Abstract
Nonsurgical and surgical endodontic treatments have a high success rate in the treatment and prevention of apical periodontitis when carried out according to standard and accepted clinical principles. Nevertheless, endodontic periapical lesions remain in some cases, and further treatment should be considered when apical periodontitis persists. Although several treatment modalities have been proposed for endodontically treated teeth with persistent apical periodontitis, there is a need for less invasive methods with more predictable outcomes. The advantages and shortcomings of existing approaches for the diagnosis and treatment of endodontic periradicular lesions are discussed in this review.
Keywords: Cone-beam computed tomography, cyst, granuloma, periradicular lesion, root canal treatment
HIGHLIGHTS.
Several methods have been proposed for treating apical periodontitis, such as root canal (re)treatment, periradicular surgery, marsupialization, decompression, and enucleation.
Cone-beam computed tomography, magnetic resonance imaging, and echography show promising results in the diagnosis of periradicular lesions.
Treatment of true cysts has remained a matter of debate, and the best possible way to treat them is still unclear.
INTRODUCTION
Root Canal Infection
The dental pulp is a sterile connective tissue protected by enamel, dentin, and cementum. Significant injury of the pulp chamber leads to inflammation and may result in pulp necrosis if left untreated. Possible scenarios that can result in periapical radiolucencies are commonly initiated either by trauma, caries, or tooth wear (1). Microorganisms might colonize the pulp tissue after it loses its blood supply as a consequence of trauma, resulting in periradicular pathosis. Pulp exposures can lead to pulp necrosis and periradicular pathosis (1). Microorganisms and their products have a pivotal role in the initiation, progression, and establishment of periradicular conditions (2, 3). With the progression of inflammation due to carious pulp exposure and invasion of microorganisms, the most likely result would be pulp necrosis. Once root canal infection is established, and pulp necrosis occurs, neither host defense nor systemic antibiotic therapy would be effective in restricting the infection due to the absence of local blood supply (4). It is possible to prevent their spread successfully through non-surgical endodontic treatment. It has been reported that the majority of endodontic bacteria are suspended in the fluids found within the root canal(s) (5); however, bacterial aggregates and biofilms tend to adhere to the root canal walls to form concentrated bacterial centers (6). Infections might spread into dentinal tubules and root canal complexities. Root canal infections can be treated through professional intervention, using endodontic procedures or extraction.
Microorganisms residing in the root canal play an essential role in the initiation and establishment of periradicular lesions, which has been proved by studies performed on rats and monkeys (2, 3). Considering the role of microorganisms in the presence of apical periodontitis, clinicians should be aware that endodontic therapy is the management of infective disease.
Teeth with inadequate root canal treatments and asymptomatic periapical (PA) lesions usually harbor obligate anaerobic microorganisms; such teeth might even have sound coronal restorations (7, 8). In this situation, the bacterial composition is similar to the infected but previously untreated teeth (7, 8). Gram-positive and facultative anaerobic microorganisms are predominant in the early stages of infection (9). Proper retreatment of these cases results in success rates of 74–82% (8, 10), comparable to those of primary non-surgical endodontic treatments, i.e., 85–94% (11). Orthograde retreatments in these cases might negate the need for periapical surgeries.
Periapical (PA) lesion
Periapical or periradicular lesions are barriers that restrict the microorganisms and prevent their spread into the surrounding tissues; microorganisms induce the PA lesions, primarily or secondarily (2, 3). The bone is resorbed, followed by substitution by a granulomatous tissue and a dense wall of polymorphonuclear leukocytes (PMN). Less commonly, there is an epithelial plug at the apical foramen to block the penetration of microorganisms into the extra-radicular tissues (5). Only a limited number of endodontic pathogens can penetrate through these barriers; however, microbial products and toxins are capable of penetrating these barriers to initiate and establish periradicular pathosis. Periapical radiolucencies are the most frequent clinical signs of these lesions (5).
The majority of periapical lesions heal subsequent to meticulous non-surgical endodontic treatments (12, 13). In order to assess the healing potential, at least a 6 (14) to 12-month (12) period after root canal treatment should be considered. It has been reported that at the 6-month visit, only half of the cases that eventually heal exhibit signs of healing (advanced and complete healing), and at the 12-month interval, 88% of these lesions exhibit signs of healing while complete healing of the PA lesion might take up to four years in some cases (12). It is advisable to follow such cases for at least 12 months before considering them as abutments (15). However, postponing the placement of coronal restoration increases the risk of tooth fracture. Remaining sound tooth structure and occlusion play an important role in this regard. Placement of a sound coronal restoration improves periapical healing (16), and delayed placement of the final restoration might lead to failure, negatively affecting the long-term survival of the teeth, which should be considered in such cases (17). It must be noted that the presence of a lesion in a radiograph should not be the only reason for commencing retreatment in teeth with proper root canal treatment. These teeth might remain in a state of asymptomatic function (18) as the incidence of flare-up is less than 6% in 20 years (18). Therefore, placement of a sound coronal restoration immediately after the completion of non-surgical endodontic treatment is highly recommended even if a follow-up period is needed to place more complicated restorations such as crowns and bridges (19).
The majority of periradicular lesions can be categorized as dental granulomas, periradicular cysts, or abscesses, which are radiolucent (20). Condensing osteitis is another entity caused by chronically inflamed pulp tissue with subsequent chronic apical periodontitis with a distinct radiographic appearance. The periradicular bone seems more radiopaque than healthy bone with occasional PDL widening (21). Histological examinations can distinguish these entities, leading to a definitive diagnosis of each category (22). The likelihood of periradicular cyst is much higher in the presence of the following conditions: (a) the periradicular lesion involving one or more teeth with necrotic pulps; (b) the lesion is ≥200 mm2; (c) aspiration yielding a straw-colored fluid or drainage of such fluid through an access; and (d) the presence of cholesterol crystals in the fluid. It has been reported that 100% of the cases were cysts with radiographic lesion sizes of ≥200 mm2 (23). Furthermore, the incidence of cysts has been reported to be 60–67% in lesions measuring 10–20 mm in diameter (24, 25). When considering the lesion volume, there is an 80% probability of a cyst if it measures >247 mm3 and a 60% probability with root displacement and a volume <247 mm3 (26). Cholesterol crystals, identifiable under a microscope, are present in 29–43% of periradicular cysts (27). These crystals are more common in periradicular cysts compared to apical granulomas (22, 27). The treatment modalities for periapical lesions include non-surgical root canal treatment, periapical surgery, or tooth extraction. If non-surgical treatment is deemed ineffective or difficult, periapical surgery is the treatment of choice. True cysts are closed pathologic entities that are separate from the apex and have an intact epithelial lining and might have a cord of epithelium that attaches them to the root apex (28, 29). They have probably become an independent entity and will likely not respond to non-surgical treatment (30). There are several irritants such as intracanal irritants and cholesterol crystals that continuously stimulate the basal stem cells of the cystic epithelium in true apical cysts (31, 32), which cannot be removed without surgery (28). Even a large periradicular lesion might directly communicate with the root canal system (28) and might heal favorably after non-surgical treatment with optimal infection control (22, 33); nevertheless, the success rate is lower than cases with smaller lesions (16) (Fig. 1).
Figure 1.
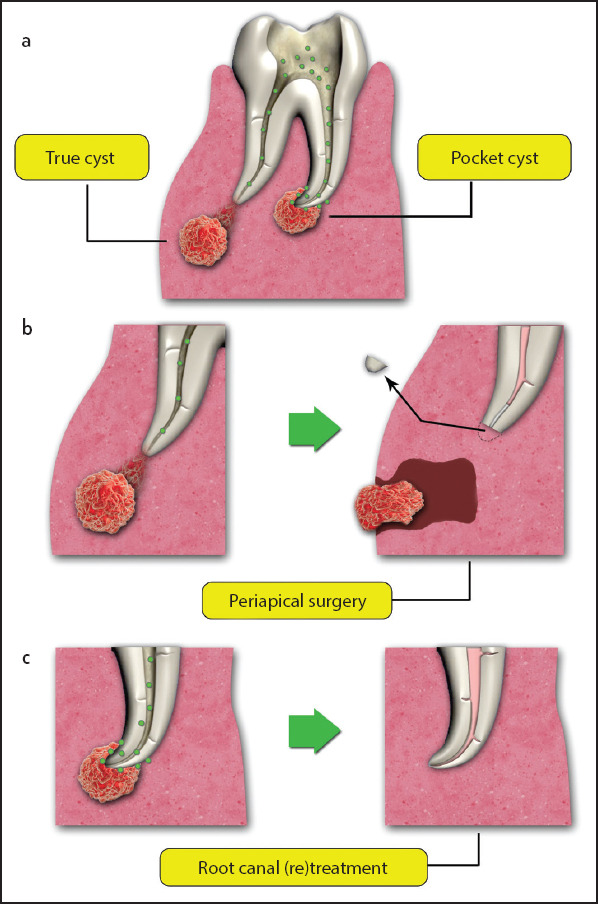
Periapical cysts are caused by the presence of infection in the root canal space and can be categorized as true or pocket cysts (a). Periradicular surgery might be needed for resolving the true cysts (b) while most of the pocket cysts are resolved after root canal (re)treatment without the need for surgical intervention (c)
Differential diagnosis of different types of endodontic-related PA lesions
The predominance and prevalence of inflammatory changes, such as granulomas and periapical cysts induced by root canal infection, have been assessed by examining periapical biopsy specimens (34). Attempts to accurately assess the nature of the periapical pathosis and diagnose the lesion have limited success before performing surgery. Although several methods have been proposed, such as periapical radiographs (35), contrast media (36), Papanicolaou smears (37), real-time ultrasound imaging (38), and albumin tests (25), these have proved inaccurate. Although the postoperative histopathological examination has remained the standard for the evaluation of the nature of the lesion (39-41), the use of other imaging systems, such as cone beam computed tomography (CBCT) with high specificity and excellent accuracy, can enhance the chance of a more accurate preoperative diagnosis (26).
It is possible to differentiate a cyst from a granuloma by density, using a CT scan (40). A cyst screening method based on specific CBCT radiologic criteria (Table 1) (42) was also proposed as a preoperative screening tool with 90.8% specificity and favorable sensitivity (58%) (26). A gray level correction technique was also applied to assess treatment outcomes (43).
TABLE 1.
Diagnostic criteria for periapical cysts based on radiologic features (42). Each feature can be seen alone or in combination with others
| Location | Apex of the involved tooth |
| Periphery | Well-defined corticated border |
| Shape | Curved or circular |
| Internal structure | Radiolucent |
| Effect on surrounding structures | Displacement and resorption of the roots with a curved outline |
| Effect on surrounding bone | Cortical plate perforation |
Granulomas are usually composed of solid soft tissue, while cysts have a semi-solid, liquefied cystic area. Therefore, the least dense area of the radiographic lesion should be measured to diagnose these lesions correctly. Measuring the gray value makes it possible to differentiate soft tissues and fluid or empty areas (43).
A periapical abscess has features similar to periapical granulomas and periapical cysts with a varying degree of peripheral cortication, which makes it difficult to distinguish them from one another (43). Cortical erosion or perforation seen in CBCT examinations and the presence of edema might provide additional information in distinguishing abscesses; however, the early stages of periapical abscesses often do not present with these characteristics (44). Although histopathologic evaluation is the definitive method of differentiating between the periapical radiolucencies of endodontic origin, it is rarely carried out as these diseases often resolve with non-surgical endodontic treatment; therefore, the differentiation between a granuloma and a cyst is not always required. Treatment of all these three lesions is either root canal (re)treatment, periradicular surgery, or extraction, or a combination of them (44).
Several studies have focused on the etiology, pathogenesis, and radiographic characteristics of scar tissue (45, 46). Fibrous scar tissue has radiographic features similar to periapical granulomas and periapical cysts. Healing by fibrous scar tissue rather than bone might occur, especially after endodontic surgery. When a considerable portion of the cortical plates and bone periosteum are destroyed, the fibrous tissue develops (47, 48) with a round, punched-out appearance (49) and no form of irritation in the area (49). It has a typical radiographic appearance and needs no further treatment (49). Although scar tissue can only be confirmed histopathologically, some radiographic features might help in identifying them. Decreasing rarefaction with an irregular outline extending angularly into the periodontal space located asymmetrically with the root apex with or without visible internal bone structures is suggestive of scar tissue. Lamina dura might be present around the apex and separate the rarefaction from the tooth (50).
The CBCT might be an accurate diagnostic tool for differentiating a solid from a fluid-filled lesion or cavity. This technique is the most accurate in the central area or at the tip of the root (51). The whole radiolucency should be scanned appropriately for the most lucent area to improve CBCT’s accuracy, i.e., the least dense area. If this area exhibits a negative grayscale value on the CBCT image, it indicates a semi-solid or fluid-filled area, either the lumen of a bay or a true cyst (cavitated lesions) (51). Although the grayscale values could be easily affected by the field of view and spatial resolution selections, hard beaming, scattering, and the number of projections (51), the CBCT technique shows lower grayscale values, indicating a cavity containing fluid and it does not reveal the epithelial lining. If it yields a positive grayscale value, the lesion is an epithelialized granuloma or a granuloma, which might help the clinician predict treatment outcome (51). The CBCT technique distinguishes between a solid lesion in the soft tissue from one with soft tissue and an area with less density, i.e., a fluid-containing cavity and a semi-solid substance in the lumen (39). CBCT is moderately accurate in making a distinction between periapical cysts and granulomas, especially in apical lesions with a minimum average diameter of 5 mm (52, 53). Periapical radiographs are only 26–48% accurate in diagnosing a periapical lesion (54). CBCT is more accurate than PA radiographs in revealing periapical pathosis (55, 56). CBCT is more accurate than PA radiographs in identifying apical periodontitis, especially when the lesions are >1.4 mm (55, 56). CBCT is a non-invasive method for differentiating periapical cysts and granulomas (39), and its ability to diagnose a cyst preoperatively is convincing (52). For detailed diagnostic tasks, such as endodontics or visualization of small bony structures, high-resolution scans are necessary (57).
One of the drawbacks of CBCT is that it might yield false-positive results of PDL widening in somewhat healthy teeth, indicating PA lesions (58); in addition, its use is controversial in some situations due to concerns related to its higher radiation dose to patients, long scanning time, and higher cost compared to conventional radiographic techniques (59). Materials with a high atomic number can affect the CBCT image quality. Inferior image quality and contrast can lead to a limited interpretation of the 3D volumes (60). One study showed that CBCT might not be a reliable diagnostic tool due to the wide range of possibilities in the diagnosis of apical pathosis, such as granulomas, granuloma-like lesions, cysts, cyst-like lesions, and other lesions (61).
According to the American Association of Endodontists (AAE), ‘CBCT should only be used when the question for which imaging is required cannot be answered adequately by lower-dose conventional radiography or alternate imaging modalities’ (62).
Magnetic resonance imaging (MRI) is an imaging modality with no radiation, which provides superb soft-tissue contrast. The use of high field strength (63), unique coil systems (64, 65), and optimal sequence techniques (66) have resulted in high-quality images, which has led to significant interest in dental MRI. Not only has the MRI been used for characterizing periapical lesions (67), but also it might be a valid and reliable non-invasive tool in differentiating apical periodontitis, periapical cysts/granulomas (68), and condensing osteitis (69). X-ray-based methods have shortcomings and limited performance in measuring the accurate lesion border. At the same time, MRI as a non-invasive diagnostic tool in apical periodontitis (68), is more accurate in this regard and gives a better estimation on the proximity of the lesion to nearby structures (70). MRI is superior to CT techniques in diagnosing soft tissue-associated pathosis in the head and neck area (71, 72), and it can be used for assessing the nature of periapical lesions (73). However, this method has some limitations. It is required to scan a longer time to have sufficient resolution (74). Visualization of enamel and dentin is challenging as they have no MRI signals. Imaging artifacts caused by metal restorations, materials with high atomic numbers, and patient movements affect the image’s clarity (75). Artifacts related to the device might occur (75).
Echography (ultrasonography), as a real-time ultrasound imaging technique, has many applications in medicine (76). It relies on the reflection of ultrasound waves. The echographic examination can be used to evaluate endodontic periradicular lesions (77). Different tissues in the body with different acoustic properties reflect the ultrasound waves differently. Bone exhibits total reflection; therefore, such a technique can only be implemented through bony windows or in areas where the architecture of bone has changed (76). Areas with different tissue types exhibit ‘dishomogeneous echo.’ It has been suggested that echography is a reliable technique that can be used as an adjunct to conventional radiography to diagnose and follow periapical lesions (77). Furthermore, it can furnish some information on the lesion size and its contents and vascularization, which can be helpful for the differential diagnosis of endodontic and other lesions affecting the maxillary bone (77). The percentage accuracy of diagnosing periradicular lesions using ultrasonography was reported to be 95.2%, which was higher than conventional radiography (47.6%) and digital radiography (55.6%) (78). Ultrasonography is a valuable tool for evaluating the nature of intra-osseous lesions in the jaws, particularly for the differential diagnosis between periradicular cysts (echogenic in grayscale) and granulomas, which puts it in a prime position to be considered as an additional imaging technique in routine dentistry and maxillofacial surgery (79). Although ultrasonography examination can help detect apical periodontitis, cysts, apical granulomas, vascular lesions, and malignancies, it is inconclusive in some cases, such as lesions containing mineralized tissue, such as an ossifying fibroma or dentigerous cyst, which could also act as a barrier to ultrasound waves passing through the tissue (79). The clinical value of ultrasonography in detecting lesions in the bone has been reported (79). It is noteworthy that ultrasonography is unable to discriminate between true and pocket cysts (79). The cortical plate requires to be eroded by the lesion to diagnose the intra-osseous lesions by ultrasound (79). (Table 2)
TABLE 2.
Techniques available for diagnosing periradicular lesions
| Diagnostic tool | Pros | Cons | Accuracy |
|---|---|---|---|
| Periapical radiograph | Non invasive Low radiation Available |
Non-differentiating Not very accurate |
47.6-55.6% |
| Histopathology | Standard procedure for differentiating radicular cysts Accurate |
Needs surgery | N/A |
| CBCT | Fast Accurate Non invasive |
High radiation False positive results Long scanning time |
>60.9% |
| MRI | Accurate, valid and reliable Non invasive Radiation free Superb soft tissue contrast |
Long scan time Imaging artifacts Patient cooperation |
More accurate than CBCT |
| Echography | Non invasive Real time image Easy Reproducible Information on the lesion size and its contents and vascularization |
Needs cortical bone perforation Inconclusive in some cases, such as lesions containing mineralised tissue |
95.2% |
Root canal treatment
The goal of endodontic treatment is to clean, shape, and seal the root canal system in three dimensions to eliminate or prevent (re)infection (80). Endodontic failure means the recurrence of clinical symptoms along with the presence of a periapical radiolucency (81). The primary root canal treatment yields predictable results and is a highly successful procedure (30, 82, 83) with a survival rate of 95% after a 4-year follow-up (84). Some findings indicate a favorable outcome; lack of pain, sinus tract, swelling, and other symptoms, with no loss of function and the presence of normal periapical tissues, which should be confirmed radiographically (85). However, failure is possible after treatment due to different microbial and non-microbial factors, such as extraradicular infections, intraradicular infections, periodontal factors, and prosthetic factors (32, 86, 87). Some systematic reviews have reported 14–16% failure rates for root canal treatment (82, 88). Researchers have attributed the lack of healing to the persistence of intraradicular infection(s) in uninstrumented root canals and dentinal tubules plus the irregularities of the root canal system (11, 30, 89, 90). Root canal treatment might fail when the treatment does not conform to acceptable standards (8, 91). Many pathosis do not respond appropriately to root canal treatment due to procedural errors, such as ledges, zipping, and perforation, because they interfere with the removal of intracanal infection(s) from uninstrumented areas (92). These areas might harbor bacteria and necrotic tissues despite the apparently radiographic adequacy of the root canal obturation (93, 94). A radiograph of a well-treated root canal does not necessarily mean thorough cleanliness or obturation of the root canal system (95). The bacteria residing in isthmuses, ramifications, deltas, irregularities, and dentinal tubules might not be affected by disinfection and cleaning during endodontic procedures (96). Furthermore, these bacteria might continue to receive their supply of nutrients in the ramifications and deltas after the treatment. The bacteria residing in dentinal tubules and isthmuses might have significantly reduced access to substrates and would be entombed due to the presence of root canal filling materials that hamper the access of bacteria to periradicular tissues (8, 9, 96). Unfortunately, some bacteria survive for long periods because they receive nutrients from residual tissues and necrotic cells. In cases where root canal obturation does not result in an adequate seal, penetration of tissue fluids provides substrates for the bacteria. When a mix of microorganisms with pathogenic capabilities reach a threshold count and gain access to the periradicular lesion, they induce inflammation in the periradicular tissues (8, 9). Failure of non-surgical endodontic treatment due to residual microorganisms only occurs when they are pathogenic, reach certain counts, and have access to the periradicular tissues to cause or maintain periradicular disease (8, 9).
Some other important factors can lead to root canal treatment failure, such as lack of coronal seal. An impervious coronal seal is essential for successful outcomes; using rubber dam while performing root canal treatment and restoration procedure, placing orifice barriers, and ensuring no leakage under previous and new restorations are highly recommended for achieving higher success rates (88, 97). Prosthetic reasons are the most common ones that lead to the extraction of endodontically treated teeth (98); other causes include non-restorable carious destruction and some endodontic related issues such as vertical root fracture (87, 99). Endodontically treated teeth in patients with periodontal disease are more than five times more susceptible to developing apical periodontitis, which may be due to higher permeability of the dentinal tubules to periodontal pathogens (100). Even occlusal contacts during working-side and protrusive movements can enhance the chance of developing new periapical lesions or perpetuating the older ones, which might be due to apical tissue inflammation, higher likelihood of marginal leakage and loss of the retentive stability of the cemented coronal restoration (101).
Antimicrobial endodontic therapy
Antimicrobial therapy in endodontics has been established on the opinion that periradicular conditions are infectious entities. Such therapies should be able to eliminate pathogenic microorganisms; in this context, highly effective antimicrobial strategies should be applied to achieve optimal outcomes (102, 103).
Several antimicrobial agents are used in endodontics, some of which have some shortcomings. Sodium hypochlorite is one of the most widely used root canal irrigation solutions with strong dissolving effects on necrotic and vital tissues and with a wide spectrum and nonspecific killing efficacy on microbes, spores, and viruses (104, 105). Chlorhexidine can be used as a root canal irrigant and intracanal medicament. However, it is unable to dissolve necrotic tissue remnants (106), and it is less effective on gram-negative than on gram-positive bacteria (107). Calcium hydroxide (CH) is the most commonly used inter-appointment dressing used for disinfecting the root canal (108), and it is effective against gram-negative species. It can perform its antibacterial effect by inactivating the membrane transport mechanisms (109).
The concept ‘lesion sterilization and tissue repair (LSTR)’ therapy (103) uses a mixture of antibacterial agents in the root canal space after instrumentation for disinfection and treatment of dentinal, pulpal, and periradicular conditions. Metronidazole has been administered as the first choice due to its broad bactericidal spectrum against anaerobes (110) commonly found in oral sites. Some bacteria in oral lesions have proved resistant to metronidazole, necessitating mixing ciprofloxacin and minocycline with metronidazole (111) to improve the efficacy of combating oral bacteria (102, 112). Some studies have confirmed the efficacy of this combination in the treatment of periradicular lesions and infected tooth structures (102, 112). This method has been clinically effective in the disinfection of immature teeth with apical periodontitis (112). It is incumbent on dentists to exercise caution in administering local or systemic antibacterial agents. Although the doses of these drugs are small when administered locally, great care is necessary for patients who are sensitive to these chemical agents and antibiotics. Furthermore, the use of antibiotics should be limited to particular situations as it can maintain and cause the spread of antibiotic resistance genes within root canal biofilms (113).
Overinstrumentation, Apexum, and GentleWave
Drainage of cystic fluid can help in the conservative management of large periapical lesions, and it is supported by histologic findings (114). The overinstrumentation technique is claimed to have clinical success in providing drainage through the canal (22, 115). This technique is based on the assumption that the periapical lesion can be a cyst. It has been suggested that overinstrumentation to 1 mm beyond the apical foramen develops an inflammatory reaction that can destroy the epithelial lining of the cyst and convert it to a granuloma (116). Also, overinstrumentation might allow and establish drainage of the cystic fluid through the canal, which might induce degeneration of the epithelial cells by strangulation (117). Further clinical studies are required to understand the validity of this procedure.
Apexum is a technique to remove or debulk periapical tissues, by using a device to remove the chronically inflamed periapical tissues via a root canal access. First animal studies testing this technique have yielded promising results in terms of its safety and efficacy (118). It does not confine the non-surgical endodontic treatment only to the elimination of the etiologic agent (microorganisms) and then to rely on the host to heal on itself (119). The removal of chronically inflamed periapical tissues improves the healing process of the lesion (118, 120). A study compared the healing process of the apexum technique with that of the conventional root canal treatment. After three months, 87% of the periapical lesions completely healed or were in the advanced stages of healing; however, with the use of the conventional treatment modality, only 22% of the cases exhibited such a characteristic. After six months, 95% of the lesions in the apexum group showed advanced or complete healing, while the conventional root canal treatment gave rise to such progress in about 39% of the cases. Therefore, the apexum protocol results in faster healing and disappearance of the PA lesion compared to the conventional root canal treatment (120). This procedure does not remove the cyst lining, if present, which can be a cause of late failure (121); therefore, late failures might occur compared to endodontic surgery. A lack of long-term follow-ups and randomized clinical trials necessitate the need for these studies to understand the effect of this procedure on treatment outcomes.
It has been reported that the use of apexum resulted in no swelling, and only a few cases experienced postoperative discomfort or mild pain (9%) (120). No patient undergoing this protocol reported any adverse outcomes; however, 31% of patients undergoing conventional root canal treatment reported some discomfort or pain. It is of utmost importance to note that during or after a conventional, open-flap, apical surgical procedure, many patients experience pain, swelling, or both, necessitating the use of analgesics after surgery. Moreover, 23% of the patients having undergone apical surgeries reported working day losses due to these symptoms (122). This method has a positive effect on the patient’s well-being, with very mild symptoms compared to conventional open-flap apical surgeries and conventional root canal treatment (120).
The apexum technique is very different from the simple overinstrumentation in conventional root canal treatment. Contrary to apexum (120), overinstrumentation results in tissue traumas and might introduce bacteria or their products into a tissue. Immunoglobulins might be directed against these antigens (123, 124), resulting in acute inflammatory responses, leading to edema and flare-up (119). The removal or s debulking of chronically inflamed periapical tissues gives rise to the elimination of mechanisms that cause flare-ups (120). However, this technique has some shortcomings; there is a risk of separation of the apexum beyond the apical foramen. Furthermore, there is a risk of over-enlargement of apical foramen, which increases the chance of extrusion of obturation materials, interappointment medicaments, and irrigation solutions, and injuring or damaging adjacent vital tissues, such as the inferior alveolar nerve or perforating the maxillary sinus.
GentleWave (Sonendo, Laguna Hills, CA, USA) is used for irrigation of the canal and generates different physiochemical mechanisms, including a broad spectrum of sound waves to clean the root canal space (125). It has superior tissue dissolution ability through the generated mechanisms (126). It has a greater ability to remove residual debris than conventional methods (127), which can enhance the speed and rate of healing of apical periodontitis (128). However, it should be noted that this technology is costly and is not available worldwide.
Non-surgical retreatment
Dentists should have thorough knowledge about the biological factors that lead to the failure of endodontic treatments. The persistence of intraradicular infection(s) is the prominent cause of such failures; therefore, retreatment of failed cases using standard protocols is of paramount importance before considering surgery. The success rate of retreatment might approach almost two thirds of the cases (10, 129). However, teeth having undergone proper root canal treatment that exhibits persistent apical periodontitis should be approached differently from the initial endodontic therapy in teeth with apical periodontitis. Some principal factors that might give rise to the persistence of apical radiolucencies in endodontically treated teeth are persistent intraradicular infection(s) remaining in the complex apical part of the root canal (93), extraradicular infection(s) (130, 131), foreign body reactions due to extruded obturating material or exogenous materials (132, 133), endogenous cholesterol crystals (89), true cysts (32, 134) and fibrous scar tissues (134). Of all these factors, microorganisms remaining in the root canal should be addressed by conventional orthograde retreatment; however, extraradicular lesions due to the bacteria remaining in the complex root canal space, true cysts, and foreign bodies are managed by periapical surgical procedures. Cholesterol crystals can be numerous in chronic periradicular lesions and are derived from the plasma lipids, disintegrating host cells, including erythrocytes, lymphocytes, plasma cells, and macrophages, in the periapical connective tissue exhibiting inflammation (135). They can be the cause of non-resolving chronic inflammation (28, 136). Unsuccessful phagocytosis of cholesterol crystals by multinucleated giant cells results in the accumulation of these cells, leading to the persistence of periradicular lesion (32, 136).
Foreign body reaction
Some cases might fail as a result of non-microbial intrinsic or extrinsic factors. In these cases, foreign body reaction in the periradicular tissues results in failure instead of microorganisms (32). A study reported a lesion that was resistant to therapy; the lesion was removed surgically, and a diagnosis of periradicular cyst was confirmed by light and electron microscopic evaluations. No microorganism was detected; therefore, the failure was attributed to a foreign body reaction against cholesterol crystals detected in the connective tissue around the cyst epithelial lining (32). Materials that might provoke a foreign body reaction in the periapical tissues are usually exogenous and include talc-contaminated gutta-percha (132), the cellulose in the paper points, cotton wool, and food items derived from vegetables, resulting in persistent periradicular lesions when they enter the periradicular tissues (28, 137). A cause-and-effect relationship between the presence of endodontic sealer material and periapical lesions has been suggested (138). Two studies reported a decrease in the success rate of root canal treatment with overfilling (91, 139), while other studies failed to find any correlation between the apical extent of root canal obturation and treatment failure (90). Also, based on previous reports, the toxicity of root canal filling materials plays a vital role in this regard (140). However, most of the materials, apart from the paraformaldehyde containing materials used for root canal obturation, are either biocompatible or are cytotoxic only before setting (141). Therefore, currently available root canal filling materials are almost unable to sustain periradicular inflammation in the absence of endodontic infections. This is further supported by the high success rate of treatment in teeth without periradicular lesions, even in the presence of overfilling (11, 90). However, the size and surface characteristics of overfilling gutta percha can alter the type of tissue reaction to the material, with fine particles of overfilling inducing impaired healing of PA lesions (142). The accumulation of macrophages around gutta percha might be an essential factor in the impairment of healing of periapical lesions when teeth are root-filled with excess material (142). These are the only non-microbial factors causing periapical lesions in endodontically treated teeth. To date, surgery has been the only technique to eliminate these agents; therefore, periapical surgery should be considered, particularly when the conventional orthograde retreatment proves ineffective.
Periapical cyst
Periradicular cysts originate from the epithelial cell rests of Malassez in the alveolus. These cells proliferate due to periapical inflammation induced by the infection of the root canal system. Periradicular cysts are more frequently encountered in the anterior maxilla, which might be explained by traumas and the presence of epithelial cells (143). A definitive diagnosis of periradicular cyst is reached only through histopathologic evaluation by serial cross-sectioning of the lesion specimen (29); in fact, conventional radiographic techniques cannot be applied for the definitive diagnosis of the cystic and non-cystic periapical lesions (20, 144). There is no strong correlation between periapical radiograph findings, such as the presence of lamina dura, and histological diagnosis of a cyst which needs serial sections (35). Two types of periradicular cysts have been defined: true cysts, with cavities that are entirely enclosed by an epithelial lining, and bay cysts or pocket cysts, with epithelium-lined cavities which communicate with the root canals (145). A study on 256 periapical lesions reported that 15% were periapical cysts, 9% of which were true cysts, and 6% were pocket cysts (29).
Contrary to true cysts that are self sufficient due to their independence from the irritants in the root canal system (28), periapical pocket cysts and granulomas might heal after non-surgical root canal treatment. In contrast, it is believed that a true periapical cyst is less likely to heal after non-surgical root canal treatment and might require periradicular surgery (146) (Fig. 1). For more than three years, follow-up studies have revealed that approximately 13% of postoperative apical lesions were true cysts (32, 134). The prevalence of cysts originating from apical periodontitis lesions has been reported to be <20% (29, 145). Periradicular cysts’ growth rate is usually slow, centrifugal, and infiltrative (147). They do not exhibit very large sizes, and patients feel no pain, except when there is an episode of acute inflammatory exacerbation. The lesions are usually discovered during routine radiographic examinations. In the case of exacerbation, the cysts enlarge, with some symptoms, including swelling, mild sensitivity, tooth mobility, and displacement. Pulp sensitivity test results are negative (146).
Morphological characteristics of the cyst cavity tend to render the host defense ineffective. The persistent egress of microorganisms and their by-products from inside the cystic lumen might be responsible for the persistence of periradicular inflammation in properly treated root canal systems (148, 149).
Biofilm
The biofilm lifestyle provides a suitable shelter for mechanisms to evade the host defense system. A biofilm is a microbial aggregate adhering to an organic or inorganic substrate and is surrounded by extracellular microbial products to form an intermicrobial matrix (150, 151). Microorganisms in the biofilm are more resistant to antimicrobial agents and host defenses than planktonic cells (150, 152). Examining teeth with failed root canal treatment revealed bacterial biofilms next to the apical foramen, with bacterial colonies within the periradicular granulomas (153). However, a low incidence (4%) of periradicular biofilms has been reported in untreated teeth with periradicular lesions (154). This might explain that the periradicular biofilm might be responsible for only a low percentage of failed cases. As periradicular biofilm and microorganisms are hardly accessible through the root canal space, the surgical approach for eliminating these invaders seems inevitable.
Extraradicular infection
Several cultural and microscopic examinations confirmed the occurrence of extraradicular infections in both treated and untreated root canals (153, 155). From a histologic point of view, there exist two types of extraradicular infection:
1. Acute periapical abscess: This is a form of purulent inflammation in the periapex in response to the egress of pathogenic bacteria from the root canal. It depends on the intraradicular infection; therefore, the extraradicular infection should subside following the (re)treatment of intraradicular infection as well as the body response (156).
2. External root surface infection: Microorganisms such as Actinomyces, Propionibacterium propionicum, and Bacteroides species (130, 131) are lodged in the periapical tissues by adhering to the apical root surface as biofilms (157) or within the inflammatory lesion body in the form of cohesive colonies (13). Since the root canal treatment strategies are within the root canal space, the microorganisms residing in the periradicular space are not amenable to disinfection procedures during non-surgical endodontic treatment (158, 159). They can overcome the defensive action of cells, molecules, and the complement system, and avoid elimination by phagocytes, through immunosuppression, altering their antigenic coats and induction of proteolysis of antibody molecules (153, 160). Seepage of periapical tissue fluids rich in glycoproteins into the root canals is a source of substrates for residual microorganisms so that they can proliferate and reach sufficient counts to induce periradicular lesions (8, 161). Overinstrumentation should be considered as another cause of failure, which displaces contaminated dentinal debris into the periradicular spaces, resulting in extraradicular inflammation and infections (133, 162). The debris can provide a shelter for microorganisms to be physically protected against host defenses, survive in lesion area, and perpetuate periradicular inflammation. This would eventually affect the healing of the lesion (133).
Periapical surgery
Periapical surgery is an endodontic therapy through a surgical flap which focuses on removing a portion of a root with anatomical complexities and undebrided canal when a complete seal cannot be achieved through orthograde non-surgical approach (163). It is undertaken to confine microorganisms in the root canal(s) by sealing the root canal apically, eliminate the most apical and more complicated part of the root canal, and remove the periapical lesion for further histological evaluation. The aim is to optimize the conditions so that the periapical tissue can heal, and the attachment apparatus can regenerate (163, 164).
The rate of successful healing of periapical surgery has been reported to range from 60% to 91% (165). Some factors might affect the outcomes of periapical surgeries. Retrofilling is a significant prognostic factor (164). The presence or absence of a root-end filling material is an essential factor in the long-term prognosis of surgical intervention. It is possible to increase the success rate by 10–13%, using retrograde obturation (166, 167).
The size of the apical lesion is another factor; there is a significantly higher healing rate in teeth with smaller (<5 mm) preoperative lesions (168, 169). The quality of the previously existing root fillings has its own impact. Teeth that have preoperative long/short root fillings exhibit higher healing rates compared to teeth with adequate root canal obturation (168, 170). This might be due to the removal of the infection (the unfilled portion of the root canal space) and irritation (extruded root fillings and infected debris) causes (132, 170). Tooth location can play its role as well. The maxillary lateral incisors have the highest healing rate by scar tissue formation (169, 171). The maxillary premolars appear to exhibit poorer outcomes compared to the anterior teeth (172). The posterior teeth have more favorable outcomes compared to the anterior teeth, with the mandibular incisors exhibiting the most unfavorable outcomes (173). The amount of alveolar bone loss can affect the outcome of surgery, too. Considerable loss of the bony plate or marginal bone has a detrimental effect on the outcomes of periapical surgeries (171, 174). Temporary restorations (167), posts (170), and crowns (175) exert deleterious effects on the outcomes of periradicular surgery.
It has been shown that the teeth undergoing conventional retreatment before periapical surgical intervention exhibit a 24% increase in success rate compared to the situation in which only periapical surgery is carried out (169); if conventional retreatment is undertaken just before the surgical procedure, the success rate can increase to as high as 90% (171, 176). According to some reports, surgical retreatments have a higher failure rate (166, 177) compared to orthograde retreatment. Surgical retreatment has limited indications, such as when the obstruction of the canal cannot be removed, or the risk of damage to the crown or restoration is tremendous and not feasible (85). Apical periodontitis lesions, which were treated surgically, healed over 12 months, with healing progress and speed comparable to those treated with non-surgical retreatment modality (178), plus there was no significant difference in healing rates in the long term. Periapical lesions heal rapidly after apical surgeries; this is an indication that the surgical removal of chronically inflamed periapical tissues might result in the formation of a fresh blood clot, which then organizes to form granulation tissue, paving the way for rapid healing (179). However, apical surgery may imply a higher risk of late failures (47, 178). Apart from periapical surgery advantages such as faster healing process and speed, it has its drawbacks; surgery affects the patient’s well-being, with swelling, pain, and discomfort being expected (122). Moreover, many anatomic locations and adjacent structures can affect the feasibility of periapical surgery due to inaccessibility or the risk of damaging adjacent anatomic structures (180). However, significant improvements have been made in endodontic surgical procedures in recent years thanks to advances in techniques, equipment, and materials. The dental operating microscope improves visibility, facilitating a better understanding of the root canal anatomy, and enabling the surgeon to perform better and more predictable apical resections. Currently, ultrasonic retrotips allow a more conservative and precise root-end preparation. These advances enable dental surgeons to attain more predictable surgical outcomes with higher success rates (180, 181).
A summary of indications for surgical treatment is presented here:
Orthograde retreatment is impossible for various reasons, including fractured instruments, ledges, blockages, and the inability to remove the root canal obturation material.
Orthograde retreatment has failed: Bacteria are residing in areas, such as isthmuses, ramifications, deltas, irregularities, and dentinal tubules, and might not be affected by endodontic disinfection procedures and might have survived the previous root canal (re)treatment (90, 182). The history of previous treatments can help the clinician make better decisions toward further treatment options.
Non-surgical retreatment is questionable or impractical, such as in cases with an extensive coronal restoration that should be sacrificed.
The patient might not accept the routine retreatment because of financial or time constraints.
A biopsy is necessary. For differential diagnosis of pathological entities, a biopsy from the periapical area may be needed. Some (non-)odontogenic lesions might mimic the periapical radiolucencies of endodontic origin.
The patients should also participate in the decision-making process. The clinician should help the patients make a sound decision by providing the relevant information (183). The patients tend to opt for treatments that the clinicians recommend (184). Effective communication between the patient and the clinician before making a decision helps avoid misunderstandings, disappointments, and litigation. However, since the recommendations are usually subjective, there is disagreement among dental practitioners over selecting the best treatment modality (185, 186). This is very important because the recommended and selected treatment modality might be lengthy, difficult, and costly. Root canal treatment, as well as retreatment, seems an appropriate and cost-effective way of preserving teeth; however, when it comes to apical surgery after failed root canal retreatment implant might be a better option (187). For example, surgery is advocated when the patient refuses complex retreatment procedures. However, the clinician should inform the patient of the possible unfavorable long-term prognosis/outcome of a surgical procedure alone without retreatment. When there is no motivation for preserving a tooth, extraction followed by the placement of an implant or preserving the gap for future implant placement might be the treatment of choice; other valid alternatives are fixed and removable restorations as well as maintaining the gap which should be discussed with the patient.
There is considerable debate over the treatment of large periapical cysts (188). The therapeutic options for these lesions cover a wide range from conventional root canal treatment plus the use of calcium hydroxide for a long time to different surgical modalities. According to some endodontists, true cysts can be managed successfully only through surgery (32), while others believe that further treatment measures should be considered (22). Endodontic treatment is not successful in all cases. However, some of the radiolucencies might be healing lesions.
A surgical approach for treating periapical lesions might be a wise approach for the treatment of large periapical cysts where non-surgical treatment is deemed ineffective or burdensome. A large periapical radiolucency, as a result of pulp necrosis due to a persistent infection, might be believed to be refractory to conventional root canal treatment and considered a cyst and requires endodontic surgery (144). However, marsupialization or tube decompression might be proper and alternative treatment modalities for large cysts (33).
It should be reiterated that although endodontic periapical surgery offers favorable initial success, there is a chance of late failure, which might be in part due to the type of retrograde filling material, the method of retro-preparation, quality of previous orthograde treatment, tooth type, position, and location, and incomplete removal of the cyst lining (121, 189).
Surgical retrograde retreatment is another option for treating teeth with postoperative apical periodontitis (190). It should be noted when orthograde retreatment fails to provide predictable outcomes or cannot be performed. It has indications and considerations similar to periapical surgery. The procedure can be performed with ultrasonic tips or hand files to clean and shape the remaining untreated part of the canal (191, 192). Promising results have been reported by using this technique (192, 193).
Marsupialization, decompression, and enucleation
The surgical treatment modalities for periradicular cysts include the enucleation of small lesions, marsupialization to decompress large cysts, and a combination of these two modalities. It is the clinician who decides to carry out surgery using a flap technique to enucleate the lesion or decompress it (194, 195). Decompression aims to relieve the pressure within a cyst, which helps it to grow. It is performed by making a small opening in the cyst and leaving it open with a drain (196, 197). Marsupialization aims at converting the cyst into a pouch, which makes the lesion depressurized (198). Marsupialization and decompression decrease the size of the lesion to facilitate its removal, with lower risks of damaging the teeth and adjacent anatomic structures (199). Enucleation is advocated because marsupialization is associated with the risk of residual cystic cells with malignant potential (200, 201). On the other hand, marsupialization is associated with a lower risk of damage to the nasal cavity floor or the maxillary sinuses and the need to subject the patient to general anesthesia. Although the marsupialization and decompression techniques aim to decrease the lesion size without periapical curettage (202, 203), they rely on patient compliance, are relatively lengthy, and do not conform to the principles of endodontic treatment, especially concerning the prevention of bacterial contamination of the oral cavity. There is no data on the percentage of periradicular cysts expected to heal using marsupialization and decompression techniques alone; however, this option should be considered when large cystic lesions are encountered (199). Furthermore, the decompression technique has been suggested in rarefaction areas adjacent to vital anatomic structures (199).
Decompression results in the drainage of periapical lesions so that they can be enucleated. Different techniques and instruments are used to drain and decompress large periapical lesions, ranging from placing a stainless steel tube into the root canal exhibiting persistent apical exudation (202, 204), which is non-surgical decompression, to placing polyvinyl or polyethylene tubes through the alveolar mucosa covering the apical lesion, which is surgical decompression (194). Nonetheless, various problems are associated with these techniques. Patients have to accept the responsibility for keeping the tubes open. Occasionally the tubes are displaced, and the patients have difficulty performing oral hygiene procedures in the area. In addition, these surgical techniques give rise to pain, swelling, and discomfort (202).
Aspiration decompression was proposed as another method to deal with periapical lesions. In cases of uninfected apical cysts, irrigation and aspiration might help the healing of the lesions. This conservative method has several advantages, such as reduced treatment time with a lower chance of iatrogenic problems and might eliminate the need for apical surgery (33).
An active decompression technique with a vacuum system has been introduced, which facilitates the drainage of the apical inflammatory fluids through the root canal, without impinging on the apical constriction (203). The vacuum system provides a negative pressure felt by the patient, which might alter the structure of the lesion. This vacuum system applies negative pressure to large periapical lesions, rapidly removing the periapical exudate through the root canal in teeth exhibiting immature apices, and is of advantage in the presence of copious suppuration (203). Active non-surgical decompression is superior to other techniques because:
Patients feel less discomfort because no surgical flaps are necessary.
There is no communication between the root canal(s) and the oral cavity, which helps control microorganisms.
There is no need for patient cooperation, contrary to surgical decompression or marsupialization.
It is relatively time-saving.
It is a minimally invasive technique because it is carried out through the root canal access without impinging on the anatomic structures, bone, or soft tissues. Moreover, the technique gives rise to proper healing (203). (Table 3)
TABLE 3.
Positive and negative aspects of different treatment modalities
| reatment modality | Pros | Cons | Success rate |
|---|---|---|---|
| Root canal treatment (RCT) | High success rate Effective on IR infections | Not effective against ER infections | 85-94% |
| Antimicrobial endodontic therapy | Effective on IR infections | Chance of hypersensitivity | These are used in combination with other techniques |
| Overinstrumentation | Providing drainage through the canal | Risk of transporting the microorganisms beyond the apical foramen | |
| Apexum | Effective against granulomas and cysts | Needs access through root canal space | |
| GentleWave | Superior tissue dissolution ability Greater ability to remove residual debris | Costly Not available worldwide | |
| Nonsurgical retreatment | Effective against IR infection | Lower success rate compared with RCT Costly in case of any needs for sacrificing previous restorations | 74-82% |
| Periapical Surgery | Effective against ER infection | Risk of damage to surrounding tissues Patient discomfort | 60-91% |
| Marsupialization, decompression, and enucleation | Management of large cyst | Time consuming Need patient cooperation | Not available |
It should be noted that many periradicular lesions are not of endodontic origin; therefore, an appropriate diagnosis is required before commencing any treatments.
CONCLUSION
Although histopathology is still the standard for the diagnosis of PA lesions, technologies such as CBCT, MRI, and echography show promising results in differentiating granulomas and cyst, which can affect the treatment strategy. There are several new treatment options available for eliminating periradicular lesions or enhancing the healing process to save teeth with persistent periapical lesions. Albeit several treatment modalities have been proposed for these teeth which have failed endodontically, there is a need for less invasive methods with more predictable outcomes. It is highly recommended that with technological advances, further minimally invasive approaches must be considered for resolving the issue of persistent apical periodontitis and true cysts to reduce the burden for patients.
Acknowledgments:
MAS is a recipient of the New Jersey Health Foundation Innovation And TechAdvance Awards. This publication is dedicated to the memory of Dr. H. Afsar Lajevardi (205), a legendry pediatrician (1953–2015). The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the affiliated organizations.
Footnotes
Conflict of interest: None declared.
Ethics Committee Approval: N/A.
Peer-review: Externally peer-reviewed.
Financial Disclosure: None declared.
Authorship contributions: Concept – M.A.S., K.K.; Design – M.A.S., K.K.; Supervision – M.A.S., K.K.; Funding - M.A.S., K.K.; Materials - M.A.S., K.K., A.T.; Data collection &/or processing – M.A.S., K.K.; Analysis and/or interpretation – K.K., M.A.S., A.T.; Literature search – M.A.S., K.K. A.T.; Writing – M.A.S., K.K.; Critical Review – M.A.S., K.K., A.T.
REFERENCES
- 1.Zaleckiene V, Peciuliene V, Brukiene V, Drukteinis S. Traumatic dental injuries:etiology, prevalence and possible outcomes. Stomatologija. 2014;16(1):7–14. [PubMed] [Google Scholar]
- 2.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 3.Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89(6):475–84. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 4.Segura-Egea JJ, Gould K, Şen BH, Jonasson P, Cotti E, Mazzoni A, et al. Antibiotics in Endodontics:a review. Int Endod J. 2017;50(12):1169–84. doi: 10.1111/iej.12741. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987;13(1):29–39. doi: 10.1016/S0099-2399(87)80089-4. [DOI] [PubMed] [Google Scholar]
- 6.Ricucci D, Siqueira JF., Jr Biofilms and apical periodontitis:study of prevalence and association with clinical and histopathologic findings. J Endod. 2010;36(8):1277–88. doi: 10.1016/j.joen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Cheung GS, Ho MW. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol. 2001;16(6):332–7. doi: 10.1034/j.1399-302x.2001.160603.x. [DOI] [PubMed] [Google Scholar]
- 8.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(1):86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 9.Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31(1):1–7. [PubMed] [Google Scholar]
- 10.de Chevigny C, Dao TT, Basrani BR, Marquis V, Farzaneh M, Abitbol S, et al. Treatment outcome in endodontics:the Toronto study--phases 3 and 4:orthograde retreatment. J Endod. 2008;34(2):131–7. doi: 10.1016/j.joen.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30(5):297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 12.Orstavik D. Time-course and risk analyses of the development and healing of chronic apical periodontitis in man. Int Endod J. 1996;29(3):150–5. doi: 10.1111/j.1365-2591.1996.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 13.Figdor D. Apical periodontitis:a very prevalent problem. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(6):651–2. doi: 10.1067/moe.2002.130322. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Lagares D, Segura-Egea JJ, Rodríguez-Caballero A, Llamas-Carreras JM, Gutiérrez-Pérez JL. Treatment of a large maxillary cyst with marsupialization, decompression, surgical endodontic therapy and enucleation. J Can Dent Assoc. 2011;77:b87. [PubMed] [Google Scholar]
- 15.Friedman S. Prognosis of initial endodontic therapy. Endod Topics. 2002;2(1):59–88. [Google Scholar]
- 16.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment:part 1:periapical health. Int Endod J. 2011;44(7):583–609. doi: 10.1111/j.1365-2591.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- 17.Yee K, Bhagavatula P, Stover S, Eichmiller F, Hashimoto L, MacDonald S, et al. Survival Rates of Teeth with Primary Endodontic Treatment after Core/Post and Crown Placement. J Endod. 2018;44(2):220–5. doi: 10.1016/j.joen.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Yu VS, Messer HH, Yee R, Shen L. Incidence and impact of painful exacerbations in a cohort with post-treatment persistent endodontic lesions. J Endod. 2012;38(1):41–6. doi: 10.1016/j.joen.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Chugal NM, Clive JM, Spångberg LS. Endodontic treatment outcome:effect of the permanent restoration. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(4):576–82. doi: 10.1016/j.tripleo.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Bhaskar SN. Oral surgery-oral pathology conference No. 17, Walter Reed Army Medical Center. Periapical lesions--types, incidence, and clinical features. Oral Surg Oral Med Oral Pathol. 1966;21(5):657–71. doi: 10.1016/0030-4220(66)90044-2. [DOI] [PubMed] [Google Scholar]
- 21.Abbott PV. Classification, diagnosis and clinical manifestations of apical periodontitis. Endod Topics. 2004;8(1):36–54. [Google Scholar]
- 22.Calişkan MK. Prognosis of large cyst-like periapical lesions following nonsurgical root canal treatment:a clinical review. Int Endod J. 2004;37(6):408–16. doi: 10.1111/j.1365-2591.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- 23.Natkin E, Oswald RJ, Carnes LI. The relationship of lesion size to diagnosis, incidence, and treatment of periapical cysts and granulomas. Oral Surg Oral Med Oral Pathol. 1984;57(1):82–94. doi: 10.1016/0030-4220(84)90267-6. [DOI] [PubMed] [Google Scholar]
- 24.Lalonde ER. A new rationale for the management of periapical granulomas and cysts:an evaluation of histopathological and radiographic findings. J Am Dent Assoc. 1970;80(5):1056–9. doi: 10.14219/jada.archive.1970.0236. [DOI] [PubMed] [Google Scholar]
- 25.Morse DR, Patnik JW, Schacterle GR. Electrophoretic differentiation of radicular cysts and granulomas. Oral Surg Oral Med Oral Pathol. 1973;35(2):249–64. doi: 10.1016/0030-4220(73)90292-2. [DOI] [PubMed] [Google Scholar]
- 26.Pitcher B, Alaqla A, Noujeim M, Wealleans JA, Kotsakis G, Chrepa V. Binary Decision Trees for Preoperative Periapical Cyst Screening Using Cone-beam Computed Tomography. J Endod. 2017;43(3):383–8. doi: 10.1016/j.joen.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 27.Browne RM. The origin of cholesterol in odontogenic cysts in man. Arch Oral Biol. 1971;16(1):107–13. doi: 10.1016/0003-9969(71)90141-5. [DOI] [PubMed] [Google Scholar]
- 28.Nair PN. New perspectives on radicular cysts:do they heal? Int Endod J. 1998;31(3):155–60. doi: 10.1046/j.1365-2591.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran Nair PN, Chevigny Pajarola G, Schroeder HE. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(1):93–102. doi: 10.1016/s1079-2104(96)80156-9. [DOI] [PubMed] [Google Scholar]
- 30.Sjogren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16(10):498–504. doi: 10.1016/S0099-2399(07)80180-4. [DOI] [PubMed] [Google Scholar]
- 31.Shear M. Cysts of the jaws:recent advances. J Oral Pathol. 1985;14(1):43–59. doi: 10.1111/j.1600-0714.1985.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 32.Nair PN, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth:a long-term post-treatment follow-up. Int Endod J. 1993;26(4):225–33. doi: 10.1111/j.1365-2591.1993.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoen MM, LaBounty GL, Strittmatter EJ. Conservative treatment of persistent periradicular lesions using aspiration and irrigation. J Endod. 1990;16(4):182–6. doi: 10.1016/S0099-2399(06)81968-0. [DOI] [PubMed] [Google Scholar]
- 34.Becconsall-Ryan K, Tong D, Love RM. Radiolucent inflammatory jaw lesions:a twenty-year analysis. Int Endod J. 2010;43(10):859–65. doi: 10.1111/j.1365-2591.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- 35.Ricucci D, Mannocci F, Ford TR. A study of periapical lesions correlating the presence of a radiopaque lamina with histological findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):389–94. doi: 10.1016/j.tripleo.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham CJ, Penick EC. Use of a roentgenographic contrast medium in the differential diagnosis of periapical lesions. Oral Surg Oral Med Oral Pathol. 1968;26(1):96–102. doi: 10.1016/0030-4220(68)90228-4. [DOI] [PubMed] [Google Scholar]
- 37.Howell FV, De la Rosa VM. Cytologic evaluation of cystic lesions of the jaws:a new diagnostic technique. J South Calif Dent Assoc. 1968;36(4):161–6. [PubMed] [Google Scholar]
- 38.Cotti E, Campisi G, Ambu R, Dettori C. Ultrasound real-time imaging in the differential diagnosis of periapical lesions. Int Endod J. 2003;36(8):556–63. doi: 10.1046/j.1365-2591.2003.00690.x. [DOI] [PubMed] [Google Scholar]
- 39.Simon JH, Enciso R, Malfaz JM, Roges R, Bailey-Perry M, Patel A. Differential diagnosis of large periapical lesions using cone-beam computed tomography measurements and biopsy. J Endod. 2006;32(9):833–7. doi: 10.1016/j.joen.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Trope M, Pettigrew J, Petras J, Barnett F, Tronstad L. Differentiation of radicular cyst and granulomas using computerized tomography. Endod Dent Traumatol. 1989;5(2):69–72. doi: 10.1111/j.1600-9657.1989.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 41.Kruse C, Spin-Neto R, Wenzel A, Kirkevang LL. Cone beam computed tomography and periapical lesions:a systematic review analysing studies on diagnostic efficacy by a hierarchical model. Int Endod J. 2015;48(9):815–28. doi: 10.1111/iej.12388. [DOI] [PubMed] [Google Scholar]
- 42.White SC, Pharoah MJ. Oral radiology-E-Book: Principles and interpretation. 7th ed. Elsevier Health Sciences; 2014. [Google Scholar]
- 43.Camps J, Pommel L, Bukiet F. Evaluation of periapical lesion healing by correction of gray values. J Endod. 2004;30(11):762–6. doi: 10.1097/01.don.0000129964.50505.b2. [DOI] [PubMed] [Google Scholar]
- 44.White SC. Cysts of the jaws. In: White SC, Pharoah MJ, editors. Oral Radiology. 4th ed. St. Louis: Mosby; 2000. [Google Scholar]
- 45.Hjorting-Hansen E, Andreasen JO. Incomplete bone healing of experimental cavities in dog mandibles. Br J Oral Surg. 1971;9(1):33–40. doi: 10.1016/s0007-117x(71)80006-9. [DOI] [PubMed] [Google Scholar]
- 46.Mascrès C, Marchand JF. Experimental apical scars in rats. Oral Surg Oral Med Oral Pathol. 1980;50(2):164–75. doi: 10.1016/0030-4220(80)90206-6. [DOI] [PubMed] [Google Scholar]
- 47.Rud J, Andreasen JO, Jensen JE. A follow-up study of 1,000 cases treated by endodontic surgery. Int J Oral Surg. 1972;1(4):215–28. doi: 10.1016/s0300-9785(72)80014-0. [DOI] [PubMed] [Google Scholar]
- 48.Molven O, Halse A, Grung B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int J Oral Maxillofac Surg. 1987;16(4):432–9. doi: 10.1016/s0901-5027(87)80080-2. [DOI] [PubMed] [Google Scholar]
- 49.Molven O, Halse A, Grung B. Incomplete healing (scar tissue) after periapical surgery--radiographic findings 8 to 12 years after treatment. J Endod. 1996;22(5):264–8. doi: 10.1016/s0099-2399(06)80146-9. [DOI] [PubMed] [Google Scholar]
- 50.Rud J, Andreasen JO, Jensen JE. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1(4):195–214. doi: 10.1016/s0300-9785(72)80013-9. [DOI] [PubMed] [Google Scholar]
- 51.Parsa A, Ibrahim N, Hassan B, Motroni A, van der Stelt P, Wismeijer D. Influence of cone beam CT scanning parameters on grey value measurements at an implant site. Dentomaxillofac Radiol. 2013;42(3):79884780. doi: 10.1259/dmfr/79884780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo J, Simon JH, Sedghizadeh P, Soliman ON, Chapman T, Enciso R. Evaluation of the reliability and accuracy of using cone-beam computed tomography for diagnosing periapical cysts from granulomas. J Endod. 2013;39(12):1485–90. doi: 10.1016/j.joen.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 53.Chanani A, Adhikari HD. Reliability of cone beam computed tomography as a biopsy-independent tool in differential diagnosis of periapical cysts and granulomas:An In vivo Study. J Conserv Dent. 2017;20(5):326–31. doi: 10.4103/JCD.JCD_124_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortensen H, Winther J, Birn H. Periapical granulomas and cysts:An investigation of 1,600 cases. Eur J Oral Sci. 1970;78(1-4):241–50. [PubMed] [Google Scholar]
- 55.Estrela C, Bueno MR, Azevedo BC, Azevedo JR, Pécora JD. A new periapical index based on cone beam computed tomography. J Endod. 2008;34(11):1325–31. doi: 10.1016/j.joen.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Tsai P, Torabinejad M, Rice D, Azevedo B. Accuracy of cone-beam computed tomography and periapical radiography in detecting small periapical lesions. J Endod. 2012;38(7):965–70. doi: 10.1016/j.joen.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Loubele M, Guerrero ME, Jacobs R, Suetens P, van Steenberghe D. A comparison of jaw dimensional and quality assessments of bone characteristics with cone-beam CT, spiral tomography, and multi-slice spiral CT. Int J Oral Maxillofac Implants. 2007;22(3):446–54. [PubMed] [Google Scholar]
- 58.Pope O, Sathorn C, Parashos P. A comparative investigation of cone-beam computed tomography and periapical radiography in the diagnosis of a healthy periapex. J Endod. 2014;40(3):360–5. doi: 10.1016/j.joen.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Ludlow JB, Davies-Ludlow LE, Brooks SL, Howerton WB. Dosimetry of 3 CBCT devices for oral and maxillofacial radiology:CB Mercuray, NewTom 3G and i-CAT. Dentomaxillofac Radiol. 2006;35(4):219–26. doi: 10.1259/dmfr/14340323. [DOI] [PubMed] [Google Scholar]
- 60.Katsumata A, Hirukawa A, Noujeim M, Okumura S, Naitoh M, Fujishita M, et al. Image artifact in dental cone-beam CT. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(5):652–7. doi: 10.1016/j.tripleo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg PA, Frisbie J, Lee J, Lee K, Frommer H, Kottal S, et al. Evaluation of pathologists (histopathology) and radiologists (cone beam computed tomography) differentiating radicular cysts from granulomas. J Endod. 2010;36(3):423–8. doi: 10.1016/j.joen.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 62.American Association of Endodontists;American Academy of Oral and Maxillofacial Radiology. Use of cone-beam computed tomography in endodontics Joint Position Statement of the American Association of Endodontists and the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):234–7. doi: 10.1016/j.tripleo.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 63.Gaudino C, Cosgarea R, Heiland S, Csernus R, Beomonte Zobel B, Pham M, et al. MR-Imaging of teeth and periodontal apparatus:an experimental study comparing high-resolution MRI with MDCT and CBCT. Eur Radiol. 2011;21(12):2575–83. doi: 10.1007/s00330-011-2209-0. [DOI] [PubMed] [Google Scholar]
- 64.Prager M, Heiland S, Gareis D, Hilgenfeld T, Bendszus M, Gaudino C. Dental MRI using a dedicated RF-coil at 3 Tesla. J Craniomaxillofac Surg. 2015;43(10):2175–82. doi: 10.1016/j.jcms.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Ludwig U, Eisenbeiss AK, Scheifele C, Nelson K, Bock M, Hennig J, et al. Dental MRI using wireless intraoral coils. Sci Rep. 2016;6:23301. doi: 10.1038/srep23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Idiyatullin D, Corum C, Moeller S, Prasad HS, Garwood M, Nixdorf DR. Dental magnetic resonance imaging:making the invisible visible. J Endod. 2011;37(6):745–52. doi: 10.1016/j.joen.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geibel MA, Schreiber E, Bracher AK, Hell E, Ulrici J, Sailer LK, et al. Characterisation of apical bone lesions:Comparison of MRI and CBCT with histological findings - a case series. Eur J Oral Implantol. 2017;10(2):197–211. [PubMed] [Google Scholar]
- 68.Juerchott A, Pfefferle T, Flechtenmacher C, Mente J, Bendszus M, Heiland S, et al. Differentiation of periapical granulomas and cysts by using dental MRI:a pilot study. Int J Oral Sci. 2018;10(2):17. doi: 10.1038/s41368-018-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ylikontiola L, Altonen M, Uhari M, Tiilikainen A, Oikarinen K. Chronic sclerosing osteomyelitis of the mandible in monozygotic twins. Int J Oral Maxillofac Surg. 1994;23(6 Pt 1):359–62. doi: 10.1016/s0901-5027(05)80055-4. [DOI] [PubMed] [Google Scholar]
- 70.Geibel MA, Schreiber ES, Bracher AK, Hell E, Ulrici J, Sailer LK, et al. Assessment of apical periodontitis by MRI:a feasibility study. Rofo. 2015;187(4):269–75. doi: 10.1055/s-0034-1385808. [DOI] [PubMed] [Google Scholar]
- 71.Kim EY, Kim HJ, Chung SK, Dhong HJ, Kim HY, Yim YJ, et al. Sinonasal organized hematoma:CT and MR imaging findings. AJNR Am J Neuroradiol. 2008;29(6):1204–8. doi: 10.3174/ajnr.A1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44(3):275–81. doi: 10.1023/a:1006308808769. [DOI] [PubMed] [Google Scholar]
- 73.Cotti E, Campisi G. Advanced radiographic techniques for the detection of lesions in bone. Endod Topics. 2004;7(1):52–72. [Google Scholar]
- 74.Drăgan OC, Fărcăşanu AŞ, Câmpian RS, Turcu RV. Human tooth and root canal morphology reconstruction using magnetic resonance imaging. Clujul Med. 2016;89(1):137–42. doi: 10.15386/cjmed-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Assaf AT, Zrnc TA, Remus CC, Schönfeld M, Habermann CR, Riecke B, et al. Evaluation of four different optimized magnetic-resonance-imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T. J Craniomaxillofac Surg. 2014;42(7):1356–63. doi: 10.1016/j.jcms.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Auer LM, van Velthoven V. Intraoperative ultrasound (US) imaging. Comparison of pathomorphological findings in US and CT. Acta Neurochir (Wien) 1990;104(3-4):84–95. doi: 10.1007/BF01842825. [DOI] [PubMed] [Google Scholar]
- 77.Cotti E, Campisi G, Garau V, Puddu G. A new technique for the study of periapical bone lesions:ultrasound real time imaging. Int Endod J. 2002;35(2):148–52. doi: 10.1046/j.1365-2591.2002.00458.x. [DOI] [PubMed] [Google Scholar]
- 78.Raghav N, Reddy SS, Giridhar AG, Murthy S, Yashodha Devi BK, et al. Comparison of the efficacy of conventional radiography, digital radiography, and ultrasound in diagnosing periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(3):379–85. doi: 10.1016/j.tripleo.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 79.Musu D, Rossi-Fedele G, Campisi G, Cotti E. Ultrasonography in the diagnosis of bone lesions of the jaws:a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):e19–29. doi: 10.1016/j.oooo.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18(2):269–96. [PubMed] [Google Scholar]
- 81.Ashley M, Harris I. The assessment of the endodontically treated tooth. Dent Update. 2001;28(5):247–52. doi: 10.12968/denu.2001.28.5.247. [DOI] [PubMed] [Google Scholar]
- 82.Torabinejad M, Anderson P, Bader J, Brown LJ, Chen LH, Goodacre CJ, et al. Outcomes of root canal treatment and restoration, implant-supported single crowns, fixed partial dentures, and extraction without replacement:a systematic review. J Prosthet Dent. 2007;98(4):285–311. doi: 10.1016/S0022-3913(07)60102-4. [DOI] [PubMed] [Google Scholar]
- 83.de Chevigny C, Dao TT, Basrani BR, Marquis V, Farzaneh M, Abitbol S, et al. Treatment outcome in endodontics:the Toronto study--phase 4:initial treatment. J Endod. 2008;34(3):258–63. doi: 10.1016/j.joen.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 84.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment:part 2:tooth survival. Int Endod J. 2011 Jul;44(7):610–25. doi: 10.1111/j.1365-2591.2011.01873.x. [DOI] [PubMed] [Google Scholar]
- 85.European Society of Endodontology. Quality guidelines for endodontic treatment:consensus report of the European Society of Endodontology. Int Endod J. 2006;39(12):921–30. doi: 10.1111/j.1365-2591.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen SC, Chueh LH, Hsiao CK, Wu HP, Chiang CP. First untoward events and reasons for tooth extraction after nonsurgical endodontic treatment in Taiwan. J Endod. 2008;34(6):671–4. doi: 10.1016/j.joen.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 87.Vire DE. Failure of endodontically treated teeth:classification and evaluation. J Endod. 1991;17(7):338–42. doi: 10.1016/S0099-2399(06)81702-4. [DOI] [PubMed] [Google Scholar]
- 88.Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment:systematic review of the literature - part 1. Effects of study characteristics on probability of success. Int Endod J. 2007;40(12):921–39. doi: 10.1111/j.1365-2591.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 89.Nair PN. On the causes of persistent apical periodontitis:a review. Int Endod J. 2006;39(4):249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 90.Lin LM, Skribner JE, Gaengler P. Factors associated with endodontic treatment failures. J Endod. 1992;18(12):625–7. doi: 10.1016/S0099-2399(06)81335-X. [DOI] [PubMed] [Google Scholar]
- 91.Engstrom B. Correlation of positive cultures with the prognosis for root canal therapy. Odontol. Rev. 1964;15(3):257–70. [PubMed] [Google Scholar]
- 92.Lopes HP, Siqueira JF., Jr . Endodontia:biologia e técnica. Brasil: Elsevier; 2015. [Google Scholar]
- 93.Nair PN, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions:a long-term light and electron microscopic follow-up study. J Endod. 1990;16(12):580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 94.Peters OA, Barbakow F, Peters CI. An analysis of endodontic treatment with three nickel-titanium rotary root canal preparation techniques. Int Endod J. 2004;37(12):849–59. doi: 10.1111/j.1365-2591.2004.00882.x. [DOI] [PubMed] [Google Scholar]
- 95.Kersten HW, Wesselink PR, Thoden van Velzen SK. The diagnostic reliability of the buccal radiograph after root canal filling. Int Endod J. 1987;20(1):20–4. doi: 10.1111/j.1365-2591.1987.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 96.Siqueira JF, Jr, de Uzeda M. Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J Endod. 1996;22(12):674–6. doi: 10.1016/S0099-2399(96)80062-8. [DOI] [PubMed] [Google Scholar]
- 97.Gillen BM, Looney SW, Gu LS, Loushine BA, Weller RN, Loushine RJ, et al. Impact of the quality of coronal restoration versus the quality of root canal fillings on success of root canal treatment:a systematic review and meta-analysis. J Endod. 2011;37(7):895–902. doi: 10.1016/j.joen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olcay K, Ataoglu H, Belli S. Evaluation of Related Factors in the Failure of Endodontically Treated Teeth:A Cross-sectional Study. J Endod. 2018;44(1):38–45. doi: 10.1016/j.joen.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 99.TouréB Faye B, Kane AW, Lo CM, Niang B, Boucher Y. Analysis of reasons for extraction of endodontically treated teeth:a prospective study. J Endod. 2011;37(11):1512–5. doi: 10.1016/j.joen.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Ruiz XF, Duran-Sindreu F, Shemesh H, García Font M, Vallés M, Roig Cayón M, et al. Development of Periapical Lesions in Endodontically Treated Teeth with and without Periodontal Involvement:A Retrospective Cohort Study. J Endod. 2017;43(8):1246–9. doi: 10.1016/j.joen.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 101.Iqbal MK, Johansson AA, Akeel RF, Bergenholtz A, Omar R. A retrospective analysis of factors associated with the periapical status of restored, endodontically treated teeth. Int J Prosthodont. 2003;16(1):31–8. [PubMed] [Google Scholar]
- 102.Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29(2):118–24. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 103.Iwaku M, Hoshino E, Kota K. How to conserve infected pulps. Tokyo, Japan: Nihon-Shika-Hyoron; 1996. Lesion sterilization and tissue repair (LSTR) therapy:new pulpal treatment. [Google Scholar]
- 104.Austin JH, Taylor HD. Behavior of hypochlorite and of chloramine-T solutions in contact with necrotic and normal tissues in vivo. J Exp Med. 1918;27(5):627–33. doi: 10.1084/jem.27.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valera MC, Silva KC, Maekawa LE, Carvalho CA, Koga-Ito CY, Camargo CH, et al. Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J Appl Oral Sci. 2009;17(6):555–9. doi: 10.1590/S1678-77572009000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30(11):785–7. doi: 10.1097/00004770-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 107.Hennessey TS. Some antibacterial properties of chlorhexidine. J Periodontal Res Suppl. 1973;12:61–7. doi: 10.1111/j.1600-0765.1973.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 108.Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24(3):119–25. doi: 10.1111/j.1365-2591.1991.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 109.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide:a critical review. Int Endod J. 1999;32(5):361–9. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 110.Ingham HR, Selkon JB, Hale JH. The antibacterial activity of netronidazole. J Antimicrob Chemother. 1975;1(4):355–61. doi: 10.1093/jac/1.4.355. [DOI] [PubMed] [Google Scholar]
- 111.Sato T, Hoshino E, Uematsu H, Noda T. In vitro antimicrobial susceptibility to combinations of drugs on bacteria from carious and endodontic lesions of human deciduous teeth. Oral Microbiol Immunol. 1993;8(3):172–6. doi: 10.1111/j.1399-302x.1993.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 112.Takushige T, Cruz EV, Asgor Moral A, Hoshino E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37(2):132–8. doi: 10.1111/j.0143-2885.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 113.Rossi-Fedele G, Scott W, Spratt D, Gulabivala K, Roberts AP. Incidence and behaviour of Tn916-like elements within tetracycline-resistant bacteria isolated from root canals. Oral Microbiol Immunol. 2006;21(4):218–22. doi: 10.1111/j.1399-302X.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 114.Fujii H, Machida Y. Histological study of therapy for infected nonvital permanent teeth with incompletely formed apices. Bull Tokyo Dent Coll. 1991;32(1):35–45. [PubMed] [Google Scholar]
- 115.Esposito JV. Apical violation in periapical “area” cases. Blasphemy or therapy? Dent Clin North Am. 1990;34(1):171–8. [PubMed] [Google Scholar]
- 116.Bhaskar SN. Nonsurgical resolution of radicular cysts. Oral Surg Oral Med Oral Pathol. 1972;34(3):458–8. doi: 10.1016/0030-4220(72)90325-8. [DOI] [PubMed] [Google Scholar]
- 117.Bender IB. A commentary on General Bhaskar's hypothesis. Oral Surg Oral Med Oral Pathol. 1972;34(3):469–76. doi: 10.1016/0030-4220(72)90326-x. [DOI] [PubMed] [Google Scholar]
- 118.Metzger Z, Huber R, Tobis I, Better H. Enhancement of healing kinetics of periapical lesions in dogs by the Apexum procedure. J Endod. 2009;35(1):40–5. doi: 10.1016/j.joen.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 119.Siqueira Jr JF. Reaction of periradicular tissues to root canal treatment:benefits and drawbacks. Endod Topics. 2005;10(1):123–47. [Google Scholar]
- 120.Metzger Z, Huber R, Slavescu D, Dragomirescu D, Tobis I, Better H. Healing kinetics of periapical lesions enhanced by the apexum procedure:a clinical trial. J. Endod. 2009;35(2):153–9. doi: 10.1016/j.joen.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 121.Rud J, Andreasen JO. A study of failures after endodontic surgery by radiographic, histologic and stereomicroscopic methods. Int J Oral Surg. 1972;1(6):311–28. doi: 10.1016/s0300-9785(72)80052-8. [DOI] [PubMed] [Google Scholar]
- 122.Kvist T, Reit C. Postoperative discomfort associated with surgical and nonsurgical endodontic retreatment. Endod Dent Traumatol. 2000;16(2):71–4. doi: 10.1034/j.1600-9657.2000.016002071.x. [DOI] [PubMed] [Google Scholar]
- 123.Baumgartner JC, Falkler WA., Jr Reactivity of IgG from explant cultures of periapical lesions with implicated microorganisms. J Endod. 1991;17(5):207–12. doi: 10.1016/S0099-2399(06)81922-9. [DOI] [PubMed] [Google Scholar]
- 124.Kettering JD, Torabinejad M, Jones SL. Specificity of antibodies present in human periapical lesions. J Endod. 1991;17(5):213–6. doi: 10.1016/S0099-2399(06)81923-0. [DOI] [PubMed] [Google Scholar]
- 125.Haapasalo M, Wang Z, Shen Y, Curtis A, Patel P, Khakpour M. Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. J Endod. 2014;40(8):1178–81. doi: 10.1016/j.joen.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 126.Haapasalo M, Shen Y, Wang Z, Park E, Curtis A, Patel P, et al. Apical pressure created during irrigation with the GentleWave™system compared to conventional syringe irrigation. Clin Oral Investig. 2016;20(7):1525–34. doi: 10.1007/s00784-015-1632-z. [DOI] [PubMed] [Google Scholar]
- 127.Molina B, Glickman G, Vandrangi P, Khakpour M. Evaluation of Root Canal Debridement of Human Molars Using the GentleWave System. J Endod. 2015;41(10):1701–5. doi: 10.1016/j.joen.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 128.Sigurdsson A, Garland RW, Le KT, Woo SM. 12-month Healing Rates after Endodontic Therapy Using the Novel GentleWave System:A Prospective Multicenter Clinical Study. J Endod. 2016;42(7):1040–8. doi: 10.1016/j.joen.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 129.Torabinejad M, Corr R, Handysides R, Shabahang S. Outcomes of nonsurgical retreatment and endodontic surgery:a systematic review. J Endod. 2009;35(7):930–7. doi: 10.1016/j.joen.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 130.Sjögren U, Happonen RP, Kahnberg KE, Sundqvist G. Survival of Arachnia propionica in periapical tissue. Int Endod J. 1988;21(4):277–82. doi: 10.1111/j.1365-2591.1988.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 131.Sundqvist G, Reuterving CO. Isolation of Actinomyces israelii from periapical lesion. J Endod. 1980;6(6):602–6. doi: 10.1016/S0099-2399(80)80021-5. [DOI] [PubMed] [Google Scholar]
- 132.Nair PN, Sjögren U, Krey G, Sundqvist G. Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. J Endod. 1990;16(12):589–95. doi: 10.1016/S0099-2399(07)80202-0. [DOI] [PubMed] [Google Scholar]
- 133.Yusuf H. The significance of the presence of foreign material periapically as a cause of failure of root treatment. Oral Surg Oral Med Oral Pathol. 1982;54(5):566–74. doi: 10.1016/0030-4220(82)90196-7. [DOI] [PubMed] [Google Scholar]
- 134.Nair PN, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(5):617–27. doi: 10.1016/s1079-2104(99)70145-9. [DOI] [PubMed] [Google Scholar]
- 135.Nair PN, Sjögren U, Sundqvist G. Cholesterol crystals as an etiological factor in non-resolving chronic inflammation:an experimental study in guinea pigs. Eur J Oral Sci. 1998;106(2 Pt 1):644–50. doi: 10.1046/j.0909-8836.1998.eos106206.x. [DOI] [PubMed] [Google Scholar]
- 136.Nair PN. Cholesterol as an aetiological agent in endodontic failures--a review. Aust Endod J. 1999;25(1):19–26. doi: 10.1111/j.1747-4477.1999.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 137.Koppang HS, Koppang R, Solheim T, Aarnes H, Stølen SO. Cellulose fibers from endodontic paper points as an etiological factor in postendodontic periapical granulomas and cysts. J Endod. 1989;15(8):369–72. doi: 10.1016/S0099-2399(89)80075-5. [DOI] [PubMed] [Google Scholar]
- 138.Love RM, Firth N. Histopathological profile of surgically removed persistent periapical radiolucent lesions of endodontic origin. Int Endod J. 2009;42(3):198–202. doi: 10.1111/j.1365-2591.2008.01500.x. [DOI] [PubMed] [Google Scholar]
- 139.Strindberg LZ. The dependence of the results of pulp therapy on certain factors-an analytical study based on radiographic and clinical follow-up examination. Acta Odontol Scand. 1956;14:1–175. [Google Scholar]
- 140.Murazabal M, Erausquin J, Devoto FH. A study of periapical overfilling root canal treatment in the molar of the rat. Arch Oral Biol. 1966;11(4):373–83. doi: 10.1016/0003-9969(66)90103-8. [DOI] [PubMed] [Google Scholar]
- 141.Barbosa SV, Araki K, Spångberg LS. Cytotoxicity of some modified root canal sealers and their leachable components. Oral Surg Oral Med Oral Pathol. 1993;75(3):357–61. doi: 10.1016/0030-4220(93)90151-s. [DOI] [PubMed] [Google Scholar]
- 142.Sjögren U, Sundqvist G, Nair PN. Tissue reaction to gutta-percha particles of various sizes when implanted subcutaneously in guinea pigs. Eur J Oral Sci. 1995;103(5):313–21. doi: 10.1111/j.1600-0722.1995.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 143.Lin LM, Huang GT, Rosenberg PA. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod. 2007;33(8):908–16. doi: 10.1016/j.joen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 144.Lalonde ER, Luebke RG. The frequency and distribution of periapical cysts and granulomas. An evaluation of 800 specimens. Oral Surg Oral Med Oral Pathol. 1968;25(6):861–8. doi: 10.1016/0030-4220(68)90163-1. [DOI] [PubMed] [Google Scholar]
- 145.Simon JH. Incidence of periapical cysts in relation to the root canal. J Endod. 1980;6(11):845–8. doi: 10.1016/S0099-2399(80)80039-2. [DOI] [PubMed] [Google Scholar]
- 146.Lin LM, Ricucci D, Lin J, Rosenberg PA. Nonsurgical root canal therapy of large cyst-like inflammatory periapical lesions and inflammatory apical cysts. J Endod. 2009;35(5):607–15. doi: 10.1016/j.joen.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 147.Asián-González E, Pereira-Maestre M, Conde-Fernández D, Vilchez I, Segura-Egea JJ, Gutiérrez-Pérez JL. Dentigerous cyst associated with a formocresol pulpotomized deciduous molar. J Endod. 2007;33(4):488–92. doi: 10.1016/j.joen.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 148.Tek M, Metin M, Sener I, Bereket C, Tokac M, Kazancioglu HO, et al. The predominant bacteria isolated from radicular cysts. Head Face Med. 2013;9:25. doi: 10.1186/1746-160X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nainani P, Sidhu GK. Radicular Cyst –An Update with emphasis on Pathogenesis. J Adv Med Dent Scie Res. 2014;2(3):97–101. [Google Scholar]
- 150.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–64. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 151.Siqueira Junior JF, Lopes HP. Periradicular biofilm:structure, implication in the endodontic failure, and treatment strategies. Rev Paul Odontol. 1998;20(6):4–6. 8. [Google Scholar]
- 152.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11(1):160–7. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 153.Tronstad L, Barnett F, Cervone F. Periapical bacterial plaque in teeth refractory to endodontic treatment. Endod Dent Traumatol. 1990;6(2):73–7. doi: 10.1111/j.1600-9657.1990.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 154.Siqueira JF, Jr, Lopes HP. Bacteria on the apical root surfaces of untreated teeth with periradicular lesions:a scanning electron microscopy study. Int Endod J. 2001;34(3):216–20. doi: 10.1046/j.1365-2591.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 155.Tronstad L, Barnett F, Riso K, Slots J. Extraradicular endodontic infections. Endod Dent Traumatol. 1987;3(2):86–90. doi: 10.1111/j.1600-9657.1987.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 156.Siqueira JF, Jr, Rôças IN, Souto R, de Uzeda M, Colombo AP. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J Endod. 2002;28(3):168–72. doi: 10.1097/00004770-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 157.Tronstad L, Kreshtool D, Barnett F. Microbiological monitoring and results of treatment of extraradicular endodontic infection. Endod Dent Traumatol. 1990;6(3):129–36. doi: 10.1111/j.1600-9657.1990.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 158.Arnold M, Ricucci D, Siqueira JF., Jr Infection in a complex network of apical ramifications as the cause of persistent apical periodontitis:a case report. J Endod. 2013;39(9):1179–84. doi: 10.1016/j.joen.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 159.Su L, Gao Y, Yu C, Wang H, Yu Q. Surgical endodontic treatment of refractory periapical periodontitis with extraradicular biofilm. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(1):e40–4. doi: 10.1016/j.tripleo.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 160.Signoretti FG, Endo MS, Gomes BP, Montagner F, Tosello FB, Jacinto RC. Persistent extraradicular infection in root-filled asymptomatic human tooth:scanning electron microscopic analysis and microbial investigation after apical microsurgery. J Endod. 2011;37(12):1696–700. doi: 10.1016/j.joen.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 161.Siqueira JF., Jr Aetiology of root canal treatment failure:why well-treated teeth can fail. Int Endod J. 2001;34(1):1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 162.Seltzer S, Soltanoff W, Smith J. Biologic aspects of endodontics. V. Periapical tissue reactions to root canal instrumentation beyond the apex and root canal fillings short of and beyond the apex. Oral Surg Oral Med Oral Pathol. 1973;36(5):725–37. doi: 10.1016/0030-4220(73)90146-1. [DOI] [PubMed] [Google Scholar]
- 163.Shabahang S American Association of Endodontics Research and Scientific Affairs Committee. State of the art and science of endodontics. J Am Dent Assoc. 2005;136(1):41–52. doi: 10.14219/jada.archive.2005.0025. [DOI] [PubMed] [Google Scholar]
- 164.Hirsch JM, Ahlström U, Henrikson PA, Heyden G, Peterson LE. Periapical surgery. Int J Oral Surg. 1979;8(3):173–85. doi: 10.1016/s0300-9785(79)80016-2. [DOI] [PubMed] [Google Scholar]
- 165.Mead C, Javidan-Nejad S, Mego ME, Nash B, Torabinejad M. Levels of evidence for the outcome of endodontic surgery. J Endod. 2005;31(1):19–24. doi: 10.1097/01.don.0000133158.35394.8a. [DOI] [PubMed] [Google Scholar]
- 166.Friedman S, Lustmann J, Shaharabany V. Treatment results of apical surgery in premolar and molar teeth. J Endod. 1991;17(1):30–3. doi: 10.1016/S0099-2399(07)80158-0. [DOI] [PubMed] [Google Scholar]
- 167.Rapp EL, Brown CE, Jr, Newton CW. An analysis of success and failure of apicoectomies. J Endod. 1991;17(10):508–12. doi: 10.1016/S0099-2399(06)81800-5. [DOI] [PubMed] [Google Scholar]
- 168.Wang N, Knight K, Dao T, Friedman S. Treatment outcome in endodontics-The Toronto Study. Phases I and II:apical surgery. J Endod. 2004;30(11):751–61. doi: 10.1097/01.don.0000137633.30679.74. [DOI] [PubMed] [Google Scholar]
- 169.Grung B, Molven O, Halse A. Periapical surgery in a Norwegian county hospital:follow-up findings of 477 teeth. J Endod. 1990;16(9):411–7. doi: 10.1016/S0099-2399(06)81882-0. [DOI] [PubMed] [Google Scholar]
- 170.Lustmann J, Friedman S, Shaharabany V. Relation of pre- and intraoperative factors to prognosis of posterior apical surgery. J Endod. 1991;17(5):239–41. doi: 10.1016/S0099-2399(06)81929-1. [DOI] [PubMed] [Google Scholar]
- 171.Rud J, Andreasen JO, Jensen JF. A multivariate analysis of the influence of various factors upon healing after endodontic surgery. Int J Oral Surg. 1972;1(5):258–71. doi: 10.1016/s0300-9785(72)80045-0. [DOI] [PubMed] [Google Scholar]
- 172.Ericson S, Finne K, Persson G. Results of apicoectomy of maxillary canines, premolars and molars with special reference to oroantral communication as a prognostic factor. Int J Oral Surg. 1974;3(6):386–93. doi: 10.1016/s0300-9785(74)80003-7. [DOI] [PubMed] [Google Scholar]
- 173.Rud J, Munksgaard EC, Andreasen JO, Rud V, Asmussen E. Retrograde root filling with composite and a dentin-bonding agent. 1. Endod Dent Traumatol. 1991;7(3):118–25. doi: 10.1111/j.1600-9657.1991.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 174.Skoglund A, Persson G. A follow-up study of apicoectomized teeth with total loss of the buccal bone plate. Oral Surg Oral Med Oral Pathol. 1985;59(1):78–81. doi: 10.1016/0030-4220(85)90120-3. [DOI] [PubMed] [Google Scholar]
- 175.Mikkonen M, Kullaa-Mikkonen A, Kotilainen R. Clinical and radiologic re-examination of apicoectomized teeth. Oral Surg Oral Med Oral Pathol. 1983;55(3):302–6. doi: 10.1016/0030-4220(83)90332-8. [DOI] [PubMed] [Google Scholar]
- 176.Molven O, Halse A, Grung B. Surgical management of endodontic failures:indications and treatment results. Int Dent J. 1991;41(1):33–42. [PubMed] [Google Scholar]
- 177.Frank AL, Glick DH, Patterson SS, Weine FS. Long-term evaluation of surgically placed amalgam fillings. J Endod. 1992;18(8):391–8. doi: 10.1016/s0099-2399(06)81226-4. [DOI] [PubMed] [Google Scholar]
- 178.Kvist T, Reit C. Results of endodontic retreatment:a randomized clinical study comparing surgical and nonsurgical procedures. J Endod. 1999;25(12):814–7. doi: 10.1016/S0099-2399(99)80304-5. [DOI] [PubMed] [Google Scholar]
- 179.Metzger Z. Macrophages in periapical lesions. Endod Dent Traumatol. 2000;16(1):1–8. doi: 10.1034/j.1600-9657.2000.016001001.x. [DOI] [PubMed] [Google Scholar]
- 180.Kim S. Principles of endodontic microsurgery. Dent Clin North Am. 1997;41(3):481–97. [PubMed] [Google Scholar]
- 181.Gray GJ, Hatton JF, Holtzmann DJ, Jenkins DB, Nielsen CJ. Quality of root-end preparations using ultrasonic and rotary instrumentation in cadavers. J Endod. 2000;26(5):281–3. doi: 10.1097/00004770-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 182.Siqueira JF, Jr, De Uzeda M, Fonseca ME. A scanning electron microscopic evaluation of in vitro dentinal tubules penetration by selected anaerobic bacteria. J Endod. 1996;22(6):308–10. doi: 10.1016/S0099-2399(96)80265-2. [DOI] [PubMed] [Google Scholar]
- 183.Friedman S. Management of post-treatment endodontic disease:a current concept of case selection. Aust Endod J. 2000;26(3):104–9. doi: 10.1111/j.1747-4477.2000.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 184.Foster KH, Harrison E. Effect of presentation bias on selection of treatment option for failed endodontic therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(5):e36–9. doi: 10.1016/j.tripleo.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 185.Aryanpour S, Van Nieuwenhuysen JP, D'Hoore W. Endodontic retreatment decisions:no consensus. Int Endod J. 2000;33(3):208–18. doi: 10.1046/j.1365-2591.1999.00297.x. [DOI] [PubMed] [Google Scholar]
- 186.Kvist T, Reit C. The perceived benefit of endodontic retreatment. Int Endod J. 2002;35(4):359–65. doi: 10.1046/j.1365-2591.2002.00486.x. [DOI] [PubMed] [Google Scholar]
- 187.Pennington MW, Vernazza CR, Shackley P, Armstrong NT, Whitworth JM, Steele JG. Evaluation of the cost-effectiveness of root canal treatment using conventional approaches versus replacement with an implant. Int Endod J. 2009;42(10):874–83. doi: 10.1111/j.1365-2591.2009.01582.x. [DOI] [PubMed] [Google Scholar]
- 188.Gallego Romero D, Torres Lagares D, GarcIa Calderón M, Romero Ruiz MM, Infante Cossio P, Gutiérrez Pérez JL. Differential diagnosis and therapeutic approach to periapical cysts in daily dental practice. Med Oral. 2002;7(1):54–8. 59–2. [PubMed] [Google Scholar]
- 189.Kruse C, Spin-Neto R, Christiansen R, Wenzel A, Kirkevang LL. Periapical Bone Healing after Apicectomy with and without Retrograde Root Filling with Mineral Trioxide Aggregate:A 6-year Follow-up of a Randomized Controlled Trial. J Endod. 2016;42(4):533–7. doi: 10.1016/j.joen.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 190.Nygaard-Östby B. Introduction to endodontics. Oslo: Scandinavian University; 1971. [Google Scholar]
- 191.Jonasson P, Ragnarsson MF. Surgical endodontic retreatment. Clin Dent Rev. 2018;2(1):17. [Google Scholar]
- 192.Reit C, Hirsch J. Surgical endodontic retreatment. Int Endod J. 1986;19(3):107–12. doi: 10.1111/j.1365-2591.1986.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 193.Jonasson P, Reit C, Kvist T. A preliminary study on the technical feasibility and outcome of retrograde root canal treatment. Int Endod J. 2008;41(9):807–13. doi: 10.1111/j.1365-2591.2008.01395.x. [DOI] [PubMed] [Google Scholar]
- 194.Freedland JB. Conservative reduction of large periapical lesions. Oral Surg Oral Med Oral Pathol. 1970;29(3):455–64. doi: 10.1016/0030-4220(70)90150-7. [DOI] [PubMed] [Google Scholar]
- 195.Neaverth EJ, Burg HA. Decompression of large periapical cystic lesions. J Endod. 1982;8(4):175–82. doi: 10.1016/S0099-2399(82)80214-8. [DOI] [PubMed] [Google Scholar]
- 196.Brøndum N, Jensen VJ. Recurrence of keratocysts and decompression treatment. A long-term follow-up of forty-four cases. Oral Surg Oral Med Oral Pathol. 1991;72(3):265–9. doi: 10.1016/0030-4220(91)90211-t. [DOI] [PubMed] [Google Scholar]
- 197.Nakamura N, Mitsuyasu T, Mitsuyasu Y, Taketomi T, Higuchi Y, Ohishi M. Marsupialization for odontogenic keratocysts:long-term follow-up analysis of the effects and changes in growth characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(5):543–53. doi: 10.1067/moe.2002.128022. [DOI] [PubMed] [Google Scholar]
- 198.Pogrel MA. Decompression and marsupialization as a treatment for the odontogenic keratocyst. Oral Maxillofac Surg Clin North Am. 2003;15(3):415–27. doi: 10.1016/S1042-3699(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 199.Martin SA. Conventional endodontic therapy of upper central incisor combined with cyst decompression:a case report. J Endod. 2007;33(6):753–7. doi: 10.1016/j.joen.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 200.Gardner AF. A survey of odontogenic cysts and their relationship to squamous cell carcinoma. Dent J. 1975;41(3):161–7. [PubMed] [Google Scholar]
- 201.Schneider LC. Incidence of epithelial atypia in radicular cysts:a preliminary investigation. J Oral Surg. 1977;35(5):370–4. [PubMed] [Google Scholar]
- 202.Tsurumachi T, Saito T. Treatment of large periapical lesions by inserting a drainage tube into the root canal. Endod Dent Traumatol. 1995;11(1):41–6. doi: 10.1111/j.1600-9657.1995.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 203.Mejia JL, Donado JE, Basrani B. Active nonsurgical decompression of large periapical lesions--3 case reports. J Can Dent Assoc. 2004;70(10):691–4. [PubMed] [Google Scholar]
- 204.Walker TL, Davis MS. Treatment of large periapical lesions using cannulization through the involved teeth. J Endod. 1984;10(5):215–20. doi: 10.1016/S0099-2399(84)80086-2. [DOI] [PubMed] [Google Scholar]
- 205.Saghiri MA, Saghiri AM. In Memoriam: Dr. Hajar Afsar Lajevardi MD, MSc, MS (1955-2015) Iranian Journal of Pediatrics. 2017;27:e8093. [Google Scholar]


