Abstract
Objective:
This study aimed to investigate the root canal system morphology of maxillary first molar mesiobuccal (MB) roots in a Brazilian sub-population using micro-computed tomography.
Methods:
Ninety-six MB roots were scanned with a micro-CT (Skyscan 1173, Bruker). Three-dimensional images were analyzed regarding the number of pulp chamber orifices, the number and classification of the canals, the presence of accessory canals in different thirds of the root as well as the number and type of apical foramina.
Results:
A single entrance orifice was found in 53.0% of the samples, two in 43.9% and only 3.1% had three orifices. The second mesiobuccal root canal (MB2) was present at some portion of the root in 87.5% of the specimens. A single apical foramen was present in 16.7%, two in 22.9%, and three or more foramina in 60.4% of the roots. Only 55.3% and 76.1% of the root canals could be arranged by Weine’s and Vertucci’s classifications, respectively.
Conclusion:
The number of orifices at the pulp chamber level could not work as a predictor of the MB2 presence. The most prevalent canal configuration was Weine type IV / Vertucci type V. The anatomical complexity of the MB root could not be entirely classified by the current most accepted classifications.
Keywords: Micro-computed tomography, maxillary molars, pulp chamber anatomy, root canal anatomy, root canal classification
HIGHLIGHTS.
Weine type IV/ Vertucci type V was the most common canal configuration.
The prevalence of MB2 is influenced by the author’s definition.
The anatomical complexity of the MB root canal could not be entirely classified.
INTRODUCTION
Failure of the endodontic therapy is related to bacterial persistence through areas unaffected by instruments and antimicrobial substances, including not only untouched walls of the main canal but also lateral canals and apical ramifications (1, 2). In this sense, the study of the internal anatomy is strongly emphasized in Endodontics and the accurate knowledge of the canal morphology and its common variations are considered an essential requirement to achieve success in the endodontic treatment (3–5).
Due to a significant prevalence of additional canals found and a large number of variations in its morphology, the endodontic treatment of the mesiobuccal (MB) root of the first maxillary molar is considered one of the biggest challenges to clinicians (6). It has generated a large number of investigations and clinical reports compared to other dental roots (6, 7). Previous studies have shown a big discrepancy in the literature regarding the prevalence of a second mesiobuccal canal (MB2) in this root not only between studies using different techniques but even in those using the same methodology (7–9). These findings could be explained by demographic and ethnic factors (10), methodological limitations and also because of the authors’ definition of what constitutes a canal (7).
The presence of the MB2 can be investigated regardless of the definition used as MB2 canal with the interrelation analysis of 2D and 3D images provided by micro-CT technology. As a non-destructive, reproducible and high-reliable methodology, micro-CT is considered the gold standard technique for in vitro studies of the root canal anatomy due to the high level of details achieved (11–16). Moreover, the different anatomies can be grouped by the clinical classification of Weine et al. (3) and by a more appropriate classification for in vitro studies developed by Vertucci (4) Table 1 (7). At the same time, due to its micrometrical scale, it enables us to examine small complexities of the root canal system as accessory canals, the apical third, and also to analyze the internal morphology in bi-dimensional and three-dimensional (3D) models (12, 13).
TABLE 1.
| Weine et al. (1969) | Vertucci (1984) | Morphologies description | |
|---|---|---|---|
| 1-1 | Type I | Type I | A single canal with one foramen |
| 2-1 | Type II | Type II | Two canals that join in the apical third |
| 1-2-1 | - | Type III | One canal that divides into two that subsequently reunite and exits as one |
| 2-2 | Type III | Type VI | Two separate canals all the way to the apex |
| 1-2 | Type IV | Type V | One canal that divides just short of the apex |
| 2-1-2 | - | Type VI | Two canals that unite in the root and then divides again at the apex |
| 1-2-1-2 | - | Type VII | One canal that divides, reunites and finally exits through two apical foramina |
| 3-3 | - | Type VIII | Three separate canals in one root |
In the anthropological field, several studies investigating the morphologies of teeth following Weine’s and Vertucci’s classifications suggested that populations from different geographic regions and ethnic backgrounds may present differences in root canal configurations (11, 13, 17, 18). However, these studies were mainly based on Caucasian, Asian, Indian or Mexican sub-populations. This knowledge cannot be applicable in a heterogeneous society, such as the Brazilian one, which has genetic contributions from the four main continental groups: Europeans, Africans, Asians, and Native South-Americans (19). To our concern, this is the first study in the literature using a micro-CT approach to evaluate the most prevalent canal configuration in the maxillary first molar mesiobuccal (MB) roots in a Brazilian sub-population following Weine’s and Vertucci’s classification systems.
Therefore, this study aimed to analyze the internal morphology of the mesiobuccal root of the maxillary first molars from 96 Brazilian individuals by quantifying the number of orifices at the pulp chamber level and the prevalence of the MB2 canal. The number of accessory canals in different thirds of the roots and the number and type of apical foramen were also determined. The most common canal configuration from a Brazilian population was established according to the classifications conceived by Weine et al. (3) and Vertucci (4).
MATERIALS AND METHODS
Specimen selection and preparation
Ninety-six healthy maxillary first molars were extracted from Brazilian subjects for reasons unrelated to the current study. Approval for the study protocol (n 347.074) was obtained from the ethical committee of the University. The patient gender and age were unknown. Only intact teeth with mature apices were selected, and any attached soft tissue and calculus was removed using ultrasonic scaler and sodium hypochlorite before the experiment.
Micro-CT scanning and 3D reconstruction
The specimens were scanned by a micro-CT system (Skyscan 1173; Bruker Co., Kontich, Belgium), using 70kV, 114 mA, 360° rotation with a step of 0.3°, a pixel size of 14.87 µm and aluminum filter (1.0 mm of thickness). The average scanning time was 30 minutes per sample. These settings provided a spatial resolution of 21.39 µm.
Images were reconstructed by the dedicated NRecon software v.1.6.9.4 (Bruker Co., Kontich, Belgium) and InstaRecon® v.1.3.9.2 (IR-CBR Server, University of Illinois Research Park, Illinois, EUA).
Analysis of the root canal morphology
CTAn v.1.14.4.1, Dataviewer, and CTVox software (Bruker Co., Kontich, Belgium) were used for 3D evaluation of internal anatomy. The volume of interest (VOI) was defined as from the first axial slice that showed the separation between the mesiobuccal and distobuccal canals, until the first slice after the MB root apex. We used the ‘ROI shrink-wrap’ tool in CTAn to define the region of interest (ROI) of each slice, and it resulted in the whole volume of the MB root. We applied an automatic thresholding method (Otsu 2D, Bruker Co., Kontich, Belgium) for segmenting the root canal system from the dentin.
Three experienced endodontists evaluated the root canal systems independently using 3D rendered volume and the full axial slices dataset. If disagreement occurred, it was solved by discussion until a consensus was reached. The following observations were recorded: (a) the number of root canal orifices in the pulp chamber; (b) the number of accessory canals in the different thirds; (c) qualification and quantification of apical foramen; (d) the number of root canals; (e) categorization of the root canal system according to the classification proposed by Weine et al. (3) and Vertucci (4) Figure 1.
Figure 1.
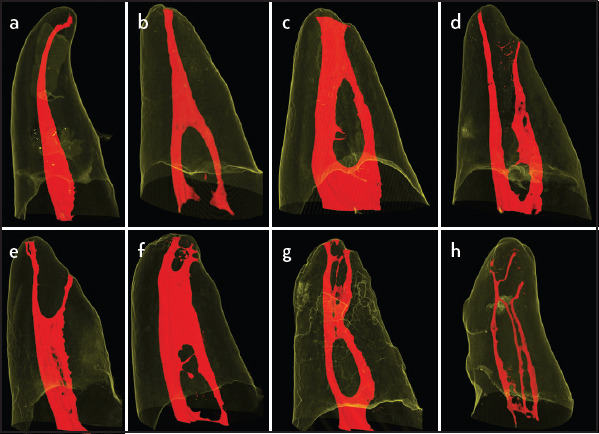
Classification of the root canal system according to Weine et al. (3) and Vertucci (4). (a) Configuration classified as Weine/Vertucci type I. (b) Weine/Vertucci type II. (c) Vertucci type III. (d) Weine type III/Vertucci type IV. (e) Weine type IV/Vertucci type V. (f) Vertucci type VI. (g) Vertucci type VII. (h) Vertucci type VIII
Accessory canals were defined as any branch of a root canal that communicates with the outer surface, and they were quantified in the different root thirds following Vertucci’s classification (5). Apical foramen was defined as a circular or oval orifice which marks the end of the canal at the external surface (5). Moreover, apical deltas were defined as a complex of branches located near the apex, where the main canal could not be distinguishable from the others (5). Finally, the reticular canals were defined as the result of three or more parallel canals in the apical third connected by inter-canals (20).
RESULTS
Number of orifices in the pulp chamber
The number of entrance orifices found at the pulp chamber level was as follows: 53.0% (n=51) of the samples had a single entrance orifice, 43.9 % (n=42) had two orifices, and 3.1% (n=3) of the teeth had three orifices (Table 2 and 3).
TABLE 2.
Number of orifices in the pulp chamber related to Weine et al. classification (3)
| Number of orifices | Weine et al. classification type | Non- classifiable | |||
|---|---|---|---|---|---|
| I (1-1) | II (2-1) | III (2-2) | IV (1-2) | ||
| 1-(53.0%) | 12.5% | - | - | 20.8% | 19.8% |
| 2-(43.9%) | - | 11.6% | 10.4% | - | 21.9% |
| 3-(3.1%) | - | - | - | - | 3.1% |
| Total | 12.5% | 11.6% | 20.8% | 10.4% | 44.8% |
| 55.3% | |||||
TABLE 3.
Number of orifices in the pulp chamber related to Vertucci’s classification (4)
| Number of Orificies | Vertucci’s classification type | Non- classifiable | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I (1-1) | II (2-1) | III (1-2-1) | IV (2-2) | V (1-2) | VI (2-1-2) | VII (1-2-1-2) | VIII (3-3) | ||
| 1-(53.0%) | 12.5% | - | 5.2% | - | 20.8% | - | 6.2% | - | 8.3% |
| 2-(43.9%) | - | 11.6% | - | 10.4% | - | 9.4% | - | - | 12.5% |
| 3-(3.1%) | - | - | - | - | - | - | - | 0.0% | 3.1% |
| Total | 12.5% | 11.6% | 5.2% | 10.4% | 20.8% | 9.4% | 6.2% | 0.0% | 23.9% |
| 76.1% | |||||||||
Canal type
The results of the different types of root canal configurations obtained can be seen in Tables 2 and 3. Most of the samples were classified as Weine IV/Vertucci type V (20.8%), followed by Weine/Vertucci type I (12.5%) and Weine/Vertucci type II (11.6%). Some of the teeth which could not be classified can be visualized in Figure 2.
Figure 2.
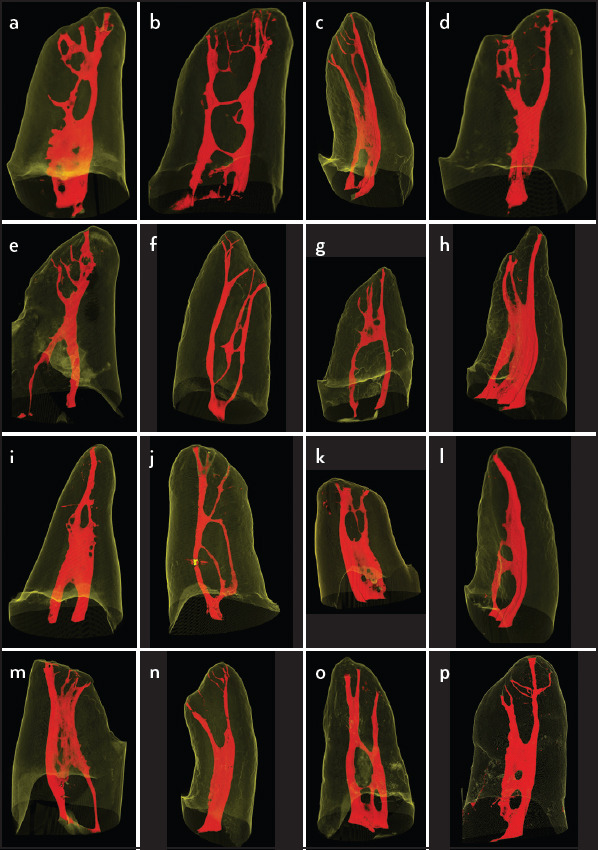
(a-p) Examples of roots that do not meet the classifications proposed by Weine et al. (3) and Vertucci (4)
Number of accessory canals
In 47.9% of the teeth an accessory canal was present (46 in 96). The apical third presented the highest percentage of accessory canals: 76.0% (57/75) in 32 samples. The middle third showed 16.0% (12/75) accessory canals in 8 specimens, with a single sample presenting three accessory canals in this third. The cervical third was the one with the smallest number of accessory canals (8.0%; 6/75) accessory canals in 6 different samples.
Number of apical foramina and configuration
Multiple apical foramina were found in 83.3% (n=80) of the roots. A single apical foramen was found in 16.7% (n=16), and two apical foramina were found in 22.9% (n=22) of the roots (Table 4). Fourteen samples (14.6%) showed apical delta (Figs. 3 and 4). In addition to this, four (4.7%) of the specimens showed reticular canals (Fig. 5).
TABLE 4.
Number of specimens and incidence of apical foramina
| Nr. of foramina | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Specimens (n) | 16 | 22 | 27 | 17 | 6 | 3 | 2 | 2 | 0 | 1 |
| Incidence | 16.7% | 22.9% | 28.1% | 17.7% | 6.3% | 3.1% | 2.1% | 2.1% | 0.0% | 1.0% |
| More than 2 foramina: 58 (60.4%) | ||||||||||
Figure 3.
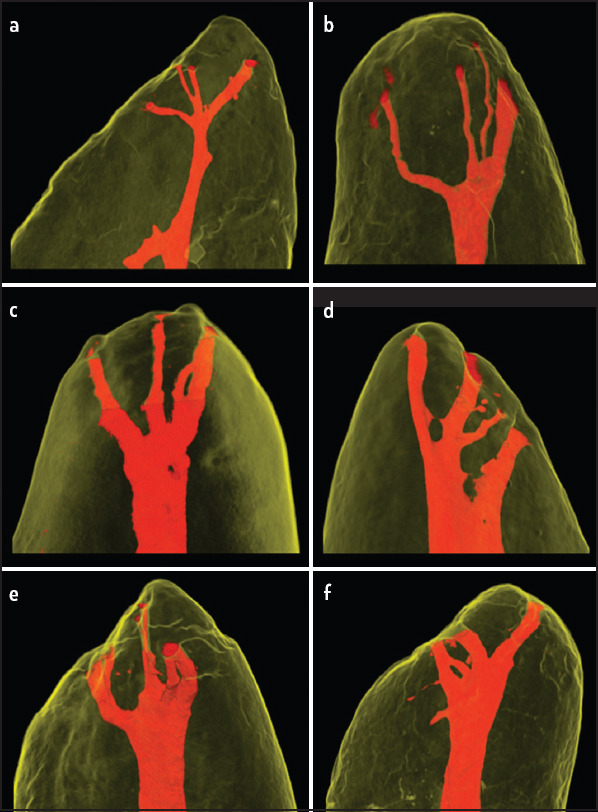
Different morphologies classified as apical deltas (a-f). The main canal cannot be differentiated from the accessory canals
Figure 4.
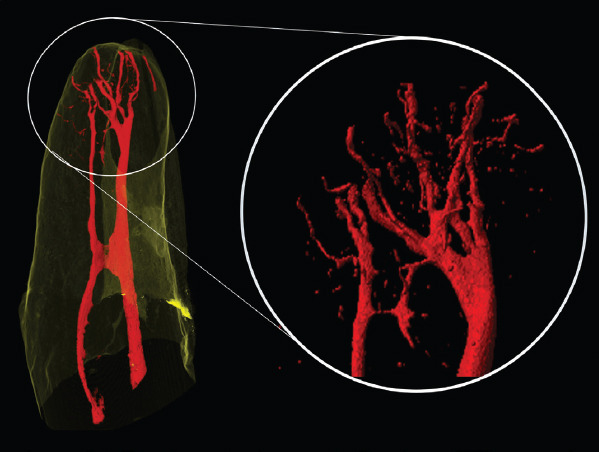
The most complex apical configuration visualized in this study classified as an apical delta. Up to ten different apical foramina can be observed
Figure 5.
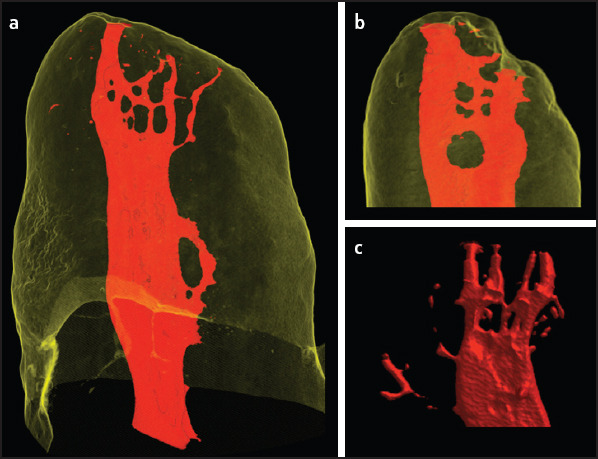
Reticular canals. (a, b) Relationship between the internal and external morphologies. (c) Internal anatomy as a 3D model
DISCUSSION
Most authors of clinical studies acknowledge the presence of the MB2 canal if an independent orifice was found on the floor of the pulp chamber (21, 22), not mentioning if the canal remained separate from the MB canal after instrumentation. Stropko (23) considered the MB2 as a separate canal if it was possible to instrument it to a depth of 3 to 4 mm after troughing. Wolcott et al. (24) included the MB2 as an extra canal if it was obturated apart from the MB canal or within 5 mm from the apex when it joined the MB. Some authors considered the existence of MB2 canal if two instruments could be placed simultaneously in the MB root to a minimum depth of 16 mm from the cusp of an entire tooth (25). Other methods to count the main canals of a given root is to quantify the presence of an MB2 in different root levels using micro-CT, CBCT, decalcification, 2D radiography, and sectioning (8, 11–13, 26–28). However, other authors failed to provide a clear definition of what they considered a canal in their reported data (7).
The present study showed a prevalence of 46.9% of MB2 orifices at the pulp chamber level, while 87.5% of the specimens presented two, three, or four canals at some portion of the root. These results are not in agreement with the common knowledge that implies that the observation of the pulp chamber floor could offer indications of the internal configuration (4, 29). Furthermore, our results suggest that the number of orifices at the pulp chamber level might not be a good predictor of the presence of the MB2.
The identification of multiple canals with micro-CT technology does not necessarily mean that they will be clinically accessible (26, 30) (Fig. 2), but it can explain the high rate of treatment failure of the MB root (3). Even with no report in this study about the occurrence of “dead-end” canals (7, 23, 31), or calcified segments (21), some samples showed constrictions, bends and pronounced curvatures that could preclude exploration with current endodontic instruments due to physical limitations (23). These MB2 canals that can only be cleaned by chemical substances are related to post-treatment apical periodontitis caused by persistent bacteria located on un-instrumented canal walls or in areas such as accessory canals and apical ramifications (1).
In this sense, our results report a higher frequency of accessory canals at the apical third where only 16.7% of the MB roots showed one and 22.9% two foramina. These results suggest that 60.4% of the apical foramina where these canals flow into would also be cleaned only by chemical substances (Fig. 6). The high prevalence of accessory canals in the apical region is in agreement with previous studies and confirms that the number of accessory canals increased in the coronal-apical direction (12, 17, 30). These apical complexities could also explain why most post-treatment apical periodontitis are caused by bacteria located in the apical root canal system (1, 2).
Figure 6.
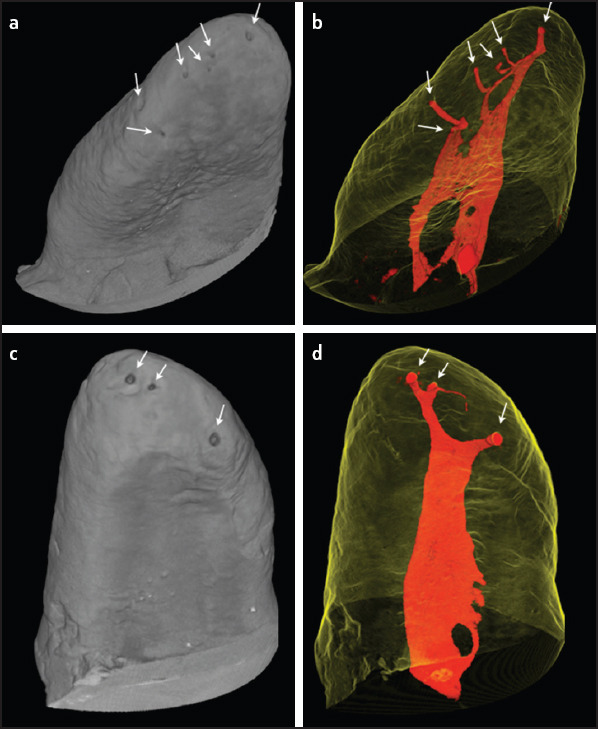
Relationship between the internal and external morphologies. a-b A representative sample with 6 apical foramina (white arrows). (a) External surface of the root. (b) The canals (in red) in relationship with the translucent external morphology. (c, d) A representative sample with 3 apical foramina (white arrows). (c) External surface of the root. (d) Root canals (in red) in relationship with the translucent external morphology
Another relevant finding of this present study is concerning the anthropological aspect of the most common configurations found in this population. Studies with uniparental markers demonstrate that the genomes of most Brazilians are mosaic, having elevated levels of genetic admixture between European, Amerindian, and Africans. This high ancestral variability suggests that each Brazilian has a singular and quite individual genome (19). Considering Weine and Vertucci classifications, our study found that the majority of specimens were classified as Weine IV/Vertucci type V (20.8%), followed by Weine/Vertucci type I (12.5%) and Weine/Vertucci type II (11.6%). Using CBCT, de Freitas et al. (28) found type I (25.7%) and type V (17.1%) the first and second most prevalent in Vertucci’s classification. Ethnic characteristics are observed by comparing these results with findings of micro-CT studies in East and South Asian, Korean, Indian, and Mexican populations, with a prevalence of Weine type III/Vertucci type IV configuration, which seems to be a typical Mongoloid trait (11, 30). In a Caucasian population study, Weine/Vertucci type II was the most prevalent (4, 12), the same for an Iranian sub-population (27).
Particular attention should be given to 44.8% and 23.9% of the samples that do not meet the classifications proposed by Weine et al. (3) and Vertucci (4), respectively Figure 2. These specimens were allocated in 16 different types. This proportion of non-classified samples is corroborated by other micro-CT studies, like the study of Verma & Love (6), where 40.0 % of the samples could not be classified by Weine’s and 30.0 % by Vertucci’s classifications. Gu et al. (32) observed that 38.6 % of the samples could be sortable by Weine et al. (3) and 16.9% by Vertucci (4) classification. In a Korean population, Kim et al. (30) found 29.2% and 17.7% non-classifiable samples by the classifications Weine et al. (3) and Vertucci (4), respectively. Thus, the results of this study, in addition to studies with a similar methodology, support the claim that Weine et al. (3) and Vertucci (4) ratings do not adequately reflect the configurations of MB root of maxillary molars presented in such detail by micro-CT methodology.
Micro-CT and CBCT offer the same characteristic by evaluating the presence of the MB2 canal in different thirds of the root but for laboratory and clinical studies respectively. Comparing our results of 87.5% of MB2 presence with CBCT in the same Brazilian population, Reis et al. (2013) (33) found 86.1% and 91.0% on the right and left side respectively while Filho et al. (2009) (26) found 37.0% of MB2. The use of different CBCT protocols, as well as the higher resolution of Micro-CT, could explain these results. The presence of isthmus and flattened canals complicates the interpretation of CBCT images making it more difficult to reach a consensus on canals identification (28). The CBCT protocols that are more suitable for clinically locate MB2 canals showed 80% of concordance with micro-CT even with the image resolution limitation (28). Consequently, CBCT could be used as a valuable auxiliary diagnostic tool for routine clinical practice.
Limitations of the present study might be that the patients’ age and gender and the location of the tooth at the time of the extraction were unknown. Unfortunately, the inclusion of these parameters were not possible and they could also explain the discrepancies observed (18). As Brazil is a continental country with diverse and mixed genotypic origins (19), future research should focus on comparing the different root canal morphologies among different ethnic groups in Brazilian sub-populations from different geographical locations.
CONCLUSION
In conclusion, this study suggests that the number of orifices in the pulp chamber might not be a reliable indicator of the presence of a second mesiobuccal canal and confirms the influence of the author’s definition on the prevalence of MB2 canals. The most prevalent type in this Brazilian population was Weine type IV/Vertucci type V, and accessory canals were higher detected at the apical third, followed by the middle and cervical thirds of the root respectively. Weine’s (3) and Vertucci’s (4) classifications do not adequately reflect the configurations of the mesiobuccal root of maxillary molars as presented in such detail by micro-CT methodology.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Ethics Committee Approval: Ethical Committee of Estácio de Sá University, Rio de Janeiro, RJ, Brazil, n 347.074.
Peer-review: Externally peer-reviewed.
Financial Disclosure: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number: 141210/2014-1) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Capes, Rio de Janeiro, Brazil (grant number: 88881.133693/2016-01).
Authorship contributions: Concept – B.C.D.S., J.C.M.O., I.C.B.L., P.L.; Design – B.C.D.S., J.C.M.O.; Supervision – I.C.B.L., P.L.; Funding - J.C.M.O., I.C.B.L.; Materials - None; Data collection &/or processing – B.C.D.S., M.S.P., C.K.G.; Analysis and/or interpretation – B.C.D.S., M.S.P., P.L., J.C.M.O.; Literature search – B.C.D.S., C.K.G.; Writing – B.C.D.S., M.S.P.; Critical Review – B.C.D.S., M.S.P., C.K.G., J.C.M.O., I.C.B.L., P.L.
REFERENCES
- 1.Antunes HS, Rôças IN, Alves FR, Siqueira JF., Jr Total and Specific Bacterial Levels in the Apical Root Canal System of Teeth with Post-treatment Apical Periodontitis. J Endod. 2015;41(7):1037–42. doi: 10.1016/j.joen.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Ricucci D, Siqueira JF., Jr Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. J Endod. 2010;36(1):1–15. doi: 10.1016/j.joen.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Weine FS, Healey HJ, Gerstein H, Evanson L. Canal configuration in the mesiobuccal root of the maxillary first molar and its endodontic significance. Oral Surg Oral Med Oral Pathol. 1969;28(3):419–25. doi: 10.1016/0030-4220(69)90237-0. [DOI] [PubMed] [Google Scholar]
- 4.Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol. 1984;58(5):589–99. doi: 10.1016/0030-4220(84)90085-9. [DOI] [PubMed] [Google Scholar]
- 5.Vertucci FJ. Root canal morphology and its relationship to endodontic procedures. Endod Top. 2005;10:3–29. [Google Scholar]
- 6.Verma P, Love RM. A Micro CT study of the mesiobuccal root canal morphology of the maxillary first molar tooth. Int Endod J. 2011;44(3):210–7. doi: 10.1111/j.1365-2591.2010.01800.x. [DOI] [PubMed] [Google Scholar]
- 7.Cleghorn BM, Christie WH, Dong CC. Root and root canal morphology of the human permanent maxillary first molar:a literature review. J Endod. 2006;32(9):813–21. doi: 10.1016/j.joen.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Domark JD, Hatton JF, Benison RP, Hildebolt CF. An ex vivo comparison of digital radiography and cone-beam and micro computed tomography in the detection of the number of canals in the mesiobuccal roots of maxillary molars. J Endod. 2013;39(7):901–5. doi: 10.1016/j.joen.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiebert BM, Abramovitch K, Rice D, Torabinejad M. Prevalence of Second Mesiobuccal Canals in Maxillary First Molars Detected Using Cone-beam Computed Tomography, Direct Occlusal Access, and Coronal Plane Grinding. J Endod. 2017;43(10):1711–5. doi: 10.1016/j.joen.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Martins JNR, Marques D, Silva EJNL, Caramês J, Mata A, Versiani MA. Second mesiobuccal root canal in maxillary molars - A systematic review and meta-analysis of prevalence studies using cone beam computed tomography. Arch Oral Biol. 2020;113:104589. doi: 10.1016/j.archoralbio.2019.104589. [DOI] [PubMed] [Google Scholar]
- 11.Park JW, Lee JK, Ha BH, Choi JH, Perinpanayagam H. Three-dimensional analysis of maxillary first molar mesiobuccal root canal configuration and curvature using micro-computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):437–42. doi: 10.1016/j.tripleo.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Somma F, Leoni D, Plotino G, Grande NM, Plasschaert A. Root canal morphology of the mesiobuccal root of maxillary first molars:a micro-computed tomographic analysis. Int Endod J. 2009;42(2):165–74. doi: 10.1111/j.1365-2591.2008.01472.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang SW, Lee JK, Lee Y, Kum KY. In-depth morphological study of mesiobuccal root canal systems in maxillary first molars:review. Restor Dent Endod. 2013;38(1):2–10. doi: 10.5395/rde.2013.38.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordinola-Zapata R, Martins JNR, Bramante CM, Villas-Boas MH, Duarte MH, Versiani MA. Morphological evaluation of maxillary second molars with fused roots:a micro-CT study. Int Endod J. 2017;50(12):1192–200. doi: 10.1111/iej.12752. [DOI] [PubMed] [Google Scholar]
- 15.Martins JNR, Ordinola-Zapata R, Marques D, Francisco H, Caramês J. Differences in root canal system configuration in human permanent teeth within different age groups. Int Endod J. 2018;51(8):931–41. doi: 10.1111/iej.12896. [DOI] [PubMed] [Google Scholar]
- 16.Peters OA, Laib A, Rüegsegger P, Barbakow F. Three-dimensional analysis of root canal geometry by high-resolution computed tomography. J Dent Res. 2000;79(6):1405–9. doi: 10.1177/00220345000790060901. [DOI] [PubMed] [Google Scholar]
- 17.Alavi AM, Opasanon A, Ng YL, Gulabivala K. Root and canal morphology of Thai maxillary molars. Int Endod J. 2002;35(5):478–85. doi: 10.1046/j.1365-2591.2002.00511.x. [DOI] [PubMed] [Google Scholar]
- 18.Martins JNR, Gu Y, Marques D, Francisco H, Caramês J. Differences on the root and root canal morphologies between Asian and White Ethnic groups analyzed by cone-beam computed tomography. J Endod. 2018;44(7):1096–104. doi: 10.1016/j.joen.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Pena SD, Bastos-Rodrigues L, Pimenta JR, Bydlowski SP. DNA tests probe the genomic ancestry of Brazilians. Braz J Med Biol Res. 2009;42(10):870–6. doi: 10.1590/s0100-879x2009005000026. [DOI] [PubMed] [Google Scholar]
- 20.Pineda F. Roentgenographic investigation of the mesiobuccal root of the maxillary first molar. Oral Surg Oral Med Oral Pathol. 1973;36(2):253–60. doi: 10.1016/0030-4220(73)90247-8. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen RB, Alyassin AM, Peters DD, Carnes DL, Lancaster J. Microcomputed tomography:an advanced system for detailed endodontic research. J Endod. 1995;21(11):561–8. doi: 10.1016/S0099-2399(06)80986-6. [DOI] [PubMed] [Google Scholar]
- 22.Buhrley LJ, Barrows MJ, BeGole EA, Wenckus CS. Effect of magnification on locating the MB2 canal in maxillary molars. J Endod. 2002;28(4):324–7. doi: 10.1097/00004770-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Stropko JJ. Canal morphology of maxillary molars:clinical observations of canal configurations. J Endod. 1999;25(6):446–50. doi: 10.1016/S0099-2399(99)80276-3. [DOI] [PubMed] [Google Scholar]
- 24.Wolcott J, Ishley D, Kennedy W, Johnson S, Minnich S. Clinical investigation of second mesiobuccal canals in endodontically treated and retreated maxillary molars. J Endod. 2002;28(6):477–9. doi: 10.1097/00004770-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Seidberg BH, Altman M, Guttuso J, Suson M. Frequency of two mesiobuccal root canals in maxillary permanent first molars. J Am Dent Assoc. 1973;87(4):852–6. doi: 10.14219/jada.archive.1973.0489. [DOI] [PubMed] [Google Scholar]
- 26.Baratto Filho F, Zaitter S, Haragushiku GA, de Campos EA, Abuabara A, Correr GM. Analysis of the internal anatomy of maxillary first molars by using different methods. J Endod. 2009;35(3):337–42. doi: 10.1016/j.joen.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Khademi A, Zamani Naser A, Bahreinian Z, Mehdizadeh M, Najarian M, Khazaei S. Root Morphology and Canal Configuration of First and Second Maxillary Molars in a Selected Iranian Population:A Cone-Beam Computed Tomography Evaluation. Iran Endod J. 2017;12(3):288–92. doi: 10.22037/iej.v12i3.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Freitas JV, Baratto-Filho F, Coelho BS, Tomazinho FSF, Crozeta BM, de Sousa Neto MD, et al. Efficacy of different cone-beam computed tomographic protocols in the identification of mesiobuccal canals of maxillary first molars:A tomographic and ex vivo study. J Endod. 2017;43(5):810–5. doi: 10.1016/j.joen.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Slowey RR. Radiographic aids in the detection of extra root canals. Oral Surg Oral Med Oral Pathol. 1974;37(5):762–72. doi: 10.1016/0030-4220(74)90142-x. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y, Chang SW, Lee JK, Chen IP, Kaufman B, Jiang J, et al. A micro-computed tomography study of canal configuration of multiple-canalled mesiobuccal root of maxillary first molar. Clin Oral Investig. 2013;17(6):1541–6. doi: 10.1007/s00784-012-0852-8. [DOI] [PubMed] [Google Scholar]
- 31.Kulild JC, Peters DD. Incidence and configuration of canal systems in the mesiobuccal root of maxillary first and second molars. J Endod. 1990;16(7):311–7. doi: 10.1016/s0099-2399(06)81940-0. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y, Lee JK, Spångberg LS, Lee Y, Park CM, Seo DG, Chang SW, et al. Minimum-intensity projection for in-depth morphology study of mesiobuccal root. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(5):671–7. doi: 10.1016/j.tripleo.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 33.Reis AG, Grazziotin-Soares R, Barletta FB, Fontanella VR, Mahl CR. Second canal in mesiobuccal root of maxillary molars is correlated with root third and patient age:a cone-beam computed tomographic study. J Endod. 2013;39(5):588–92. doi: 10.1016/j.joen.2013.01.003. [DOI] [PubMed] [Google Scholar]


