophysiol. 2005;93(1):189–200. doi: 10.1152/jn.00221.2004. [DOI] [PubMed] [Google Scholar]
- Maurer C., Mergner T., Peterka R.J. Multisensory control of human upright stance. Exp. Brain Res. 2006;171:231–250. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- Mickle K.J., Munro B.J., Steele J.R. Gender and age affect balance performance in primary school-aged children. J. Sci. Med. Sport. 2011;14:243–248. doi: 10.1016/j.jsams.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Muehlbauer T., Kuehnen M., Granacher U. Inline skating for balance and strength promotion in children during physical education. Percept. Mot. Skills. 2013;117(3):665–681. doi: 10.2466/30.06.PMS.117x29z9. [DOI] [PubMed] [Google Scholar]
- Nagymáté G., Takács M., Kiss R.M. Does bad posture affect the standing balance? Cogent Med. 2018;5(1):1503778. [Google Scholar]
- Nault M.L., Allard P., Hinse S., Le Blanc R., Caron O., Labelle H., Sadeghi H. Relations between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2002;27:1911–1917. doi: 10.1097/00007632-200209010-00018. [DOI] [PubMed] [Google Scholar]
- Nolan L., Grigorenko A., Thorstensson A. Balance control: sex and age differences in 9- to 16-year-olds. Dev. Med. Child Neurol. 2005;47:449–454. doi: 10.1017/s0012162205000873. [DOI] [PubMed] [Google Scholar]
- Opstoel K., Pion J., Elferink-Gemser M., Hartman E., Willemse B., Philippaerts R., Lenoir M. Anthropometric characteristics, physical fitness and motor coordination of 9 to 11 year old children participating in a wide range of sports. PloS One. 2015;10(5):e0126282. doi: 10.1371/journal.pone.0126282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion J., Segers V., Fransen J., Debuyck G., Deprez D., Haerens L. Generic anthropometric and performance characteristics among elite adolescent boys in nine different sports. Eur. J. Sport Sci. 2015;15(5):357–366. doi: 10.1080/17461391.2014.944875. [DOI] [PubMed] [Google Scholar]
- Page P. Sensorimotor training: a “global” approach for balance training. J. Bodyw. Mov. Ther. 2006;10(1):77–84. [Google Scholar]
- Paniccia M., Wilson K.E., Hunt A., Keightley M., Zabjek K., Taha T., Reed N. Postural stability in healthy child and youth athletes: the effect of age, sex, and concussion-related factors on performance. Sports Health. 2018;10(2):175–182. doi: 10.1177/1941738117741651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka R. Sensorimotor integration in human postural control. J. Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Peterka R.J., Loughlin P.J. Dynamic regulation of sensorimotor integration in human postural control. J. Neurophysiol. 2004;91:410–423. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- Polastri P.F., Barela J.A. Adaptive visual Re-weighting in children’s postural control. PloS One. 2013;8 doi: 10.1371/journal.pone.0082215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punakallio A. Balance abilities in different aged workers in physically demanding jobs. J. Occup. Rehabil. 2003;13:33–43. doi: 10.1023/a:1021845823521. [DOI] [PubMed] [Google Scholar]
- Reeves N.P., Narendra K.S., Cholewicki J. Spine stability: the six blind men and the elephant. Clin. Biomech. (Bristol, Avon) 2007;22:266–274. doi: 10.1016/j.clinbiomech.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B.L., Myers J.B., Lephart S.M. Sensorimotor system measurement techniques. J. Athl. Train. 2002;37:85–98. [PMC free article] [PubMed] [Google Scholar]
- Ruhe A., Fejer R., Walker B. The test-retest reliability of centre of pressure measures in bipedal static task conditions--a systematic review of the literature. Gait Posture. 2010;32:436–445. doi: 10.1016/j.gaitpost.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Ruivo R.M., Pezarat-Correia P., Carita A.I. Intrarater and interrater reliability of photographic measurement of upper-body standing posture of adolescents. J. Manip. Physiol. Ther. 2015;38:74–80. doi: 10.1016/j.jmpt.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Schmitz C., Martin N., Assaiante C. Building anticipatory postural adjustment during childhood: a kinematic and electromyographic analysis of unloading in children from 4 to 8 years of age. Exp. Brain Res. 2002;142:354–364. doi: 10.1007/s00221-001-0910-y. [DOI] [PubMed] [Google Scholar]
- Schwesig R., Kluttig A., Leuchte S., Becker S., Schmidt H., Esperer H.D. The impact of different sports on posture regulation. Sportverletz. Sportschaden. 2009;23:148–154. doi: 10.1055/s-0028-1109576. [DOI] [PubMed] [Google Scholar]
- Schwesig R., Fischer D., Kluttig A. Are there changes in postural regulation across the lifespan? Somatosens. Mot. Res. 2013;30(4):167–174. doi: 10.3109/08990220.2013.779245. [DOI] [PubMed] [Google Scholar]
- Smith A.W., Ulmer F.F., Wong D.P. Gender differences in postural stability among children. J. Hum. Kinet. 2012;33:25–32. doi: 10.2478/v10078-012-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa A.S., Silva A., Tavares J.M. Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: a review. Somatosens. Mot. Res. 2012;29:131–143. doi: 10.3109/08990220.2012.725680. [DOI] [PubMed] [Google Scholar]
- Steindl R., Kunz K., Schrott-Fischer A., Scholtz A.W. Effect of age and sex on maturation of sensory systems and balance control. Dev. Med. Child Neurol. 2006;48:477–482. doi: 10.1017/S0012162206001022. [DOI] [PubMed] [Google Scholar]
- Stolinski L., Kozinoga M., Czaprowski D., Tyrakowski M., Cerny P., Suzuki N., Kotwicki T. Two-dimensional digital photography for child body posture evaluation: standardized technique, reliable parameters and normative data for age 7-10 years. Scoliosis Spinal Disord. 2017;12:38. doi: 10.1186/s13013-017-0146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianides G.A., Dalleau G., Begon M., Rivard C.H., Allard P. Pelvic morphology, body posture and standing balance characteristics of adolescent able-bodied and idiopathic scoliosis girls. PloS One. 2013;8 doi: 10.1371/journal.pone.0070205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbecque E., Vereeck L., Hallemans A. Postural sway in children: a literature review. Gait Posture. 2016;49:402–410. doi: 10.1016/j.gaitpost.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Willigenburg N.W., Kingma I., Van Dieën J.H. Center of pressure trajectories, trunk kinematics and trunk muscle activation during unstable sitting in low back pain patients. Gait Posture. 2013;38(4):625–630. doi: 10.1016/j.gaitpost.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Winter D.A., Patla A.E., Frank J.S. Assessment of balance control in humans. Med. Prog. Technol. 1990;16:31–51. [PubMed] [Google Scholar]
- Wirth B., Knecht C., Humphreys K. Spine Day 2012: spinal pain in Swiss school children- epidemiology and risk factors. BMC Pediatr. 2013;13:159. doi: 10.1186/1471-2431-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott M.H., Shumway-Cook A. Changes in posture control across the life span--a systems approach. Phys. Ther. 1990;70:799–807. doi: 10.1093/ptj/70.12.799. [DOI] [PubMed] [Google Scholar]
- Wrotniak B.H., Epstein L.H., Dorn J.M., Jones K.E., Kondilis V.A. The relationship between motor proficiency and physical activity in children. Pediatrics. 2006;118(6):e1758–e1765. doi: 10.1542/peds.2006-0742. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Smith C.E., Suzuki Y., Kiyono K., Tanahashi T., Sakoda S., Morasso P., Nomura T. Universal and individual characteristics of postural sway during quiet standing in healthy young adults. Phys. Rep. 2015;3(3) doi: 10.14814/phy2.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]