Abstract
India is the second most populous country in the world with a population of nearly 1.3 billion, comprising 20% of the global population. There are an estimated 37.5 million cases of asthma in India, and recent studies have reported a rise in prevalence of allergic rhinitis and asthma.
Overall, 40–50% of paediatric asthma cases in India are uncontrolled or severe. Treatment of allergic rhinitis and asthma is sub-optimal in a significant proportion of cases due to multiple factors relating to unaffordability to buy medications, low national gross domestic product, religious beliefs, myths and stigma regarding chronic ailment, illiteracy, lack of allergy specialists, and lack of access to allergen-specific immunotherapy for allergic rhinitis and biologics for severe asthma. High quality allergen extracts for skin tests and adrenaline auto-injectors are currently not available in India. Higher postgraduate specialist training programmes in Allergy and Immunology are also not available.
Another major challenge for the vast majority of the Indian population is an unacceptably high level of exposure to particulate matter (PM)2.5 generated from traffic pollution and use of fossil fuel and biomass fuel and burning of incense sticks and mosquito coils.
This review provides an overview of the burden of allergic disorders in India. It appraises current evidence and justifies an urgent need for a strategic multipronged approach to enhance quality of care for allergic disorders. This may include creating an infrastructure for education and training of healthcare professionals and patients and involving regulatory authorities for making essential treatments accessible at subsidised prices. It calls for research into better phenotypic characterisation of allergic disorders, as evidence generated from high income western countries are not directly applicable to India, due to important confounders such as ethnicity, air pollution, high rates of parasitic infestation, and other infections.
Abbreviations: A&I, Allergy and Immunology; AB-NHPS, Ayushman Bharath National Health Protection Scheme; ABPA, Allergic Bronchopulmonary Aspergillosis; AD, Atopic Dermatitis; BTS, British Thoracic Society; CME, Continuing Medical Education; COPD, Chronic Obstructive Pulmonary Disease; DALY, Disability Associated Life Years; DBPCFC, Double Blind Placebo Controlled Food Challenge; ELISA, Enzyme Linked Immunosorbent Assay; ETS, Environmental Tobacco Smoke; GDP, Gross Domestic Product; GINA, Global Initiative for Asthma; ICAAI, Indian College of Allergy Asthma and Applied Immunology; IHDS, Indian Human Development Survey; INR, Indian Rupees; ISAAC, International Study of Asthma and Allergies in Childhood; NMBA, Neuromuscular blocking agents; PAFs, Population Attributable Factors; SAFS, Severe Asthma and Fungal Sensitisation; SCIT, Subcutaneous Injection Immunotherapy; SLIT, Sublingual Immunotherapy; SPT, Skin Prick Test; USA, United States of America; USD, United States Dollars; WHO, World Health Organization
Introduction
India has a population of nearly 1.3 billion, and this translates to 20% of the global population. India has a great diversity with respect to its environment, rates and types of infections, socio-economic strata within population, diet, culture, language, religious beliefs, literacy, aero-biology, and climate. Allergy and allergic disorders result from a complex interaction between genetic, environmental, and multiple lifestyle factors; thus, a country such as India offers an excellent platform to investigate epidemiology and natural course of allergic diseases.
Most published literature regarding atopy and allergic conditions comes from high-income English speaking countries, which may not be directly applicable to low-middle income countries such as India due to fundamental differences in genetic, environmental, and lifestyle factors. There is emerging evidence regarding a rising prevalence of allergic and other immune-mediated diseases in India and other countries in South Asia.1, 2, 3, 4, 5
This review aims to provide an overview of allergies and allergic conditions in India, with a focus on disease burden, gaps in evidence, and current barriers and challenges for patients, their caregivers, and healthcare professionals in the delivery of an evidenced-based high quality asthma and allergy care.
Disease burden
Asthma
India has a substantial burden of respiratory disorders, with chronic obstructive pulmonary disease (COPD) and asthma comprising the vast majority.6 Salvi et al., in a 1 day point-prevalence study (POSEIDON study6) conducted across 880 Indian cities and towns, in 204,912 patients, analysed data on 554,146 reasons for visit to a doctor. Respiratory symptoms were the most common cause of presentation to a physician in 51% of all patients included in the study and in 65% of paediatric age group, and obstructive airways disease was as one of the most common diagnosis reported.6 The Global Burden of Disease study 1990–2016 reported an estimate of 37.9 million (35·7–40·2) cases of asthma in 2016.7 The Death and Disability Associated Life Years (DALY) per case of asthma in India was 2.4-fold higher than the global average.7 The second round of Indian Human Development Survey (IHDS-II) was conducted in 2011–12 and involved all states and union territories except Lakshadweep and Andaman and Nicobar Islands, and it included 204,568 participants from 42,152 homes in 1503 villages and 971 urban sites.8 The authors reported that the overall asthma prevalence had increased from 41.9/1000 to 54.9/1000 population between 2004–5 and 2011-12.8 The prevalence was higher in poorer Indian states, and higher in Northern states with low rates in North Eastern states.8 The odds of reporting asthma was significantly higher amongst participants with lower literacy, lower socio-economic background, and those belonging to homes using unclean fuels. The authors went on to compute Population Attributable Fractions (PAFs) to estimate overall and risk factor specific burden of asthma in 2015.8 Out of an estimated 65 million cases a staggering burden of 80%, 78%, and 52% was attributable to use of fire wood, kerosene, and cow dung cakes as fuels, respectively.8 This translated to avoidable cases amounting to 16 million and 11 million, respectively, if firewood and kerosene/cow dung were not used.8
The data on prevalence of asthma and asthma symptoms among children in India are from the International Study of Allergy and Asthma in Children (ISAAC) studies.1,3 In comparison to high-income English speaking countries, India had a relatively lower prevalence. Overall prevalence rates of “ever asthma” in children aged 6–7 years and 13–14 years were 3.5% and 4.5%, respectively. The prevalence of asthma symptoms in the older age group was 6.0% for wheeze, 9.5% for "wheeze on exertion", and 14% for nocturnal cough. Prevalence rates from the capital city of Chandigarh, in Punjab state were lower at 3.5% for asthma, 4.2% for wheeze, and 8% for "exertional wheeze" and nocturnal cough in the age group 13–14 years. The phase III ISAAC study reported a prevalence of 7–20%. Paramesh reported a rising prevalence of asthma in a hospital-based outpatient study over a period of 20 years (1979–1999).9 The prevalence rate rose from 9% to 29.5% over a span of 20 years and was influenced by demographic changes.
In a recent study, the authors extrapolated an average yearly expense to the overall prevalence of asthma.10 Even at a lower prevalence rate of around 2%, the estimated yearly economic burden in India was a whopping INR 71 billon (~USD 7 billion) per year. This should be interpreted in conjunction with India's per capita GDP ($2172 USD). The overall cost could be reduced significantly if a "shared care" concept between the doctors and peripheral health workers was implemented, especially in rural areas of India.11 Health insurance is not well established in India. Nearly 70% of the population pay out-of-pocket for both inpatient and outpatient care including medications. This results in a huge strain on the population, particularly for those belonging to the lower socio-economic group. Only 2 states out of 28 Indian states have introduced free distribution of basic asthma medications in all government medical institutions, where 70% of the public seek care.12
Risk factors
It is recognised that allergic disorders are influenced by multiple environmental and lifestyle factors. India has undergone major socioeconomic changes in the new millennium, at least in part due to greater international travel and westernisation within society, and a very high burden of indoor and outdoor air pollution. Environmental Tobacco Smoke (ETS) is a major issue both in rural and urban homes. A study carried out in Chandigarh, Punjab state, reported that adolescents living with parents who were smokers had a higher incidence and morbidity due to asthma compared to households with a non-smoking environment.13
India has one of the highest burdens of air pollution globally.14 The main sources are vehicular exhausts, biomass fuels, and fossil fuels. Nearly 55.5% of the population use solid fuels, as high as 75% in some states. Nearly 77% of the Indian population is exposed to an annual weighted mean particulate matter (PM)2.5 levels of >40mcg/m3, a threshold recommended by National Ambient Air Quality Standards, which exceeds the limit set by the World Health Organization (WHO) (<10mcg/m3).14 PM2.5 levels are particularly high (>125 mcg/m3), in New Delhi, Uttar Pradesh, Bihar, and Haryana. Exposure to PM2.5 is associated with an adverse impact on respiratory diseases including asthma and COPD, as well as vascular events and mortality.14 India had nearly 26% of global air pollution Disability Adjusted Life Years (DALYS) in 2017.14
Salvi et al reported in the Asia Pacific-Asthma Insights and Management (AP-AIM) study that India had the lowest score for asthma management.15 The common triggers for asthmatics in India were dust (49%) and air pollution (49%), while only 5% reported pollen as a trigger. They reported high rates of oral corticosteroid use and only 36% and 50% of Indian asthmatics used preventer and rescue inhalers, with a majority preferring an oral route of asthma medication. This study clearly highlighted that asthma management in India remains suboptimal, with a significant proportion of patients experiencing uncontrolled disease that results in poor quality of life. There is a need for an urgent review of this situation and initiation of active measures at local as well as national levels to improve asthma care in India. In a multicentre study involving 73,605 adults, Aggarwal et al. reported risk factors for asthma including female gender, older age, urban setting, lower socioeconomic group, atopy, family history, and ETS exposure.16
High rates of sensitisation (51%) to aspergillus and allergic bronchopulmonary aspergillosis (ABPA; 38%) has been reported amongst Indian patients admitted with acute severe asthma to intensive care units.17 Also, children with evidence of sensitisation to fungi had higher rates of severe asthma compared to those without sensitisation (17.6% and 2.4% respectively).18 Agarwal et al reported estimates of ABPA and severe asthma with fungal sensitisation (SAFS) in the Indian adult population based on 2011 census.19 ABPA was 0.12–6.09 million cases and SAFS 0.52–1.21 million.19
WHO's "best buy" interventions did not include asthma management in its costing scenarios. Better evidence of effective asthma management in diverse health systems and contexts and proper delivery of care to patients from lower socioeconomic groups are required. The "essential medications" as per WHO guidelines does not include basic asthma medications such as inhaled corticosteroids. A comprehensive health insurance plan announced by the present government in India called Ayushman Bharat-National Health Protection Scheme (AB-NHPS; https://www.india.gov.in/spotlight/ayushman-bharat-national-health-protection-mission) covers only hospitalisations for poor patients, and not outpatient care. This could limit its role in contributing to the care of patients suffering from allergic disorders.
Allergic rhinitis
The prevalence of allergic rhinitis has gradually risen in India in the last 2 decades.20 This was greater in older children aged 13–14 years with relatively smaller increase in 6–7 year-old children as reported in ISAAC phase-I and phase-III studies.1,4,21 Nasal symptoms were present in 12.5% and 18.6% of 6–7 year-old and 13–14 year-old children, respectively, in ISAAC phase-I and 12.9% and 23.6% in ISAAC phase-III studies, respectively. Prevalence of rhinoconjunctivitis showed a similar pattern of increase over the same period.4,20 Allergic rhinitis and asthma coexist in 70–80% of Indian patients.22,23 Allergic rhinitis has been reported to have a significant adverse impact on health-related quality of life in Indian patients.22,23
Common aeroallergens of relevance to allergic rhinitis and asthma in India include house dust mite, cockroach, pollen, and mould spores.22 A seminal study from Eastern India revealed that 96% of patients with naso-bronchial allergy showed sensitisation to house dust mite with predominant mites being Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Blomia tropicalis at 75.06%, 63.72%, and 72% respectively.24 Similar studies from Western, Northern, and Southern India revealed somewhat lower rates of sensitisation, although D. pteronyssinus was the major offending allergen.25,26 Dominance of D. pteronyssinus sensitisation is further validated by another recent study from Eastern India.27 In a study conducted amongst asthmatic children aged ≥5 years, common sensitisers included housefly antigen (36.7%), rice grain dust (31%), cockroach (18%), and house dust mite (8%).28 Important cockroach species are Blatella germanica and Perplanata Americana. Some interesting differences have been reported in allergic rhinitis phenotypes between "sneezers and runners" versus "blockers", the latter showing significantly higher rates of sensitisation to dust mites and fungi as opposed to the former group which showed higher rates of pollen sensitisation.22 Amongst aspergilli, A. flavus showed the highest rate of sensitisation in allergic rhinitis.22
There are two pollen seasons in India, firstly tree pollen between February–April and grass pollen between September and December.22 Allergenically important pollens are Prosopis juliflora, Ricinus communis, Morus, Mallotus, Alnus, Quercus, Cedrus, Argemone, Amaranthus, Chenopodium, Holoptelea, Brassica, Cocos, Cannabis, Parthenium, Cassia, and grasses.29 The local vegetation determines the predominant pollen in the ambient air of that region, thus leading to aerobiological diversity.30
Table-1 summarises common aero-allergens of relevance to India.
Table 1.
Important pollen grains and air borne fungal spores in India
| POLLEN GRAINS30,66 | ||
|---|---|---|
| NAME OF PLANT | PEAK POLLINATION SEASON | POLLINATION PATTERN |
| Poaceae | Oct–Nov | Seasonal |
| Trema orientalis | Nov–Mar | Seasonal |
| Carica papaya | Nov–May | Seasonal |
| Aegle mermelos | Mar–June | Seasonal |
| Parthenium | Perennial | |
| Cyperaceae | Perennial | |
| Catharanthus roseus | Mar–June | Seasonal |
| Ricinus communis | Sept–Mar | Seasonal |
| Xanthium strumarium | Aug–Nov | Seasonal |
| Cheno Amaranthaceae | Perennial | |
| Cocos nucifera | Perennial | |
| Ocimum basilicum | May–June | Seasonal |
| Moringa oleifera | Dec–Mar | Seasonal |
| Areca catechu | Nov–Mar | Seasonal |
| Tridax procumbens | Sept–May | Perennial |
| Phoenix sylvestris | Nov–Apr | Seasonal |
| Helianthus annus | Perennial | |
| Azadirachta indica | Dec–May | Seasonal |
| Achyranthes aspera | Sept–Mar | Seasonal |
| Lantana camara | Sept–May | Perennial |
| Polygonum plebelum | Dec–June | Seasonal |
| Eclipta alba | Perennial | |
| Albizia lebbeck | Feb–June | Seasonal |
| Vernonia cinerea | Perennial | |
| Artocarpus heterophyllus | Mar–July | Seasonal |
| Adhetoda varica | Mar–July | Seasonal |
| Eucalyptus citriodora | Apr–Oct | Seasonal |
| Borassus flabellifur | Feb–June | Seasonal |
| Psidium guajava | Mar–June | Seasonal |
| Cassia sophera | May–July | Seasonal |
| Delonix regia | Feb–June | Seasonal |
| Lagerstroemia reginae | Mar–June | Seasonal |
| Peltophorum pterocarpum | Mar–Aug | Seasonal |
| Mangifera indica | Jan–Apr | Seasonal |
|
Alstonia scholaris |
Sept–Nov |
Seasonal |
| FUNGAL SPORES66,67 | ||
|
NAME OF FUNGUS |
PEAK SPORING SEASON |
|
| Alternaria spp | Early June | |
| Aspergilli/Penicilli | Oct–Nov | |
| Chaetomium sp | Perennial | |
| Cladosporium sp | Dec | |
| Coprinus sp | Perennial | |
| Curvularia sp | July–Aug | |
| Drechslera hawaiiensis | July–Aug | |
| Nigrospora sphaerica | Late summer | |
Atopic dermatitis
The prevalence of atopic dermatitis (AD) is wide ranging (3.0–20.5%) globally as per ISAAC studies.1,31,32 Comparison of data between ISAAC phase I and III has revealed that worldwide prevalence of AD is rising, especially in younger children.31,32 The prevalence of AD in India is lower in comparison to other countries as per ISAAC phase I and III study reports. Importantly, there has not been any significant increase in prevalence of AD in India. All the Indian participating centres in ISAAC phase I study (except Kottayam in Kerala) reported a 12-month period prevalence between 2.4% and 6%. In ISAAC phase III, most of the participating Indian centres showed low prevalence rates (<5%) of AD, similar to phase I data.1,32
Hospital-based studies from Northern and Eastern part of the country reported low prevalence among dermatology outpatients at 0.42% and 0.55%, respectively.33 Prevalence rates were much lower than those reported in ISAAC studies, probably due to strict criteria (Hannifin and Rajka criteria) for diagnosis of AD adopted for the evaluation of the skin lesions by specialist doctors.
Racial differences in prevalence has been reported, with higher rates in Africa and Oceania, in contrast to lower rates in India and Eastern and Northern Europe. Filaggrin loss-of-function mutations occur in up to 50% of European and 27% of Asian patients with AD, and they are 6 times less common in African American than in European American patients.34
Food allergy
Cohort studies from The Isle of Wight, UK, have shown that prevalence of peanut allergy has risen during the last 2-3 decades.35 In a population survey involving 38,480 children in the United States, prevalence of food allergy was reported amongst 8%, with multiple food allergies in nearly a third of this population.36 The most common food allergies reported were peanut, milk, and shellfish.36 In a cross-sectional study involving adults from 15 countries in the European community respiratory health survey, 12% of the participants reported adverse reactions (allergy and intolerance) to food.37 This was however not confirmed with allergy testing and food challenges. In contrast to high income countries, a recently conducted EuroPrevall-INCO survey revealed food allergy to be 0.14% among children from 2 participating Indian centres.38 Interestingly, sensitisation rate was quite high (19.1%) to food allergens in Indian children in this survey.38 In other words, despite high sensitisation rates, the prevalence of clinically relevant food allergies was extremely low. Despite high consumption of peanuts in different foods in India, peanut allergy in children was found to be quite low (0.03%), although peanut sensitisation was high at 6.3%.38 Health Nuts study from Australia showed that the odds of peanut allergy was three-fold greater amongst children born to parents born in East Asia as opposed to children born to parents born in Australia.39
The EuroPrevall-INCO study in an adult population conducted in 2 centres in Karnataka state showed a very high sensitisation rate of 26.5%, whereas rates of "probable food allergy" were low at 1.2% (see Table- 2 for details).40 Further research is needed to investigate why prevalence of clinically relevant food allergy is low in India despite high rates of sensitisation, in contrast to relatively high rates of allergic rhinitis and chronic asthma.
Table 2.
Food allergen sensitisation patterns in India
| Study, Author and Year | Place, Hospital, Community, Sample size (N) | Prevalence of sensitisation to Foods | Comments |
|---|---|---|---|
| Patil SP et al.68 2001 | Allergy clinic, Bombay, Western India, N = 1400 | Chickpea and other legumes were tested | Self-reported chickpea allergy - 59.; History and SPT positive for chickpea – 41; DBPCFC positive for chickpea - 31 |
| Kumari D et al.69 2006 | Tertiary care hospital, New Delhi, North India, N = 816 | Blackgram | Self-reported black gram – 35/816.; History and SPT positive for black gram 16/35–12.1%; History and specific IgE to black gram – 14/35; DBPCFC – 4/14 subjects where it was performed |
| Kumar R70 et al., 2006 | Tertiary care hospital, New Delhi, North India, N = 216 | 20 foods, Rice, Blackgram, Lentils, Citrus fruits, Pea, Maize, Lima bean, French bean | Sensitisation rates: Rice – 11.1%; Black gram – 10.1%; Lentils – 9.7%; Citrus fruits – 9.2%; Pea – 6%; Maize – 6%; Lima bean – 5.5%; French bean - 5.5% |
| Kumar R et al.71 2007 | Tertiary care hospital, New Delhi, North India, N = 1200 | Rice | Self-reported Rice allergy – 165/1200; History and SPT positive for rice; 20/165–12.1%; History and specific IgE to rice – 13/165; DBPCFC – 6/10 subjects where it was performed |
| Harish Babu et al.72 2008 | Community, Mysore, South India, Adults N = 741 | Only eggplant was tested | Self-reported eggplant allergy −9.2%; History and SPT positive for eggplant – 4.3%; History and specific IgE to eggplant – 0.8% |
| Misra A et al.73 2008 | Hospital, Lucknow, North India, N = 76 | Legumes | Sensitisation to legumes 30; History and SPT positive 6 |
| Mandal J et al.74 2009 | Allergy unit in a tertiary care hospital, Kolkotta, Eastern India, N = - 684 | Cereals, milk, egg, fish, fruits and vegetables | Sensitisation to Prawn – 53.5%; Banana – 40.6%; Brinjal – 45.6%; Wheat – 28.4%; Egg – 34.9%; Milk – 20.5% |
| Kumar R et al.75 2010 | Tertiary care hospital, New Delhi, North India, N = 1860 | Rice, Black gram, Lentil, Citrus fruits, Pea, Maize, Banana | Sensitisation rates: Rice 6.2%; Black gram 5.9%; Lentil 5.5%; Citrus fruits 5.3%; Pea 3.8%; Maize 3.8%;Banana 3.6% |
| Dey D et al.76 2014 | Clinic in Kolkota, Eastern India, N = 5161 | 46 foods Banana, Brinjal, Lentils, Wheat, Egg | Sensitisation rates: Bananas 32.4%; Brinjal – 29.4%; Lentils – 10.4%;Wheat - 21.7%; Egg – 23.1% |
| Mahesh PA et al.,40 2016 | Randomly selected General population from Mysore and Bangalore (Part of an International study – Europrevall), AdultsScreened N = 10,931, Case Control Study, N = 587 | 24 Europrevall priority foods fish, cow's milk, egg, mustard seed, soya bean, peanut,lentil, wheat, buckwheat, walnut, poppy seed, melon, sunflower,corn, banana, sesame, shrimp, tomato, kiwi, carrot,celery, apple, peach and hazelnut) | % Sensitisation and probable allergy: Wheat – 11.93% and 0.02% ;Peanut – 8.73% and 0.0%; Egg – 2.56% and 0.05%; Milk – 2.71% and 0.50%; Fish – 0.5% and 0.0% ;Shrimp – 15.53% and 0.0%; Apple – 7.27% and 0.50% (Sensitisation rates were measured by specific IgE and probable allergy needed a positive history of allergic symptoms within 2 h of consumption of the food and either a positive specific IgE or SPT) |
| Chogtu B et al.77 2017 | University teaching Hospital in Karnataka, N = 2219 | 31 foods Red gram, Green gram, Red kidney beans, Wheat, Egg | Sensitisation rates: Red gram 12.6%; Green gram – 12.5%; Red kidney beans – 10.9%; Wheat - 9.6%; Egg – 6.9% |
| Gobinaath T R et al.78 2018 | School children urban and rural schools, N = 350 | 10 foods Prawn, Peanut, Fish, Milk, Banana | Sensitisation rates: Prawn – 17.7% (urban), 5.7% (rural); Peanut – 19.6% (urban), 10.4% (rural); Fish - 17.7% (urban), 5.7% (rural); Milk - 17.7% (urban), 5.2% (rural); Banana – 2.5% (urban), 1.0% (rural) |
| Li J et al.,38 2019 | Randomly selected general population from Mysore and Bangalore (Part of an International study – Europrevall, which screened 35,549, in China, India and Russia), Children screened N = 5677, Case control study, N = 450 | 25 Europrevall priority foods hen's egg, cow's milk, peanut, soy, hazelnut, walnut, celery, kiwi, apple, peach, sesame, mustard, wheat,fish, shrimp, buckwheat, corn, carrot, rice, tomato, melon, banana, lentils, sunflower seeds and poppy seeds. | % Sensitisation and probable allergy: Wheat – 6.7% and 0.0%; Peanut – 6.3% and 0.0% Egg – 1.7% and 0.05%; Milk – 2.1% and 0.0%; Fish – 0.4% and 0.0%; Shrimp – 10.3% and 0.0%; Apple – 4.2% and 0.0%, (Sensitisation rates were measured by specific IgE and probable allergy needed a positive history of allergic symptoms within 2 h of consumption of the food and either a positive specific IgE or SPT) |
Table-2 summarises studies conducted in India investigating common food allergen sensitisation patterns.
Drug allergy
Adverse drug reactions (ADRs) are broadly classified into immunological and non-immunological with the vast majority being non-immunological.41 Reports from western countries suggest that up to 30% of ADRs in western countries are allergic or pseudo-allergic.42 Available reports from India suggest that cutaneous ADRs occur in 2–5% of inpatients.43, 44, 45, 46 A study conducted at a dermatology out-patient department reported that the common offending drug groups implicated in ADRs included antimicrobials (34%), anticonvulsants (33%), and antiinflammatory drugs (21.5%).47 Other less frequent drugs were antipsychotics, antidepressants, antihypertensives, oral contraceptives, antidiabetics, insulin, vaccines, radiocontrast media, pancreatic enzyme supplements, homeopathic, and ayurvedic preparations. The most common offending drugs were carbamazepine (16.3%), phenytoin (15%), and cotrimoxazole (13.5%).45 The under-reporting of ADRs is a major challenge in India.48 Although, there are approximately 90 ADR monitoring centres in India, their functional rate is modest at 56.5%.48
The lack of knowledge and awareness about the Pharmacovigilance Programme of India (PvPI), lack of a robust framework and infrastructure, including regulatory authority involvement, population burden, workload, and gaps in knowledge and skill in allergy amongst healthcare professionals, were found to be some of the factors responsible for underreporting of ADRs.49 A recent study by the WHO reported a wide gradient between ADR reporting rates between high-income countries and low-income countries.50 There is an urgent need to strengthen ADR reporting mechanisms in the Indian healthcare system. However, setting up an infrastructure with a central facility collating this crucial information is challenging in a low-middle income country such as India with substantial disparity in healthcare delivery between government and corporate sectors and between rural and urban centres.
Anaphylaxis
Epidemiological data regarding anaphylaxis are lacking in India. A recent single centre study in South India reported that knowledge of anaphylaxis and its management was suboptimal amongst junior doctors and medical and nursing students.51 The true estimates of incidence are not available, probably due to poor recognition and poor reporting and coding systems in Indian healthcare systems. A systematic review on drug-induced anaphylaxis in India from published studies between 1998 and 2013 reported reactions occurring in peri-operative settings (53.7%), wards (20.4%), and home (11%) with the main culprits being antimicrobials (18.5%), nonsteroidal antiinflammatory drugs (13%), and neuromuscular blockers (13%).52 In a survey conducted amongst 242 Indian anaesthetists, 67% had encountered peri-operative anaphylaxis.53 However, only 10% had requested for allergy tests, with only 38% having access to allergy testing.53 Interestingly, opioids were the most commonly implicated agents, as this is seldom reported as a trigger in the western literature.54,55 This needs cautious interpretation as an allergy specialist's input is critical for all cases, and this is currently lacking. The second most common group of drugs implicated were neuromuscular blocking agents (NMBAs) followed by others including colloids, antimicrobials, and blood products53
A study carried out in Birmingham, UK reported that South Asian British children were at a higher risk of severe anaphylaxis as opposed to Caucasian white British children.56 Similarly, it has been reported that Asian children born in Australia were at a higher risk of food and non-food related anaphylaxis in comparison to non-Asian children born in Australia.57 Higher rates of atopic dermatitis and food allergy have been reported amongst children born in Australia to Asian-born mothers than non-Asian children and similarly Asian immigrant children in the United States showed a higher risk of atopic dermatitis and food allergy.57,58 These studies highlight a role for gene-environment interaction, and further multicentre studies are warranted in the native Indian population for a meaningful comparison of data reported amongst immigrant Indian population in English speaking countries.
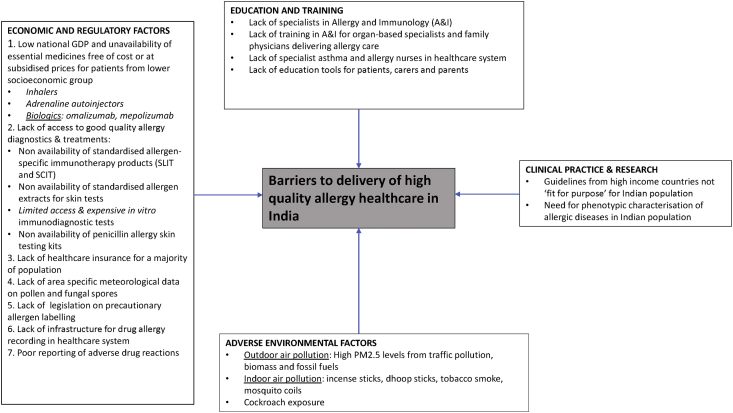
Current barriers (Fig. 1)
Fig. 1.
Barriers to delivery of high-quality allergy healthcare in India
Education and training
There are several gaps in the state of training and practice of allergy in India. Some of the key contributing factors are discussed as follows: Allergy & Immunology (A&I) does not have recognised specialty status in India. There are no postgraduate training programmes in A&I offered by the medical council of India. However, it has offered a diploma programme in A&I and outlined the objectives, skill sets, competencies, detailed syllabus, training, and assessment methods, for which there are not many takers. Allergic diseases are managed by specialists in internal medicine, pulmonary medicine, oto-rhino-laryngology, general paediatrics, and dermatology departments. The A&I societies in India such as the Indian College of Allergy Asthma and Applied Immunology (ICAAI) and Indian Academy of Allergy (IAA) have endeavoured to address the need through conferences, CMEs, and workshops. Some institutions have offered short training programmes for several decades. However, none of these courses are recognised by the Medical Council of India to date.
Lack of India-specific scientific data and guidelines
Current management protocols in India are largely based on guidelines adapted from data generated in western countries such as the GINA and the British Thoracic Society (BTS) guidelines and may not be "fit for purpose" in the Indian clinical practice. There is paucity of strong and real-world studies to confirm the adaptability of these guidelines to the Indian population. Parasitic infestations are common in India. A community study in children showed that 1 in 3 may have a parasitic infestation.59 Due to higher rates of parasitic infestations in India, there may be a higher peripheral blood eosinophils count in comparison to the western population. Indians are also high serum total IgE producers, similar to other African and Asian populations. The only study of serum total IgE in the general population in India was from the Mysore-Bangalore region in South India and demonstrated a mean IgE of 522.19 IU/ml in adults as compared to mean levels of 40.8 IU/ml in the western population.40,60 Furthermore, infectious diseases such as tuberculosis and filariasis are also known to drive peripheral blood eosinophilia, and they cause an elevated serum total IgE.61, 62, 63
Lack of aerobiological data
Most important issues impeding good clinical practice for an allergist in India is unavailability of local aerobiological data regarding pollens and fungal spores. A vast majority of the population in India reside in areas where there has never been an aerobiological study.64 This makes it challenging for the allergists to select an aeroallergen panel that is relevant to the region.
Poor asthma control and likely causes
As stated previously, the AP-AIM study observed that the majority of the asthmatics in India had poorly controlled disease, 89% of the subjects used oral corticosteroids at an average of 10.5 times a year, and most subjects preferred oral medications to inhalers.15 Key issues responsible for poor asthma management include social factors, religious beliefs, taboos, feeling that inhalers are "too powerful" and may cause side effects, the fear of habituation or even addiction to these medications, costs of medications, and the ease of use of oral medications, especially among illiterate subjects. Another important reason for poor compliance is the belief that medications need to be taken for relief of symptoms only and not for control and symptom prevention. Most doctors are not able to spend time to counsel their patients due to heavy workload. The lack of asthma nurses and nurse practitioners and the scarcity of respiratory therapists and other trained healthcare workers make it challenging to train asthmatics and their caregivers on self-management. The high rates of illiteracy in many places and linguistic diversity in India also make it challenging to develop education tools for patients. Linguistic (about 22 languages and 720 dialects) and socio-cultural diversity make developing a standardised educational tool in India a challenging task.
Lack of appropriate diagnostics
Another major barrier is unavailability of standardised allergen extracts (both aeroallergens and food allergens). None of the allergens available currently in the Indian market are standardised, resulting in variations, making clinical interpretation difficult. Christopher et al reported that the allergen extracts of D. pteronyssinus and D. farinae manufactured in India were far less potent than US FDA approved allergen extracts.65 There are difficulties in obtaining a 1 mm tip skin prick test lancet, so allergists employ a 3 mm lancet or a 26-gauge disposable needle for testing. Similarly, drug allergy tests are not standardised, and kits for penicillin allergy testing are not available. Few centres offer serum specific IgE testing, and many laboratories operate multiple collection points for blood collection and transport to a central facility. ImmunoCAP and ELISA kits are available, but most of these are for European pollens that are probably relevant only to the Himalayan belt. Comprehensive component resolved diagnostics (CRDs) tests are not available in India. More research is needed to develop CRDs for pollens and foods relevant to India.
Lack of access to medications
Most of the allergen-specific immunotherapy products available in the Indian market are aqueous extracts that are not standardised. Sublingual immunotherapy is not easily accessible and is not yet approved by the Government of India. Most of the allergy testing and allergen specific immunotherapy, although economical in high income countries, are still not affordable for patients belonging to lower socioeconomic groups in India, as most people do not have health insurance. Adrenaline auto-injectors are also not available in the Indian market. Since the shelf-life is only 12 months, they are only sourced by the distributor, on order in some of the metropolitan cities, but are quite expensive and unaffordable for patients belonging to lower socio-economic strata. At present, patients are advised to carry pre-drawn adrenaline in a 1 ml tuberculin syringe or a 2 ml syringe for self-management of anaphylaxis. This does not meet basic international health and safety standards and poses a risk to the user.
Inconsistent precautionary food allergy labelling
Another important challenge for patients is inconsistent precautionary food allergy labelling. This is not a legislative requirement in India, and it is compounded by poor awareness of food allergy and risk of anaphylaxis for consumers. A significant proportion of the population consume foods from street vendors who are beyond the ambit of regulations and appropriate precautions to be followed. There is substantial risk of cross contamination of allergens and risk of anaphylaxis.
Future directions
Allergic disorders constitute a global health concern, with India being no exception. Although the prevalence rates are relatively lower in India compared to high-income countries, the overall disease burden, based on various estimates, is in the order of several million cases. However, as described in previous sections, there are major challenges to the provision of high-quality asthma and allergy care.
How to best respond? First, there is a need to raise awareness of this growing epidemic, within the medical community and amongst national policy makers and the public at large. Educational tools should be created and disseminated in multiple languages via multiple platforms including social media, and patient support organisations need to disseminate knowledge and skills to patients and caregivers. In this regard, partnership with regional, national, and international allergy societies may be beneficial.
Second, there is a need to convince the federal and the state governments to allocate more funding for allergy research. Long-term epidemiologic studies are required to better understand the burden of allergic disorders in the Indian population and delineate specific endotypes and phenotypes. Such research will inevitably help identify potential gaps and barriers to care, formulate primary and secondary prevention strategies, and establish evidence-based clinical guidelines specific to Indian scenarios. In addition, further aerobiological studies regarding local pollen and fungal spores are needed to better characterise allergic sensitisation profiles of various communities across India.
Third, from a public health policy perspective, initiatives to reduce exposure to ETS and PM2.5 should be encouraged, namely reduction of biomass and fossil fuels and the use of incense/dhoop sticks and mosquito coils. Widespread adoption of such measures can potentially prevent, or at least reduce, the morbidity and mortality of asthma, especially among children.
Fourth, access to quality care and affordable life-saving medications should be a top priority. To that end, there is a need to appeal to the Medical Council of India to formally recognise allergy as an independent specialty and establish adequate postgraduate training programs in medical schools and hospitals. It is imperative that India trains highly skilled allergists equipped to diagnose and manage an increasing number of patients with complex allergic disorders in a cost-effective manner. Establishing partnerships between the government and the pharmaceutical industry to subsidise cost of life-saving medications to low-income patients would be helpful.
Finally, one of the reasons for the relatively low prevalence of allergic disorders in India compared to high income western countries may be explained by lifestyle and environmental factors contributing to biodiversity. The potential to flatten the allergy epidemic curve by protective measures such as nature conservation, pesticide avoidance, lifestyle adaptations, etc., should be considered going forward.
Submission declaration
This manuscript is not being considered elsewhere at present. The work described has not been published previously; it is not under consideration for publication elsewhere. The manuscript is approved by all authors. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder.
External funding
None.
Consent for publication
All authors agreed to publication of the work.
Contributions
MTK conceived the idea. All authors contributed to the writing and review of manuscript. All authors have approved this manuscript.
Availability of data and materials
N/A.
Ethical approval
N/A.
Declaration of Competing Interest
MTKs department received educational grant from ALK Abello, MEDA, Thermo Fisher and other companies for annual PracticAllergy course in recent years. MTK received funds from ALK Abello to attend EAACI conference. Other authors have no conflict of interest to declare.
Acknowledgements
N/A.
Footnotes
Full list of author information is available at the end of the article.
References
- 1.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351(9111):1225–1232. PubMed PMID: 9643741. [PubMed] [Google Scholar]
- 2.Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. PubMed PMID: 12239261. [DOI] [PubMed] [Google Scholar]
- 3.Lai C.K., Beasley R., Crane J. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64(6):476–483. doi: 10.1136/thx.2008.106609. PubMed PMID: 19237391. [DOI] [PubMed] [Google Scholar]
- 4.Bjorksten B., Clayton T., Ellwood P., Stewart A., Strachan D., Group I.P.I.S. Worldwide time trends for symptoms of rhinitis and conjunctivitis: phase III of the international study of asthma and allergies in childhood. Pediatr Allergy Immunol. 2008;19(2):110–124. doi: 10.1111/j.1399-3038.2007.00601.x. PubMed PMID: 17651373. [DOI] [PubMed] [Google Scholar]
- 5.Prideaux L., Kamm M.A., De Cruz P.P., Chan F.K., Ng S.C. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27(8):1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. PubMed PMID: 22497584. [DOI] [PubMed] [Google Scholar]
- 6.Salvi S., Apte K., Madas S. Symptoms and medical conditions in 204 912 patients visiting primary health-care practitioners in India: a 1-day point prevalence study (the POSEIDON study) Lancet Glob Health. 2015;3(12):e776–e784. doi: 10.1016/S2214-109X(15)00152-7. PubMed PMID: 26566749. [DOI] [PubMed] [Google Scholar]
- 7.Salvi S., Anil Kumar G., Dhaliwal R.S. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6(12):e1363–e1374. doi: 10.1016/S2214-109X(18)30409-1. PubMed PMID: 30219316; PubMed Central PMCID: PMCPMC6227385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P., Ram U. Patterns, factors associated and morbidity burden of asthma in India. PloS One. 2017;12(10) doi: 10.1371/journal.pone.0185938. PubMed PMID: 29073132; PubMed Central PMCID: PMCPMC5657621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paramesh H. Epidemiology of asthma in India. Indian J Pediatr. 2002;69(4):309–312. doi: 10.1007/bf02723216. PubMed PMID: 12019551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koul P.A., Dhar R. Economic burden of asthma in India. Lung India. 2018;35(4):281–283. doi: 10.4103/lungindia.lungindia_220_18. PubMed PMID: 29970764; PubMed Central PMCID: PMCPMC6034365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy K., Sastry J.G. 2005. NCMH Background Papers-Burden of Disease in India. National Commission on Macroeconomics and Health Ministry of Health & Family Welfare, Government of India. New Delhi. Economic Burden of Asthma; pp. 251–263. [Google Scholar]
- 12.Singh S., Sharma B.B., Sharma S.K., Sabir M., Singh V., investigators Ic Prevalence and severity of asthma among Indian school children aged between 6 and 14 years: associations with parental smoking and traffic pollution. J Asthma. 2016;53(3):238–244. doi: 10.3109/02770903.2015.1087558. PubMed PMID: 26365004. [DOI] [PubMed] [Google Scholar]
- 13.Gupta D., Aggarwal A.N., Kumar R., Jindal S.K. Prevalence of bronchial asthma and association with environmental tobacco smoke exposure in adolescent school children in Chandigarh, north India. J Asthma. 2001;38(6):501–507. doi: 10.1081/jas-100105871. PubMed PMID: 11642417. [DOI] [PubMed] [Google Scholar]
- 14.India State-Level Disease Burden Initiative Air Pollution C. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the Global Burden of Disease Study 2017. Lancet Planet Health. 2019;3(1):e26–e39. doi: 10.1016/S2542-5196(18)30261-4. PubMed PMID: 30528905; PubMed Central PMCID: PMCPMC6358127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvi S.S., Apte K.K., Dhar R. Asthma Insights and management in India: lessons learnt from the Asia pacific - asthma Insights and management (AP-AIM) study. J Assoc Phys India. 2015;63(9):36–43. PubMed PMID: 27608865. [PubMed] [Google Scholar]
- 16.Aggarwal A.N., Chaudhry K., Chhabra S.K. Prevalence and risk factors for bronchial asthma in Indian adults: a multicentre study. Indian J Chest Dis Allied Sci. 2006;48(1):13–22. PubMed PMID: 16482947. [PubMed] [Google Scholar]
- 17.Agarwal R., Nath A., Aggarwal A.N., Gupta D., Chakrabarti A. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses. 2010;53(2):138–143. doi: 10.1111/j.1439-0507.2008.01680.x. PubMed PMID: 19207831. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A., Singh M., Chakrabarti A., Mathew J.L., Rawat A. Correlation between fungal sensitisation in childhood persistent asthma and disease severity. Mycoses. 2018;61(3):195–200. doi: 10.1111/myc.12726. PubMed PMID: 29110335. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R., Denning D.W., Chakrabarti A. Estimation of the burden of chronic and allergic pulmonary aspergillosis in India. PloS One. 2014;9(12) doi: 10.1371/journal.pone.0114745. PubMed PMID: 25478929; PubMed Central PMCID: PMCPMC4257713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrika D. Allergic rhinitis in India: an overview. International Journal of Otorhinolaryngology and Head and Neck Surgery. 2017;3(1):1–6. [Google Scholar]
- 21.Singh S., Sharma B.B., Salvi S. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Res J. 2018;12(2):547–556. doi: 10.1111/crj.12561. PubMed PMID: 27663282. [DOI] [PubMed] [Google Scholar]
- 22.Shah A., Pawankar R. Allergic rhinitis and co-morbid asthma: perspective from India -- ARIA Asia-Pacific Workshop report. Asian Pac J Allergy Immunol. 2009;27(1):71–77. PubMed PMID: 19548632. [PubMed] [Google Scholar]
- 23.Mahesh P.A., Jayaraj B.S., Prabhakar A.K., Chaya S.K., Vijaysimha R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J Med Res. 2013;137(1):87–94. PubMed PMID: 23481056; PubMed Central PMCID: PMCPMC3657904. [PMC free article] [PubMed] [Google Scholar]
- 24.Saha G.K. House dust mite allergy in Calcutta, India: evaluation by RAST. Ann Allergy. 1993;70(4):305–309. PubMed PMID: 7682041. [PubMed] [Google Scholar]
- 25.Mahesh P.A., Kummeling I., Amrutha D.H., Vedanthan P.K. Effect of area of residence on patterns of aeroallergen sensitization in atopic patients. Am J Rhinol Allergy. 2010;24(5):e98–e103. doi: 10.2500/ajra.2010.24.3529. PubMed PMID: 21244724. [DOI] [PubMed] [Google Scholar]
- 26.Doshi A., Tripathi D.M. Early house dust mite sensitivity in Mumbai children. Indian J Pediatr. 2016;83(5):386–390. doi: 10.1007/s12098-015-1952-7. PubMed PMID: 26666904. [DOI] [PubMed] [Google Scholar]
- 27.Dey D., Mondal P., Laha A. Sensitization to common aeroallergens in the atopic population of West Bengal, India: an investigation by skin prick test. Int Arch Allergy Immunol. 2019;178(1):60–65. doi: 10.1159/000492584. PubMed PMID: 30257248. [DOI] [PubMed] [Google Scholar]
- 28.Raj D., Lodha R., Pandey A. Aeroallergen sensitization in childhood asthmatics in northern India. Indian Pediatr. 2013;50(12):1113–1118. doi: 10.1007/s13312-013-0304-9. PubMed PMID: 23999673. [DOI] [PubMed] [Google Scholar]
- 29.Singh A.B., Kumar P. Aeroallergens in clinical practice of allergy in India. An overview. Ann Agric Environ Med. 2003;10(2):131–136. PubMed PMID: 14677902. [PubMed] [Google Scholar]
- 30.Singh A.B., Mathur C. An aerobiological perspective in allergy and asthma. Asia Pac Allergy. 2012;2(3):210–222. doi: 10.5415/apallergy.2012.2.3.210. PubMed PMID: 22872824; PubMed Central PMCID: PMCPMC3406301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odhiambo J.A., Williams H.C., Clayton T.O., Robertson C.F., Asher M.I., Group I.P.T.S. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124(6):1251–1258 e23. doi: 10.1016/j.jaci.2009.10.009. PubMed PMID: 20004783. [DOI] [PubMed] [Google Scholar]
- 32.Williams H., Stewart A., von Mutius E. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121(4):947–954 e15. doi: 10.1016/j.jaci.2007.11.004. PubMed PMID: 18155278. [DOI] [PubMed] [Google Scholar]
- 33.Dhar S. Atopic dermatitis: Indian scenario. Indian J Dermatol Venereol Leprol. 1999;65(6):253–257. PubMed PMID: 20921678. [PubMed] [Google Scholar]
- 34.Brunner P.M., Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(5):449–455. doi: 10.1016/j.anai.2018.11.015. PubMed PMID: 30465859. [DOI] [PubMed] [Google Scholar]
- 35.Grundy J., Matthews S., Bateman B., Dean T., Arshad S.H. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110(5):784–789. doi: 10.1067/mai.2002.128802. PubMed PMID: 12417889. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R.S., Springston E.E., Warrier M.R. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17. doi: 10.1542/peds.2011-0204. PubMed PMID: 21690110. [DOI] [PubMed] [Google Scholar]
- 37.Woods R.K., Abramson M., Bailey M., Walters E.H. International prevalences of reported food allergies and intolerances. Comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991-1994. Eur J Clin Nutr. 2001;55(4):298–304. doi: 10.1038/sj.ejcn.1601159. PubMed PMID: 11360135. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Ogorodova L.M., Mahesh P.A. Comparative study of food allergies in children from China, India, and Russia: the EuroPrevall-INCO surveys. J Allergy Clin Immunol Pract. 2019 doi: 10.1016/j.jaip.2019.11.042. PubMed PMID: 31857266. [DOI] [PubMed] [Google Scholar]
- 39.Koplin J.J., Peters R.L., Ponsonby A.L. Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014;69(12):1639–1647. doi: 10.1111/all.12487. PubMed PMID: 25041549. [DOI] [PubMed] [Google Scholar]
- 40.Mahesh P.A., Wong G.W., Ogorodova L. Prevalence of food sensitization and probable food allergy among adults in India: the EuroPrevall INCO study. Allergy. 2016;71(7):1010–1019. doi: 10.1111/all.12868. PubMed PMID: 27297800. [DOI] [PubMed] [Google Scholar]
- 41.Mirakian R., Ewan P.W., Durham S.R. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39(1):43–61. doi: 10.1111/j.1365-2222.2008.03155.x. PubMed PMID: 19128352. [DOI] [PubMed] [Google Scholar]
- 42.Demoly P., Bousquet J. Epidemiology of drug allergy. Curr Opin Allergy Clin Immunol. 2001;1(4):305–310. doi: 10.1097/01.all.0000011031.16814.e0. PubMed PMID: 11964705. [DOI] [PubMed] [Google Scholar]
- 43.Uppal R., Jhaj R., Malhotr a.S. Adverse drug reactions among in patients in a north Indian referral hospital. Natl Med J India. 2000;13:16–18. [PubMed] [Google Scholar]
- 44.Pudukadan D., Thappa D.M. Adverse cutaneous drug reactions: clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol. 2004;70(1):20–24. PubMed PMID: 17642552. [PubMed] [Google Scholar]
- 45.Jhaj R., Uppal R., Malhotra S., Bhargava V.K. Cutaneous adverse reactions in in-patients in a tertiary care hospital. Indian J Dermatol Venereol Leprol. 1999;65(1):14–17. PubMed PMID: 20885028. [PubMed] [Google Scholar]
- 46.Noel M.V., Sushma M., Guido S. Cutaneous adverse drug reactions in hospitalized patients in a tertiary care centre. Indian J Pharmacol. 2004;36:292–295. [Google Scholar]
- 47.Chatterjee S., Ghosh A., Barbhuiya J., Dey S.K. Adverse cutaneous drug reactions: a one year survey at a dermatology outpatient clinic of a tertiary care hospital. Indian J Pharmacol. 2006;38:429–431. [Google Scholar]
- 48.Tandon V.R., Mahajan V., Khajuria V., Gillani Z. Under-reporting of adverse drug reactions: a challenge for pharmacovigilance in India. Indian J Pharmacol. 2015;47(1):65–71. doi: 10.4103/0253-7613.150344. PubMed PMID: 25821314; PubMed Central PMCID: PMCPMC4375822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S.A., Goyal C., Chandel N., Rafi M. Knowledge, attitudes, and practice of doctors to adverse drug reaction reporting in a teaching hospital in India: an observational study. J Nat Sci Biol Med. 2013;4(1):191–196. doi: 10.4103/0976-9668.107289. PubMed PMID: 23633861; PubMed Central PMCID: PMCPMC3633276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aagaard L., Strandell J., Melskens L., Petersen P.S., Holme Hansen E. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to VigiBase. Drug Saf. 2012;35(12):1171–1182. doi: 10.1007/BF03262002. doi: 10.2165/11631940-000000000-00000 10.1007/bf03262002. PubMed PMID: 23072620. [DOI] [PubMed] [Google Scholar]
- 51.Drupad H.S., Nagabushan H. Level of knowledge about anaphylaxis and its management among health care providers. Indian J Crit Care Med. 2015;19(7):412–415. doi: 10.4103/0972-5229.160288. PubMed PMID: 26180434; PubMed Central PMCID: PMCPMC4502494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel T.K., Patel P.B., Barvaliya M.J., Tripathi C.B. Drug-induced anaphylactic reactions in Indian population: a systematic review. Indian J Crit Care Med. 2014;18(12):796–806. doi: 10.4103/0972-5229.146313. PubMed PMID: 25538414; PubMed Central PMCID: PMCPMC4271279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi R., Sharma B., Sood J., Sehgal R., Chugh P. Anaphylaxis during anaesthesia: Indian scenario. Indian J Anaesth. 2017;61(5):387–392. doi: 10.4103/ija.IJA_80_17. PubMed PMID: 28584347; PubMed Central PMCID: PMCPMC5444216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishna MT, York M, Chin T, et al Multi-centre retrospective analysis of anaphylaxis during general anaesthesia in the United Kingdom: aetiology and diagnostic performance of acute serum tryptase. Clin Exp Immunol. 178(2):399-404. doi: 10.1111/cei.12424. Epub 2014/07/30.PubMed PMID: 25070464; PubMed Central PMCID: PMC4233389. [DOI] [PMC free article] [PubMed]
- 55.Mertes P.M., Laxenaire M.C., Alla F. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999-2000. Anesthesiology. 2003;99(3):536–545. doi: 10.1097/00000542-200309000-00007. Epub 2003/09/10. PubMed PMID: 12960536. [DOI] [PubMed] [Google Scholar]
- 56.Buka R.J., Crossman R.J., Melchior C.L. Anaphylaxis and ethnicity: higher incidence in British South Asians. Allergy. 2015;70(12):1580–1587. doi: 10.1111/all.12702. PubMed PMID: 26214068. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., Allen K.J., Suaini N.H.A., Peters R.L., Ponsonby A.L., Koplin J.J. Asian children living in Australia have a different profile of allergy and anaphylaxis than Australian-born children: a State-wide survey. Clin Exp Allergy. 2018;48(10):1317–1324. doi: 10.1111/cea.13235. PubMed PMID: 30025179. [DOI] [PubMed] [Google Scholar]
- 58.Dunlop J.H., Keet C.A. Allergic diseases among asian children in the United States. J Allergy Clin Immunol. 2019 doi: 10.1016/j.jaci.2019.08.009. PubMed PMID: 31449913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisht D., Verma A.K., Bharadwaj H.H. Intestinal parasitic infestation among children in a semi-urban Indian population. Tropenmed Parasitol. 2011;1(2):104–107. doi: 10.4103/2229-5070.86946. PubMed PMID: 23508675; PubMed Central PMCID: PMCPMC3593484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gergen P.J., Arbes S.J., Jr., Calatroni A., Mitchell H.E., Zeldin D.C. Total IgE levels and asthma prevalence in the US population: results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2009;124(3):447–453. doi: 10.1016/j.jaci.2009.06.011. PubMed PMID: 19647861; PubMed Central PMCID: PMCPMC2758573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prakash Babu S., Narasimhan P.B., Babu S. Eosinophil polymorphonuclear leukocytes in TB: what we know so far. Front Immunol. 2019;10:2639. doi: 10.3389/fimmu.2019.02639. PubMed PMID: 31798582; PubMed Central PMCID: PMCPMC6868031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yong A.J., Grange J.M., Tee R.D. Total and anti-mycobacterial IgE levels in serum from patients with tuberculosis and leprosy. Tubercle. 1989;70(4):273–279. doi: 10.1016/0041-3879(89)90021-4. PubMed PMID: 2516671. [DOI] [PubMed] [Google Scholar]
- 63.Ohrui T., Zayasu K., Sato E., Matsui T., Sekizawa K., Sasaki H. Pulmonary tuberculosis and serum IgE. Clin Exp Immunol. 2000;122(1):13–15. doi: 10.1046/j.1365-2249.2000.01291.x. PubMed PMID: 11012611; PubMed Central PMCID: PMCPMC1905744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh A.B. Glimpse of clinical aerobiology in India: an overview. Glob J Oto. 2017;12(3):555840. [Google Scholar]
- 65.Christopher D.J., Ashok N., Ravivarma A. Low potency of Indian dust mite allergen skin prick test extracts compared to FDA-approved extracts: a double-blinded randomized control trial. Allergy Rhinol (Providence) 2018;9 doi: 10.1177/2152656718796746. 2152656718796746. PubMed PMID: 30263870; PubMed Central PMCID: PMCPMC6156211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosal K., Pandey N., Bhattacharya S.G. Biomonitoring of pollen grains of a river bank suburban city, Konnagar, Calcutta, India, and its link and impact on local people. Ann Agric Environ Med. 2015;22(2):236–242. doi: 10.5604/12321966.1152072. PubMed PMID: 26094515. [DOI] [PubMed] [Google Scholar]
- 67.Das S., Gupta-Bhattacharya S. Enumerating outdoor aeromycota in suburban West Bengal, India, with reference to respiratory allergy and meteorological factors. Ann Agric Environ Med. 2008;15(1):105–112. PubMed PMID: 18581987. [PubMed] [Google Scholar]
- 68.Patil S.P., Niphadkar P.V., Bapat M.M. Chickpea: a major food allergen in the Indian subcontinent and its clinical and immunochemical correlation. Ann Allergy Asthma Immunol. 2001;87(2):140–145. doi: 10.1016/S1081-1206(10)62209-0. PubMed PMID: 11527247. [DOI] [PubMed] [Google Scholar]
- 69.Kumari D., Kumar R., Sridhara S., Arora N., Gaur S.N., Singh B.P. Sensitization to blackgram in patients with bronchial asthma and rhinitis: clinical evaluation and characterization of allergens. Allergy. 2006;61(1):104–110. doi: 10.1111/j.1398-9995.2006.00990.x. PubMed PMID: 16364164. [DOI] [PubMed] [Google Scholar]
- 70.Kumar R., Singh B.P., Srivastava P., Sridhara S., Arora N., Gaur S.N. Relevance of serum IgE estimation in allergic bronchial asthma with special reference to food allergy. Asian Pac J Allergy Immunol. 2006;24(4):191–199. PubMed PMID: 17348241. [PubMed] [Google Scholar]
- 71.Kumar R., Srivastava P., Kumari D. Rice (Oryza sativa) allergy in rhinitis and asthma patients: a clinico-immunological study. Immunobiology. 2007;212(2):141–147. doi: 10.1016/j.imbio.2006.11.006. PubMed PMID: 17336834. [DOI] [PubMed] [Google Scholar]
- 72.Harish Babu B.N., Mahesh P.A., Venkatesh Y.P. A cross-sectional study on the prevalence of food allergy to eggplant (Solanum melongena L.) reveals female predominance. Clin Exp Allergy. 2008;38(11):1795–1802. doi: 10.1111/j.1365-2222.2008.03076.x. PubMed PMID: 18681854. [DOI] [PubMed] [Google Scholar]
- 73.Misra A., Prasad R., Das M., Dwivedi P.D. Prevalence of legume sensitization in patients with naso-bronchial allergy. Immunopharmacol Immunotoxicol. 2008;30(3):529–542. doi: 10.1080/08923970802135294. PubMed PMID: 18618316. [DOI] [PubMed] [Google Scholar]
- 74.Mandal J., Das M., Roy I., Chatterjee S., Barui N.C., Gupta-Bhattacharya S. Immediate hypersensitivity to common food allergens: an investigation on food sensitization in respiratory allergic patients of Calcutta, India. World Allergy Organ J. 2009;2(1):9–12. doi: 10.1097/WOX.0b013e318194c0de. PubMed PMID: 23282888; PubMed Central PMCID: PMCPMC3650995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar R., Kumari D., Srivastava P. Identification of IgE-mediated food allergy and allergens in older children and adults with asthma and allergic rhinitis. Indian J Chest Dis Allied Sci. 2010;52:217–224. [PubMed] [Google Scholar]
- 76.Dey D., Ghosh N., Pandey N., Gupta Bhattacharya S. A hospital-based survey on food allergy in the population of Kolkata, India. Int Arch Allergy Immunol. 2014;164(3):218–221. doi: 10.1159/000365629. PubMed PMID: 25138428. [DOI] [PubMed] [Google Scholar]
- 77.Chogtu B., Magaji N., Magazine R., Acharya P.R. Pattern of allergen sensitivity among patients with bronchial asthma and/or allergic rhinosinusitis in a tertiary care centre of southern India. J Clin Diagn Res. 2017;11(8):OC01–OC4. doi: 10.7860/JCDR/2017/26973.10328. PubMed PMID: 28969175; PubMed Central PMCID: PMCPMC5620816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gobinaath T.R., Anand Arokiaraj L. Food allergy and allergens associated with bronchial asthma among school children in an urban and rural area of Puducherry, India: a cross-sectional study. Int J Contemp Pediatr. 2018 Jul;5(4):1623–1625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.