Abstract
Few studies have described chimeric antigen receptor–modified T cell (CAR-T) therapy for central nervous system (CNS) B-cell acute lymphocytic leukemia (B-ALL) patients due to life-threatening CAR-T-related encephalopathy (CRES) safety issues. In this study, CAR-Ts targeting CD19 with short hairpin RNA (shRNA)-IL-6 gene silencing technology (ssCART-19s) were prepared. We conducted a phase 1 clinical trial (ClinicalTrials.gov number, NCT03064269). Three patients with relapsed CNS B-ALL were enrolled, conditioned with the fludarabine and cyclophosphamide for lymphocyte depletion and infused with ssCART-19s for three consecutive days. Clinical symptoms and laboratory examinations were monitored. After ssCART-19 treatment, three patients' symptoms resolved almost entirely. Brain leukemic infiltration reduced significantly based on magnetic resonance imaging (MRI), and there were no leukemic blasts in cerebrospinal fluid (CSF), which was confirmed by cytological and molecular examinations. Additionally, increases in the levels of cytokines and immune cells were observed in the CSF of all patients. Only grade 1 cytokine release syndrome (CRS) manifesting as fever was noted in patients. In conclusion, CAR-Ts with shRNA-IL-6 gene knockdown migrated into the CNS, eradicated leukemic cells and elevated cytokines in CSF with mild, acceptable side effects.
Abbreviations: CAR-T, chimeric antigen receptor–modified T cell; CNS, central nervous system; shRNA, short hairpin RNA; CSF, cerebrospinal fluid; CRES, CAR-T-related encephalopathy; CRS, cytokine release syndrome; ssCART-19, CAR-Ts targeting CD19 with short hairpin RNA (shRNA)-IL-6 gene silencing
Introduction
The central nervous system (CNS) acute lymphocytic leukemia (ALL) accounts for more than 30% of all ALL relapses and confers a poor prognosis [1,2]. Since cranial or craniospinal radiotherapy (CRT) for CNS ALL has been almost abandoned due to its long-term side effects [[3], [4], [5]], performing allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a good choice for treating CNS ALL due to the lower relapse rate [6]. Even in this setting, the incidence of CNS relapse is as high as 13% in patients with a history of CNS involvement before transplantation compared with the overall incidence of 4% in the entire cohort [6]. Therefore, CNS ALL is a great challenge in clinical practice.
Chimeric antigen receptor–modified T cells (CAR-Ts) targeting CD19 have been demonstrated to be highly efficacious for treating relapsed and refractory (R/R) B-cell hematological malignancies in a series of clinical studies [7]. More importantly, CAR-Ts were shown to persist in cerebrospinal fluid (CSF) for a long period of time [[8], [9], [10], [11]]. It is reasonable to consider applying CAR-Ts to treat CNS ALL. Unfortunately, lethal neurotoxicity or CAR-T-related encephalopathy (CRES) caused by CAR-T treatment has halted some CD19 CAR-T trials, and there are no effective ways to manage and control these side effects [12]. Therefore, the limitations of CAR-T therapy in CNS B-cell ALL (B-ALL) remain.
It is commonly accepted that the rapid and strong elevation of cytokine levels, especially IL-6 and IL-1, in CNS B-ALL is the main cause of life-threatening clinical outcomes in CAR-T treatment of R/R B-ALL with CNS invasion. Tocilizumab has been commonly used for the management of high IL-6-mediated severe cytokine release syndrome (CRS); however, it is not useful for managing severe CRES because tocilizumab cannot pass through the blood brain barrier (BBB) to the CNS.
Here, we report three CNS B-ALL patients who were successfully treated with short hairpin RNA (shRNA)-IL-6-modified anti-CD19 CAR-Ts (referred to as ssCART-19s) to prevent or/and reduce CRES development. All the CNS B-ALL patients in this study achieved complete remission (CR) without severe CRS, and no CRES was noted regardless of whether ssCART-19s were intravenously or intrathecally administered. This is the first report showing that shRNA-IL6-modified CART-19s can be successfully used to treat CNS B-ALL patients without severe CRES. Thus, ssCART-19s might be a novel therapeutic strategy for CNS B-ALL as well as other hematological malignancies with CNS involvement.
Patients and methods
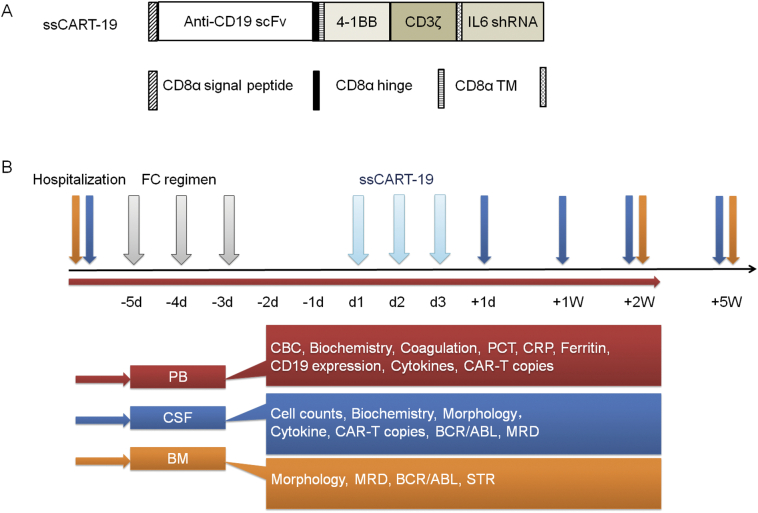
Manufacture of ssCAR-T19s
Patient 1 and 3 T cells were enriched from donor leukapheresis, patient 2 and 4 T cells were enriched from their own leukapheresis, and were all isolated using anti-CD3 magnetic beads (Miltenyi, Biotec, Bergisch-Gladbach, Germany). T cells were then stimulated with anti-CD3/CD28 (Miltenyi, Biotec, Bergisch-Gladbach, Germany) monoclonal antibodies. The cells were transduced with recombinant lentiviral vectors encoding the CD19-specific CAR, made up of an anti-CD19 single chain variable fragment (scFv), a 4-1BB costimulatory moiety and a CD3zeta activation domain with an IL-6 shRNA element (Fig. 1A). CAR-Ts were cultured in AIM-V media (Gibco, NY, USA) supplemented with 10% autologous serum, 100 IU/mL IL-2, 5 ng/mL IL-7, and 5 ng/mL IL-15 for 12 days. The transduction efficiency; % of CD3+, CD4 over CD8 ratios; and sterility (bacteria, endotoxin and mycoplasma) were analyzed before the release of the products.
Fig. 1.
The treatment courses of CAR-T therapy and clinical assessments at time points.
A Schematic structure of the CD19 CAR vector containing the anti-human CD19 scFv (FMC63) linked to 4-1BB costimulatory domains and a CD3-ζ signaling domain with shRNA against IL-6.
B Patients received the intravenous infusion of ssCART-19s at a dose of 5 × 106 cells per kg for 3 consecutive days after the FC regimen. Patients were evaluated by blood, CSF, BM and MRI examinations during hospitalization (shown in bars of various colors).
Abbreviations: CBC, complete blood count; PCT, procalcitonin; CRP, C-reactive protein; MRD, minimal residual disease; STR, short tandem repeats.
Study population
We generated ssCART-19s (Fig. 1A) and conducted an institutional review board–approved phase 1 clinical trial (ClinicalTrials.gov number, NCT03064269) at the First Affiliated Hospital of Soochow University. The trial was designed to assess the safety and feasibility of anti-CD19 CAR-T therapy in patients with CNS CD19+ B-ALL. The enrolled patients met the inclusion criteria (shown in Supplementary information).
Case 1
Patient 1 was a 36-year-old man who received a diagnosis of CD19-positive B-ALL transformation from chronic myelogenous leukemia (CML) with BCR/ABL fusion transcripts in May 2013. After chemotherapies with a TKI, morphologic remission was achieved, but the T315I BCR/ABL mutation was positive, and blast cells were found in CSF. Then, he received 6 cycles of intrathecal chemotherapies until blasts were absent in CSF. Subsequently, he underwent allo-HSCT from an unrelated donor in November 2013 and experienced sustained remission. However, the BCR/ABL transcript was detected again in March 2015 and continued to rise despite the withdrawal of immunosuppressants. He presented seizures, consciousness and CNS impairment and visual loss in December 2016. A mass of blast cells was found in CSF but the BM was negative.
Case 2
Patient 2 was a 48-year-old woman diagnosed with Ph chromosome-positive B-ALL in 2013. She remained in remission after undergoing induction and consolidation chemotherapies during 2013–2015, and oral imatinib was used afterwards. In November 2016, she began to experience headache, vomiting and blurred vision, and these symptoms worsened to visual loss half month later. No lesions were found in the brain by MRI scans. Examinations revealed the existence of blast cells in CSF but not in the BM. The blast cells in CSF became negative after high-dose methotrexate (HD-MTX) therapy and one course of intrathecal chemotherapy, but BCR/ABL transcripts remained positive.
Case 3
Patient 3 was a 48-year-old woman diagnosed with B-ALL in 2016. One month after consolidation treatment, she relapsed and underwent salvage transplantation from a matched sibling in 2017. She remained in remission until 4 months later when the transcript copies of the WT1 gene significantly increased. She discontinued cyclosporin, and the WT1 gene transcript copies declined to 80–150/10,000 abl copies, but skin GVHD occurred. After short-course oral prednisone administration, skin GVHD was controlled. Eight months after transplantation, she experienced double vision. Physical examinations showed left abducent paralysis, and MRI showed a mass in the sellar region (Fig. 2C). Flow cytometry analysis showed that the MRD was 3.1% in CSF but was negative in the BM, indicating leukemia in the CNS.
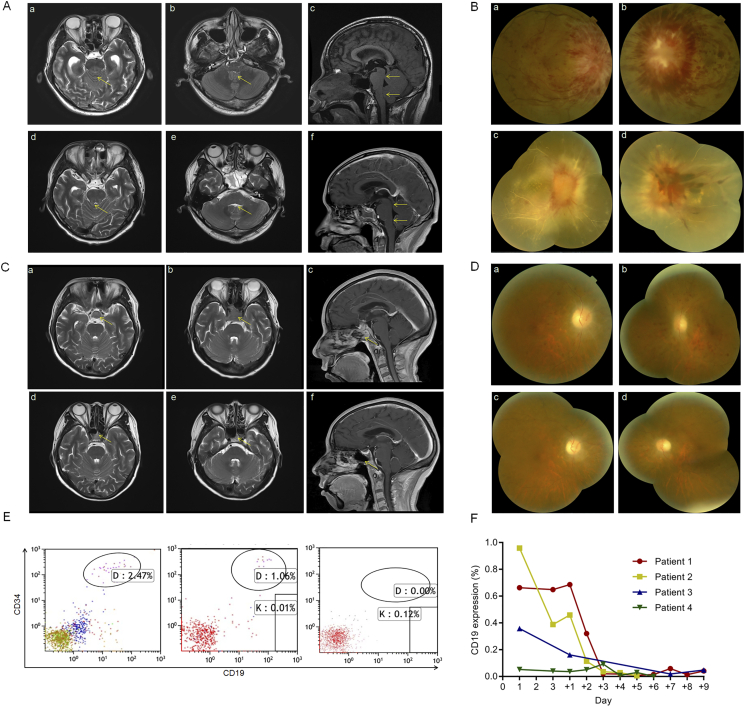
Fig. 2.
The clinical responses before and after ssCART-19 infusions in patients.
A (a) The T2-Weighted imaging (T2WI) signal of the pontine parenchyma in patient 1 was slightly high (arrowhead). (b–c) The end of the central aqueduct and four ventricle outlets became narrow (arrows), secondary to mild obstructive hydrocephalus. (d–f) One month after treatment, the pontine dorsal swelling in patient 1 was significantly reduced, the T2WI signal was nearly normal, and the degree of stenosis decreased at the end of the central aqueduct and at the outlet of the fourth ventricle.
B (a–b) The fundus showed papillary edema and a flame-shaped hemorrhage on the surface of the optic disc and nearby retina. The height of the optic disc was over the scale of optical coherence tomography (OCT). These photos were taken when patient 1 was admitted. (c–d) The optic disc edema subsided, the hemorrhage was absorbed in the right eye, and all the vessels of the retina became white-line-like. Bleeding clots in the left eye covered the optic disc. These photos were taken one month after infusion in patient 1.
C (a–c) T2WI signals showed pituitary tumors in the sellar region (arrows) when patient 3 was enrolled. (d–f) Tumors were significantly reduced and almost disappeared on day +35 after CAR-T therapy (arrows).
D (a–b) The optic disc showed edema and was pale. A large amount of punctate bleeding was observed on the retina. These photos were taken when patient 2 was admitted. (c–d) The optic disc edema was alleviated, and the bleeding decreased. These photos were taken one week after infusion in patient 2.
E MRD of ALL was evaluated in CSF in patient 3 by flow cytometric analysis on days −5, +1 and +7.
F Flow cytometry analysis of PB from patients after CAR-T infusion using antibodies against CD3 and CD19.
Study design
Patients were first evaluated with BM puncture, lumbar puncture, MRI scans, blood tests, and physical examinations performed by hematological and neurological physicians. Patients were then conditioned with fludarabine (30 mg/m2 per day) and cyclophosphamide (300 mg/m2 per day) on days −5, −4, and −3. Two days after conditioning, patients received ssCART-19 infusions at a total dose of 5 × 106 cells per kg, given for 3 consecutive days (patient 2 also received one intrathecal infusion at a dose of 1 × 107 cells). The patients underwent a series of regular tests (Fig. 1B) at particular time points to monitor the response and side effects. The median follow-up was 2.5 years.
Results
Clinical responses and evaluations
We first assessed the clinical responses in these patients. These patients' conditions (including consciousness and CNS impairments) significantly improved after ssCART-19 infusion along with dehydration and antiepileptic treatments (Table 1). The seizures in patient 1 were effectively controlled and did not deteriorate further during therapy, the brain edema decreased significantly one month after therapy (Fig. 2A). Severe papilledema with retinal hemorrhage was detected by fundoscopy in both patient 1 and patient 2 and was gradually alleviated (Fig. 2B, D), even though the visual loss was permanent. The size of the mass in the sellar region in patient 3 had significantly reduced based on magnetic resonance imaging (MRI) (Fig. 2C).
Table 1.
Summary of patient characteristics, clinical responses and toxicities after anti-CD19 CAR-T therapy in four patients.
| Patients | Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Age | 36 | 48 | 48 | 65 | ||||
| Sex | Male | Female | Female | Male | ||||
| ECOG | 4 | 2 | 2 | 1 | ||||
| Prior therapies | Induction therapy: MVP + imatinib Cycles of intrathecal injection Unrelated-HSCT |
Induction therapy: DVLP + imatinib Cycles of consolidation therapy Cycles of intrathecal injection HD-MTX,HD-MTX + VP + imatinib |
Induction therapy: VDCP Cycles of consolidation therapy Sib-HSCT |
Induction therapy: IVP + nilotinib | ||||
| Relapsed/refractor y status | 1st CNS relapse postinduction therapy within 2 months; 2nd CNS relapse post-HSCT within 2 years | CNS relapse postconsolidation therapy + imatinib within 2 years | CNS relapse post-sib-HSCT within 4 months | In remission | ||||
| Time (months) to progression or last follow-up | 3 months to relapse | CR in 12 months | CR in 10 months | CR until now | ||||
| Clinical response | Before | After | Before | After | Before | After | Before | After |
| Complaints | Headache, dizziness, fatigue, epileptic seizures, impaired consciousness | Visual loss | Headache, vomiting and visual loss | Visual loss | Double vision | Normal | Normal | Normal |
| Symptoms | Visual loss in both eyes, bilateral pupillary light reflex disappeared, left central facial palsy, bilateral Babinski's sign positive, limb muscle strength decreased | Visual loss in both eyes, left central facial palsy disappeared, bilateral Babinski's sign became negative, limb muscle strength improved (upper) | Normal | Normal | Left abducent paralysis | Normal | Normal | Normal |
| GCS score | 9 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| NIHSS score | 21 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Barthel index | 0 | 50 | 100 | 100 | 100 | 100 | 100 | 100 |
| BM | CR | CR | CR | CR | CR | CR | CR | CR |
| Molecular | BCR/ABL 4294/10,000 abl copies |
BCR/ABL <10/10,000 abl copies |
BCR/ABL 33/10,000 abl copies |
BCR/ABL 35/10,000 abl copies |
MRD 2.5 × 10−4 by flow cytometry | MRD 0 × 10−4 by flow cytometry | MRD 5.7 × 10−4 by flow cytometry | MRD 0 × 10−4 by flow cytometry |
| CSF | Positive | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Molecular | BCR/ABL positive | BCR/ABL positive | BCR/ABL positive | BCR/ABL negative | MRD 2.47% by flow cytometry | MRD 0 × 10−2 by flow cytometry | BCR/ABL negative | BCR/ABL negative |
| MRI | Signal in the pontine parenchyma was slightly high and the end of the central aqueduct and four ventricle | The pontine dorsal swelling was significantly reduced and the degree of stenosis reduced | Negative | Negative | Pituitary tumor in the sellar region | Tumor almost disappeared | N/A | N/A |
| CRS grade | 1 | 1 | 1 | 1 | ||||
| ICE score | 9 | 10 | 10 | 10 | ||||
| ICANS grade | 1 | 1 | 1 | 1 | ||||
The CD19 expression in all patients decreased dramatically since ssCAR19 infusion (Fig. 2F). Numerous blasts in patient 1 and BCR/ABL fusion gene transcripts in patient 2 disappeared in CSF after a single infusion of ssCART-19s based on examinations with a microscope with a high-powered lens and quantitative polymerase chain reaction (qPCR) (Table 1). However, disease recurred at the cellular and gene levels in patient 1 and 2, three months and 65 days respectively after the first infusion. For salvage treatment and to prolong the patient survival time, we administered one intrathecal injection of ssCART-19s a dose of 1 × 107 to patient 2 after adequate medical communications with the medical panel and family. The BCR/ABL fusion gene transcript became negative without any complications or patient discomfort. Similar responses were obtained in patient 3, showing the minimal residual disease (MRD) (Fig. 2E) dramatically decreased.
The response to ssCART-19 therapy in the bone marrow (BM) was also evaluated. Although all patients were in remission in the BM when they were admitted to the hospital, the MRD in patient 1 as detected by qPCR significantly decreased, and all patients had sustained negative results when they were discharged. In addition, the donor chimerism in patient 1 increased from 92% to 97.7%. These dramatic clinical responses in patient 1 were sustained for 3 months after ssCART-19 infusion. Unfortunately, this patient's disease eventually recurred when the patient chose to stop therapy. Patient 2 was taking imatinib and remained good living without relapse until one year after ssCART-19 therapy and died of pulmonary infections associated with leukopenia. Patient 3 had sustained remission, but unfortunately 10 months after therapy lung GVHD occurred and died of subsequent lung infection.
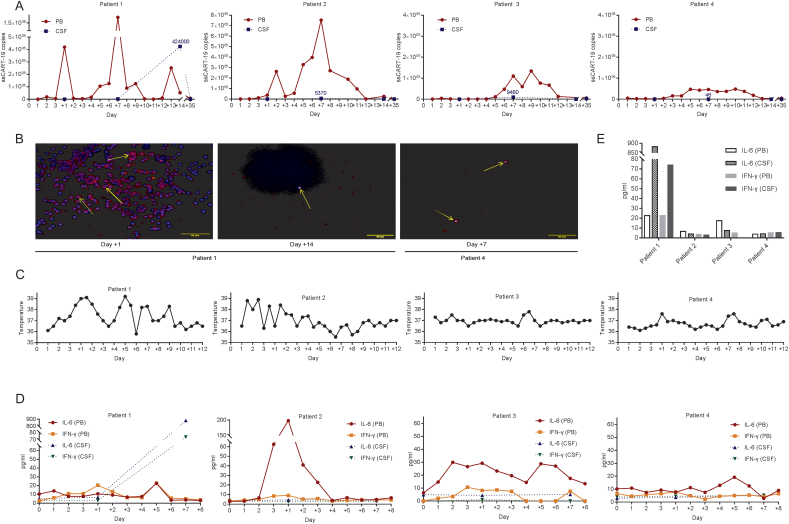
ssCART-19 proliferation and persistence
After the intravenous infusion of CAR-Ts, the number of ssCART-19s in serum peaked on approximately day +1 and day +7 (Fig. 3A). Consistent with the second proliferation peak in serum, a delayed expansion peak was detected in CSF on approximately day +14 that was elevated more than 5 logs in patient 1, while the expansion profile in CSF in patients 2 and 3 presented a relatively lower elevation on approximately day +7. These cells were present and persisted in CSF for at least two weeks in patients 1, 2 and 3, as detected by immunofluorescence and qPCR (Fig. 3B). However, compared to the high levels of ssCART-19s in serum 2 months after infusions in patients 1, 2 and 3, these cells were barely detectable in CSF one month after infusion.
Fig. 3.
CAR-T expression and cytokine levels in serum and CSF after infusion.
A Expansion and persistence of CAR-T cells in PB and CSF were assessed with quantitative real-time polymerase chain reaction (qRT-PCR).
B Cells in the CSF of patient 1 were stained by immunofluorescence on days +1 and + 14, and cells in the CSF of patient 4 were stained on day +7 after infusion. CAR-Ts were identified by PE immunofluorescence in the cytoplasm (yellow arrows), and the nucleus was labeled by DAPI.
C The lines represent the patients' temperature in degrees centigrade (°C) per 12-hour period.
D The trends of IL-6 and IFN-γ concentrations in PB were monitored each day. These circulating inflammatory cytokines in CSF were also tested upon hospitalization and on days +1 and +7 by lumbar puncture.
E The bars represent the concentrations of IL-6 and IFN-γ in PB and CSF on day +7.
The proliferation of CAR-Ts was accompanied by an increase in inflammatory cytokines in serum and CSF compared to the baseline levels in patients 1, 2 and 3 (Fig. 3D). These cytokines included interleukins 2, 4, 6, 10, and 17a, interferon-γ and tumor necrosis factor α. The increase in cytokine levels, such as interleukin-6, in CSF paralleled the proliferation level of CAR-Ts and the tumor burden, which ranged widely from 0.5 to 38 times the baseline serum levels. Interestingly, the isolated mass in the brain parenchyma in patient 3 seemed to cause a smaller increase in cytokines and CAR-Ts in CSF compared to the increase observed in patients 1 and 2.
Since dramatic clinical responses to ssCART-19 therapy were achieved in 3 CNS ALL patients, we were interested in the migration features of CAR-Ts into CSF and in the proliferative features of CAR-Ts in the CSF of patients with different tumor burdens. We next studied these features in a patient with remission in the BM without CNS involvement. Patient 4 was a 65-year-old man diagnosed with ALL with Philadelphia (Ph) chromosome positivity in January 2017 without CNS involvement. He was treated with induction chemotherapy plus a tyrosine kinase inhibitor (TKI) and entered remission. One month later, he was enrolled in another institutional review board approved phase 1 clinical trial (ClinicalTrials.gov number, NCT03984968), and received an infusion of ssCART-19s. In patient 4, the expansion profile of ssCART-19 cells had one peak in serum on approximately day +5 and a detectable expansion in CSF, but the cells increased by only 1 log (Fig. 3A). Moreover, the CAR-T proliferation level in CSF rapidly decreased in one week, although a high level of expansion persisted in serum. Meanwhile, the cytokine level and temperature were quite stable in patient 4. Similarly, there was almost no obvious proliferation of ssCART-19s in CSF in patient 2 after the second administration of ssCART-19s by intrathecal injection. A higher tumor burden was correlated with a more vigorous CAR-T expansion in serum as well as in CSF.
Safety and adverse-event profile
The intravenous infusion and intrathecal injection of ssCART-19s were safely performed at a maximum dose of 5 × 106 CAR-Ts/kg and 1 × 107 cells/kg, respectively. These treatments were associated with CRS of no higher than grade 2. No aggravation of CRES was found, and a grade 1 immune effector cell-associated encephalopathy (ICE) score [13] and immune effector cell-associated neurotoxicity syndrome (ICANS) score [13] were observed in all patients. No GVHD or severe infections occurred during ssCART-19 treatment and hospitalization.
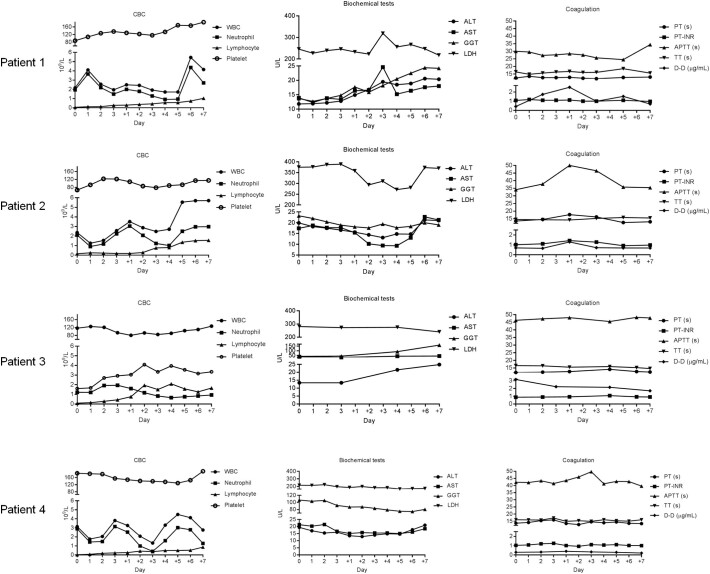
Patients had fevers after the intravenous infusion of CAR-Ts (Fig. 3C). When patient 2 received the intrathecal injection of CAR-Ts to eradicate MRD in CSF, no fever occurred, probably due to the lower tumor burden in CSF that was present after the first CAR-T therapy. Complete blood count (CBC), routine biochemistry, and coagulation tests were performed after CAR-T therapy, and the results were normal except for neutropenia that resolved with G-CSF management (Supplementary Fig. 1).
Supplementary Fig. 1.
CBCs, including the concentrations of white blood cells, lymphocytes, neutrophils and platelets, and biochemical and coagulation tests were performed after ssCART-19 infusion. Abbreviations: ALT, glutamic-pyruvic transaminase(U/L); AST, glutamic oxaloacetic transaminase (U/L); GGT, gamma-glutamyl transpeptidase (U/L); LDH, lactate dehydrogenase (U/L); PT, prothrombin time (s); PT-INR, international standardized ratio of prothrombin time; APTT, activated partial thromboplastin time (s); TT, thrombin time (s); DD, D-dimer (μg/mL).
In summary, adverse events defined as CRS were observed within 10 days after ssCART-19 infusions. These events included headaches, generalized fatigue and fevers. Concomitant medications that might have influenced this safety profile included divalproex at a dose of 400 mg three times a day and mannitol (125 mL twice a day) for seizure control and acetaminophen as needed.
Discussion
The administration of CAR-Ts to treat CNS B-ALL remains contraindicated, especially with intrathecal injections, due to potentially uncontrollable, life-threatening CRES events. The pathophysiological mechanism underlying CRES remains to be determined. Previous studies suggest that severe neurologic toxicities are associated with high serum levels of IL-6 and IL-15 [9] and the disruption of the BBB [14]; other organ dysfunctions might also contribute to encephalopathy. The anti-IL-6 receptor monoclonal antibody tocilizumab can alleviate CRS without influencing CAR-T therapeutic efficacy but failed to prevent delayed neurotoxicity due to penetrability [10,15]. Based on these results, we generated ssCART-19s, and our previous study indicated that ssCART-19s reduced the secretion of CRS-implicated cytokines, including IL-6, without affecting target cell killing in vitro or in a preclinical animal mode [16]. Recently, Norelli et al. demonstrated that monocyte-derived IL-1 and IL-6 are required for CRS and neurotoxicity in response to CAR-Ts [17]. The authors noted that CAR-Ts produce negligible levels of IL-6 upon tumor recognition in vitro and that IL-1 precedes IL-6 production. In previous unpublished studies from our group, CAR-Ts secreted IL-6 upon CAR activation, which triggered IL-6 secretion by monocytes in vitro. Nathan Singh et al. recently performed coculture experiments combining monocyte-lineage cells, T cells and targets and reported that negligible IL-6 was detected in the absence of antigen-presenting cells (APCs) or in the absence of CAR-Ts, but a low level was produced in the absence of targets (T cells and immature dendritic cells (DCs) alone) [18]. In this study, ssCART-19s eliminated leukemia cells in CNS B-ALL without causing CRES or severe CRS, which was less severe than the responses reported in response to regular CAR-T therapies. Our data do not contradict a previous study; rather, they show the feasibility of a treatment to alleviate CRS and emphasize another important role of the IL-6 released from CAR-Ts in CRS and CRES development and cascades. Furthermore, these studies raise another question: whether CAR-Ts or monocytes upstream in the initiation of CRS and CRES, this question needs to be investigated in the future.
In the present study, we confirmed that ssCART-19s migrated into the CNS in patients 1, 2, and 3 by qPCR and immunofluorescence techniques. Interestingly, we also detected a slight elevation (10-fold) in CAR-T cells in CSF concurrent with the expansion in serum in patient 4, who did not have CNS involvement. Thus, CAR-T cells could migrate and persist in CSF regardless of whether CNS ALL is present or whether blasts exist in the BM, which provides a foundation for CNS ALL treatment with CAR-Ts. We also observed that CAR-Ts in peripheral blood (PB) presented much higher proliferation levels than those in CSF (Fig. 3A). The greater expansion of ssCART-19s in PB may be related to the burden of CD19+ B cells in the circulation compared to the CSF. In addition, the time of the peak expansion in CSF is consistent with that in serum, which confirms that ssCART-19s migrate into CSF through the BBB. However, localized cytokine levels were much higher in CSF than in the PB in patient 1, indicating that despite the presence of cells in the CNS, no neurotoxicity developed, which further shows the safety and feasibility of CNS ALL treatment with CAR-Ts.
It is widely accepted that a higher tumor burden characterized by a higher proportion of blasts in the BM or higher CD19+ cell counts is correlated with more vigorous CAR-T cell expansion, more severe CRS and neurotoxicity [10,11]. As shown in patients 1 and 2, our results showed a similar correlation between the tumor burden and CAR-T expansion or cytokine levels (Fig. 3A, D). Notably, there was a significantly moderate response in patient 3, with relatively lower cytokine and CAR-T levels compared to those in patient 1 (Fig. 3E), despite both patients having visible tumors that were no longer visible on MRI after ssCART-19 therapy. These results suggest that intravenous infusions may target both separate cells in CSF and cell masses in the brain parenchyma. In terms of patient-specific factors, ALL is more strongly associated with the development of severe CRS and neurotoxicity than non-Hodgkin lymphoma [19]. Based on the present results, we hypothesize that cytokine and CAR-T are determined by the tumor burden in CSF but not in solid brain tissue. This hypothesis has been rarely described in isolated CNS ALL; further investigations should be performed and may provide evidence for clinical therapy.
Hamdi reported that the CNS relapses occurred at a median of 231 days in CNS leukemia patients after receiving allo-HSCT [6]. It is noteworthy, among 6 patients with no post-HSCT CNS therapy, the median time was 72.5 days. Compared to allo-HSCT strategy for CNS relapse, ssCART-19 seems to show comparative effect in prolonging RFS (relapse free survival) in these cases. Although three patients effectively achieved remission with ssCART-19 infusion, patient 1 recurred after several months. Inadequate T cell function related to cycles of chemotherapies, overwhelming disease burden, heterogeneity of the CSF penetrability by CAR-Ts and the environment of the CNS as a sanctuary may have caused recurrence [9]. The dose escalation of CAR-T infusion is being performed in a phase I study and needs to be followed up and analyzed. Future treatments will require more than a single infusion or the use of bivalent chimeric antigen receptors (CARs) targeting both CD19 and CD22 to eliminate MRD [20].
Researchers have attempted the intrathecal infusion of donor lymphocytes to treat CNS ALL relapse [21] and the intracranial infusion of anti-IL13Rα2 CAR-Ts to treat glioblastoma [22]. Both studies indicated that the intrathecal or intracranial infusion of allogeneic T cells or CAR-Ts with the concurrent use of antiepileptic drugs was safe and feasible options for treating CNS tumors. In the present study, patient 2 received the intrathecal infusion of anti-CD19 CAR-Ts at a set dosage to determine the CAR-T cell dose that migrated into the CNS to improve the treatment efficacy; this dosage was proven to be feasible and safe and needs to be further confirmed in more clinical studies.
Conclusion
In summary, based on our research, CAR-Ts can migrate into the CNS, eradicate leukemic cells, and elevate cytokine levels in CSF with mild, acceptable side effects. More studies are still required to validate the advantages of ssCARTs compared to conventional CAR-Ts in the future.
The following are the supplementary data related to this article.
Supplementary material
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We acknowledge all members of the study team, the patients and their families.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81970138, 81570138, 81873449), Translational Research Grant of NCRCH (grant no. 2020ZKMB05), Jiangsu Province “333” project (grant no. BRA2018391), Jiangsu Provincial Medical Youth Talent Program (grant no. QNRC2016719), a C class sponsored project from Six Talent Peaks Project in Jiangsu Province (grant no. 2016-WSN-123) and Gusu Key Medical Talent Program (grant No. GSWS2019007).
Authors' contributions
Li-Yun Chen: Investigation, Methodology, Writing - Original Draft, Writing - Review & Editing. Li-Qing Kang: Methodology, Formal analysis, Resources. Hai-Xia Zhou: Validation, Investigation, Resources. Han-Qing Gao: Validation, Visualization. Xue-Fei Zhu: Validation, Visualization. Nan Xu: Formal analysis. Lei Yu: Methodology, Writing - Original Draft, Supervision. De-Pei Wu: Supervision, Project administration. Sheng-Li Xue: Conceptualization, Investigation, Writing - Original Draft, Writing - Review & Editing, Funding acquisition. Ai-Ning Sun: Supervision, Funding acquisition.
Ethics approval and consent to participate
The research protocol referenced in this manuscript and the whole study were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and followed the tenets of the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from the patient in the study.
Consent for publication
The participants have given express consent for publication of their cases and the associated images. All personal information has been made anonymous.
Availability of data and material
Data are available on reasonable request.
Contributor Information
De-Pei Wu, Email: wudepei@suda.edu.cn.
Sheng-Li Xue, Email: slxue@suda.edu.cn.
Ai-Ning Sun, Email: sunaining@suda.edu.cn.
References
- 1.Salzer W.L., Devidas M., Carroll W.L., Winick N., Pullen J., Hunger S.P. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the children's oncology group. Leukemia. 2010;24(2):355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan S., Wade R., Moorman A.V., Mitchell C., Kinsey S.E., Eden T.O.B. Temporal changes in the incidence and pattern of central nervous system relapses in children with acute lymphoblastic leukaemia treated on four consecutive Medical Research Council trials, 1985–2001. Leukemia. 2010;24(2):450–459. doi: 10.1038/leu.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waber D.P., Turek J., Catania L., Stevenson K., Robaey P., Romero I. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL consortium protocol 95-01. J. Clin. Oncol. 2007;25(31):4914–4921. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 4.Howard S.C., Pui C.H. Endocrine complications in pediatric patients with acute lymphoblastic leukemia. Blood Rev. 2002;16(4):225–243. doi: 10.1016/s0268-960x(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 5.Hijiya N., Hudson M.M., Lensing S., Zacher M., Onciu M., Behm F.G. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA J. Am. Med. Assoc. 2007;297(11):1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 6.Hamdi A., Mawad R., Bassett R., di Stasi A., Ferro R., Afrough A. Central nervous system relapse in adults with acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014;20(11):1767–1771. doi: 10.1016/j.bbmt.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J.H., Geyer M.B., Brentjens R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127(26):3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle C.J., Hanafi L.-A., Berger C., Gooley T.A., Cherian S., Hudecek M. CD19 CAR-T cells of defined CD4(+): CD8(+) composition in adult B cell ALL patients. J. Clin. Investig. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 14.Santomasso B., Park J.H., Riviere I., Mead E., Halton E., Diamonte C. Biomarkers associated with neurotoxicity in adult patients with relapsed or refractory B-ALL (R/R B-ALL) treated with CD19 CAR T cells. J. Clin. Oncol. 2017;35 [Google Scholar]
- 15.Park J.H., Riviere I., Gonen M., Wang X., Senechal B., Curran K.J. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang L., Tang X., Zhang J., Li M., Xu N., Qi W. Interleukin-6-knockdown of chimeric antigen receptor-modified T cells significantly reduces IL-6 release from monocytes. Experimental Hematology & Oncology. 2020;9(1) doi: 10.1186/s40164-020-00166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature Medicine. 2018;24(6):739. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 18.Singh N., Hofmann T.J., Gershenson Z., Levine B.L., Grupp S.A., Teachey D.T. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy. 2017;19(7):867–880. doi: 10.1016/j.jcyt.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brudno J.N., Kochenderfer J.N. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegde M., Corder A., Chow K.K.H., Mukherjee M., Ashoori A., Kew Y. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther. 2013;21(11):2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagisawa R., Nakazawa Y., Sakashita K., Saito S., Tanaka M., Shiohara M. Intrathecal donor lymphocyte infusion for isolated leukemia relapse in the central nervous system following allogeneic stem cell transplantation: a case report and literature review. Int. J. Hematol. 2016;103(1):107–111. doi: 10.1007/s12185-015-1902-1. [DOI] [PubMed] [Google Scholar]
- 22.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data are available on reasonable request.