Abstract
The performance of multiparametric magnetic resonance imaging (mpMRI) and subsequent biopsy in monitoring prostate cancer in men on active surveillance (AS) have not been defined clearly. In this systematic review, we aimed to review current literature about the usage of MRI examination in men with low-risk prostate cancer during active surveillance. For that, we searched seven databases to include all studies reporting magnetic resonance imaging in the AS of low-risk prostate cancer. We finally included 11 studies with 1237 patients included. Our results showed an adequate sensitivity and specificity of both modalities to detect disease progression; including disease upgrading and upstaging. However, the performance in the prediction of unfavorable disease was inferior to the detection of upgrading and upstaging. In terms of MRGB, the previous literature agreed on the superiority of using a combination of different biopsy schemes to get a better progression section. Noteworthy, mp-MRI and MRGB had a good predictive value limited to the first year, with TRUSGB showing a superior role in detecting patients with a GS ≥ 7, after that. In conclusion, both of mpMRI and MRGB have shown an adequate performance on assessing disease progression in the AS of low-risk prostate cancer patients. They can be used for disease staging and grading for successful treatment planning.
Keywords: MRI, MRGB, Active surveillance, Prostate cancer
- HIGHLIGHTS
-
•
In comparison to the literature, few papers discuss the benefit of MRI screening in low-risk prostate cancer groups.
-
•
Biopsy is considered more invasive than MRI, thus reducing the burden of such methods on the patients.
-
•
PSA values can be misinterpreted especially that it can rise in other diseases such as Benign Prostatic Hyperplasia.
1. Introduction
During the past decade, massive improvement has been implicated for more understanding of the epidemiology, diagnosis, and treatment of non-communicable diseases among different worldwide populations. Prostate cancer is a disease of men and considered to be a global health issue among the clinical society that interferes with the men's quality of life [1]. Prostate cancer is prevalent in most of the populations with a rising incidence over the past decade across most of the countries. An analysis of 43 populations revealed that the incidence of prostate cancer was the highest in the United States of America (USA) while the lowest incidence was reported in Asian countries [2]. The disease usually affects elderly populations compared to the youngest ones with the highest incidence in men after 60 years old [2].
Diagnosis of prostate cancer is essential for the prevention of long term complications especially mortality if the management was not appropriate [3]. Prostate-specific antigen (PSA) was presented for many years as a widely used laboratory parameter for the diagnosis of prostate cancer and its progression through the continuous rise of it is titer [1]. However, recent research inquired about the specificity of PSA in prostate cancer diagnosis especially with the PSA rise in certain diseases such as benign prostatic hyperplasia (BPH) [4]. Moreover, the invasive method of prostate cancer diagnosis by obtaining prostate biopsy was considered to be non-beneficial especially in asymptomatic patients and associated with several complications such as pain and hematospermia [5].
The strategy of active surveillance (AS) of prostate cancer entails a way for expectantly managing selected men with possible curative treatments in cases of disease progression [6,7]. Low-risk prostate cancer men, who are amenable to the AS, are identified using favorable preoperative parameters including clinical stage, tumor extent, prostate volume, and PSA [[8], [9], [10]]. However, all of these parameters have shown different limitations and accuracy deficiencies; including the re-classification risks, repeated biopsies complications, and the potential missing of the curability window [11]. Though, magnetic resonance imaging (MRI) technique was adopted as a non-invasive technique for prostate cancer diagnosis and for estimating it is progression [12]. MRI was found to be as effective as traditional methods and in some studies was reported to be superior to PSA and biopsy techniques [13,14]. In this systematic review, we aimed to review current literature about the usage of MRI examination in men with low-risk prostate cancer during active surveillance.
2. Methods
2.1. Search strategy and study selection
We performed this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-analyses statement (PRISMA) recommendations [15]. After collecting the appropriate keywords for developing a search term “(prostate cancer) AND (active surveillance) AND (MRI OR magnetic resonance imaging)”, we performed the systematic search for collecting relevant studies. We also performed a manual search for missed papers using the methods of Vasser and colleagues [16].
The search term was used through seven databases reported as the following: Pubmed, Google Scholar, Scopus, Web of Science, The New York Academy of Medicine (NYAM), Virtual health library (VHL), and the System for Information on Grey Literature in Europe.
Studies should be to meet the following inclusion criteria [1]: original studies [2]; assessing the value of MRI in the AS of low-risk prostate cancer [3]; patients older than 18 years [4]; the target assessment outcomes included the performance of multiparametric MRI (mpMRI) in the prediction of the disease progression (upstaging, upgrading, and unfavorable disease), which is the main outcome, the prediction ability of MRI when combined with biopsy (MR-guided biopsy), and how unnecessary MR-guided biopsies should be reduced. [5]; published in the last 5 years. We did not imply restrictions to study design, the language of the included papers, and the race of the included patients. The exclusion criteria were [1]: no report of the desired outcomes [2], intermediate and high risk of prostate cancer [3], published before 2016 [4], animal and in vitro studies and duplicate studies”.
The rationale for this 5-year limitation is mainly to give an updated piece of literature (used in many studies before [[17], [18], [19], [20]]), avoid the changing incidence and prevalence rates over years (which would affect screening results) [[21], [22], [23]], and the effect of rapidly developing MRI techniques, sequences and prostate imaging reporting/data system updates [24,25]. Moreover, the availability and access to diagnostic and health-care services as well as recommendations regarding prostate cancer screening are changing over the years [23].
The steps of title and abstract screening and full-text screening were done by five reviewers. A senior author was responsible for solving the conflicts between the five reviewers.
2.2. Data extraction
Three authors made a pilot extraction of few included studies for constructing a data extraction sheet. Then, another five reviewers retrieved the needed data from each of the included papers. A senior author was responsible for solving conflicts between the three extractors.
2.3. Risk of bias
The Institutes of Health (NIH) quality assessment tool is a widely used tool for measuring the quality of evidence [26]. Based on the included studies, we have used the tool of cross-sectional and the cohort studies reported in the NIH. The disagreement was solved through discussion between the five reviewers.
3. Results
3.1. Search results
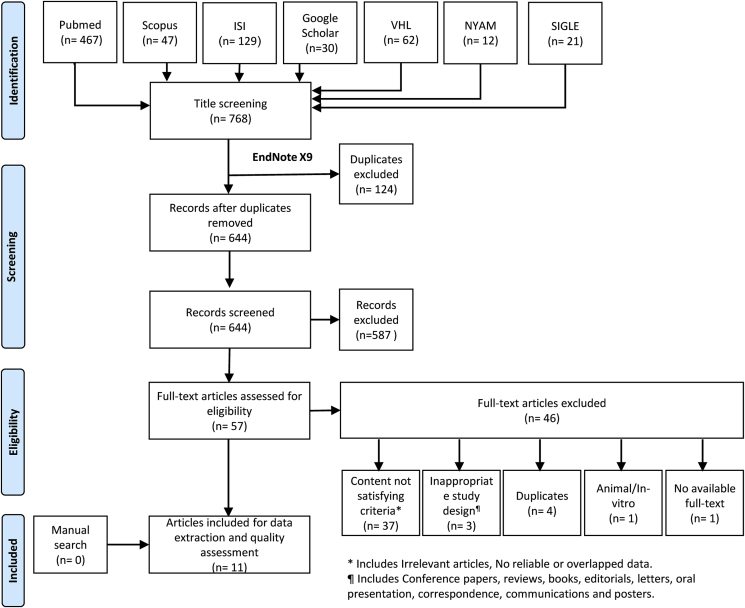
768 reports resulted from the database search. 644 were screened using the title and abstract screening method followed by the screening of 57 full texts for retrieving the relevant papers. We found 11 studies (Fig. 1). No studies were found after performing a manual search.
Fig. 1.
PRISMA flowchart of study search and selection process.
3.2. Study characteristics and quality of the included studies
The total sample size was 1237. There were 6 prospective cohorts and 5 retrospective cohorts. Age was reported in all studies; while only one study did not report the criteria for the diagnosis of low-risk prostate cancer. All included studies had fair quality with reporting most of the main items (Table 1).
Table 1.
Characteristics of the included studies.
| Reference ID | Study design | Sample size | Mean age (SD) | Definition of low-risk prostate cancer | Quality assessment |
|---|---|---|---|---|---|
| Chen/2018/Singapore [27] | Prospective cohort | 19 | 65.4 (4.9) | prostate specific antigen (PSA) ≤10 ng/mL, Gleason score ≤6, clinical stage ≤ T2a) | Fair |
| Alberts/2017/Netherlands [28] | Prospective cohort | 210 | 65.4# | Gleason score 3 + 3 | Fair |
| Almeida/2016/Italy [29] | Prospective cohort | 73 | 63 (5.9) | 1) men should have a histologically proven adenocarcinoma of the prostate, and they should be fit for possible curative treatment, be willing to attend the follow-up visits, and should not have received former therapy [2]; clinical stage T1c/T2 [3]; GS ≤ 6 [4]; ≤ 2 positive biopsy cores [5]; PSA ≤ 10 ng/mL [6]; PSAD ≤ 0.2 ng/mL/ml. | Fair |
| Borkowetz/2017/Germany [30] | Retrospective cohort | 83 | 73# | ≤cT2c, ≤2 cores with proven cancer, Gleason score ≤6 (3 + 3), prostate specific antigen (PSA) density <0.2 ng/mL2 and PSA <10 ng/mL. |
Fair |
| Hamoen/2018/Netherlands [31] | Prospective cohort | 111 | 64# | PSA density <0.2 ng/mL/ml, clinical stage cT1c–cT2c, and GS 3 + 3 and 2 positive biopsy cores at initial TRUSGB were | Fair |
| Hashimoto/2012/Japan [32] | Retrospective cohort | 11 | 65# | NR | Fair |
| Hsiang/2019/USA [12] | Retrospective cohort | 122 | 63# | grade group [GG] 1 | Fair |
| Osses/2020/Netherlands [33] | Retrospective cohort | 111 | 66# | ISUP grade 1 | Fair |
| Ploussard/2019/France [11] | Retrospective cohort | 143 | 64.4 | GG 1, T1–T2 disease and PSA ≤ 10 | Fair |
| Schoots/2018/Netherlands [34] | Prospective cohort | 331 | 67# | (GS 3 + 3) | Fair |
| Vos/2016/Canada [13] | Prospective cohort | 23 | 65 | Gleason score ≤ 6, and either clinical stage ≤ T2a or PSA ≤ 10 ng/mL. | Fair |
# = median, NR = not reported.
4. Role of multiparametric magnetic resonance imaging (mpMRI) in predicting disease progression
4.1. Prediction of disease upgrading
In a study by Hsiang et al., 44.3% of men who performed serial mpMRI examinations showed a progression in the subsequent imaging [12]. The parameters of performance in detecting the disease upgrade were: 41.3% sensitivity, 54.8% specificity, 22.2 positive predictive value (PPV), and 75% negative predictive value (NPV) [12]. In Almeida et al., the mpMRI showed a reasonable performance of sensitivity (76%) in detecting disease upgrading; however, there was no statistically significant correlation between clinical/pathological features and disease upgrading [29] (Table 2).
Table 2.
Performance of multiparametric magnetic resonance imaging (mpMRI) progression by different criteria and clinical data for prediction of disease upgrading, compared to the final pathology data.
| % Sensitivity (95% CI) | % Specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) | % Accuracy (95% CI) | Odds ratio (95% CI) | AUC (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| PSA density ≥0.15 at the follow-up biopsy (12) | 44.8 (26.4–64.3) | 72 (61.7–80.8) | 33.3 (22.9–45.6) | 80.7 (74.6–85.6) | 65.5 (56.4–73.9) | 2 (0.8–4.9) | 0.58 (0.46–0.70) | |
| mpMRI any progression (12) | 41.3 (23.5–61) | 54.8 (44.1–65.1) | 22.2 (14.9–31.7) | 75 (67.7–81) | 51.6 (42.4–60.7) | 0.8 (0.3–1.9) | 0.51 (0.39–0.63) | |
| mpMRI lesion number progression (12) | 17.2 (5.8–35.7) | 79.5 (69.9–87.2) | 20.8 (9.7–39.1) | 75.5 (71.7–78.9) | 64.7 (55.5–73.1) | 0.8 (0.2–2.4) | 0.51 (0.39–0.63) | |
| PI-RADS score | PI-RADS score progression (12) | 31 (15.2–50.8) | 77.4 (67.5–85.4) | 30 (18.1–45.3) | 78.2 (73.3–82.4) | 66.3 (57.2–74.6) | 1.5 (0.6–3.8) | 0.54 (0.41–0.66) |
| (2–3 vs. 4–5) (29) | 76 | 43 | 52 | 68 | - | - | - | |
| mpMRI index doubling (12) | 20.6 (7.9–39.7) | 68.8 (58.3–78) | 17.1 (8.7–30.9) | 73.5 (68.8–77.8) | 57.3 (48.1–66.2) | 0.5 (0.2–1.5) | 0.55 (0.43–0.66) | |
| Clinical stage (29) | 15a | 80 | 38 | 53 | - | - | - | |
| Imaging-based index of suspicion (Score 1-5) (13) | 58.3 | 81.8 | - | - | - | - | - | |
| Clinical Grade (33) | ISUP grade ≥2 | 85.71 (69.74-95.19) | 56.58 (44.71-67.92) | 47.62 (40.48-54.85) | 89.58 (78.86-95.20) | 65.77 (56.16-74.51) | - | - |
| ISUP grade ≥2 + cribriform growth/intraductal carcinoma Pca | 82.35 (56.57-96.20) | 47.87 (37.46-58.43) | 22.22 (17.57-27.70) | 93.75 (84.02-97.72) | 53.15 (43.45-62.69) | - | - | |
| ISUP grade ≥3 PCa | 80.00 (28.36-99.49) | 44.34 (34.69-54.31) | 6.35 (4.06-9.79) | 97.92 (88.94-99.64) | 45.95 (36.45-55.67) | - | - | |
| Positive core (29) | 36a | 53 | 39 | 50 | - | - | - | |
| BMI, kg/m2 (29) | Cut-off 25 | 85a | 28 | 49 | 69 | - | - | - |
| Cut-off 30 | 27a | 85 | 60 | 59 | - | - | - | |
AUC = area under the curve; CI = confidence interval; mpMRI = multiparametric magnetic resonance imaging; BMI = body mass index; NPV = negative predictive value; PIRADS = Prostate Imaging Reporting and Data System; PPV = positive predictive value; PSA = prostate-specific antigen.
Sensitivity (as reported in the study); ISUP =International Society of Urological Pathology.
In Vos et al., the detection of prostate cancer at baseline, through MRI imaging, was not adequate, with only 43.5% sensitivity [13]. On the other hand, the prediction of disease upgrading showed better performance with a sensitivity of 58.3% and specificity of 81.8% [13]. The performance of mpMRI progression by different criteria, to predict disease upgrading, is presented in Table 2.
Schoots et al. found that 25% of men on MRI-AS showed upgrading from Gleason score (GS) 3 + 3; out of them, 71% upgraded to GS 3 + 4, 16% to GS 4 + 3, and 13 to GS ≥ 4 + 4 [34]. Additionally, in patients with a suspicious MRI index lesion, 41% of them showed upgrading from GS 3 + 3 to GS 3 + 4 or higher, 22% of Prostate Imaging Reporting and Data System (PIRADS)-3 lesions upgraded to GS 3 + 4, and 8% of PIRADS-3 upgraded to GS 4 + 3 [34].
Noteworthy, the mpMRI ability to detect the upgrading in AS of prostate cancer patients remained stable in patients with testosterone replacement therapy, without biopsy progression [32].
4.2. Prediction of disease upstaging
The mpMRI showed an appropriate sensitivity (92%) to detect disease upstaging, with a higher NPV compared to upgrading (96% Vs. 68%) [29]. Moreover, disease upstaging was significantly correlated to patients’ age, clinical stage, and visible disease [29]. Vos et al., also found an adequate sensitivity (100%) of mpMRI to detect disease upstaging, however, the specificity was lower, down to 30% [13] (Table 3).
Table 3.
Performance of multiparametric magnetic resonance imaging (mpMRI) progression by different criteria and clinical data for prediction of disease upstaging, compared to the final pathology data.
| % Sensitivity |
% Specificity |
% PPV |
% NPV |
||
|---|---|---|---|---|---|
| Clinical stage [29] | 38 | 87 | 38 | 87 | |
| Imaging-based index of suspicion (Score 1–5) | 1–5 [13] | 100 | 30 | – | – |
| 1–2 [31] | – | – | – | 85 | |
| ≥ 3 [31] | 71a | – | – | – | |
| Positive core [29] | 54 | 60 | 23 | 86 | |
| PIRADS (2–3 vs. 4–5) [29] | 92 | 40 | 25 | 96 | |
| BMI, kg/m2 [29] | Cut-off 25 | 85 | 23 | 23 | 88 |
| Cut-off 30 | 38 | 83 | 33 | 86 | |
BMI: body mass index; NPV = negative predictive value; PIRADS = Prostate Imaging Reporting and Data System; PPV = positive predictive value.
Sensitivity.
In the same context, Hamoen et al. adopted an imaging-based index of suspicion (Score 1 to 5) to evaluate the MRI role [31]. They found that patients with score ≤2 had an NPV of 85% for detecting disease upstaging, compared to a sensitivity of 71% in patients with scores ≥3 [31] (Table 3). The same study highlighted that mp-MRI and MR-guided biopsy (MRGB) had a good predictive value limited to the first year; however, transrectal ultrasound-guided biopsy (TRUSGB) showed a superior role in detecting patients with a GS ≥ 7, following the first year [31].
4.3. Prediction of unfavorable disease
The unfavorable disease was defined as the presence of upgrading and/or upstaging, and PIRADS score >3. The mpMRI showed an intermediate sensitivity (76%) to detect unfavorable disease, with a specificity of 44% and PPV of 58%. The unfavorable disease had a lower NPV compared to upstaging and upgrading (64% Vs. 96% Vs. 68%) [29]. Additionally, the unfavorable disease was significantly correlated to PIRADS-5 [29] (Table 4).
Table 4.
Performance of multiparametric magnetic resonance imaging (mpMRI) progression by different criteria and clinical data for prediction of unfavorable disease, compared to the final pathology data [29].
| % Sensitivity | % Specificity | % PPV | % NPV | ||
|---|---|---|---|---|---|
| Clinical stage | 16 | 81 | 46 | 48 | |
| Positive core | 38 | 53 | 45 | 45 | |
| PIRADS (2–3 vs. 4–5) | 76 | 44 | 58 | 64 | |
| BMI, kg/m2 | Cut-off 25 | 84 | 28 | 54 | 63 |
| Cut-off 30 | 27 | 86 | 67 | 53 | |
BMI: body mass index; NPV = negative predictive value; PIRADS = Prostate Imaging Reporting and Data System; PPV = positive predictive value.
4.4. Role of MR-guided biopsy (MRGB) in predicting disease progression
A combination of mp-MRI and MRGB would is of additional value in the AS process of prostate cancer patients, especially during the first year [31]. This combination re-classified 23% of the patients, with 60% of the re-classified due to GS increase [31]. In the same context, with PSA-density (PSA-D) cut-off 0.15 ng/mL2, all PIRADS-3 lesion with upgrades to GS ≥ 3 + 4 were detected in patients with PSA-D ≥0.15 ng/mL2 [34]. The number of positive MRIs with GS outcome of MRGB stratified to PI-RADS and PSA-D is summarized in Table 5.
Table 5.
Number of positive MRIs with Gleason score outcome of MRI-targeted biopsies, stratified to PI-RADS and PSA-density.
| Schoots/2018/Netherlands [34] |
Alberts/2017/Netherlands [28] |
|||
|---|---|---|---|---|
| PSA Density (N = 198) |
PSA Density (N = 210) |
|||
| <0.15 | ≥0.15 | <0.15 | ≥0.15 | |
| PI-RADS | ||||
| 3 | 36% | 64% | 44% | 56% |
| 4 | 43% | 57% | 37% | 63% |
| 5 | 28% | 72% | 21% | 79% |
| Gleason score (GS) | ||||
| No PCa | 62% | 38% | 49% | 51% |
| GS 3 + 3 | 46% | 54% | – | – |
| GS 3 + 4 | 22% | 78% | 19% | 81% |
| GS 4 + 3 | 8% | 92% | – | – |
| GS ≥ 4 + 4 | 18% | 82% | – | – |
| GS ≥ 3 + 4 | 20% | 80% | – | – |
| GS ≥ 4 + 3 | 12% | 88% | 7% | 93% |
PIRADS = Prostate Imaging Reporting and Data System; PSA-D = prostate specific antigen-density; PCa = prostate cancer.
In terms of biopsy scheme used to assess prostate cancer upstaging and upgrading, targeted biopsies alone would miss 21.7% of cancer lesion; out of them, 16.7% are of grade group (GG) ≥ 3 [11]. However, a combination of targeted and systematic biopsies would lower the risk of GG ≥ 3 disease by 39%, compared with targeted biopsies alone [11]. Noteworthy, the biopsy scheme did not have a significant effect on the upstaging rates, even with the combination of targeted and systematic biopsies [11]. Borkowetz et al. reported similar results for combination biopsies, where a combination of MRI/ultrasound-fusion biopsy and systematic biopsy, in patients undergoing AS for prostate cancer, outperformed both modalities alone [30]. The combination scheme detected upgrading in 71% if the patients compared to 64% and 59% of MRI/ultrasound-fusion and systematic biopsies, respectively [30]. Another suggested combination scheme is the MRI-targeted and transperineal template biopsies, which detected disease upgrading in 26.3% of the patients, outperforming any of the two types alone [27].
4.5. Strategies to reduce unnecessary MRGB
In 59% of men with suspicious MRI lesions, MRGB did not show upgrading. Those biopsies could be considered unnecessary and harmful, especially in patients with PIRADS-3 (70% GS 3 + 3 or no prostate cancer) [34]. Similarly, 64% of PIRADS-4 and 34 of PIRADS-5 MRGBs were found to be unnecessary [34]. For that, Schoots et al. have suggested some possible strategies to reduce this possible harm as seen in Table 6.
Table 6.
Possible strategies to reduce targeting biopsies in low-risk men in active surveillance [34].
| No targeted biopsy | Targeted biopsy | No targeted biopsy | Targeted biopsy | |
|---|---|---|---|---|
| MRI index lesions | Threshold csPCa: GS ≥ 3 + 4 | Threshold csPCa: GS ≥ 4 + 3 | ||
| Stratification into PSAD <0.15 and ≥ 0.15 ng/mL2 | ||||
| PI-RADS 3 | P3 and PSA-D <0.15 | P3 and PSAD ≥0.15 | P3 and PSA-D <0.15 | P3 and PSAD ≥0.15 |
| PI-RADS 4 | P4 and any PSAD | P4 and PSA-D <0.15 | P4 and PSAD ≥0.15 | |
| PI-RADS 5 | P5 and any PSAD | P5 and any PSAD | ||
| Stratification into PSAD <0.20 and ≥ 0.20 ng/mL2 | ||||
| PI-RADS 3 | P3 and PSA-D <0.20 | P3 and PSAD ≥0.20 | P3 and PSA-D <0.20 | P3 and PSAD ≥0.20 |
| PI-RADS 4 | P4 and any PSAD | P4 and any PSAD | ||
| PI-RADS 5 | P5 and any PSAD | P5 and any PSAD | ||
PIRADS = Prostate Imaging Reporting and Data System; PSA-D = prostate specific antigen-density; csPCa, clinically significant prostate cancer; GS, Gleason score.
5. Discussion
The trials to use MRI to identify tumor locations in prostate cancer have started as early as the 1980s, using T1-weighted and T2-weighted images which lacked sensitivity and specificity [35]. The role of mpMRI was traditionally confined to prostate cancer staging and was typically done following biopsy to assess the possibility of different treatment modalities [36]. Recently, the function of mpMRI expanded to include tumor identification, monitoring disease during AS, and follow-up of the patients [36].
In the current study, we are presenting different aspects of mpMRI and MRGB performance as a part of the AS process. Our results showed an adequate sensitivity and specificity of both modalities to detect disease progression; including disease upgrading and upstaging. Moreover, the mpMRI ability to detect the progression in AS of prostate cancer patients remained stable in patients with testosterone replacement therapy, without biopsy progression. However, the performance in the prediction of unfavorable disease was inferior to the detection of upgrading and upstaging. In terms of MRGB, the previous literature agreed on the superiority of using a combination of different biopsy schemes to get a better progression section. Noteworthy, mp-MRI and MRGB had a good predictive value limited to the first year, with TRUSGB showing a superior role in detecting patients with a GS ≥ 7, after that.
Prostate cancer traditional identification is done using TRUSGB; nevertheless, it showed a low detection rate of 27%–44%, over-diagnosis of non-significant lesions, and missing some important ones, especially in the anterior portion of the prostate [[37], [38], [39]]. In addition to the aforementioned advantages in mpMRI performance, it can be used to target the identified lesion, either by MRGB or MRI/ultrasound-fusion biopsies [40,41]. Both of MRGB and MRI/ultrasound-fusion biopsies have higher accuracy when compared to TRUSGB alone [[42], [43], [44], [45]]. A previous systematic review showed that MRGB is superior to TRUSGB with a third fewer biopsy indicated and a 10% fewer detection of clinically insignificant lesions [46]. This was also confirmed by other studies that found a reduced missing of the clinically significant lesions using MRGB compared to TRUSGB [47], with a tumor detection rate of 70.1% in MRGB, compared to only 13.1% for TRUSGB [48].
In terms of assessing disease aggressiveness and staging, mpMRI showed a higher performance and accuracy in staging localized prostate cancer, when compared to the Partin table [49]. In the same context, MRI T2w imaging and dynamic contrast enhanced-MRI have shown high accuracy in staging prostate cancer and identifying tumors extending beyond prostate boundaries (T3 stage) [50,51]. Using mpMRI can also help in choosing treatment strategy in patients with low-risk prostate cancer to help with planning radiotherapy and surgery [52]. Moreover, mpMRI can be used to assess tumor volume, extension, and location, which is useful information to guide focal therapy [53]. Although some evidence is present on how mpMRI may miss some secondary satellite lesions, further examination of these lesions concluded that they were low-grade and significantly small ones [54].
The current study has some limitations affecting the generalizability of conclusions. A few studies did not provide a detailed definition of low-risk prostate cancer and there is some relevant heterogeneity among those who did. Although all studies concluded the usefulness and added value of MRI in AS, the performance of mpMRI and MRGB is variable among different studies.
6. Conclusion
Both of mpMRI and MRGB have shown an adequate performance on assessing disease progression in the AS of low-risk prostate cancer patients. They can be used for disease staging and grading for successful treatment planning.
Sources of funding
None.
Ethical approval
As per hospital Ethical Committee protocol, Systematic reviews and Meta analyses do not need approval as they are considered pre-approved.
Consent
Non applicable.
Author contribution
All authors were part of the study design, data collection, data analysis, interpretation, writing, editing, language proofing and resource checking of the paper.
Registration of Research Studies
Name of the registry: Research Registry.
Unique Identifying number or registration ID: reviewregistry921.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/register-now#user-systematicreviewmeta-analysesregistry/registerasystematicreviewmeta-analysidetails/5ed7b051f7f53c0015528bf8/.
Guarantor
Sultan Zaher Alshehri.
Omar Safar Alshahrani.
Provenance and peer review
Not commissioned, externally peer reviewed.
Please state any conflicts of interest
The authors declare no conflict of interest.
Please state any sources of funding for your research
No funding was granted.
Ethical approval
As per hospital Ethical Committee protocol, Systematic reviews and Meta analyses do not need approval as they are considered pre-approved.
Consent
Non applicable.
Author contribution
All authors were part of the study design, data collection, data analysis, interpretation, writing, editing, language proofing and resource checking of the paper.
Registration of research studies
1.Name of the registry: Research Registry.
2.Unique Identifying number or registration ID: reviewregistry921.
3.Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/register-now#user-systematicreviewmeta-analysesregistry/registerasystematicreviewmeta-analysidetails/5ed7b051f7f53c0015528bf8/
Guarantor
Sultan Zaher Alshehri.
Omar Safar Alshahrani.
Declaration of competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Ministry of Health and Aseer Hospital for providing us with the necessary access to make this review possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.07.015.
Contributor Information
Sultan Zaher Alshehri, Email: alshehri1491433@gmail.com, alshehris949@gmail.com.
Omar Safar Alshahrani, Email: omar2725@hotmail.com.
Nazal Ahmed Almsaoud, Email: Nazalahmad88@gmail.com.
Muhammad Ahmad Al-Ghamdi, Email: mohd.alghamdi1410@gmail.com.
Abdulaziz Mohammed Alqahtani, Email: dr.a.aziz555@gmail.com.
Muath Mohammed Almurayyi, Email: moathjony@gmail.com.
Ali Salem Autwdi, Email: aliautwdi@gmail.com.
Saeed Ahmed Al-Ghamdi, Email: damat707@gmail.com.
Mohammed Mesadef Zogan, Email: Zoogan@gmail.com.
Abdulrahim Mohammed Alamri, Email: da7m36@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.UPST Force. Screening for prostate cancer: US preventive services task force recommendation statement. Ann. Intern. Med. 2008;149(3):185. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C.K., Check D.P., Lortet‐Tieulent J., Laversanne M., Jemal A., Ferlay J. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int. J. Canc. 2016;138(6):1388–1400. doi: 10.1002/ijc.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamdy F.C., Donovan J.L., Lane J., Mason M., Metcalfe C., Holding P. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 4.Dhanasekaran S.M., Barrette T.R., Ghosh D., Shah R., Varambally S., Kurachi K. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 5.Loeb S., Vellekoop A., Ahmed H.U., Catto J., Emberton M., Nam R. Systematic review of complications of prostate biopsy. Eur. Urol. 2013;64(6):876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Tosoian J.J., Carter H.B., Lepor A., Loeb S. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat. Rev. Urol. 2016;13(4):205–215. doi: 10.1038/nrurol.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klotz L. Active surveillance for prostate cancer: patient selection and management. Curr. Oncol. 2010;17(Suppl 2):S11–S17. doi: 10.3747/co.v17i0.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klotz L., Vesprini D., Sethukavalan P., Jethava V., Zhang L., Jain S. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2015;33(3):272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 9.Bokhorst L.P., Valdagni R., Rannikko A., Kakehi Y., Pickles T., Bangma C.H. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur. Urol. 2016;70(6):954–960. doi: 10.1016/j.eururo.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Welty C.J., Cowan J.E., Nguyen H., Shinohara K., Perez N., Greene K.L. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J. Urol. 2015;193(3):807–811. doi: 10.1016/j.juro.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 11.Ploussard G., Beauval J.B., Lesourd M., Almeras C., Assoun J., Aziza R. Performance of systematic, MRI-targeted biopsies alone or in combination for the prediction of unfavourable disease in MRI-positive low-risk prostate cancer patients eligible for active surveillance. World J. Urol. 2020;38(3):663–671. doi: 10.1007/s00345-019-02848-x. [DOI] [PubMed] [Google Scholar]
- 12.Hsiang W., Ghabili K., Syed J.S., Holder J., Nguyen K.A., Suarez-Sarmiento A. European urology focus; 2019. Outcomes of Serial Multiparametric Magnetic Resonance Imaging and Subsequent Biopsy in Men with Low-Risk Prostate Cancer Managed with Active Surveillance. [DOI] [PubMed] [Google Scholar]
- 13.Vos L.J., Janoski M., Wachowicz K., Yahya A., Boychak O., Amanie J. Role of serial multiparametric magnetic resonance imaging in prostate cancer active surveillance. World J. Radiol. 2016;8(4):410–418. doi: 10.4329/wjr.v8.i4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberts A.R., Roobol M.J., Drost F.J.H., van Leenders G.J., Bokhorst L.P., Bangma C.H. Risk‐stratification based on magnetic resonance imaging and prostate‐specific antigen density may reduce unnecessary follow‐up biopsy procedures in men on active surveillance for low‐risk prostate cancer. BJU Int. 2017;120(4):511–519. doi: 10.1111/bju.13836. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):28. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassar M., Atakpo P., Kash M.J. Manual search approaches used by systematic reviewers in dermatology. J. Med. Libr. Assoc.: JMLA. 2016;104(4):302. doi: 10.3163/1536-5050.104.4.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunotto M., Zarate A.M., Bono A., Barra J.L., Berra S. Risk genes in head and neck cancer: a systematic review and meta-analysis of last 5 years. Oral Oncol. 2014;50(3):178–188. doi: 10.1016/j.oraloncology.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Grassi A., Carulli C., Innocenti M., Mosca M., Zaffagnini S., Bait C. New trends in anterior cruciate ligament reconstruction: a systematic review of national surveys of the last 5 years. Joints. 2018;(3):177–187. doi: 10.1055/s-0038-1672157. 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abyaa A., Khalidi Idrissi M., Bennani S. Learner modelling: systematic review of the literature from the last 5 years. Educ. Technol. Res. Dev. 2019;67(5):1105–1143. [Google Scholar]
- 20.Chen H.L., Chen X.Y., Wu J. The incidence of pressure ulcers in surgical patients of the last 5 years: a systematic review. Wounds : Comp. Clin. Res. Pract. 2012;24(9):234–241. [PubMed] [Google Scholar]
- 21.Culp M.B., Soerjomataram I., Efstathiou J.A., Bray F., Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020;77(1):38–52. doi: 10.1016/j.eururo.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Rawla P. Epidemiology of prostate cancer. World J. Oncol. 2019;10(2):63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taitt H.E. Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am. J. Men's Health. 2018;12(6):1807–1823. doi: 10.1177/1557988318798279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee A., Harmath C., Oto A. Abdominal Radiology; 2020. New Prostate MRI Techniques and Sequences. [DOI] [PubMed] [Google Scholar]
- 25.Steiger P., Thoeny H.C. Prostate MRI based on PI-RADS version 2: how we review and report. Canc. Imag. 2016;16(1):9. doi: 10.1186/s40644-016-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Heart L., Institute B. National Institutes of Health, Department of Health and Human Services; Bethesda: 2014. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies; pp. 103–111. [Google Scholar]
- 27.Chen K., Tay K.J., Law Y.M., Aydin H., Ho H., Cheng C. Outcomes of combination MRI-targeted and transperineal template biopsy in restaging low-risk prostate cancer for active surveillance. Asian J. Urol. 2018;5(3):184–193. doi: 10.1016/j.ajur.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberts A.R., Roobol M.J., Drost F.H., van Leenders G.J., Bokhorst L.P., Bangma C.H. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int. 2017;120(4):511–519. doi: 10.1111/bju.13836. [DOI] [PubMed] [Google Scholar]
- 29.Almeida G.L., Petralia G., Ferro M., Ribas C.A., Detti S., Jereczek-Fossa B.A. Role of multi-parametric magnetic resonance image and PIRADS score in patients with prostate cancer eligible for active surveillance according PRIAS criteria. Urol. Int. 2016;96(4):459–469. doi: 10.1159/000444197. [DOI] [PubMed] [Google Scholar]
- 30.Borkowetz A., Renner T., Platzek I., Toma M., Herout R., Baunacke M. Evaluation of magnetic resonance imaging/ultrasound-fusion biopsy in patients with low-risk prostate cancer under active surveillance undergoing surveillance biopsy. Urol. Int. 2018;100(2):155–163. doi: 10.1159/000486041. [DOI] [PubMed] [Google Scholar]
- 31.Hamoen E.H.J., Hoeks C.M.A., Somford D.M., van Oort I.M., Vergunst H., Oddens J.R. Value of serial multiparametric magnetic resonance imaging and magnetic resonance imaging-guided biopsies in men with low-risk prostate cancer on active surveillance after 1 Yr follow-up. Eur. Urol. Focus. 2019;5(3):407–415. doi: 10.1016/j.euf.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto T., Rahul K., Takeda T., Benfante N., Mulhall J.P., Hricak H. Prostate magnetic resonance imaging findings in patients treated for testosterone deficiency while on active surveillance for low-risk prostate cancer. Urol. Oncol. 2016;34(12):530. doi: 10.1016/j.urolonc.2016.07.004. e9-.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osses D.F., Drost F.H., Verbeek J.F.M., Luiting H.B., van Leenders G., Bangma C.H. Prostate cancer upgrading with serial prostate magnetic resonance imaging and repeat biopsy in men on active surveillance: are confirmatory biopsies still necessary? BJU Int. 2020 doi: 10.1111/bju.15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoots I.G., Osses D.F., Drost F.H., Verbeek J.F.M., Remmers S., van Leenders G. Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease. Transl. Androl. Urol. 2018;7(1):132–144. doi: 10.21037/tau.2017.12.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson J., Lawrentschuk N., Frydenberg M., Thompson L., Stricker P. The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int. 2013;112(Suppl 2):6–20. doi: 10.1111/bju.12381. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y., Reynolds H.M., Parameswaran B., Wraith D., Finnegan M.E., Williams S. Multiparametric MRI and radiomics in prostate cancer: a review. Australas. Phys. Eng. Sci. Med. 2019;42(1):3–25. doi: 10.1007/s13246-019-00730-z. [DOI] [PubMed] [Google Scholar]
- 37.Babaian R.J., Toi A., Kamoi K., Troncoso P., Sweet J., Evans R. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J. Urol. 2000;163(1):152–157. [PubMed] [Google Scholar]
- 38.Presti J.C., Jr., O'Dowd G.J., Miller M.C., Mattu R., Veltri R.W. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J. Urol. 2003;169(1):125–129. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 39.Eskew L.A., Bare R.L., McCullough D.L. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J. Urol. 1997;157(1):199–202. discussion -3. [PubMed] [Google Scholar]
- 40.Zamecnik P., Schouten M.G., Krafft A.J., Maier F., Schlemmer H.P., Barentsz J.O. Automated real-time needle-guide tracking for fast 3-T MR-guided transrectal prostate biopsy: a feasibility study. Radiology. 2014;273(3):879–886. doi: 10.1148/radiol.14132067. [DOI] [PubMed] [Google Scholar]
- 41.Vourganti S., Rastinehad A., Yerram N., Nix J., Volkin D., Hoang A. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J. Urol. 2012;188(6):2152–2157. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villers A., Puech P., Mouton D., Leroy X., Ballereau C., Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J. Urol. 2006;176(6 Pt 1):2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Delongchamps N.B., Rouanne M., Flam T., Beuvon F., Liberatore M., Zerbib M. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011;107(9):1411–1418. doi: 10.1111/j.1464-410X.2010.09808.x. [DOI] [PubMed] [Google Scholar]
- 44.Lemaitre L., Puech P., Poncelet E., Bouyé S., Leroy X., Biserte J. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur. Radiol. 2009;19(2):470–480. doi: 10.1007/s00330-008-1153-0. [DOI] [PubMed] [Google Scholar]
- 45.Puech P., Potiron E., Lemaitre L., Leroy X., Haber G.P., Crouzet S. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74(5):1094–1099. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- 46.Moore C.M., Robertson N.L., Arsanious N., Middleton T., Villers A., Klotz L. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur. Urol. 2013;63(1):125–140. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui M.M., Rais-Bahrami S., Turkbey B., George A.K., Rothwax J., Shakir N. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. J. Am. Med. Assoc. 2015;313(4):390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe Y., Terai A., Araki T., Nagayama M., Okumura A., Amoh Y. Detection and localization of prostate cancer with the targeted biopsy strategy based on ADC map: a prospective large-scale cohort study. J. Magn. Reson. Imag. : JMRI. 2012;35(6):1414–1421. doi: 10.1002/jmri.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Augustin H., Fritz G.A., Ehammer T., Auprich M., Pummer K. Accuracy of 3-Tesla magnetic resonance imaging for the staging of prostate cancer in comparison to the Partin tables. Acta radiol. (Stockholm, Sweden. 1987;50(5):562–569. doi: 10.1080/02841850902889846. 2009. [DOI] [PubMed] [Google Scholar]
- 50.Renard-Penna R., Rouprêt M., Comperat E., Ayed A., Coudert M., Mozer P. Accuracy of high resolution (1.5 tesla) pelvic phased array magnetic resonance imaging (MRI) in staging prostate cancer in candidates for radical prostatectomy: results from a prospective study. Urol. Oncol. 2013;31(4):448–454. doi: 10.1016/j.urolonc.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Fütterer J.J., Heijmink S.W., Scheenen T.W., Jager G.J., Hulsbergen-Van de Kaa C.A., Witjes J.A. Prostate cancer: local staging at 3-T endorectal MR imaging--early experience. Radiology. 2006;238(1):184–191. doi: 10.1148/radiol.2381041832. [DOI] [PubMed] [Google Scholar]
- 52.Barentsz J.O., Richenberg J., Clements R., Choyke P., Verma S., Villeirs G. ESUR prostate MR guidelines. Eur. Radiol. 2012;22(4):746–757. doi: 10.1007/s00330-011-2377-y. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenkrantz A.B., Scionti S.M., Mendrinos S., Taneja S.S. Role of MRI in minimally invasive focal ablative therapy for prostate cancer. AJR Am. J. Roentgenol. 2011;197(1):W90–W96. doi: 10.2214/AJR.10.5946. [DOI] [PubMed] [Google Scholar]
- 54.Tan N., Margolis D.J., Lu D.Y., King K.G., Huang J., Reiter R.E. Characteristics of detected and missed prostate cancer foci on 3-T multiparametric MRI using an endorectal coil correlated with whole-mount thin-section histopathology. AJR Am. J. Roentgenol. 2015;205(1):W87–92. doi: 10.2214/AJR.14.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.