Summary
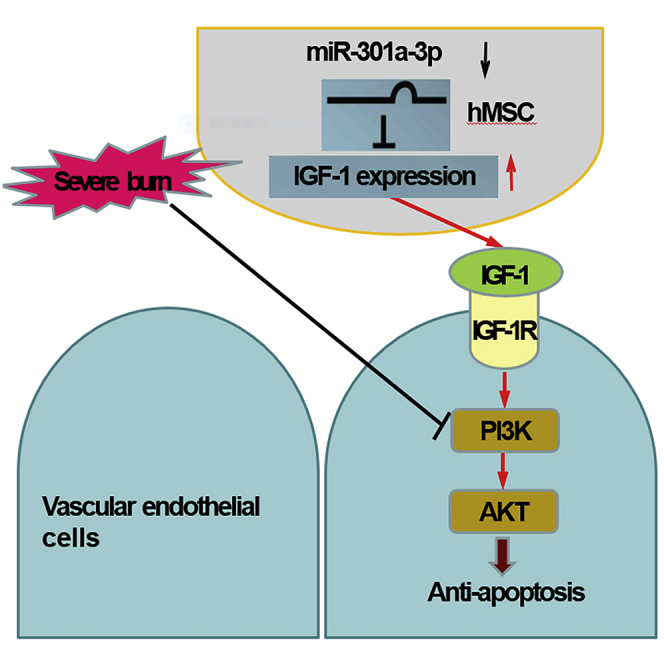
Vascular endothelium dysfunction plays a pivotal role in the initiation and progression of multiple organ dysfunction. The mesenchymal stem cell (MSC) maintains vascular endothelial barrier survival via secreting bioactive factors. However, the mechanism of human umbilical cord MSC (hMSC) in protecting endothelial survival remains unclear. Here, we found IGF-1 secreted by hMSC suppressed severe burn-induced apoptosis of human umbilical vein endothelial cells (HUVECs) and alleviated the dysfunction of vascular endothelial barrier and multiple organs in severely burned rats. Severe burn repressed miR-301a-3p expression, which directly regulated IGF-1 synthesis and secretion in hMSC. Down-regulation of miR-301a-3p decreased HUVECs apoptosis, stabilized endothelial barrier permeability, and subsequently protected against multiple organ dysfunction in vivo. Additionally, miR-301a-3p negatively regulated PI3K/Akt/FOXO3 signaling through IGF-1. Taken together, our study highlights the protective function of IGF-1 against the dysfunction of multiple organs negatively regulated by miR-301a-3p, which may provide the theoretical foundation for further clinical application of hMSC.
Subject Areas: Clinical Genetics, Cellular Therapy, Stem Cells Research
Graphical Abstract
Highlights
-
•
IGF-1 secreted by hMSC suppressed severe burn-induced apoptosis of HUVECs
-
•
miR-301a-3p directly regulated IGF-1 synthesis and secretion in hMSC
-
•
DomiR-301a-3p protected against multiple organ dysfunction
-
•
miR-301a-3p regulated PI3K/Akt/FOXO3 signaling through hMSC-secreted IGF-1
Clinical Genetics; Cellular Therapy; Stem Cells Research
Introduction
Severe burn is a complex and severe traumatic injury with high mortality in the acute phase (Auger et al., 2017; Liu et al., 2016). Vascular endothelial barrier dysfunction induced by severe burn, synergism of excessive inflammation, and serious metabolic disturbance are important contributors for the development of multiple organ dysfunction, such as acute kidney injury (AKI), cardiac dysfunction, and multiple organ failure (MOF) (Lam et al., 2018; Haines et al., 2017). It has been demonstrated that apoptosis of vascular endothelial cells induced by severe burn is one of the main concerns responsible for vascular endothelial barrier dysfunction at the early stage (Tian et al., 2015). Therefore, reducing vascular endothelial cells apoptosis may benefit patients with endothelial barrier disorder and multiple organ dysfunction post severe burn.
Because of its lower immunogenicity and higher proliferation rate, human umbilical cord mesenchymal stem cell (hMSC) is considered as a perfect candidate for stem cell-based therapy and regenerative medicine (Matthay et al., 2017; Lee and Wang, 2017; Laroye et al., 2017). Increasing evidence has shown that hMSC can protect the endothelial barrier function and effectively treat ill surgical patients who develop multiple organ dysfunction post severe burn and traumatic brain injury (TBI) (Dorronsoro and Robbins, 2013; Pati et al., 2015; Menge et al., 2012). The beneficial effects of hMSC are partially explained by secretion of bioactive factors, such as anti-apoptotic factors, immunomodulation factors, antioxidant factors, and exosomes (Yin et al., 2019; Temnov et al., 2018; Beltran et al., 2015). Among these bioactive factors, insulin-like growth factor (IGF-1) is an important anti-apoptotic factor that regulates cell survival (Li et al., 2017; Song et al., 2016). Bake et al. showed that IGF-1 could be beneficial to the endothelial barrier function at the earliest phase of ischemia (Bake et al., 2019). Pati et al. suggested that bone marrow MSC preserved vascular endothelial integrity in the lungs after hemorrhagic shock (Pati et al., 2011). Our previous study also showed that IGF-1 was one of the most important factors secreted by hMSC in eliminating apoptosis in severely burned wound (Liu et al., 2014). However, the mechanism of IGF-1 secreted by hMSC on protecting endothelial survival and function remains unclear.
MicroRNAs (miRNAs) are 21–24 nucleotides noncoding RNAs that negatively regulate the expression of genes by binding to the 3' untranslated regions (3′ UTRs) (Zhao and Srivastava, 2007; Kertesz et al., 2007; Wu and Belasco, 2008). MiRNAs participate in multiple cellular processes, including differentiation and organogenesis, as well as a broad array of repair and regeneration mechanisms of MSC (Huang et al., 2013; Lakshmipathy and Hart, 2008; Wen et al., 2012). For example, miR-126 up-regulation promotes mesenchymal stem cell (MSC) secretome and ischemic angiogenesis and improves cardiac function (Huang et al., 2013).
FOXO3a is a major downstream effector of phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway in regulating endothelial cell survival and vascular endothelial function (Tian et al., 2015; Ren et al., 2016; Li and Zheng, 2019; Yao et al., 2019; Huang et al., 2015; Yan et al., 2019). Burns induce inactivation of PI3K/Akt and dephosphorylation of FOXO3a, which translocates into the nucleus and triggers apoptosis-related gene expression (Yu et al., 2016). Several evidences have demonstrated that stem cell transplantation improves cardiac function through the Akt survival pathway in diabetic cardiomyopathy (Yan and Singla, 2013). In addition, IGF-1 released from adipose stem cells protects against 6-hydroxydopamine-induced neurotoxicity/neuronal cell death via PI3K/Akt and downstream signaling pathway (Wang et al., 2014).
Based on the published studies and our previous results, this study was designed to investigate the potential mechanism of the therapeutic effect of hMSC involved in the endothelial apoptosis and dysfunctions of endothelial barrier at early stage post severe burn.
Results
IGF-1 Secreted from hMSC Reduces Severe Burn-Induced Apoptosis of Endothelial Cells
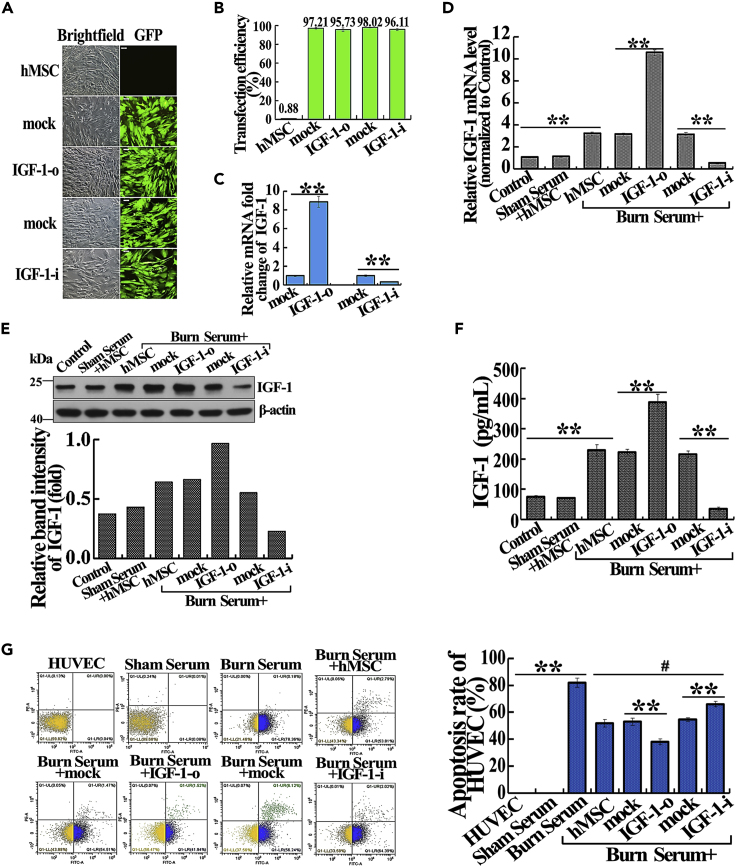
To investigate the role of hMSC-secreted IGF-1 on severe burn-induced apoptosis of endothelial cells, we overexpressed and knocked down IGF-1 in hMSC and assessed human umbilical vein endothelial cell (HUVEC) apoptosis in the severely burned serum culture system. Flow cytometry assay showed that transfection efficiency was comparable in Lenti-overexpress vehicle (mock), Lenti-IGF-1-overexpression (IGF-1-o), Lenti-siRNA vehicle (mock), and Lenti-IGF-1-siRNA (IGF-1-i) groups (Figures 1A and 1B ). Compared with the corresponding mock groups, the mRNA expression of IGF-1 in IGF-1-o and IGF-1-i groups was significantly increased or decreased, respectively (Figure 1C). After cultured with or without burn serum, the mRNA, protein, and secreted levels of IGF-1 in burn serum + hMSC group were remarkably higher than those in control and sham serum + hMSC groups. Consistently, the mRNA, protein, and secreted levels of IGF-1 were significantly higher and lower in IGF-1-o and IGF-1-i groups than in the corresponding mock groups, respectively (Figures 1D–1F).
Figure 1.
Suppressive Effect of IGF-1 Secreted from hMSC on Severe Burn-Induced HUVEC Apoptosis In Vitro
(A) Representative photographs revealed transfection efficiency with mock, IGF-1-o, mock, IGF-1-i in hMSC (magnification: 100×). Visible green fluorescence protein (GFP) expression that >96.77% of the cells were successfully infected.
(B) Transfection efficiencies of mock, IGF-1-o, mock, IGF-1-i groups were evaluated by flow cytometry assay.
(C) The relative mRNA fold change of IGF-1 in hMSC after transfection with mock, IGF-1-o, mock, IGF-1-i were detected by real-time PCR.
(D) The relative mRNA levels of IGF-1 in different treatment groups were detected by real-time PCR.
(E) The protein expression level of IGF-1 in different treatment groups was detected by western blotting.
(F) The IGF-1 level in cultural supernatant of different treatment groups was detected by ELISA assay.
(G) Apoptosis rates of HUVEC in different treatment groups were detected by the flow cytometry assay. The quantitative analyses were presented in the corresponding histogram.
Values are represented as mean ± SD (n = 6), ∗p < 0.05, ∗∗p < 0.01. The hMSC, mock, IGF-1-o, mock, or IGF-1-i was defined as untransfected, Lenti-overexpression vehicle transfected, Lenti-IGF-1-overexpression transfected, Lenti-siRNA vehicle transfected, and Lenti-IGF-1-siRNA transfected, respectively.
To investigate the paracrine effect of hMSC on severe burn-induced HUVEC apoptosis, hMSC and HUVEC were co-cultured in a trans-well co-culture system, treated with DMEM-high glucose containing 20% severely burned rat serum for 24 h. HUVEC cells were subjected to apoptosis analysis. Apoptosis was boosted in the burn serum group as compared with control and sham serum groups, whereas it was obviously reduced after co-culturing with hMSC (Figure 1G). In addition, IGF-1 overexpression further decreased apoptosis of HUVEC, whereas IGF-1 knockdown increased apoptosis compared with the corresponding mock groups.
MiR-301a-3p Down-Regulation Is Induced by Severe Burn and Acts as a Direct Regulator of IGF-1 in hMSC
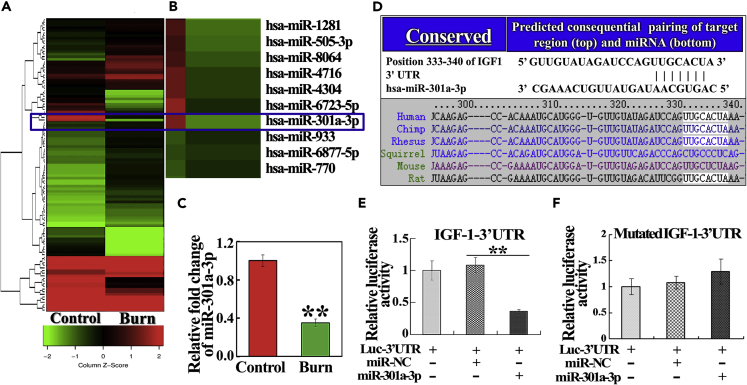
To identify dysregulated miRNAs induced by burn in hMSC, miRNA array analysis was performed in hMSCs, which were maintained in a co-culture system of severe burn serum and sham serum (control) for 24 h. The results showed that 31 miRNAs were downregulated and 81 were upregulated (fold change >2). Among them, miR-301a-3p was the top-ranked downregulated miRNA (Figures 2A and 2B). Reduction of miR-301a-3p induced by burn was also confirmed by real-time PCR assay (Figure 2C). Based on TargetScan and miRanda, we predicted that IGF-1 was a direct target for miR-301a-3p, which was highly conserved across species (Figure 2D). We therefore performed a luciferase assay to evaluate the direct interaction between miR-301a-3p and IGF-1 mRNA. The luciferase activity was inhibited when miR-301a-3p mimics were co-transfected with luciferase reporters containing the predicted binding region of the wild-type 3′ UTR of IGF-1 (Figure 2E). However, the luciferase activity remained unchanged when a plasmid with a mutated sequence was used instead (Figure 2F). These results suggest that miR-301a-3p is suppressed by burn and directly targets IGF-1.
Figure 2.
Severe Burn-Induced Down-Regulation of miR-301a-3p Regulated Directly on IGF-1 Protein Translation in hMSC
(A) Hierarchical clustering of miRNAs expressed differentially in hMSC of the control and burn groups. The scale bar at the bottom depicts standard deviation from the mean.
(B) Enlarged view of miRNAs with significantly differential expression was shown.
(C) The relative fold change of miR-301a-3p in hMSC of control and burn groups were detected by real-time PCR assay.
(D) Bioinformatics predicted miR-301a-3p-binding sites in IGF-1. Partial sequences of miR-301a-3p and sequences of its binding sites in the 3′ UTR of IGF-1 across various species are shown.
(E and F) Luciferase activity derived from a reporter containing a wide-type (E) or mutant (F) 3′ UTR of IGF-1 co-transfected with miR-301a-3p or miR-NC, respectively. All values are represented as mean ± SD (n = 3), ∗∗p < 0.01.
The miR-301a-3p Regulating hMSC-Secreted IGF-1 Reduces Severe Burn-Induced HUVEC Apoptosis
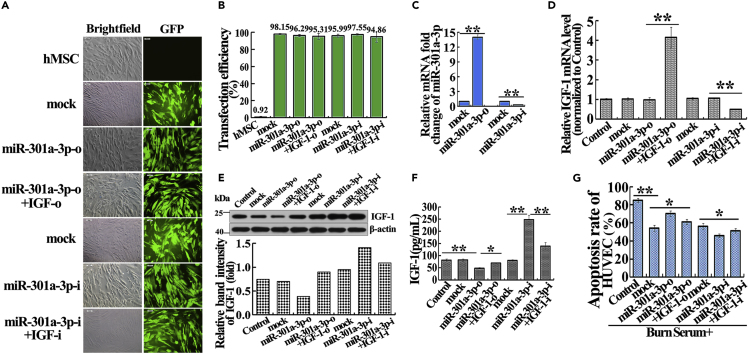
To determine whether miR-301a-3p modulates severe burn-induced HUVEC apoptosis by regulating hMSC-secreted IGF-1, hMSCs were transfected with Lenti-mimic vehicle (mock), Lenti-miR-301a-3p mimic (miR-301a-3p-o), Lenti-miR-301a-3p mimic combined Lenti-IGF-1-overexpression (miR-301a-3p-o + IGF-1-o), Lenti-inhibitor vehicle (mock), Lenti-miR-301a-3p inhibitor (miR-301a-3p-i), and Lenti-miR-301a-3p inhibitor combined Lenti-IGF-1-siRNA (miR-301a-3p-i + IGF-1-i). The transfection efficiency was comparable among these groups (Figures 3A and 3B). MiR-301a-3p overexpression and inhibition significantly increased and decreased the expression level of miR-301a-3p, respectively (Figure 3C). MiR-301a-3p overexpression obviously repressed the protein expression and secretion of IGF-1. By contrast, downregulation of miR-301a-3p enhanced the protein and secreted abundance of IGF-1 (Figures 3E and 3F). IGF-1 knockdown and overexpression resulted in downregulation and upregulation of IGF-1, respectively (Figures 3D–3F). However, the relative IGF-1 mRNA levels in miR-301a-3p-o and miR-301a-3p-i groups exhibited no significant changes compared with the corresponding mock groups (Figure 3D). These data suggest that miR-301a-3p regulated IGF-1 at the post-transcriptional level.
Figure 3.
miR-301a-3p Negatively Regulating hMSC-Secreted IGF-1 Reduces Severe Burn-Induced HUVEC Apoptosis In Vitro
(A) Representative photographs demonstrated successful transfection with mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i in hMSC (magnification: 100×). Visible green fluorescence protein (GFP) expression that >96.36% of the cells were successfully infected.
(B) Transfection efficiencies of mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i groups were evaluated by flow cytometry assay.
(C) The relative expression levels of miR-301a-3p in hMSC after transfection with mock, miR-301a-3p-o, mock, and miR-301a-3p-i were detected by real-time PCR.
(D) The relative IGF-1 mRNA levels in hMSC of control, mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i groups were detected by real-time PCR.
(E) The relative IGF-1 protein expression levels in hMSC of control, mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i groups were detected by western blotting.
(F) The IGF-1 levels in cultural supernatant of control, mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i groups were detected by ELISA assay.
(G) Apoptosis rates of HUVEC in control, mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i groups were detected by flow cytometry assay in the severely burned serum culture system. The quantitative analyses were presented in the corresponding histogram. Values are represented as mean ± SD (n = 6), ∗p < 0.05, ∗∗p < 0.01. Control, mock, miR-301a-3p-o, miR-301a-3p-o + IGF-1-o, mock, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i were defined as untransfected, Lenti-mimics vehicle transfected, Lenti-miR-301a-3p mimics transfected, Lenti-miR-301a-3p mimics and Lenti-IGF-1-overexpress co-transfected, Lenti-inhibitors vehicle transfected, Lenti-miR-301a-3p inhibitors transfected, and Lenti-miR-301a-3p inhibitors and Lenti-IGF-1-siRNA co-transfected, respectively.
Next, the hMSC and HUVEC co-culture system was used to evaluate the effect of miR-301a-3p on HUVEC apoptosis induced by burn. Compared with the burn serum group, other groups reduced HUVEC apoptosis rates (Figure 3G). miR-301a-3p overexpression led to enhance HUVEC apoptosis, which was reversed by IGF-1 ectopic expression (Figure 3G). Oppositely, reduction of miR-301a-3p suppressed HUVEC apoptosis, which was reversed by IGF-1 inhibition (Figure 3G). Taken together, down-regulation of miR-301a-3p in hMSC reduces burn-induced HUVEC apoptosis by upregulating IGF-1 expression.
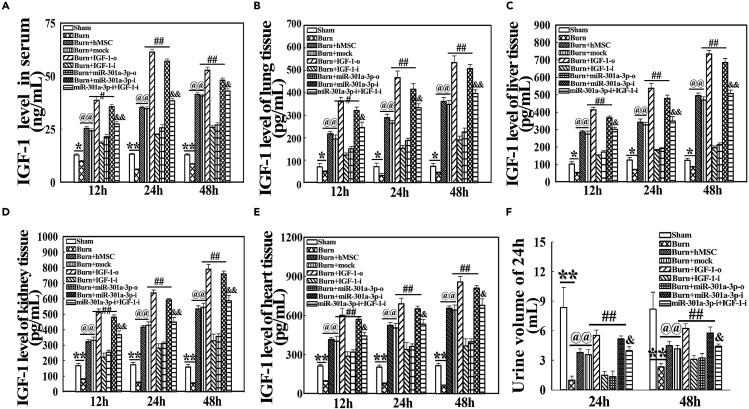
The miR-301a-3p Regulates IGF-1 Levels in Serum and Vital Organs of Severely Burned Rats
To further determine if miR-301a-3p regulates IGF-1 levels in vivo, severely burned rats were immediately injected with hMSC, which was co-transfected with IGF-1 and miR-301a-3p. IGF-1 levels in serum and vital organs were detected at 12, 24, and 48 h post severe burn. IGF-1 levels in serum and vital organs, such as lung, liver, kidney, and heart, were decreased in the burn group, whereas they were increased in the burn + hMSC and burn + mock groups, compared with those in the sham group (p < 0.05). Moreover, they were markedly higher in burn + IGF-1-o and burn + miR-301a-3p-i groups and lower in burn + IGF-1-i and burn + miR-301a-3p-o groups than in the burn + mock group (p < 0.05). They were also significantly decreased in miR-301a-3p-i + IGF-1-i groups compared with burn + miR-301a-3p-i groups and were comparable with burn + mock groups (p < 0.05) (Figures 4A–4F). The results suggest that down-regulation of miR-301a-3p mediates IGF-1 expression and secretion in multiple organs and serum of severely burned rats.
Figure 4.
The miR-301a-3p Regulates IGF-1 Levels in Serum and Vital Organs of Severely Burned Rats
(A–E) IGF-1 levels in serum (A) and vital organs, such as lung (B), liver (C), kidney (D), and heart (E), in rats transfected with miR-301a-3p were detected by ELISA assay at 12, 24, and 48 h post severe burn, respectively. The down-regulation expression of miR-301a-3p mediated IGF-1 secretion from the transplanted hMSC in serum and vital organs, such as lung, liver, kidney, and heart, of severely burned rats.
(F) The urine volume was collected and recorded at 24 h post severe burn. Values are represented as mean ± SD (n = 8). ∗p < 0.05 and ∗∗p < 0.01 compared with sham groups, respectively. @p < 0.05 and @@p < 0.01 compared with burn group, respectively. #p < 0.05 and ##p < 0.01 compared with burn + mock group, respectively. &p < 0.05 and &&p < 0.01 compared with miR-301a-3p-i group, respectively. The mock, IGF-1-o, IGF-1-i, miR-301a-3p-o, miR-301a-3p-i or miR-301a-3p-i + IGF-1-i groups were defined as transplanted hMSC transfected with Lenti-GFP vehicle, Lenti-IGF-1-overexpress, Lenti-IGF-1-siRNA, Lenti-miR-301a-3p mimics and Lenti-miR-301a-3p inhibitors, Lenti-miR-301a-3p inhibitors combined Lenti-IGF-1-siRNA co-transfected, respectively.
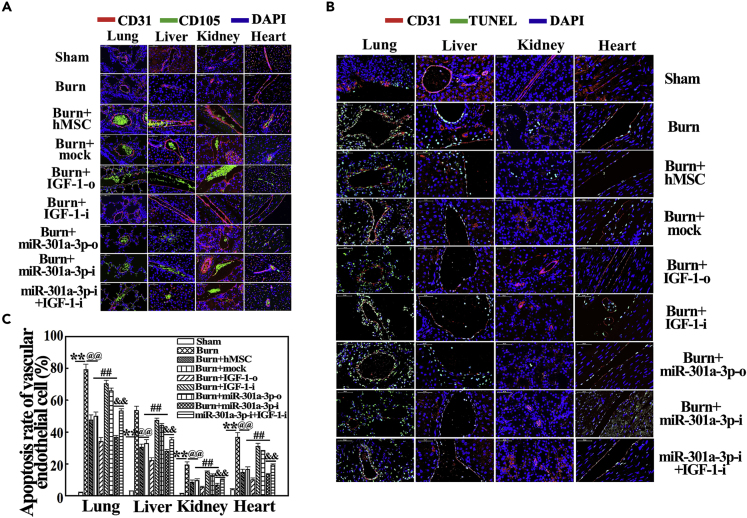
MiR-301a-3p Negatively Regulating hMSC-Secreted IGF-1 Inhibits Vascular Endothelial Apoptosis in Vital Organs in Severely Burned Rats
Based on the above results, we next investigated the effect of miR-301a-3p-mediated IGF-1 secretion from hMSC on vascular endothelial apoptosis of vital organs in severely burned rats. As shown in Figure 5A, the transplanted cells of burn + mock, burn + IGF-1-o, burn + IGF-1-i, burn + miR-301a-3p-o, burn + miR-301a-3p-i, and burn + miR-301a-3p-i + IGF-1-i groups migrated into the lung, liver, kidney, and heart, which were accumulated in the blood vessels of these vital organs at 24 h post severe burn. The vascular endothelial apoptosis of vital organs was not observed in sham groups but was significantly increased in the burn group (p < 0.01) (Figure 5B). hMSC and IGF-1 both obviously suppressed the vascular endothelial apoptosis of vital organs (p < 0.01) (Figures 5B and 5C). Moreover, compared with the burn + mock group, the apoptosis was further decreased in burn + IGF-1-o and burn + miR-301a-3p-i groups but was markedly increased in burn + IGF-1-i and burn + miR-301a-3p-o groups (p < 0.05) (Figures 5B and 5C). The decreased apoptosis in burn + miR-301a-3p-i groups was reversed by IGF-1 inhibition (p < 0.05) (Figures 5B and 5C). Collectively, IGF-1 expression directly regulated by miR-301a-3p protects vital organs from burn-induced apoptosis.
Figure 5.
Distribution of Transplanted hMSC with IGF-1/miR-301a-3p Overexpression and Knockdown and Their Effects on Apoptosis of Vascular Endothelial Cell in Vital Organs of Rats at 24 h Post Severe Burn
(A) Representative photographs showed that hMSC transfected with mock, IGF-1-o, IGF-1-i, miR-301a-3p-o, miR-301a-3p-i, and miR-301a-3p-i + IGF-1-i were mainly distributed in blood vessels of lung, liver, kidney, and heart tissues in rats at 24 h post severe burn.
(B) MiR-301a-3p negatively regulating hMSC-secreted IGF-1 reduced vascular endothelial apoptosis of vital organs, such as lung, liver, kidney, and heart, in severely burned rats at 24 h post injury.
(C) Quantitative analysis of apoptosis rate is shown in the corresponding histogram. Values are represented as mean ± SD (n = 8). ∗p < 0.05 and ∗∗p < 0.01 compared with sham group, respectively. @p < 0.05 and @@p < 0.01 compared with burn group, respectively. #p < 0.05 and ##p < 0.01 compared with burn + mock group, respectively. &p < 0.05 and &&p < 0.01 compared with miR-301a-3p-i group, respectively.
MiR-301a-3p Regulating hMSC-Secreted IGF-1 Reduces Vascular Permeability of Vital Organs in Severely Burned Rats
Then we evaluated the therapeutic effect of miR-301a-3p-mediated IGF-1 secretion from hMSC on vascular permeability of vital organs, including lung, liver, kidney, and heart in rats at 12, 24, and 48 h post severe burn by detecting water content ratio and Evans Blue (EB) content. The water content ratios and the EB content of lung, liver, kidney, and heart were increased in the burn group but were decreased in the burn + hMSC group compared with the sham group (p < 0.05). IGF-1 up-regulation and miR-301a-3p down-regulation suppressed the water content ratio and the EB content. Opposite results were observed in IGF-1 inhibition and miR-301a-3p overexpression groups (p < 0.05). IGF-1 down-regulation enhanced the inhibition effect of miR-301a-3p inhibitors on water content ratio and the EB content (Tables 1 and 2).
Table 1.
Water Content Ratio of Vital Organs (mean ± SD, %, n = 8)
| Groups | Parameter |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung |
Liver |
Kidney |
Heart |
|||||||||
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | 12h | 24 h | 48 h | |
| Sham | 63.15 ± 1.54 | 64.46 ± 0.77 | 63.94 ± 1.68 | 70.01 ± 1.54 | 71.16 ± 1.34 | 70.74 ± 1.38 | 78.48 ± 1.02 | 77.25 ± 2.74 | 78.58 ± 1.20 | 60.85 ± 1.39 | 61.18 ± 0.96 | 60.98 ± 1.02 |
| Burn | 90.19 ± 1.39 ∗∗ |
96.57 ± 2.08 ∗∗ |
85.92 ± 1.14 ∗∗ |
92.07 ± 0.62 ∗∗ |
97.24 ± 1.51 ∗∗ |
86.02 ± 2.03 ∗∗ |
85.19 ± 0.98∗∗ | 90.58 ± 1.63 ∗∗ |
82.72 ± 2.69 | 81.46 ± 1.64 ∗∗ |
85.36 ± 2.16∗∗ | 76.45 ± 1.11 ∗∗ |
| Burn + hMSC | 76.24 ± 0.88 @@ |
80.3 ± 1.15 @@ |
73.09 ± 1.20 @@ |
82.95 ± 1.03 @@ |
84.87 ± 1.29 @@ |
78.81 ± 1.38 @@ |
80.19 ± 0.71 @@ |
83.44 ± 0.95 @@ |
78.72 ± 1. 90 | 70.05 ± 1.12 @@ |
74.80 ± 1.09@@ | 68.38 ± 1.06 @@ |
| Burn + mock | 76.97 ± 0.51 @@ |
81 .24 ± 1.04 @@ |
74.71 ± 2.45 @@ |
83.39 ± 0.88 @@ |
85.95 ± 0.97 @@ |
78.15 ± 1.20 @@ |
80.50 ± 1.13 @@ |
83.73 ± 0.78 @@ |
78.53 ± 1.47 | 70.96 ± 0.63 @@ |
74.99 ± 0.54 @@ |
68.85 ± 0.71 @@ |
| Burn + IGF-o | 68.24 ± 1.49 ## |
72.36 ± 1.81 # |
67.07 ± 1.07 ## |
76.15 ± 1.03 ## |
78.01 ± 1.06 ## |
74.09 ± 0.74 # |
78.12 ± 0.85 | 79.51 ± 0.96 # |
78.37 ± 0.96 | 65.49 ± 0.41 # |
67.66 ± 0.90 ## |
64.12 ± 0.55 # |
| Burn + IGF-i | 86.03 ± 0.76 ## |
91.97 ± 0.74 ## |
81.99 ± 0.88 ## |
89.05 ± 0.56 ## |
93.00 ± 1.35 ## |
83.98 ± 0.97 # |
83.22 ± 0.78 | 88.73 ± 0.54 # |
81.93 ± 1.50 | 78.90 ± 0.84 ## |
82.82 ± 0.45 ## |
73.07 ± 1.44 # |
| Burn + miR-301a-3p-o | 84.12 ± 0.85 ## |
88.86 ± 1.31 ## |
79. 24 ± 0.92 # |
87.29 ± 1.04 # |
91.81 ± 0.77 ## |
82.57 ± 2.16 | 82.86 ± 0.49 | 87.24 ± 1.06 # |
80.64 ± 3.35 | 77.13 ± 0.72 ## |
80.79 ± 0.58 ## |
72.88 ± 1.57 |
| Burn + miR-301a-3p-i | 70.28 ± 0.83 # |
74.27 ± 0.59 ## |
69.33 ± 1.20 # |
77.48 ± 0.90 ## |
80.35 ± 0.59 # |
75.64 ± 1.11 | 79.46 ± 0.81 | 80.02 ± 0.49 # |
79.11 ± 0.72 | 66.98 ± 0.55 # |
69.04 ± 0.86 ## |
65.03 ± 0.98 # |
| miR-301a3p-i +IGF-1-i |
75.52 ± 1.12 & |
79.91 ± 0.84 & |
72.94 ± 1.05 & |
81.93 ± 0.74 & |
84.36 ± 0.78 & |
77.83 ± 1.00 | 81.3 ± 0.92 | 83.08 ± 0.72 & |
79.48 ± 0.82 | 69.77 ± 0.79 & |
72.85 ± 0.92 & |
67.14 ± 0.56 |
MiR-301a-3p negatively regulating hMSC-secreted IGF-1 reduces water content ratio of vital organs in severely burned rats (mean ± SD, n = 8). ∗p < 0.05 and ∗∗p < 0.01 compared with sham group, respectively. @p < 0.05 and @@p < 0.01 compared with burn group, respectively. #p < 0.05 and ##p < 0.01 compared with burn + mock group, respectively. &p < 0.05 and &&p < 0.01 compared with burn + miR-301a-3p-i group, respectively.
Table 2.
Evans Blue Content in Supernatant of Vital Organs (mean ± SD, μg/mL, n = 8)
| Groups | Parameter |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung |
Liver |
Kidney |
Heart |
|||||||||
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | |
| Sham | 10.87 ± 0.93 | 11.26 ± 0.82 | 10.95 ± 1.16 | 11.81 ± 0.66 | 11.23 ± 0.22 | 10.93 ± 0.50 | 10.52 ± 0.81 | 10.11 ± 0.26 | 10.00 ± 0.79 | 14.14 ± 0.85 | 14.18 ± 1.02 | 13.78 ± 1.01 |
| Burn | 56.55 ± 2.80 ∗∗ |
62.18 ± 3.33 ∗∗ |
40.74 ± 0.95 ∗∗ |
32.63 ± 0.58 ∗∗ |
40.60 ± 0.35 ∗∗ |
25.92 ± 1.09 ∗∗ |
23.48 ± 1.05 ∗∗ |
35.56 ± 1.04 ∗∗ |
18.61 ± 0.83 ∗∗ |
27.81 ± 1.00 ∗∗ |
38.23 ± 2.05 ∗∗ |
20.92 ± 0.56 ∗ |
| Burn + hMSC | 28.96 ± 0.92 @@ |
37.69 ± 1.85 @@ |
21.80 ± 1.09 @@ |
19.90 ± 0.73 @@ |
23.77 ± 0.94 @@ |
16.48 ± 1.20 @@ |
16.01 ± 0.48 @@ |
21.79 ± 1.04 @@ |
13.50 ± 0.62 @ |
18.52 ± 1.13 @@ |
22.70 ±1.10@@ | 15.54 ± 1.02 @ |
| Burn + mock | 29.03 ± 1.01 @@ |
38.51 ± 2.49 @@ |
22.93 ± 0.61 @@ |
20.36 ± 0.85 @@ |
24.05 ± 0.68 @@ |
16.96 ± 0.85 @@ |
16.24 ± 0.77 @@ |
22.33 ± 0.50 @@ |
13.92 ± 1.14@ | 19.04 ± 0.95 @@ |
23.08 ± 0.94 @@ |
15.69 ± 0.95 @ |
| Burn + IGF-o | 19.85 ± 1.35 ## |
23.06 ± 1.70 ## |
14.44 ± 0.70 ## |
13.93 ± 0.57 ## |
15.46 ± 0.41 ## |
12.65 ± 0.76 # |
12.71 ± 0.54 # |
15.54 ± 0.89 # |
10.87 ± 1.03 # |
14.60 ± 0.47 # |
16.52 ± 0.78 ## |
14.05 ± 1.19 |
| Burn + IGF-i | 47.96 ± 2.96 ## |
55.58 ± 1.36 ## |
35.59 ± 0.88 ## |
27.80 ± 0.72 ## |
35.79 ± 0.90 ## |
21.80 ± 0.88 # |
20.76 ± 0.32 # |
31.73 ± 0.92 ## |
16.35 ± 1.50 | 25.01 ± 0.92 # |
35.05 ± 0.60 ## |
19.04 ± 0.87 # |
| Burn + miR-301a-3p-o | 45.78 ± 1.04 ## |
52.70 ± 1.81 ## |
33. 37 ± 1..04 ## |
25.94 ± 1.19 # |
32.81 ± 1.42 ## |
20.97 ± 1.30 # |
19.81 ± 0.20 # |
29.11 ± 0.18 # |
15.89 ± 0.77 | 23.99 ± 0.73 # |
33.73 ± 1.39 ## |
18.97 ± 1.03 # |
| Burn + miR-301a-3p-i | 22.06 ± 0.67 ## |
25.93 ± 0.62 ## |
16.80 ± 0.65 ## |
15.20 ± 0.71 # |
17.18 ± 0.85 ## |
13.08 ± 1.12 # |
13.29 ± 1.40 | 17.02 ± 0.75 # |
11.43 ± 0.99 | 15.50 ± 1.09 # |
17.42 ± 0.71 ## |
15.12 ± 0.91 |
| miR-301a3p-i +IGF-1-i |
26.55 ± 1.14 & |
33.79 ± 1.26 && |
19.82 ± 0.70 & |
18.71 ± 1.08 & |
21.89 ± 1.21 & |
15.24 ± 0.93 | 14.89 ± 0.50 | 20.14 ± 0.37 & |
13.79 ± 0.51 | 17.38 ± 1.25 | 21.35 ± 1.12 & |
16.33 ± 1.79 |
MiR-301a-3p negatively regulating hMSC-secreted IGF-1 reduces Evans blue content in supernatant of vital organs in severely burned rats (mean ± SD, n = 8). ∗p < 0.05 and ∗∗p < 0.01 compared with sham group, respectively. @p < 0.05 and @@p < 0.01 compared with burn group, respectively. #p < 0.05 and ##p < 0.01 compared with burn + mock group, respectively. &p < 0.05 and &&p < 0.01 compared with burn + miR-301a-3p-i group, respectively.
MiR-301a-3p Negative Regulating hMSC-Secreted IGF-1 Improves the Function Parameters of Vital Organs in Severely Burned Rats
We also evaluated the therapeutic effects of miR-301a-3p-mediated IGF-1 secretion from hMSC on function parameters of lung, liver, kidney, and heart in rats at 24 h post severe burn. Lung function was determined by examining the parameters of inspiratory resistance (Ri), expiratory resistance (Re), compliance (Cl), peak expiratory flow (PEF), and maximal voluntary ventilation (MVV). Liver function was checked by measuring Alanine Transaminase (ALT) and Aspartate Transaminase (AST). Kidney function was determined by detecting urine volume, creatinine (Crea), and Urea. Heart function was determined by measuring Troponin, Creatine Kinase (CK), MB isoform of CK (CK-MB), and Lactate Dehydrogenase (LDH) and a-hydroxybutyrate Dehydrogenase (HBDH). We showed that the multiple organ functions were deteriorated the in the burn group but significantly improved in the burn + hMSC group compared with the sham group (p < 0.05). Compared with the burn + mock group, they were greatly improved in burn + IGF-1-o and burn + miR-301a-3p-i groups and markedly deteriorated in burn + IGF-1-i and burn + miR-301a-3p-o groups (p < 0.05). Moreover, compared with the burn + miR-301a-3p-i group, these parameters were worsened again in the miR-301a-3p-i + IGF-1-i group, and close to burn + mock group (Table 3) (p < 0.05). Taken together, these data comprehensively confirmed the therapeutic effects of miR-301a-3p-mediated IGF-1 secretion on vascular endothelial apoptosis, vascular permeability, and function parameters of vital organs, such as lung, liver, kidney, and heart in rats at very early stages post severe burn.
Table 3.
Function Parameters of Vital Organs (mean ± SD, n = 8)
| Groups | Parameter |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Function |
Liver Function |
Kidney Function |
Heart Function |
|||||||||||
| Inspiratory Resistance (Ri, cm H2O/mL.s) | Expiratory Resistance (Re, cm H2O/mL.s) | Compliance (Cl, mL/cm H2O) | The Peak Expiratory Flow (PEF, mL/min) | Maximal Voluntary Ventilation (MVV, mL) | ALT (U/L) | AST (U/L) | Urea (mmol/L) | Crea (mmol/L) | Troponin (pg/mL) | CK (U/L) | CK-MB (U/L) | LDH (U/L) | HBDH (U/L) | |
| Sham | 1.80 ± 0.09 | 1.37 ± 0.05 | 0.28 ± 0.02 | 38.09 ± 1.67 | 194.46 ± 3.27 | 39.51 ± 3.73 | 133.49 ± 32.18 | 4.55 ± 0.64 | 16.5 ± 0.71 | 210.24 ± 11.57 | 1,439.5 ± 140.12 | 1,520.5 ± 186.34 | 1,402 ± 138.63 | 469.50 ± 60.93 |
| Burn | 5.45 ± 0.13 ∗∗ |
4.48 ± 0.09 ∗∗ |
0.11 ± 0.03 ∗ |
20.24 ± 2.04 ∗∗ |
121.07 ± 2.38 ∗∗ |
210.33 ± 8.57 ∗∗ |
892.41 ± 56.33 ∗∗ |
13.63 ± 0.71 ∗∗ |
27.48 ± 0.63 ∗∗ |
320.78 ± 15.35 ∗∗ |
5,741.26 ± 137.51 ∗∗ |
3,620.97 ± 91.29 ∗∗ |
3,431.17 ± 165.74 ∗∗ |
1,183.82 ± 166.27 ∗∗ |
| Burn + hMSC | 3.31 ± 0.40 @@ |
2.70 ± 0.38 @@ |
0.20 ± 0.02 @ |
31.72 ± 1.15 @@ |
163.96 ± 1.85 @@ |
113.10 ± 4.18 @@ |
542.39 ± 20.05 @@ |
8.59 ± 0.50 @@ |
22.18 ± 0.77 @ |
259.39 ± 8.70 @@ |
2,952.80 ± 73.15 @@ |
2,490.38 ± 56.64 @@ |
2,172.86 ± 72.10 @@ |
721.95 ± 38.17 @@ |
| Burn + mock | 3.54 ± 0.18 @@ |
2.99 ± 0.17 @@ |
0.20 ± 0.04 | 31.38 ± 0.93 @@ |
163..81 ± 2.04 @@ |
114.67 ± 9.36 @@ |
544.85 ± 34.41 @@ |
8.76 ± 0.88 @@ |
22.96 ± 0.49 @ |
261.46 ± 9.98 @@ |
2,967.96 ± 62.97 @@ |
2,518.10 ± 90.01 @@ |
2,221.33 ± 85.71 @@ |
742.92 ± 41.24 @@ |
| Burn + IGF-o | 2.19 ± 0.17 # |
1.84 ± 0.56 # |
0.26 ± 0.02 | 36.49 ± 0.68 # |
182.75 ± 3.66 ## |
68.72 ± 6.35 ## |
357.33 ± 28.80 ## |
5.94 ± 0.27 ## |
18.01 ± 0.36 # |
229.32 ± 10.27 # |
2054.76 ± 90.29 ## |
1903.48 ± 103.4 ## |
1,696.57 ± 68.84 ## |
585.71 ± 18.48 ## |
| Burn + IGF-i | 4.73 ± 0.20 ## |
4.17 ± 0.38 ## |
0.13 ± 0.05 | 23.57 ± 0.51 # |
132.07 ± 2.03 ## |
187.27 ± 9.59 ## |
825.48 ± 36.10 ## |
11.09 ± 0.45 # |
25.51 ± 0.14 # |
294.21 ± 13.52 # |
4,562.19 ± 115.26 ## |
3,360.51 ± 57.32 ## |
3,074.95 ± 109.12 ## |
1,003.92 ± 42.77 ## |
| Burn + miR-301a-3p-o | 4.28 ± 0.11 ## |
3.86 ± 0.20 ## |
0.14 ± 0.03 | 24.49 ± 1.25 # |
136.71 ± 3.12 ## |
178.68 ± 7.44 ## |
784.52 ± 20.45 ## |
10.54 ± 0.62 # |
24.82 ± 0.53 # |
289.65 ± 3.26 # |
4,357.34 ± 197.05 ## |
3,283.94 ± 45.15 ## |
2,918.33 ± 70.31 ## |
984 ± 25.91 ## |
| Burn + miR-301a-3p-i | 2.55 ± 0.16 # |
2.05 ± 0.23 # |
0.25 ± 0.04 | 35.72 ± 1.03 | 179.96 ± 1.94 ## |
75.35 ± 3.82 ## |
371.19 ± 26.71 ## |
6.34 ± 0.19 ## |
19.15 ± 0.79 # |
234.05 ± 9.51 # |
2,211.38 ± 79.67 ## |
2075.37 ± 42.71 ## |
1743.22 ± 91.29 ## |
610.11 ± 20.10 ## |
| miR-301a3p-i +IGF-1-i |
3.09 ± 0.25 & |
2.52 ± 0.24 & |
0.22 ± 0.03 | 32.02 ± 1.09 | 167.24 ± 1.51 & |
100.72 ± 13.59 && |
481.48 ± 29.28 && |
7.94 ± 0.52 & |
21.95 ± 0.54 & |
250.29 ± 4.03 & |
2,708.84 ± 35.44 && |
2,326.95 ± 76.74 & |
2075.02 ± 79.63 && |
697.73 ± 26.38 & |
MiR-301a-3p negatively regulating hMSC-secreted IGF-1 improves function parameters of vital organs in severely burned rats (mean ± SD, n = 8). ALT, alanine transaminase; AST, aspartate transaminase, Crea, creatinine; CK, creatine kinase; CK-MB, MB isoform of CK; LDH, lactate dehydrogenase; HBDH, a-hydroxybutyrate dehydrogenase. ∗p < 0.05 and ∗∗p < 0.01 compared with sham group, respectively. @p < 0.05 and @p < 0.01 compared with burn group, respectively. #p < 0.05 and #p < 0.01 compared with burn + mock group, respectively. &p < 0.05 and &&p < 0.01 compared with burn + miR-301a-3p-i group, respectively.
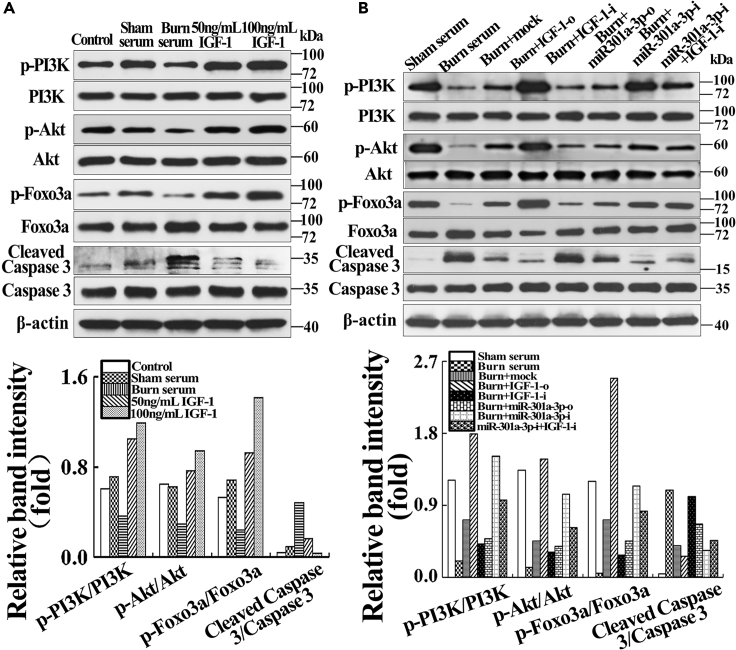
MiR-301a-3p Negatively Regulates hMSC-Secreted IGF-1 to Activate PI3K/Akt/FOXO3 Signal in Endothelial Cells
To elucidate the potential mechanism, we examined PI3K/Akt signaling using western blotting. The phosphorylation of PI3K, AKT, and Foxo3a was normalized to their protein expression. Cleaved caspase 3 was normalized to caspase 3. Compared with control and sham serum groups, the p-PI3K/PI3K, p-Akt/Akt, and p-FOXO3a/FOXO3a were decreased in the burn serum group but increased in IGF-1treated groups in a dose-dependent manner (Figure 6A) (p < 0.05). In addition, the p-PI3K/PI3K, p-Akt/Akt, and p-FOXO3a/FOXO3a of the burn serum group were lower than in the sham serum group, and those of the burn + mock group were markedly higher than in the burn group, but markedly lower than in the sham serum group (p < 0.05). They were remarkably increased in burn + IGF-1-o and burn + miR-301a-3p-i groups, but obviously decreased in burn + IGF-1-i and burn + miR-301a-3p-o groups compared with burn + mock group (p < 0.05). Moreover, IGF-1 inhibition suppressed the expression of p-PI3K/PI3K, p-Akt/Akt, and p-FOXO3a/FOXO3a (Figure 6B) (p < 0.05). By contrast, the ratio of cleaved Caspase 3 to Caspase 3 showed opposite change tendency relative to p-PI3K/PI3K, p-Akt/Akt, and p-FOXO3a/FOXO3a (Figures 6A and 6B). The data confirmed that miR-301a-3p regulation of hMSC-secreted IGF-1 activated PI3K/Akt/FOXO3a signal of vascular endothelial cells in severe burn microenvironment in vitro.
Figure 6.
Effects of IGF-1 and hMSC Transfected with IGF-1/miR-301a-3p Overexpression and Knockdown on PI3K/Akt/FOXO3a Signaling of Endothelial Cells in Severely Burned Serum Culture System
(A) Severe burn impaired activation of PI3K/Akt and its downstream effector FOXO3a, and IGF-1 activated PI3K/Akt/FOXO3a in a dose-dependent manner. The quantitative analysis was reported in the corresponding histogram.
(B) MiR-301a-3p negatively regulated hMSC-secreted IGF-1 inhibited PI3K/Akt/FOXO3a signaling of endothelial cells in the severely burned serum culture system; three samples in each group were applied for analysis. The quantitative analysis was reported in the corresponding histogram.
Discussion
Burn is a common form of traumatic injury with high mortality and morbidity. The vascular endothelium injury leading to vascular endothelial barrier dysfunction, which is essential for the initiation of shock at the early stage and progression of multiple organ dysfunction at the advanced stage. Recently, the therapeutic effects of hMSC have been demonstrated on some severe traumas and serious injuries through stabilizing endothelial barrier function (Walker et al., 2010; Bruno et al., 2012; Gatti et al., 2011; Walter et al., 2014; Yang et al., 2015; Meng et al., 2019). We previously found that hMSC alleviated cells apoptosis of wound and organ tissues post severe burn by secreting bioactive factors (Liu et al., 2016; Bake et al., 2019). In this study, we found that hMSC significantly reduced severe burn-induced vascular endothelial apoptosis, protected against endothelial barrier disorder and multiple organ dysfunction by secreting IGF-1. Severe burn-induced down-regulation of miR-301a-3p directly increased IGF-1 synthesis and secretion in hMSC, hence reducing vascular endothelial apoptosis and protecting against dysfunction of endothelial barrier and multiple organs. We also demonstrated that miR-301a-3p negatively regulated hMSC-secreted IGF-1 to activate PI3K/Akt/FOXO3 signaling in endothelial cells. Overall, we clarified the potential mechanism of hMSC on the endothelial apoptosis and dysfunctions of endothelial barrier at early stage post severe burn.
As a key mitogenic and anti-apoptosis factor, IGF-1 exhibits pro-survival and repairing effect on endothelial barrier, which is susceptible to ectopic expression and dysfunction induced by excessive inflammation, serious metabolic disturbance, and other systematic disorders after severe burn or trauma (Aman et al., 2016; Bake et al., 2019; Davis et al., 2006). Our precious study showed that IGF-1 was a pivotal bioactive factor secreted by hMSC in alleviating cell apoptosis after severe burn (Liu et al., 2014). Here we demonstrated that IGF-1 secreted by hMSC displayed a strong anti-apoptotic activity and therapeutic effects. IGF-1 reduced severe burn-induced vascular endothelial apoptosis, protected against endothelial barrier disorder, and multiple organ dysfunction in vitro and in vivo. It has been shown that bone marrow-derived MSC has beneficial effects on tubular cell repair in acute kidney injury by producing IGF-1 (Imberti et al., 2007). In rats with myocardial infarction, human cardiac stem cells with IGF-1 overexpression enhanced myocardial repair by improving the long-term survival of transplanted cells and the surrounding myocardium (Jackson et al., 2015). Therefore, based on this, IGF-1 secreted by the transplanted hMSC not only protects against apoptosis of the vascular endothelial and dysfunction of endothelial barrier but also exerts a self-protection function in the organic microenvironment post severe burn.
Increasing evidences have reported that MSC exerts their beneficial influences on repair and regeneration of injured organs through miRNA-mediated bioactive factors production and secretion (Lakshmipathy and Hart, 2008; Wen et al., 2012). We herein attempted to profile the microRNAs induced by burn to regulate hMSC-secreted IGF-1. We found that severe burn induced the down-regulation of miR-301a-3p. IGF-1 was a direct target for miR-301a-3p. In fact, miR-301a-3p negatively regulated IGF-1 protein translation and secretion in hMSC. Inhibition of miR-301a-3p in hMSC reduced the apoptosis of HUVECs in vitro as well as the apoptosis of vascular endothelial cells in vivo, thereby promoting the endothelial barrier permeability of lung, liver, kidney, and heart and eventually improving the function parameters of these organs in severely burned rats. Therefore, this study provided strong evidences supporting that miR-301a-3p directly regulated the production of IGF-1 from hMSC, which functions as a key upstream repair mechanism of hMSC's therapeutic effect on stabilizing endothelial barrier function. Antagonizing miR-301a-3p is a promising strategy to enhance IGF-1 release, which may be a helpful therapy for dysfunction of endothelial barrier and multiple organs. Meanwhile, our team profiled the dysregulated miRNAs after severe burn and its relationship with the biological characteristics of hMSC, which could augment the opportunities to safely pursue better therapeutic modalities during MSC-based therapy in severely burned patients who develop multiple organ dysfunction, even failure.
Finally, we clarified the anti-apoptosis mechanism of IGF-1 secreted by hMSC, which is the downstream signaling of hMSC on protecting against vascular endothelial apoptosis and endothelial barrier dysfunction. PI3K/Akt/FoxO3a signaling participates in regulating cell survival after severe burns. We also showed that severe burn impaired PI3K/Akt activation and increased total FOXO3a protein and cleaved caspase 3 levels, subsequently resulting in apoptosis of vascular endothelial cells. IGF-1 activated PI3K/Akt signaling, increased p-FOXO3a/FOXO3a level, and decreased cleaved Caspase 3/Caspase 3 level. Furthermore, we found that hMSC with IGF-1 overexpression and miR-301a-3p inhibitors remarkably increased PI3K and Akt phosphorylation and the ratio of p-FOXO3a to FOXO3a and decreased the ratio of cleaved Caspase 3 to Caspase 3. On the contrary, hMSC with IGF-1 siRNA and miR-301a-3p mimics could remarkably decrease PI3K and Akt phosphorylation and the ratio of p-FOXO3a/FOXO3a and increase the ratio of cleaved Caspase 3 to Caspase 3. These data confirmed miR-301a-3p negatively regulating hMSC-secreted IGF-1 reduced severe burn-induced vascular endothelial apoptosis via activating PI3K/Akt/FOXO3a signaling. And studies showed that phosphorylation of FOXOs by Akt inhibited transcriptional functions of FOXOs and contributed to cell survival, growth, and proliferation, thereby protecting against injured tissue or organ function (Zhang et al., 2011, 2019; Tucka et al., 2014). In addition, FOXO3 was also considered an evolutionarily conserved longevity factor, and FOXO3-activated vascular cells exhibited delayed aging and increased resistance to injury, even FOXO3-enhanced vascular cells could promote vascular regeneration in a mouse model of ischemic injury (Flachsbart et al., 2017; Martins et al., 2016; Yan et al., 2019).
Conclusion
In summary, these comprehensive data suggested that down-regulation of miR-301a-3p directly regulated hMSC-secreted IGF-1 to reduce severe burn-induced vascular endothelial apoptosis and protected against dysfunction of endothelial barrier and multiple organs via activating PI3K/Akt/FOXO3a signaling. Moreover, our results not only confirmed the potential mechanism involved in the therapeutic effect of hMSC on the endothelial apoptosis and dysfunctions of endothelial barrier at the early stage post severe burn but also might provide the theoretical foundation for further clinical applications of hMSC application into burn areas.
Limitations of the Study
Our study demonstrated the functional role of miR-301a-3p that directly regulated hMSC-secreted IGF-1 to protect against dysfunction of multiple organs in vivo and in vitro. Although we found the therapeutic effect of hMSC through downregulation of miR-301a-3p expression, the precise role of PI3K/Akt/FOXO3a signaling in mediating the therapeutic effect of hMSC needs further study.
Resource Availability
Lead Contact
Further information and requests for resources and regents will be provided by the Lead Contact, Jiake Chai (cjk304@126.com).
Materials Availability
This study did not involve any new unique reagents or resources.
Data and Code Availability
All data are included in the published article and the Supplemental Information, and any additional information will be available from the lead contact upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by research projects of National Natural Science Foundation of China (81701900, 81571894, 81772067), Nursery Fund of PLA General Hospital (17KMM28), the First Affiliated Hospital to the PLA General Hospital Science Research Foundation (2017FC-304M-CXYY-01) and Translational Medicine Foundation of PLA General Hospital (2017TM-029) and Major Project of Military Logistical Support Department (AWS15J003 and ALB19J001).
Author Contributions
L.L., H.Y., X.H., H.S.: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; J.C.: conception and design, financial support, data interpretation, and final approval of the manuscript; H.D., Y. Chang, L.Y., Y.W., S.H., X.W., X.Y., Y. Chi, W.L., Q.W., H.W., H.B., X.S., S.L.: provision of study material, data analysis, and interpretation.
Declaration of Interests
The authors declare that they have no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101383.
Supplemental Information
References
- Aman J., Weijers E.M., van Nieuw Amerongen G.P., Malik A.B., van Hinsbergh V.W. Using cultured endothelial cells to study endothelial barrier dysfunction: challenges and opportunities. Am. J. Physiol. Lung Cell Mol Physiol. 2016;311:L453–L466. doi: 10.1152/ajplung.00393.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger C., Samadi O., Jeschke M.G. The biochemical alterations underlying post-burn hypermetabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2633–2644. doi: 10.1016/j.bbadis.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S., Okoreeh A., Khosravian H., Sohrabji F. Insulin-like Growth Factor (IGF)-1 treatment stabilizes the microvascular cytoskeleton under ischemic conditions. Exp. Neurol. 2019;311:162–172. doi: 10.1016/j.expneurol.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran S.R., Svoboda K.K., Kerns D.G., Sheth A., Prockop D.J. Anti-inflammatory protein tumor necrosis factor-alpha-stimulated protein 6 (TSG-6) promotes early gingival wound healing: an in vivo study. J. Periodontol. 2015;86:62–71. doi: 10.1902/jop.2014.140187. [DOI] [PubMed] [Google Scholar]
- Bruno S., Grange C., Collino F., Deregibus M.C., Cantaluppi V., Biancone L., Tetta C., Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.E., Hsieh P.C., Takahashi T., Song Q., Zhang S., Kamm R.D., Grodzinsky A.J., Anversa P., Lee R.T. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl. Acad. Sci. U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorronsoro A., Robbins P.D. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther. 2013;4:39. doi: 10.1186/scrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F., Dose J., Gentschew L., Geismann C., Caliebe A., Knecht C., Nygaard M., Badarinarayan N., Elsharawy A., May S. Identification and characterization of two functional variants in the human longevity gene FOXO3. Nat. Commun. 2017;8:2063. doi: 10.1038/s41467-017-02183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S., Bruno S., Deregibus M.C., Sordi A., Cantaluppi V., Tetta C., Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transpl. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- Haines R.J., Wang C.Y., Yang C.G.Y., Eitnier R.A., Wang F., Wu M.H. Targeting palmitoyl acyltransferase ZDHHC21 improves gut epithelial barrier dysfunction resulting from burn-induced systemic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313:G549–G557. doi: 10.1152/ajpgi.00145.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Zhu X., Hu X.Q., Fang Z.F., Tang L., Lu X.L., Zhou S.H. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int. J. Mol. Med. 2013;31:484–492. doi: 10.3892/ijmm.2012.1200. [DOI] [PubMed] [Google Scholar]
- Huang L., Wang F., Wang Y., Cao Q., Sang T., Liu F., Chen S. Acidic fibroblast growth factor promotes endothelial progenitor cells function via Akt/FOXO3a pathway. PLoS One. 2015;10:e0129665. doi: 10.1371/journal.pone.0129665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberti B., Morigi M., Tomasoni S., Rota C., Corna D., Longaretti L., Rottoli D., Valsecchi F., Benigni A., Wang J. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J. Am. Soc. Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Jackson R., Tilokee E.L., Latham N., Mount S., Rafatian G., Strydhorst J., Ye B., Boodhwani M., Chan V., Ruel M. Paracrine engineering of human cardiac stem cells with insulin-like growth factor 1 enhances myocardial repair. J. Am. Heart Assoc. 2015;4:e002104. doi: 10.1161/JAHA.115.002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz M., Iovino N., Unnerstall U., Gaul U., Segal E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- Lakshmipathy U., Hart R.P. Concise review: MicroRNA expression in multipotent mesenchymal stromal cells. Stem Cells. 2008;26:356–363. doi: 10.1634/stemcells.2007-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam N.N., Hung T.D., Hung D.K. Acute respiratory distress syndrome among severe burn patients in a developing country: application result of the berlin definition. Ann. Burns Fire Disasters. 2018;31:9–12. [PMC free article] [PubMed] [Google Scholar]
- Laroye C., Gibot S., Reppel L., Bensoussan D. Concise review: mesenchymal stromal/stem cells: a new treatment for sepsis and septic shock? Stem Cells. 2017;35:2331–2339. doi: 10.1002/stem.2695. [DOI] [PubMed] [Google Scholar]
- Lee W.Y., Wang B. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J. Orthop. Translat. 2017;9:76–88. doi: 10.1016/j.jot.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li S., Wang X., Wang H. Esculetin induces apoptosis of SMMC-7721 cells through IGF-1/PI3K/Akt-mediated mitochondrial pathways. Can. J. Physiol. Pharmacol. 2017;95:787–794. doi: 10.1139/cjpp-2016-0548. [DOI] [PubMed] [Google Scholar]
- Li J., Zheng J. Theaflavins prevent cartilage degeneration via AKT/FOXO3 signaling in vitro. Mol. Med. Rep. 2019;19:821–830. doi: 10.3892/mmr.2018.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Song H., Duan H., Chai J., Yang J., Li X., Yu Y., Zhang X., Hu X., Xiao M. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci. Rep. 2016;6:30121. doi: 10.1038/srep30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Yu Y., Hou Y., Chai J., Duan H., Chu W., Zhang H., Hu Q., Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9:e88348. doi: 10.1371/journal.pone.0088348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R., Lithgow G.J., Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Pati S., Lee J.W. Concise review: mesenchymal stem (stromal) cells: biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells. 2017;35:316–324. doi: 10.1002/stem.2551. [DOI] [PubMed] [Google Scholar]
- Meng S.S., Guo F.M., Zhang X.W., Chang W., Peng F., Qiu H.B., Yang Y. mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J. Cell Biochem. 2019;120:3637–3650. doi: 10.1002/jcb.27642. [DOI] [PubMed] [Google Scholar]
- Menge T., Zhao Y., Zhao J., Wataha K., Gerber M., Zhang J., Letourneau P., Redell J., Shen L., Wang J. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl Med. 2012;4:161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S., Gerber M.H., Menge T.D., Wataha K.A., Zhao Y., Baumgartner J.A., Zhao J., Letourneau P.A., Huby M.P., Baer L.A. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S., Pilia M., Grimsley J.M., Karanikas A.T., Oyeniyi B., Holcomb J.B., Cap A.P., Rasmussen T.E. Cellular therapies in trauma and critical care medicine: forging new frontiers. Shock. 2015;44:505–523. doi: 10.1097/SHK.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Mu J., Ma J., Gong J., Li J., Wang J., Gao T., Zhu P., Zheng S., Xie J., Yuan B. Selenium inhibits homocysteine-induced endothelial dysfunction and apoptosis via activation of AKT. Cell Physiol Biochem. 2016;38:871–882. doi: 10.1159/000443041. [DOI] [PubMed] [Google Scholar]
- Song C.L., Liu B., Diao H.Y., Shi Y.F., Zhang J.C., Li Y.X., Liu N., Yu Y.P., Wang G., Wang J.P., Li Q. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget. 2016;7:39740–39757. doi: 10.18632/oncotarget.9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temnov A., Astrelina T., Rogov K., Moroz B., Lebedev V., Nasonova T., Lyrshchikova A., Dobrynina O., Deshevoy Y., Melerzanov A. Use of paracrine factors from stem cells to treat local radiation burns in rats. Stem Cells Cloning. 2018;11:69–76. doi: 10.2147/SCCAA.S164630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian K.Y., Liu X.J., Xu J.D., Deng L.J., Wang G. Propofol inhibits burn injury-induced hyperpermeability through an apoptotic signal pathway in microvascular endothelial cells. Braz. J. Med. Biol. Res. 2015;48:401–407. doi: 10.1590/1414-431X20144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucka J., Yu H., Gray K., Figg N., Maguire J., Lam B., Bennett M., Littlewood T. Akt1 regulates vascular smooth muscle cell apoptosis through FoxO3a and Apaf1 and protects against arterial remodeling and atherosclerosis. Arterioscler Thromb. Vasc. Biol. 2014;34:2421–2428. doi: 10.1161/ATVBAHA.114.304284. [DOI] [PubMed] [Google Scholar]
- Walker P.A., Shah S.K., Jimenez F., Gerber M.H., Xue H., Cutrone R., Hamilton J.A., Mays R.W., Deans R., Pati S. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp. Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Ware L.B., Matthay M.A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014;2:1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao Z., Gong J., Zhou S., Peng H., Shatara A., Zhu T.Z., Meltzer R., Du Y., Gu H. Adipose stem cells-conditioned medium blocks 6-hydroxydopamine-induced neurotoxicity via the IGF-1/PI3K/AKT pathway. Neurosci. Lett. 2014;581:98–102. doi: 10.1016/j.neulet.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Wen Z., Zheng S., Zhou C., Yuan W., Wang J., Wang T. Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: microRNAs as novel regulators. J. Cell Mol Med. 2012;16:657–671. doi: 10.1111/j.1582-4934.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Belasco J.G. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol. Cell. 2008;29:1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Yan B., Singla D.K. Transplanted induced pluripotent stem cells mitigate oxidative stress and improve cardiac function through the Akt cell survival pathway in diabetic cardiomyopathy. Mol. Pharm. 2013;10:3425–3432. doi: 10.1021/mp400258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Li Q., Wang L., Lu P., Suzuki K., Liu Z., Lei J., Li W., He X., Wang S. FOXO3-Engineered human ESC-derived vascular cells promote vascular protection and regeneration. Cell Stem Cell. 2019;24:447–461.e8. doi: 10.1016/j.stem.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Yang Y., Chen Q.H., Liu A.R., Xu X.P., Han J.B., Qiu H.B. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Jia H., Wang G., Ma Y., Sun W., Li P. miR-297 protects human umbilical vein endothelial cells against LPS-induced inflammatory response and Apoptosis. Cell Physiol Biochem. 2019;52:696–707. doi: 10.33594/000000049. [DOI] [PubMed] [Google Scholar]
- Yin S., Ji C., Wu P., Jin C., Qian H. Human umbilical cord mesenchymal stem cells and exosomes: bioactive ways of tissue injury repair. Am. J. Transl Res. 2019;11:1230–1240. [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Li X., Liu L., Chai J., Haijun Z., Chu W., Yin H., Ma L., Duan H., Xiao M. miR-628 promotes burn-induced skeletal muscle atrophy via targeting IRS1. Int. J. Biol. Sci. 2016;12:1213–1224. doi: 10.7150/ijbs.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang L., Peng L., Tian X., Qiu X., Cao H., Yang Q., Liao R., Yan F. Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway. J. Cell Mol. Med. 2019;23:4829–4838. doi: 10.1111/jcmm.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Srivastava D. A developmental view of microRNA function. Trends Biochem. Sci. 2007;32:189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the published article and the Supplemental Information, and any additional information will be available from the lead contact upon request.