Summary
Photoelectrochemical CO2 reduction into syngas (a mixture of CO and H2) provides a promising route to mitigate greenhouse gas emissions and store intermittent solar energy into value-added chemicals. Design of photoelectrode with high energy conversion efficiency and controllable syngas composition is of central importance but remains challenging. Herein, we report a decoupling strategy using dual cocatalysts to tackle the challenge based on joint computational and experimental investigations. Density functional theory calculations indicate the optimization of syngas generation using a combination of fundamentally distinctive catalytic sites. Experimentally, by integrating spatially separated dual cocatalysts of a CO-generating catalyst and a H2-generating catalyst with GaN nanowires on planar Si photocathode, we report a record high applied bias photon-to-current efficiency of 1.88% and controllable syngas products with tunable CO/H2 ratios (0–10) under one-sun illumination. Moreover, unassisted solar CO2 reduction with a solar-to-syngas efficiency of 0.63% is demonstrated in a tandem photoelectrochemical cell.
Subject Areas: Catalysis, Electrochemical Energy Conversion, Nanomaterials
Graphical Abstract
Highlights
-
•
Combined experimental and theoretical investigations were performed
-
•
A record high applied bias photon-to-current efficiency of 1.88% was achieved
-
•
The CO/H2 ratio in the syngas product can be controllably tuned in a wide range
-
•
Unassisted syngas generation was proved in a tandem photoelectrochemical cell
Catalysis; Electrochemical Energy Conversion; Nanomaterials
Introduction
Photoelectrochemical (PEC) CO2 reduction represents a promising route to store intermittent solar energy into clean and sustainable chemical fuels, while simultaneously reducing atmospheric carbon emissions at ambient conditions (White et al., 2015; Zhang et al., 2018; Shan et al., 2019; Feng et al., 2020; Zhou et al., 2020). In aqueous solutions, CO2 reduction reaction (CO2RR) is difficult to compete with hydrogen evolution reaction (HER), since HER is kinetically more feasible owing to a two-electron transfer process and the high proton concentration in aqueous environment (Zhang et al., 2014; Qiu et al., 2015; Landers et al., 2018). In this context, the generation of syngas (a mixture of CO and H2) from a combination of CO2RR and HER renders the opportunity to utilize side product H2 and leverages well-established industrial processes (e.g., Fischer-Tropsch technology) for synthesizing long hydrocarbons and liquid fuels (Sheng et al., 2017).
In practical applications to upgrade syngas into downstream products, the ratio of CO/H2 plays a key role in tuning the product selectivity (e.g., 1:1, 1:2, and 1:3 for hydroformylation, methanol synthesis, and methanation reaction, respectively) (Kang et al., 2014; Foit et al., 2017; He et al., 2018; Ross et al., 2019; Lee et al., 2019). In industrial technology, the CO/H2 ratio was adjusted by use of the (reverse) water-gas shift process, which is costly and has a large carbon footprint. Therefore, it is desirable to develop an efficient PEC syngas generation system that can deliver controllable CO/H2 ratio. To date, a variety of photocathodes, such as p-Si (Kong et al., 2016; Song et al., 2017; Chu et al., 2016, 2018; Li et al., 2019), ZnTe (Jang et al., 2014, 2015), Cu2O (Schreier et al., 2015b; Deng et al., 2019b), and perovskite (Andrei et al., 2020), usually in conjunction with a CO2RR cocatalyst (e.g., Au, Ag, and molecular metal-complex), have been developed for PEC CO2 reduction into CO/syngas. However, it remains challenging to develop efficient syngas production with widely controllable CO/H2 ratio, as a single catalyst with a monofunctional site usually cannot provide balanced and simultaneously optimized CO2RR and HER activity. Herein, we report a decoupling strategy using spatially separated dual cocatalysts to tackle the challenge, on the basis of theoretical calculations and experimental verifications. By rationally assembling a Au CO-generating site and a Pt H2-generating site at the tip and side of GaN nanowires, respectively, a record high applied bias photon-to-current efficiency (ABPE) of 1.88% was achieved on planar Si photocathode. In addition, the CO/H2 ratio in the syngas product can be controllably tuned in a wide range between 1:99 and 10:1 by simply varying the composition of dual cocatalysts. We also construct a bias-free solar syngas generation device in a tandem PEC cell with a solar-to-syngas (STS) efficiency of 0.63%.
Results and Discussion
Theory-Guided Design of Enhanced Syngas Generation by a Decoupling Strategy
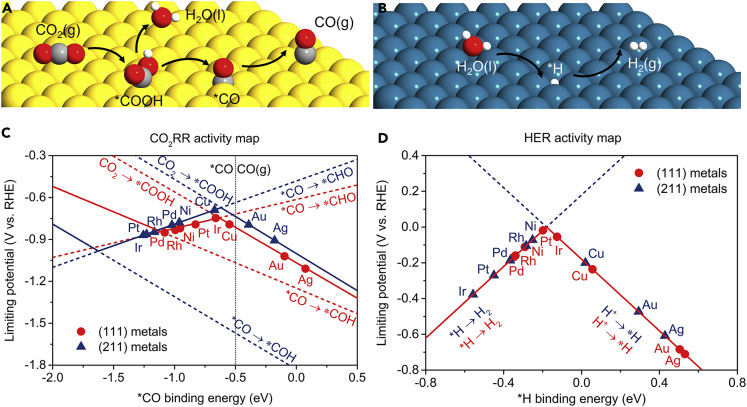
Density functional theory (DFT) studies were conducted first to gain insight into the design of high-performing catalyst for syngas generation from CO2 reduction at an atomistic level. CO2RR to CO proceeds via reaction intermediates of ∗COOH and ∗CO that bind metal surfaces through a carbon atom (Figure 1A), whereas HER takes place via H∗ intermediate (Figure 1B). The binding energies of key reaction intermediates, ∗COOH and ∗CO in the CO2RR to CO, and ∗H in HER, are reaction descriptors to determine the corresponding catalytic activity (Hansen et al., 2013; Bagger et al., 2017). In addition, two ∗CO protonation steps to form ∗COH and ∗CHO were also considered as they are possible potential-determining steps that compete with CO desorption step (Shi et al., 2014). The volcano plot can be obtained according to the Sabatier principle, since the optimal catalyst binds the reaction intermediates neither too strongly nor too weakly (Peterson and Nørskov, 2012; Liu et al., 2017). Figures 1C and 1D show the theoretical predicted volcano plots of CO2RR and HER activity versus the binding energy of ∗CO for eight face-centered cubic (fcc) transition metals, including H2-generating ones (Pt, Ir, Rh, Ni, Pd), CO-generating ones (Au, Ag), and hydrocarbon-generating one (Cu) (Hori, 2018). The uniformity of the fcc crystal structure allows us to perform a better comparison of catalytic activity among various metals. In addition, we consider both (111) and (211) facets to represent terrace and step sites, respectively. The detailed fitting data of scaling relations are available in Figures S1 and S2. The free energy corrections for adsorbed and non-adsorbed species are listed in Table S1.
Figure 1.
Theory-Guided Design of Enhanced Syngas Generation by Decoupling Strategy
Schematic of reaction paths for (A) CO2RR to CO and (B) HER. Theoretical predicted volcano plots of (C) CO2RR activity versus ∗CO binding energy and (D) HER activity versus ∗H binding energy on (111) and (211) facets of different fcc metals. The black vertical dashed line shows the equilibrium potential of ∗CO/CO. Red and blue solid lines show the potential-limiting steps for CO2RR and HER as the ∗CO/∗H binding energy varies on the (111) and (211) facets, respectively, whereas dashed lines show associated elementary steps for CO2RR and HER.
Among the fcc metals screened, it is found that Au and Ag locate on the right of the ∗CO/CO equilibrium line (black vertical dashed line), indicating facile CO desorption due to the weak binding energy of ∗CO. However, both Au and Ag display a low HER activity with a limiting potential larger than 0.4 eV as their bindings with ∗H are too weak. In contrast, Pt and Ni locate very near the top of the HER volcano plot with optimal binding of the ∗H intermediate, but both locate on the left of the ∗CO/CO equilibrium line that favors further reduction of ∗CO to other downstream products owing to stronger bindings with ∗CO. The above findings demonstrate that it is challenging to develop efficient PEC syngas generation with optimal activity for both CO and H2 evolution using a single monofunctional site, suggesting the necessity to decouple the two reactions using dual catalytic sites. As Au (Pt) is predicted to be more active for CO evolution (H2 evolution) than that of Ag (Ni) in the DFT calculations, we selected Au and Pt as representative CO-generating and H2-generating cocatalysts to demonstrate the decoupling strategy for enhanced PEC syngas generation.
Design and Characterization of Photoelectrocatalyst with Dual Cocatalysts
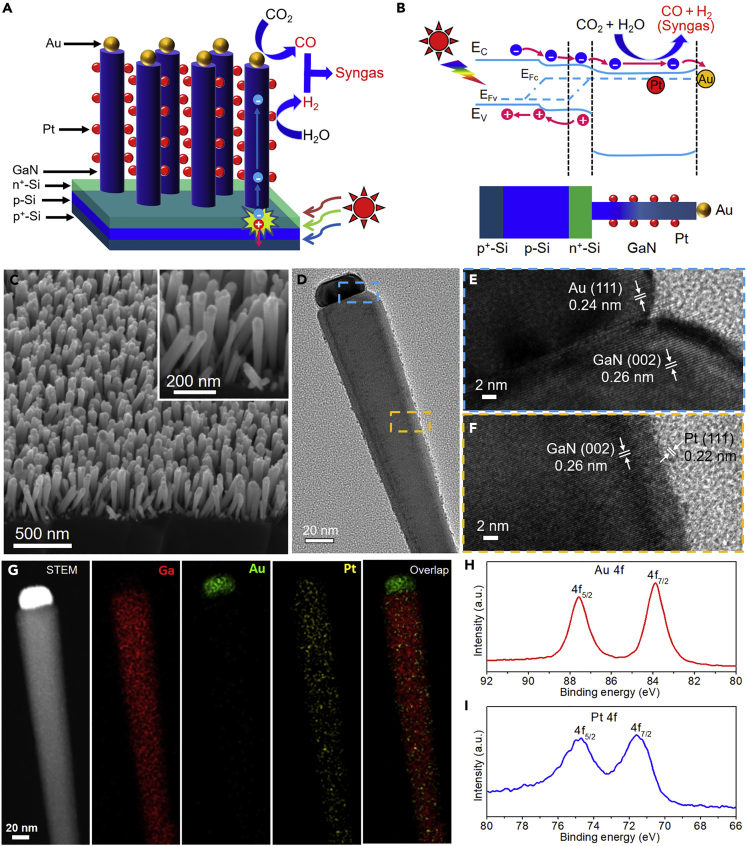
Experimentally, we integrated spatially separated dual cocatalysts of a Au CO-generating site and a Pt H2-generating site with a material platform of GaN nanowire arrays on n+-p Si (abbreviated as AuPtx/GaN/n+-p Si, where x denotes the Pt/Au molar ratio). The schematic design and energy band diagram of AuPtx/GaN/n+-p Si are illustrated in Figures 2A and 2B. The GaN/n+-p Si platform takes advantage of the strong light-harvesting of Si (bandgap of 1.1 eV) and efficient electron extraction/transportation effect as well as large surface area provided by GaN nanowires (Vanka et al., 2018; Zhou et al., 2018). The nanowires allow high mass loadings of electrocatalyst and enhance the light absorption with decreased light reflection (Deng et al., 2019a). Significantly, the optical absorption and electrochemical reaction are decoupled spatially and functionally in the multi-dimensional structure, providing a unique platform to tune the product distribution by simply varying the cocatalyst composition. Under light illumination, the narrow bandgap of n+-p Si junction is readily photoexcited by the major portion of solar spectrum to generate electron-hole pairs for the reactions. The light absorption of GaN nanowires is negligible owing to its large bandgap of 3.4 eV. Because the conduction band edges of GaN and Si are closely aligned and both Si and GaN are heavily n-type doped, the photogenerated electrons can be readily migrated from Si to GaN (Vanka et al., 2018).
Figure 2.
Design and Characterization of Photoelectrocatalyst with Dual Cocatalysts
(A and B) (A) Schematic and (B) energy band diagram of AuPtx/GaN/n+-p Si photocathode (not drawn to scale), showing the generation of syngas under sunlight illumination.
(C–F) (C) 45°-tilted SEM, (D) TEM, (E) and (F) HRTEM images of AuPt0.2/GaN/n+-p Si sample. The HRTEM images of (E) and (F) are obtained from the blue and yellow boxed areas in (D), respectively. See also Figure S4.
(G–I) (G) STEM-EDX elemental mapping images. XPS of (H) Au 4f and (I) Pt 4f.
The sample was synthesized in two main steps. First, GaN nanowire arrays were grown on n+-p Si wafer by molecular beam epitaxy. Next, Au nanoparticles were decorated at the tip of GaN nanowires using e-beam evaporation followed with thermal annealing, and Pt nanoparticles were anchored on the side of GaN nanowires by photodeposition using H2PtCl6 as Pt precursor (see Methods and Figure S3 for synthesis and details). The spatial separation of dual cocatalysts, i.e., Au on the polar surface (top surface) and Pt on the nonpolar surface (side surface) of GaN nanowires, could controllably align the CO evolution rate and H2 evolution rate in a balanced manner to achieve tunable syngas composition.
The morphology and chemical component of AuPt0.2/GaN/n+-p Si sample were examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX) mapping, X-ray photoelectron spectroscopy (XPS), and inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis. The cross-sectional SEM image (Figure 2C) indicates an average length of 350 nm and a diameter of 40 nm for the GaN nanowires and a size ranging from 20 to 40 nm for Au tips. TEM image (Figure 2D) indicates the Au-tipped structure and 1–2 nm Pt nanoparticles on the side surface of GaN nanowire. High-resolution (HR) TEM images in Figures 2E and 2F, and Figure S4 show the (111) facets of Au and Pt and (002) lattice plane of GaN, indicating the nanowire growth along the c-axis direction. XRD results confirmed the (111) plain of Au and (002) plane of GaN (Figure S5). The absence of XRD peaks of Pt nanoparticles is likely due to its low content and small crystalline size of 1–2 nm. The scanning transmission electron microscopy EDX (STEM-EDX) elemental mapping of a single nanowire (Figure 2G) confirmed the Au-tipped structure and Pt distributed uniformly on the lateral surface of nanowire. The XPS analysis in Figures 2H and 2I indicates metallic Au (Au0) and Pt (Pt0). ICP-AES analysis reveals the loading amounts of Au and Pt in AuPt0.2/GaN/n+-p Si were 8.5 and 1.7 nmol cm−2, respectively, with Au/Pt molar ratio of 5. By varying the introduced amount of Pt precursor, AuPtx/GaN/n+-p Si samples with different cocatalyst ratios (AuPt0.1, AuPt0.2, AuPt0.4) were prepared, with the loading amounts determined by ICP-AES analysis (Table S2). The TEM images of AuPtx/GaN/n+-p Si with different cocatalyst compositions are shown in Figure S6, showing Au located at the tip and the well dispersion of Pt nanoparticles across the nanowire.
Realization of Efficient and Tunable PEC Syngas Generation
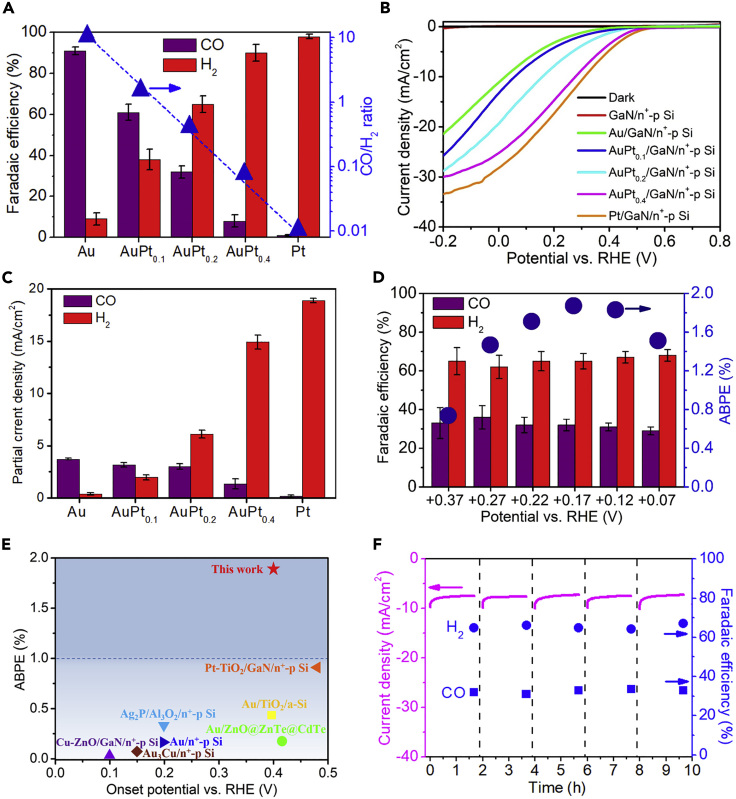
PEC studies of AuPtx/GaN/n+-p Si photocathodes were investigated in CO2-saturated 0.5 M KHCO3 solution (pH 7.5) under air mass 1.5 global (AM 1.5G) standard one-sun illumination (100 mW cm−2) in a three-electrode configuration. Figure 3A shows the Faradaic efficiencies (FEs) for CO and H2 on AuPtx/GaN/n+-p Si photocathodes with different cocatalyst compositions at an applied potential of +0.17 V versus reversible hydrogen electrode (RHE). Hereafter, all the PEC potentials are reported with respect to the RHE unless otherwise specified. The corresponding chronoamperometry data are shown in Figure S7. Dominant CO evolution and H2 evolution were detected on Au/GaN/n+-p Si and Pt/GaN/n+-p Si, respectively. With the increase of x from 0.1 to 0.4 in AuPtx/GaN/n+-p Si, an increased FE for H2 evolution with concurrently decreased FE for CO evolution was observed. By systematically tuning the cocatalyst composition, the ratio of CO/H2 could be tailored in a wide range from 1:99 to 10:1. In particular, AuPt0.2/GaN/n+-p Si produced syngas with a CO/H2 ratio of 1:2, which is a desirable composition for methanol and hydrocarbon fuels synthesis (Foit et al., 2017). As for all the samples, a unity FE was obtained for the cogeneration of CO and H2, with no other gaseous or liquid products detected.
Figure 3.
Realization of Efficient and Tunable PEC Syngas Generation
(A) FEs for CO (purple bars) and H2 (red bars), and CO/H2 ratio of AuPtx/GaN/n+-p Si photocathodes at +0.17 V versus RHE.
(B) J-V curves.
(C) Partial current density for CO (purple bars) and H2 (red bars) of AuPtx/GaN/n+-p Si photocathodes at +0.17 V versus RHE.
(D) FEs for CO (purple bars) and H2 (red bars), and ABPE of AuPt0.2/GaN/n+-p Si as a function of applied potential.
(E) Performance comparison of AuPt0.2/GaN/n+-p Si with state-of-the-art photocathodes for PEC CO2 reduction into CO/syngas.
(F) Chronoamperometry data and FEs for CO and H2 of AuPt0.2/GaN/n+-p Si photocathode at +0.17 V versus RHE. The dashed lines indicate cleaning of photoelectrode with DI water and purging of PEC chamber with CO2. Experimental conditions: CO2-saturated 0.5 M KHCO3 aqueous solution (pH 7.5), AM 1.5G one-sun illumination (100 mW cm−2). Error bars represent one standard deviation of multiple independent measurements.
Figure 3B shows the current-potential (J-V) curves of different photocathodes. Compared with the negligible photocurrent density of bare GaN/n+-p Si, Au/GaN/n+-p Si displays a photocurrent density of 21 mA cm−2 at −0.2 V with an onset potential of ∼0.4 V. With the incorporation of Pt, the onset potential shifts gradually to ∼0.5 V, and the photocurrent increases owing to high activity of Pt for HER. The partial current densities of CO and H2 at +0.17 V are shown in Figure 3C. With the increase of Pt content, the partial current density for H2 increases, whereas CO was kept nearly unchanged with the increase of Pt/Au ratio up to 0.2, indicating the access of balanced and simultaneously high CO2RR and HER activity in AuPt0.2/GaN/n+-p Si sample.
It is worth noting that the spatial separation of CO evolution and H2 evolution sites is critical to controllably tune syngas composition with a wide ratio of CO/H2. A control experiment using Pt(NH3)4Cl2 as Pt precursor obtained sample with Pt nanoparticles located on the polar surface of GaN nanowire in close proximity to Au (Figure S8). In contrast to the spatially separated dual cocatalysts, the mixture of cocatalysts produces a dominant H2 evolution with FEs over 90% and low controllability of syngas composition as a function of cocatalyst composition (Figure S9). It is a highly competitive process between adjacent Pt and Au for electron transfer, which favors the HER on Pt owing to its kinetic feasibility. The different photodeposition behaviors using different Pt precursors are ascribed to the stronger adsorption of PtCl62− anions on GaN surface than Pt(NH3)42+ cations (Figure S10). Pt nanoparticles were preferentially deposited on the sidewall of GaN nanowires using H2PtCl6 as Pt precursor owing to a sorption-determined deposition mechanism (Wenderich et al., 2014). In contrast, Pt was photodeposited on the tip of nanowire in close proximity with Au where electrons reside when using Pt(NH3)4Cl2 as Pt precursor owing to negligible adsorption of Pt(NH3)42+ cations on nanowire (Li et al., 2013, 2020).
Figure 3D shows the FEs for CO and H2 on AuPt0.2/GaN/n+-p Si at an applied potential between +0.37 and +0.07 V. The CO/H2 ratio was kept at nearly 1:2 in the PEC potential range investigated. The ABPE at different applied potentials were calculated according to the measured photocurrent density and FEs for CO and H2 (Equation 7, presented in Transparent Methods). The ABPE reached a maximum of 1.88% at +0.17 V, which is more than two times higher than that of state-of-the-art photocathodes (Figure 3E, and the details including the reference sources can be found in Table S3). The durability of AuPt0.2/GaN/n+-p Si photocathode was also investigated, as shown in Figure 3F. Both the photocurrent density and product selectivity remain unchanged for a period of 10 h. In addition, the SEM, TEM, and XPS analysis of AuPt0.2/GaN/n+-p Si sample after the PEC reaction show no change of GaN nanowires and Au-Pt cocatalysts (Figure S11). The turnover number for CO (TONCO), defined as the ratio of the evolved CO amount (40 μmol) to the amount of Au catalyst (1.7 nmol, calculated from the catalyst loadings of 8.5 nmol cm−2 and electrode sample area of 0.2 cm2), was calculated to be 23,500. However, this value is a low limit because the calculation is based on the 30 nm bulk Au catalyst instead of only the relevant surface sites. The surface atom ratio of Au NPs with 30 nm size was calculated to be ∼5% using theoretical Au nanoparticle model. By considering only the surface sites, the TONCO was calculated to be 470,000, which is the highest reported value to date for PEC CO/syngas production from CO2RR to our knowledge (Chu et al., 2018; Rosser et al., 2016; Kumagai et al., 2017). In addition, the turnover frequency for CO (TOFCO) was calculated to be 56,400 h−1 considering a duration time of 500 min. A comparison of TOF values was shown in Table S4, showing the TOFCO value reported in this work is comparable with most of the state-of-the-art catalysts for (photo)electrochemical CO2 reduction into CO. The mass activity of syngas production for AuPt0.2/GaN/n+-p Si photocathode at +0.17 V was calculated ∼4.7 A/mg, which is one or two orders of magnitude higher than the conventional planar Au-based CO/syngas generation system (Li et al., 2019; Feng et al., 2015; Sun et al., 2017). The large surface-to-volume ratio of GaN nanowire allows high-density catalytic sites with a significantly reduced loading amount compared with the planar structure.
To understand the role of GaN nanowires, control experiments using AuPt0.2/n+-p Si planar sample in the absence of GaN nanowires were performed. The J-V curve of AuPt0.2/n+-p Si planar sample displays a low photocurrent density (0.5 mA cm−2 at +0.17 V) and a poor onset potential of 0.25 V (Figure S12). Meanwhile, the FE for CO of AuPt0.2/n+-p Si is 9% at +0.17 V, which is much lower than that of 32% for AuPt0.2/GaN/n+-p Si (Figure S13). These results indicate the critical role of GaN nanowires as a superior structural scaffold to enhance the PEC performance. To demonstrate that the generated CO from CO2 reduction, isotopic experiment using 13CO2 was conducted. The signal at m/z = 29 assigned to 13CO appeared in the gas chromatography-mass spectrometry analysis and no signal of 12CO was detected (Figure S14). In addition, blank test performed in Ar-purged Na2SO4 aqueous solution showed no formation of CO, confirming the CO product originated from the reduction of CO2.
Demonstration of Unassisted Overall Syngas Generation in a Tandem PEC Cell
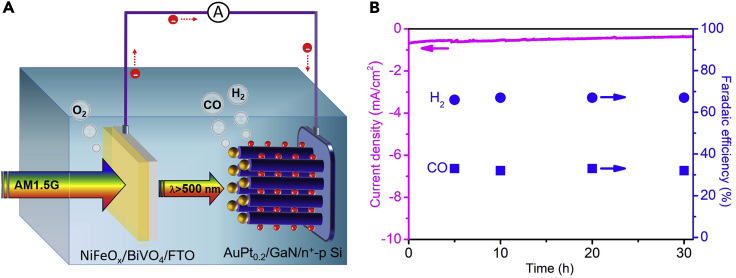
We constructed an unassisted overall solar CO2 reduction device by pairing the AuPt0.2/GaN/n+-p Si photocathode with a BiVO4-based photoanode to form a p/n tandem PEC cell. BiVO4 photoanode was chosen owing to its demonstrated relatively high photocurrent with an early onset potential (0.2–0.3 V versus RHE) (Kim and Choi, 2014; Kuang et al., 2017; Luo et al., 2011). The configuration of the device is shown in Figure 4A. In this configuration, the photovoltage is the sum of the two parts, and the longer wavelength photons that are not absorbed by the front BiVO4 absorber are transmitted and harvested by the bottom Si absorber. The maximum operating current density of the device was predicted to be 1.2 mA cm−2 by overlapping the J-V curves of photoanode and photocathode in a three-electrode cell (Figure S14). This nonzero operating point indicates the unassisted overall solar CO2 reduction could occur, which was confirmed in a two-electrode cell. Figure 4B shows the time course of the photocurrent and FEs for CO and H2 generated by the tandem PEC cell over a period of ∼30 h. A stable CO/H2 ratio of 1:2 with an average photocurrent density of ∼0.5 mA cm−2 was attained under no applied bias, which corresponds to an STS efficiency of 0.63%. The efficiency is higher than recently reported values in a PEC cell without external bias (Li et al., 2019; Andrei et al., 2020). Although the efficiency is lower than that reported in photovoltaic-electrolysis (PV-E) system (Schreier et al., 2015a, 2017; Urbain et al., 2017; Zhang et al., 2019; Kim et al., 2019; Cheng et al., 2020), the PEC approach integrates the light harvesting and electrochemical process of PV-E process into a single and monolithic device via a direct semiconductor-electrolyte interface, providing potential advantages over the PV-E system in terms of cost and complexity. And it is expected that the STS efficiency of the PEC system could be enhanced further by employing better photoanode with higher photocurrent at the low bias region, which would improve the overlap of J-V curves between photocathode and photoanode.
Figure 4.
Demonstration of Unassisted Overall Syngas Generation in a Tandem PEC Cell
(A) Schematic illustration of the tandem cell, consisting of AuPt0.2/GaN/n+-p Si as the photocathode and NiFeOx/BiVO4 as the photoanode without bias.
(B) Unassisted syngas generation in a two-electrode tandem cell under AM 1.5G one-sun illumination (100 mW cm−2).
Conclusion
In summary, we have demonstrated the decoupling of CO2RR and HER using dual cocatalysts to overcome the efficiency bottleneck and composition uncontrollability of PEC syngas generation from aqueous CO2. By spatially assembling a Au CO-generating cocatalyst and a Pt H2-generating cocatalyst on the polar and nonpolar surfaces of GaN nanowires, respectively, a record ABPE of 1.88% was achieved on planar Si photocathode. In addition, the CO/H2 ratio in the syngas mixture was controllably tuned in a wide range between 1:99 and 10:1 with a total unity Faradaic efficiency, by simply varying the composition of dual cocatalysts. And an STS efficiency of 0.63% without the application of an external bias was demonstrated in a tandem PEC cell as well. This work provides a promising route for the rational design of high-performance PEC syngas generation with controllable composition from aqueous CO2 reduction.
Limitations of the Study
Although this study has demonstrated a decoupling strategy to enhance the syngas generation from photoelectrochemical CO2 reduction, the overall syngas generation performance was largely limited by the CO evolution part, because CO2RR requires a higher potential than the HER as shown in Figures 1C and 1D. The absolute HER activity was controlled by using rational amount of Pt to align with CO evolution rate for achieving meaningful syngas composition in this study. Further improvement of the syngas generation performance is expected if better catalyst for CO2RR to CO is applied.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Zetian Mi (ztmi@umich.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The work was conducted at McGill University and the Michigan Center for Materials Characterization at the University of Michigan (NSF #DMR-0723032). We greatly acknowledge the financial support from Emissions Reduction Alberta (ERA), McGill Engineering Doctoral Award, and National Sciences and Engineering Research Council (NSERC) Discovery grant (grant # RGPIN-2017-05187). We also thank Supercomputer Consortium Laval UQAM McGill and Eastern Quebec for providing computing power and the National Natural Science Foundation of China for Excellent Young Scholar (51822604).
Author Contributions
S.C., R.T.R., and P.G. prepared and characterized the sample and performed PEC test. P.O. performed DFT calculations. P.O. and J.S. analyzed calculation results. R.T.R., R.W., and H.N.T. conducted the nanowires growth. S.Z. and H.Z. contributed to result analysis and discussions. The manuscript was written by S.C. P.O., J.S., and Z.M. with contributions from other co-authors.
Declaration of Interests
The authors declare no competing interest.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101390.
Contributor Information
Sheng Chu, Email: schu@seu.edu.cn.
Jun Song, Email: jun.song2@mcgill.ca.
Zetian Mi, Email: ztmi@umich.edu.
Supplemental Information
References
- Andrei V., Reuillard B., Reisner E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite-BiVO4 tandems. Nat. Mater. 2020;19:189–194. doi: 10.1038/s41563-019-0501-6. [DOI] [PubMed] [Google Scholar]
- Bagger A., Ju W., Varela A.S., Strasser P., Rossmeisl J. Electrochemical CO2 reduction: a classification problem. ChemPhysChem. 2017;18:3266–3273. doi: 10.1002/cphc.201700736. [DOI] [PubMed] [Google Scholar]
- Cheng W.-H., Richter M.H., Sullivan I., Larson D.M., Xiang C., Brunschwig B.S., Atwater H.A. CO2 reduction to CO with 19% efficiency in a solar-driven gas diffusion electrode flow cell under outdoor solar illumination. ACS Energy Lett. 2020;5:470–476. [Google Scholar]
- Chu S., Fan S.Z., Wang Y.J., Rossouw D., Wang Y.C., Botton G.A., Mi Z. Tunable syngas production from CO2 and H2O in an aqueous photoelectrochemical cell. Angew. Chem. Int. Ed. 2016;55:14260–14264. doi: 10.1002/anie.201606424. [DOI] [PubMed] [Google Scholar]
- Chu S., Ou P., Ghamari P., Vanka S., Zhou B., Shih I., Song J., Mi Z. Photoelectrochemical CO2 reduction into syngas with the metal/oxide interface. J. Am. Chem. Soc. 2018;140:7869–7877. doi: 10.1021/jacs.8b03067. [DOI] [PubMed] [Google Scholar]
- Deng J., Su Y., Liu D., Yang P., Liu B., Liu C. Nanowire photoelectrochemistry. Chem. Rev. 2019;119:9221–9259. doi: 10.1021/acs.chemrev.9b00232. [DOI] [PubMed] [Google Scholar]
- Deng X., Li R., Wu S., Wang L., Hu J., Ma J., Jiang W., Zhang N., Zheng X., Gao C. Metal-organic framework coating enhances the performance of Cu2O in photoelectrochemical CO2 reduction. J. Am. Chem. Soc. 2019;141:10924–10929. doi: 10.1021/jacs.9b06239. [DOI] [PubMed] [Google Scholar]
- Feng J.Y., Huang H.T., Yan S.C., Luo W.J., Yu T., Li Z.S., Zou Z.G. Non-oxide semiconductors for artificial photosynthesis: progress on photoelectrochemical water splitting and carbon dioxide reduction. Nano Today. 2020;30:100830. [Google Scholar]
- Feng X., Jiang K., Fan S., Kanan M.W. Grain-boundary-dependent CO2 electroreduction activity. J. Am. Chem. Soc. 2015;137:4606–4609. doi: 10.1021/ja5130513. [DOI] [PubMed] [Google Scholar]
- Foit S.R., Vinke I.C., de Haart L.G.J., Eichel R.A. Power-to-syngas: an enabling technology for the transition of the energy system? Angew. Chem. Int. Ed. 2017;56:5402–5411. doi: 10.1002/anie.201607552. [DOI] [PubMed] [Google Scholar]
- Hansen H.A., Varley J.B., Peterson A.A., Norskov J.K. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J. Phys. Chem. Lett. 2013;4:388–392. doi: 10.1021/jz3021155. [DOI] [PubMed] [Google Scholar]
- He R., Zhang A., Ding Y., Kong T., Xiao Q., Li H., Liu Y., Zeng J. Achieving the widest range of syngas proportions at high current density over cadmium sulfoselenide nanorods in CO2 electroreduction. Adv. Mater. 2018;30:1705872. doi: 10.1002/adma.201705872. [DOI] [PubMed] [Google Scholar]
- Hori Y. vol. 42. Springer; 2018. pp. 89–189. (Modern Aspects of Electrochemistry). [Google Scholar]
- Jang J.-W., Cho S., Magesh G., Jang Y.J., Kim J.Y., Kim W.Y., Seo J.K., Kim S., Lee K.-H., Lee J.S. Aqueous-solution route to zinc telluride films for application to CO2 reduction. Angew. Chem. Int. Ed. 2014;53:5852–5857. doi: 10.1002/anie.201310461. [DOI] [PubMed] [Google Scholar]
- Jang Y.J., Jang J.W., Lee J., Kim J.H., Kumagai H., Lee J., Minegishi T., Kubota J., Domen K., Lee J.S. Selective CO production by Au coupled ZnTe/ZnO in the photoelectrochemical CO2 reduction system. Energy Environ. Sci. 2015;8:3597–3604. [Google Scholar]
- Kang P., Chen Z., Nayak A., Zhang S., Meyer T.J. Single catalyst electrocatalytic reduction of CO2 in water to H2+CO syngas mixtures with water oxidation to O2. Energy Environ. Sci. 2014;7:4007–4012. [Google Scholar]
- Kim B., Seong H., Song J.T., Kwak K., Song H., Tan Y.C., Park G., Lee D., Oh J. Over a 15.9% Solar-to-CO conversion from dilute CO2 streams catalyzed by gold nanoclusters exhibiting a high CO2 binding affinity. ACS Energy Lett. 2019;5:749–757. [Google Scholar]
- Kim T.W., Choi K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science. 2014;343:990–994. doi: 10.1126/science.1246913. [DOI] [PubMed] [Google Scholar]
- Kong Q., Kim D., Liu C., Yu Y., Su Y., Li Y., Yang P.D. Directed assembly of nanoparticle catalysts on nanowire photoelectrodes for photoelectrochemical CO2 reduction. Nano Lett. 2016;16:5675–5680. doi: 10.1021/acs.nanolett.6b02321. [DOI] [PubMed] [Google Scholar]
- Kuang Y.B., Jia Q.X., Ma G.J., Hisatomi T., Minegishi T., Nishiyama H., Nakabayashi M., Shibata N., Yamada T., Kudo A. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy. 2017;2:16191. [Google Scholar]
- Kumagai H., Sahara G., Maeda K., Higashi M., Abe R., Ishitani O. Hybrid photocathode consisting of a CuGaO2 p-type semiconductor and a Ru(II)-Re(I) supramolecular photocatalyst: non-biased visible-light-driven CO2 reduction with water oxidation. Chem. Sci. 2017;8:4242–4249. doi: 10.1039/c7sc00940b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers A.T., Fields M., Torelli D.A., Xiao J., Hellstern T.R., Francis S.A., Tsai C., Kibsgaard J., Lewis N.S., Chan K. The predominance of hydrogen evolution on transition metal sulfides and phosphides under CO2 reduction conditions: an experimental and theoretical study. ACS Energy Lett. 2018;3:1450–1457. [Google Scholar]
- Lee J.H., Kattel S., Jiang Z., Xie Z., Yao S., Tackett B.M., Xu W., Marinkovic N.S., Chen J.G. Tuning the activity and selectivity of electroreduction of CO2 to synthesis gas using bimetallic catalysts. Nat. Commun. 2019;10:3724. doi: 10.1038/s41467-019-11352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.C., Wang T., Liu B., Chen M.X., Li A., Zhang G., Du M.Y., Wang H., Liu S.F., Gong J.L. Photoelectrochemical CO2 reduction to adjustable syngas on grain-boundary-mediated a-Si/TiO2/Au photocathodes with low onset potentials. Energy Environ. Sci. 2019;12:923–928. [Google Scholar]
- Li R., Zhang F., Wang D., Yang J., Li M., Zhu J., Zhou X., Han H., Li C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013;4:1432. doi: 10.1038/ncomms2401. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang L., Liu Y., Shao C., Gao Y., Fan F., Wang J., Li J., Yan J., Li R., Li C. Surface polarity-induced spatial charge separation boosting photocatalytic overall water splitting on GaN nanorod arrays. Angew. Chem. Int. Ed. 2020;59:935–942. doi: 10.1002/anie.201912844. [DOI] [PubMed] [Google Scholar]
- Liu X., Xiao J., Peng H., Hong X., Chan K., Norskov J.K. Understanding trends in electrochemical carbon dioxide reduction rates. Nat. Commun. 2017;8:15438. doi: 10.1038/ncomms15438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W.J., Yang Z.S., Li Z.S., Zhang J.Y., Liu J.G., Zhao Z.Y., Wang Z.Q., Yan S.C., Yu T., Zou Z.G. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011;4:4046–4051. [Google Scholar]
- Peterson A.A., Nørskov J.K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett. 2012;3:251–258. [Google Scholar]
- Qiu J., Zeng G.T., Ha M.A., Ge M.Y., Lin Y.J., Hettick M., Hou B.Y., Alexandrova A.N., Javey A., Cronin S.B. Artificial photosynthesis on TiO2-passivated InP nanopillars. Nano Lett. 2015;15:6177–6181. doi: 10.1021/acs.nanolett.5b02511. [DOI] [PubMed] [Google Scholar]
- Ross M.B., Li Y., De Luna P., Kim D., Sargent E.H., Yang P.D. Electrocatalytic rate alignment enhances syngas generation. Joule. 2019;3:1–8. [Google Scholar]
- Rosser T.E., Windle C.D., Reisner E. Electrocatalytic and solar-driven CO2 reduction to CO with a molecular manganese catalyst immobilized on mesoporous TiO2. Angew. Chem. Int. Ed. 2016;55:7388–7392. doi: 10.1002/anie.201601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M., Curvat L., Giordano F., Steier L., Abate A., Zakeeruddin S.M., Luo J., Mayer M.T., Grätzel M. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 2015;6:7326. doi: 10.1038/ncomms8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M., Gao P., Mayer M.T., Luo J.S., Moehl T., Nazeeruddin M.R., Tilley S.D., Gratzel M. Efficient and selective carbon dioxide reduction on low cost protected Cu2O photocathodes using a molecular catalyst. Energy Environ. Sci. 2015;8:855–861. [Google Scholar]
- Schreier M., Héroguel F., Steier L., Ahmad S., Luterbacher J.S., Mayer M.T., Luo J., Grätzel M. Solar conversion of CO2 to CO using earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy. 2017;2:17087. [Google Scholar]
- Shan B., Vanka S., Li T., Troian-Gautier L., Brennaman M.K., Mi Z., Meyer T.J. Binary molecular-semiconductor p–n junctions for photoelectrocatalytic CO2 reduction. Nat. Energy. 2019;4:290–299. [Google Scholar]
- Sheng W., Kattel S., Yao S., Yan B., Liang Z., Hawxhurst C.J., Wu Q., Chen J.G. Electrochemical reduction of CO2 to synthesis gas with controlled CO/H2 ratios. Energy Environ. Sci. 2017;10:1180. [Google Scholar]
- Shi C., Hansen H.A., Lausche A.C., Norskov J.K. Trends in electrochemical CO2 reduction activity for open and close-packed metal surfaces. Phys. Chem. Chem. Phys. 2014;16:4720–4727. doi: 10.1039/c3cp54822h. [DOI] [PubMed] [Google Scholar]
- Song J.T., Ryoo H., Cho M., Kim J., Kim J.G., Chung S.Y., Oh J. Nanoporous Au thin films on Si photoelectrodes for selective and efficient photoelectrochemical CO2 reduction. Adv. Energy Mater. 2017;7:1601103. [Google Scholar]
- Sun K., Cheng T., Wu L., Hu Y., Zhou J., Maclennan A., Jiang Z., Gao Y., Goddard W.A., 3rd, Wang Z. Ultrahigh mass activity for carbon dioxide reduction enabled by gold-iron core-shell nanoparticles. J. Am. Chem. Soc. 2017;139:15608–15611. doi: 10.1021/jacs.7b09251. [DOI] [PubMed] [Google Scholar]
- Urbain F., Tang P., Carretero N.M., Andreu T., Gerling L.G., Voz C., Arbiol J., Morante J.R. A prototype reactor for highly selective solar-driven CO2 reduction to synthesis gas using nanosized earth-abundant catalysts and silicon photovoltaics. Energy Environ. Sci. 2017;10:2256–2266. [Google Scholar]
- Vanka S., Arca E., Cheng S., Sun K., Botton G.A., Teeter G., Mi Z. High efficiency Si photocathode protected by multifunctional GaN nanostructures. Nano Lett. 2018;18:6530–6537. doi: 10.1021/acs.nanolett.8b03087. [DOI] [PubMed] [Google Scholar]
- Wenderich K., Klaassen A., Siretanu I., Mugele F., Mul G. Sorption-determined deposition of platinum on well-defined platelike WO3. Angew. Chem. Int. Ed. 2014;53:12476–12479. doi: 10.1002/anie.201405274. [DOI] [PubMed] [Google Scholar]
- White J.L., Baruch M.F., Pander J.E., III, Hu Y., Fortmeyer I.C., Park J.E., Zhang T., Liao K., Gu J., Yan Y. Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem. Rev. 2015;115:12888–12935. doi: 10.1021/acs.chemrev.5b00370. [DOI] [PubMed] [Google Scholar]
- Zhang H., Ming J., Zhao J., Gu Q., Xu C., Ding Z., Yuan R., Zhang Z., Lin H., Wang X. High-Rate, Tunable syngas production with artificial photosynthetic cells. Angew. Chem. Int. Ed. 2019;58:7718–7722. doi: 10.1002/anie.201902361. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhao Z.J., Wang T., Gong J. Nano-designed semiconductors for electro- and photoelectro-catalytic conversion of carbon dioxide. Chem. Soc. Rev. 2018;47:5423–5443. doi: 10.1039/c8cs00016f. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-J., Sethuraman V., Michalsky R., Peterson A.A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 2014;4:3742–3748. [Google Scholar]
- Zhou B., Kong X., Vanka S., Chu S., Ghamari P., Wang Y., Pant N., Shih I., Guo H., Mi Z. Gallium nitride nanowire as a linker of molybdenum sulfides and silicon for photoelectrocatalytic water splitting. Nat. Commun. 2018;9:3856. doi: 10.1038/s41467-018-06140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Ou P., Pant N., Cheng S., Vanka S., Chu S., Rashid R.T., Botton G., Song J., Mi Z. Highly efficient binary copper-iron catalyst for photoelectrochemical carbon dioxide reduction toward methane. Proc. Natl. Acad. Sci. U S A. 2020;117:1330–1338. doi: 10.1073/pnas.1911159117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.