Highlights
-
•
Transdiagnostic evidence for neural disruption in psychotic hallucinations.
-
•
Hallucinations associated increased connectivity in auditory association cortex.
-
•
Brain regions for auditory-verbal language comprehension linked to hallucinations.
-
•
Interhemispheric connectivity alterations related to subgroup-specific findings.
-
•
Hallucinations link to auditory rs-connectivity tested in 243 psychosis patients.
Abbreviations: AUD, core auditory cortex; AAC, unimodal auditory association cortex; BDP, bipolar disorder with psychotic features; B-SNIP, Bipolar Schizophrenia Network for Intermediate Phenotypes study
Keywords: Hallucination, Psychosis, Functional connectivity, rs-fMRI (Resting State fMRI), Schizophrenia, Bipolar
Abstract
Background
Auditory hallucinations are prevalent across the major psychotic disorders, but their underlying mechanism is poorly understood. Limited prior work supports a hypothesis of altered auditory/language brain systems. To more definitively assess this, we examined whether alterations in resting state connectivity of auditory and language cortices are associated with hallucination severity in a large sample of individuals in the schizo-bipolar spectrum.
Methods
Whole brain resting state connectivity of auditory and language cortex (primary auditory cortex, unimodal auditory association cortex, Wernicke’s area [speech and heteromodal association cortex] and Broca’s area [speech production motor]) was evaluated for 243 subjects with schizophrenia, schizoaffective, or bipolar disorder with psychosis and 186 healthy controls from the Bipolar Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Regression analyses were conducted to evaluate whether resting state connectivity of auditory and language cortex was a significant predictor of current overall hallucination severity (information about specific modality of hallucinations experienced was not available).
Results
Increased connectivity between lower and higher order regions of left temporal-parietal auditory/language processing cortex was associated with worse hallucination severity for all psychosis patients. Additionally, within bipolar subjects, increased interhemispheric connectivity between higher order temporal-parietal auditory/language regions was related to greater hallucination severity. When patients were categorized by B-SNIP biomarker-based Biotype groups, interhemispheric connectivity between left auditory association cortex and right core auditory cortex was related to greater hallucination severity for Biotype 1 patients. Exploratory analyses resulted in different patterns of connectivity of auditory/language cortex in patients and controls, unrelated to current hallucination severity.
Conclusions
Although the findings cannot be precisely attributed to auditory hallucination severity or possible differences in such experiences between groups, increased connectivity among the left hemisphere auditory and receptive language cortex may represent a significant factor contributing to hallucination severity across psychotic disorders, and additional subgroup specific connectivity alterations may also be present.
1. Introduction
Auditory hallucinations are highly prevalent in psychotic disorders, yet they remain poorly understood. Commonly experienced as voices, auditory hallucinations are often uncontrollable and usually negative, features that make them a significant source of distress. In about one-third of individuals with schizophrenia who have auditory hallucinations, the symptom is antipsychotic medication treatment resistant, and even when treatment is considered efficacious, it may not correspond to complete resolution (Shergill et al., 1998, Sommer et al., 2012). Hence, increased understanding of hallucinations may improve our understanding of psychotic disorders in a manner that is urgently needed.
Neurocognitive models of auditory hallucinations have been proposed that implicate abnormality in auditory and/or language processing neural systems in the left hemisphere, consistent with the dominant auditory/verbal nature of the symptom. The models include hypersensitivity or activation of auditory cortex including primary and association auditory cortex, and/or adjacent polymodal association cortex of the inferior parietal lobe, dysregulation of receptive auditory cortex by disrupted top-down influences especially from frontal speech areas, altered interhemispheric communication, misattribution of internal speech, and improper engagement of episodic memory and emotion systems (Aleman et al., 2003, Ford and Mathalon, 2005, Allen et al., 2008, Hugdahl, 2009, Frith, 2005, Jones, 2010, Steinmann et al., 2014, Ćurčić-Blake et al., 2017).
Neuroimaging studies have supported the general hypothesis of auditory hallucinations being associated with altered auditory and language systems and their regulation. Structural neuroimaging meta-analyses have found reduced grey matter volume in the right superior temporal gyrus associated with greater hallucination severity (Barta et al., 1990, Palaniyappan et al., 2012, Modinos et al., 2013). Meta-analyses of active hallucination studies, in which participants report hallucinatory experiences in real time during functional neuroimaging, have reported activation in Broca’s area and auditory cortex in individuals with schizophrenia and other psychosis-spectrum conditions (Jardri et al., 2011, Kompus et al., 2011, Kühn and Gallinat, 2012, van Lutterveld et al., 2013, Zmigrod et al., 2016). While such “hallucination-capture” work has been useful, it is limited by small nonrepresentative samples of those insightful enough to report auditory hallucinations occurring in the moment. Further, many of these studies focus on diagnoses or traits related to, but not including, the major psychotic diagnoses. Hence, how well these findings generalize to the schizo-bipolar disorder spectrum is unclear.
Another approach is to assess whether auditory/language system alterations can be discerned in resting state brain activity in association with hallucination severity. Resting state functional magnetic resonance imaging (rs-fMRI) allows for characterization of patterns of intrinsic connectivity of neural systems. These may be altered in ways related to auditory hallucinations or an increased vulnerability to experiencing them. Seed-based connectivity analysis is a common rs-fMRI analysis method that assesses brain networks connected to selected brain regions (seeds). Limited work using this approach to study hallucinations has implicated altered connectivity in left posterior temporal regions mediating language perception, as well as inferior frontal, parietal, limbic and striatal regions, but with mixed increased or decreased connectivity findings (Gavrilescu et al., 2010, Shinn et al., 2013, Oertel-Knöchel et al., 2014, Zhang et al., 2018). The inconsistency may be due in part to dissimilar methodologies, varying samples (e.g., ever vs. never hallucinators, current vs. not current hallucinators, non-ill voice hearers, etc.) and small samples (none report >30 subjects in a group). Assessment approaches for hallucinations are also inconsistent, ranging from binary to quantitative severity ratings. In sum, prior work has been promising in terms of supporting a role for altered auditory/language systems underlying auditory hallucinations in major psychotic disorders, but more work is needed.
A useful way forward may be to study auditory/language connectivity in a larger, representative sample. Such an opportunity exists in the Bipolar Schizophrenia Network for Intermediate Phenotypes (B-SNIP) study (Tamminga et al., 2014), a multi-site study of schizophrenia, schizoaffective, and bipolar disorder with psychotic features (BDP). This data can align with prior work that has focused primarily on schizophrenia and can extend it to bipolar disorder. Phenomenologically, there have been more similarities than differences noted for auditory hallucinations when comparing schizophrenia, schizoaffective disorder, and BDP (Toh et al., 2015, Waters and Fernyhough, 2017). As these groups have significant biological and clinical overlap including treatment response (Badner and Gershon, 2002, Purcell et al., 2009, Keshavan et al., 2011), the same neural circuitry alterations may underlie hallucinations across these disorders. B-SNIP has proposed a set of three subgroups within the schizo-bipolar spectrum, called Biotypes 1–3, based on neurobiological distinctions (Clementz et al., 2016). Different patterns of neural alterations may be associated with auditory hallucinations in these subgroups, in parallel to their other neurophysiological differences. The present study aimed to assess the association of hallucination severity to auditory/language neural system connectivity and to test for both common and group-specific alterations (diagnostic groups and B-SNIP Biotype groups). We also tested for connectivity differences of auditory/language regions in patients with the psychotic disorders relative to healthy controls to characterize the full spectrum of connectivity alterations of these regions irrespective of hallucination severity.
2. Methods
2.1. Participants
The recruitment and assessment approaches used in the B-SNIP study are described in detail elsewhere (Tamminga et al., 2013, Tamminga et al., 2014). Psychosis patients were clinically characterized, and all subjects had a panel of biomarkers assessed including the neuroimaging data reported here. The psychosis patients and healthy volunteers were recruited using local advertising. Inclusion and exclusion criteria were age 18–60, ability to provide written informed consent, estimated IQ > 60, no current substance abuse disorders or major neurological/cognitive/cerebrovascular-affecting disorders, and no significant head trauma history. Healthy controls had no personal history of any psychotic disorder or first-degree relative with schizophrenia, schizoaffective disorder, or mood disorder.
2.2. Ethics statement
The study was approved by the Biological Sciences Division IRB at the University of Chicago, and written informed consent was obtained for all research participants.
2.3. Clinical assessments
Trained clinical raters confirmed DSM-IV diagnosis of schizophrenia, schizoaffective, or BDP using the SCID-IV (First et al., 1996). Raters also administered the Positive and Negative Syndrome Scale (PANSS) which assesses the severity of a range of psychotic symptoms in the last week (Kay et al., 1987). For hallucination severity, we used the score for the “Hallucinatory Behavior” PANSS item 3 (score range 1–7). While the score is not specific to any hallucination sensory modality, it references auditory hallucination aspects in its rating criteria more frequently than other sensory modalities, and it is reasonable to assume the majority of variance represents auditory hallucinations given they are the most common hallucination sensory modality among the three disorders (Baethge et al., 2005, Shinn et al., 2012).
2.4. Imaging data acquisition and preprocessing
Details of resting state and accompanying T1-weighted structural scans for this study have been described elsewhere (Meda et al., 2014). Briefly, subjects underwent a 5-minute rs-fMRI scan in 3 T scanners with closely aligned acquisition parameters. Subjects were instructed to remain still, stay awake, and keep their eyes focused on a crosshair for the scan’s duration. Wakefulness was confirmed with the subjects following the scan. To allow for scanner stabilization, the initial 6 images were discarded. Using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012), the time series was aligned, slice-time corrected, normalized to MNI space, and smoothed at 8 mm, with 2 × 2 × 2 mm resampled voxel size. Denoising (per aCompCor as implemented in CONN) included regression of white matter and CSF signal, scrubbed volumes, motion + 1st order derivatives, a linear and 2nd order polynomial drift term, and applying a bandpass filter (0.008–0.1 Hz) (Goto et al., 2015). Subjects with visually identified artifacts and scan-to-scan composite motion >3 mm were excluded from analyses.
2.5. Auditory/language cortex connectivity maps
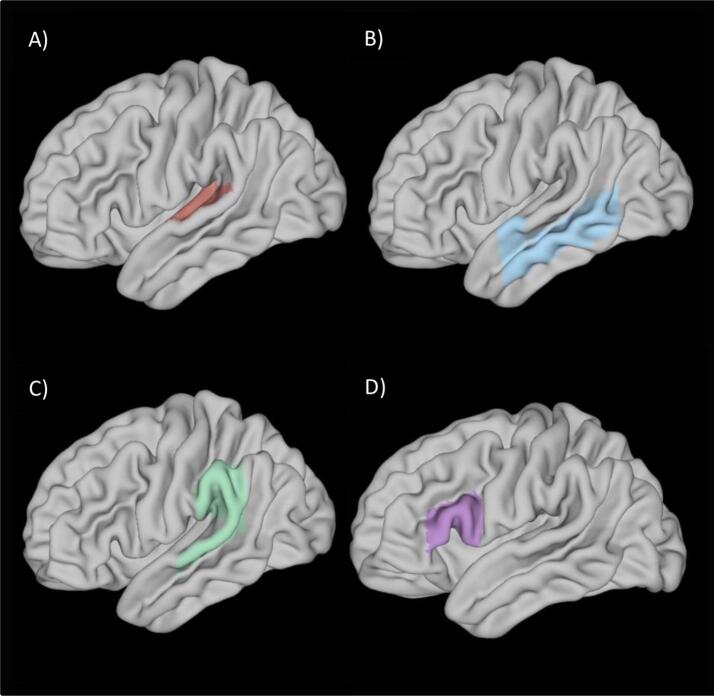
We created whole brain connectivity maps for auditory/language cortex for each subject. Seeds for connectivity maps were selected based on recent auditory/language system reviews. Seeds were left and right core auditory cortex (AUD) (Moerel et al., 2014) encompassing cortex traditionally considered primary and unimodal auditory association cortex (Heschel’s gyrus, planum polare, planum temporale), extended left and right unimodal auditory association cortex (AAC; anterior superior temporal gyrus, middle temporal gyrus), Broca’s speech motor area (left inferior frontal gyrus pars triangularis, left pars operculum) and Wernicke’s area which encompassed cortex specialized for phonological representation as well as heteromodal association functions (left posterior superior temporal gyrus, left supramarginal gyrus, left parietal operculum) (Ardila et al., 2016, Tremblay and Dick, 2016, Binder, 2017). Masks for each seed were generated using the Harvard Oxford Probabilistic Atlas thresholded at 25% (Fig. 1), yielding masks comprised of voxels estimated to be 25% or higher likelihood of belonging to each intended region. We selected a 25% threshold to be on the side of inclusivity across varying individual anatomy of the subjects (Mazziotta et al., 1995). Timeseries across voxels within each mask were averaged and then correlated with all remaining voxels in the brain to create a whole brain functional connectivity map for each seed region. Maps were converted to Fisher z- scores.
Fig. 1.
Resting State Connectivity Seeds. Harvard-Oxford atlas regions used for each: A) Core auditory cortex (AUD): Planum temporale, Planum polare, Heschl’s gyrus. B) Unimodal Auditory Association Cortex (AAC): Superior temporal gyrus, anterior division; Middle temporal gyrus, anterior division; Middle temporal gyrus, posterior division; Middle temporal gyrus, temporo-occipital division. C) Wernicke’s Area: Superior temporal gyrus, posterior division; Supramarginal gyrus, anterior division; Supramarginal gyrus, posterior division; Parietal operculum. D) Broca’s Area: Inferior frontal gyrus pars triangularis and Inferior frontal gyrus pars opercularis.
2.6. Group analysis
Using the CONN toolbox, each set of connectivity z-score maps was entered into a separate multiple linear regression with the PANSS hallucination severity item score as an independent predictor variable. Age, sex, recruitment site, antipsychotic medication dose expressed as chlorpromazine equivalents (CPZ) (Andreasen et al., 2010) and motion via the mean framewise displacement (FDpower; Power et al., 2012) were additional predictors of no interest. We evaluated results for a main effect across all patients, and for interaction with group (separate analyses used diagnostic group or Biotype group). Significance was set at p < 0.05, familywise corrected, obtained by using a voxel threshold of p ≤ 0.001 and cluster pFWE < 0.0083, accounting for six seed connectivity analyses being conducted. Regions with significant group interaction effects were followed up with pairwise comparisons. Finally, comparison of the six connectivity map sets between patient groups and healthy controls were conducted using ANCOVAs (age, sex, site, FDpower covariates) as supplemental analyses.
3. Results
3.1. Demographic and clinical characteristics
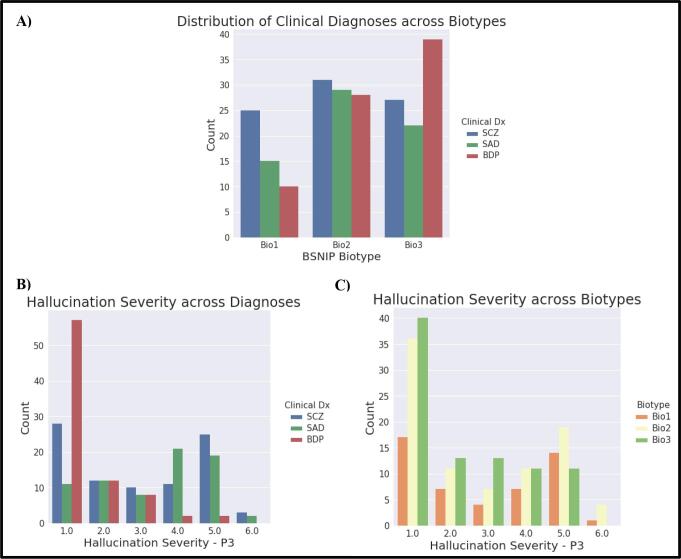
In total, 243 psychosis patients and 186 healthy controls were included, while 39 patients and 27 healthy controls from the BSNIP study who had imaging data were excluded due either to excessive motion or image artifacts (see Supplemental Table 1). All diagnoses were distributed across BSNIP Biotypes (Fig. 2a). Each subgroup spanned a range of hallucination severity (Table 1; Fig. 2b, c; see also Supplemental Fig. 1).
Fig. 2.
Sample Distributions. A) Distribution of clinical diagnoses across BSNIP Biotypes. B) Distribution of PANSS P3 Hallucination Severity score across diagnoses (Dx) and C) Biotypes. Abbreviations: BPD – Bipolar Disorder, Bio1 – Biotype 1, Bio2 – Biotype 2, Bio3 – Biotyp3, Dx – Diagnosis, HC – Healthy Controls, P3 – Hallucination severity rating, SCZ – Schizophrenia, SADP – Schizoaffective Disorder.
Table 1.
Demographics and Clinical Characteristics for Included Subjects.
| SCZ | SAD | BPD | HC | P-value | Post-hoc | |
|---|---|---|---|---|---|---|
| N | 89 | 73 | 81 | 186 | ||
| Male/Female | 32/57 | 29/44 | 22/59 | 72/114 | <0.001 | *a, d, e |
| Avg. Age (y) | 33.5 (11.4) | 37.6 (11.7) | 35.5 (12.4) | 37.8 (12.4) | 0.031 | *a |
| Avg. Daily CPZ | 438.0 (375.5) | 451.5 (387.9) | 269.0 (322.9) | 0.002 | *e, f | |
| PANSS Hallucination | 3.0 (1.7) | 3.4 (1.5) | 1.5 (1.0) | <0.001 | *e, f | |
| PANSS Positive | 16.9 (5.5) | 18.4 (4.3) | 12.9 (4.1) | <0.001 | *e, f | |
| PANSS Negative | 15.8 (6.1) | 15.9 (4.4) | 12.2 (3.7) | <0.001 | *e, f | |
| PANSS General | 31.7 (9.0) | 36.2 (8.6) | 29.2 (7.7) | <0.001 | *d, f | |
| PANSS Total | 64.4 (17.9) | 70.6 (14.8) | 54.4 (12.4) | <0.001 | *d, e, f | |
| GAF | 49.1 (12.7) | 46.7 (10.4) | 59.4 (13.0) | 85.9 (6.8) | <0.001 | * a, b, c, e, f |
| mFDpower (mm) | 0.18 (0.11) | 0.25 (0.15) | 0.60 (0.41) | 0.17 (0.10) | <0.001 | *b, d, f |
| Biotype 1 | Biotype 2 | Biotype 3 | HC |
|||
| N | 50 | 88 | 88 | 186 | ||
| Male/Female | 26/24 | 34/54 | 42/46 | 72/114 | 0.310 | NS |
| Avg. Age (y) | 34.0 (10.8) | 35.8 (12.0) | 34.9 (11.8) | 37.8 (12.4) | 0.147 | NS |
| Avg. Daily CPZ | 523.1 (405.2) | 441.3 (433.7) | 279.5 (258.4) | <0.001 | *k, l | |
| PANSS Hallucination | 2.9 (1.7) | 2.8 (1.8) | 2.3 (1.5) | 0.120 | NS | |
| PANSS Positive | 16.7 (5.6) | 16.3 (5.5) | 15.1 (4.4) | 0.209 | NS | |
| PANSS Negative | 16.1 (5.3) | 15.3 (5.2) | 13.2 (4.9) | 0.008 | *k, l | |
| PANSS General | 32.0 (9.2) | 33.5 (9.5) | 30.7 (7.9) | 0.160 | NS | |
| PANSS Total | 64.8 (17.0) | 65.1 (17.6) | 59.1 (14.1) | 0.062 | NS | |
| GAF | 47.6 (11.2) | 49.6 (13.8) | 55.8 (13.1) | 85.9 (6.8) | <0.001 | *g, h, i, k, l |
| mFDpower (mm) | 0.18 (0.10) | 0.25 (0.15) | 0.18 (0.11) | 0.17 (0.10) | <0.001 | * h, j, l |
Abbreviations: BPD – Bipolar Disorder, Bio1 – Biotype 1, Bio2 – Biotype 2, Bio3 – Biotyp3, CPZ – Chlorpromazine Equivalents, mFDpower – mean Framewise Displacement power, GAF – Global Assessment of Function, HC – Healthy Control, NS – Not Significant, PANSS -Positive and Negative Syndrome Scale, SAD – Schizoaffective Disorder, SCZ – Schizophrenia. Post-Hoc tests were Bonferroni-corrected. Values in parentheses are standard deviations.
a SCZ vs HC
b SAD vs HC
c BPD vs HC
d SCZ vs BPD
e SCZ vs SAD
f SAD vs BPD
g Bio1 vs HC
h Bio2 vs HC
i Bio3 vs HC
j Bio1 vs Bio2
k Bio1 vs Bio3
l Bio2 vs Bio3
3.2. Hallucination associated connectivity
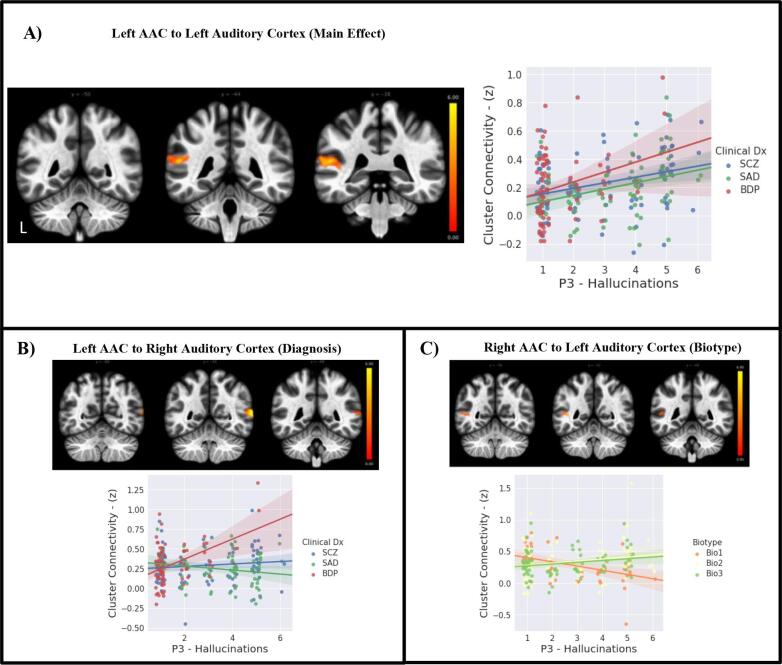
Greater hallucination severity was predicted by greater connectivity between left AAC and a cluster including portions of left core auditory cortex and Wernicke’s Area (Table 2, Fig. 3a). When diagnosis group was included in the analysis, there was an interaction for connectivity of the left AAC to a cluster that was in more posterior portions of right unimodal auditory association cortex. Post-hoc analyses revealed that greater hallucination severity was associated with greater connectivity between these brain regions for the bipolar group compared to the schizoaffective group (Fig. 3b). Next, Biotype group was used in the analysis. There was an interaction for Biotype group with connectivity of the right AAC to a cluster including portions of left AAC and Wernicke’s area. Post-hoc analyses revealed greater hallucination severity associated with lower connectivity between these brain regions for Biotype 1 than for Biotype 2 (Fig. 3c).
Table 2.
Auditory/Language Cortex Connectivity Associated with Hallucination Severity (top) or Distinguishing Patients from Healthy Controls (bottom).
| Cluster Size (8 mm3 voxels) | Peak Voxel (x, y, z) | Location of Cluster | pFWE | |
|---|---|---|---|---|
| Connectivity associated with Hallucination Severity in Patients | ||||
| Main Effect | ||||
| Left AAC seed | 439 | −54, −44, 18 | Left PT-PO-SMG | 0.000300 |
| Biotype Interaction | ||||
| Right AAC seed | 265 | −44, −52, 10 | Left MTG-SMG-PT | 0.003759 |
| *Bio1 vs Bio2 | 416 | −44, −54, 08 | Left MTG-SMG-AG-PT | 0.014000 |
| Diagnosis Interaction | ||||
| Left AAC seed | 273 | 66, −54, 10 | Right MTG-AG | 0.002981 |
| *SADP vs BDP | 354 | 66, −54, 10 | Right MTG-AG | 0.001435 |
| Connectivity Differences between Patients vs Healthy Controls | ||||
| Main Effect | ||||
| Right AUD seed | 267 | −50, −60, 38 | Left Angular Gyrus | 0.007474 |
| Right AAC seed | 634 | −36, −30, 40 | Left Posterior Central Gyrus | 0.000015 |
| Diagnosis F-test | ||||
| Left AAC seed | 232 | 30, −96, −02 | Right Occipital Pole | 0.005091 |
| *HC vs SAD | 316 | 30, −94, −02 | Right Occipital Pole | 0.020000 |
| Right AAC seed | 262 | −42–24, 34 | Left Post-Central Gyrus | 0.002625 |
| *HC vs SCZ | 378 | −44, −22, 36 | Left Post-Central Gyrus | 0.000686 |
Abbreviations: AAC – Auditory Association Cortex, AG - Angular Gyrus, AUD – Core Auditory Cortex, BPD – Bipolar Disorder, Bio1 – Biotype 1, Bio2 – Biotype 2, Bio3 – Biotyp3, HC – Healthy Controls, MTG – Middle Temporal Gyrus, pFWE – p-value Family Wise Error corrected, PO – Planum Operculum, PT – Planum Temporale, SCZ – Schizophrenia, SAD – Schizoaffective Disorder, SMG – Superior Marginal Gyrus. *Pairwise comparison, among all possible pairwise comparisons, with significant finding spatially overlapping cluster with significant interaction.
Fig. 3.
Auditory-Language Connectivity Associated with Hallucination Severity. Scatterplots depict extracted mean connectivity from each cluster for each group, fit linearly with a 95% confidence interval for display purposes. Left side of brain images depicts left hemisphere. A) Main Effect: Greater hallucination severity in psychosis patients predicts increased connectivity of left AAC to adjacent portions of left auditory cortex. B) Interaction with Diagnosis: Greater hallucination severity in BPD relative to SAD predicts increased connectivity of left AAC to right auditory cortex. C) Interaction of Biotype: Greater hallucination severity in Biotype 1 relative to Biotype 2 predicts reduced connectivity of right AAC to posterior portions of left auditory/language cortex. Abbreviations: AAC – Auditory Association Cortex, BPD – Bipolar Disorder with Psychosis, Bio1 – Biotype 1, Bio2 – Biotype 2, Bio3 – Biotyp3, Dx – Diagnosis, P3 – Hallucination severity rating, SCZ – Schizophrenia, SAD – Schizoaffective Disorder.
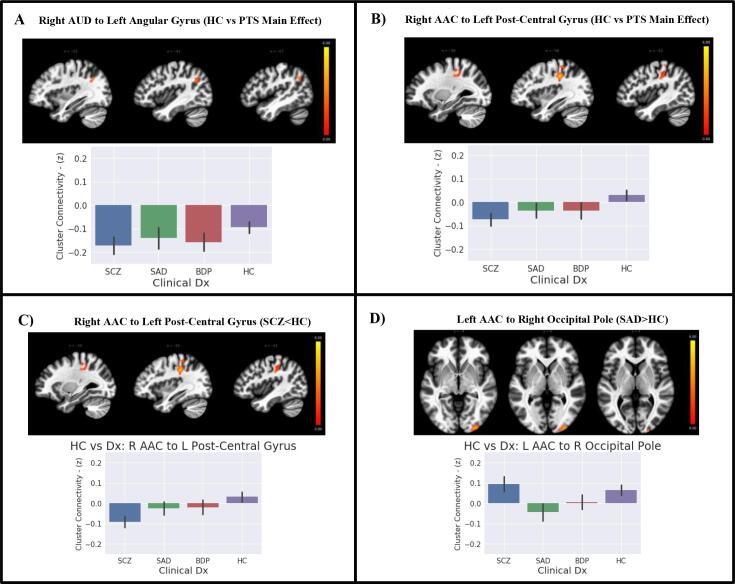
3.3. Connectivity differences of auditory/language cortex between psychosis patients and healthy controls
Results of ANCOVAs to compare the connectivity maps of psychosis patients to those of healthy controls (Table 2) revealed that patients had significantly decreased connectivity between right AUD and a cluster in the left angular gyrus/lateral occipital heteromodal cortex (Fig. 4a) and right AAC to left post-central gyrus (Fig. 4b). When assessing for interaction with group status, only the schizophrenia group had significantly decreased connectivity of right AAC to the post-central gyrus compared to healthy controls (Fig. 4c). This cluster spatially overlapped the main effect cluster of lower connectivity between right AAC and post-central gyrus. There was an additional cluster showing interaction with group, where the schizoaffective group had significantly increased connectivity of right AAC to the right occipital pole compared to healthy controls (Fig. 4d). There was no interaction with Biotype group status.
Fig. 4.
Auditory-Language Connectivity in Psychosis Patients Compared to Healthy Controls. Bar plots shows extracted mean connectivity for respective significant clusters. Error bars are 95% confidence intervals. A) Main Effect: Significant decrease in connectivity of right AUD with left angular gyrus in all patients relative to controls. B) Main Effect: Significant decrease in connectivity of right AAC with left post-central gyrus in psychosis patients relative to healthy controls. C) Diagnosis Effect: Significant decrease in connectivity of right AAC with left post-central gyrus for schizophrenia patients relative to healthy controls (highly overlapping cluster shown in B). D) Diagnosis Main Effect: Significant decrease in connectivity of left AAC with occipital pole in schizoaffective patients relative to healthy controls. Abbreviations: AAC – Auditory Association Cortex, AUD – Core Auditory Cortex, BPD – Bipolar Disorder, Dx – Diagnosis, HC – Healthy Control, PTS – Patients, SCZ – Schizophrenia, SAD – Schizoaffective Disorder.
3.4. Interest in some of the “covariates of no interest”
We conducted supplemental analyses to explore for the influence of factors that were used as covariates, but whose association with the data may shed further light on the findings. First, we assessed antipsychotic medication association with auditory/language connectivity. We conducted six additional regressions (one for each seed) in the total patient sample using the connectivity maps to predict CPZ, using all other covariates as in the main analyses. Results were negative except for one cluster not overlapping with other findings (the cluster showed that greater CPZ associated with stronger connectivity between left AUD and right occipital lobe; pFWE = 0.003; x = 12, y = -76, z = 42; 296 8 mm3 voxels). Next, given interest in sex differences within psychosis (Abel et al., 2010), we assessed for any by re-running the original main regressions of hallucination severity and connectivity in the patients, but instead of sex as a covariate, we added it as a factor. We did not find any significant sex effect on the association between hallucination severity and connectivity.
4. Discussion
This study aimed to investigate the relationship of hallucination severity to resting state connectivity of cortex involved in auditory and language processing using the largest transdiagnostic sample to date. It also sought to investigate potential heterogeneity of these relationships in accordance with psychosis subgroups. We suggest three primary observations from our study. 1) Across all results, connectivity of unimodal auditory association cortex to other unimodal as well as polymodal temporal-parietal auditory/language regions, and not Broca’s or other frontal areas, was associated with hallucination severity. 2) There was a common increase in connectivity within left hemisphere temporal-parietal auditory/receptive language regions for all psychosis patients as hallucination severity increased, which is consistent with the more common auditory-verbal nature of hallucinations given left hemisphere specialization for language. 3) There were additional subgroup-specific findings involving interhemispheric connectivity alterations.
4.1. Transdiagnostic observations: increased left hemisphere auditory/language connectivity
Within our full psychosis sample, connectivity of the auditory/language cortex was associated with hallucination severity, as predicted. Specifically, we found greater hallucination severity was related to stronger functional connectivity of the left auditory unimodal association cortex seed with an ipsilateral cluster of voxels including lower order core auditory cortex and an adjacent portion of heteromodal association cortex. This is consistent with the hypothesis that the aberrant perceptual experience of hallucinations, most frequently auditory/verbal in nature, may be due to hyperactivity in left hemisphere brain areas responsible for auditory and language processing. Our results implicate cortex that processes more basic features of sound (core auditory cortex), higher order unimodal and language-specific processing (auditory association cortex) and integration of auditory information with other modalities in heteromodal cortex (located in posterior superior temporal gyrus and inferior parietal lobule). These regions normally co-activate when listening to voices. Abnormally high correlation in the activity of these regions may yield an experience of hearing sounds, and perhaps voices in particular, in the absence of external stimuli.
This result is consistent with prior “hallucination capture” studies showing increased activity in these areas during self-reported hallucinations (Jardri et al., 2011), and extends it to the general, chronically ill population. It is also consistent with Shinn et al. (2013), who conducted a similar analysis of resting state connectivity that included covarying for antipsychotic medication. Shinn et al. reported additional associations to hallucinations not found in the present study, such as in Broca’s area. However, the Shinn et al study relied on liberal statistical thresholding, which puts it at risk for false positives (Eklund et al, 2016). Overall, our finding serves as the most substantive confirmation to date that hyper-connectivity of left hemisphere auditory/language systems is involved in hallucinations in psychotic disorders. As there were no connectivity alterations from frontal or other regions such as insula with the association cortex we studied, the finding supports an interpretation of a receptive/perceptual system pathophysiology, rather than a problem of poor regulation or aberrant input from executive or other modulating regions, at least as can be detected with the connectivity analysis approach we used.
4.2. Subgroup effects
When comparing groups by diagnosis for connectivity patterns associating with hallucination severity, the BDP group had unique findings. These observations are fairly novel, as no functional imaging study, to our knowledge, has directly addressed hallucinations in bipolar disorder previously. Greater hallucination severity was associated with greater connectivity of the left AAC to a cluster including right middle temporal and angular gyrus, including the right hemisphere homologue of Wernicke’s area. This generally confirms the suggestion that auditory hallucinations may occur due to altered connectivity of auditory/language areas and extends it to cross-hemisphere auditory cortex hyperconnectivity for BDP. A recent review concluded there is evidence to support more interhemispheric alteration in bipolar disorder than in schizophrenia, though this was based on white matter studies (O’Donoghue et al., 2017). Some have reported interhemispheric functional alterations in bipolar disorder (Reite et al., 2009, Wang et al., 2015), but have not addressed these in relation to symptom severity. We note that while this observation for BDP was in relation to schizoaffective disorder, the general pattern was one in which schizophrenia and schizoaffective had near-zero connectivity values between the areas, whereas BDP had increased connectivity.
In further subgroup analyses, we assessed whether any connectivity alterations may be specific to BSNIP Biotype groups. Biotype groups were constructed previously in this sample based on cognitive and electrophysiological measures separate from MRI data (Clementz et al., 2016). Further, each Biotype group is comprised of individuals from each of the diagnostic groups, offering an alternative subgrouping organization relative to traditional diagnostic categorization. We found that for Biotype 1, greater hallucination severity was associated with decreased connectivity between the left and right hemisphere unimodal auditory processing cortices. Specifically, there was lower connectivity between the right AAC seed and a cluster including left unimodal auditory association cortex. This decreased connectivity for Biotype 1 associating with increased hallucination severity was significantly different from the connectivity pattern for Biotype 2, which had an increasing trend in connectivity strength as hallucination severity increased. Biotype 3 was not distinguished from the other two groups but displayed an increasing connectivity trend between these regions like Biotype 2. Biotype 1 is characterized by more severe deficits overall relative to the other Biotypes - more pervasive gray matter loss, more profound cognitive impairment, and reduced sensorimotor responsivity compared to Biotype 2 and Biotype 3 (Clementz et al., 2016, Ivleva et al., 2017, Tamminga et al., 2017). It is therefore conceivable that lower interhemispheric neural synchrony may be implicated as an etiology of auditory hallucinations that is most pronounced in patients categorized as Biotype 1.
The different patterns of connectivity for diagnostic and Biotype subgroups may account for heterogeneity in prior reports of auditory/language connectivity associated with hallucinations. Prior resting state fMRI studies have been in small samples, not likely to represent the heterogeneity that is present. Moreover, no prior work has assessed for subgroups effects that may parse heterogeneity in hallucination-associated alteration. In sum, the transdiagnostic finding of increased connectivity within left hemisphere receptive auditory/language cortex, along with the subgroup findings, support a conclusion that either increased connectivity, or a mix of increased and decreased connectivity, of auditory cortices is implicated in hallucination severity.
4.3. Auditory cortex connectivity alterations unrelated to hallucination severity
To supplement our symptom-connectivity observations, we sought to determine whether auditory connectivity is abnormal in relation to healthy subjects, an analysis irrespective of hallucination severity. We found significant differences between psychosis patients and controls, but none of these findings overlapped with those showing hallucination severity association (see Supplemental Fig. 2 demonstrating lack of spatial overlap between results of hallucination severity analyses and analyses of patients vs. healthy controls). There was a cluster of more strongly negative connectivity values for patients relative to controls between right AUD and left angular gyrus/lateral occipital lobe, indicating anti-correlation between the seed and the cluster. There was also greater negative connectivity of right AAC to left post-central gyrus, largely accounted for by the schizophrenia patients, and greater negative connectivity of right ACC to posterior occipital cortex for schizoaffective disorder patients relative to healthy subjects. Our additional analyses of antipsychotic dose associations with auditory cortex connectivity were largely negative, indicating the group differences in connectivity were unlikely to have been driven by antipsychotic medication dose effects. These findings generally fit with prior observations of reduced connectivity in functional neural systems in psychosis patients (Baker et al., 2014, Amico et al., 2017), including association of auditory cortex connectivity alterations with positive psychosis symptoms (Rotarska-Jagiela et al., 2010), and auditory, visual, and somatosensory neural system connectivity alterations (Skåtun et al., 2017).
It is worth highlighting that more commonly, studies seek differences between a patient and a control group on some measure, such as connectivity, and then seek correlation of any deviation found with symptom severity, often in a post-hoc manner. Had we approached our study with such a strategy, we would not have identified hallucination severity correlates. As there are no pathophysiological markers consistently associating with psychosis symptoms, we speculate this could be due to over reliance on group differences rather than potentially more sensitive approaches such as used here.
5. Limitations
Limitations to this study include, firstly, the measurement of hallucinations via a single item of the PANSS, which assesses hallucination severity within the last week. Although auditory hallucinations are by far the most common modality, the PANSS hallucination item is not limited to considering auditory hallucinations. Hence, we were unable to determine what proportion of the sample experienced auditory hallucinations, and whether this differed between diagnostic groups. Future studies using more in-depth and sensory-domain specific hallucination assessments will be beneficial. Another dimension of this limitation is that the findings may actually be meaningful with respect to a wider range of hallucination modalities given that it is more the rule than the exception for those in the schizo-bipolar spectrum to report lifetime experience of hallucinations in additional sensory modalities beyond auditory; however, we do not have such information from the subjects in this study to tease apart such effects. Another limitation is that although no functional imaging study, to our knowledge, has directly addressed hallucinations in bipolar disorder previously, the findings presented here should be considered tentative given the smaller number with greater symptomatic activity.
5.1. Conclusion
This study provides the most robust investigation to date of hallucination severity and auditory/language resting state connectivity in psychosis. We found that auditory/ language association areas are dysconnectivity hotspots associated with hallucinations, corroborating the pathogenic association of these regions in several prior, smaller resting state studies, as well as structural or activity-based studies of auditory hallucinations (e.g., Ford et al., 2009). The results also support both a common and a heterogeneous neurobiology for hallucinations in psychosis, depending on Biotype and diagnostic subgroup.
Data and code availability statement: The BSNIP1 dataset analyzed in this study is publicly available for download in the National Institute of Mental Health Data Archive (NDA; https://nda.nih.gov). Data was processed and analyzed with publicly available software CONN v18b utilizing Matlab 2017b and SPM12 (revision 6778).
Funding
This work was supported by the National Institutes of Health grants MH077851, MH077945, MH078113, MH077852, MH077862, MH077851, MH077945, MH078113, MH103366, MH103368, T3GM007281.
CRediT authorship contribution statement
Victoria T. Okuneye: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Shashwath Meda: Investigation, Methodology, Validation, Writing - review & editing. Godfrey D. Pearlson: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Brett A. Clementz: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Matcheri S. Keshavan: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Carol A. Tamminga: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Elena Ivleva: Conceptualization, Investigation, Methodology, Project administration, Resources. John A. Sweeney: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Elliot S. Gershon: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Sarah K. Keedy: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the study participants for their contributions, along with the many dedicated members of the BSNIP Consortium who helped administer the study. The analyses were completed in part with resources provided by the University of Chicago’s Research Computing Center. We thank Haley Church for proofing and helping prepare the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102358.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abel K.M., Drake R., Goldstein J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry. 2010;22(5):417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Aleman A., Böcker K.B., Hijman R., de Haan E.H., Kahn R.S. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr. Res. 2003;64(2–3):175–185. doi: 10.1016/S0920-9964(03)00060-4. [DOI] [PubMed] [Google Scholar]
- Allen P., Larøi F., McGuire P.K., Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 2008;32(1):175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Amico F., O’Hanlon E., Kraft D., Oertel-Knöchel V., Clarke M., Kelleher I., Power E. Functional connectivity anomalies in adolescents with psychotic symptoms. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0169364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A., Bernal B., Rosselli M. How localized are language brain areas? A review of Brodmann areas involvement in oral language. Arch. Clin. Neuropsychol. 2016;31(1):112–122. doi: 10.1093/arclin/acv081. [DOI] [PubMed] [Google Scholar]
- Badner J.A., Gershon E.S. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol. Psychiatry. 2002;7(4):405. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Baethge C., Baldessarini R.J., Freudenthal K., Streeruwitz A., Bauer M., Bschor T. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136–145. doi: 10.1111/j.1399-5618.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- Baker J.T., Holmes A.J., Masters G.A., Yeo B.T., Krienen F., Buckner R.L., Öngür D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta P.E., Pearlson G.D., Powers R.E., Richards S.S., Tune L.E. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am. J. Psychiatry. 1990 doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Binder J.R. Current controversies on Wernicke’s area and its role in language. Curr Neurol Neurosci Rep. 2017;17(8):58. doi: 10.1007/s11910-017-0764-8. [DOI] [PubMed] [Google Scholar]
- Clementz B.A., Sweeney J.A., Hamm J.P., Ivleva E.I., Ethridge L.E., Pearlson G.D., Keshavan M.S., Tamminga C.A. Identification of distinct psychosis biotypes using brain-based biomarkers. Am. J. Psychiatry. 2016;173(4):373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćurčić-Blake B., Ford J.M., Hubl D., Orlov N.D., Sommer I.E., Waters F., Aleman A. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog. Neurobiol. 2017;148:1–20. doi: 10.1016/j.pneurobio.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. User’s guide for the structured clinical interview for DSM-IV axis I Disorders—Research version. [Google Scholar]
- Ford J.M., Roach B.J., Jorgensen K.W., Turner J.A., Brown G.G., Notestine R., Mathalon D.H. Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophr. Bull. 2009;35(1):58–66. doi: 10.1093/schbul/sbn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.M., Mathalon D.H. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int. J. Psychophysiol. 2005;58(2–3):179–189. doi: 10.1016/j.ijpsycho.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Frith C. The neural basis of hallucinations and delusions. C.R. Biol. 2005;328(2):169–175. doi: 10.1016/j.crvi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Gavrilescu M., Rossell S., Stuart G.W., Shea T.L., Innes-Brown H., Henshall K., Egan G.F. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol. Med. 2010;40(7):1149–1158. doi: 10.1017/S0033291709991632. [DOI] [PubMed] [Google Scholar]
- Goto, M., Abe, O., Miyati, T., Yamasue, H., Gomi, T., Takeda, T., 2015. Head motion and correction methods in resting-state functional MRI. Magnet. Resonance Med. Sci. rev-2015. [DOI] [PMC free article] [PubMed]
- Ivleva E.I., Clementz B.A., Dutcher A.M., Sara Arnold J.M., Jeon-Slaughter Haekyung, Aslan Sina, Witte Bradley. Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol. Psychiatry. 2017;82(1):26–39. doi: 10.1016/j.biopsych.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K. “Hearing voices”: Auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand. J. Psychol. 2009;50(6):553–560. doi: 10.1111/j.1467-9450.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jones S.R. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr. Bull. 2010;36(3):566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opfer L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan M.S., Morris D.W., Sweeney J.A., Pearlson G., Thaker G., Seidman L.J., Tamminga C. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr. Res. 2011;133(1–3):250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K., Westerhausen R., Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 2012;38(4):779–786. doi: 10.1093/schbul/sbq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J.C., Toga A.W., Evans A., Fox P., Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) NeuroImage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Meda S.A., Ruaño G., Windemuth A., O’Neil K., Berwise C., Dunn S.M., Keshavan M.S. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. 2014;111(19):E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Costafreda S.G., Van Tol M.J., McGuire P.K., Aleman A., Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex. 2013;49(4):1046–1055. doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Moerel M., De Martino F., Formisano E. An anatomical and functional topography of human auditory cortical areas. Front. Neurosci. 2014;8:225. doi: 10.3389/fnins.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue S., Holleran L., Cannon D.M., McDonald C. Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: A selective review of structural network analyses using diffusion MRI. J. Affect. Disord. 2017;209:217–228. doi: 10.1016/j.jad.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Oertel-Knöchel V., Knöchel C., Matura S., Stäblein M., Prvulovic D., Maurer K., van de Ven V. Association between symptoms of psychosis and reduced functional connectivity of auditory cortex. Schizophr. Res. 2014;160(1–3):35–42. doi: 10.1016/j.schres.2014.10.036. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Balain V., Radua J., Liddle P.F. Structural correlates of auditory hallucinations in schizophrenia: A meta-analysis. Schizophr. Res. 2012;137(1–3):169–217. doi: 10.1016/j.schres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O’Donovan M.C., Sullivan P.F., Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite M., Teale P., Rojas D.C., Reite E., Asherin R., Hernandez O. MEG auditory evoked fields suggest altered structural/functional asymmetry in primary but not secondary auditory cortex in bipolar disorder. Bipolar Disord. 2009;11(4):371–381. doi: 10.1111/j.1399-5618.2009.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knöchel V., Uhlhaas P.J., Vogeley K., Linden D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., Murray R.M., McGuire P.K. Auditory hallucinations: A review of psychological treatments. Schizophr. Res. 1998;32:137–150. doi: 10.1016/S0920-9964(98)00052-8. [DOI] [PubMed] [Google Scholar]
- Shinn A.K., Pfaff D., Young S., Lewandowski K.E., Cohen B.M., Öngür D. Auditory hallucinations in a cross-diagnostic sample of psychotic disorder patients: a descriptive, cross-sectional study. Compr. Psychiatry. 2012;53(6):718–726. doi: 10.1016/j.comppsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn A.K., Baker J.T., Cohen B.M., Öngür D. Functional connectivity of left Heschl's gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr. Res. 2013;143(2–3):260–268. doi: 10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skåtun K.C., Kaufmann T., Doan N.T., Alnæs D., Córdova-Palomera A., Jönsson E.G., Andreassen O.A. Consistent functional connectivity alterations in schizophrenia spectrum disorder: a multisite study. Schizophr. Bull. 2017;43(4):914–924. doi: 10.1093/schbul/sbw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I.E., Slotema C.W., Daskalakis Z.J., Derks E.M., Blom J.D., van der Gaag M. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr. Bull. 2012;38(4):704–714. doi: 10.1093/schbul/sbs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann S., Leicht G., Mulert C. Interhemispheric auditory connectivity: structure and function related to auditory verbal hallucinations. Front. Hum. Neurosci. 2014;8:55. doi: 10.3389/fnhum.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga C.A., Ivleva E.I., Keshavan M.S., Pearlson G.D., Clementz B.A., Witte B., Sweeney J.A. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am. J. Psychiatry. 2013;170(11):1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Pearlson G., Keshavan M., Sweeney J., Clementz B., Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: Outcomes across the psychosis continuum. Schizophr. Bull. 2014;40(Suppl. 2):131–137. doi: 10.1093/schbul/sbt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga Carol A., Pearlson Godfrey D., Stan Ana D., Gibbons Robert D., Padmanabhan Jaya, Keshavan Matcheri, Clementz Brett A. Strategies for advancing disease definition using biomarkers and genetics: The Bipolar and Schizophrenia Network for Intermediate Phenotypes. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 2017;2(1):20–27. doi: 10.1016/j.bpsc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Toh W.L., Thomas N., Rossell S.L. Auditory verbal hallucinations in bipolar disorder (BD) and major depressive disorder (MDD): A systematic review. J. Affect. Disord. 2015;184:18–28. doi: 10.1016/j.jad.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Tremblay P., Dick A.S. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016;162:60–71. doi: 10.1016/j.bandl.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhong S., Jia Y., Zhou Z., Wang B., Pan J., Huang L. Interhemispheric resting state functional connectivity abnormalities in unipolar depression and bipolar depression. Bipolar Disord. 2015;17(5):486–495. doi: 10.1111/bdi.12315. [DOI] [PubMed] [Google Scholar]
- Waters F., Fernyhough C. Hallucinations: a systematic review of points of similarity and difference across diagnostic classes. Schizophr. Bull. 2017;43(1):32–43. doi: 10.1093/schbul/sbw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- van Lutterveld R., Diederen K.M., Koops S., Begemann M.J., Sommer I.E. The influence of stimulus detection on activation patterns during auditory hallucinations. Schizophr. Res. 2013;145(1–3):27–32. doi: 10.1016/j.schres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang W., Li S., Wang X., Gong Y., Yao L., Xiao Y., Lui S. Abnormal dynamic functional connectivity between speech and auditory areas in schizophrenia patients with auditory hallucinations. NeuroImage: Clinical. 2018;19:918–924. doi: 10.1016/j.nicl.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmigrod L., Garrison J.R., Carr J., Simons J.S. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 2016;69:113–123. doi: 10.1016/j.neubiorev.2016.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.