Abstract
Background
Multidrug-resistant tuberculosis (MDR-TB) is an increasing problem worldwide, and 24% occurs in India. Linezolid is associated with improved MDR-TB treatment outcomes but causes significant side-effects and drug susceptibility testing (DST) is rarely available. This study assessed whether clinical factors could predict linezolid resistance.
Methods
An observational cohort of adults and adolescents with MDR-TB at a tertiary care hospital in Mumbai, India was analyzed for clinical, laboratory, and radiographic findings associated with linezolid resistance.
Results
In total, 343 MDR-TB patients had linezolid DST performed, and 23 (6.7%) had linezolid-resistant MDR-TB. Univariable analysis associated linezolid resistance with underweight (odds ratio (OR)–1.07, 95% confidence interval (CI):1.01–1.12); number of previous providers (OR:1.03, 95% CI:1.00–1.05); previous treatment with linezolid (OR:1.12, 95% CI:1.06–1.05), bedaquiline (OR:1.55, 95% CI:1.22–1.98), or clofazimine (OR:1.08 95% CI:1.03–1.16); cavitary disease (OR:1.10, 95% CI:1.04–1.16) and percent lung involvement (OR:1.02, 95% CI:1.01–1.03) on radiograph. DST associated linezolid resistance with resistance to fluoroquinolones (OR:1.08, 95% CI:1.01–1.14), injectables (OR:1.09, 95% CI:1.03–1.15), ethionamide (OR:1.09, 95% CI:1.03–1.15), and PAS (OR:1.13, 95% CI:1.06–1.21). In multivariate analysis, only prior linezolid and percent lung involvement were associated with linezolid resistance.
Conclusion
To maximize treatment benefits while minimizing toxicity, DST remains an important tool to identify linezolid resistance.
Keywords: MDR-TB, Drug susceptibility testing, Linezolid, Drug resistance, India
1. Introduction
Tuberculosis (TB) is the leading cause of death from a single infectious agent.[1] Approximately 10 million people contract TB every year, 27% of which occur in India. Reported rates of multidrug resistant (resistance to isoniazid and rifampin, “MDR”) and extensively drug resistant (MDR resistant to fluoroquinolones and second-line injectable drugs, “XDR”) are rising, affecting 558,000 people worldwide in 2017. Nearly half were in 3 countries, with 24% of reported drug-resistant TB found in India.[1] People can develop MDR/XDR-TB through either inadequate treatment or primary infection with drug-resistant strains of Mycobacterium tuberculosis (“Mtb,” the bacterium that causes TB), as occurs for most resistant TB in South Africa.[2] While many countries have developed guidelines for treatment of MDR/XDR-TB, additional resistance such as that found in Mumbai often requires an individualized approach to find drugs to which a particular isolate is susceptible.[3], [4] Constructing treatment regimens for resistant TB is difficult, requiring a balance of efficacy, toxicity, and expense with limited trial evidence available to support specific regimens for complex resistance. In this context, significant attention has been paid to new and repurposed drugs such as linezolid.[5]
Linezolid is an oxazolidinone antibiotic with potent bacteriostatic activity, good penetration into lungs, infected tissue, and CSF,[6], [7] and is associated with improved TB treatment outcomes.[8] Following early clinical successes, WHO guidelines have prioritized linezolid, and linezolid is a component of several ongoing clinical trials of TB treatment.[5], [9], [10], [11] Unfortunately, linezolid therapy is also complicated by serious side effects including painful peripheral neuropathy, vision-threatening optic neuritis, myelosuppression, and drug interactions.[12] In addition, linezolid resistance–while rare–has been reported in multiple countries.[13], [14], [15] Few mycobacterial laboratories can perform drug susceptibility testing (“DST”) for linezolid, so most TB patients receive linezolid without confirmed susceptibility. In this context, it would be helpful to know which clinical factors should lead clinicians to suspect linezolid resistance and prioritize DST. To maximize the benefit to patients of linezolid treatment and minimize unnecessary toxicity, we performed a cross-sectional analysis of MDR-TB patients in Mumbai, India to determine if clinical factors present at the start of treatment were associated with linezolid resistance.
2. Methods
2.1. Study setting
The study was carried out at the P.D. Hinduja National Hospital and Medical Research Centre (“Hinduja Hospital”), a private, tertiary care hospital in Mumbai, India with an outpatient chest clinic and a mycobacteriology laboratory that performs extensive DST. The outpatient clinic sees ~ 3,000 adults annually, and the lab processes > 32,000 samples for Mtb each year. In addition to smear, Xpert MTB/RIF, line probe assays, and pyrosequencing, phenotypic testing at Hinduja Hospital includes mycobacteria growth indicator tube (MGIT) DST for isoniazid, rifampin, pyrazinamide, ethambutol, ofloxacin, moxifloxacin, amikacin, kanamycin, capreomycin, ethionamide, para-aminosalicylic acid (“PAS”), clofazimine, and linezolid (1 μg/mL).[16]
2.2. Study design
From October 20, 2015 to July 20, 2019, adults and adolescents ≥ 15 years old seeking care for MDR-TB at the Hinduja Hospital chest clinic were enrolled in an observational clinical cohort approved by the Institutional Review Boards of Hinduja Hospital and Johns Hopkins University. After providing written informed consent or (for participants 15–18 years old) written guardian consent and participant assent, each participant’s medical records were reviewed. Variables collected included participant demographics (age, sex, self-reported tobacco use, height, weight, and known TB contacts), site of TB (defined as pulmonary or extrapulmonary, not mutually exclusive), diagnosis history (public or private sector, number of prior providers, and months between symptom onset and TB diagnosis), self-reported history of prior TB (defined as completion of treatment followed by ≥ 6 symptom-free months), treatment prescribed prior to enrolment, laboratory and imaging studies (glycosylated hemoglobin, HIV test results, percentage of lung affected and presence of a cavity on chest radiograph using a standardized scoring system[17]) and DST results (resistant or susceptible to each drug tested). Diabetes status was defined as either self-reported diagnosis at the time of enrolment or documented glycosylated hemoglobin > 6.5% during study participation. DST results were considered equivalent if they were performed at Hinduja Hospital or if formal reports including drug concentrations were available from another lab.
2.3. Statistical analysis
Data were collected on paper forms, entered in a Microsoft Access database (Office Professional 365, Microsoft Corporation, Redmond, Washington), and analyzed in R (version 3.5.3, R Core Team, Vienna, Austria). Participants were analyzed if linezolid resistance testing was performed (Fig. 1). Frequency tables were constructed for stratified analysis of each characteristic by linezolid resistance status. Due to the observational nature of this study, not every participant had complete laboratory or radiographic records available for review. In such cases, the denominator is defined as the number with available data for each variable. Differences in proportions of categorical variables were compared by Fisher exact tests and differences in continuous variables were compared by student t-tests with p-values < 0.05 considered statistically significant.
Fig. 1.
Observational Cohort Study Schema. Participants were recruited from a tertiary care chest clinic in Mumbai. Adults and adolescents with multidrug resistant tuberculosis (“MDR-TB,” resistant to rifampin and isoniazid) were recruited to an observational cohort. All participants with linezolid resistance test results available were included for analysis in this manuscript.
Univariable and multivariable logistic regression models were constructed to measure associations between each characteristic and linezolid resistance. Independent variables were modelled continuously (age, body mass index (height in meters / weight in kg2, “BMI”), months from symptom to diagnosis, medical providers before enrolment, percent lung involvement on initial chest radiograph) and categorically (all others). TB requires treatment with multiple drugs simultaneously and treatments are provided based on pre-specified regimens.[18] For model simplification, BMI was dichotomized as underweight (<18.5 kg/m2) or not, and treatment prior to enrolment and resistance profiles were categorized according to WHO drug categories from 2018.[5] Classes assessed included “first line drugs” (isoniazid, rifampin, pyrazinamide, and ethambutol), fluoroquinolones (ofloxacin, levofloxacin, or moxifloxacin at this site) other group A drugs (linezolid and bedaquiline); “second-line injectable drugs” (amikacin, kanamycin, or capreomycin, whose priority status was reduced after many participants were treated), “group B drugs” (cycloserine and clofazimine); “additional group C drugs” (only ethionamide and PAS, as injectables, pyrazinamide, and ethambutol are presented separately, while delamanid and carbapenem susceptibility was not tested), and “salvage drugs” (amoxicillin/clavulanate and clarithromycin), which are no longer recommended for TB. Multivariable models were constructed based on literature review and exploratory data analysis and included factors known to be associated with treatment outcomes (site of disease, underweight, extent of pulmonary disease, additional drug resistance), potential confounders (age, sex, number of prior providers, prior treatment). Multivariable models were tested for collinearity by variance inflation factor (VIF), with variables reassessed for inclusion in the final model if VIF was ≥ 2.
3. Results
During the study period, a total of 598 participants with MDR-TB were enrolled. Of these, 343 completed DST for linezolid and 23 were resistant (6.7% of those with DST results, 3.8% of total, Fig. 1). Participants tested for linezolid resistance were young with a median age of 27 (interquartile range, “IQR:” 21–35) and were predominantly female (215 participants, 62.7%). Most had pulmonary TB (258 participants, 75.2%), were diagnosed in the public sector (282, 86.5%) and saw a median of 2 other providers before study enrolment (IQR: 1–3). HIV was uncommon (2 participants, 0.6%), while 31 participants (9.0%) reported tobacco use and 32 had diabetes (21.2% of 151 participants tested, 9.3% of total). Prior TB was reported by 99 (28.9%) participants and 103 (30.0%) reported a household contact with TB. Underweight was common (39.9% of 328 participants with data), as was cavitary lung disease on chest radiograph (53.2% of 310 participants with radiographs). Of the clinical features assessed, lower BMI, prior treatment with linezolid, bedaquiline, clofazimine, amoxicillin/clavulanate, and clarithromycin were more frequent among participants with linezolid resistance than with linezolid susceptible TB. Similarly, resistance to fluoroquinolones, injectable drugs, and group C drugs, and more extensive lung involvement on chest radiograph (percent of lung involved and presence of cavity) were more frequent among those with linezolid resistance (Table 1, Fig. 2). While not statistically significant, pulmonary disease and prior fluoroquinolone treatment were also more common among participants with linezolid resistance.
Table 1.
Clinical characteristics of 343 patients with multidrug-resistant tuberculosis (MDR-TB) and linezolid drug susceptibility test results.
| Participants with Linezolid Resistance (N = 23) | Participants with Linezolid Susceptibility (N = 320) | p-value1 | |
|---|---|---|---|
| Clinical Features | |||
| Age in Years, Median (IQR)2 | 26.0 (21.5–38.0) | 27.0 (21.0–35.0) | 0.708 |
| Female Sex, N (%) | 13 (56.5) | 202 (63.1) | 0.514 |
| Pulmonary TB,3 N (%) | 21 (91.3) | 237 (74.1) | 0.079** |
| Extrapulmonary TB,3 N (%) | 2 (8.7) | 71 (22.2) | 0.186 |
| Body Mass Index (kg/m2), Median (IQR)2 | 17.4 (15.8–20.3) | 19.8 (16.4–23.4) | 0.003* |
| Underweight (<18.5 kg/m2) | 13 (65.0) | 118 (38.3) | 0.031 |
| Months from Symptom to Diagnosis, Median (IQR)2 | 1.0 (1.0–1.8) | 1.0 (1.0–2.0) | 0.234 |
| Number of Medical Providers Before Susceptibility, Median (IQR)2 | 3.0 (1.5–4.5) | 2.0 (1.0–3.0) | 0.129 |
| Previous Episode of Tuberculosis, N (%)4 | 4 (17.4) | 95 (29.7) | 0.243 |
| Known Contact with TB, N (%)4 | 7 (30.4) | 96 (30.0) | 1.000 |
| Self-Reported Tobacco Use (Current or Former), N (%)4 | 1 (4.3) | 30 (9.4) | 0.708 |
| Diabetes, N (%)4 | 2 (25.0) | 30 (21.0) | 0.677 |
| HIV Positive, N (%)4 | 0 (0.0) | 2 (0.8) | 1.000 |
| Works in Health Care Sector, N (%)4 | 0 (0.0) | 18 (5.6) | 0.621 |
| Prior Treatments | |||
| Previous Treatment with First-Line Drugs (Isoniazid, Rifampin, Pyrazinamide, and Ethambutol), N (%) | 14 (60.9) | 243 (75.9) | 0.133 |
| Previous Treatment with a Fluoroquinolone, N (%) | 15 (65.2) | 149 (46.6) | 0.089** |
| Previous Treatment with Linezolid, N (%) | 14 (60.9) | 79 (24.7) | <0.001* |
| Previous Treatment with Bedaquiline, N (%) | 2 (8.7) | 2 (0.6) | 0.024* |
| Previous Treatment with a Group B Drug (Clofazimine or Cycloserine), N (%) | 15 (65.2) | 134 (41.9) | 0.048* |
| Previous Treatment with a Second-line Injectable Drug (Amikacin, Kanamycin, or Capreomycin), N (%) | 12 (52.2) | 127 (39.7) | 0.275 |
| Previous Treatment with Another Group C Drug (Ethionamide, Prothionamide, or PAS), N (%) | 13 (56.5) | 136 (42.5) | 0.199 |
| Previous Treatment with a Salvage Drug (Clarithromycin, Amoxicillin/Clavulanate), N (%) | 11 (47.8) | 58 (18.1) | 0.002* |
| Drug Resistance | |||
| Resistant to First-Line Drugs (Isoniazid, Rifampin, Pyrazinamide, or Ethambutol), N (%)4 | 23 (100.0) | 302 (94.4) | 0.621 |
| Resistant to Fluoroquinolones (Ofloxacin or Moxifloxacin), N (%)4 | 22 (95.7) | 234 (73.1) | 0.013* |
| Resistant to Clofazimine, N (%)4 | 1 (4.3) | 4 (1.3) | 0.300 |
| Resistant to a Second-line Injectable Drug (Amikacin, Kanamycin, or Capreomycin), N (%) | 12 (52.2) | 81 (25.3) | 0.013* |
| Resistant to Another Group C Drug (Ethionamide or PAS), N (%)4 | 21 (91.3) | 202 (63.1) | 0.006* |
| Radiographic Findings | |||
| % Lung Involvement on Initial Chest Radiograph, Median (IQR)2 | 25.0 (10.0–35.0) | 10.0 (0.0–30.0) | 0.044* |
| Bilateral Disease on Chest Radiograph, N (%) | 2 (10.0)4 | 21 (7.2)4 | 0.651 |
| Cavitary Disease on Chest Radiograph, N (%) | 18 (90.0)4 | 147 (50.7)4 | <0.001* |
5This row presents only resistance to ethionamide and PAS, as the other group C drugs are either represented in other rows (ethambutol and pyrazinamide are first-line drugs, and amikacin is a second-line injectable drug) or are not tested at the study site.
p-value indicates t-test for continuous variables and Fisher’s exact test for categorical variables
IQR – interquartile range
Pulmonary and extrapulmonary TB not mutually exclusive categories
Due to the observational nature of this cohort, not all participants answered all questions, completed susceptibility testing, radiography, or the entire treatment course. Percentage reflects number of participants with data available, rather than denominator of the entire study group.
p-value < 0.05
p-value 0.05–0.10
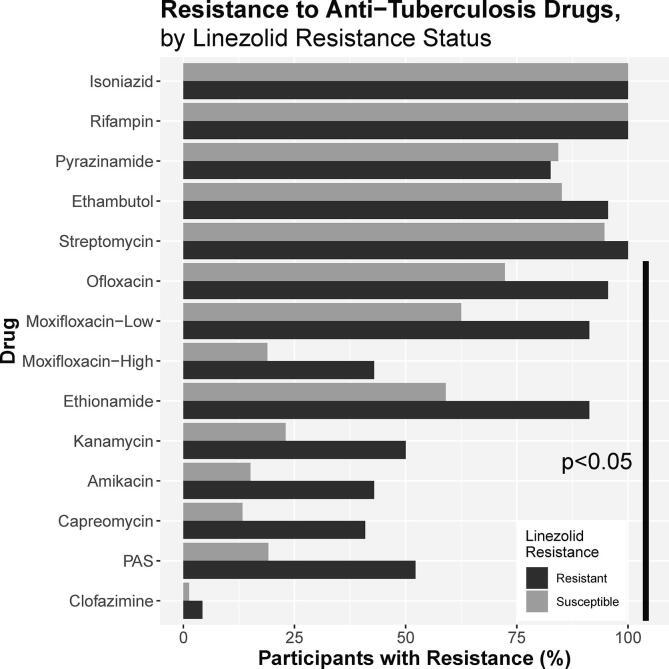
Fig. 2.
Resistance to Anti-Tuberculosis Drugs by Linezolid Resistance Status. Rates of resistance to additional drugs on drug susceptibility testing by mycobacterial growth indicator tube identified frequent resistance to other drugs. Rates of resistance to amikacin, kanamycin, capreomycin, ofloxacin, moxifloxacin, ethionamide, and PAS were all statistically significantly different according to linezolid resistance status (p < 0.05).
Regression analysis found higher odds of linezolid resistance among participants who were underweight (odds ratio (“OR”):1.07, 95% confidence interval (“CI”):1.01–1.12), were seen by a greater number of medical providers before diagnosis (OR:1.03, 95% CI:1.00–1.05), were previously treated with group A (OR:1.12, 95% CI:1.06–1.19 for linezolid, OR:1.55, 95% CI:1.22–1.98 for bedaquiline), group B (OR:1.06, 95% CI:1.01–1.12), or salvage drugs (OR:1.12, 95% CI:1.05–1.20, Table 2). Linezolid resistance was also associated with resistance to fluoroquinolones (OR: 1.08, 95% CI:1.01–1.14), injectable drugs (OR:1.09, 95% CI:1.03–1.15), ethionamide (OR:1.09, 95% CI:1.03–1.15), and PAS (OR:1.13, 95% CI:1.06–1.21). In addition, extent of pulmonary disease was associated with odds of linezolid resistance, either measured by percent lung involvement (OR:1.02, 95% CI:1.01–1.03) or cavitary lung disease (OR:1.10, 95% CI:1.04–1.16). While not statistically significant, increased odds of linezolid resistance were also associated with pulmonary TB (OR 1.06, 95% CI 1.00–1.13) and prior fluoroquinolone treatment (OR 1.05, 95% CI 0.99–1.10). Importantly, a history of prior TB, duration of symptoms before starting treatment, known TB contact, tobacco use, comorbid diabetes, and employment in the health sector were not associated with linezolid resistance. Adjusted multivariable regression analysis found that only prior linezolid treatment (OR:1.10, 95% CI:1.02–1.20) and percent lung involvement on chest radiograph (OR:1.02, 95% CI:1.00–1.04) were significantly associated with linezolid resistance.
Table 2.
Unadjusted and Adjusted Odds of Linezolid Resistance.
| Variable | Unadjusted Odds Ratio (95% CI1) | p-value | Adjusted Odds Ratio (95% CI1) | p-value |
|---|---|---|---|---|
| Clinical Features | ||||
| Age in Years (10-Year Increments) | 1.00 (0.98–1.03) | 0.655 | 1.00 (0.97–1.03) | 0.979 |
| Female Sex | 0.98 (0.93–1.04) | 0.528 | 0.98 (0.91–1.04) | 0.467 |
| Pulmonary TB | 1.06 (1.00–1.13) | 0.065** | 0.96 (0.88–1.05) | 0.404 |
| Underweight (<18.5 kg/m2) | 1.07 (1.01–1.12) | 0.018* | 1.01 (0.94–1.09) | 0.727 |
| Months from Symptom to Diagnosis | 1.00 (0.99–1.01) | 0.441 | ||
| Number of Medical Providers Before Enrolment | 1.03 (1.00–1.05) | 0.024* | 1.00 (0.97–1.03) | 0.888 |
| Previous Episode of Tuberculosis | 0.96 (0.91–1.02) | 0.210 | ||
| Known Contact with TB | 1.00 (0.94–1.06) | 0.965 | ||
| Self-Reported Tobacco Use (Current or Former) | 0.96 (0.88–1.06) | 0.418 | ||
| Diabetes | 1.01 (0.93–1.11) | 0.788 | ||
| HIV Positive | 0.93 (0.66–1.33) | 0.703 | ||
| Works in Health Care Sector | 0.93 (0.83–1.05) | 0.244 | ||
| Prior Treatments | ||||
| Previous Treatment with First-Line Drugs (Isoniazid, Rifampin, Pyrazinamide, and Ethambutol) | 0.95 (0.89–1.01) | 0.108 | 0.95 (0.87–1.02) | 0.168 |
| Previous Treatment with a Fluoroquinolone | 1.05 (0.99–1.10) | 0.084** | ||
| Previous Treatment with Linezolid | 1.12 (1.06–1.19) | <0.001* | 1.10 (1.02–1.20) | 0.020* |
| Previous Treatment with Bedaquiline | 1.55 (1.22–1.98) | <0.001* | ||
| Previous Treatment with a Group B Drug (Clofazimine or Cycloserine) | 1.06 (1.01–1.12) | 0.029* | ||
| Previous Treatment with a Second-line Injectable Drug (Amikacin, Kanamycin, or Capreomycin) | 1.03 (0.98–1.09) | 0.240 | ||
| Previous Treatment with Another Group C Drug (Ethionamide, Prothionamide, or PAS) | 1.04 (0.98–1.09) | 0.191 | ||
| Previous Treatment with a Salvage Drug (Clarithromycin, Amoxicillin/Clavulanate) | 1.12 (1.05–1.20) | 0.001* | ||
| Drug Resistance | ||||
| Resistant to First-Line Drugs (Isoniazid, Rifampin, Pyrazinamide, or Ethambutol) | 1.07 (0.95–1.21) | 0.244 | ||
| Resistant to Fluoroquinolones (Ofloxacin or Moxifloxacin) | 1.08 (1.01–1.14) | 0.016* | 1.01 (0.94–1.09) | 0.803 |
| Resistant to Clofazimine2 | 1.14 (0.91–1.43) | 0.242 | ||
| Resistant to a Second-line Injectable Drug (Amikacin, Kanamycin, or Capreomycin) | 1.09 (1.03–1.15) | 0.005* | 1.01 (0.93–1.10) | 0.793 |
| Resistant to Another Group C Drug (Ethionamide or PAS)3 | 1.08 (1.02–1.14) | 0.006* | 1.07 (1.00–1.15) | 0.060** |
| Radiographic Findings | ||||
| Lung Involvement on Chest Radiograph (10% Increments) | 1.02 (1.01–1.03) | 0.008* | 1.02 (1.00–1.04) | 0.034* |
| Cavitary Disease on Chest Radiograph | 1.10 (1.04–1.16) | 0.001* | 1.06 (0.98–1.15) | 0.120 |
| Bilateral Disease on Chest Radiograph | 1.02 (0.92–1.14) | 0.650 |
CI: confidence interval
Susceptibility testing for cycloserine is not routinely performed. This represents all susceptibility testing for group B drugs.
This row presents only resistance to ethionamide and PAS, as the other group C drugs are either represented in other rows (ethambutol and pyrazinamide are first-line drugs, and amikacin is a second-line injectable drug) or are not tested at the study site.
p-value < 0.05
p-value 0.05–0.10
4. Discussion
This large, single-site study of 343 participants with MDR-TB and linezolid resistance testing had several important findings. Among this young, female-predominant MDR-TB cohort with high rates of prior treatment and known TB contacts, 6.7% of Mtb isolates that completed DST were resistant to linezolid. We found increased odds of linezolid resistance among participants with more severe disease indicated by underweight, extent of lung disease on chest radiograph, and cavitary lung disease. Increased odds of linezolid resistance were also associated with extent of prior treatment indicated by number of previous medical providers and prior treatments received. Similarly, odds of resistance were increased in association with resistance to other drugs, particularly among pre-XDR and XDR Mtb isolates, as well as those that were resistant to either ethionamide or PAS. Importantly, a history of prior TB, duration of illness prior to treatment, known TB contact, tobacco use, and comorbid diabetes were not associated with odds of linezolid resistance. In multivariable analysis, only percent of lung involvement on chest radiograph and prior linezolid treatment remained significant predictors of linezolid resistance.
As rates of MDR- and XDR-TB rise, clinicians in high-burden areas will need improved tools to determine which patients are most likely to benefit from new and repurposed drugs like linezolid. Between excellent distribution throughout the body,[6], [7] its in-vitro synergy with bedaquiline, fluoroquinolones, amikacin, and clofazimine,[19], [20], [21] and its association with improved treatment outcomes,[22] there are many reasons to include linezolid in treatment regimens for drug-resistant TB. At the same time linezolid is often prescribed for several months outside of clinical trials (median 300 days in meta-analysis), and neurologic and hematologic toxicities impacting nearly 60% of treated patients.[23], [24] A recent study of 26 weeks of bedaquiline, pretomanid, and linezolid (1200 mg total daily) demonstrated favorable outcomes among 90% of participants, though 81% developed peripheral neuropathy and only 15% completed treatment without linezolid interruption or dose reduction.[25] In the absence of clinical means to predict horrible side effects like optic neuropathy,[26] it is important to identify resistance promptly and appropriately adjustment treatment strategy to prevent unnecessary harm.
Unfortunately, increased use has not been associated with increased global DST capacity for linezolid, so many new prescriptions will occur without confirmation of linezolid susceptibility. This is important, considering increasing recognition of circulating linezolid resistance. The rate of linezolid resistance identified among Mtb isolates in this study (6.7% of tested isolates) is similar to rates reported in other areas with high prevalence of drug resistance such as Beijing (5.6%) and Karachi (5.9%), though other studies have reported linezolid resistance rates as high as 10.8% among MDR-TB isolates.[14], [15], [27] While the number of participants that developed linezolid resistance during treatment is too low in this study for extensive analysis, the importance of testing is further underscored by the published association between linezolid resistance and treatment failure.[28]
Few data on clinical predictors of linezolid resistance are available. In our study, while the minority of participants who received linezolid treatment prior to enrolment were found to have linezolid-resistant isolates (14 out of 93, 15.1%), prior treatment with linezolid was associated with increased odds of linezolid resistance in both univariable and multivariable regression models. This is consistent with previous reports of linezolid resistance among patients previously treated with linezolid.[29] Similarly, in univariable analysis (but not multivariable analysis), we saw increased odds of linezolid resistance associated with resistance to fluoroquinolones, second-line injectable drugs, ethionamide, and PAS. While these results were not significant in multivariable analysis, an association between resistance to linezolid and resistance to fluoroquinolones and injectables has been reported before, though not in association with ethionamide, and PAS resistance.[30] In sensitivity analysis, we did not find significant correlation between resistance to various TB drugs (Pearson correlation coefficient 0.16–0.34 between each drug assessed), however it is reasonable to expect linezolid resistance to follow resistance to first-line drugs, fluoroquinolones, and injectable drugs.[31], [32] This is also consistent with the tendency of clinicians to add more drugs to a treatment regimen as resistance is identified,[28] which may explain our findings associating linezolid resistance with a greater number of providers and treatments prior to study enrolment – particularly salvage therapies like amoxicillin/clavulanate and clarithromycin. Finally, cavitary lung disease was more common among participants with linezolid resistance in this study. Linezolid treatment of MDR-TB has been associated specifically with cavity closure,[33] so it is possible that the increased rates of cavitary disease among those with linezolid-resistant isolates reflects failure of response to prior treatment.
Our study has several limitations. This single-site study occurred in Mumbai, where circulating drug resistance is common. We have previously reported high rates of resistance to these drugs even among untreated study participants.[4] In this setting, it is possible that the rates of drug resistance identified, the frequency with which DST is performed, and the resulting prescription of other drugs prior to enrollment are higher than among populations with lower rates of drug resistance. Additionally, this cross-sectional dataset did not have sufficient power to report on factors impacting final treatment outcomes, as only 3 participants (0.9%) developed linezolid resistance and most participants have not been followed long-enough to determine final outcome. Our longitudinal clinical experience with linezolid including side effect profiles has been previously published.[34] As more data are available from this and other studies,[9], [10], [11] a deeper analysis of the findings of this study with final treatment outcomes will be helpful to guide linezolid treatment decisions. Similarly, the lack of association in this study between linezolid resistance and either prior episodes of TB or known contacts with TB may reflect the frequency of circulating drug resistance rather than new drug resistance developing within study participants during prior treatment. These data may not be generalizable to people with HIV, who represented < 1% of this cohort and have been underrepresented in many published studies of linezolid treatment efficacy.[35] Finally, the reported rate of resistance (7%) is alarming, but may not reach the threshold at which all programs would initiate universal DST,[36] particularly in light of variation in choice of companion drugs. Considering heterogeneity of experiences between published studies of linezolid, additional data from multiple clinical settings is warranted to determine the population threshold at which universal DST would need to be implemented to prevent linezolid resistance-associated treatment failure or mortality.
The results of this study indicate that clinical data alone have limited association with linezolid resistance test results, with relatively small increases in adjusted odds of linezolid resistance (1.02–1.10). In order to provide patients with the benefits of linezolid treatment without exposing them to unnecessary toxicity, DST for linezolid remains and important tool. While 7% of participants were found to have linezolid-resistant TB, no readily measured clinical correlates other than prior linezolid treatment and extent of lung disease were associated with linezolid resistance. DST remains an important tool to identify resistance for patients planning TB treatment with linezolid.
CRediT authorship contribution statement
J.A. Tornheim: Conceptualization, Funding acquisition, Project administration, Methodology, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. E. Intini: Methodology, Formal analysis, Writing - original draft, Writing - review & editing. A. Gupta: Conceptualization, Funding acquisition, Supervision, Methodology, Visualization, Writing - review & editing. Z.F. Udwadia: Conceptualization, Supervision, Methodology, Investigation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
JAT was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH NIAID awards number K23AI135102 and R21AI122922), the UJMT Fogarty Global Health Fellows Program (NIH Fogarty International Center, NINDS, NIMH, NHBLI and NIEHS award number D43 TW009340), and the Johns Hopkins University School of Medicine Clinician Scientist Career Development Award. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design, analysis, manuscript preparation, or decision to publish this manuscript. EI was supported by the Italian Respiratory Society (SIP/IRS). The authors would like to acknowledge Prof. L Richeldi for his support of EI during her short-term scholarship at the Department of Respiratory Medicine to P.D. Hinduja Hospital and Medical Research Centre in Mumbai.
References
- 1.WHO. Global Tuberculosis Report 2018. Geneva: WHO, 2018. p. 277.
- 2.Shah N.S., Auld S.C., Brust J.C., Mathema B., Ismail N., Moodley P. Transmission of Extensively Drug-Resistant Tuberculosis in South Africa. The New England journal of medicine. 2017;376:243–253. doi: 10.1056/NEJMoa1604544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udwadia Z.F., Mullerpattan J.B., Shah K.D., Rodrigues C.S. Possible impact of the standardized Category IV regimen on multidrug-resistant tuberculosis patients in Mumbai. Lung India. 2016;33:253–256. doi: 10.4103/0970-2113.180800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udwadia Z.F., Tornheim J.A., Ganatra S., DeLuca A., Rodrigues C.S., Gupta A. Few eligible for the newly recommended short course MDR-TB regimen at a large Mumbai private clinic. BMC Infect Dis. 2019;19:94. doi: 10.1186/s12879-019-3726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. 2018:9. [Google Scholar]
- 6.Tsona A., Metallidis S., Foroglou N., Selviaridis P., Chrysanthidis T., Lazaraki G. Linezolid penetration into cerebrospinal fluid and brain tissue. J Chemother. 2010;22:17–19. doi: 10.1179/joc.2010.22.1.17. [DOI] [PubMed] [Google Scholar]
- 7.Strydom N., Gupta S.V., Fox W.S., Via L.E., Bang H., Lee M. Tuberculosis drugs' distribution and emergence of resistance in patient's lung lesions: A mechanistic model and tool for regimen and dose optimization. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad N., Ahuja S.D., Akkerman O.W., Alffenaar J.C., Anderson L.F., Baghaei P. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet (London, England) 2018;392:821–834. doi: 10.1016/s0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov Safety and Efficacy of Various Doses and Treatment Durations of Linezolid Plus Bedaquiline and Pretomanid in Participants With Pulmonary TB, XDR-TB, Pre- XDR-TB or Non-responsive/Intolerant MDR-TB (ZeNix). Available at: https://clinicaltrials.gov/ct2/show/NCT03086486 Accessed 7/24/2020.
- 10.ClinicalTrials.gov Evaluating Newly Approved Drugs for Multidrug-resistant TB (endTB). Available at: https://clinicaltrials.gov/ct2/show/record/NCT02754765 Accessed 7/24/2020.
- 11.Lee M., Mok J., Kim D.K., Shim T.S., Koh W.J., Jeon D. Delamanid, linezolid, levofloxacin, and pyrazinamide for the treatment of patients with fluoroquinolone-sensitive multidrug-resistant tuberculosis (Treatment Shortening of MDR-TB Using Existing and New Drugs, MDR-END): study protocol for a phase II/III, multicenter, randomized, open-label clinical trial. Trials. 2019;20:57. doi: 10.1186/s13063-018-3053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Paolo A., Malacarne P., Guidotti E., Danesi R., Del Tacca M. Pharmacological issues of linezolid: an updated critical review. Clin Pharmacokinet. 2010;49:439–447. doi: 10.2165/11319960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Richter E., Rusch-Gerdes S., Hillemann D. First linezolid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2007;51:1534–1536. doi: 10.1128/aac.01113-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang Y.u., Zong Z., Huo F., Jing W., Ma Y., Dong L., Li Y., Zhao L., Fu Y., Huang H. In Vitro Drug Susceptibility of Bedaquiline, Delamanid, Linezolid, Clofazimine, Moxifloxacin, and Gatifloxacin against Extensively Drug-Resistant Tuberculosis in Beijing, China. Antimicrob Agents Chemother. 2017;61(10) doi: 10.1128/AAC.00900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed I., Jabeen K., Inayat R., Hasan R. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob Agents Chemother. 2013;57:2522–2525. doi: 10.1128/aac.02020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues C., Jani J., Shenai S., Thakkar P., Siddiqi S., Mehta A. Drug susceptibility testing of Mycobacterium tuberculosis against second-line drugs using the Bactec MGIT 960 System. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2008;12:1449–1455. doi: 10.1128/jcm.00652-10. [DOI] [PubMed] [Google Scholar]
- 17.Ralph A.P., Ardian M., Wiguna A., Maguire G.P., Becker N.G., Drogumuller G. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65:863–869. doi: 10.1136/thx.2010.136242. [DOI] [PubMed] [Google Scholar]
- 18.RNTCP. Guidelines on Programatic Management of Drug-Resistant Tuberculosis in India 2017. New Delhi, India: Revised National Tuberculosis Control Programme, Central TB Division, 2017. p. 320.
- 19.Zou L., Liu M., Wang Y., Lu J., Pang Y. Determination of in vitro synergy between linezolid and other antimicrobial agents against Mycobacterium tuberculosis isolates. Tuberculosis (Edinburgh, Scotland) 2015;95:839–842. doi: 10.1016/j.tube.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Santos NCS, Scodro RBL, de Almeida AL, Baldin VP, Nakamura de Vasconcelos SS, Siqueira VLD, Caleffi-Ferracioli KR, Campanerut-Sa PAZ, Cardoso RF. Combinatory activity of linezolid and levofloxacin with antituberculosis drugs in Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland) 2018;111:41-44. doi: 10.1016/j.tube.2018.05.005. [DOI] [PubMed]
- 21.de Miranda Silva C, Hajihosseini A, Myrick J, Nole J, Louie A, Schmidt S, Drusano GL. Effect of Linezolid plus Bedaquiline against Mycobacterium tuberculosis in Log Phase, Acid Phase, and Nonreplicating-Persister Phase in an In Vitro Assay. Antimicrobial agents and chemotherapy 2018;62 doi: 10.1128/aac.00856-18. [DOI] [PMC free article] [PubMed]
- 22.Jeon D.S., Kim D.H., Kang H.S., Hwang S.H., Min J.H., Kim J.H. Survival and predictors of outcomes in non-HIV-infected patients with extensively drug-resistant tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2009;13:594–600. [PubMed] [Google Scholar]
- 23.Bolhuis M.S., van der Werf T.S., Kerstjens H.A.M., de Lange W.C.M., Alffenaar J.C., Akkerman O.W. Treatment of MDR-TB using therapeutic drug monitoring: first experiences with sub-300 mg linezolid dosages using in-house made capsules. The European respiratory journal. 2019 doi: 10.1183/13993003.00580-2019. [DOI] [PubMed] [Google Scholar]
- 24.Sotgiu G., Centis R., D'Ambrosio L., Alffenaar J.W., Anger H.A., Caminero J.A. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. The European respiratory journal. 2012;40:1430–1442. doi: 10.1183/09031936.00022912. [DOI] [PubMed] [Google Scholar]
- 25.Conradie F., Diacon A.H., Ngubane N., Howell P., Everitt D., Crook A.M. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. The New England journal of medicine. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S., Das M., Laxmeshwar C., Jonckheere S., Thi S.S., Isaakidis P. Linezolid-Associated Optic Neuropathy in Drug-Resistant Tuberculosis Patients in Mumbai, India. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0162138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z., Pang Y., Wang Y., Liu C., Zhao Y. Beijing genotype of Mycobacterium tuberculosis is significantly associated with linezolid resistance in multidrug-resistant and extensively drug-resistant tuberculosis in China. Int J Antimicrob Agents. 2014;43:231–235. doi: 10.1016/j.ijantimicag.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Wasserman S., Louw G., Ramangoaela L., Barber G., Hayes C., Omar S.V. Linezolid resistance in patients with drug-resistant TB and treatment failure in South Africa. The Journal of antimicrobial chemotherapy. 2019;74:2377–2384. doi: 10.1093/jac/dkz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimenkov D.V., Nosova E.Y., Kulagina E.V., Antonova O.V., Arslanbaeva L.R., Isakova A.I. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. The Journal of antimicrobial chemotherapy. 2017;72:1901–1906. doi: 10.1093/jac/dkx094. [DOI] [PubMed] [Google Scholar]
- 30.Huang T.S., Liu Y.C., Sy C.L., Chen Y.S., Tu H.Z., Chen B.C. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis complex isolated in Taiwan over 10 years. Antimicrob Agents Chemother. 2008;52:2226–2227. doi: 10.1128/aac.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen K.A., Abeel T., Manson McGuire A., Desjardins C.A., Munsamy V., Shea T.P. Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium tuberculosis Isolates from KwaZulu-Natal. PLoS Med. 2015;12 doi: 10.1186/s13073-015-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun G., Luo T., Yang C., Dong X., Li J., Zhu Y. Dynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patients. J Infect Dis. 2012;206:1724–1733. doi: 10.1093/infdis/jis601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang S., Yao L., Hao X., Zhang X., Liu G., Liu X. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. The European respiratory journal. 2015;45:161–170. doi: 10.1183/09031936.00035114. [DOI] [PubMed] [Google Scholar]
- 34.Udwadia ZF, Sen T, Moharil G. Assessment of linezolid efficacy and safety in MDR- and XDR-TB: an Indian perspective. The European respiratory journal 2010;35:936-938; author reply 938-940. doi: 10.1183/09031936.00132009. [DOI] [PubMed]
- 35.Agyeman A.A., Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2016;15:41. doi: 10.1186/s12941-016-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daneman N., Low D.E., McGeer A., Green K.A., Fisman D.N. At the threshold: defining clinically meaningful resistance thresholds for antibiotic choice in community-acquired pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46:1131–1138. doi: 10.1086/529440. [DOI] [PubMed] [Google Scholar]