Graphical abstract
Toxicological study of aqueous-methanol solvent fraction of methanol extract of Dacryodes edulis leaves.
Keywords: Medicinal plants, Dacryodes edulis, LD50, Subacute toxicity, Haematology
Highlights
-
•
Toxicity of aqueous-methanol solvent fraction of methanol extract of Dacryodes edulis leaves was evaluated.
-
•
The oral median lethal dose (LD50) was greater than 5000 mg/kgbw –practically non-toxic.
-
•
Erythrocytic indices – RBC, HB, HCT – were significantly decreased.
-
•
No renal or liver toxicity observed on treating (200−600 mg/kgbw) for 28- days.
-
•
Mitotic depression and chromosomal aberrations were observed in the Allium cepa model.
Abstract
Dacryodes edulis (G. Don) H.J. Lam) is the most popular species under the genus Dacryodes. It is well known for its nutritional and ethno-medicinal uses in South-eastern and South-western Nigeria. This study was aimed to evaluate the toxicity of the aqueous-methanol fraction of crude methanol extract of Dacryodes edulis leaves (AMDE). The test rats were randomized to groups of single oral treatment of AMDE (10−5000 mg/kgbw) for the acute toxicity study. They were monitored for obvious signs of behavioural change and mortality. For the subacute toxicity study, the rats were randomized to three daily treatment groups (of 200, 400 and 600 mg/kgbw of AMDE) for 28 days. The fourth group (control) received 2.5 %v/v DMSO. At the end of the experiment, blood samples were collected for hematology and clinical chemistry evaluation. The histopathology of the livers and kidneys were assessed using the excised organs. The cytotoxicity and genotoxicity of AMDE were also evaluated using Allium cepa model. The result showed that acute administration of AMDE, up to a dose of 5000 mg/kgbw did not result in mortality of the test rats. The observed median lethal dose (LD50) was greater than 5000 mg/kgbw. The subacute oral administration of AMDE for 28 days showed no significant (p > 0.05) effect on liver function, kidney function indices, organ - body weight ratio, but significantly (p < 0.05) decreased erythrocytic indices: red blood cells, haematocrit, and haemoglobin at 600 mg/kgbw. The Allium cepa assay revealed a non-significant reduction in mitotic index and low chromosomal aberrations of the treated groups. In conclusion, the aqueous-methanol solvent fraction of methanol extract of Dacryodes edulis leaves, AMDE is relatively safe. However, there are strong indications that it may contain compounds that are cytotoxic and reduces erythrocytic indices including red blood cells at high doses. Thus, adequate care should be taken in dosing and administering the extract to avert anaemic condition.
1. Introduction
In olden times – back at least 60,000 years -, man had depended on plants, animals, microorganisms (terrestrial and marine) for curing and managing diseases [1]. This body of knowledge and practice has progressively become a well-organized medical system – traditional medicine. It has attracted huge research interests, despite the tremendous development in modern medicine in most parts of the world [1]. According to WHO, the estimates of African and Asian population that are reliant on traditional medicine is about 80 %, while in the developed world, the situation is not so different: about 70–80 % of the population utilize at least one form of complementary or alternative medicine to date [2]. Traditional medicine practice in recent times still enjoys a continual rise in patronage; this is ascribed to the fact that they are considered to be cheap, effective, accessible, socially acceptable, and most of all safe. This assumed inherent safety status usually stems from a long history of use and a misconception that the terms “natural” and “safe” are almost synonymous. However, several studies have demonstrated some toxicological risks that are associated with the use of some vegetable species and herbs, which are natural [[3], [4], [5]]. It is of interest to ethnopharmacologists that despite the age of traditional medicine practice globally, it is still plagued by lack of strong unified regulatory guidelines and techniques for validating the efficacy and safety and also ensuring standard best practices in the production and distribution - an advantage the conventional modern medicine holds relatively [2]. Thus, it is important to test and standardize various herbal formulations used to manage myriads of diseases in traditional medicine practice using modern scientific techniques [6]. Dacryodes is a plant genus that is currently comprised of about 70–80 species of trees in the family of Burseraceae. It derives its generic name from "Dacruom", a Greek term for “tear (drop)”- this is in reference to the resin droplets from the bark of the tree [7]. Members of the genus grow as small medium-sized trees. They are distributed naturally in tropical America, (22 species and 14 undescribed species), South and Central Africa (18 species) and South East Asia (18 species) [7]. Notable species in this genus include D. edulis, D. rostrata, D. buettneri, D. klaineana and D. hexandara. There is still dearth of information on most of the species [8]. Dacryodesedulis (G. Don) H.J. Lam) is the most popular species under the genus. The name was derived from the term “edible” denoting its nutritional relevance [9]. It is called Safoutier in French; African pear, Bush butter, Bush fruit, or Native pear in English; Atanga in Gabon. In Nigeria, it is commonly called Ube and Eleme in the southeastern and western parts respectively [10,11]. Different parts of the plant are used by local populations of West and Central Africa for nutrition and ethno-medicine [12]. The fruits are eaten raw, boiled, or roasted and the other parts (bark, leaves, stem, and roots) are employed for other purposes in different forms [13]. Nutritive oil for cooking and preparation of livestock feeds is sourced from the fruit [14]. The bark is used to treat wounds, skin diseases, dysentery, debility, and tonsillitis [12] while the pulp oil and leaves in decoction are used to ameliorating gastrointestinal discomfort of different sorts [11]. Decoctions of the leaves are also used to manage hypertension, treat malaria, general weakness, and ease labour pain [11] and diabetes mellitus [15]. The use of this plant in a wide range of traditional medical practices has necessitated several scientific studies to evaluate their therapeutic potentials [12]. The antioxidant activity of the leaves and oil [16]; antimicrobial and antibacterial activity of the seeds [17]; Haematopoetic, anti-cardiovascular and anti-depranocytary activities of the seeds and leaves have all been reported [18]. Nevertheless, there is a dearth of information and data on the safety of the extracts of this plant. Most evidence of the safety of the fruit had come from the long historical use for nutrition, but this assumption cannot be made for other parts of the plant that have found great use in traditional medicine. Thus, this study (based on the premise discussed) is aimed to evaluate the toxicity (acute, sub-acute, cyto- and geno-toxicity) of the aqueous-methanol solvent fraction of methanol extract of Dacryodes edulis leaves (AMDE). In a previous study, the methanol extract/solvent fractions of Dacryodes edulis leaves had been characterized and shown to have significant hypoglycaemic and antioxidant activities. The aqueous-methanol solvent fraction (quite rich in phenolics, flavonoids and saponins) was reported to exhibit the most significant activity [19]; thus, this extract was chosen for this study.
2. Materials and methods
2.1. Chemicals
All the solvents (n-hexane, methanol, ethyl acetate, DMSO) used were of analytical grade and were purchased from Loba Chemie (India); the kits for electrolytes were purchased from Spectrum Diagnostics (Egyptian Company for Biotechnology, Cairo Egypt); the other biochemical assay kits were purchased from Randox Diagnostics LTD (UK).
2.2. Experimental animals
Thirty-nine (39) male albino wistar rats, aged 8–10 weeks and weighing 150−180 g were purchased from the National Institute for Trypanosomosis and Onchocerciasis Research, Vom, Jos, Plateau State, Nigeria. The animals were housed and maintained at room temperature (26−29 °C on a 12-h light: 12-h dark cycle). They were placed on standard commercial feed and clean water ad libitum and allowed to acclimatize to the animal house environment for two weeks. All procedures involving animals were performed following the internationally accepted principles for laboratory animal use and care and approved by POLAC Ethics Committee (Animal Research), Nigeria Police Academy Wudil, Kano State, Nigeria (Ref No: PEC/AR17/0102).
2.3. Plant materials
Dacryodes edulis leaves were obtained in May, 2017, from the University of Nigeria Nsukka campus in Enugu State. The plant was identified and authenticated (voucher No:UNNHN0601) by a Taxonomist, - Felix Nwafo - in the Department of Plant Science and Biotechnology, University of Nigeria, Nsukka, Enugu State, Nigeria. The leaves were shade-dried for two weeks at room temperature (26−29 °C)
2.4. Preparation of plant extracts
The crude extract and solvent fractions were prepared in series of batches using a method described by Teke et al. [20] with little modifications. A portion (500 g) of the air-dried leaves were ground in to coarse powder and then macerated in 2.5 l methanol (98.95 %) for seventy two hours in glass cylinders. The extract was filtered with Whatman No.1 filter paper, and concentrated in vacuo at 50 °C using a rotary evaporator to obtain the crude extracts Dacryodes edulis (yielded 99.50 g (19.9 %)). The solvent fractions were obtained by solvent/solvent partitioning of the crude extract in a seperatory funnel. A portion of the concentrated crude extract (40.28 g) was dissolved with 200 ml of methanol and then partitioned into 700 ml of n-hexane in a separation funnel. The mixture was allowed to stand for 12 h until a clearly separated two phases were formed (upper and lower phases). The upper (n-hexane) phase was collected and concentrated (at 40 °C in rotary evaporator) as n-hexane solvent fraction. This was repeated three times. Then, the residual phase (methanol) was subsequently concentrated (at 50 °C using a rotary evaporator), re-constituted in 200 ml of aqueous-methanol (55:45 v/v) solvent and partitioned into a 700 ml of ethyl acetate. This was also repeated three times as described above for n-hexane. The denser lower (aqueous-methanol) phase was collected and concentrated (by evaporation in an oven at 50 °C) as the aqueous-methanol fraction while the upper (ethyl acetate) phase was collected and concentrated (at 40 °C in rotary evaporator) as the ethyl acetate fraction. The aqueous-methanol solvent fraction was stored in a brown airtight bottle at 4 °C in a refrigerator for the toxicological study.
2.5. Acute toxicity study
The acute toxicity (LD50) study of AMDE was carried out in two phases using Lorke’s method [21].The extract was prepared in dimethyl sulfoxide (DMSO; 2.5 % v/v). A total of fifteen albino wistar rats were used. In the first phase, twelve (12) of the wistar rats were randomized into four (4) groups of three (3) rats each. The groups were administered 10, 100, and 1000 mg/kg body weight of the extract orally and the vehicle (DMSO (2.5 % v/v)) to the fourth group which served as control. They were monitored for 24 h for mortality, significant behavioural, and physical changes. In the second phase, the last set of three (3) wistar rats were used. They were randomized to three higher doses of the extract- 1600, 2900, and 5000 mg/kg bw – and monitored again for 24 h for mortality.
The LD50 was calculated as the geometric mean of D0 and D100 by the formula:
Where D0 is the highest dose that gave no mortality and D100 is the lowest dose that produced mortality.
2.6. Subacute oral toxicity of AMDE
The oral subacute toxicity was carried out as described in Zakaria et al. [22]. Twenty-four wistar rats weighing 150−180 g were used. The rats were randomly divided into four (4) groups of six (6) rats each and the AMDE extract was prepared in 2.5 % v/v DMSO and administered as follows;
Control: Wistar rats administered 10 ml/kgbw of 2.5 %v/v DMSO
AMDE1: Wistar rats200 mg/kgbw of AMDE
AMDE2: Wistar rats400 mg/kgbw of AMDE
AMDE3: Wistar rats600 mg/kgbw of AMDE
Key- AMDE: Aqueous-methanol solvent fraction of the crude extract of Dacryodesedulis.
The extract was administered orally and daily to the groups for 28days. They were observed daily for mortality and any sign of behavioural, and physical changes. At the end of the treatment, the animals were anaesthetized in an airtight chloroform fume chamber, following a 12-h fast. Blood samples were collected via cardiac puncture into plain and EDTA-containing tubes for biochemical and haematological analyses, respectively. The blood samples collected in EDTA-tubes were immediately used for haematological assays while that of the non-heparinized tubes were allowed to clot under room temperature for 15−30 min and then centrifuged at 3000 × g, 4 °C for 10 min. The serum obtained was stored at −20 °C for biochemical analysis. After the blood collection procedure, the animals were sacrificed by cervical dislocation and dissected. The organs (spleen, heart, liver, kidneys, and lungs) were excised, rinsed in 0.9 % saline, and weighed individually. The relative organ weight (ROW) for each organ was determined using the following formula;
The kidneys and livers for histopathological examination were then fixed in 10 % formalin.
2.7. Hematological and biochemical analysis
Hematological parameters were determined using automated Beckman Coulter (Beckman Coulter Inc. Brea CA, USA). The following haematological indices were assayed: white blood cell (WBC) count, lymphocytes (LYMPH), MID cells (MID), granulocyte (GRAN), haemoglobin (HGB), red blood cell (RBC) count, haematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW-CV), RDW-SD, platelet count (PLT), mean platelet volume (MPV), Platelet distribution width (PDW), and plateletcrit (PCT).
2.8. Biochemical analysis
Biochemical analysis was conducted using diagnostic kits: Randox and Spectrum diagnostic kits according to the manufacturers’ manuals. The biochemical parameters assayed included: Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Alkaline phosphatase, Albumin, Bilirubin for liver function and Creatinine, Urea, Electrolytes (Sodium (Na+), Potassium (K+), Chloride (Cl−) for renal function.
2.9. Histopathological assessment
Histopathological examinations were carried out on the excised and fixed (10 % formalin) liver and kidney tissues of the test rats. They were dehydrated in gradual ethanol concentrations (50–100 %), cleared in toluene and embedded in paraffin. Prior to photomicroscope observation, they were prepared into sections (4–6 μM thick) and stained with Hematoxylin and Eosin (H-E) dye. The stained sections were eventually observed with Leica DMTSO microscope at a power magnification of x100 and photographed with Leica ICC50 HD camera [23].
2.10. Cytotoxicity/genotoxicity study using Allium cepa model
The cytotoxic/genotoxicity effect of AMDE extract was carried out according to the protocol of Fiskesjo, [24]. Healthy onion bulbs (Allium cepa) of similar sizes were purchased from the local market. They were submerged in water for 10–12 h to soften their scales. The outer scales were removed carefully to avoid damaging the root primordial and subsequently, the bulbs were kept in freshwater to prevent the root primordial from drying and also to identify the viable bulbs. Five (5) viable onion bulbs were randomly selected and set up for each concentration. Different concentrations of AMDE were prepared (200, 500, 800 and 1100 mg/100 ml) respectively alongside the negative control (tap water). The selected viable onion bulbs were then placed on 100 ml of each of the solutions (different concentration of AMDE, and the control(s)) - such that the primordial was wholly submerged - and then incubated at normal room temperature (26−27 °C) for 72 h. At every 24 h, the root lengths for each onion bulb were measured using a ruler and the incubating solution changed. On the third day, the percentage root growth inhibition relative to the negative control was computed from the weighted root length averages for each AMDE concentration and the control to determine an effective concentration (EC50: 802.60 mg/100 ml) – A concentration that resulted in 50 % inhibition/retardation of the root growth (supplementary Table A.1). To evaluate the cyto-/genotoxicity of AMDE, the procedure above was repeated using AMDE concentrations of 100 mg–1000 mg/100 ml (1/8, 3/8, 3/4, and 4/3 of the EC50), the negative control and positive control (methyl methane sulfonate (1 mg/100 ml)). On the third day (72 h), the root tips were excised with scissors and then fixed in an acetic acid-alcohol mixture (3:1). The root tips were transferred to a clean, plain glass slide and a drop of 1.0 N hydrochloric acid was added to dehydrate and soften the tissue for easy maceration for 2 min. A dissection needle was then used to macerate the root tip after which a drop of acetic orcein stain (2.2 g of orcein powder in 45cm3 of glacial acetic acid and made up to 100 cm3 with distilled water) was added for 15−20 min. The slide was placed neatly on the slide content and with a slight pressure, the tip of cells were squashed in between. The edges were sealed with nail hardener before viewing microscopically for the various mitotic stages and chromosomal aberrations.
2.11. Statistical analysis
All the statistical analysis was performed using SPSS- Statistical Package for the Social Science version 16. The data were expressed as mean ± SEM. Test of statistical difference was carried out using Analysis of variance (ANOVA). p < 0.05 was considered to be statistically significant.
3. Results
3.1. Acute toxicity of AMDE
The result of the oral acute toxicity of AMDE is presented in Table 1. The oral administration of the solvent fraction at doses of 1000 mg/kgbw-5000 mg/kgbw showed no marked, adverse effect on the test animals. No death was recorded except for a decline in the normal activity of the animals administered the 5000 mg/kgbw dose. The median lethal dose (LD50) was determined to be greater than 5000 mg/kgbw. Overall, there was no marked indication of toxicity or physiological changes.
Table 1.
Acute toxicity of AMDE.
| Extract | Dose (mg/kgbw) | Mortality | Behavioural indices of toxicity |
|---|---|---|---|
| AMDE | 10 | 0/3 | None |
| 100 | 0/3 | None | |
| 1000 | 0/3 | None | |
| 1600 | 0/1 | None | |
| 2900 | 0/1 | None | |
| 5000 | 0/1 | Corner sitting/ reduced activity |
3.2. Sub-acute toxicity of AMDE
3.2.1. Effect on organ-body weight ratio
The effect of AMDE on relative organ weight ratio is presented in Fig. 1. The result shows no significant difference in the relative organ-body weight ratios (p > 0.05) of the control group and the treated groups.
Fig. 1.
Effect of AMDE on organ-body weight ratio of treated wistar rats expressed in percentage.
AMDE1: Group administered with AMDE (200 mg/kgbw), AMDE2: Group administered with AMDE (400 mg/kgbw), AMDE3: Group administered with AMDE (600 mg/kgbw). Values are mean ± SEM, n = 6. Superscripts: Values bearing the same superscript are not statistically different (p>0.05).
3.2.2. Effect on haematology
The effect of AMDE on haematological indices is presented in Table 2. The result showed there was no significant (p < 0.05) difference between the control and the treated group for the following indices - WBC, LYMPH, MID, GRAN, MCV, MCH, RDW, PLT, MPV PDW and PCT. A significantly (p < 0.05) low red blood cells, haematocrit, and haemoglobin level were observed in in the group treated with the highest dose of 600 mg/kgbw.
Table 2.
Effect of AMDE on haematological indices of the treated wistar rats.
| Control | AMDE1 | AMDE2 | AMDE3 | |
|---|---|---|---|---|
| WBC (x109/L) | 9.0 ± 0.2 | 10.4 ± 0.5 | 10.8 ± 0.7 | 10.3 ± 0.6 |
| LYMPH (%) | 77.3 ± 2.8 | 83.0 ± 4.6 | 72.0 ± 4.5 | 83.3 ± 5.0 |
| MID (%) | 10.0 ± 0.9 | 7.7 ± 0.3 | 7.0 ± 0.3 | 9.3 ± 0.2 |
| GRAN (%) | 9.5 ± 0.5 | 9.3 ± 0.6 | 7.7 ± 0.8 | 8.3 ± 0.2 |
| HGB (g/dL) | 17.9 ± 1.0 | 16.0 ± 2.3 | 15.1 ± 0.6 | 14.1 ± 0.6a |
| RBC (x1012/L) | 9.0 ± 0.4 | 8.3 ± 0.4 | 7.8 ± 0.1 | 7.2 ± 0.3a |
| HCT (%) | 53.3 ± 3.4 | 49.4 ± 6.9 | 47.1 ± 1.9 | 42.1 ± 1.5a |
| MCV (fL) | 58.6 ± 3.5 | 59.9 ± 3.3 | 55.4 ± 2.6 | 56.7 ± 0.5 |
| MCH (pg) | 19.8 ± 1.4 | 19.3 ± 0.8 | 20.3 ± 1.0 | 19.1 ± 0.5 |
| MCHC (g/dL) | 33.4 ± 1.5 | 32.4 ± 0.7 | 32.1 ± 0.3 | 33.4 ± 1.0 |
| RDW-SD (fL) | 36.6 ± 2.9 | 33.3 ± 1.0 | 35.3 ± 3.0 | 33.4 ± 1.0 |
| PLT (x109/L) | 426.7 ± 74.6 | 452.00 ± 60.2 | 495.3 ± 79.4 | 415.7 ± 52.6 |
| MPV (fL) | 8.7 ± 0.1 | 8.60 ± 0.2 | 8.7 ± 0.3 | 8.9 ± 0.2 |
| PDW | 14.6 ± 0.5 | 14.43 ± 0.5 | 14.9 ± 0.1 | 15.1 ± 0.1 |
| PCT (%) | 0.4 ± 0.0 | 0.39 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
AMDE1: Group administered with AMDE (200 mg/kgbw), AMDE2: Group administered with AMDE (400 mg/kgbw), AMDE3: Group administered with AMDE (600 mg/kgbw). Values are mean ± SEM, n = 6. Superscripts: Values bearing superscript (a) across a row are statistically different from that of control (p > 0.05). WBC: White Blood Cell Count, LYMPH: Lymphocytes, MID: MID cells, GRAN: Granulocyte, HGB: Total haemoglobin, RBC: Red blood cell count, HCT: Haematocrit, MCV: Mean Corpuscular Volume, MCH: Mean Corpuscular Haemoglobin, MCHC: Mean Corpuscular Haemoglobin Concentration, RDW-CV: Red Cell Distribution Width, PLT: Platelet count, MPV: Mean Platelet Volume, PDW: Platelet Distribution Width, PCT: Plateletcrit.
3.2.3. Effect of AMDE on liver and kidney function indices
The effect of AMDE on liver and kidney function indices are presented in Table 3. The result shows the oral administration of the solvent fraction, AMDE for 28 days produced no significant (p > 0.05) difference in the liver and kidney function indices in both control and treated groups. AST, ALT, ALP, albumin, creatinine, urea, sodium, chloride and potassium were not significantly different (p > 0.05) in the control group and treated group. The chloride level in groups treated with 400 mg/kgbw and 600 mg/kgbw were higher relative to the control however it was not statistically significant.
Table 3.
Effect of AMDE on the liver and kidney function indices of the treated wistar rats.
| Control | AMDE1 | AMDE2 | AMDE3 | |
|---|---|---|---|---|
| *AST(U/L) | 83.20 ± 2.94a | 88.70 ± 1.27 a | 82.60 ± 6.90 a | 80.40 ± 7.90 a |
| *ALT(U/L) | 58.80 ± 2.30 b | 61.40 ± 2.60 b | 59.00 ± 3.20b | 59.40 ± 1.80 b |
| *ALP(U/L) | 65.60 ± 12.70 c | 56.40 ± 3.10 c | 59.10 ± 7.00c | 65.70 ± 10.00 c |
| *TOTAL BILIRUBIN (mg/dl) |
0.74 ± 0.07d | 0.72 ± 0.05d | 0.68 ± 0.07d | 0.67 ± 0.04d |
| *DIRECT BILIRUBIN (mg/dl) |
0.33 ± 0.03e | 0.32 ± 0.02e | 0.30 ± 0.03e | 0.29 ± 0.02e |
| *ALBUMIN (g/dl) |
6.07 ± 0.24f | 6.05 ± 0.24f | 6.08 ± 0.28f | 6.05 ± 0.45f |
|
#CREATININE (mg/dl) |
1.04 ± 0.03g | 1.10 ± 0.03g | 1.08 ± 0.08g | 0.94 ± 0.04g |
| #UREA(mg/dl) | 59.9 ± 1.06h | 61.02 ± 7.10h | 56.90 ± 5.60h | 53.50 ± 6.80h |
|
#SODIUM (mEq/L) |
296.58 ± 87.90i | 297.42 ± 65.25i | 294.50 ± 41.61i | 294.33 ± 23.23i |
|
#CHLORIDE (mEq/L) |
109.63 ± 10.08 j | 102.22 ± 8.25 j | 126.54 ± 21.49 j | 116.07 ± 24.38j |
|
#POTASSIUM (mEq/L) |
7.176 ± 0.10 k | 6.838 ± 0.21 k | 8.081 ± 0.23 k | 5.975 ± 0.22 k |
AMDE1: Group administered with AMDE (200 mg/kgbw), AMDE2: Group administered with AMDE (400 mg/kgbw), AMDE3: Group administered with AMDE (600 mg/kgbw). Values are mean ± SEM, n = 6. Superscripts: Values bearing the same superscript across a row are not statistically different (p>0.05).* Liver function indices, # Kidney function indices.
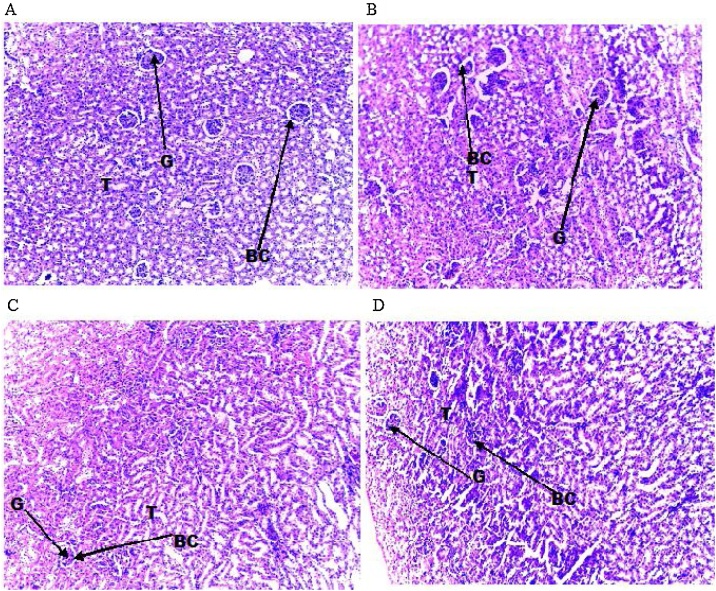
3.2.4. Effect of AMDE on liver and kidney histology
The effect of the subacute oral administration of AMDE on liver and kidney histology of treated wistar rats are presented in Fig. 2, Fig. 3. The result showed that both the control and treated group showed unremarkable liver and kidney tissues. The liver histology showed normal hepatocytes and central vein. The kidney histology also showed normal renal architecture with glomerulus, Bowman’s capsule and proximal convoluted tubules. There was no sign of marked distortion of tissue or tissue architecture in the treated group when compared with the control group.
Fig. 2.
Effect of aqueous-methanol solvent fraction of Dacryodes edulis (AMDE) on the liver histology of the treated albino wistar rats (H&E, Mag x 100).
A (control): liver histology of control group showing normal unremarkable liver tissue. B (AMDE1): liver histology of the group administered with AMDE (200 mg/kgbw) showing normal liver tissue. C (AMDE2): liver histology of the group administered with AMDE (400 mg/kgbw) showing relatively normal liver architecture. D (AMDE3): liver histology of the group administered with AMDE (600 mg/kgbw) showing normal liver architecture. HP: Hepatocytes, CV: Central vein.
Fig. 3.
Effect of aqueous-methanol solvent fraction of Dacryodes edulis (AMDE) on kidney histology of the treated albino wistar rats (H&E, Mag x 100).
A (control): Kidney histology of control group showing unremarkable renal tissue with normal glomerular surrounded by Bowman’s capsule. B (AMDE1): Kidney histology of the group administered with AMDE (200 mg/kgbw) showing normal renal tissue. C (AMDE2): Kidney histology of the group administered with AMDE (400 mg/kgbw) showing relatively normal renal architecture. D (AMDE3): Kidney histology of the group administered with AMDE (600 mg/kgbw) showing normal renal tissue. G: Glomerulus, BC: Bowman’s capsule, T: Tubules.
3.3. Cytotoxicity and genotoxicityAMDE
3.3.1. Effect on Mitotic index (cytotoxic effect)
The effect of AMDE on the cytogeny of Allium cepa model is presented in Fig. 4. The result showed a non-significant, dose-dependent, decline in the mitotic indices (MI) of the groups treated with AMDE relative to negative control. The positive control showed a significantly (p < 0.05) lower MI value. None of the groups showed an MI value less than 50 % (sublethal threshold) or 22 % (lethal threshold) of the positive control except the positive control.
Fig. 4.
Effect of AMDE on mitotic index of Allium cepa.
AMDE100: Group incubated in AMDE (100 mg/100 ml), AMDE300: Group incubated in AMDE (300 mg/100 ml), AMDE600: Group incubated in AMDE (600 mg/100 ml), AMDE1000: Group incubated in AMDE (1000 mg/100 ml). Control (−): Group incubated in distil water, Control (+): Group incubated in methyl methane sulfonate (1 mg/100 ml). Values are expressed as mean ± SEM, n = 500. Superscripts: Values bearing superscript “a” are statistically different (p>0.05).
3.3.2. Effect on chromosomal structure
The effect of AMDE on chromosomal structure is presented in Table 4. The result showed chromosomal aberrations ranging from 4.10 to 5.30% for the groups treated with AMDE and 8.85 % for the positive control. The observed prominent chromosomal aberrations include: attached, bridged, stickiness and vagrant chromosomes. C-mitosis, laggard, and multipolar were less frequent (Fig. 5A and B).
Table 4.
Effect of AMDE on the chromosomal structure of Allium cepa.
| Test Groups | Attached | Bridged | C-mitosis | Stickiness | Vagrant | Laggard | Multi polar |
Total CA (CA in %) |
|---|---|---|---|---|---|---|---|---|
| Control(−) | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 4(0.8) |
| AMDE100 | 5 | 8 | 0 | 7 | 5 | 0 | 0 | 25(5.30) |
| AMDE300 | 4 | 5 | 2 | 6 | 4 | 0 | 3 | 24(5.10) |
| AMDE600 | 4 | 5 | 0 | 7 | 5 | 1 | 0 | 22(4.90) |
| AMDE1000 | 3 | 5 | 0 | 6 | 4 | 0 | 0 | 18(4.10) |
| Control(+) | 7 | 9 | 5 | 9 | 5 | 3 | 6 | 44(8.85) |
AMDE100: Group incubated in AMDE (100 mg/100 ml), AMDE300: Group incubated in AMDE (300 mg/100 ml), AMDE600: Group incubated in AMDE (600 mg/100 ml), AMDE1000: Group incubated in AMDE (1000 mg/100 ml). Control (−): Group incubated in distil water, Control (+): Group incubated in methyl methane sulfonate (1 mg/100 ml).
Fig. 5.
A: Normal chromosome structure of the control Allium cepa specimens. a) Anaphase b) Telophase c) Prophase d) Metaphase.
B: Different chromosomal aberration observed in the treated Allium cepa specimens. a) Attached b) Bridged c) C-mitosis d) Stickiness e) vagrant f) Laggard h) Multipolar chromosomes.
4. Discussion
Median lethal dose (LD50) is a quantitative index of acute toxicity, which is usually determined in the preliminary step of evaluating the safety/toxicity of drugs, compounds, and medicinal plants [25]. It provides information on the nature of toxicity and the basis for the classification and dosage design of a substance or drug [22]. In this study, oral administration of AMDE up to a dose of 5000 mg/kgbw did not result in mortality (Table 1), nevertheless, some little behavioural changes such as decline in level of activity relative to the control group were observed. Thus, the LD50 was greater than 5000 mg/kgbw and this classifies the extract as relatively non-toxic according to Hodger and Sterners's toxicity scale [26]. There is a dearth of literature on the acute toxicity of Dacryodes edulis leave - extracts till date; however, a recent study by Okolo et al. [27] have reported an LD50 of 3500 mg/kgbw for n-hexane extract of fruits of Dacryodes edulis; which is at variance with this present study. The observed difference in LD50 of the two studies could be because the two studies investigated two different parts of the plant (fruit and leaves) using different solvents/extraction procedures. Thus, this is expected to impact their phytochemical and toxicity profiles. As previously reported by Joseph et al. [28]. They opined that different extracts of the same plant could present with varying toxicological outcomes in the same animal model or human.
Data from subacute and subchronic toxicity studies usually depict the undesirable effects of long-term use and exposure of an experimental animal model or human subjects (in cases of natural or occupational exposure) to substances. This can specifically identify organ-specific toxicity and bioaccumulation potentials of a compound or drug [22,29] via relative organ weight, bio-enzyme markers, haematology, and histology of specific organs. Relative organ weight (heart, liver, kidney, spleen, and lungs) flux as an index of toxicity usually depicts an upset in normal pathophysiology of a model animal or damage of a specific organ which physical expression could be changes in the histology, relative sizes of the organ and the induction of organ-specific enzymes [30]. In this study, the result revealed that the relative organ weight of vital organs like heart, liver, spleen, kidney, and lung of all the treated groups did not differ significantly (p > 0.05) from the control. This suggests that the extract did not exert any effect that perturbed normal physiological and anatomical function of the test animals and as such, the structural integrity of the organs was not gravely perturbed (Fig. 1).
The liver is the organ tasked with metabolism and biotransformation of foreign compounds in animals. Therefore, it is a major target for every xenobiotic that gains access to the system. Some of these foreign compounds may cause injury to the liver resulting in the induction and release of liver enzymes and proteins in magnitude higher than the basal level [31]. Thus, significant alterations (particularly increase) in the levels and activities of serum liver function enzymes (ALT, AST, and ALP), particularly ALT, are indicative of injury on hepatocytes [31]. Serum proteins such as albumin are synthesized by the liver and the relative level could provide information on the synthetic capability of the liver. A decline in serum level of albumin usually indicates a perturbation in the capacity of the liver to synthesize proteins [32]. In this study, there was no significant (p > 0.05) difference in the activities of ALT, AST, and ALP in the control group and all the treated groups (Table 3). This is an indication that the subacute administration of AMDE at doses 200−600 mgkgbw does not adversely affect hepatocellular functions. The result further showed that there was no significant (p > 0.05) alteration in the protein (albumin) level between the control group and the treated groups (Table 3). This shows that AMDE at the doses studied (200−600 mg/kgbw) does not affect hepatocellular secretory and synthetic functions. Serum urea, creatinine, and electrolytes are considered sensitive kidney function indices [33]. An upsurge in their levels indicates anomalies in glomerular filtration or kidney function [22]. Creatinine level is a good index of glomerular filtration rate while urea level is connected with kidney diseases and urinary tract problems. Electrolytes are indicators of renal tubular functions [34]. In this study, serum urea, creatinine and electrolytes (Na+, K+, and H−) were not affected by the subacute treatment with AMDE (Table 3). This indicates that AMDE did not provoke any significant renal dysfunction at or within the doses studied. These results (liver and kidney function) are further corroborated by the histopathological assessment of the liver and kidney. Both organs (liver and kidney) showed unremarkable tissues relative to the control: an indicator that the structural integrity of the organs was not significantly affected. Thus, the serum level or activities of the organ-specific biochemical parameters (enzymes and proteins) were preserved.
The haematopoetic system is very sensitive to chemicals and foreign compounds at elevated doses. Experimental evidences have shown that exposure to certain plants, drugs, and compounds significantly alter haematological parameters. Thus, it is an important index of toxicity with high predictive potential for human toxicity [35]. The result of this study indicated that there was no significant (p > 0.05) alteration in most of the haematological indices (WBC, LYMPH, MID, GRAN, MCV, MCH, RDW, PLT, MPV, PDW, and PCT) studied except for erythrocytic indices, HGB, RBC, and HCT which showed a marginal but significant (p < 0.05) decline in the group treated with the highest dose of 600 mg/kgbw (Table 3). This result suggests that AMDE may contain compounds that destroy erythrocytes or interfere adversely with erythropoetic process in the bone marrow. Plants rich in saponin and tannin have been suggested to be capable of destroying red blood cells in vivo [18,36]. Interestingly, AMDE has been reported to be highly rich in saponins and tannins [19]. Similar findings have been reported by Ufelle et al. [18] in a related study. Their study demonstrated that oral administration of methanol extract of seeds of Dacryodes edulis for 30 days significantly (p < 0.05) reduced erythrocytic indices – red blood cells, haemoglobin and haematocrit – at doses of 200 mg/kgbw and 400 mg/kgbw. The decline in haematocrit is a consequence of reduced net RBCs which may be due to its lytic destruction, inhibition of the production process or both. Haemoglobin production may also have been impacted by the destruction and reduced production of RBCs.
The Allium cepa test model has been reported to be an efficient bio-indicator of potential cytotoxicity and geno-toxicity for first-line screening [37]. This assay is quite simple, cost-effective, and highly sensitive with a less laborious protocol [38]. It has been used in monitoring the potential toxicity of plant extracts, environmental pollutants, and chemicals [37,39]. In this study, the potential cytotoxicity and geno-toxicity of the aqueous methanol solvent fraction of methanol-crude extract of Dacryodes edulis leaves (AMDE) using mitotic index (MI) and chromosomal aberration (CA) were evaluated. MI flux is a measure of altered frequency of cell division and is a strong indicator of deranged cell proliferation [40]. Mitotic index decline below 50 % (cytotoxic limit) and 22 % of the control are reported to have sub-lethal and lethal effect on test organisms respectively [[41], [42], [43]]. But the result of this study showed that the MIs for all the treatment groups (though declined) were way above the 50 % mark (relative to the negative control) with exception of the positive control (32.90 % of negative control MI) (Fig. 4). This indicates that the effect of AMDE at the concentrations studied is not significantly lethal. However, the dose-dependent nature of the MI decline shows a trend suggestive of possible lethal effects with higher concentrations. This finding is similar to the reports of Ribeiro et al. [44] in a study with Hancornia specios latex using Allium cepa root model. The latex showed significantly lower MI with an increasing concentration of the latex.
Chromosomal aberrations (CAs) are changes in chromosomal structure resulting from a break or exchange of chromosomal materials [45]. Induction of micronucleus and other chromosomal aberrations are generally considered an indication of geno-toxicity [[46], [47], [48]]. The result of this study revealed a level of chromosomal aberrations ranging from 4.10 to 5.30%) (Table 4). This was much less than that of the positive control but higher than the negative control. Chromosomal aberrations such as attached, bridged sticky, and vagrant chromosomes were observed more frequently (Fig. 5A and B). The presence of chromosomal aberrations in the groups treated with AMDE also suggests the potential to exert geno-toxicity. However, the decline in % CA with higher concentrations of AMDE indicates a counter effect. This may be due to the presence of some classes of phytochemicals that could exert cyto-protective effect at optimal concentrations such as antioxidants. Similar findings have been made by Ihegboro et al. [49] and Bertãoet al. [50]. According to these studies, extract with high antioxidant capacity exhibited lower cytotoxicity/geno-toxicity potential.
As mentioned earlier, there is still a paucity of reports on the toxicity of extracts of different parts of Dacryodes edulis. Nonetheless, Ajibesin [9], in a comprehensive review of Dacryodes edulis plant in 2012, opined that “to date, no part of this plant has been reported to be toxic at the doses/concentrations they have been used. Most cases of toxicity reported could have stemmed from handling and preparation of the plant in the form they are used”. He made particular reference to heavy metal toxicity possibly resulting from preparatory procedures [9]. However, this study has been able to demonstrate the potential toxicity of the leaves of this plant. The aqueous-methanol fraction of methanol extract of Dacryodes edulis leaves, AMDE; causes significant decrease in eythrocytic indices; which is indicative of potential anaemic condition with prolonged administration or increase in administered doses. The plausible mechanism of action of this, could be via inhibition of the erythropoetic process in the bone marrows and cellular destruction of red blood cells (which by its very nature is quite susceptible to lytic action) by phytochemicals such as flavonoids saponins and tannins – the major bioactive classes of compounds in the leaves of Dacryodes edulis [19]. Furthermore, a similar observation was made by Prajitha and Thoppil [51] in their study. They demonstrated that extracts of Amaranthus spinosus L. showed cytotoxic and apoptotic activities of in Allium cepa and human erythrocytes models. The extract modified the cytoskeleton and membrane of RBC via blebbing. A phenomenon that is consistent with apoptosis in nucleated cell. This may not be so different with this present study.
In conclusion, the present study revealed that AMDE exhibited no significant toxicological effect with respect to liver functions, kidney functions, and organ - body weight ratios on tested models. However a decline in erythrocytic indices in wistar rat model and non-lethal mitotic depressive and geno-toxic activities (in Allium cepa model) at high concentrations were recorded. Thus, exercising caution in the use of this extract is advised. Further study should be carried out to establish safe and effective doses for the extract and also to assess other types of toxicity such as reproductive toxicity and mutagenicity using other models.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Chimaobi J. Ononamadu: Conceptualization, Investigation, Writing - original draft. Adamu J. Alhassan: Methodology, Supervision. Aminu Ibrahim: Methodology, Validation. Abdullahi A. Imam: Formal analysis, Validation. Godwin O. Ihegboro: Investigation. Alowonle T. Owolarafe: Writing - review & editing. Obiajulu C. Ezeigwe: Formal analysis. Mohammed K. Atiku: Supervision, Writing - review & editing. Mohammed S. Sule: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors appreciate Hajia Hauwa Yakubu, ASP Jonathan Halima H, ASP Unah Paul and ASP Olua Moses, all of Department of Biochemistry & Forensic Science Nigeria Police Academy, Wudil for providing the required technical assistance and support for the success of this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.07.007.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Yuan H., Qianqian M.Q., Li Y.L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(559):1–18. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neergheen-Bhujun V.S. Underestimating the toxicological challenges associated with the use of herbal medicinal products in developing countries. BioMed Res. Int. 2013:1–9. doi: 10.1155/2013/804086. Article ID 804086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adewale O.B., Onasanya A., Anadozie S.O., Abu M.F., Akintan I.A., Ogbole C.J., Olayide I., Afolabi O.B., Jaiyesimi K.F., Ajiboye B.O., Fadaka A.O. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J. Ethnopharmacol. 2016;188:153–158. doi: 10.1016/j.jep.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Bello I., Bakkouri A.S., Tabana Y.M., Al-Hindi B., Al-Mansoub M.A., Mahmud R., Asmawi M.Z. Acute and sub-acute toxicity evaluation of the methanolic extract of Alstonia scholaris stem bark. Med. Sci. 2016;4(1):4. doi: 10.3390/medsci4010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rokaya M.B., Uprety Y., Poudel R.C., Timsina B., Munzbergova Z., Asselin H., Tiwari A., Shrestha S.S., Sigdel S.R. Traditional uses of medicinal plants in gastrointestinal disorders in Nepal. J. Ethnopharmacol. 2014;158:221–229. doi: 10.1016/j.jep.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Unuofin J.O., Otunola G.A., Afolayan A.J. Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia Less. in Wistar rats. J. Integr. Med. 2018;16(5):335–341. doi: 10.1016/j.joim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Onana J.M.A. synoptic revision of Dacryodes (Burseraceae) in Africa, with a new species from Central Africa. Kew Bull. 2008;63(3):385–400. doi: 10.1007/s12225-008-9064-4. [DOI] [Google Scholar]
- 8.Tee L.H., Yang B., Nagendra K.P., Ramanan R.N., Sun J., Chan E.-S., TiTey B., Azlan A., Ismail A., Lau C.Y., Jiang Y. Nutritional compositions and bioactivities of Dacryodes species: a review. Food Chem. 2014;165:247–255. doi: 10.1016/j.foodchem.2014.05.084. [DOI] [PubMed] [Google Scholar]
- 9.Ajibesin K.K. Dacryodes edulis (G.Don) H.J. Lam: a review on its medical, phytochemicals and economical properties. Res. J. Med. Plant. 2011;5(1):32–41. [Google Scholar]
- 10.Orwa C., Mutua A., Kindt R., Jamnadass R., Anthony S. 2009. Agroforestry Database: a Tree Reference and Selection Guide Version 4.0.http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp [Google Scholar]
- 11.Omonhinmin A.C. Ethnobotany of Dacryodes edulis (G. Don) H.J. Lam in Southern Nigeria 1: practices and applications among the Yoruba speaking people. Ethnobot. Res. Appl. 2012;10:175–184. [Google Scholar]
- 12.Tene T.O., Fouodjouo M., Seukep A.J., Ngouafong T.F. A review on traditional uses, phytochemical and pharmacological profiles, spiritual and economic values, and toxicity of Dacryodes edulis (G. Don) H.J. LAM. J. Drug Deliv. Ther. 2016;6(5):84–90. doi: 10.22270/jddt.v6i5.1276. [DOI] [Google Scholar]
- 13.Jirovetz L., Buchbauer G., Stoyanova A.S., Georgiev E.V., Damianova S.T. Composition, quality control and antimicrobial activity of the essential oil of long time stored dill (Anethum graveolens L.) seeds from Bulgaria. J. Agric. Food Chem. 2003;18(51):3854–3857. doi: 10.1021/jf030004y. [DOI] [PubMed] [Google Scholar]
- 14.Ikhuoria E., Maliki U.M. Characterization of avocado pear (Persea americana) and African pear (Dacryodes edulis) extracts. Afr. J. Biotechnol. 2007;6(7):950–952. http://www.academicjournals.org/AJB [Google Scholar]
- 15.Erukainure O.L., Mopuri R., Oyebode O.A., Koorbanally N.A., Islam M.S. Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed. Pharmacother. 2017;96:37–47. doi: 10.1016/j.biopha.2017.09.106. [DOI] [PubMed] [Google Scholar]
- 16.Omonhinmin A.C., Uche A.I. Assessment on in vitro antioxidant properties of Dacryodes edulis and Ficus exasperate as antimalarial plants. Asian Pac. J. Trop. Dis. 2013;3(4):294–300. doi: 10.1016/S2222-1808(13)60072-9. [DOI] [Google Scholar]
- 17.Omogbai B.A., Eneh T.O. Antibacterial activity of Dacryodes edulis seed extracts on food borne pathogens. Bayero J. Pure Appl. Sci. 2011;4(1):17–21. doi: 10.4314/bajopas.v4i1.3. [DOI] [Google Scholar]
- 18.Ufelle S., Ukaejiofo E., Achukwu P., Eluke B., Ghasi S., Neboh E. Potential haemopoietic effects of Dacryodes edulis seeds extract in Wistar rats. Int. J. Healthcare Sci. 2015;3(1):413–416. [Google Scholar]
- 19.Ononamadu C.J., Alhassan A.J., Aminu A., Imam A.A., Ihegboro G.O., Owolarafe T.A., Sule M.S. Methanol-extract/fractions of Dacryodes edulis leaves ameliorate hyperglycemia and associated oxidative stress in streptozotocin-induced diabetic wistar rats. J. Evid. Integr. Med. 2019;24:1–12. doi: 10.1177/2515690X19843832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teke G.N., Lunga P.K., Wabo H.K., Kuiate J.-R., Vilarem G., Giacinti G., Kikuchi S.H., Oshima Y. Antimicrobial and antioxidant properties of methanol extract, fractions and compounds from the stem bark of Entada abyssinica Stend ex A. Satabie. BMC Complement. Altern. Med. 2011;11:57–65. doi: 10.1186/1472-6882-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 22.Zakaria Z.A., Rahim H.F.A., Mohtarrudin N., Kadir A.A., Cheema M.S., Ahmad Z., Mooi C.S., Md Tohid S.F. Acute and sub-chronic oral toxicity studies of methanol extract of Clinacanthus nutans in mice. Afr. J. Tradit. Complement. Altern. Med. 2016;13(2):210–222. doi: 10.4314/ajtcam.v13i2.25. [DOI] [Google Scholar]
- 23.Hassan S.W., Ladan M.J., Dogondaji R.A., Umar R.A., Bilbis L.S., Hassan L.G., Ebbo A.A., Matazu I.K. Phytochemical and toxicological studies of aqueous leaves extracts of Erythrophleum africanum. Pak. J. Biol. Sci. 2007;10:3815–3821. doi: 10.3923/pjbs.2007.3815.3821. https://scialert.net/abstract/?doi=pjbs.2007.3815.3821 [DOI] [PubMed] [Google Scholar]
- 24.Fiskesjo G. Allium cepa test for screening chemicals: evaluation of cytologic parameters. In: Wang W., Gorsruch J.W., Hughes J.S., editors. Plants for Environmental Studies. CRC, Lewis publishers; Boca Raton, New York: 1997. pp. 308–333. 1997. [Google Scholar]
- 25.Png X.W., Akowuah G.A., Chin J.H. Acute oral toxicity study of Clinacanthus nutans in mice. Int. J. Pharm. Sci. Res. 2012;3(11):4202–4205. doi: 10.13040/IJPSR.0975-8232. [DOI] [Google Scholar]
- 26.Hodge A., Sterner B. Canadian Center for Occupational Health and Safety. 2005. Toxicity classes.http://www.ccohs.ca/oshanswers/chemicals/id50.htm (Accessed 30th April, 2020) [Google Scholar]
- 27.Okolo C.A., Ejere V.C., Chukwuka C.O., Ezeigbo I.I., Nwibo D.D., Okorie A.N. Hexane extract of Dacryodes edulis fruits possesses anti-diabetic and hypolipidaemic potentials in alloxan diabetes of rats. Afr. J. Tradit. Complement. Altern. Med. (AJTCAM) 2016;13(4):132–144. doi: 10.21010/ajtcam.v13i4.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph I.E., Jaja I.F., Boyi A.H., Olugbenga O.M. Comparative effects of methanol and oil extracts of Ocimum gratissimum on testicular morphology and epididymal sperm reserve of adult male albino rats (Wistar strain) Toxicol. Rep. 2019;6:1127–1134. doi: 10.1016/j.toxrep.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research Council (NRC) National Academics Press; USA: 2006. Toxicity Testing for Assessment of Environmental Agents: Interim Report; p. 34. [Google Scholar]
- 30.P’ng X.W., Akowuah G.A., Chin J.H. Evaluation of the sub-acute oral toxic effect of methanol extract of Clinacanthus nutans leaves in rats. J. Acute Dis. 2012;2(1):29–32. doi: 10.1016/S2221-6189(13)60090-6. [DOI] [Google Scholar]
- 31.Ogunmoyole T., Adeyeye R.I., Olatilu B.O., Akande O.A., Agunbiade O.J. Multiple organ toxicity of Datura stramonium seed extracts. Toxicol. Rep. 2019;6:983–989. doi: 10.1016/j.toxrep.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasekh H.R., Nazari P., Kamli-Nejad M., Hosseinzadeh L. Acute and subchronic oral toxicity of Galega officinalis in rats. J. Ethnopharmacol. 2008;166(1):21–26. doi: 10.1016/j.jep.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Musila M.N., Ngai D.N., Mbiri J.W., Njagi S.M., Mbinda W.M. Acute and sub-chronic oral toxicity study of methanolic extract of Caesalpinia volkensii (Harms) J. Drug Metab. Toxicol. 2017;8:222–230. doi: 10.4172/2157-7609.1000222. [DOI] [Google Scholar]
- 34.Gowda S., Desai P.B., Kulkarni S.S., Hull V.V., Math A.A.K., Vernekar S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2009;2(4):170–173. [PMC free article] [PubMed] [Google Scholar]
- 35.Olayode O.A., Daniyan M.O., Olayiwola G. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J. Tradit. Complement. Med. J. Tradit. Complement. Med. 2019 doi: 10.1016/j.jtcme.2019.05.001. (accepted article in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elekofehinti O.O., Adanlawo I.G., Fakoya A. Solanum anguivi saponins inhibit basal erythropoeisis in Rattus novergicus. Asian J. Pharm. Health Sci. 2012;2(3):416–419. [Google Scholar]
- 37.Barbério A., Barros L., Voltolini J.C., Mello M.L.S. Evaluation of the cytotoxic and genotoxic potential of water from the River Paraíba do Sul, in Brazil, with the Allium cepa L. test. Braz. J. Biol. 2009;69(3):837–842. doi: 10.1590/S1519-69842009000400010. [DOI] [PubMed] [Google Scholar]
- 38.Khora S.S., Panda K.K., Panda B.B. Genotoxicity of tetrodotoxin from puffer fish tested in root meristem cells of Allium cepa. Mutagenesis. 1997;12(4):265–269. doi: 10.1093/mutage/12.4.265. [DOI] [PubMed] [Google Scholar]
- 39.Souza L.F.B., Laughinghouse I.V.H.D., Pastori T., Tedesco M., Kuhn A.W., Canto-Dorow T.S., Tedesco S.B. Genotoxic potential of aqueous extracts of Artemisia verlotorum on the cell cycle of Allium cepa. Int. J. Environ. Stud. 2010;67(6):871–877. doi: 10.1080/00207233.2010.520457. 2010. [DOI] [Google Scholar]
- 40.Marcano L., Carruyo I., Campo A.D., Montiel X. Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environ. Res. 2004;94(2):221–226. doi: 10.1016/S0013-9351(03)00121-X. [DOI] [PubMed] [Google Scholar]
- 41.Sharma C.B.S.R. Plant meristems as monitors of genetic toxicity of environmental chemicals. Curr. Sci. 1983;52:1000–1002. https://www.jstor.org/stable/24086355 [Google Scholar]
- 42.Panda B.B., Sahu U.K. Induction of abnormal spindle function and cytokinesis inhibition in mitotic cells of Allium cepa by the organophosphorus insecticide fensulfothion. Cytobios. 1985;42:147–155. [Google Scholar]
- 43.Antosiewicz D. Analysis of the cell cycle in the root meristem of Allium cepa under the influence of Ledakrin. Folia Histochemicaet Cytobiologica. 1990;26:79–96. [PubMed] [Google Scholar]
- 44.Ribeiro T.P., Sousa T.R., Arruda A.S., Peixoto N., Gonçalves P.J., Almeida L.M. Evaluation of cytotoxicity and genotoxicity of Hancornia speciosa latex in Allium cepa root model. Braz. J. Biol. 2016;76(1):245–249. doi: 10.1590/1519-6984.20114. [DOI] [PubMed] [Google Scholar]
- 45.Celik T.A., Aslanturk O.S. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium Test. J. Biomed. Biotechnol. 2010:1–8. doi: 10.1155/2010/189252. Article ID 189252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dash S., Panda K.K., Panda B.B. Biomonitoring of low levels of mercurial derivatives in water and soil by Allium micronucleus assay. Mutat. Res. 1988;203:11–21. doi: 10.1016/0165-1161(88)90003-9. [DOI] [PubMed] [Google Scholar]
- 47.Grover I.S., Kaur S. Genotoxicity of wastewater sample from sewage and industrial effluent detected by the Allium root anaphase aberration and micronucleus assays. Mutat. Res. 1999;426(2):183–188. doi: 10.1016/S0027-5107(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 48.Chandra S., Chauhan L.K.S., Murthy R.C., Saxena P.N., Pande P.N., Gupta S.K. Comparative biomonitoring of leachates from hazardous solid waste of two industries using Allium test. Sci. Total Environ. 2005;347:46–52. doi: 10.1016/j.scitotenv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Ihegboro G.O., Ononamadu C.J., Owolarafe T.A., Afor E., Zaharadeen I.K. Antioxidants in plant extracts may contribute to the modulation of their toxicity: an Insight with Allium cepa model. NISEB. J. 2018;18(2):92–104. [Google Scholar]
- 50.Bertão M.R., Moraes M.C., Palmieri D.A., Silva L.P., Gonçalves da Silva R.M. Cytotoxicity, genotoxicity and antioxidant activity of extracts from Capsicum spp. Res. J. Med. Plants. 2016;10:265–275. doi: 10.3923/rjmp.2016.265.275. [DOI] [Google Scholar]
- 51.Prajitha V., Thoppil J.E. Cytotoxic and apoptotic activities of extract of Amaranthus spinosus L. in Allium cepa and human erythrocytes. Cytotechnology. 2017;69:123–133. doi: 10.1007/s10616-016-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.