Summary
The human malaria parasite Plasmodium vivax remains vastly understudied, mainly due to the lack of suitable laboratory models. Here, we report a humanized mouse model to test interventions that block P. vivax parasite transition from liver stage infection to blood stage infection. Human liver-chimeric FRGN huHep mice infected with P. vivax sporozoites were infused with human reticulocytes, allowing transition of exo-erythrocytic merozoites to reticulocyte infection and development into all erythrocytic forms, including gametocytes, in vivo. In order to test the utility of this model for preclinical assessment of interventions, the invasion blocking potential of a monoclonal antibody targeting the essential interaction of the P. vivax Duffy Binding Protein with the Duffy antigen receptor was tested by passive immunization. This antibody inhibited invasion by over 95%, providing unprecedented in vivo evidence that PvDBP constitutes a promising blood stage vaccine candidate and proving our model highly suitable to test blood stage interventions.
Subject Areas: Microbiology, Model Organism, Parasitology
Graphical Abstract
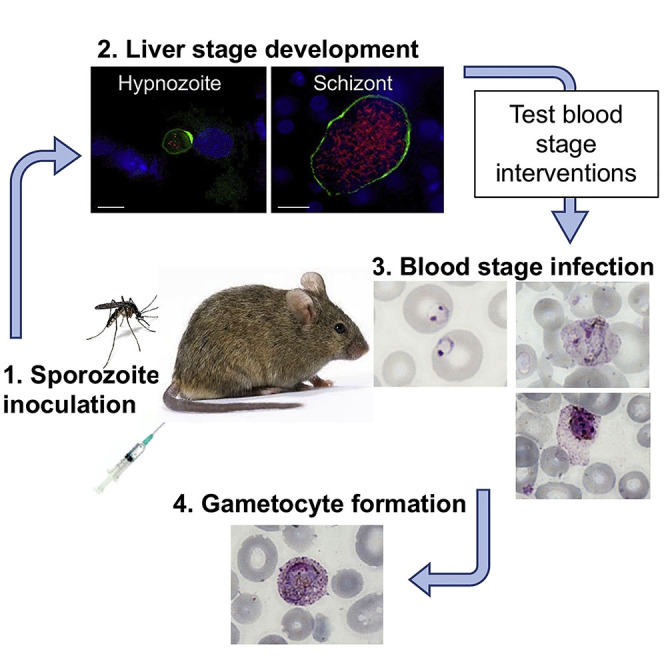
Highlights
-
•
A humanized mouse model allows P. vivax liver stage to blood stage transition
-
•
This model is highly suitable to test P. vivax blood stage interventions in vivo
-
•
P. vivax parasites can commit to sexual development as they emerge from the liver
Microbiology; Model Organism; Parasitology
Introduction
Plasmodium vivax (P. vivax) and Plasmodium falciparum (P. falciparum) are the leading causes of malaria worldwide, putting nearly half the world's population at risk of infection. P. falciparum causes the majority of malaria-associated morbidities and mortalities in sub-Saharan Africa, whereas P. vivax is geographically most widespread (Gething et al., 2011; Shretta et al., 2017). Its endemicity throughout tropical as well as temperate climate zones is attributed to the parasite's ability to form dormant liver stages, hypnozoites, which can activate weeks or months after the primary infection, leading to repeated onset of blood stage infection and recurring transmission (Adams and Mueller, 2017; White et al., 2014; White, 2011).
The establishment of a continuous in vitro culture system for the blood stages of P. falciparum more than 40 years ago (Trager, 1977) has revolutionized insights into parasite biology and malaria pathogenesis, allowing genetic manipulation (Goswami et al., 2019) as well as extensive omics studies (Cowell and Winzeler, 2019) and evaluation of novel interventions (Cowell and Winzeler, 2019; Mogire et al., 2017). In contrast, research on P. vivax greatly lags behind and despite more than a century of efforts (Bass and Johns, 1912; Noulin et al., 2013), a continuous in vitro culture system has yet to be established. Unlike P. falciparum, which infects red blood cells (RBCs) of all stages of maturity, P. vivax preferentially, if not exclusively, infects reticulocytes expressing the surface marker CD71 (Gruszczyk et al., 2018; Malleret et al., 2015). These cells are produced in the bone marrow and released into circulation as they mature, where they constitute only 0.5% to 1.5% of all RBCs (Ney, 2011). Reticulocytes can be enriched by magnetic beads or density gradient centrifugation, but robust growth of P. vivax parasites even in pure reticulocyte preparations cannot routinely be observed in vitro (Bermudez et al., 2018).
The lack of an in vitro culture system also prevents the generation of gametocytes, limiting our current knowledge of P. vivax gametocytogenesis. Since the full P. vivax life cycle cannot be maintained in the laboratory, research relies on patient samples. This is a great obstacle for any P. vivax research and largely limits the progress that can be achieved in the field. Furthermore, it necessitates the use of a different field isolate for every experiment, complicating assay optimization and often leading to great inter-experimental variation due to differences between the strains. Clearly, the lack of a continuous in vitro culture system impedes basic research on P. vivax erythrocytic stages and there is an urgent need for relevant models to test disease interventions.
Recently, the use of human liver-chimeric mice has opened up new avenues for research on P. vivax liver stages. Fah−/−Rag2−/−IL2rg−/− mice transplanted with primary human hepatocytes (FRG KO huHep) (Azuma et al., 2007) are highly susceptible to infection with P. vivax sporozoites and support full P. vivax liver stage development as well as the formation and activation of hypnozoites (Mikolajczak et al., 2015). Backcrossing of FRG mice to the non-obese diabetic (NOD) background (FRGN KO) additionally makes these mice more suitable for repopulation with human red blood cells. This increased tolerance of human cells is due to a NOD strain-derived polymorphism in the signal-regulatory protein alpha (SIRPα), leading to improved engagement of SIRPα expressed on mouse phagocytes with its ligand CD47, ubiquitously expressed on the transferred human cells, thereby providing a more efficient “don't-eat-me signal” (Kwong et al., 2014; Yamauchi et al., 2013).
Here, we show that FRGN KO huHep mice support P. vivax liver stage development with formation of exo-erythrocytic merozoites that efficiently infect infused human reticulocytes, allowing reproducible transition from liver stage infection to blood stage infection. We provide evidence that this model fills the gap of an urgently needed small animal model that allows testing of P. vivax erythrocytic stage interventions.
Results
Successful Transition of P. vivax Liver Stage Parasites to Blood Stage Parasites
A pivotal transition point in the malaria life cycle occurs when parasites emerging from the liver infect the first red blood cells. We have shown previously that P. vivax liver stage development in FRG KO huHep mice is completed 9–10 days post sporozoite infection and culminates with the release of exo-erythrocytic merozoites from the liver into the blood stream (Mikolajczak et al., 2015). Here, we developed this model further in order to allow exo-erythrocytic merozoite infection of reticulocytes and asexual blood stage development. We modified our protocol previously developed for P. falciparum (Foquet et al., 2018) using immunomodulation with Clodronate liposomes and Cyclophosphamide to deplete mouse macrophages and mouse neutrophils, respectively, which prevents clearance of infected human red blood cells. We combined this immunomodulation protocol in FRGN KO huHep mice with intravenous (i.v.) injections of enriched human reticulocyte preparations on days 9 and 10 post P. vivax sporozoite infection (Figure 1A). In order to also decrease the potential innate immune response in the host that is caused by liver infection (Liehl et al., 2015; Miller et al., 2014), Clodronate Liposomes and Cyclophosphamide were injected intraperitoneally (i.p.) on days 3 and 7 post infection.
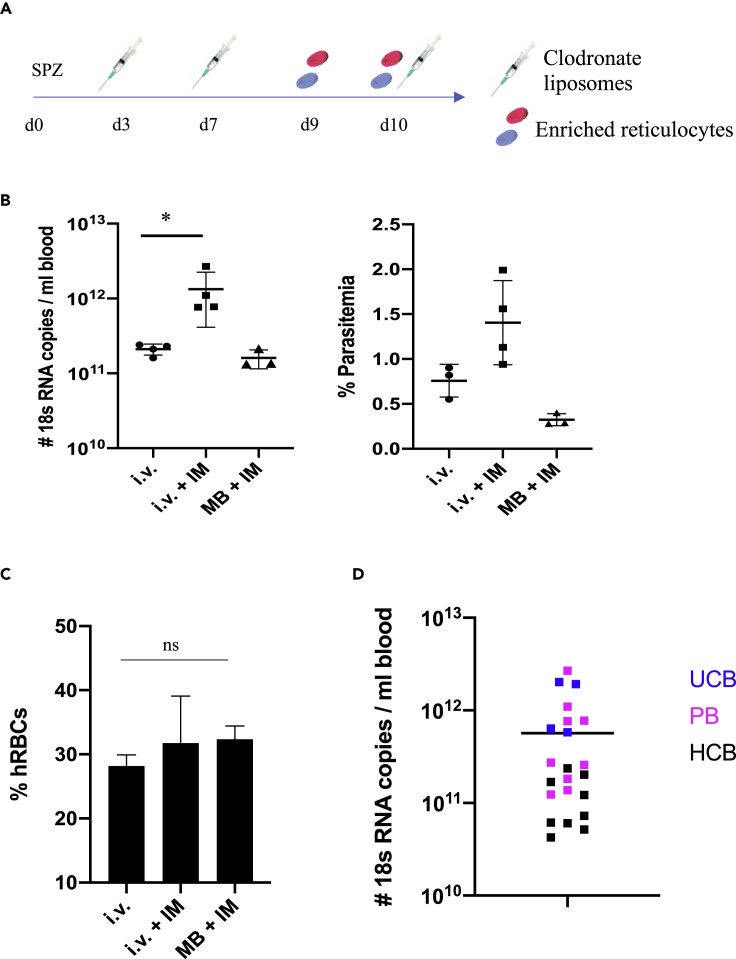
Figure 1.
Reticulocyte Invasion by Exo-Erythrocytic Merozoites
(A) FRGN KO huHep mice were infected with P. vivax sporozoites either by intravenous injection of salivary gland sporozoites or by mosquito bite (50 mosquitos per mouse). Three days post infection, mice received 150 μL Clodronate liposomes and 150 mg/kg Cyclophosphamide intraperitoneally. These injections were repeated on days 7 and 10 post infection. On days 9 and 10 post infection, 400 μL enriched human reticulocytes at 50% hematocrit was injected intravenously to provide a pool of target cells for parasites egressing from the liver.
(B) Mice were randomized into three groups of which two groups were infected by injection of 0.9 million sporozoites (i.v.) and one group was infected by the bite of 50 mosquitos (MB). One group did not receive any immunomodulation (IM). P. vivax 18S RNA was measured by qRT-PCR, and parasitemia was counted on thin blood smears 5 h after the second reticulocyte injection. Parasites were detected in all groups, and the i.v. + IM protocol led to a significant increase in parasites detected. Each dot represents one mouse. Shown is mean ± SD. Statistics: Mann-Whitney test; ∗p = 0.0286.
(C) IFAs were performed on thin blood smears staining for human Glycophorin A. Thousand cells were counted per slide and the percentage of human red blood cells was calculated. No significant difference between the groups was observed. Shown is mean ± SD.
(D) The copy number of P. vivax 18S RNA measured by qRT-PCR 5 h after the second reticulocyte injection is shown for five independent experiments. The different blood sources used are marked in different colors. Parasites were detected in every mouse analyzed, independent of the blood source used. Mean is shown.
UCB, umbilical cord blood; PB, peripheral blood; HCB, blood from patients with hemochromatosis.
We first assessed whether this protocol supports liver infection and transition of P. vivax exo-erythrocytic merozoites to blood stage infection using either i.v. injection of sporozoites or infection by mosquito bite, the latter to test whether the natural route of infection is feasible with Anopheles dirus (An. dirus) mosquitoes. In addition, we directly assessed the benefits of the immunomodulation with Clodronate liposomes and Cyclophosphamide. We infected a cohort of eleven FRGN KO huHep mice with P. vivax sporozoites. Eight FRGN KO huHep mice were i.v. infected with 0.9 million sporozoites isolated from An. dirus salivary glands. Four FRGN KO huHep mice were treated with Clodronate liposomes and Cyclophosphamide, whereas the other group of four mice remained untreated prior to reticulocyte injection. Three additional FRGN KO huHep mice were infected by the bites of 50 P. vivax-infected An. dirus mosquitoes and treated with Clodronate liposomes and Cyclophosphamide as described above.
On day 10 post infection, 4 hours after the second reticulocyte injection, asexual blood stage parasites could be detected on Giemsa-stained thin blood smears of all i.v.-infected FRGN KO huHep mice and, notably, also in the FRGN KO huHep mice infected by mosquito bite. At this time point, parasitemia as high as 2% was observed in the group infected intravenously and treated with Clodronate liposomes and Cyclophosphamide. The immunomodulatory treatment significantly increased the amount of parasite 18S RNA detected by qRT-PCR over the untreated group, supporting the benefit of this treatment (Figure 1B). Therefore, this protocol was used for all further experiments. Levels of human red blood cell (hRBC) reconstitution were comparable between the groups, reaching more than 30% blood chimerism after the second reticulocyte injection (Figure 1C). Notably, the significantly lower blood stage parasitemia observed in the group of mice not receiving any immunomodulation was not caused by increased clearance of all hRBCs, indicating a selective clearance of infected hRBCs by phagocytic cells and neutrophils.
P. vivax parasites preferentially, if not exclusively, infect reticulocytes and not mature RBCs. This necessitates the enrichment of large volumes of these rare cells. We therefore set out to determine the most suitable source of reticulocytes. We used three different sources of blood, namely, peripheral blood from healthy donors, umbilical cord blood as well as peripheral blood from patients with hemochromatosis. Peripheral blood from healthy donors has low reticulocyte counts (∼0.5%), whereas reticulocyte counts in umbilical cord blood can reach up to 5%. Patients with hemochromatosis, an iron-overload disease, undergo frequent phlebotomy as their standard treatment, which over time leads to increased reticulocyte counts of around 3%. Reticulocytes were enriched by density gradient centrifugation, which yielded red blood cell preparations containing 30%–60% reticulocytes, as determined by New Methylene Blue staining. In each of five independent experiments using different reticulocyte sources and following the protocol depicted in Figure 1A, P. vivax asexual blood stage parasites were consistently seen on thin blood smears on days 9 and 10 post sporozoite infection and high levels of P. vivax 18S RNA were detected by qRT-PCR independent of the reticulocyte source used (Figure 1D). Throughout these experiments, a total of 22 FRGN KO huHep mice and five different P. vivax field isolates were used. The protocol described here therefore allows efficient and reproducible transition of P. vivax field isolates from liver stage infection to blood stage infection.
P. vivax Asexual Blood Stages Develop Normally in FRGN KO huHep/huRetic Mice
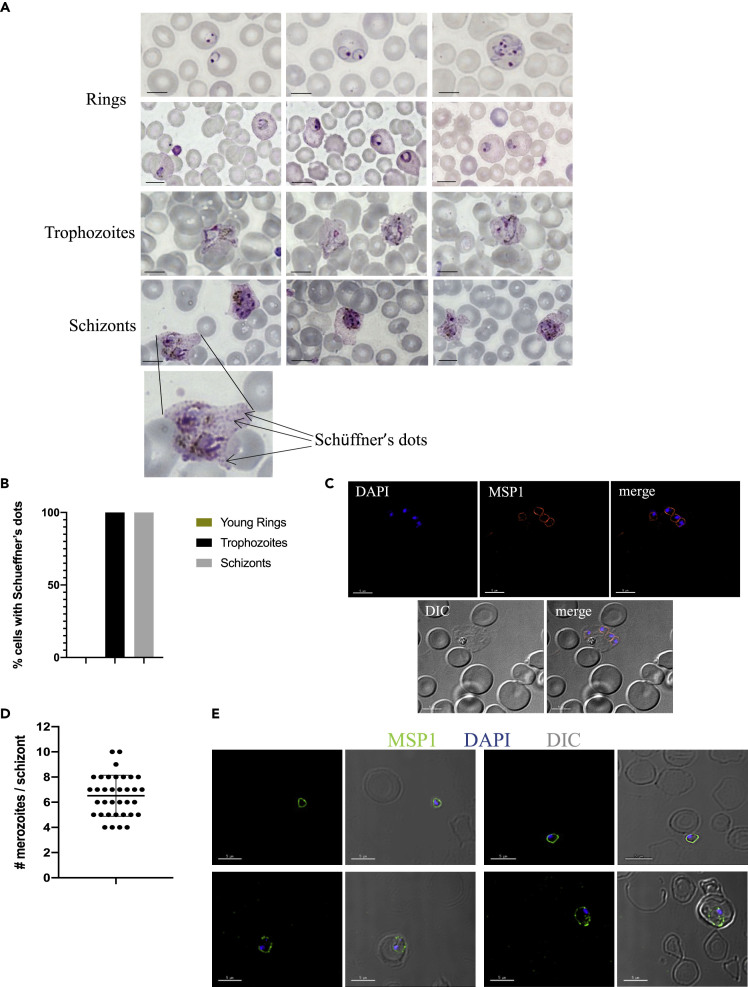
Next, we wanted to assess whether the P. vivax exo-erythrocytic merozoites released from the liver of FRGN KO huHep mice could initiate normal intra-erythrocytic development. We performed Giemsa stains of thin blood smears at various time points after transition, and all asexual parasite forms could be detected between days 9 and 12 post infection. Typical morphological features described for P. vivax-infected RBCs from human infections (Adams and Mueller, 2017; Aikawa et al., 1975; Suwanarusk et al., 2004) were readily observed: red blood cells infected by multiple ring stage parasites; Schüffner's dots; an increase in size and deformation of the infected RBC, leading to the typical ameboid shape (Figure 2A). Host cells infected with parasites at different developmental stages, including early ring stages 4 h after the first reticulocyte infection and mature schizonts, were analyzed for the presence of Schüffner's dots. None of the early ring stage-infected reticulocytes showed the development of Schüffner's dots, whereas these were observed on all trophozoite and schizont-infected cells (Figure 2B). Schizonts were detected at 50 h after the first reticulocyte injection, indicating that the parasites developed within their normal 48-h life cycle. In order to investigate whether schizonts mature fully and release infectious merozoites, we performed immunofluorescence assays (IFAs) using antibodies against merozoite surface protein 1 (MSP1). This staining revealed fully segmented schizonts (Figure 2C). The number of merozoites per schizont was quantified, and on average, a schizont contained 6.5 merozoites (Figure 2D). Remarkably, free merozoites as well as invaded ring stage parasites could be detected as late as day 14 post infection (5 days post first reticulocyte injection), indicating the release of infectious erythrocytic merozoites and the subsequent invasion of new target red blood cells (Figure 2E).
Figure 2.
Asexual Blood Stage Parasite Morphology in FRGN KO huHep Mice
(A) Mice were infected with P. vivax sporozoites, and parasites were transitioned to blood stages following the protocol described in Figure 1A. Giemsa-stained thin blood smears were analyzed at various time points between day 9 (5 h after the first reticulocyte injection) and day 12 post infection. All asexual parasite forms were seen, and the morphology was comparable with parasites seen on blood smears from P. vivax-infected patients. Scale bars represent 5 μm.
(B) Host cells infected with parasites at different developmental stages, including early ring stages 4 h after the first reticulocyte infection and mature schizonts, were analyzed for the presence of Schüffner's dots. None of the early ring stage-infected reticulocytes showed any Schüffner's dots, whereas these were observed on all trophozoite and schizont-infected cells.
(C) IFAs were performed on thin blood smears from day 12 post infection and stained with anti-MSP1 and DAPI. Intact MSP1 staining of fully segmented schizonts could be observed. Scale bars represent 5 μm.
(D) Thin blood smears from two individual experiments were stained with anti-MSP1 and DAPI. The quantity of merozoites per schizont was counted. On average, 6.5 merozoites per schizont were detected. Each dot represents one schizont. Mean ± SD is shown.
(E) IFAs were performed on thin blood smears from day 14 post infection and stained with anti-MSP1 and DAPI. Free merozoites as well as invaded ring stage parasites were observed. Scale bars represent 5 μm.
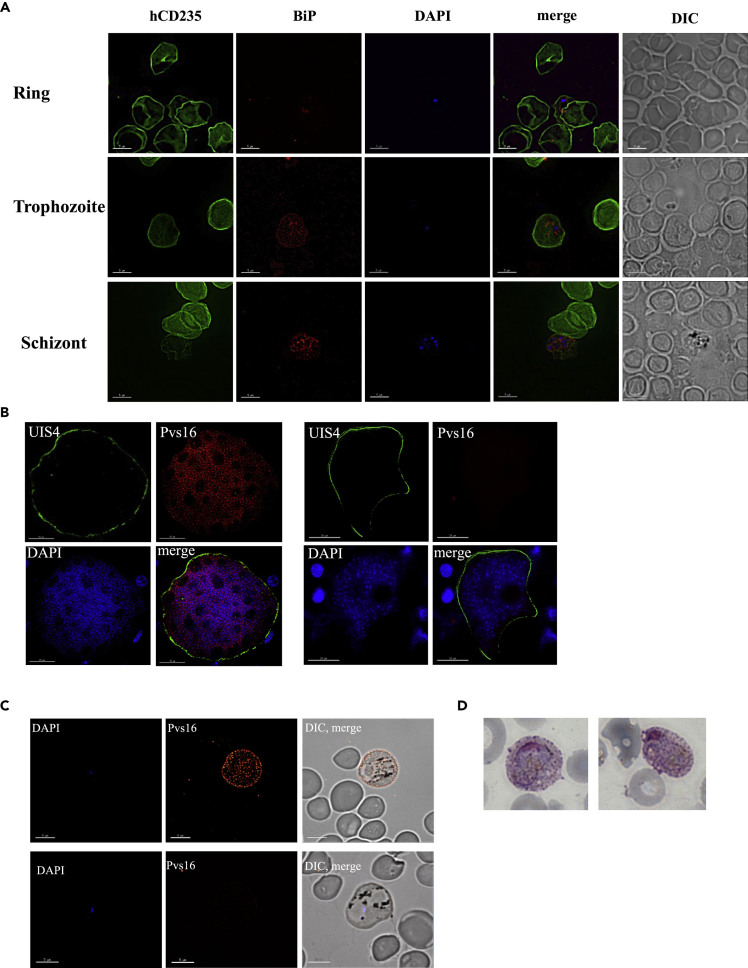
In order to confirm that the merozoites exclusively infect red blood cells of human origin, we performed IFAs using antibodies to the parasite marker BiP and the human RBC marker CD235a. We examined 50 infected RBCs at different time points post transition. At any time point, parasites were exclusively found in CD235a-positive cells (Figure 3A), indicating that no successful invasion of mouse RBCs had occurred.
Figure 3.
Parasites Exclusively Infect Human Red Blood Cells and a Subset of Parasites Express the Gametocyte Antigen Pvs16
(A) To determine whether parasites can also be observed in mouse red blood cells, thin blood smears from different time points post infection were stained with anti-CD235a and anti-BiP, a parasite protein located in the endoplasmic reticulum. Nucleic acid was visualized with DAPI. Fifty infected cells were imaged, and all parasite forms were only detected in human cells. Scale bars represent 5 μm.
(B) Liver sections from day 9 post sporozoite infection were stained for Pvs16, and a subset of exoerythrocytic schizonts expressing this gametocyte antigen was observed. Scale bars represent 15 μm.
(C) IFAs were performed on thin blood smears from day 11 post infection; staining for Pvs16 and a subset of parasites expressing this gametocyte marker was detected. Scale bars represent 5 μm.
(D) Gametocytes were detected on Giemsa-stained thin blood smears.
See also Figure S1.
We further utilized the FRGN KO huHep/huRetic model to investigate P. vivax gametocytogenesis in vivo. IFAs on liver sections from P. vivax sporozoite-infected FRG KO huHep mice revealed a subset of exo-erythrocytic schizonts that stained positive for the immature gametocyte marker Pvs16, 9 days post infection (Figure 3B). In contrast, IFAs staining for the mature gametocyte marker and one of the leading candidate antigens for a transmission blocking vaccine, Pvs230, revealed no expression (data not shown). In order to confirm these findings, we performed RT-PCR using RNA isolated from P. vivax sporozoite-infected FRG KO huHep livers 8 days post infection. We successfully amplified Pvs16, but not Pvs230, confirming the results obtained by IFAs (Figure S1). IFAs on blood smears obtained 11 days post sporozoite infection, approximating a time point ≤2 days post onset of blood stage infection, also revealed a subset of parasites expressing Pvs16 (Figure 3C), indicating the presence of sexual stages very early post onset of blood stage parasitemia. This presence of gametocytes was confirmed by Giemsa-stained thin blood smears (Figure 3D).
Inhibition of Reticulocyte Invasion by an Antibody Targeting the Essential P. vivax Duffy Binding Protein Interaction with Its Receptor
Assays currently available to test interventions targeting P. vivax asexual blood stages are extremely limited (Armistead and Adams, 2018), and no small animal model has been developed for this purpose. In order to assess whether the FRGN KO huHep/huRetic model can fill this gap, we measured the in vivo invasion-inhibitory efficacy of a candidate antibody biologic targeting the essential interaction between P. vivax Duffy Binding Protein (PvDBP) and its receptor the Duffy Antigen Receptor for Chemokines (DARC). PvDBP is the leading blood stage vaccine candidate for this parasite species (Singh et al., 2018). It has previously been shown that immunization with the receptor binding domain of PvDBP (PvDBPII) leads to the generation of antibodies that can inhibit the interaction between recombinant DBP and recombinant DARC (de Cassan et al., 2015; Grimberg et al., 2007; Moreno et al., 2008; Payne et al., 2017; Singh et al., 2018). Furthermore, the presence of high-level PvDBPII-specific binding inhibitory antibodies after natural infection is associated with protection against P. vivax infection (He et al., 2019; King et al., 2008). In a recent study, ten human monoclonal antibodies (mAbs) from a PvDBP-immunized volunteer were cloned and tested for their blocking activity using the DBP-DARC interaction. One mAb potently inhibited invasion in vitro using P. knowlesi parasites expressing P. vivax DBP (Rawlinson et al., 2019).
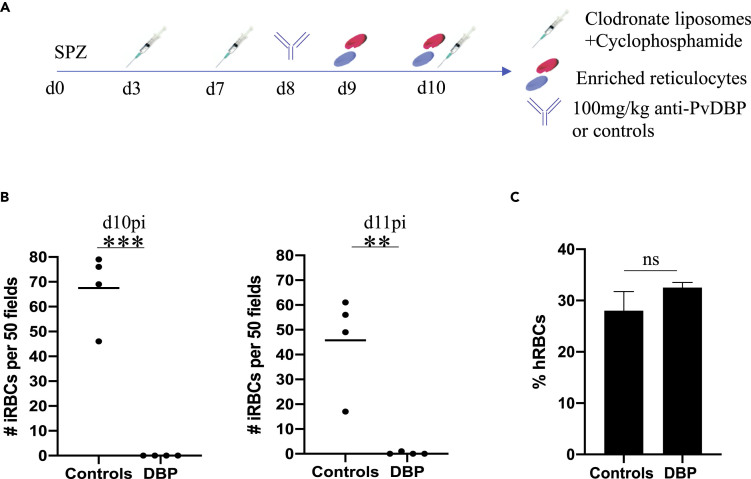
Here we tested a version of this antibody (“anti-PvDBP”) for invasion-inhibitory activity against P. vivax merozoites in vivo, whereby we infected eight FRGN KO huHep mice i.v. with 0.8 million P. vivax sporozoites. Mice were randomized into two groups with four mice each. We followed our protocol described above and outlined in Figure 4A. On day 8 post infection and 24 h before the first reticulocyte injection, each group was passively transferred with 100 mg/kg of anti-PvDBP mAb, a control antibody or PBS. Serum antibody levels were measured immediately before the first reticulocyte injection and revealed high serum concentrations of mAb (Figure S2). Giemsa-stained thin blood smears were generated 5 and 24 h after the second reticulocyte injection, and the number of parasites within 50 fields of view was quantified by microscopy (Figure 4B). Strikingly, PvDBP mAb showed potent inhibition of P. vivax RBC invasion, with no intracellular parasites observed in this group of mice, in contrast to the control group in which on average 67.5 intracellular parasites were observed. During the course of the infection, two of the four mice from this group remained blood smear negative and only a single parasite each was seen on the remaining two mice. There was no significant difference in the level of red blood cell chimerism between the groups (Figure 4C).
Figure 4.
Inhibition of Invasion by a Monoclonal Antibody Targeting the Interaction of PvDBP with Its Receptor DARC
(A) Mice were infected with 0.87 million P. vivax sporozoites. Immunomodulation was performed as described in Figure 1A. On day 8, mice were randomized into two groups. Four mice were intraperitoneally injected with 100 mg/kg anti-PvDBP. Four mice served as controls, of which two were injected with 100 mg/kg anti-P. yoelii CSP and two mice were injected with 200 μL PBS. Enriched reticulocytes were injected intravenously on days 9 and 10 post infection.
(B) Thin blood smears were performed 5 h after the second reticulocyte injection (d10pi) and 24 h after the second reticulocyte injection (d11pi). Infected red blood cells were enumerated in 50 fields. The control mice showed a mean of 67.5 parasites on day 10pi, whereas no parasites were seen in the group of mice passively transferred with anti-PvDBP. On day 11pi, one parasite was seen in one of the mice treated with anti-PvDBP, whereas the other three mice of this group remained blood smear negative. Each dot represents one mouse. Mean is shown. Statistics: unpaired t test; ∗∗∗p = 0.0001; ∗∗p = 0.0037.
(C) IFAs were performed on thin blood smears from day 10pi, staining for human CD235a. Thousand cells were counted per slide, and the percentage of human red blood cells was calculated. There was no significant difference between the groups. Shown is mean with SD.
See also Figure S2.
This experiment demonstrates that the FRGN KO huHep/huRetic mouse model is suitable to test P. vivax intervention strategies in vivo, thereby complementing the currently available assays with an urgently needed small animal model. It is the only model in which interventions can be tested against the first wave of merozoites egressing from the liver, the parasite population that constitutes the essential transition from liver to blood and that a vaccine will have to target in order to prevent onset of blood stage infection, clinical disease, and onward parasite transmission.
Discussion
Research on P. vivax erythrocytic stages is greatly impeded by the lack of a continuous in vitro culture system and robust preclinical models to assess interventions. Here, we report the development of the first small animal model for investigation of P. vivax blood stage interventions. Liver-chimeric FRGN KO huHep mice were infected using the natural route by mosquito bite or i.v. injection of salivary gland sporozoites. Liver stage development and exo-erythrocytic schizogony resulted in the release of exo-erythrocytic merozoites that were able to infect infused human reticulocytes. This is the only model that recapitulates the parasite's physiological developmental cycle, combining sporozoite infection by mosquito bite and full liver stage development with the transition of exo-erythrocytic merozoites to blood stage infection. These features cannot currently be modeled in vitro. The unique advantage of this model over the existing ex vivo invasion assay (Russell et al., 2011) is that it provides the exclusive opportunity to test viability and infectivity of exo-erythrocytic merozoites and interventions that block the first stage of red blood cell infection by the parasite. The reproducibility of the described protocol for five different field isolates is especially remarkable and highlights the utility of this model.
To date, the only in vivo model system for P. vivax blood stages has been the non-human primate (NHP) model. Aotus or Saimiri species are susceptible to infection with NHP-adapted strains of P. vivax (Joyner et al., 2015). Although these models can produce informative data, there are difficulties that arise with their use. First, research using NHPs is limited owing to ethical concerns. The advancement of alternative model systems should greatly reduce the need to use NHPs for research purposes. Second, NHPs are not susceptible to infection with P. vivax field isolates. The parasite strains used for NHP studies had to be adapted in order to allow replication. It is unknown to what extent the biological features of these adapted strains are equivalent to P. vivax field isolates. For example, recent de novo assembly of a P. vivax field isolate genome identified large genomic regions including predicted protein-coding genes implicated to play a role in RBC invasion that were absent in the SalI adapted reference strain, which has been passaged extensively in NHPs (Hester et al., 2013).
Throughout our experiments, three different sources of blood were used, namely, cord blood, peripheral blood from healthy donors, and blood from patients with hemochromatosis. Although in our experiments reticulocytes from all three sources were invaded by merozoites with comparable efficiency, there are differences that need to be considered when deciding which blood source to use. Cord blood typically has higher reticulocyte counts but is expensive, and the volumes obtained are generally low. Furthermore, earlier reports suggested that Plasmodium parasites do not develop normally in red blood cells containing fetal hemoglobin (Wilson et al., 1977). These data have been challenged by more recent study results that show that P. falciparum and P. vivax parasites invade cord blood-derived RBCs as well as RBCs from peripheral blood (Archer et al., 2019; Roobsoong et al., 2015). Concordantly, the invasion capacity of the parasites in our model was not reduced in reticulocytes originating from cord blood. Conversely, obtaining access to large volumes of peripheral blood from healthy donors is facile, but reticulocyte counts are typically low. Therefore, we used blood from hemochromatosis patients. These patients undergo frequent phlebotomy as a treatment for iron overload disease and therefore have increased reticulocyte counts. Blood volumes of about 500 mL are drawn, making this an ideal source for large volumes of reticulocyte-rich blood.
Stage transition is a powerful feature of a model to test P. vivax erythrocytic vaccine candidates. In order to prevent disease and transmission, an erythrocytic stage vaccine ideally would not only prevent continuous blood stage infection but should effectively prevent the onset of asexual blood stage infection by blocking the first generation of merozoites that egress from the liver. Stage transition cannot robustly be modeled in vitro and is therefore a unique advantage of the described model over current ex vivo models. To this end, we used the FRGN KO huHep/huRetic model to test a monoclonal antibody against the interaction of the leading blood stage vaccine candidate PvDBP with its receptor DARC. We show that this antibody potently blocks the first wave of reticulocyte invasion by exo-erythrocytic merozoites, which proves the system highly suitable to test blood stage interventions. Although the ex vivo invasion assay described by Russell et al. (2011) provides the opportunity to test invasion inhibitory potential of antibodies, it has clearly been demonstrated that in vitro inhibitory activity of antibodies does not necessarily correlate with inhibitory activity seen in vivo (Kisalu et al., 2018; Sack et al., 2017). Therefore, the in vivo model described here is an important advance over the available ex vivo invasion assay.
Natural and experimental infections of humans have shown that P. vivax gametocytes are detectable as early as 3 days post onset of blood stage parasitemia. These observations have led to the hypothesis that a subset of P. vivax liver stage schizonts are pre-programmed to become gametocytes and therefore sexual stages already emerge with the first round of parasites egressing from the liver, allowing transmission before the onset of clinical symptoms (Bousema and Drakeley, 2011). Furthermore, gene expression data combined with mathematical modeling suggest that P. vivax transmission to mosquitoes is possible at the very first blood stage cycle and can therefore only be prevented if the first erythrocytic infection step is blocked (Adapa et al., 2019). Here, we provide the first direct in vivo evidence that a subset of exo-erythrocytic schizonts express the sexual stage marker Pvs16, but not the mature gametocyte marker Pvs230, and therefore exo-erythrocytic merozoites might be preprogrammed to become gametocytes in the first cycle of blood stage infection. This is in accordance with recent in vitro data in which infection of primary human hepatocytes with P. vivax sporozoites revealed Pvs16-expression on liver stage schizonts 8 days post infection (Roth et al., 2018). Importantly, the onset of blood stage parasitemia in the FRGN KO huHep/huRetic model further allowed us to follow the expression of gametocyte markers in erythrocytic stages. We detected Pvs16-expression on a subset of erythrocytic stage parasites as early as ≤2 days post onset of blood stage infection, suggesting that sexual stages can emerge within the first intra-erythrocytic replication cycle. Pvs16 is expressed early in gametocytogenesis and in line with the detection of Pvs16-expressing parasites ≤2 days post onset of blood stage infection, we observed gametocytes by Giemsa stain 1 day later, starting 3 days post onset of blood stage infection. Blocking liver to blood transition of P. vivax as demonstrated herein might thus offer the additional benefit of preventing parasite transmission. Clearly, these findings are of epidemiological importance and provide an intriguing example of the broad applicability of the FRGN KO huHep/huRetic model to answer highly translational as well as basic biological questions.
Future studies will be aimed at utilizing the P. vivax humanized mouse model to test not only erythrocytic but also pre-erythrocytic stage intervention strategies. The FRGN KO huHep/huRetic model will allow assessment of passive immunization against pre-erythrocytic stages by determining parasite liver burden, but importantly, it will also allow measurement of the onset of blood stage patency and the delay or absence thereof as an additional, highly sensitive readout for the efficacy of a pre-erythrocytic intervention. It is questionable whether a single-stage subunit vaccine alone will be able to completely prevent infection, and thus, combination multi-stage vaccine approaches have to be considered (Tham et al., 2017). Since the FRGN KO huHep/huRetic model combines the natural route of infection, liver stage development and transition to parasite blood stages, it will provide the unique opportunity to test combination pre-erythrocytic stage and blood stage vaccine approaches, for which to date there is no available assay system.
In summary, we have developed a novel humanized mouse system that will serve as a valuable preclinical model to test interventions against P. vivax and will contribute to the accelerated discovery and development of novel vaccine and drug candidates.
Limitations of the Study
The liver-chimeric mouse model described here is a highly specialized mouse model that requires careful handling owing to its immunocompromised nature. The additional depletion of mouse macrophages and mouse neutrophils with Clodronate liposomes and Cyclophosphamide makes the mice increasingly susceptible to bacterial infections, if not handled with required care.
Any work utilizing P. vivax sporozoites is challenging owing to the requirement of patient blood to infect mosquitoes in order to obtain these stages. This requires great logistical effort by laboratories in endemic countries. This, together with the complex nature of the mouse model used, requires careful assessment of the minimal group size necessary to achieve statistical significance.
The described model is a powerful tool for the transition of P. vivax liver stage to blood stage infection and testing of interventions against the first generation of merozoites egressing from the liver in vivo. Further refinement of the model will potentially allow continuous maintenance of asexual blood stage infection as well as parasite transmission to mosquitoes.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stefan Kappe (stefan.kappe@seattlechildrens.org).
Materials Availability
The study did not generate new unique reagents.
Data and Code Availability
Any data generated in this study is included in the published article.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the insectary team at Mahidol University for the supply of P. vivax infected mosquitoes and Yecuris Corp. for providing FRGN KO huHep mice. We thank Bloodworks Northwest and all donors for providing cord blood and blood units from patients with hemochromatosis. We also thank John Adams for the MSP1 antibody and Tomoko Ishino for Pvs16 and Pvs230 polyclonal serum. We thank Dr. Ashley Vaughan for critical reading of the manuscript and helpful discussions.
This work was funded by the German Research Foundation (fellowship SCHA2047/1-1 to CS) as well as the U.S. Department of Defense (award #W81XWH-17-1-0482 to SAM) and in part by the UK Medical Research Council (MRC) Confidence in Concept (MC_PC_16056) to M.B. T.A.R. held a Wellcome Trust Research Training Fellowship (108734/Z/15/Z), and S.J.D. is a Jenner Investigator and a Wellcome Trust Senior Fellow (106917/Z/15/Z).
Author Contributions
C.S. planned, performed, and analyzed experiments and wrote the manuscript; W.R. and N.K. performed experiments and analyzed data; M.B., T.A.R., and S.J.D. generated and provided the anti-DBP antibody biologic; D.N.S., O.T., and N.D. provided the anti-PyCSP control antibody and performed and analyzed ELISAs to determine serum antibody concentrations; C.P. and S.C.M. performed qRT-PCR analysis; D.G., L.M.R., and S.Y.K. performed laboratory work; W.R., N.K., and J.S. provided P. vivax-infected mosquitos; E.L.F., S.A.M., J.S., and S.H.I.K. supervised the work, analyzed data, and contributed to discussions; S.H.I.K. edited the manuscript; the final manuscript was edited and approved by all authors.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101381.
Supplemental Information
References
- Adams J.H., Mueller I. The biology of Plasmodium vivax. Cold Spring Harb Perspect. Med. 2017;7:a025585. doi: 10.1101/cshperspect.a025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adapa S.R., Taylor R.A., Wang C., Thomson-Luque R., Johnson L.R., Jiang R.H.Y. Plasmodium vivax readiness to transmit: implication for malaria eradication. BMC Syst. Biol. 2019;13:5. doi: 10.1186/s12918-018-0669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M., Miller L.H., Rabbege J. Caveola--vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P cynomolgi. Unique structures related to Schuffner's dots. Am. J. Pathol. 1975;79:285–300. [PMC free article] [PubMed] [Google Scholar]
- Archer N.M., Petersen N., Duraisingh M.T. Fetal hemoglobin does not inhibit Plasmodium falciparum growth. Blood Adv. 2019;3:2149–2152. doi: 10.1182/bloodadvances.2019000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead J.S., Adams J.H. Advancing research models and technologies to overcome biological barriers to Plasmodium vivax control. Trends Parasitol. 2018;34:114–126. doi: 10.1016/j.pt.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Paulk N., Ranade A., Dorrell C., Al-Dhalimy M., Ellis E., Strom S., Kay M.A., Finegold M., Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat. Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C.C., Johns F.M. The cultivation of malarial plasmodia (Plasmodium vivax and Plasmodium falciparum) in vitro. J. Exp. Med. 1912;16:567–579. doi: 10.1084/jem.16.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez M., Moreno-Perez D.A., Arevalo-Pinzon G., Curtidor H., Patarroyo M.A. Plasmodium vivax in vitro continuous culture: the spoke in the wheel. Malar. J. 2018;17:301. doi: 10.1186/s12936-018-2456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T., Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell A.N., Winzeler E.A. Advances in omics-based methods to identify novel targets for malaria and other parasitic protozoan infections. Genome Med. 2019;11:63. doi: 10.1186/s13073-019-0673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cassan S.C., Shakri A.R., Llewellyn D., Elias S.C., Cho J.S., Goodman A.L., Jin J., Douglas A.D., Suwanarusk R., Nosten F.H. Preclinical assessment of viral vectored and protein vaccines targeting the duffy-binding protein region II of Plasmodium vivax. Front. Immunol. 2015;6:348. doi: 10.3389/fimmu.2015.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foquet L., Schafer C., Minkah N.K., Alanine D.G.W., Flannery E.L., Steel R.W.J., Sack B.K., Camargo N., Fishbaugher M., Betz W. Plasmodium falciparum liver stage infection and transition to stable blood stage infection in liver-humanized and blood-humanized FRGN KO mice enables testing of blood stage inhibitory antibodies (Reticulocyte-Binding protein homolog 5) in vivo. Front. Immunol. 2018;9:524. doi: 10.3389/fimmu.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething P.W., Van Boeckel T.P., Smith D.L., Guerra C.A., Patil A.P., Snow R.W., Hay S.I. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasit. Vectors. 2011;4:92. doi: 10.1186/1756-3305-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D., Minkah N.K., Kappe S.H.I. Designer parasites: genetically engineered Plasmodium as vaccines to prevent malaria infection. J. Immunol. 2019;202:20–28. doi: 10.4049/jimmunol.1800727. [DOI] [PubMed] [Google Scholar]
- Grimberg B.T., Udomsangpetch R., Xainli J., McHenry A., Panichakul T., Sattabongkot J., Cui L., Bockarie M., Chitnis C., Adams J. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med. 2007;4:e337. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszczyk J., Kanjee U., Chan L.J., Menant S., Malleret B., Lim N.T.Y., Schmidt C.Q., Mok Y.F., Lin K.M., Pearson R.D. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science. 2018;359:48–55. doi: 10.1126/science.aan1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W.Q., Shakri A.R., Bhardwaj R., Franca C.T., Stanisic D.I., Healer J., Kiniboro B., Robinson L.J., Guillotte-Blisnick M., Huon C. Antibody responses to Plasmodium vivax Duffy binding and Erythrocyte binding proteins predict risk of infection and are associated with protection from clinical Malaria. PLoS Negl. Trop. Dis. 2019;13:e0006987. doi: 10.1371/journal.pntd.0006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester J., Chan E.R., Menard D., Mercereau-Puijalon O., Barnwell J., Zimmerman P.A., Serre D. De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl. Trop. Dis. 2013;7:e2569. doi: 10.1371/journal.pntd.0002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner C., Barnwell J.W., Galinski M.R. No more monkeying around: primate malaria model systems are key to understanding Plasmodium vivax liver-stage biology, hypnozoites, and relapses. Front. Microbiol. 2015;6:145. doi: 10.3389/fmicb.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.L., Michon P., Shakri A.R., Marcotty A., Stanisic D., Zimmerman P.A., Cole-Tobian J.L., Mueller I., Chitnis C.E. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc. Natl. Acad. Sci. U S A. 2008;105:8363–8368. doi: 10.1073/pnas.0800371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisalu N.K., Idris A.H., Weidle C., Flores-Garcia Y., Flynn B.J., Sack B.K., Murphy S., Schon A., Freire E., Francica J.R. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018;24:408–416. doi: 10.1038/nm.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong L.S., Brown M.H., Barclay A.N., Hatherley D. Signal-regulatory protein alpha from the NOD mouse binds human CD47 with an exceptionally high affinity-- implications for engraftment of human cells. Immunology. 2014;143:61–67. doi: 10.1111/imm.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehl P., Meireles P., Albuquerque I.S., Pinkevych M., Baptista F., Mota M.M., Davenport M.P., Prudencio M. Innate immunity induced by Plasmodium liver infection inhibits malaria reinfections. Infect. Immun. 2015;83:1172–1180. doi: 10.1128/IAI.02796-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B., Li A., Zhang R., Tan K.S., Suwanarusk R., Claser C., Cho J.S., Koh E.G., Chu C.S., Pukrittayakamee S. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood. 2015;125:1314–1324. doi: 10.1182/blood-2014-08-596015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak S.A., Vaughan A.M., Kangwanrangsan N., Roobsoong W., Fishbaugher M., Yimamnuaychok N., Rezakhani N., Lakshmanan V., Singh N., Kaushansky A. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe. 2015;17:526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.L., Sack B.K., Baldwin M., Vaughan A.M., Kappe S.H.I. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7:436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Mogire R.M., Akala H.M., Macharia R.W., Juma D.W., Cheruiyot A.C., Andagalu B., Brown M.L., El-Shemy H.A., Nyanjom S.G. Target-similarity search using Plasmodium falciparum proteome identifies approved drugs with anti-malarial activity and their possible targets. PLoS One. 2017;12:e0186364. doi: 10.1371/journal.pone.0186364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A., Caro-Aguilar I., Yazdani S.S., Shakri A.R., Lapp S., Strobert E., McClure H., Chitnis C.E., Galinski M.R. Preclinical assessment of the receptor-binding domain of Plasmodium vivax Duffy-binding protein as a vaccine candidate in rhesus macaques. Vaccine. 2008;26:4338–4344. doi: 10.1016/j.vaccine.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P.A. Normal and disordered reticulocyte maturation. Curr. Opin. Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noulin F., Borlon C., Van Den Abbeele J., D'Alessandro U., Erhart A. 1912-2012: a century of research on Plasmodium vivax in vitro culture. Trends Parasitol. 2013;29:286–294. doi: 10.1016/j.pt.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Payne R.O., Silk S.E., Elias S.C., Milne K.H., Rawlinson T.A., Llewellyn D., Shakri A.R., Jin J., Labbe G.M., Edwards N.J. Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies. JCI Insight. 2017;2:e93683. doi: 10.1172/jci.insight.93683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson T.A., Barber N.M., Mohring F., Cho J.S., Kosaisavee V., Gerard S.F., Alanine D.G.W., Labbe G.M., Elias S.C., Silk S.E. Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralizing vaccine-induced human antibody. Nat. Microbiol. 2019;4:1497–1507. doi: 10.1038/s41564-019-0462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobsoong W., Tharinjaroen C.S., Rachaphaew N., Chobson P., Schofield L., Cui L., Adams J.H., Sattabongkot J. Improvement of culture conditions for long-term in vitro culture of Plasmodium vivax. Malar. J. 2015;14:297. doi: 10.1186/s12936-015-0815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A., Maher S.P., Conway A.J., Ubalee R., Chaumeau V., Andolina C., Kaba S.A., Vantaux A., Bakowski M.A., Thomson-Luque R. A comprehensive model for assessment of liver stage therapies targeting Plasmodium vivax and Plasmodium falciparum. Nat. Commun. 2018;9:1837. doi: 10.1038/s41467-018-04221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Suwanarusk R., Borlon C., Costa F.T., Chu C.S., Rijken M.J., Sriprawat K., Warter L., Koh E.G., Malleret B. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood. 2011;118:e74–e81. doi: 10.1182/blood-2011-04-348748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack B.K., Mikolajczak S.A., Fishbaugher M., Vaughan A.M., Flannery E.L., Nguyen T., Betz W., Jane Navarro M., Foquet L., Steel R.W.J. Humoral protection against mosquito bite-transmitted Plasmodium falciparum infection in humanized mice. NPJ Vaccin. 2017;2:27. doi: 10.1038/s41541-017-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shretta R., Liu J., Cotter C., Cohen J., Dolenz C., Makomva K., Newby G., Menard D., Phillips A., Tatarsky A. Malaria elimination and eradication; Chapter 12. In: Holmes K.K., Bertozzi S., Bloom B.R., Jha P., editors. Major Infectious Diseases, 3rd ed. The International Bank for Reconstruction and Development / The World Bank; 2017. [PubMed] [Google Scholar]
- Singh K., Mukherjee P., Shakri A.R., Singh A., Pandey G., Bakshi M., Uppal G., Jena R., Rawat A., Kumar P. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccin. 2018;3:48. doi: 10.1038/s41541-018-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanarusk R., Cooke B.M., Dondorp A.M., Silamut K., Sattabongkot J., White N.J., Udomsangpetch R. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis. 2004;189:190–194. doi: 10.1086/380468. [DOI] [PubMed] [Google Scholar]
- Tham W.H., Beeson J.G., Rayner J.C. Plasmodium vivax vaccine research - we've only just begun. Int. J. Parasitol. 2017;47:111–118. doi: 10.1016/j.ijpara.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Trager W. Cultivation of Plasmodium falciparum. Arch. Pathol. Lab. Med. 1977;101:277–278. [PubMed] [Google Scholar]
- White M.T., Karl S., Battle K.E., Hay S.I., Mueller I., Ghani A.C. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. Elife. 2014;3:e04692. doi: 10.7554/eLife.04692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.J., Pasvol G., Weatherall D.J. Invasion and growth of Plasmodium falciparum in different types of human erythrocyte. Bull. World Health Organ. 1977;55:179–186. [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Takenaka K., Urata S., Shima T., Kikushige Y., Tokuyama T., Iwamoto C., Nishihara M., Iwasaki H., Miyamoto T. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121:1316–1325. doi: 10.1182/blood-2012-06-440354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data generated in this study is included in the published article.