Highlights
-
•
We assessed the relationships between MRI measurements and clinical symptoms in PD.
-
•
Using longitudinal data (3 year follow-up) from 50 PD patients and 45 controls.
-
•
SN atrophy and WMHs were associated with additional motor deficits in PD patients.
-
•
WMHs and hippocampal atrophy were associated with cognitive deficit in PD patients.
-
•
Both grey and white matter damage contribute to motor and cognitive deficits in PD.
Keywords: Parkinson’s disease, White matter hyperintensities, Grey matter atrophy, Deformation based morphometry, Scoring by Nonlocal Image Patch Estimator
Abstract
Introduction
Previous studies have found associations between grey matter atrophy and white matter hyperintensities (WMH) of vascular origin with cognitive and motor deficits in Parkinson’s disease (PD). Here we investigate these relationships in a sample of PD patients and age-matched healthy controls.
Methods
Data included 50 PD patients and 45 age-matched controls with T1-weighted and FLAIR scans at baseline, 18-months, and 36-months follow-up. Deformation-based morphometry was used to measure grey matter atrophy. SNIPE (Scoring by Nonlocal Image Patch Estimator) was used to measure Alzheimer’s disease-like textural patterns in the hippocampi. WMHs were segmented using T1-weighted and FLAIR images. The relationship between MRI features and clinical scores was assessed using mixed-effects models. The motor subscore of the Unified Parkinson's Disease Rating Scale (UPDRSIII), number of steps in a walking trial, and Dementia Rating Scale (DRS) were used respectively as measures of motor function, gait, and cognition.
Results
Substantia nigra atrophy was significantly associated with motor deficits, with a greater impact in PDs (p < 0.05). Hippocampal SNIPE scores were associated with cognitve decline in both PD and controls (p < 0.01). WMH burden was significantly associated with cognitive decline and increased motor deficits in the PD group, and gait deficits in both PD and controls (p < 0.03).
Conclusion
While substantia nigra atrophy and WMH burden were significantly associated with additional motor deficits, WMH burden and hippocampal atrophy were associated with cognitive deficits in PD patients. These results suggest an additive contribution of both grey and white matter damage to the motor and cognitive deficits in PD.
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by motor (bradykinesia, tremor, muscular rigidity, impaired postural reflexes, and gait dysfunction) and non-motor deficits (REM sleep behaviour disorder, autonomic dysfunction, neuropsychiatric symptoms, cognitive impairment and dementia) (Jankovic, 2008). Two key pathological features define PD: degeneration of the nigrostriatal dopaminergic system and accumulation of misfolded α-synuclein aggregates spreading in a stereotypical caudal-to-rostral pattern (Smith et al., 2019). Atrophy in the basal ganglia, particularly in the substantia nigra, subthalamic nucleus, nucleus accumbens, putamen, caudate nucleus, and internal and external globus pallidus, has been associated with motor and cognitive symptoms and longitudinal disease progression in PD (Zeighami et al., 2015, Zeighami et al., 2019).
In addition to the typical PD pathology, co-morbid Alzheimer’s disease (AD) pathology has been observed in 20–30% of PD patients (with an even higher prevalence in patients with dementia) (Smith et al., 2019) and atrophy in the hippocampi has also been related to slowing of gait and cognitive impairment in PD patients (Summerfield et al., 2005, Gee et al., 2017, Kandiah et al., 2014, Callisaya et al., 2013).
White matter hyperintensities (WMHs) are areas of increased signal on T2-weighted or Fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) sequences. WMHs of presumed vascular origin are common findings in the aging population and are attributed to an interplay of ischemic, inflammatory, and protein deposition processes. Hypertension and blood pressure fluctuations are associated with WMHs (Mok and Kim, 2015). In non-PD elderly individuals, WMHs have been associated with global cognitive impairment, executive dysfunction, rigidity, and gait disorders (Gunning-Dixon and Raz, 2000, Dadar et al., 2019, Baezner et al., 2008, Camarda et al., 2018, Prins et al., 2005, Rosso et al., 2017). Given that some of the symptoms associated with WMHs in non-PD individuals overlap with PD features, one might hypothesize that coexistence of WMHs in PD patients would lead to even greater cognitive and motor deficits. However, WMH associations with cognitive and motor symptoms are less well established and consistent in PD, although some recent studies report such relationships in both de novo (Dadar et al., 2018a) and later stage PD patients (Kandiah et al., 2014, Callisaya et al., 2013, de Schipper et al., 2019, Pozorski et al., 2019, Toda et al., 2019, Stojkovic et al., 2018, Veselỳ and Rektor, 2016). Methodological differences in assessment of WMHs might explain some of these inconsistensies in the findings, where more recent studies use automated tools to measure WMH volumes (Dadar et al., 2017, Schmidt et al., 2012), while previous studies used semi-quantitative visual rating scales (Fazekas et al., 2002, Scheltens et al., 1993), known to be less sensitive and more variable.
The aim of this study was to take advantage of our longitudinal sample of PD and control participants for whom MRI and clinical assessments (including assessments of vascular risk factors and relevant PD and non-PD medications) were available at baseline, month 18, and moth 36 follow-up visits, in order to investigate the impact of grey matter atrophy and textural changes, alongside WMH, on cognitive and motor symptoms in PD when compared to non-PD controls, as well as determine the strength of these relationships. We hypothesized that the greater the grey matter atrophy in substantia nigra regions and the hippocampus, together with a higher WMH burden, the greater the motor and cognitive deficits in PD patients when compared with age-matched normal controls.
2. Materials and methods
2.1. Subjects
Data included 50 subjects with PD (meeting UK brain bank criteria according to a movement disorders specialist) and 45 age-matched non-PD controls. Subjects were assessed at baseline, 18 and 36 months. Out of 50 PD participants, 42 (84%) and 39 (78%) completed month 18 and month 36 follow-up visits, respectively. Out of 45 control participants, 43 (95%) and 41 (91%) completed month 18 and month 36 follow-up visits, respectively. Baseline age, sex, education and other demographic features were measured. As previously described (Camicioli et al., 2011), general and neurological examinations were performed at each follow-up by a neurologist (RC), and included the Unified Parkinson's Disease Rating Scale (UPDRS), a gait assessment (steps taken to traverse 9.1 m), mini-mental state exam (MMSE), and the Dementia Rating Scale (DRS). Levodopa equivalent dose calculation and dementia assessment were performed as previously described (Camicioli et al., 2011). In short, the study neurologist and research assistant conducted separate interviews to obtain patient and caregiver-derived Clinical Dementia Rating (CDR) scores (Morris, 1991, Camicioli and Majumdar, 2010). Diagnosis of dementia (at month 36 visit) was defined as cognitive impairment in two domains with functional impairment due to cognitive decline, based on all available information, and independent of neuroimaging assessment. Memory impairment was not necessary for a diagnosis of dementia. Participants with significant cognitive or functional decline (on the MMSE, DRS, or CDR scores) or who declined below age‐ and education‐based cutoff thresholds were classified with dementia (i.e. worsening from baseline with impairment in two or more domains on the CDR, a 3‐point change on the modified MMSE (Molloy and Standish, 1997) or a 6‐point change on the DRS‐II Brown et al., 1999) beyond what would be accepted on the basis of reliability for these measures (>1 SD). Baseline scores were not used in determining final dementia classification. The classification was also consistent with MDS criteria (McDermott et al., 2018).
Assessments of PD patients were performed in the ON state. Vascular risk factors were obtained at baseline. In brief, medical history and medications were reviewed for hypertension, diabetes, hyperlipidimia and ischemic disease at each visit. Postural vital signs (including supine and standing blood pressure) were obtained at each assessment. Blood was obtained at baseline and included measurement of fasting glucose and lipids as well as homocysteine, and Apolipoprotein E genotyping at the clinical laboratory at the University of Alberta Hospital (Camicioli et al., 2009, Bouchard et al., 2008). The study was approved by the Health Research Ethics Board of the University of Alberta Pro00001182.
2.2. MRI acquisition
All participants received MRI scans on a Siemens Sonata 1.5 T system. T1-weighted images were acquired using a 3D magnetization prepared rapid acquisition gradient echo sequence (MPRAGE) (TR = 1800 ms, TE = 3.2 ms, TI = 1100 ms, 1 average, flip angle = 15°, field of view (FOV) = 256 mm, image matrix = 256 × 256, 128 slices, 1.5 mm slices). Native spatial resolution was 1 × 1 × 1.5 mm3 which was zero-filled to 0.5 × 0.5 × 1.5 mm3 . Axial FLAIR images were also acquired (TR = 8000 ms, TE = 99 ms, 2 averages, FOV = 220 mm, 25 slices, 5 mm slice thickness) oriented to the inferior margin of the corpus callosum.
2.3. MRI preprocessing
All T1-weighted and FLAIR images were preprocessed in three steps: noise reduction (Coupe et al., 2008), intensity non-uniformity correction (Sled et al., 1998), and intensity normalization into range [0–100]. Using a 6-parameter rigid registration, the FLAIR and T1-weighted images were linearly coregistered. The T1-weighted images were first linearly (Dadar et al., 2018b) and then nonlinearly (Avants et al., 2008) registered to the MNI-ICBM152 average template (Manera et al., 2020).
2.4. Grey matter DBM measurements
Deformation-based morphometry (DBM) is used to identify macroscopic anatomical changes within the population by spatially normalizing T1-weighted MRIs so that they all conform to the same stereotaxic space. Cross-sectional non-linear registration to the MNI-ICBM152 template (version 2009c) resulted in a deformation field sampled on a 1mm3 grid for each subject and timepoint. DBM maps were calculated by taking the Jacobian determinant of the inverse deformation field (Ashburner and Friston, 2000). Jacobian determinant values reflect the relative volume of the voxel to the MNI-ICBM152 template; i.e. a value of 1 indicates similar volume to the same region in the template, values lower than one indicate volumes smaller than the corresponding region in the template, while values higher than one indicate volumes that are larger than the corresponding region in the template. Therefore, a decrease in the Jacobian determinant values for a specific region between two timepoints can be interpreted as reduction in the structure volume, i.e. atrophy.
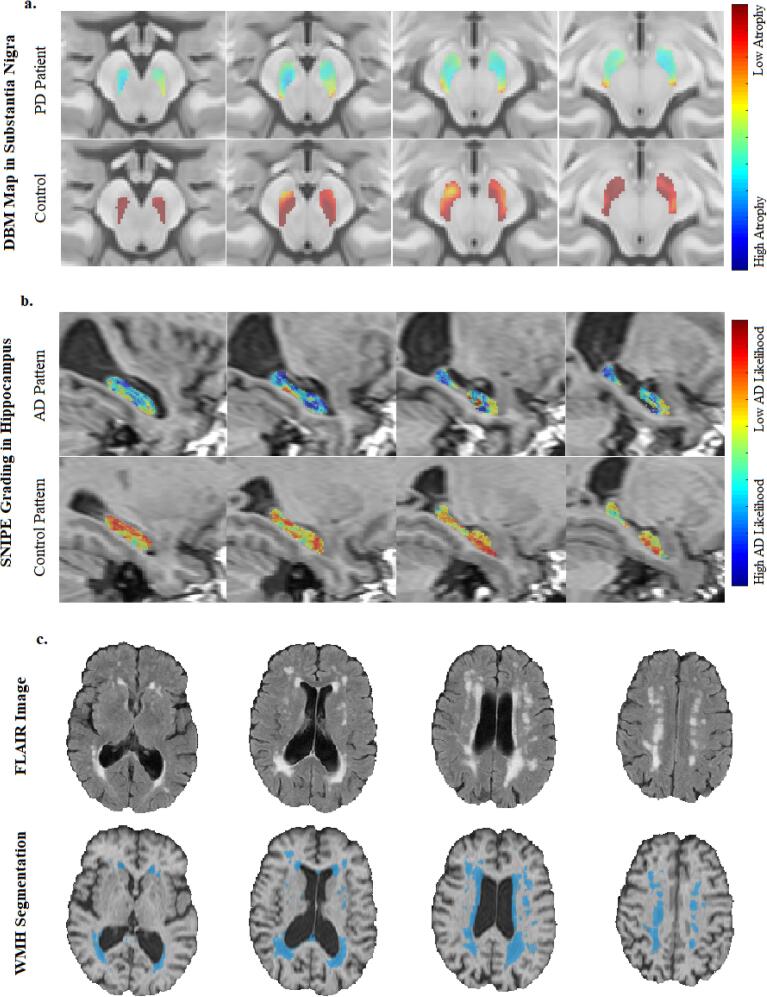
The CerebrA atlas of cortical grey matter regions was used to calculate average grey matter atrophy in 80 cortical regions (Manera et al., 2020). CerebrA atlas is based on the DKT atlas (Desikan et al., 2009), nonlinearly registered to the latest version of the MNI-ICBM152 template (with greater anatomical detail), and followed by manual edditing. Similarly, Xiao et al. atlas of deep grey matter regions was used to calculate average subcortical grey matter atrophy in 16 subcortical regions (Xiao et al., 2017) (e.g. Fig. 1a for substantia nigra). Both atlases are based on the latest MNI-ICBM152 template, which was also used for calculating the nonlinear registrations for the DBM analysis, ensuring higher anatomical accuracy and sensitivity in the extracted DBM measurements.
Fig. 1.
Extracted MRI Measurements: a. DBM maps in the substantia nigra mask for a PD and a control participant, b. hippocampal SNIPE grading for an AD-like and a control-like participant, and c. axial FLAIR scans and corresponding WMH segmentations. MRI = Magnetic Resonance Imaging. FLAIR = FLuid-Attenuated Inversion Recovery. WMH = White Matter Hyperintensity. DBM = Deformation Based Morphometry. PD = Parkinson’s Disease. SNIPE = Scoring by Nonlocal Image Patch Estimator. AD = Alzheimer’s Disease.
2.5. SNIPE grading in the hippocampi
An estimate of the level of pathology related to Alzheimer’s disease was obtained by applying Scoring by Nonlocal Image Patch Estimator (SNIPE) to the preprocessed T1-weighted images for all timepoints (Coupé et al., 2012). SNIPE assigns a similarity metric to each voxel in the hippocampus, indicating how much that voxel's surrounding patch resembles similar patches in a library of patients with probable Alzheimer’s disease or cognitively healthy controls (Fig. 1b). SNIPE workflow can be described in three main steps: 1) Template preselection: N/2 subjects from each training library (i.e. Alzheimer’s disease patients and controls) that are closest to the subject are preselected using sum of the squared differences over the initialization mask (a bounding box including the hippocampus) as the distance metric. 2) Scoring: For each voxel in the initialization mask, the surrounding patch is compared with all the patches from the N preselected training templates, and 3) Feature extraction: the final SNIPE grading score is an average of all the voxels inside the hippocampal region, providing an estimate of the confidence that this image is similar to those of individuals with probable Alzheimer’s disease. Positive SNIPE grading scores indicate normal appearing hippocampi, whereas negative scores indicate presence of AD-like atrophy.
2.6. WMH measurements
The WMHs were segmented using a previously validated automatic multi-modality segmentation technique that combines a set of location and intensity features obtained from a library of manually segmented scans with a Random Forest classifier to detect WMHs (Dadar et al., 2017, Dadar, et al., 2017) (Fig. 1c). The intensity features include voxel intensity, the probability of a specific intensity value being WMH (PWMH) or non-WMH (Pnon-WMH), and the ratio of these two probabilities for both T1-weighted and FLAIR images. The spatial features include a spatial WMH probability map indicating the probability of a voxel at a specific location being a WMH and the average intensity of a non-WMH voxel at that specific voxel location for T1-weighted and FLAIR images. After preprocessing and co-registration of all available modalities, these spatial and intensity features are calculated for each modality (i.e. in this case, T1-weighted and FLAIR). The Random Forest classifier is then trained using these features to segment the WMHs. The library used in this study was generated based on data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) since the FLAIR images had similar acquisition protocols. WMH load was defined as the volume of the voxels identified as WMH in the standard space (in cm3) and are thus normalized for head size. WMH volumes were log-transformed to achieve normal distribution.
2.7. Quality assessment
The quality of all the registrations and segmentations were visually assessed and cases that did not pass this quality control were discarded (N = 3, due to acquisition artifacts such as motion). All MRI processing, segmentation and quality control steps were blinded to clinical outcomes.
2.8. Statistical analyses
Paired t-tests or Chi-squared tests were used to assess differences between PD patients and controls in the baseline demographics and clinical variables. Mixed-effects models were used to assess the relationship between vascular risk factors and MRI measurements, controlling for age, sex, and use of relevant medications. Mixed-effects models were also used to investigate group differences in the MRI measurements (eq.1) as well as the associations between longitudinal MRI and clinical variables (eq.2). To assess the relationship between change in the MRI measurements during the time of the study and a diagnosis of dementia at the last visit (m36) in the PD group, age was split into age at baseline (AgeBL) and time from the baseline (TimeFromBL) visit (Eq. (3)).
| (1) |
| (2) |
| (3) |
where Cohort denotes a categorical variable contrasting PD patients and controls, and Measure:Cohort denotes an interaction term between the measure of interest and Cohort, reflecting differences in the slope of change in the variable of interest between the two groups. Dementia_m36 denotes a categorical variable contrasting a diagnosis of dementia at 36 months (i.e. last visit). Clinical Variable denotes the clinical variable of interest such as DRS or UPDRSIII scores. Subject ID was entered as a categorical random variable. To control for the impact of PD medications on motor symptoms, models assessing motor performance (i.e. UPDRSIII and gait measurements) also included Levodopa equivalent dose as a continuous random variable. All continuous variables were z-scored prior to the analyses. All demographic and clinical comparisons, associations with vascular risk factors, and atlas based results were corrected for multiple comparisons using False Discovery Threshold (FDR) technique with a significance threshold of 0.05.
3. Results
Table 1 summarizes the baseline demographics and clinical characteristics of the controls and PD patients used in this study. There were no significant differences in demographic variables. Compared to controls, PD patients had significantly lower standing systolic blood pressure (p = 0.01) and greater drop (from sitting to standing position) in both systolic and diastolic blood pressure (p < 0.002), and significantly higher homocysteine levels (p < 0.0001) at baseline. As expected, PD patients also had significantly higher baseline UPDRSIII scores (p < 0.0001) than the controls. Compared to the female participants, the male participants had significantly lower HDL baseline cholesterol levels in both control and PD groups (p < 0.002). The control male participants also had significantly lower baseline LDL and total cholesterol levels compared to the female control participants (p < 0.01). Table S1 in the supplemetary materials summarizes the clinical and MRI characteristics for PD and control participants separately for each follow-up visit.
Table 1.
Descriptive statistics for the subjects enrolled in this study. Data are numbers (N) or mean ± standard deviation. Significant P values after FDR correction (corrected P < 0.05) are indicated in bold font.
| Variable | Control | PD | P value |
|---|---|---|---|
| Age (years) | 71.61 ± 4.99 | 71.48 ± 4.66 | 0.90 |
| N (Male/Female) | 45 (25/20) | 50 (29/21) | 0.89 |
| Education (years) | 15 ± 3.15 | 13.9 ± 2.97 | 0.09 |
| Hypertension | 32/13 | 36/14 | 0.85 |
| Diabetes | 39/2 | 39/4 | 0.52 |
| Focal Ischemic Signs (No/Yes) | 29/13 | 25/21 | 0.03 |
| Heart Disease (No/Yes) | 35/6 | 38/5 | 0.88 |
| BMI | 26.86 ± 4.46 | 27.37 ± 5.22 | 0.61 |
| Supine BP Diastolic | 82.38 ± 9.30 | 83.80 ± 12.22 | 0.54 |
| Supine BP Systolic | 135.63 ± 13.26 | 135.94 ± 17.93 | 0.93 |
| Standing BP Diastolic | 85.41 ± 9.32 | 81.30 ± 12.74 | 0.09 |
| Standing BP Systolic | 135.09 ± 13.45 | 124.72 ± 25.05 | 0.01 |
| Sitting BP Diastolic | 84.49 ± 9.42 | 82.26 ± 9.76 | 0.27 |
| Sitting BP Systolic | 135.51 ± 12.61 | 129.92 ± 14.85 | 0.06 |
| BP Fall Diastolic | −3.32 ± 7.79 | 3.82 ± 13.02 | 0.002 |
| BP Fall Systolic | −1.29 ± 9.58 | 9.69 ± 16.10 | 0.0001 |
| Total Cholesterol | 5.18 ± 1.19 | 4.69 ± 0.87 | 0.02 |
| LDL Cholesterol | 3.22 ± 0.96 | 2.85 ± 0.77 | 0.04 |
| HDL Cholesterol | 1.33 ± 0.41 | 1.33 ± 0.30 | 0.92 |
| Homocysteine | 10.46 ± 2.60 | 13.68 ± 3.76 | <0.0001 |
| Triglycerides | 1.37 ± 0.68 | 1.14 ± 0.46 | 0.06 |
| Fasting Glucose | 5.55 ± 1.03 | 5.50 ± 0.64 | 0.74 |
| APOE: E3/E3, E3/E4, E2/E3, E2/E44 = E4/E4) | 28, 9, 5, 2 | 37, 5, 6, 1 | 0.64 |
| UPDRSIII | 1.90 ± 2.79 | 16.89 ± 8.40 | <0.0001 |
| Gait Trial Steps | 16.82 ± 3.32 | 21.12 ± 14.00 | 0.05 |
| MMSE | 28.45 ± 1.60 | 28.04 ± 1.77 | 0.26 |
| Total DRS | 138.56 ± 3.57 | 137.56 ± 4.78 | 0.28 |
| Hoehn and Yahr | – | 2.2 ± 0.67 | – |
BMI = Body Mass Index. BP = Blood Pressure. LDL = Low Density Lipoprotein. HDL = High Density Lipoprotein. APOE = Apolipoprotein E. UPDRS = Unified Parkinson's Disease Rating Scale. MMSE = MiniMental State Examination. DRS = Dementia Rating Scale.
3.1. Vascular risk factors
Table 2 shows the associations between vascular risk factors and the longitudinal MRI measures in controls and PD patients, controlling for age and sex. To decrease the number of analyses, left and right hippocampal SNIPE scores and substantia nigra DBM values were averaged. For each risk factor, use of relevant medication was included as a binary categorical covariate in the model (e.g. blood pressure medication for blood pressure measurements, diabetes medication for fasting glucose, cholesterol medication for cholesterol measurements, etc.) to ensure that the results are not driven by the medication usage.
Table 2.
Association between substantia nigra DBM, SNIPE grading, and WMH load and vascular risk factors. Data are numbers (N) or mean ± standard deviation. Significant P values after FDR correction (corrected P < 0.05) are indicated in bold font.
| Cohort | Control |
PD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | SN DBM |
SNIPE HC |
WMH |
SN DBM |
SNIPE HC |
WMH |
||||||
| Variable | TStat | PValue | TStat | PValue | TStat | PValue | TStat | PValue | TStat | PValue | TStat | PValue |
| Hypertension | −0.60 | 0.55 | −0.67 | 0.50 | 0.63 | 0.53 | 2.40 | 0.02 | 0.62 | 0.53 | 0.04 | 0.96 |
| Diabetes | −1.57 | 0.12 | 0.68 | 0.49 | 2.97 | 0.004 | −0.85 | 0.40 | −1.08 | 0.07 | 0.05 | 0.96 |
| Ischemia | −0.36 | 0.72 | 0.11 | 0.91 | 1.55 | 0.12 | 0.38 | 0.71 | −0.53 | 0.59 | 0.28 | 0.77 |
| Heart Disease | −0.53 | 0.60 | −0.32 | 0.74 | −0.65 | 0.51 | 0.95 | 0.34 | −0.76 | 0.55 | −1.15 | 0.25 |
| BMI | −0.52 | 0.60 | −0.16 | 0.87 | −1.42 | 0.15 | −0.30 | 0.76 | −0.53 | 0.60 | −1.10 | 0.27 |
| Supine BP Diastolic | 0.55 | 0.58 | 0.74 | 0.46 | −0.27 | 0.78 | 2.48 | 0.01 | 0.28 | 0.77 | 0.97 | 0.33 |
| Supine BP Systolic | −0.01 | 0.99 | 0.37 | 0.71 | 0.02 | 0.98 | 1.89 | 0.06 | 0.49 | 0.62 | 1.71 | 0.24 |
| Standing BP Diastolic | −1.86 | 0.06 | −0.44 | 0.66 | 1.55 | 0.12 | 3.06 | 0.002 | −0.59 | 0.55 | 0.52 | 0.60 |
| Standing BP Systolic | −1.88 | 0.06 | −0.42 | 0.67 | 1.77 | 0.08 | 1.40 | 0.16 | −0.25 | 0.80 | 0.59 | 0.55 |
| Sitting BP Diastolic | −0.27 | 0.79 | −0.52 | 0.61 | −0.01 | 0.99 | 2.29 | 0.02 | −0.05 | 0.96 | −0.36 | 0.71 |
| Sitting BP Systolic | −0.66 | 0.50 | −0.25 | 0.80 | 1.07 | 0.28 | 1.96 | 0.05 | 0.90 | 0.37 | 0.07 | 0.94 |
| BP Fall Diastolic | 1.58 | 0.12 | 1.37 | 0.17 | −0.34 | 0.73 | 0.06 | 0.95 | 0.62 | 0.53 | 0.71 | 0.48 |
| BP Fall Systolic | 1.61 | 0.11 | 1.02 | 0.28 | −0.95 | 0.34 | 0.41 | 0.68 | −0.09 | 0.92 | 0.91 | 0.36 |
| Total Cholesterol | 1.43 | 0.15 | −1.29 | 0.20 | −2.06 | 0.04 | 1.94 | 0.05 | 2.04 | 0.04 | −2.01 | 0.04 |
| LDL Cholesterol | 1.59 | 0.11 | −1.68 | 0.10 | −2.07 | 0.04 | 1.77 | 0.08 | 1.70 | 0.09 | −2.24 | 0.02 |
| HDL Cholesterol | −0.88 | 0.37 | 3.06 | 0.002 | −0.32 | 0.75 | 0.17 | 0.85 | 1.33 | 0.18 | 0.50 | 0.61 |
| Homocysteine | −0.60 | 0.55 | −0.49 | 0.62 | 1.02 | 0.30 | 0.34 | 0.70 | 0.80 | 0.40 | −0.37 | 0.70 |
| Triglyceride | 0.99 | 0.32 | −2.71 | 0.007 | −0.40 | 0.68 | 1.40 | 0.17 | 0.33 | 0.74 | −0.91 | 0.36 |
| Fasting Glucose | −0.28 | 0.77 | −0.52 | 0.60 | 2.84 | 0.006 | −0.25 | 0.80 | 1.62 | 0.10 | 0.08 | 0.91 |
DBM = Deformation Based Morphometry. SNIPE = Scoring by Nonlocal Image Patch Estimator. HC = Hippocampus. SN = Substantia Nigra. BMI = Body Mass Index. BP = Blood Pressure. LDL = Low Density Lipoprotein. HDL = High Density Lipoprotein. APOE = Apolipoprotein E. UPDRS = Unified Parkinson's Disease Rating Scale. MMSE = MiniMental State Examination. DRS = Dementia Rating Scale.
Substantia nigra DBM scores were not associated with any of the risk factors in the controls. In the PD group, hypertension, higher diastolic blood pressure (in all supine, standing, and sitting positions), higher systolic blood pressure in a sitting position, and higher total cholesterol levels were associated with higher DBM scores in the substantia nigra (p < 0.05), however, only associations with diastolic blood pressure in a standing position survived FDR correction. In the control group, higher HDL cholesterol levels and lower triglyceride levels were associated with higher hippocampal SNIPE scores, indicating lower levels of AD-related atrophy (p < 0.007). The control participants with a history of diabetes and higher fasting glucose levels had significantly greater WMH loads (p < 0.006).
3.2. Group comparisons of longitudinal MRI measures (Model 1)
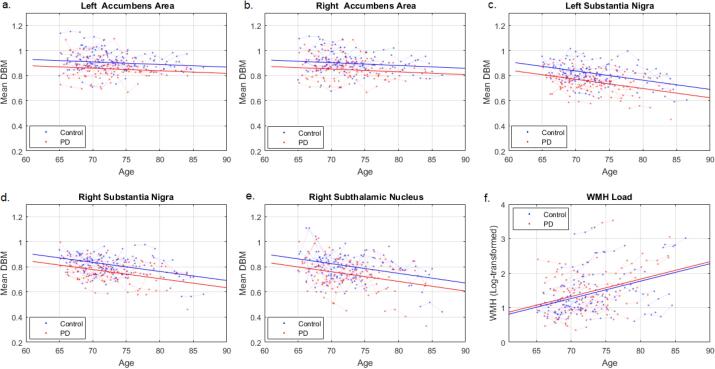
After FDR correction, mean DBM values were significantly lower in PD patients compared to controls in left (t = −3.37, uncorrected p = 0.0001, Fig. 2a) and right accumbens areas (t = −3.18, uncorrected p = 0.001, Fig. 2b), left (t = −4.19, uncorrected p < 0.0001, Fig. 2c) and right (t = −3.95, uncorrected p = 0.0001, Fig. 2d) substantia nigra, and right subthalamic nucleus (t = −3.23, uncorrected p = 0.001, Fig. 2e). Mean DBM values in left and right substantia nigra and right subthalamic nucleus also significantly decreased with age in both controls and PD patients (uncorrected p < 0.0001). In both controls and PD patients, WMH burden significantly increased with age (t = 7.71, p < 0.0001, Fig. 2e). There were no significant group differences in WMH loads (t = 0.49, p = 0.62).
Fig. 2.
Group differences in average grey matter DBM values and WMH load. DBM = Deformation Based Morphometry. PD = Parkinson’s Disease. WMH = White Matter Hyperintensities.
3.3. Presence of dementia at 36 months
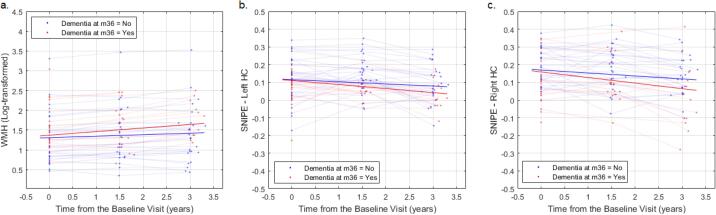
Out of the 39 PD patients that completed month 36 visits, 19 (48.7%) were diagnosed with dementia. Controlling for age at baseline and sex, WMH load significantly increased in PD patients that were diagnosed with dementia at 36 months (t = 3.58, p = 0.0005), compared to those that remained stable (Fig. 3a, longitudinal assessment, Model 3). There was also a marginally significant decrease in the right hippocampal SNIPE grading scores (t = −1.69, p = 0.09, Fig. 4c). No control participant met the criteria for a diagnosis of dementia at month 36.
Fig. 3.
Comparing the change in the WMH load and the hippocampal SNIPE grading scores in PD patients that are diagnosed with dementia at 36 months and those that remain stable. SNIPE = Scoring by Nonlocal Image Patch Estimator.
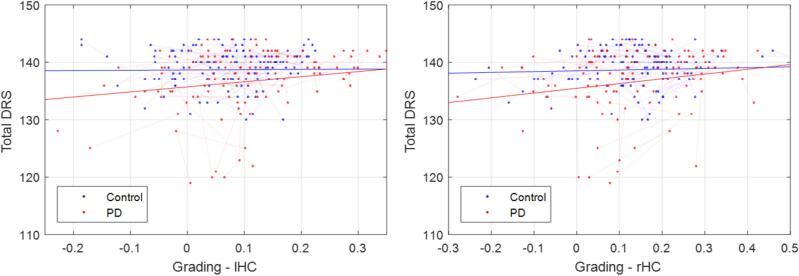
Fig. 4.
Association between Total DRS and mean SNIPE grading scores in left and right hippocampi. DRS = Dementia Rating Scale. SNIPE = Scoring by Nonlocal Image Patch Estimator. PD = Parkinson’s Disease.
3.4. Total dementia rating scale
Controlling for age and sex, we did not find a significant association between WMH load and Total DRS score in either group (longitudinal assessment, Model 2). Total DRS significantly decreased with age (t = −2.23, p < 0.04) and decrease in hippocampal SNIPE grading in the PD group (tLeftHC = 2.83, pLeftHC = 0.005, tRightHC = 2.55, pRighHC = 0.01), with a marginal interaction (Fig. 4, tLeftHC = −1.80, pRighHC = 0.07, tLeftHC = −1.58, pRighHC = 0.10). We also performed a second level analysis, assessing the associations with individual DRS subscores (Fig. S1, Table S2). Memory, attention, and initiation preservation subscores showed similar relationships with hippocampal SNIPE grading scores, while conceptualization and constructions were not significantly associated. To ensure that these associations were not just downstream effects of PD-related pathologies, we repeated the analysis, including UPDRSIII, step count in the gait trial, and substantia nigra atrophy as covariates in the models. The obtained results were similar in terms of estimated t statistics and p values.
3.5. Motor symptoms
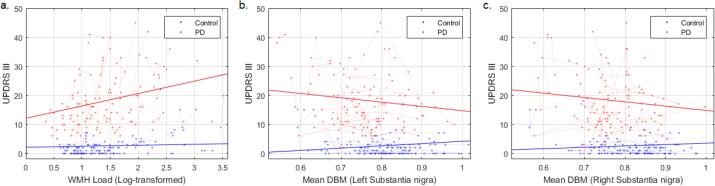
Controlling for age and sex, higher WMH load was significantly associated with higher UPDRSIII scores in PD (Fig. 5a, t = 3.41, p = 0.0008), but not in controls (t = 0.26, p = 0.79), with a significant interaction (t = 2.30, p = 0.02). UPDRSIII also significantly increased with age (t = 4.75, p < 0.00001) and decrease in SN DBM (Fig. 5b-c, tLeftSN = −2.02, tRightSN = −1.88, p < 0.05), with a significantly greater impact in PD (tLeftSN = −2.10, tRightSN = −1.73, p < 0.04, longitudinal assessment, Model 2).
Fig. 5.
Association between UPDRSIII, WMH burden and mean DBM scores in left and right substantia nigra. UPDRS = Unified Parkinson's Disease Rating Scale. WMH = White Matter Hyperintensities. DBM = Deformation Based Morphometry.
3.6. Gait
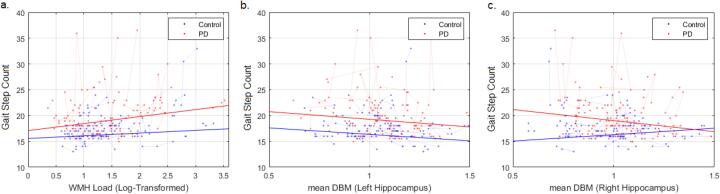
The number of steps in the walking trial significantly increased with age (t = 2.50, p = 0.01) and increased WMH load (t = 2.13, p = 0.03) in both groups (Fig. 6a). There was no significant interaction (t = 0.95, p = 0.34). An increased step count was significantly associated with a decrease in the mean DBM in the right hippocampus in the PD group (Fig. 6c, t = −2.02, p = 0.04) with a significant interaction (t = 2.18, p = 0.03), but not the left (Fig. 6b, p > 0.11, longitudinal assessment, Model 2). Controlling for age and sex, there was no significant association between mean DBM in the substantia nigra and gait in either controls or PD patients (p > 0.5).
Fig. 6.
Association between gait, WMH burden and mean DBM scores in left and right hippocampi. WMH = White Matter Hyperintensities. DBM = Deformation Based Morphometry.
4. Discussion
In this study, we investigated the impact of grey matter and white matter pathology, in a longitudinal sample of PD patients and age-matched controls. In addition to the overall pattern of increased atrophy associated with age in both controls and PD patients, we found significantly greater bilateral atrophy in the substantia nigra and accumbens areas as well as the right subthalamic nucleus in the PD patients. These findings are in line with the pathologic hallmarks of PD and the previous imaging studies in the literature (Zeighami et al., 2015, Boucetta et al., 2016).
Also in line with previous studies, atrophy in the right hippocampus was associated with slowing in gait as well as future cognitive decline in PD patients (Summerfield et al., 2005) (Fig. 3c and 6c). In addition, bilateral hippocampal atrophy was associated with poorer cognitive performance in both groups, with a marginally greater impact in the PD patients (Fig. 4). The hippocampus is involved in spatial memory as well as sensorimotor integration, contributing to both gait and cognitive function. Loss of hippocampal integrity is a well-established contributor to cognitive decline in the aging population. This is also in line with previous studies reporting contribution of Lewy body and possibly coexisting Alzheimer’s disease pathology to dementia in PD patients (Smith et al., 2019). Furthermore, to ensure that these associations were not just downstream effects of PD-related pathologies, we repeated the analysis, including UPDRSIII, gait step count, and substantia nigra atrophy as covariates in the models, and obtained similar relationships between total DRS score and hippocampal atrophy.
Consistent with previous findings, PD patients had significantly greater atrophy in the bilateral substantia nigra (Zeighami et al., 2015, Boucetta et al., 2016). Substantia nigra atrophy increased with age and was associated with poorer motor perfomance in the PD patients (Fig. 3), as also reported in previous studies (Zeighami et al., 2015, Zeighami et al., 2019). Substantia nigra contains dopamine neurons whose degeneration accounts for all the key clinical features of PD. The sensitivity of DBM (which is based only on T1w MRI) in detecting substantia nigra atrophy in PD is yet another clinically important finding in this study.
WMH volume increased with age in both controls and PD patients (Fig. 2e). Similar to the majority of previous studies which report no disease-specific differences in WMH loads in PD patients and age-matched normal controls, we did not find a significant difference in WMH burden between controls and PD patients (Dadar et al., 2018a, Pozorski et al., 2019, Acharya et al., 2007, Dalaker et al., 2009). However, increase in WMH burden was associated with greater cognitive decline and motor and gait deficits, as previously reported in the literature (Kandiah et al., 2014, Callisaya et al., 2013, Dadar et al., 2018a, de Schipper et al., 2019, Pozorski et al., 2019, Toda et al., 2019, Stojkovic et al., 2018, Veselỳ and Rektor, 2016) (Figs. 3a, 5a, and 6a, respectively). Taken together, our results support the conclusion that even though PD patients do not present with greater WMH loads than normal aging individuals, comorbid existence of WMHs exacerbates the cognitive and motor sysmptoms in PD by aggravating the already defective neuronal connections.
Few studies have investigated the impact of longitudinal progression of WMHs on cognition in PD. We found that PD patients that were diagnosed with dementia at the last visit (after three years) had greater WMH load increase compared to those that remained stable. This is in line with another previous study, which found periventriventricular WMH progression at 30-month follow-up to be associated with conversion to dementia (Gonzalez-Redondo et al., 2012).
Compared to controls, our PD patients had significantly higher homocysteine levels. This is in line with previous studies, reporting higher homocysteine levels in PD patients, particularly those treated with L-Dopa (Blandini et al., 2001). Unlike previous studies, we did not find an association between hypertension and WMH burden (Dadar et al., 2018a, Jimenez-Conde et al., 2010) (Table 2). This might be due to the relatively smaller sample size or that the blood presure levels were well-controlled in both controls and PD patients in our sample (Table 1). Also, WMH burden was associated with diabetes and higher fasting glucose levels in the control group, but not the PD patients. This might also be due to the small sample size or the patient selection process.
Previous studies investigating the impact of MRI measures on cognitive and motor symptoms in PD have mostly been cross sectional in their WMH and GM assessments (Zeighami et al., 2015, Dadar et al., 2018a, de Schipper et al., 2019, Toda et al., 2019, Stojkovic et al., 2018, Dadar, et al., 2020, Zeighami et al., 2017, Fang et al., 2020, Wan et al., 2019), are mainly from the Parkinson's Progression Markers Initiative (PPMI) study which includes only de novo PD patients at baseline (Zeighami et al., 2015, Zeighami et al., 2019, Dadar et al., 2018a, Dadar, et al., 2020, Zeighami et al., 2017, Chahine et al., 2019), and most don’t assess the impact of grey matter and white matter measures in the same setting (Zeighami et al., 2015, Zeighami et al., 2019, de Schipper et al., 2019, Pozorski et al., 2019, Toda et al., 2019, Stojkovic et al., 2018, Dadar, et al., 2020, Zeighami et al., 2017, Wan et al., 2019, Chahine et al., 2019). In comparison, there are several strengths to the present study. Longitudinal MRI and clinical data were consistently acquired from the patients and age-matched controls across all visits, allowing us to investigate longitudinal changes and relationships in the measures. DBM, SNIPE, and WMH measurements were estimated using extensively validated and widely used automated methods (Zeighami et al., 2015, Zeighami et al., 2019, Dadar et al., 2019, Dadar et al., 2018a, Dadar et al., 2017, Coupé et al., 2012, Dadar, et al., 2017, Boucetta et al., 2016), allowing refined and accurate assessment of the grey matter and white matter pathology in the same population. We had extensive assessments of vascular risk factors, which enabled us to investigate their associations with MRI measurements of interest. Relevant medication (both PD and non-PD) was included as covariates in the models. The main limitation of our study concerns its relatively small sample size. It should also be noted that patients with dementia or a history of stroke at baseline were excluded from this study, leading to a select patient group. Replication in larger cohorts are therefore necessary to further confirm these findings.
In conclusion, this study suggests that grey matter atrophy and WM lesions contribute to the cognitive decline and motor deficits in PD. In accordance with previous studies, we found that PD patients had greater bilateral substantia nigra atrophy compared to controls, contributing to poorer motor performance. Hippocampal atrophy also affected both cognition and gait in the PD patients. Together with the evidence from previous studies, our results suggest that even though WMH burden does not differ between PD patients and age-matched otherwise healthy individuals, it aggravates the cognitive and motor symptoms in PD patients. Since many risk factors that are associated with WMHs and their progression are potentially modifiable through treatment and lifestyle changes, the impact of these lesions on cognitive and motor function in PD pinpoints a need for assessment and treatment of these risk factors with a higher priority in PD patients. Given that currently, early intervention and preventive strategies are the key elements in preventing cognitive decline in the aging populations, PD patients might also benefit from systematic cardiovascular risk modification.
Funding
This study was supported in part by the Canadian Consortium on Neurodegeneration in Aging (CCNA 137794, www.ccna-ccnv.ca). CCNA is supported by a grant from the Canadian Institutes of Health Research with funding from several partners. Original data acquisition was funded by an operating grant (RC) from CIHR (MOP 67132).
CRediT authorship contribution statement
Mahsa Dadar: Conceptualization, Formal analysis, Writing- original draft. Myrlene Gee: Data curation, Writing-review & editing. Ashfaq Shuaib: Conceptualization, Writing-review & editing. Simon Duchesne: Conceptualization, interpretation of the data, Writing-review & editing. Richard Camicioli: Conceptualization, interpretation of the data, Writing-review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102353.
Contributor Information
Mahsa Dadar, Email: mahsa.dadar.1@ulaval.ca.
Myrlene Gee, Email: myrlene@ualberta.ca.
Ashfaq Shuaib, Email: shuaib@ualberta.ca.
Simon Duchesne, Email: simon.duchesne@fmed.ulaval.ca.
Richard Camicioli, Email: rcamicio@ualberta.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Smith C. Neuropathology of dementia in patients with Parkinson’s disease: a systematic review of autopsy studies. J. Neurol. Neurosurg. Psychiatry. 2019;90:1234–1243. doi: 10.1136/jnnp-2019-321111. [DOI] [PubMed] [Google Scholar]
- Zeighami Y. Network structure of brain atrophy in de novo Parkinson’s disease. eLife. 2015;4 doi: 10.7554/eLife.08440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeighami Y. Assessment of a prognostic MRI biomarker in early de novo Parkinson’s disease. NeuroImage Clin. 2019;24 doi: 10.1016/j.nicl.2019.101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch. Neurol. 2005;62:281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- Gee M. Regional volumetric change in Parkinson’s disease with cognitive decline. J. Neurol. Sci. 2017;373:88–94. doi: 10.1016/j.jns.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Kandiah N. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:1203–1208. doi: 10.1016/j.parkreldis.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Callisaya M.L. Brain structural change and gait decline: a longitudinal population-based study. J. Am. Geriatr. Soc. 2013;61:1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- Mok V., Kim J.S. Prevention and management of cerebral small vessel disease. J. Stroke. 2015;17:111. doi: 10.5853/jos.2015.17.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Dadar M., Maranzano J., Ducharme S., Collins D.L. White matter in different regions evolves differently during progression to dementia. Neurobiol. Aging. 2019;76:71–79. doi: 10.1016/j.neurobiolaging.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Baezner H. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- Camarda C. Association between cerebral Small vessel disease, measures of brain atrophy and mild Parkinsonian signs in neurologically and cognitively healthy subjects aged 45–84 years: a cross-sectional study. Curr. Alzheimer Res. 2018;15:1013–1026. doi: 10.2174/1567205015666180702111110. [DOI] [PubMed] [Google Scholar]
- Prins N.D. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- Rosso A.L. Contributors to poor mobility in older adults: integrating white matter hyperintensities and conditions affecting other systems. J. Gerontol. Ser. Biomed. Sci. Med. Sci. 2017;72:1246–1251. doi: 10.1093/gerona/glw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson’s disease patients. NeuroImage Clin. 2018;20:892–900. doi: 10.1016/j.nicl.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Schipper L.J. Age-and disease-related cerebral white matter changes in patients with Parkinson’s disease. Neurobiol. Aging. 2019;80:203–209. doi: 10.1016/j.neurobiolaging.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Pozorski V. Cross-sectional and longitudinal associations between total and regional white matter hyperintensity volume and cognitive and motor function in Parkinson’s disease. NeuroImage Clin. 2019;23 doi: 10.1016/j.nicl.2019.101870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K., Iijima M., Kitagawa K. Periventricular White Matter Lesions Influence Gait Functions in Parkinson’s Disease. Eur. Neurol. 2019;81:120–127. doi: 10.1159/000499908. [DOI] [PubMed] [Google Scholar]
- Stojkovic T. Exploring the relationship between motor impairment, vascular burden and cognition in Parkinson’s disease. J. Neurol. 2018;265:1320–1327. doi: 10.1007/s00415-018-8838-3. [DOI] [PubMed] [Google Scholar]
- Veselỳ B., Rektor I. The contribution of white matter lesions (WML) to Parkinson’s disease cognitive impairment symptoms: A critical review of the literature. Parkinsonism Relat. Disord. 2016;22:S166–S170. doi: 10.1016/j.parkreldis.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Dadar M. Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. NeuroImage. 2017;157:233–249. doi: 10.1016/j.neuroimage.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Fazekas F. CT and MRI Rating of White Matter Lesions. Cerebrovasc. Dis. 2002;13:31–36. doi: 10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- Scheltens P.H. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J. Neurol. Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- Camicioli R. Ventricular dilatation and brain atrophy in patients with Parkinson’s disease with incipient dementia. Mov. Disord. 2011;26:1443–1450. doi: 10.1002/mds.23700. [DOI] [PubMed] [Google Scholar]
- Morris J.C. The clinical dementia rating (cdr): Current version and. Young. 1991;41:1588–1592. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Camicioli R., Majumdar S.R. Relationship between mild cognitive impairment and falls in older people with and without Parkinson’s disease: 1-Year Prospective Cohort Study. Gait Posture. 2010;32:87–91. doi: 10.1016/j.gaitpost.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Molloy D.W., Standish T.I. A guide to the standardized Mini-Mental State Examination. Int. Psychogeriatr. 1997;9:87–94. doi: 10.1017/s1041610297004754. [DOI] [PubMed] [Google Scholar]
- Brown G.G. Validity of the Dementia Rating Scale in assessing cognitive function in Parkinson’s disease. J. Geriatr. Psychiatry Neurol. 1999;12:180–188. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- Manera A.L., Dadar M., Fonov V., Collins D.L. Mindboggle-101 atlas for MNI-ICBM152 template. Scientific Data. 2020;7(1):1–9. doi: 10.1038/s41597-020-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott K.L., Fisher N., Bradford S., Camicioli R. Parkinson’s disease mild cognitive impairment classifications and neurobehavioral symptoms. Int. Psychogeriatr. 2018;30:253–260. doi: 10.1017/S1041610217002265. [DOI] [PubMed] [Google Scholar]
- Camicioli R.M., Bouchard T.P., Somerville M.J. Homocysteine is not associated with global motor or cognitive measures in nondemented older Parkinson’s disease patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2009;24:176–182. doi: 10.1002/mds.22227. [DOI] [PubMed] [Google Scholar]
- Bouchard T.P. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiol. Aging. 2008;29:1027–1039. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Coupe P. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging. 2008;27:425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. Med. Imaging IEEE Trans. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Dadar M., Fonov V.S., Collins D.L., Initiative A.D.N. A comparison of publicly available linear MRI stereotaxic registration techniques. NeuroImage. 2018;174:191–200. doi: 10.1016/j.neuroimage.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Desikan R.S. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer’s disease. Brain. 2009;awp123 doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinson׳s disease cohort. Data Brief. 2017;12:370–379. doi: 10.1016/j.dib.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé P. Scoring by nonlocal image patch estimator for early detection of Alzheimer’s disease. NeuroImage Clin. 2012;1:141–152. doi: 10.1016/j.nicl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar, M. et al. Validation of a Regression Technique for Segmentation of White Matter Hyperintensities in Alzheimer’s Disease. IEEE Trans. Med. Imaging (2017). [DOI] [PubMed]
- Boucetta S. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson’s disease. Sci. Rep. 2016;6 doi: 10.1038/srep26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya H.J., Bouchard T.P., Emery D.J., Camicioli R.M. Axial signs and magnetic resonance imaging correlates in Parkinson’s disease. Can. J. Neurol. Sci. 2007;34:56–61. doi: 10.1017/s0317167100005795. [DOI] [PubMed] [Google Scholar]
- Dalaker T.O. Brain atrophy and white matter hyperintensities in early Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009;24:2233–2241. doi: 10.1002/mds.22754. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Redondo R. The impact of silent vascular brain burden in cognitive impairment in Parkinson’s disease. Eur. J. Neurol. 2012;19:1100–1107. doi: 10.1111/j.1468-1331.2012.03682.x. [DOI] [PubMed] [Google Scholar]
- Blandini F. Plasma homocysteine and l-DOPA metabolism in patients with parkinson disease. Clin. Chem. 2001;47:1102–1104. [PubMed] [Google Scholar]
- Jimenez-Conde J. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41:437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar, M. et al. White matter hyperintensities mediate impact of dysautonomia on cognition in Parkinson’s disease. Mov. Disord. Clin. Pract. (2020). [DOI] [PMC free article] [PubMed]
- Zeighami Y. A clinical-anatomical signature of Parkinson’s Disease identified with partial least squares and magnetic resonance imaging. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.12.050. [DOI] [PubMed] [Google Scholar]
- Fang E. Differentiating Parkinson’s disease motor subtypes using automated volume-based morphometry incorporating white matter and deep gray nuclear lesion load. J. Magn. Reson. Imaging. 2020;51:748–756. doi: 10.1002/jmri.26887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. Exploring the association between Cerebral small-vessel diseases and motor symptoms in Parkinson’s disease. Brain Behav. 2019;9 doi: 10.1002/brb3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine L.M. Modifiable vascular risk factors, white matter disease and cognition in early Parkinson’s disease. Eur. J. Neurol. 2019;26:246–e18. doi: 10.1111/ene.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.