Abstract
Worldwide, several hospitals in different regions and countries have been affected with Corona Virus Disease-19 (COVID-19). All medical specialties including gastroenterology are impacted by COVID-19. Here, we review the bidirectional comorbidity of chronic gastrointestinal (GI) disorders and COVID-19, including the incidence and outcome of COVID-19 in individuals with various GI disorders and the impact of COVID-19 on the course and outcome of the underlying (or comorbid) GI disorders. Currently, there is no evidence that COVID-19 is more (or less) frequent in comorbid GI disorders. It is also reassuring that the outcome of COVID-19 is unaffected by the underlying GI disorder or its treatment, though potential concerns remain in regard to the use of immunomodulatory treatments in inflammatory bowel disease (IBD) and liver transplant recipients. Despite these concerns, there is now agreement among experts that ongoing immunomodulatory treatments may not be interrupted in individuals with IBD during the COVID-19 pandemic. Caution, however, may be exercised with the use of corticosteroids in the management of IBD. In addition, COVID-19 does not appear to impact the manifestations, course, outcome, and treatment of comorbid GI disorders, e.g. IBD. Decompensation of liver cirrhosis is, however, possible during COVID-19 episodes. A direct concern, however, might relate to the potential transmission of the virus through fecal microbiota transplants.
Keywords: Comorbidity, Corona Virus, COVID-19, Crohn disease, Diarrhea, Immunosupression, Inflammatory bowel disease, Pandemic, Ulcerative colitis
Introduction
The occurrence of a large cluster of cases of pneumonia was first reported from Wuhan in Hubei Province, China, in early December 2019 [1]. Very rapidly, the outbreak metamorphosed into a local epidemic and eventually, a global pandemic and public health emergency [2]. The causative agent was found to be a novel beta coronavirus, namely, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) as it shared considerable similarity to the SARS-COV, responsible for the SARS syndrome in 2003. The WHO later renamed the infection as Corona Virus Disease-19 (COVID-19) [2]. The pathogenicity of COVID-19 is thought to be related to the angiotensin-converting enzyme (ACE) type 2 receptor, expressed in the airway epithelium, vascular, kidney, and small intestinal cells [3].
As of May 29, 2020, the estimated number of positive patients is over 6 million with a mortality of 366,812. Within a relatively short period, there has been a surge of publications on the clinical, and imaging patterns, virus behavior, or biology. An initial PubMed search on April 07, 2020, using the terms COVID-19 and coronavirus yielded 2780 and 16,725 citations, respectively. A similar search on May 29, whilst revising this article, yielded 16,907 and 47,294 citations, respectively. Hence, we recognize the rapidity with which new information is being generated and acknowledge that the standpoints presented in this review might change in the times to come.
The cardinal manifestations of COVID-19 include fever, dry cough, fatigue, and myalgias [4]. Gastrointestinal (GI) manifestations are not uncommon and rarely might be the sole presenting manifestation of SARS-CoV-2 infection [5–8]. These have been systematically reviewed elsewhere, and an updated meta-analysis of the gastroenterological manifestations of COVID-19 appears in this very issue of the Indian Journal of Gastroenterology [9]. Hence, we choose not to discuss GI manifestations of COVID-19 here and the reader is referred to the accompanying paper for further information. On a different note, there is concern that the course, outcome, and treatment of certain GI disorders, e.g. inflammatory bowel disease (IBD), are modified by COVID-19 [10]. Lastly, the flood of COVID-19 to hospitals in several locations has had implications on the operation of several GI procedures and treatment and in a larger unforeseen manner of routine GI practice [11]. Here, we review published literature from PubMed to address the following two concerns: (i) Are people with GI disorders at an altered risk or severity of developing COVID-19? and (ii) Does the occurrence of intercurrent COVID-19 impact the manifestations, course, outcome, and treatment of premorbid GI disorders? Also, we chose not to address the feasibility, indication, and adaptations to GI endoscopic procedures as several professional bodies across different countries and regions have proposed interim guidelines, largely based on expert consensus, and the reader is referred to these for detailed understanding and adherence [12, 13].
Are people with GI disorders at an altered risk or severity of developing COVID-19?
SARS-CoV-2 RNA has been isolated from stool specimens [5, 14, 15]. The isolation of viral RNA from stool samples correlates with abdominal pain but not to other GI symptoms [16]. Anecdotally, the virus isolation from feces follows isolation from the throat or the respiratory tract by 2–3 days but more experience is required to confirm this observation. In convalescent individuals, i.e. those in whom fever has resolved with two consecutive nasopharyngeal swabs negative for reverse transcriptase polymerase chain reaction (RT-PCR), the virus might persist in stool specimens for several days [17, 18]. The familial clustering of cases, observed anecdotally, adds support to and might indeed be the result of transmission by the fecal route [19]. Transmission by the fecal route might be more important in infected children, particularly those who have not acquired bowel control [20, 21]. Virus isolation from stool specimens raises several issues: (1) it opens up the possibility of diagnosis using fecal samples, thereby potentially reducing false-negative results if nasal and throat swabs are negative; (2) it also opens up the possibility of transmission via the fecal route; (3) it also precludes the potential of contamination and spread of infection during colonoscopic and proctoscopic examinations [18]; and (4) it raises the speculation that certain inflammatory bowel disorders might alter the risk of acquiring or the severity of COVID-19. Below, we discuss the clinical implications of the fourth concern.
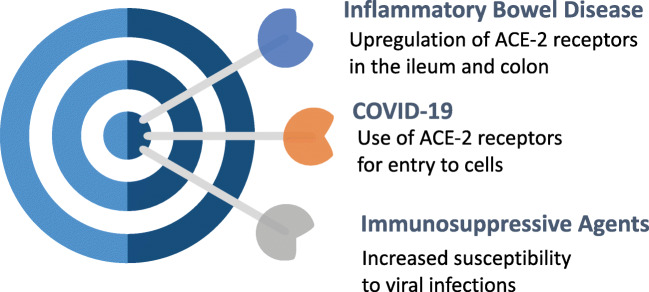
The pathogenicity of COVID-19 agent in relation to the GI tract has been recently reviewed elsewhere [22]. Briefly, an S protein component of the virus interacts with cell surface angiotensin-converting enzyme type 2 (ACE-2) receptors to enter human host cells [23]. The ACE-2 receptors are expressed abundantly in the nasal epithelium and upper and lower respiratory tract epithelial cells [3]. In addition, ACE-2 receptors are also highly expressed in the human GI tract epithelium, particularly the terminal ileum and fairly in the colon [24]. Studies have documented an increased expression of ACE-2 in histological samples of IBD [25]. Lastly, there is an upregulation of trypsinases, which might aid the solubilization of viral S protein to aid the assimilation of the virus in the human host cells [22]. The findings have stirred speculation that individuals with IBD might be more prone to COVID-19 and, when infected, more likely to have severe manifestations, e.g. requirement for intensive care, mechanical ventilation, and even death (Fig. 1). Alternatively, studies have demonstrated a massive increase in the expression of several cytokines in individuals with severe COVID-19, referred to as the cytokine storm [26]. It has been, therefore, surmised that immunomodulatory agents used in the treatment of IBD might favorably modulate the cytokine expression and shield COVID-19 infected individuals from serious manifestations.
Fig. 1.
Cartoon depicting the possible relationship between inflammatory bowel disease and Corona Virus Disease-19 (COVID-19). ACE-2 angiotensin-converting enzyme type 2
The use of angiotensin-converting enzyme–blocking agents, e.g. for the control of hypertension, is thought to upregulate the expression of ACE-2 receptors and may predispose individuals with COVID-19 to more severe manifestations with poor outcome [27, 28]. Anecdotally, the occurrence of hemorrhagic colitis in an individual with COVID-19, who was previously using lisinopril, has been described [29].
Initial reports of a large number of cases from Wuhan and other locations in China did not identify IBD as one of the predisposing conditions for COVID-19 or its more severe manifestations [5, 30]. Likewise, IBD did not figure in a systematic review of premorbid conditions associated with COVID-19 [31]. The concern of an altered risk, however, fueled investigators from different parts of the world to join hands to initiate a collaborative voluntary online registry, known as the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion-inflammatory bowel disease (IBD) database (https://covidibd.org) (SECURE-IBD). As of May 31, 2020, 1302 cases of COVID-19 in IBD have been registered from 46 countries, though most cases have been volunteered from the USA. Of them, 30% required hospitalization, 6% required intensive care, 5% required mechanical ventilation, and 3% died. Overall, therefore, the need for mechanical ventilation and mortality due to COVID-19 does not seem to be increased among individuals with IBD. To date, three cases in the registry have been contributed from India, of whom one died. Interestingly, in contrast to the bimodal peak of the age distribution of IBD, over 57% of the patients in the SECURE-IBD registry are younger than 50 years of age [32]. Individuals over 60 years represent only 21% of the global cohort. In contrast, 46% of those who died in the registry were over 60 years of age. These data are consistent with the age trends on mortality associated with COVID-19 [33]. Preliminary data gathered from the website depicts the high case fatality rates (of 10%) among users of oral or parenteral corticosteroids. A sizable proportion of the cohort was on anti-tumor necrosis factor with or without other immunosuppressive agents and the case fatality rate in this subgroup was less than 2%.
Once published, the SECURE-IBD data are likely to drive treatment recommendations for IBD during the COVID-19 pandemic. Currently, however, most scientific bodies recommend the continuation of ongoing therapies including immunosuppressants as well as biological agents during the period of COVID-19 pandemic [34–36]. If an individual with IBD requires new treatment/s (e.g. due to a flare-up or new-onset disease), then the risks and benefits of the treatments, especially corticosteroids, should be carefully discussed in order to arrive at a mutual decision between the physician and patient. If an individual with IBD develops COVID-19, immunosuppressants may be carefully withheld during the period of active infection and reinstituted as soon as microbiological clearance has been demonstrated. Surgical treatments with the exception of emergent procedures are best deferred until after the pandemic wanes [37]. Needless to add, all individuals with IBD should maintain high levels of safety precautions required in general for the prevention of COVID-19, including the use of masks, hand washing, social distancing, and avoiding face-to-face visits to hospitals. Physicians involved in IBD care should be mindful of the high mortality risks associated with older age and other comorbidities, e.g. hypertension and diabetes, and should likewise advise their patients.
Other than IBD, data on the comorbidity of COVID-19 and GI disorders are limited [5, 30]. Preliminary data from China pointed towards an increased likelihood of comorbid chronic liver disease in COVID-19-infected individuals with GI symptoms and signs [15, 16]. The National Health Commission study of 1099 COVID-19-infected individuals from China found that the frequency of HBsAg positivity in this population was 2.1% [30]. The SECURE registries invited data collection for celiac disease, eosinophilic gastroenteritis, chronic liver disease, and liver transplant in association with COVID-19 (https://covidibd.org/other-registries/). The data for each of these conditions are sparse in comparison with IBD. The SECURE-Cirrhosis collects data on chronic liver disease (both cirrhotic and non-cirrhotic; liver transplant included) from the Americas, Japan, Korea, and China and works in tandem with the European Association for the Study of the Liver COVID-Hep registry, which likewise collects data from the rest of the world. A combined up-to-date dataset can be accessed at https://covidcirrhosis.web.unc.edu. Towards the end of May 2020, there is information on 710 cases including 124 liver transplant recipients (https://covidcirrhosis.web.unc.edu/updates-and-data/). Case fatality rates have been the highest (over 30%) in those with hepatic cirrhosis and hepatic transplant recipients (17%) but low in those with non-cirrhotic chronic liver disease (7%) (https://covidcirrhosis.web.unc.edu/files/2020/05/Weekly-Data-Update-26-May-2020-1.png). The current issue of the journal presents a single-center study on COVID-19 from Delhi, India on this issue [38].
Hepatic transplant recipients present special concerns from the standpoint of vulnerability to COVID-19 (see Table 1 for mortality outcomes in different centers). A single-center report described 6 COVID-19 infection episodes among 150 transplant recipients, of whom 3 died [39]. Those who died were over 65 years of age and had undergone transplant over 10 years ago. Early data from the two combined international registries (up to April 20, 2020) included 39 individuals with 9 (24%) deaths [40]. Age, duration of transplant, and treatment agents used did not appear to influence mortality risk. More data from the registry accrued over time would, however, clarify the issue.
Table 1.
Mortality outcomes in reported cases of Corona Virus Disease-19 (COVID-19) among liver transplant centers in different countries and the combined international registries*
| Author/s | Country | Number of cases | Mortality (%) |
|---|---|---|---|
| Gruttadauria et al. [47] | Italy | 24 | 5 (21%) |
| Fernandez-Ruiz et al. [48] | Spain | 6 | 2 (33%) |
| Pereira et al. [49] | USA | 13 | NA |
| Tschopp et al. [50] | Switzerland | 5 | 0 |
| Bhoori et al. [39] | Italy | 6 | 3 (50%) |
| Webb et al.** | Multicenter registry | 122 | 23 (19%) |
*Isolated case reports are excluded from this Table
**The multicenter international registry is updated up to May 26, 2020 (https://covidcirrhosis.web.unc.edu/)
Does the occurrence of intercurrent COVID-19 impact the manifestations, course, outcome, and treatment of premorbid GI disorders?
Theoretically though, even with the pathophysiological inter-relatedness of COVID-19 and other GI disorders through the ACE-2 receptors, there are scanty evidence to suggest that the occurrence of intercurrent COVID-19 influences the manifestations of pre-existing chronic GI disorders [41, 42]. In the two combined international registries of the 288 cases with hepatic cirrhosis included until May 26, 2020, 43% experienced decompensation in the form of increased ascites, spontaneous bacterial peritonitis, or worsening encephalopathy grades during COVID-19 episodes. Variceal hemorrhage was reported in only about 1%. Worsening jaundice was reported in nearly 18% of patients in the registry. A preliminary analysis of the registry data (until April 20, 2020) demonstrated an association between decompensation of liver cirrhosis and mortality [43]. Most deaths in individuals with liver cirrhosis and COVID-19 episodes were still attributed to respiratory failure with only 12% of deaths being liver-related. Nonetheless, treating physicians should be mindful of the possibility of decompensation of liver cirrhosis during COVID-19 episodes. In the current issue of the journal, a single-center study from Delhi, India showed poor outcome of cirrhosis patients with COVID-19 [38].
An isolated case of hemorrhagic colitis in an individual with COVID-19, who was previously using lisinopril, has been described [29]. It would be expected that some individuals with IBD might experience flare-ups as natural events during their disease course [44]. These should, however, not be attributed to co-incident COVID-19. Lastly, however, the isolation of the virus from fecal samples has implications for fecal microbiota transplants [45, 46]. Although presently undocumented, COVID-19 might potentially spread during fecal microbiota transplants, and hence, it might be prudent to demonstrate negative viral RT-PCR multiple times in donor samples before accepting fecal samples for transplant.
Our understanding of the manifestations and comorbidities of COVID-19 are rapidly evolving and are likely to advance as more data emerge. At the moment, however, healthcare systems across the world are overwhelmed by care provision to critically ill individuals with COVID-19. This precludes systematic analyses of clinical manifestations especially in relation to comorbid conditions. The creation of web-based international platforms by GI specialists to pool in rare presentations and the effect of COVID-19 on rare premorbid GI disorders will be immensely useful in sorting out some of the issues and is a welcome step. Many unanswered questions will perhaps be suitably addressed with time.
Compliance with ethical standards
Conflict of interest
ANSB, VM, RM, and AS declare that they have no conflict of interest.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coronavirus: China’s first confirmed Covid-19 case traced back to November 17, 2019. South China Morning Post.
- 2.Coronavirus Disease (COVID-19) - events as they happen [Internet]. [cited 2020 June 29]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 3.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus ADME, Voort PHJ, Mulder DJ, Goor H. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. [DOI] [PubMed]
- 6.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. 10.1001/jama.2020.4344. [DOI] [PubMed]
- 9.Kumar A, Arora A, Sharma P, et al. Gastrointestinal and hepatic manifestations of Corona Virus Disease-19 and their relationship to severe clinical course: A systematic review and meta-analysis. Indian J Gastroenterol. 2020;39. 10.1007/s12664-020-01058-3. [DOI] [PMC free article] [PubMed]
- 10.Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection. J Crohns Colitis. 2020:jjaa061. [DOI] [PMC free article] [PubMed]
- 11.Repici A, Maselli R, Colombo M, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192–7. [DOI] [PMC free article] [PubMed]
- 12.Ang TL, Li JW, Vu CK, et al. Chapter of gastroenterologists professional guidance on risk mitigation for gastrointestinal endoscopy during COVID-19 pandemic in Singapore. Singapore Med J. 2020. 10.11622/smedj.2020050. [DOI] [PMC free article] [PubMed]
- 13.Chiu PWY, Ng SC, Inoue H, Reddy DN, Ling Hu E, Cho JY, Ho LKY, Hewett DG, Chiu HM, Rerknimitr R, Wang HP, Ho SH, Seo DW, Goh KL, Tajiri H, Kitano S, Chan FKL. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92:612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotfis K, Skonieczna-Żydecka K. COVID-19: gastrointestinal symptoms and potential sources of SARS-CoV-2 transmission. Anaesthesiol Intensive Ther. 2020;52:171–2. [DOI] [PMC free article] [PubMed]
- 19.Qian G, Yang N, Ma AHY, et al. A COVID-19 transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis. 2020. 10.1093/cid/ciaa316. [DOI] [PMC free article] [PubMed]
- 20.Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020;16:251–9. [DOI] [PMC free article] [PubMed]
- 21.Hong H, Wang Y, Chung HT, Chen CJ. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. 2020;61:131–2. [DOI] [PMC free article] [PubMed]
- 22.Neurath MF. Covid-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao S, Lau A, So HC. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;43:1416–1426. doi: 10.2337/dc20-0643. [DOI] [PubMed] [Google Scholar]
- 24.Burgueño JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, Santander AM, Brito N, Damas OM, Deshpande A, Kerman DH, Zhang L, Gao Z, Ban Y, Wang L, Pignac-Kobinger J, Abreu MT. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26:797–808. doi: 10.1093/ibd/izaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairbrass KM, Hoshen D, Gracie DJ, Ford AC. Effect of ACE inhibitors and angiotensin II receptor blockers on disease outcomes in inflammatory bowel disease. Gut. 2020:gutjnl-2020-321186. [DOI] [PubMed]
- 26.Bindoli S, Felicetti M, Sfriso P, Doria A. The amount of cytokine-release defines different shades of Sars-Cov2 infection. Exp Biol Med (Maywood). 2020;245:970–6. [DOI] [PMC free article] [PubMed]
- 27.Albini A, Di Guardo G, Noonan DM, Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med. 2020;19;1–8. [DOI] [PMC free article] [PubMed]
- 28.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho A, Alqusairi R, Adams A, et al. SARS-CoV-2 gastrointestinal infection causing haemmorrhagic colitis; implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115:942–6. [DOI] [PMC free article] [PubMed]
- 30.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–1590. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CC, Wang CY, Wang YH, et al. Global epidemiology of coronavirus disease 2019: disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55:105946. [DOI] [PMC free article] [PubMed]
- 34.Hanzel J, Ma C, Marshall JK, Feagan BG, Jairath V. Managing inflammatory bowel disease during COVID-19: summary of recommendations from gastrointestinal societies. Clin Gastroenterol Hepatol. 2020;18:2143–6. [DOI] [PMC free article] [PubMed]
- 35.Turner D, Huang Y, Martín-de-Carpi J, Aloi M, Focht G, Kang B, Zhou Y, Sanchez C, Kappelman MD, Uhlig HH, Pujol-Muncunill G, Ledder O, Lionetti P, Dias JA, Ruemmele FM, Russell RK. COVID-19 and paediatric inflammatory bowel diseases: global experience and provisional guidance (march 2020) from the Paediatric IBD Porto group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2020;70:727–733. doi: 10.1097/MPG.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: Expert commentary. Gastroenterology. 2020;S0016-5085(20)30482-0. [DOI] [PMC free article] [PubMed]
- 37.Remzi FH, Panis Y, Spinelli A, Kotze PG, Mantzaris G, Söderholm JD, d’Hoore A, Bemelman WA, Yamamoto T, Pemberton JH, Tiret E, Øresland T, Fleshner P. International organization for the Study of Inflammatory Bowel Disease recommendations for surgery in patients with inflammatory bowel disease during the COVID-19 pandemic. Dis Colon Rectum. 2020;63:870–873. doi: 10.1097/DCR.0000000000001718. [DOI] [PubMed] [Google Scholar]
- 38.Shalimar, Elhence A, Vaishnav M, et al. Poor Outcomes in Patients with Cirrhosis and COVID-19. Indian J Gastroenterol. 2020; 39. 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed]
- 39.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong J, Young BE, Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69:1144–1145. doi: 10.1136/gutjnl-2020-321051. [DOI] [PubMed] [Google Scholar]
- 42.Ungaro RC, Sullivan T, Colombel JF, Patel G. What should gastroenterologists and patients know about COVID-19. Clin Gastroenterol Hepatol. 2020;18:1409–1411. doi: 10.1016/j.cgh.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;S0168-8278(20)30305-6. [DOI] [PMC free article] [PubMed]
- 44.Rosen MH, Axelrad J, Hudesman D, Rubin DT, Chang S. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis. 2020;26:971–973. doi: 10.1093/ibd/izaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sood A, Singh A, Mahajan R, Midha V, Mehta V, Gupta YK, Narang V, Kaur K. Acceptability, tolerability, and safety of fecal microbiota transplantation in patients with active ulcerative colitis (AT&S Study) J Gastroenterol Hepatol. 2020;35:418–424. doi: 10.1111/jgh.14829. [DOI] [PubMed] [Google Scholar]
- 46.Ianiro G, Mullish BH, Kelly CR, Sokol H, Kassam Z, Ng SC, Fischer M, Allegretti JR, Masucci L, Zhang F, Keller J, Sanguinetti M, Costello SP, Tilg H, Gasbarrini A, Cammarota G. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruttadauria S; Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, The Italian Society of Organ Transplantation (SITO). Preliminary Analysis of the Impact of the Coronavirus Disease 2019 Outbreak on Italian Liver Transplant Programs. Liver Transpl. 2020;26(7):941–944. [DOI] [PMC free article] [PubMed]
- 48.Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, González E, Polanco N, Folgueira MD, Lalueza A, Lumbreras C, Aguado JM. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS, Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infection in solid organ transplant recepients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;10.1111/ajt.16062. 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed]