Abstract
Background
Although ST-segment elevation (STE) has been used synonymously with acute coronary occlusion (ACO), current STE criteria miss nearly one-third of ACO and result in a substantial amount of false catheterization laboratory activations. As many other electrocardiographic (ECG) findings can reliably indicate ACO, we sought whether a new ACO/non-ACO myocardial infarction (MI) paradigm would result in better identification of the patients who need acute reperfusion therapy.
Methods
A total of 3000 patients were enrolled in STEMI, non-STEMI and control groups. All ECGs were reviewed by two cardiologists, blinded to any outcomes, for the current STEMI criteria and other subtle signs. A combined ACO endpoint was composed of peak troponin level, troponin rise within the first 24 h and angiographic appearance. The dead or alive status was checked from hospital records and from the electronic national database.
Results
In non-STEMI group, 28.2% of the patients were re-classified by the ECG reviewers as having ACO. This subgroup had a higher frequency of ACO, myocardial damage, and both in-hospital and long-term mortality compared to non-STEMI group. A prospective ACOMI/non-ACOMI approach to the ECG had superior diagnostic accuracy compared to the STE/non-STEMI approach in the prediction of ACO and long-term mortality. In Cox-regression analysis early intervention in patients with non-ACO-predicting ECGs was associated with a higher long-term mortality.
Conclusions
We believe that it is time for a new paradigm shift from the STEMI/non-STEMI to the ACOMI/non-ACOMI in the acute management of MI. (DIFOCCULT study; ClinicalTrials.gov number, NCT04022668.)
Keywords: Coronary occlusion, Electrocardiogram, Myocardial infarction, Percutaneous coronary intervention, ST-segment elevation
Abbreviations: ACO, acute coronary occlusion; CABG, coronary artery by-pass grafting; ECG, electrocardiogram; GRACE, The Global Registry of Acute Coronary Events; MI, myocardial infarction; PCI, percutaneous coronary intervention; STE, ST-segment elevation
1. Introduction
Acute reperfusion of the occluded coronary arteries is one of the most impressive advancements in the whole history of medicine [1]. Prior to the discovery of thrombolytics, clinicians had to observe the patients while they were completing their myocardial infarction (MI) and then used to classify them according to whether their subsequent electrocardiogram (ECG) developed the Q-waves. After large-scale thrombolytic trials showed a clear survival benefit, especially in patients showing ST-segment elevation (STE), the paradigm shifted from this passive “Q-wave/non-Q-wave MI” to more active “STE-MI/non-STE-MI” [2], [3]. Although these studies had not utilized coronary angiography, the term “STEMI” spontaneously became synonymous with acute coronary occlusion or near-occlusion (ACO) that necessitates acute reperfusion and it continues to be used as such in the international guidelines [4], [5], [6], [7], [8], [9]. Surprisingly, such a connection has never been explored in a dedicated trial.
In reality, STEMI criteria have a limited diagnostic accuracy for ACO, causing a substantial amount of false catheterization laboratory activations [7], [8], [9], more importantly, missing nearly one-third of ACO [10], [11], [12], [13], [14], [15], [16] and causing this unfortunate group of patients, labeled as non-STEMI, to be deprived of emergent reperfusion therapy, just as they were in the old days of Q-wave/non-Q-wave MI approach.
Accordingly, several authors called for a new paradigm shift from STEMI/non-STEMI to ACO-MI/non-ACO-MI [17], [18], [19], as ACO can be reliably recognized with the help of many other ECG findings, such as minor STE not fulfilling STEMI criteria [18], STE disproportionate to preceding QRS [19], [20], unusual patterns with contiguous leads showing opposite ST deviations [21] and some patterns not showing STE at all [22], [23]. However, it is uncertain whether this new approach would result in better identification of the patients who need acute reperfusion therapy and/or whether the ECG has sufficient diagnostic power to go beyond established STEMI criteria. The objective of this study is to provide answers to these critical questions.
2. Methods
2.1. Patient population
The study was undertaken at Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital, Istanbul, a tertiary center for primary percutaneous coronary intervention (PCI). An institutional review board approval was obtained.
Between May 2017 and December 2018, adult patients admitted to the emergency department with a clinical picture suggestive of acute coronary syndrome were scanned (by B.Ş.). Consecutive patients with an initial diagnosis of MI according to the fourth universal definition of MI, who had a technically adequate admission ECG, were enrolled in STEMI and non-STEMI groups, according to whether their admission ECGs fulfilling STEMI criteria or not, until 1000 patients were allocated to both groups [4]. A computer-generated random date list was used to enroll another 1000 patients as the control group, who had been excluded for acute coronary syndrome with serial unchanging ECGs and negative serial troponins for at least 12-hours after beginning of symptoms. To define the respective frequency of each individual clinical presentation (STEMI, non-STEMI, control), we also reckoned the total admission rates during the allocation period for weighted analysis.
2.2. Study protocol
Baseline characteristics were obtained via chart review. GRACE risk score at admission was calculated retrospectively [24]. Troponin I Abbott c4100i (Abbott Diagnostics, Chicago, IL, USA) was used as the troponin assay. Admission troponin was defined as the first troponin obtained at the emergency department or catheterization laboratory; before, during or immediately after cardiac catheterization. In addition to peak troponin level, a 24 to 48-hour troponin level was also sought, as it was shown to be better correlated with infarct size [25]. Coronary angiograms were performed via femoral route. Routine echocardiographic assessment was performed in the first 24-hours of admission, predominantly after revascularization. All patients were given guideline-recommended contemporary treatment.
All ECGs were randomly sorted and then reviewed by two cardiologists (E.A., M.A.Ş.), who were blinded to the angiographic and clinical outcomes. After the calculation of interobserver variability, a final composite evaluation by two reviewers was undertaken. Any disagreement was resolved with the help of a third cardiologist (M.D.). Also, one of the ECG reviewers (E.A.) reviewed all ECGs twice, three months apart, for the assessment of intra-observer variability. Using 12-lead information and a set of predefined ECG findings [18], [19], [20], [21], [22], [23] the reviewers also attempted to predict whether an ACO was present and classified ECGs into a diagnostic category as defined previously (Table S1) [26]. If present and needed, ECGs with additional leads were also included in the analysis. Type Ic ECGs (Fig. S1) were allowed to be reclassified as type 1b, if additional criteria were positive [27], [28], [29], [30]. An ECG was only deemed to be compatible with ACO when it is classified as type 1a or 1b.
The coronary angiograms were reviewed by two interventional cardiologists (Ö.Y., E.B.), who were blinded to the ECGs. Any disagreement was resolved by a third cardiologists’ opinion (C.Y.K.). The presence of culprit lesion was defined based on several angiographic properties including appearance, presence of angiographic thrombus or critical stenosis with less than Thrombolysis in Myocardial Infarction 3 flow. In the case of total occlusion, the absence of collaterals and easiness of crossing the lesion with guidewire were also used in the decision about the acute or chronic nature of total occlusion.
2.3. Endpoints
Because the artery may spontaneously open by the time of the angiogram [31] or total occlusion may be chronic in nature, we defined a composite ACO using following criteria: (1) total occlusion or presence of culprit lesion on angiography with a peak troponin I level equal to or greater than 1.0 ng/ml plus an at least 20% rise within 24 h [4] or (2) a highly elevated peak troponin (greater than 5.0 ng/mL), which was shown to be correlated with ACO [32] or (3) cardiac arrest before any troponin rise has been documented with supporting clinical evidence of possible ACO [4]. Each individual’s vital status was checked from hospital records for all cause in-hospital mortality and from the electronic national database for all-cause long-term mortality.
2.4. Statistical analysis
We estimated that the enrollment of 963 patients in non-STEMI group would provide the study with a statistical power of 95% to detect a relative excess mortality rate of 5% in ACOMI subgroup (from 10% to 15%) with the use of a two-sided test at the 0.05 level, with a frequency prediction of 25% for ACOMI. Fewer patients would be necessary to detect a 10% difference in the area under curve (AUC, from 0.700 to 0.770) for the comparison of diagnostic accuracy of two approaches in predicting ACO and long-term mortality (267 and 383, respectively).
Baseline characteristics were summarized using standard descriptive statistics. Comparisons of relevant parameters between groups were performed by chi-square, Mann-Whitney U, Kruskal-Wallis H test, one-way ANOVA and student t-test, as appropriate. Patients with missing values were excluded pairwise from analyses. Kaplan-Meier analysis was performed to determine the cumulative long-term mortality rates in different ECG subgroups, which were then compared using the log-rank test. A Cox-regression model was used to perform a survival analysis according to intervention timing and revascularization status. Baseline characteristics with a P-value of 0.05 or less in the univariate analysis were included and a step-down procedure was applied for the selection of final covariates. A Cohen's κ test was run to determine the intra- and inter-observer agreement for ECG classifications. The sensitivity, specificity and diagnostic accuracy of STE/non-STE or ACO/non-ACO-ECG approaches were calculated using receiver operating characteristics analysis. These calculations were repeated after weighing cases for the total number of hospital admissions. Statistical analyses were performed with SPSS (version 24.0; SPSS Inc., Chicago, IL) and MedCalc Software (version 18.2.1 [Evaluation version]; MedCalc Software, Ostend, Belgium).
3. Results
During the study period, there were 1152 STEMI and 2353 non-STEMI admissions to the emergency department, whereas 15,510 patients with a clinical picture suggestive of acute coronary syndrome were ruled-out by serial troponin follow-up. One-thousand patients of each presentation group were included. Baseline characteristics of the patients were summarized at Table S2. Missingness rate was very low for primary objectives.
3.1. Detection of ACO in non-STEMI group
In non-STEMI group, 282 patients (28.2%) were re-classified by the ECG reviewers as having ACO on the basis of their type 1b ECGs. Intra-observer (κ = 0.944; 95% confidence interval [CI], 0.934 to 0.954; P < 0.001) and inter-observer agreement (κ = 0.834; 95% CI, 0.818 to 0.850; P < 0.001) for detecting these subtle ECGs was very good. The reason for type 1b ECG was minor STE with reciprocal ST-depression in 215 (76.2%) (Fig. S2) [18], hyperacute T-waves or de Winter’s pattern in 35 (12.4%) (Fig. S3) [22], [23], subtle anterior STE in 18 (6.3%) [19], [20] and nonconsecutive STE in 14 (4.9%) of the patients [21]. The reason for less pronounced ECG changes in this group may partly be explained by the more limited infarct size as indicated by the lower 24 to 48-hour troponin I level (5.703 [IQR 19.347] ng/ml vs. 32.990 [43.356] ng/ml in STEMI-group, P < 0.001) and higher left ventricular ejection fraction (50% [IQR 20%] vs. 45% [20%], respectively; P < 0.001), and/or involvement of an electrocardiographically silent area as indicated by the more frequent involvement of the circumflex artery as the infarct-related artery (27.9% vs. 17.8%, respectively, P = 0.001).
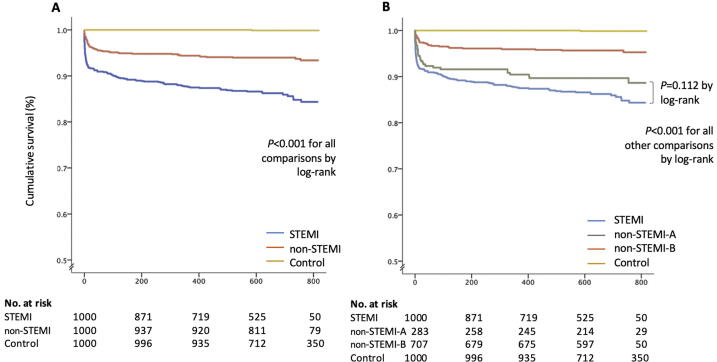
When this subgroup of all Non-STEMI was classified as “non-STEMI subgroup-A” and the remaining non-STEMI patients were reclassified as “non-STEMI subgroup-B”, non-STEMI subgroup-A was more similar to the STEMI group rather than to non-STEMI subgroup-B in terms of the frequency of ACO (85.3% in STEMI group vs. 60.9% in non-STEMI subgroup-A, P < 0.001 and 25.3% in non-STEMI subgroup-B, P for difference with non-STEMI subgroup-A < 0.001) and in terms of myocardial damage measured, as by 24- to 48-hour troponin I (32.990 [IQR 43.356] ng/ml vs. 5.703 [19.347] ng/ml, P < 0.001 and 0.622 [3.112] ng/ml, P < 0.001; respectively), although their baseline characteristics were similar (Table 1). More importantly, in-hospital and long-term mortality rates in non-STEMI subgroup-A was similar to STEMI group (8.3% vs. 5.0%, P = 0.073 and 13.7% vs. 10.6%, P = 0.188, respectively), but significantly higher than non-STEMI subgroup-B (5.0%, vs 1.8%, P = 0.009 and 10.6% vs. 4.4%, P = 0.001, respectively) (Table 2 and Fig. 1).
Table 1.
Baseline characteristics*.
| STEMI (N = 1000) |
NSTEMI (N = 1000) |
Control (N = 1000) |
P-value† | ||
|---|---|---|---|---|---|
| Characteristic | NSTEMI Subgroup A (n = 282) |
NSTEMI Subgroup B (n = 718) |
|||
| Age - years | 61 ± 13 | 61 ± 13 | 61 ± 13 | 48 ± 16 | 0.383 |
| Male sex – no. (%) | 757 (76) | 200 (71) | 466 (65) | 646 (65) | 0.069 |
| Medical history – no./total no. (%) | |||||
| Hypertension | 481 (48) | 156 (55) | 418 (58) | 195 (20) | 0.405 |
| Diabetes | 292 (29) | 107 (38) | 264 (37) | 83 (8) | 0.730 |
| Dyslipidemia | 276 (27) | 262 (36) | 207 (20) | 207 (21) | 0.826 |
| Current smoker | 514 (51) | 123 (44) | 292 (41) | 483 (48) | 0.371 |
| Prior MI | 185 (19) | 74 (26) | 200 (28) | 89 (9) | 0.628 |
| Prior PCI | 150 (15) | 54 (19) | 159 (22) | 111 (11) | 0.298 |
| Prior CABG | 55 (5) | 30 (11) | 66 (9) | 63 (6) | 0.485 |
| Clinical parameters | |||||
| Systolic blood pressure - mmHg | 136 ± 33 | 146 ± 34 | 146 ± 28 | 139 ± 24 | 0.971 |
| Heart rate – min.−1 | 80 (27) | 81 (24) | 81 (26) | 77 (19) | 0.618 |
| ECG to PCI time – min. | 40 (42) | 360 (2834) | 2760 (4800) | N/A | <0.001 |
| Killip Class, – no. (%) | 0.386 | ||||
| 1 | 906 (91) | 260 (92) | 672 (94) | 1000 (1 0 0) | |
| 2 | 16 (2) | 8 (3) | 10 (1) | 0 (0) | |
| 3 | 36 (4) | 8 (3) | 30 (4) | 0 (0) | |
| 4 | 35 (4) | 6 (2) | 5 (1) | 0 (0) | |
| GRACE risk score | 147 (42) | 142 (33) | 142(41) | 129 (44) | 0.573 |
| Laboratory investigations | |||||
| Creatinine – mg/dl | 0.8 (0.3) | 0.9 (0.3) | 0.9 (0.4) | 0.8 (0.2) | 0.260 |
| Hemoglobin – g/dl | 13.7 (2.5) | 13.3 (2.7) | 13.3 (2.7) | 13.9 (2.5) | 0.908 |
| Admission troponin I – ng/ml | 3.462 (19.555) | 0.700 (3.119) | 0.356 (1.546) | 0.002 (0.003) | <0.001 |
CABG, coronary artery by-pass grafting; ECG, electrocardiogram; GRACE, Global Registry of Acute Coronary Events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Values are mean ± standard deviation or median (interquartile range).
P value is for comparisons between non-STEMI group-A and -B.
Table 2.
Distribution of coronary involvement endpoints across groups.
| STEMI (N = 1000) |
non-STEMI (N = 1000) |
Control (N = 1000) |
P-value* | ||
|---|---|---|---|---|---|
| Subgroup A (n = 282) |
Subgroup B (n = 718) |
||||
| ECG type – no. (%) | <0.001 | ||||
| 1a | 767 (77) | 0 (0) | 0 (0) | 0 (0) | |
| 1b | 0 (0) | 282 (1 0 0) | 0 (0) | 16 (2) | |
| 1c | 123 (12) | 0 (0) | 3 (1) | 64 (6) | |
| 1d | 110 (11) | 0 (0) | 0 (0) | 6 (1) | |
| 2 | 0 (0) | 0 (0) | 296 (41) | 54 (5) | |
| 3 | 0 (0) | 0 (0) | 318 (44) | 260 (26) | |
| 4 | 0 (0) | 0 (0) | 101 (14) | 600 (60) | |
| Troponin level – ng/dl | |||||
| Admission troponin | 3.463 (19.550) | 0.700 (3.119) | 0.356 (1.546) | 0.002 (0.003) | <0.001 |
| 24–48 h troponin | 32.990 (43.356) | 5.703 (19.347) | 0.622 (3.112) | 0.002 (0.003) | <0.001 |
| Peak troponin | 34.873 (42.473) | 6.893 (20.803) | 1.135 (4.589) | 0.002 (0.003) | <0.001 |
| 20% increase within first 24–48 h | 739/851 (57) | 213/261 (82) | 347/705 (49) | 7/1000 (1) | <0.001 |
| Angiographic involvement – no./total no. (%) | |||||
| LMCA | 34/909 (4) | 18/246 (7) | 23/574 (4) | 0/2 (0) | 0.931 |
| LAD | 578/909 (64) | 174/246 (71) | 302/574 (53) | 1/2 (50) | <0.001 |
| Cx | 377/909 (41) | 137/246 (56) | 279/574 (49) | 1/2 (50) | 0.121 |
| RCA | 523/909 (58) | 137/246 (56) | 275/574 (47) | 1/2 (50) | <0.001 |
| IRA – no./total no. (%) | 0.009 | ||||
| LMCA | 9/872 (1) | 6/215 (3) | 4/425 (1) | N/A | |
| LAD | 369/872 (42) | 83/215 (39) | 138/425 (33) | N/A | |
| Cx | 155/872 (18) | 60/215 (28) | 134/425 (32) | N/A | |
| RCA | 325/872 (37) | 61/215 (28) | 105/425 (21) | N/A | |
| Culprit plaque | 818/890 (92) | 136/226 (60) | 166/534 (31) | N/A | <0.001 |
| Angiographic ACO | 558/909 (61) | 73/245 (30) | 95/574 (17) | N/A | <0.001 |
| Echocardiography | |||||
| Ejection fraction – % | 45 (20) | 50 (20) | 50 (20) | 60(5) | <0.001 |
| Composite ACO endpoint – no./total no. (%) | |||||
| 833/977 (85) | 170/279 (61) | 179/708 (25) | 0/1000 (0) | <0.001 | |
| Mortality – no./total no. (%) | |||||
| In-hospital mortality | 83/1000 (8) | 14/282 (5) | 13/718 (2) | 0/1000 (0) | <0.001 |
| Long-term mortality | 135/986 (14) | 29/274 (11) | 31/699 (4) | 1/1000 (0) | <0.001 |
| Follow-up, days | 610 (3 8 1) | 676 (1 7 7) | 688(1 5 3) | 781 (3 5 1) | <0.001 |
ACO, acute coronary occlusion; Cx, circumflex artery; ECG; electrocardiogram; LAD, left anterior descending artery; LMCA, left main coronary artery; IRA, infarct-related artery; RCA, right coronary artery.
P values for comparisons among the first three groups, since P-value is always < 0.001 when the control group is included.
Fig. 1.
Cumulative survival according to presentation groups. Kaplan-Meier estimates of the cumulative survival according to groups are presented, first non-ST-elevation myocardial infarction (STEMI) group as a whole (Panel A), and then as divided into two according to the presence of an ACO-predicting ECG (non-STEMI-A) or not (non-STEMI-B) (Panel B).
3.2. STEMI/non-STEMI vs. ACOMI/non-ACOMI approach to the ECG
The ECG reviewers prospectively classified 35.6% (1070/3000) of ECGs as STEMI and 35.5% (1066/3000) of ECGs as ACOMI; 25.6% (769/3000) being shared in the both MI definitions. Both unweighted and weighted (corrected for admission rates of STEMI/non-STEMI/control) sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively) of STEMI/non-STEMI and ACOMI/non-ACOMI approaches for ACO and long-term mortality were presented in Table 3. The diagnostic accuracy of the ACOMI/non-ACOMI approach was superior to the STEMI/non-STEMI approach in three out of four comparisons.
Table 3.
Sensitivity, specificity, positive and negative predictive values of both approaches for acute coronary occlusion and long-term mortality.*
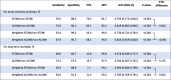 |
ACO, acute coronary occlusion; AUC, area under curve; CI, confidence interval; MI, myocardial infarction; NPV, negative predictive value; PPV, positive predictive value; STE, ST-segment elevation.
Weighted values were corrected for the real admission rates of each group (STEMI, non-STEMI, and control).
3.3. The effect of intervention timing according to ECG
Since the main difference between two approaches originates from ECG subtypes covered by these definitions, we sought to compare ECG subtypes according to early (ECG-to-PCI time less than 120 min) and late (ECG-to-PCI time equal or greater than 120 min) coronary intervention. In Cox-regression models for comparing mortality according to intervention timing or whether the patient underwent revascularization, the models only included baseline GRACE score as a covariate, since GRACE risk score single-handedly outperformed the inclusion of the baseline factors, including age, gender, systolic blood pressure, heart rate, creatinine, etc. Early intervention in patients with type 1a ECG was associated with a significantly lower long-term mortality compared to late intervention after controlling for baseline GRACE risk score (HR, 0.47; 95% CI 0.23 to 0.97; P = 0.042), however, this was not valid for the patients with type 1b (HR, 0.85; 95% CI 0.26 to 2.75; P = 0.796) or other ECG subtypes. When other ECG subgroups were combined and compared with type 1a and type 1b ECGs, early intervention in non-ACO-predicting ECGs was associated with an increased long-term mortality risk, even after correcting for baseline GRACE risk scores (HR, 2.81; 95% CI 1.10 to 7.12; P = 0.030) (Table S3). Coronary revascularization, either with PCI or coronary artery by-pass grafting (CABG), reduced mortality dramatically from 47.1% (32/68) to 12.0% (83/690) in type 1a ECGs (HR 0.21, 95% CI 0.12 to 0.35; P < 0.001). It did not reach statistical significance in patients with type 1b ECGs (14.7%[14/95] vs. 7.7% [15/196]; HR 1.07, 95% CI 0.40 to 2.84, P = 0.887) and others (2.1%[29/1329] vs. 4.3% [23/531]; HR 1.86, 95% CI 0.99 to 3.48, P = 0.051), despite showing a trend to the opposite directions. The results were similar when only patients with composite ACO endpoint were included.
4. Discussion
Our results indicate that ACOMI/non-ACOMI approach can consistently recognize a high-risk subgroup in the non-STEMI population having a higher frequency of ACO, larger infarct size, and higher short- and long-term mortality. Less pronounced ECG changes in this group may be partly explained by the more limited infarct size and/or involvement of an electrocardiographically silent area, but this was not always the case.
The ACOMI/non-ACOMI approach has a significantly superior diagnostic accuracy in the prediction of ACO and long-term mortality compared to the STEMI/non-STEMI approach. Furthermore, since STEMI criteria do not exclude spontaneously reperfused or subacute MIs (type 1d ECGs), and many of these patients have positive composite ACO endpoint and high mortality rates despite theoretically not necessitating acute reperfusion, the real impact of the new paradigm might be underestimated.
An interesting finding came forward when we attempted to further delineate this difference by examining the relationship between intervention timing and mortality according to ECG subgroups. Early intervention (ECG-to-PCI time < 120 min.) in patients with type 1a ECG was associated with significantly lower long-term mortality compared to late intervention (ECG-to-PCI time ≥ 120 min.), even after controlling for baseline GRACE risk score. For type 1b ECGs, mortality seems unchanged according to the intervention timing. Coronary revascularization, either with PCI or coronary artery by-pass grafting (CABG), reduced mortality in type 1a ECGs but it did not reach statistical significance in patients with type 1b ECGs. These findings should be interpreted with caution because some of the patients with subtle ECG changes had a large MI and, for the time being, there is no reliable way of differentiating these patients from the patients with a more limited area-at risk that would negate the benefits of early intervention. Moreover, this is a post hoc analysis and our study is not adequately powered to detect a modest benefit from early intervention over late intervention.
On the other hand, non-ACO-predicting ECG types showed significant harm with early intervention. This previously unreported finding is interesting, but not unexpected. Revascularization in stable coronary artery disease is known to be associated with better outcomes in patients with moderate-to-severe ischemia, but with worse prognosis in patients with mild or no ischemia [33], [34], [35]. Although we did not attempt to measure ischemic area in our patients, a non-ACO-predicting ECG may be a reflection of a limited area-at-risk and less benefit from emergent revascularization. Furthermore, the presence of destabilized plaque and/or vulnerable myocardium, and time lag of adjunctive therapy may create an even more susceptible environment to periprocedural myocardial injury. Therefore, a critical amount of salvageable myocardium may be necessary for gaining benefit from intervention during an acute ischemic event. Further studies are needed to clarify this issue.
In addition to our results, we believe that there are other reasons compelling the need for a transformation from STEMI/non-STEMI to ACOMI/non-ACOMI paradigm. Universally recommended STEMI criteria come from the studies designed for discriminating biomarker-positive MI (mainly CK-MB) from benign-variant STE, in which neither coronary angiography had been utilized nor ACO had been sought for [36], [37], [38], [39]. Our study, for the first time, validates the diagnostic accuracy of 20-year-old STEMI concept, with STE as a surrogate for the physiologic reality of ACO, which is really meant by the term “STEMI”. However, the term STEMI restricts our thinking to the point that it is only the ST-segment that matters for the reperfusion decision, with no consideration of any other ECG variables, such as the preceding QRS-complex, the T-wave, or even the morphology of ST-segment itself. Recent studies clearly indicated that any STE should be interpreted in the context of other ECG variables [19], [20]. Additionally, the term STEMI is somewhat self-contradictory, since a patient without STE on ECG, but ACO on the angiogram, is still classified as non-STEMI. But ACOMI/non-ACOMI definition is not limited to ECG and permits retrospective reclassification of these patients. Lastly, it is important to note that our data indicate this new paradigm still misses 17.9% ACOs in the non-STEMI group. These patients are currently regarded as a high-risk subgroup of non-STEMI, but presumably have the same underlying pathophysiology with STEMI patients. Labeling these patients as non-STEMI potentially hinders the discovery of new ECG patterns or the quest for better diagnostic approaches. Moreover, the diagnosis of ACO-MI is not limited to the ECG, as it may also be made with other modalities when the ECG is nondiagnostic, but clinical suspicion is high; such as other biomarkers, echocardiography, computed tomography, or conventional angiography itself.
Our study has several limitations. This is a retrospective study and susceptible to bias. Intra- and interobserver agreement on ECG classifications may change significantly according to the experience of ECG interpreters. An obstacle to the widespread application of the ACOMI/non-ACOMI concept is its dependence on better ECG interpreting skills, which may be hard to achieve in the real clinical world, but this is an unavoidable necessary step for improvement. Improved computer interpretation algorithms, especially use of neural networks [40], may partly overcome this issue. As a universally agreed definition of ACO is not present, we created our own arbitrary definition. STEMI/non-STEMI/control ratio shows variation among hospitals, therefore calculated PPV and NPV may change accordingly. Although all patients were intended to take guideline-recommended contemporary treatment, this process was not controlled and the timing, the duration and the percentage of used drugs might have influenced the outcomes. We divided intervention timing according to ECG-to-PCI time, but this was not a randomized process, and the decision to undergo catheterization early or late might be influenced by multiple factors, although correction for baseline risk was performed.
In conclusion, ECG can reliably detect ACO in patients not fulfilling STEMI criteria. The ACOMI/non-ACOMI approach results in better identification of ACO and long-term mortality compared to the STEMI/non-STEMI approach. On the other hand, undergoing catheterization before scrutinizing the subtleties of ECG may be equally hazardous as waiting for full-blown STEMI criteria to develop. We believe that it is time for a new paradigm shift from STEMI/non-STEMI to ACOMI/non-ACOMI in the acute management of MI.
CRediT authorship contribution statement
Emre K.Aslangera: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Özlem Yıldırımtürk: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing - review & editing. Barış Şimşek: Data curation, Methodology, Project administration, Validation, Visualization, Writing - review & editing. Emrah Bozbeyoğlu: Data curation, Methodology, Project administration, Validation, Visualization, Writing - review & editing. Mustafa Aytek Şimşek: Data curation, Methodology, Project administration, Validation, Visualization, Writing - review & editing. Can Yücel Karabay: Data curation, Methodology, Project administration, Validation, Visualization, Writing - review & editing. Stephen W. Smith: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Muzaffer Değertekina: Data curation, Methodology, Project administration, Validation, Visualization, Writing - review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100603.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Van deWerf F. The history of coronary reperfusion. Eur. Heart J. 2014;35:2510–2515. doi: 10.1093/eurheartj/ehu268. [DOI] [PubMed] [Google Scholar]
- 2.Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomized trials of more than 1000 patients. Lancet 1994; 343: 311-22 [PubMed]
- 3.Braunwald E., Antman E.M., Beasley J.W. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) J. Am. Coll. Cardiol. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K., Alpert J.S., Jaffe A.S. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 5.O'Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez B., James S., Agewall S. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 7.McCabe J.M., Armstrong E.J., Kulkarni A. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention-capable centers: a report from the Activate-SF registry. Arch. Intern. Med. 2012;172:864–871. doi: 10.1001/archinternmed.2012.945. [DOI] [PubMed] [Google Scholar]
- 8.Larson D.M., Menssen K.M., Sharkey S.W. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 9.Kontos M.C., Kurz M.C., Roberts C.S. An evaluation of the accuracy of emergency physician activation of the cardiac catheterization laboratory for patients with suspected ST-segment elevation myocardial infarction. Ann. Emerg. Med. 2010;55:423–430. doi: 10.1016/j.annemergmed.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt C., Lehmann G., Schmieder S., Karch M., Neumann F.J., Schömig A. Diagnosis of acute myocardial infarction in angiographically documented occluded infarct vessel: limitations of ST-segment elevation in standard and extended ECG leads. Chest. 2001;120:1540–1546. doi: 10.1378/chest.120.5.1540. [DOI] [PubMed] [Google Scholar]
- 11.Koyama Y., Hansen P.S., Hanratty C.G., Nelson G.I., Rasmussen H.H. Prevalence of coronary occlusion and outcome of an immediate invasive strategy in suspected acute myocardial infarction with and without ST-segment elevation. Am. J. Cardiol. 2002;90:579–584. doi: 10.1016/s0002-9149(02)02559-6. [DOI] [PubMed] [Google Scholar]
- 12.Abbas A.E., Boura J.A., Brewington S.D., Dixon S.R., O'Neill W.W., Grines C.L. Acute angiographic analysis of non-ST-segment elevation acute myocardial infarction. Am. J. Cardiol. 2004;94:907–909. doi: 10.1016/j.amjcard.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Khan A.R., Golwala H., Tripathi A. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur. Heart J. 2007;38:3082–3089. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 14.Wang T.Y., Zhang M., Fu Y. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am. Heart J. 2009;157:716–723. doi: 10.1016/j.ahj.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Pride Y.B., Tung P., Mohanavelu S. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depressions: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) substudy. JACC Cardiovasc Interv. 2010;3:806–811. doi: 10.1016/j.jcin.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Martí D., Mestre J.L., Salido L. Incidence, angiographic features, and outcomes of patients presenting with subtle ST-elevation myocardial infarction. Am. Heart J. 2014;168:884–890. doi: 10.1016/j.ahj.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Phibbs B., Nelson W. Differential classification of acute myocardial infarction into ST- and non-ST segment elevation is not valid or rational. Ann Noninvasive Electrocardiol. 2010;15:191–199. doi: 10.1111/j.1542-474X.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda D.F., Lobo A.S., Walsh B., Sandoval Y., Smith S.W. New Insights Into the Use of the 12-Lead Electrocardiogram for Diagnosing Acute Myocardial Infarction in the Emergency Department. Can. J. Cardiol. 2018;34:132–145. doi: 10.1016/j.cjca.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Smith S.W., Khalil A., Henry T.D. Electrocardiographic differentiation of early repolarization from subtle anterior ST-segment elevation myocardial infarction. Ann. Emerg. Med. 2012;60:45–56. doi: 10.1016/j.annemergmed.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Aslanger E., Yıldırımtürk Ö., Bozbeyoğlu E. A Simplified Formula Discriminating Subtle Anterior Wall Myocardial Infarction from Normal Variant ST-Segment Elevation. Am. J. Cardiol. 2018;122(8):1303–1309. doi: 10.1016/j.amjcard.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Durant E., Singh A. Acute first diagonal artery occlusion: a characteristic pattern of ST elevation in noncontiguous leads. Am. J. Emerg. Med. 2015;33(1326):e3–e5. doi: 10.1016/j.ajem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 22.De Winter R.J., Verouden N.J., Wellens H.J., Wilde A.A. A new ECG sign of proximal LAD occlusion. N. Engl. J. Med. 2008;359:2071–2073. doi: 10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 23.Verouden N.J., Koch K.T., Peters R.J. Persistent precordial “hyperacute” T-waves signify proximal left anterior descending artery occlusion. Heart. 2009;95:1701–1706. doi: 10.1136/hrt.2009.174557. [DOI] [PubMed] [Google Scholar]
- 24.Fox K.A., Dabbous O.H., Goldberg R.J. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333(7578):1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boden H., Ahmed T.A., Velders M.A. Peak and fixed-time high-sensitive troponin for prediction of infarct size, impaired left ventricular function, and adverse outcomes in patients with first ST-segment elevation myocardial infarction receiving percutaneous coronary intervention. Am. J. Cardiol. 2013;111:1387–1393. doi: 10.1016/j.amjcard.2013.01.284. [DOI] [PubMed] [Google Scholar]
- 26.Smith S.W. ST-elevation acute myocardial infarction: a critical but difficult electrocardiographic diagnosis. Acad. Emerg. Med. 2001;8:382–385. doi: 10.1111/j.1553-2712.2001.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith S.W., Dodd K.W., Henry T.D., Dvorak D.M., Pearce L.A. Diagnosis of ST-Elevation Myocardial Infarction in the Presence of Left Bundle Branch Block using the ST-Elevation to S-Wave Ratio in a Modified Sgarbossa Rule. Ann. Emerg. Med. 2012;60:766–776. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 28.Klein L.R., Shroff G.R., Beeman W., Smith S.W. Electrocardiographic criteria to differentiate acute anterior ST-elevation myocardial infarction from left ventricular aneurysm. Am. J. Emerg. Med. 2015;33:786–790. doi: 10.1016/j.ajem.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Bischof J.E., Worrall C., Thompson P., Marti D., Smith S.W. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am. J. Emerg. Med. 2016;34:149–154. doi: 10.1016/j.ajem.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong E.J., Kulkarni A.R., Bhave P.D. Electrocardiographic criteria for ST-elevation myocardial infarction in patients with left ventricular hypertrophy. Am. J. Cardiol. 2012;110:977–983. doi: 10.1016/j.amjcard.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Karwowski J., Gierlotka M., Gąsior M. Relationship between infarct artery location, acute total coronary occlusion, and mortality in STEMI and NSTEMI patients. Pol Arch Intern Med. 2017;127:401–411. doi: 10.20452/pamw.4018. [DOI] [PubMed] [Google Scholar]
- 32.Dumas F., Manzo-Silberman S., Fichet J. Can early cardiac troponin I measurement help to predict recent coronary occlusion in out-of-hospital cardiac arrest survivors? Crit. Care Med. 2012;40:1777–1784. doi: 10.1097/CCM.0b013e3182474d5e. [DOI] [PubMed] [Google Scholar]
- 33.Hachamovitch R., Hayes S.W., Friedman J.D., Cohen I., Berman D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single-photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 34.Hachamovitch R., Rozanski A., Shaw L.J. Impact of ischemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur. Heart J. 2011;32:1012–1024. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 35.Shaw L.J., Berman D.S., Maron D.J. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 36.Menown I.B.A., Mackenzie G., Adgey A.A.J. Optimizing the initial 12-lead electrocardiographic diagnosis of acute myocardial infarction. Eur. Heart J. 2000;21:275–283. doi: 10.1053/euhj.1999.1748. [DOI] [PubMed] [Google Scholar]
- 37.Surawicz B., Parikh S.R. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J. Am. Coll. Cardiol. 2002;40:1870–1876. doi: 10.1016/s0735-1097(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 38.Wu J., Kors J.A., Rijnbeek P.R. Normal limits of the electrocardiogram in Chinese subjects. Int. J. Cardiol. 2003;87:37–51. doi: 10.1016/s0167-5273(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 39.Macfarlane P.W., Browne D., Devine B. Modification of ACC/ESC criteria for acute myocardial infarction. J. Electrocardiol. 2004;37:98–103. doi: 10.1016/j.jelectrocard.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Smith S.W., Walsh B., Grauer K. A deep neural network learning algorithm outperforms a conventional algorithm for emergency department electrocardiogram interpretation. J Electrocardiol. 2019;52:88–95. doi: 10.1016/j.jelectrocard.2018.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.