We evaluated two commonly used methods to estimate maximum metabolic rate (MMR) in fishes (measured via oxygen consumption rate). The ‘chase method’, where fish are chased to exhaustion and transferred to a chamber for post-exercise measurement of oxygen consumption rate, underestimated MMR by ~20% when compared against using a swim tunnel respirometer. The difference was consistent across body sizes, temperatures and two species—each of which has important implications when using these measurements to inform conservation planning.
Keywords: Climate change, metabolic scope, oxygen uptake, ram ventilation, thermal biology
Abstract
Experimental biologists now routinely quantify maximum metabolic rate (MMR) in fishes using respirometry, often with the goal of calculating aerobic scope and answering important ecological and evolutionary questions. Methods used for estimating MMR vary considerably, with the two most common methods being (i) the ‘chase method’, where fish are manually chased to exhaustion and immediately sealed into a respirometer for post-exercise measurement of oxygen consumption rate (ṀO2), and (ii) the ‘swim tunnel method’, whereby ṀO2 is measured while the fish swims at high speed in a swim tunnel respirometer. In this study, we compared estimates for MMR made using a 3-min exhaustive chase (followed by measurement of ṀO2 in a static respirometer) versus those made via maximal swimming in a swim tunnel respirometer. We made a total of 134 estimates of MMR using the two methods with juveniles of two salmonids (Atlantic salmon Salmo salar and Chinook salmon Oncorhynchus tshawytscha) across a 6°C temperature range. We found that the chase method underestimated ‘true’ MMR (based on the swim tunnel method) by ca. 20% in these species. The gap in MMR estimates between the two methods was not significantly affected by temperature (range of ca. 15–21°C) nor was it affected by body mass (overall range of 53.5–236 g). Our data support some previous studies that have suggested the use of a swim tunnel respirometer generates markedly higher estimates of MMR than does the chase method, at least for species in which a swim tunnel respirometer is viable (e.g. ‘athletic’ ram ventilating fishes). We recommend that the chase method could be used as a ‘proxy’ (i.e. with a correction factor) for MMR in future studies if supported by a species-specific calibration with a relevant range of temperatures, body sizes or other covariates of interest.
Introduction
The use of respirometry to estimate metabolic rate in fishes has recently grown in popularity, with researchers interested in relating metabolic rate to intra- and inter-specific variation in life history, behaviour and responses to global changes (e.g. Myles-Gonzalez et al. 2015; Speers-Roesch et al. 2018; Montgomery et al. 2019). Amidst debate about its ecological relevance (Farrell 2016; Jutfelt et al. 2018), researchers continue to use aerobic scope (AS) to quantify thermal niche in ectotherms in the context of the threat posed by climate change. AS is the capacity of an animal to supply oxygen to tissues in excess of the supply required to maintain homeostasis while at rest, calculated as the difference between standard metabolic rate (SMR, the minimum or ‘resting’ rate of oxygen consumption) and maximum metabolic rate (MMR, measured as the maximum mass-specific rate of oxygen consumption, ṀO2). All animals require some AS to enable critical activities like locomotion, digestion, growth and reproduction (Fry 1947; Farrell 2016).
A critical challenge in quantifying AS in fishes is to accurately estimate MMR. The challenges in estimating MMR in fishes contrast with estimating SMR, which can be achieved with static intermittent-flow respirometers and best practices that are well established and applicable to most fishes (Clark et al. 2013; Chabot et al. 2016). The use of a swim tunnel respirometer, an apparatus pioneered more than 50 years ago (Brett 1964), is considered the ‘gold standard’ for measuring MMR in fishes. However, not all fishes will swim volitionally in a swim tunnel for long enough periods to measure ṀO2 (Norin and Clark 2016). Another hurdle is that swim tunnels are relatively expensive to purchase or build and time-consuming to operate—a trial for a single fish can take hours (Hvas and Oppedal 2019). In contrast, it is relatively straightforward to manually ‘chase’ a fish to exhaustion (e.g. for 3 min) and then transfer it into a static respirometry chamber for immediate post-chase measurement of ṀO2. The chase method allows for high throughput; a single researcher can, in practice, generate many estimates of MMR in a short period of time (e.g. using several static respirometers operating in parallel, Norin et al. 2016). However, the chase method assumes the fish reaches MMR during brief exhaustive exercise and remains there for some period thereafter, because post-exercise is when ṀO2 is measured with this approach (but see Zhang et al. 2020). That assumption implies that, once transferred to the respirometer, the fish uses its full capacity for oxygen delivery to tissues (i.e. MMR) to repay its oxygen debt and to stave off potentially lethal intracellular acidosis (Wood et al. 1983).
There are a handful of published studies that have focused on directly comparing methods for estimating MMR (Roche et al. 2013; Rummer et al. 2016; Hvas and Oppedal 2019; Little et al. 2020; Zhang et al. 2020). Understanding the differences between the chase and swim tunnel methods may be useful in interpreting the many papers that have already made claims about the MMR and AS of their fish and in helping direct future research. In this study, we used 2-year-old juveniles of two salmonids (Atlantic salmon Salmo salar and Chinook salmon Oncorhynchus tshawytscha) across a 6º C range of temperatures (ca. 15–21º C) to assess the difference in MMR estimates with a swim tunnel respirometer versus estimating MMR in a static respirometer immediately after an exhaustive chase protocol (hereafter, the ‘chase method’).
Salmon were good models for this work because they are well suited to swim tunnel respirometers and are ram ventilators (Steffensen 1985). We hypothesized that the swim tunnel respirometer would allow fish to achieve higher oxygen uptake (i.e. their ‘true’ MMR; in line with the findings of Hvas and Oppedal 2019; Roche et al. 2013; Rummer et al. 2016) thanks to ram ventilation (Clark et al. 2013), which stops the moment the fish is transferred to a static respirometer because of the cessation of directed flow through the mouth and across the gills. Alternatively, higher oxygen uptake in the swim tunnel respirometer could be driven partly or entirely by higher tissue O2 demand rather than a higher capacity to supply O2. We were also interested in assessing whether any differences in MMR estimates between the two methods were consistent across species, temperatures and body sizes. Our results are relevant to studies that measure MMR and AS in fishes with the goal of making ecological or evolutionary inferences relevant to conservation (e.g. Eliason et al. 2011; Kelly et al. 2014).
Materials and methods
Animals and acclimation conditions
All experimental procedures were approved by the University of Windsor Animal Care Committee following guidance set by the Canadian Council on Animal Care (U. Windsor AUPP #17-07 and #19-08). Gametes were collected from Chinook salmon spawning in the Credit River, ON, Canada (43.5813°N, 79.7085°W), in October of 2015 and transported ca. 340 km by road to the Freshwater Restoration Ecology Centre (FREC) in LaSalle, ON, Canada (42.2360°N, 83.1047°W). At FREC, 24 families of fertilized eggs were then created (24 females and 24 males). The fertilized eggs were incubated in recirculating vertical stack incubators using dechlorinated municipal water that was continuously aerated, filtered and kept at 10°C using thermostat-controlled chillers. Replicate groups of each family were housed separately within the incubators and during early post-hatch rearing (at the fry stage, full yolk sack absorption) transferred to 35-L tanks. Once fish grew to ca. 2 g body mass they were transferred to 850-L tanks where all families were mixed together (temperatures ranging between 10°C and 16°C due to seasonal fluctuations). The rearing tanks were connected to a recirculation system using dechlorinated municipal water that was continuously aerated and filtered and whose temperature was regulated with a thermostat-controlled chiller. Fish were fed using commercial aquaculture pellets (ca. 1% body weight per day). Of the Chinook salmon reared in FREC, 40 were used in this experiment [mean: 92.5 g, range: 53.5–166 g; mean: 20.3 cm fork length (FL), range: 17.5–24.2 cm] between 25 September 2017 and 23 October 2017. Of these, fish were split between two rearing tanks (n = 20 per temperature) each set to a different temperature (temperature range of 15–17°C or 20–21°C) beginning three days before the experiment began.
Atlantic salmon were reared in the same way and in the same facility as were the Chinook salmon (described above), from 2017 to 2019, and experiments occurred between 11 May 2019 and 2 August 2019 (mean: 141.3 g, range: 78–236 g; mean: 21.1 cm FL, range: 17.4–25.3 cm). One difference from the Chinook salmon was that the Atlantic salmon gametes came from hatchery rather than wild fish. Among the fish we used in 2019 were an additional seven Chinook salmon (41–195 g, 16.9–23.9 cm FL) of the same age as the Atlantic salmon (2 years), which allowed us to add to our data on Chinook salmon collected two years prior. All fish for the 2019 experiment (Atlantic salmon) were injected with a passive integrated transponder (PIT tags were 0.032 g, 8.4 mm long, mini HPT8 tags; www.biomark.com) at the start of the experiment so that individuals could be identified over time without impacting their growth or metabolism (Reemeyer et al. 2019). PIT tags were injected into the body cavity using an N165 needle after fish were mildly anaesthetized via immersion (for 2–3 min) in a 75 mg L−1 bath of tricaine methanesulfonate (MS-222, buffered with 150 mg L−1 sodium bicarbonate). Following PIT tagging, fish were held in a tank (same 850-L tanks as described above) set at 15–16°C from 7 May 2019 to 3 July 2019 and moved to a second tank on 3 July 2019 set to 18–20°C, where they remained through 2 August 2019 when data collection finished for this study.
Estimation of MMR with swim tunnel respirometry
The same swim tunnel respirometer (hereafter, ‘swim tunnel’; Loligo Systems, Viborg, Denmark; www.loligosystems.com) was used throughout the study as a means of estimating MMR. The swim tunnel had an effective volume of 28.85 L and an impeller connected to a control box that allowed us to modulate the speed of the water against which the fish had to swim in the 46 cm long × 14 cm deep × 14 cm wide working section. Dissolved oxygen (DO) was recorded inside the swim tunnel with an optical oxygen sensor (PreSens, Regensburg, German) connected via a fibre optic cable to a WITROX 1 oxygen meter (Loligo Systems, Viborg, Denmark; www.loligosystems.com). Oxygen was recorded at 1 hz in mg L−1 and the sensor was re-calibrated (100% air saturation one-point) before each trial. Temperature inside the swim tunnel was also recorded via the WITROX 1 system, which used that temperature value to automatically adjust recorded oxygen values in real time. A ‘flush line’ continuously flushed the swim tunnel with water from an adjacent tank containing the static respirometers (see below), which in turn was aerated and continuously exchanging water with the fish’s home tank (same temperature). Leak tests were done to confirm that the swim tunnel was perfectly sealed (no oxygen exchange with the surrounding water bath) when the flush line was closed (i.e. to enable measurement of ṀO2, see below).
The swim tunnel protocol began with the fish being sealed into the swim tunnel, with the flush line open, and given a minimum of 5 min to recover (at ca. 0.4 FLs s−1) before a practice swim began (following Lee et al. 2003). The practice swim involved ramping the water speed up to ca. 1.75–2.00 FL s−1 over the first ca. 5 min and letting the fish continue at that speed until a total of 15 min was reached. The fish then was left to recover at ca. 0.4 FL s−1 for 45 min (Lee et al. 2003) before we carried out a Umax (maximum swimming speed) swim protocol. The protocol was designed to elicit MMR (following Clark et al. 2011, Raby et al. 2016) in a relatively short amount of time as compared with a traditional Ucrit (critical swimming speed) protocol, which involves increasing the swimming speed more gradually (ca. 2.5 h total time per fish with our protocol vs. ca. 4–6 h if we had used a Ucrit protocol). Our Umax method began by increasing the water speed every 1–2 min until the fish began exhibiting intermittent burst-and-glide swimming behaviour, or until the fish began to struggle to continue swimming, with frequent bouts of ‘resting’ against the grid at the downstream end of the working section. To discourage fish from resting, a bright light shone on the downstream end of the working section and the upstream part of the working section was covered in dark plastic. In addition, the metal grid at the downstream end of the working section was occasionally electrified (switched on for 1 s) with ca. 8 V to motivate the fish to swim. Once the fish appeared to be swimming at close to maximum capacity and was able to sustain that speed for ≥2 min, the swim tunnel was sealed by closing a valve on the flush line, allowing measurement of ṀO2 to begin. At that point, the water speed was further increased (typically by a further 0.5–1.0 FL s−1) and then modulated as needed to encourage the fish to swim as fast as possible without having to rest on the grid at the downstream end of the working section. The tunnel was typically sealed for ca. 15–30 min during the Umax swim depending primarily on the size of the fish: smaller fish used less oxygen, requiring a longer period to establish a sufficient decline in DO. After the measurement period was complete, the fish was removed from the tunnel and weighed (nearest 0.5 g) and measured (FL, nearest mm). Fish were then transferred back to their home tank, used for static respirometry or sacrificed for dissection for a separate study. With the flush line back open, DO was then allowed to return to 100% air saturation. Once DO and temperature re-stabilized, the chamber was re-sealed to measure background respiration with the water speed set to ca. 55–60 cm s−1 (the speed at which the ṀO2 measurement typically began).
Swimming speed from the trial is reported here as the maximum speed (Umax, in FL s−1) the fish was able to maintain for ≥2 min during the ṀO2 measurement period. Water speeds (in cm s−1) were calibrated against impeller motor settings using a handheld digital flow meter with a vane wheel (flowtherm NT, hontzsch flow measuring technology; Waiblingen, Germany; https://www.hoentzsch.com/en/). Swimming speeds were not corrected for the solid blocking effect of the fish because the fish took up <10% of the cross-sectional area of the working section (Jones et al. 1974).
Estimating MMR with static respirometry
We attempted to elicit MMR by manually chasing fish and then sealing them into a static respirometer. Our protocol involved chasing fish for three min around a 1-m diameter circular tank filled to a depth of ca. 20 cm with the fish’s acclimation water (matching temperature). Fish were vigorously encouraged to swim by repeatedly startling the fish (with bare hands), splashing around the fish’s tail and occasionally tapping or gently grabbing the fish. After the chase ended, fish were transferred into respirometers to begin measuring ṀO2. A range of times are typically used for exhaustive chase protocols; we used 3 min because we found that it was sufficient to ensure fish became exhausted (stopped responding to contact) and it has been a commonly used duration in previous studies (Clark et al. 2012; Hvas and Oppedal 2019; Little et al. 2020). Eliciting MMR in this way is sometimes followed by 1–2 min of air exposure prior to sealing the fish into the respirometer (Clark et al. 2012; Roche et al. 2013). In our case, the fish was exposed to air for ca. 10 s during transfer to the respirometer. We avoided a longer air exposure because it could cause lamellae to partially collapse (Cook et al. 2015) and thereby reduce oxygen uptake capacity in the short term, reducing ṀO2. For example, Clark et al. (2012) used 3 min chase +1 min air and their fish did not reach ṀO2,max until 3–6 hours after entry into the respirometer.
In 2017 (Chinook salmon), we used four static respirometers that were custom-built using clear polycarbonate. The respirometers were 42 cm long × 20.2 cm wide × 19 cm deep inside but four water-sealed (non-porous) blocks were used in each to reduce the volume to a total of 12.26 L to better match the size of the fish (body mass: mean, 95.1 g; range, 53.5–166 g). An external recirculation loop with an inline pump ensured the chamber remained well mixed. An optical oxygen sensor (OXROB10, PyroScience, Aachen, Germany) was inserted into the recirculation loop of each chamber and connected to a four-channel Firesting O2 system (PyroScience, Aachen, Germany). A temperature sensor was inserted into one of the four chambers via its standpipe and connected to the Firesting O2 unit, allowing the software to adjust DO (recorded in mg L−1 at 0.2 Hz) for small changes in water temperature. Each probe was re-calibrated to 100% air saturation before each trial. A single pump flushed water from the surrounding 241 cm long × 91 cm wide × 31 cm deep water bath into all four chambers (water bath continuously recirculating water with the home tank of the fish, i.e. matching acclimation temperatures). In addition, there was a valve in the flush line connected to each chamber, such that the chamber could be sealed immediately upon the fish being inserted into the chamber (after the 3 min chase). The lid that opened at the top of each chamber, allowing us to insert/remove fish for these chambers, used 10 bolts, tightened with wingnuts, to seal the O-ring between the lid and chamber. The median time elapsed between the end of the exhaustive chase and the sealing of the fish in the chamber to begin ṀO2 measurement was 1 min 33 s (range of 1 min 13 s–2 min 52 s) for these four respirometers. For the post-chase MMR measurement, once DO had declined in a chamber to ca. 75–85% air saturation, generating a linear decline in DO, the flush line valve was manually re-opened. For some fish in 2017, we estimated MMR using both the swim tunnel and chase methods (N = 16; swim tunnel used prior to chase method in 9 of 16 cases, median time gap of 23.8 h between the two measurements; range, 16.1–51.3 h), but additional animals were used to generate an estimate of MMR using only the chase method (N = 20) or using only the swim tunnel (N = 4).
In the 2019 experiments (primarily with Atlantic salmon), we used eight 5.66-L respirometers, custom-built using polypropylene food containers (37.5 × 14.7 × 12.7 cm), made with a water-tight, snap-on lid that allowed the chamber to be quickly sealed once the fish was inside. For these chambers, the median time from the end of the chase to the sealing of the chamber was 39 s (17 s–1 min 24 s), slightly quicker than for the larger chambers used in 2017 (see above). Other elements of these smaller chambers, and the overall protocol, were the same as the larger chambers used in 2017 (see above). In the second part of the 2019 experiments (the higher temperature treatment), we used seven of the 5.66-L respirometers and one of the larger respirometers from 2017 to accommodate the largest individuals (effective volume of 13.4 L). In both 2017 and 2019, multiple tests confirmed that oxygen was unable to leak into the chambers when the flush pump was off for ṀO2 measurements. All fish were weighed (nearest 0.5 g) after their respirometry trial was complete and were typically then returned to their home tank (some fish were immediately transferred to the swim tunnel respirometer). In 2019, we made 19 paired estimates on Atlantic salmon (chase and swim tunnel methods on the same fish, plus one Chinook salmon), 38 additional estimates using only the chase method (plus six for Chinook salmon) and one additional tunnel-only estimate. For the 19 paired Atlantic salmon estimates, the swim tunnel method was used before the chase method in 8 of 19 cases (the reverse for the remaining cases), and the median time gap between measurements was 71.3 h (range, 2.6 h—16 days).
Data analyses
Data were brought into RStudio (v. 1.1.383; RStudio Team 2016) for inspection and analyses (R v. 3.4.2; Team 2017). The start and end of the sealed period (the ṀO2 measurement) during Umax swimming (swim tunnel method), or immediately following exhaustive exercise (chase method) was marked for analysis for each fish with the ‘locator’ function in R. The marked segment of data (a negative slope with DO on the y-axis with time in s on the x-axis) was used for estimation of MMR. We then fit many overlapping regressions to the DO data that began and ended at successive 5 s increments, starting from the beginning of the sealed period. We repeated those steps using regressions that were 2, 3, 4, 5, 6 and 7 min in length (as applicable, i.e. some ṀO2 measurement periods were < 7 min). We also ran a linear regression on the entire ṀO2 measurement period, which we confined to a maximum of the first 8 min for the static respirometry trials. The reason for this latter step with static respirometry data was that we a priori expected ṀO2 to decline with time elapsed since the end of the exhaustive chase (and some ṀO2 measurements were >8 min for the fish tested in the larger respirometers). Indeed, this expectation is why researchers typically try to seal their fish into respirometers as quickly as possible following exhaustive exercise and often report the time required to do so (as we have done here, and see Hvas and Oppedal 2019; Little et al. 2020; Norin et al. 2016).
To estimate MMR (in mg O2 kg−1 min−1) we used the steepest slope of any linear regression with an R2 of >0.95 (of any length, i.e. 2 min, 3 min, etc.). In total, this meant that 134 estimates of MMR (across species and protocols) were derived from 27 439 linear regressions on the static respirometry data and 55 433 regressions on the swim tunnel data (note: the swim tunnel measurement periods generally needed to be much longer because of the higher water volume compared to the static respirometry chambers, allowing for a greater number of linear regressions to be run on those data). For comparison, we also used the slope of the linear regression of the entire sealed measurement cycle (but only up to the first 8 min for static respirometry, see above) to calculate more conservative MMR estimates. MMR was calculated (as ṀO2) using the following formula:
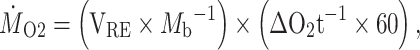 |
where VRE is the effective respirometer volume (in L, after removing the volume of the fish), Mb is the body mass of the fish (kg) and ΔO2 t−1 is the rate (in mg O2 s−1) of decline of DO (i.e. the slope of the regression) after removing the corresponding value for background respiration. Background ṀO2 was measured after the fish was removed from the chamber. The background respiration value was based on the slope of the best fit regression of the entire background measurement, which typically was based on a ca. 10–20-min-long background measurement (without a fish) for static respirometers (matching to the exact chamber) and ca. 20 min for swim tunnel respirometry trials. For static respirometry, median background respiration was 1.2% of fish MMR (range: 0.03%–16.1%, calculated as 100 × [ṀO2,background/ṀO2,fish]; median: 0.11 mg O2 kg−1 min−1, range: 0.004–0.76 mg O2 kg−1 min−1 with fish body mass standardized to the species mean). For the swim tunnel, ṀO2,background was a median of 4.3% of ṀO2,fish (range: 1.1%–9.9%; median of 0.54 mg O2 kg−1 min−1 with fish body mass standardized, range: 0.04–1.20 O2 kg−1 min−1).
Data files associated with this manuscript can be viewed on figshare (DOI: https://doi.org/10.6084/m9.figshare.11342435.v1).
Statistics
We were primarily interested in the effect of estimation method (swim tunnel vs. chase) on MMR but also interactions with temperature (mean temperature in °C during the ṀO2 measurement) and body mass (g). We built separate linear mixed effects models for each of the species (fish ID as a random effect, R package ‘nlme’; Pinheiro et al. 2017) using backwards model selection, beginning with all one-way interactions (body mass, temperature, estimation method), and sequentially removing the least significant model terms (keeping any with P < 0.05) using the ‘drop1’ function in R (i.e. nested model comparisons using likelihood ratio tests). Among the swim tunnel fish, we also fit a linear model with water temperature, species, and Umax as fixed effects to assess whether among-individual (or between-species) differences in swimming performance were associated with MMR. We used F-tests to compare variances among groups and incorporated variance structures into models where necessary (i.e. to meet model assumptions).
For estimates of MMR made using static respirometry, we were interested in assessing whether time elapsed since the end of the chase was an important factor. To do so, we first used a linear model to assess whether the time gap between the steepest decline in DO [i.e. the regression with the steepest slope (R2 > 0.95) used to calculate MMR] and the end of the exhaustive chase was predictive of the magnitude of MMR (species and time gap, in s, as fixed effects). This allowed us to test the prediction that, across individuals, the highest ṀO2 values would tend to occur relatively close in time to when the chase ended. To further test that prediction, we used a generalized additive mixed model (GAMM; function ‘gamm’ in package ‘mgcv’, Wood 2011) to assess the effect of time since the end of the chase on all ṀO2 estimates from the many linear regressions that were run (i.e. not only the one that was assigned the label of ‘MMR’). We predicted that ṀO2 would decline with time elapsed since the start of the ṀO2 measurement, especially after the first 2–3 min (additive models allow for non-linear effects of time). To do so, we calculated ṀO2 from every 2 min linear regression (run sequentially on the data in 5 s time steps, described above). We focused this analysis on the 2019 static respirometry data with the smaller respirometers because DO was being recorded in 2019 at a higher frequency (every 2 s rather than every 5 s), allowing high R2 values across most of the 2 min regressions (we kept all ṀO2 values with slopes of R2 > 0.85 for this analysis; N = 2463 of 2498 overall). The GAMM involved a time smoother (in seconds), a random effect of fish ID, and a temporal autocorrelation structure (‘corARMA’). Additional steps we took to assess the effect of time elapsed post-exercise on MMR are detailed in the Supplementary Information.
Models and their parameters are reported here as ‘significant’ based on α = 0.05, but we focus our interpretation more on the strength and size of effects given the pitfalls of relying heavily on P values (Halsey et al. 2015). Model assumptions were checked via qq-plots and by inspecting plots of model residuals (against fitted values and all fixed factors). For mixed effects models, we report marginal and condition R2 values calculated using the R package ‘MuMIn’ (Barton 2018).
Results
Across a ca. 6°C range of temperatures in two salmonids, we found that a manual chase protocol paired with static respirometry generated lower estimates of MMR than did the use of a swim tunnel respirometer (Fig. 1). The effect remained whether MMR was calculated using the steepest decline in DO for any 2+ min of the measurement period with an R2 > 0.95 (Fig. 1; statistics in Tables 1 and 2) or if we more conservatively used the slope of the entire ṀO2 measurement (up to the first 8 min for static respirometers; Fig. S1, Tables S1–S2). In either case, the swim tunnel generated estimates of MMR that were ca. 20% higher than using the chase method (all P ≤ 0.02 effects of estimation method; statistics in Tables 1–2, S1–S2). The magnitude of the difference was not significantly different between the two species based on a comparison of model-estimated effect sizes (i.e. effect sizes for estimation method in the different models described in Tables 1 and 2). The effect of method also appeared to be unaffected by temperature or body size (i.e. no interactions with treatment, statistics in Tables 1 and 2). Across both methods, temperature did have a weak, positive effect on MMR in Chinook salmon (but not in Atlantic salmon; Fig. 2, Tables 1 and 2), while we detected a negative effect of body mass on MMR in Atlantic salmon (but not in Chinook salmon; Fig. 3).
Fig. 1.
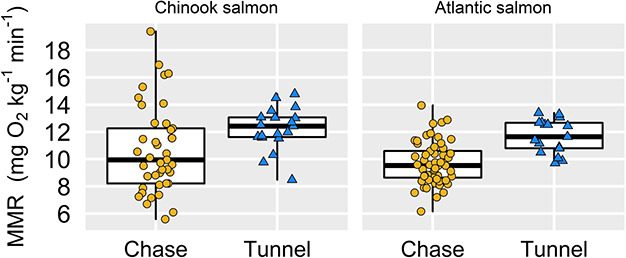
Species- and method-specific estimates of MMR based on using the linear regression with the steepest slope (of regressions with R2 > 0.95) shows that the chase method underestimates MMR (P ≤ 0.02 for all cases; statistics in Tables S1–S2). (The thick middle line in each boxplot indicates the median, the box represents the middle 50% of the distribution and the whiskers extend to the most extreme data points (all raw data points overlaid). Sample sizes are as follows (boxplots from left to right): 41, 19, 57, 17.)
Table 1.
Parameters and their statistical significance for a linear mixed model predicting MMR in Chinook salmon was higher for fish tested in the swim tunnel and that there was also a positive effect of water temperature
| Parameter | Value ± S.E. | df | t | P |
|---|---|---|---|---|
| Intercept | 4.11 ± 2.36 | 44 | 1.75 | 0.09 |
| Method (tunnel) | 1.39 ± 0.53 | 14 | 2.61 | 0.02 |
| Temperature (°C) | 0.36 ± 0.13 | 14 | 2.86 | 0.01 |
*Here, MMR was calculated using the steepest slope of any linear regression with an R2 > 0.95 (data corresponding to Fig. 2 in the paper).
Note: this model also had a random effect of fish ID (some fish were tested using both methods) and a variance structure that allowed the model to satisfy the assumption of equal variances between the two treatments.
Variance was significantly higher in the chase group for Chinook salmon (F-test; F = 4.17, P = 0.002); addition of the variance structure markedly improved the model residuals.
Backward selection was used to arrive at this model, starting with all two-way interactions among methods, body mass and water temperature (see Methods).
Marginal R2 (fixed effects) = 0.10; Condition R2 (fixed plus random effects) = 0.20.
Table 2.
Parameters and their statistical significance for a linear mixed model predicting MMR in Atlantic salmon was higher for fish tested in the swim tunnel and that there was also a negative effect of body mass.
| Parameter | Value ± S.E. | df | t | P |
|---|---|---|---|---|
| Intercept | 11.09 ± 0.63 | 42 | 17.73 | <.001 |
| Method (tunnel) | 2.20 ± 0.41 | 29 | 5.39 | <.001 |
| Body mass (g) | −0.01 ± 0.004 | 29 | −2.35 | 0.03 |
Here, MMR was calculated using the steepest slope of any linear regression with an R2 > 0.95 (data and model fits corresponding to Fig. 3 in the paper).
Note: this model also had a random effect of fish ID.
Backward selection was used to arrive at this model, starting with all two-way interactions among method, body mass, and water temperature (see Methods).
Marginal and condition model R2 = 0.30.
Fig. 2.
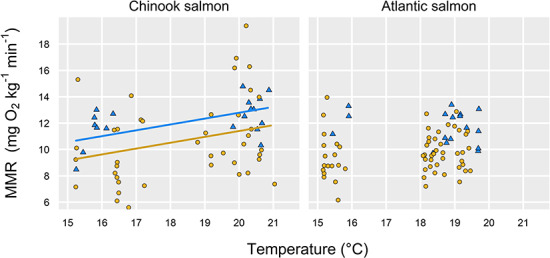
MMR estimates for Chinook salmon (left) and Atlantic salmon (right) as a function of estimation method (blue triangles, swim tunnel; yellow circles, chase method) and temperature; there was a positive overall effect of temperature for Chinook salmon (P = 0.01, with different intercepts for each estimation method, Table 1). Temperature was not a significant driver of MMR for Atlantic salmon (Table 2). Both panels represent MMR calculated using the steepest slope with R2 > 0.95 of any 2+ min regression for each fish’s MMR measurement period (see Methods).
Fig. 3.
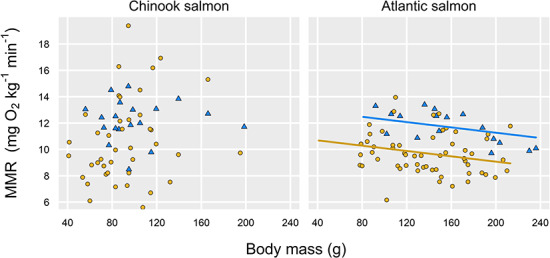
MMR estimates for Chinook salmon (left) and Atlantic salmon (right) as a function of estimation method (blue triangles, swim tunnel; yellow circles, chase method) and body mass; there was a negative effect of body mass for Atlantic salmon (P = 0.03, with different intercepts for each estimation method, Table 2). Body mass was not a significant driver of MMR for Chinook salmon (Table 1). Both panels use MMR calculated using the steepest slope with R2 > 0.95 of any 2+ min regression for each fish’s MMR measurement period (see Methods).
In the Chinook salmon data, fish ID accounted for the same amount of variance (ca. 10%) in the data as did the fixed effects (estimation method and water temperature), indicating some level of within-individual repeatability between the two methods (based on a comparison of condition and marginal R2 values for a mixed model whose random effect was fish ID; Table 1). In the Atlantic experiment, which had a somewhat different design with respect to the timing of repeated measures (see Discussion), fish ID explained none of the variance in the data (Table 2).
The chase method led to higher variance in MMR than did the swim tunnel method (F40,18 = 4.17, P = 0.002) but only for Chinook salmon for which we used the R2 > 0.95% method for selecting the steepest regression slope to calculate MMR (Fig. 1). In Atlantic salmon (F56,16 = 1.58, P = 0.31, Fig. 1), and for Chinook salmon when we switched to using a longer period to calculate MMR (data in Fig. S1), the difference in variance between methods disappeared (F40,19 = 1.05, P = 0.97).
For the chase method, there was a weak tendency for ṀO2 to decline with time elapsed since the cessation of exhaustion exercise. In a GAMM smoother modelling the effect of time (P = 0.02, Fig. S2), there appeared to be a subtle decline in ṀO2 over time, but the model explained almost none of the variance in the data (R2 = −0.013). Among our estimates of MMR made using the chase method (Fig. 1), there was a tendency for assigned values of MMR to be lower if they occurred later relative to the end of the chase (in seconds) but the trend did not rise to statistical significance [t = −1.74, P = 0.09; species effect (P = 0.02) also included in model; overall model R2 = 0.08]. Visualizing the MMR estimates as a function of time elapsed since the end of exercise for both species similarly revealed no effect of time when examining the first 9 min after entry into the respirometer (see Fig. S3 and associated statistics).
In Atlantic salmon whose MMR was estimated with static respirometry, all of the estimates we used came from slopes that were 2 min in duration, but the exact time relative to when the respirometer was closed varied widely (median 110 s after the chamber was sealed, range 0 to 370 s). For Chinook salmon, which were tested in larger chambers with relatively weaker mixing of the water and a lower recording frequency (0.2 Hz cf. 0.5 Hz for Atlantic salmon), the steepest regression slope with an R2 > 0.95 varied in length from 2 min (the minimum we attempted to use, n = 17 of 41) to 7 min (n = 4 of 41), with a median of 3 min for the slope duration used to calculate MMR (Fig. S5–S6). For Chinook salmon, the slope for the ‘best estimate’ of MMR began 0 to 725 s after the chamber was sealed for measurement of MMR, with a median of 185 s. Additional text and plots in the online supplement provide further exploration of the relationships between regression length, time elapsed since the end of the chase, R2 and the resulting MMR estimate for the two species (Fig. S3–S6).
In the swim tunnel, Chinook salmon reached ca. 14% higher Umax (FL s−1) than did Atlantic salmon (species-level means of 3.38 vs. 2.96 FL s−1, t1,34 = 2.53, P = 0.02). However, for fish tested in the swim tunnel, there was no between-species difference in absolute MMR after controlling for a modest overall effect of water temperature on MMR (t2,33 = 2.63, P = 0.01) and a clear effect of Umax on MMR across species (t2,33 = 4.45, P < 0.001, overall model R2 = 0.39; Fig. 4).
Fig. 4.
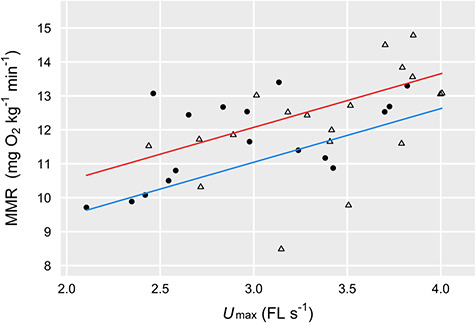
MMR for Chinook salmon (open triangles) and Atlantic salmon (solid circles), which tended to be higher (across species, P < 0.001) in fish that reached a higher maximum swimming speed during measurement of ṀO2 (same data in Fig. 1). There was a modest positive effect (across species) of water temperature (P = 0.02); model fits based on 16°C (blue) and 20°C (red) are shown.
Discussion
We found that the use of an exhaustive chase protocol paired with static respirometry underestimated MMR in juvenile Chinook and Atlantic salmon. Given that the ‘chase method’ is commonly used to estimate MMR in fishes (e.g. Healy and Schulte 2012; Kelly et al. 2014; Norin et al. 2016), our results help to highlight that researchers should proceed with caution when labelling their data as MMR if they have used this method. However, an important finding was that the gap between the ‘gold standard’ swim tunnel method and the chase method appeared to be unaffected by water temperature (across a ca. 6°C range), body mass, or species. That finding suggests researchers working with salmonids may be able to use the chase method to generate a proxy for MMR when examining the effects of body size or temperature on AS, given that the measurement ‘error’ associated with this method may not have an allometric or thermal bias.
While the approach of using the chase method as a proxy for ‘true’ MMR and AS at the group level (e.g. for a body size or thermal treatment) may be appropriate, more evidence is needed about within-individual consistency across estimation methods before that approach could be used in the study of among-individual variation (Norin and Malte 2011). A subset of the animals in this study was tested using both methods. We did find some support for the idea that fish with high MMR in the chase protocol also have high MMR in the swim tunnel respirometer: in our Chinook salmon experiment, the random effect of fish ID explained as much variance as both fixed effects combined (temperature and estimation method, ca. 10% each). In that experiment, the two estimates were made relatively close together in time on the same fish (range of 16–51 h, mean of 24 h). In the Atlantic salmon experiment, the fish were PIT tagged allowing us to track fish ID over time and because of logistical constraints associated with other experiments being conducted in parallel (data not reported here), fish were typically not tested using both methods on the same day. The median time gap between the two estimates (at a matching temperature treatment) in Atlantic salmon was 70 h (min = 2.6 h, max = 16 days), and we found no clear evidence for repeatability across methods. That time gap may have led to small deviations in body mass or other aspects of physiological state of the fish, leading to lower within-individual repeatability (White et al. 2013). More broadly, there is some evidence that MMR may be a less repeatable trait than is SMR (reviewed in Norin and Clark 2016). A future study focused on examining within-individual repeatability for MMR would benefit from multiple repeated measurements for each estimation method.
There has been some important previous work assessing the differences in MMR estimates among different methods. An analysis of data extracted from the literature suggested that MMR is not sensitive to methodology, but that analysis did not focus on experiments specifically designed to address methodology (Killen et al. 2017). In contrast to our results, Little et al. (2020) did not detect differences among methods, including those we used here, in mature coho salmon (Oncorhynchus kisutch) at a single temperature (9°C). In other cases, researchers who have set out to look for differences in MMR between swim tunnel respirometry and the chase method have typically found them. Roche et al. (2013) conducted a study with 10 Scolopsis bilineata (two-lined monacle bream, a coral reef fish) and found that a 3 min chase +1 min air treatment underestimated MMR by ca. 1.7 mg O2 kg−1 min−1 (ca. 22% lower), similar to our study. Rummer et al. (2016) extended upon that work in four additional coral reef fishes (5–11 individuals per species) and found that the chase method consistently underestimated MMR across species but that the gap size varied among species (from ca. 2–22%). Hvas and Oppedal (2019) compared a 3 min chase protocol (identical to the present study) to swim tunnel respirometry, using Atlantic salmon that were ca. 50–75% larger than ours (body mass, all at 13°C). That study provides an especially useful comparison because their post-chase ṀO2 measurements were made in a swim tunnel (at a slow water speed of < 0.5 FL s−1) rather than in a static respirometer, meaning the fish were able to receive some ram ventilation (Hvas and Oppedal 2019). They found a 50% increase (from 5.6 to 8.5 mg O2 kg−1 min−1) in MMR when comparing their chase method to Ucrit swimming. While that increase in MMR was high in relative terms, the numerical increase of 2.9 mg O2 kg−1 min−1 was only slightly higher than in our study (Fig. 2). The estimates of MMR in Hvas and Oppedal (2019) were lower than in our study on the same species because the fish were larger and the water temperatures were lower. Zhang et al. (2020) recently introduced a static respirometer design that allows the fish to be ‘chased’ with a probe while inside a static chamber, a technique that generated MMR estimates equivalent to those obtained with a swim tunnel (using juveniles of another salmonid, Oncorhynchus mykiss). In their study, they also found that post-exercise MMR in fish transferred rapidly to respirometers (ca. 10 s) was 18% lower than ‘true’ MMR, a nearly identical effect size as in the present study (Zhang et al. 2020).
What are we measuring with these two approaches?
Swimming maximally (the swim tunnel method) and recovering from exhaustive exercise (the chase method) are functionally different processes, which may help explain why estimates made using these two methods can differ. Depending on the species and research question, one method might be more ecologically relevant than the other. The Hvas and Oppedal (2019) study suggests ram ventilation is not a mechanism behind the differences in MMR between the two methods (chase vs. swim tunnel) because their fish were receiving gentle ram ventilation during post-chase ṀO2 measurements (cf. our study where fish were in a static respirometer). Likewise, Little et al. (2020) found ṀO2 to be markedly lower during recovery from Umax or Ucrit swimming even while fish received gentle ram ventilation in a swim tunnel. These findings from the literature might appear to invalidate our hypothesis about ram ventilation being responsible for fish reaching higher ṀO2 in a swim tunnel respirometer (cf. static respirometry, where fish rely on buccal-opercular pumping, which may provide less capacity for O2 uptake). However, it is possible that the high swimming speeds achieved during Ucrit or Umax are needed to detect an O2-uptake benefit of ram ventilation (Steffensen 1985).
Another reason the chase method may underestimate MMR is because it involves eliciting primarily anaerobic burst swimming and then measuring its immediate aftermath, as opposed to measuring post-exercise ṀO2 for high-speed sustained (aerobic) swimming. However, ṀO2 measured immediately after the end of a Ucrit swim is typically well below MMR (e.g. Reidy et al. 2000). Moreover, in Atlantic cod (Gadus morhua), post-exercise ṀO2 is actually higher for fish manually chased to exhaustion than for fish exercised with a Ucrit protocol (Reidy et al. 1995). It may be that regardless of the type of exercise, the species, or the context, the moment exercise ends, fish reduce their ṀO2 to below maximum (e.g. to 80–90% of maximum), leaving some AS available for other functions while they recover, rather than remaining at MMR to power a rapid recovery. Indeed, post-exercise oxygen consumption can be a prolonged affair and occur in stages (e.g. 8–16 h in Atlantic salmon at 12°C, Zhang et al. 2018) whereby ṀO2 falls rapidly over the first 30–60 min after exercise and then declines slowly thereafter. Thus, while ram ventilation may facilitate higher O2 uptake, differences in MMR between methods could be largely driven by O2 tissue demand dropping the moment exercise (i.e. muscle contraction) stops.
Methodological considerations
Advocates of using the chase method to estimate MMR might argue that for it to be successful, the fish should be sealed into the respirometer extremely rapidly after exercise ends (e.g. within 10 s), given that ṀO2 typically begins to decline quickly. Along the same lines, one might also predict that MMR estimation should focus on the first 2–3 min of the decline in O2 after the chamber is sealed. Based on our data we cannot rule out the possibility that higher estimates from the chase method may be possible with a faster transfer to the respirometer (e.g. 10 s) than we achieved, combined with a shorter measurement window (e.g. 30–60 s). However, we were unable to find any evidence that estimation of MMR is highly sensitive to these issues. Our fish were sealed into respirometers to begin measuring ṀO2 as rapidly as possible (ca. 30–120 s depending on the respirometer design, see Methods). Thereafter, we found little evidence of any decline in ṀO2 over the first 3–6 min of the measurement based on rolling 2-min regressions used to calculate ṀO2 (Fig. S2). Similarly, a comparison of ṀO2 estimates from the first 1–3 min after exercise to 5–7 min after exercise revealed no differences based on histograms and statistical tests (see Supplementary Information including Fig. S3). Simply selecting the steepest regression slope with an R2 of >0.95 revealed that the best MMR estimates did not necessarily occur near the start of the ṀO2 measurement. Thus, while we agree that sealing the fish into the respirometer rapidly is a good step to take, along with best practices for static respirometry (e.g. strong mixing, no leaks, accounting for background respiration, Clark et al. 2013), including robust methods to calculate MMR (see Zhang et al. 2019; Little et al. 2020), deviations of 1–2 min in the time it takes to seal fish into the chamber did not appear to be responsible for the chase method generated a lower estimate of MMR. Further emphasizing this conclusion, Zhang et al. (2020) were able to transfer their juvenile O. mykiss to static respirometers in as little as 10 s and found that their post-exercise measurements similarly underestimated MMR.
There was substantial variation in MMR using both methods. Using our method to calculate MMR (steepest slope with R2 > 0.95), there was significantly more variance for Chinook salmon using the chase method than for the swim tunnel method (Fig. 1). For those fish, we were using larger respirometers and a lower recording frequency than in 2019 (Atlantic salmon). As a result, we believe the fact that some MMR estimates were relatively high for those data was partially an artefact of inferior mixing (same power recirculation pump—600 L h−1, but a larger volume chamber) and the lower recording frequency when compared against the Atlantic salmon data. The effects of these differences in static respirometry methods between the Atlantic salmon and Chinook salmon experiments are explored in detail in the Supplementary Information. As noted in Results, when we switched to calculating MMR using the full slope of the sealed cycle (Fig. S1) the difference in variance disappeared while the gap between the two methods remained.
In the case of the swim tunnel respirometer, some of the among-individual variation in MMR was predicted by variation in swimming speed (Umax, Fig. 6). We have no way of knowing from our data whether the relationship between MMR and Umax was causal. For example, does physiological capacity for oxygen uptake drive swimming performance, or vice versa, or are these traits simply associated via a common physiological mechanism (e.g. at the cellular level)? It is also plausible that behavioural differences (i.e. motivation) led some fish to swim faster than others, resulting in differences in oxygen uptake. One key difference between the chase and swim tunnel methods is that we were able to use mild electric shock to motivate the fish in the swim tunnel, a technique that has long been standard with swim tunnel respirometry (e.g. Steffensen et al. 1984; Farrell et al. 2003; Eliason et al. 2011). In the manual chase protocol, we relied on visual and mild physical stimuli (i.e. contact from the hands of researchers) to motivate fish to swim. Based on our personal observations running a small number of pilot trials in the swim tunnel without the electrifiable grid, the maximum speeds fish reached without it were much lower—a decrease in swimming speed would decrease ṀO2 and therefore any estimate of MMR (e.g. Fig. 6).
Summary and recommendations
Taken together with most of the existing literature (Roche et al. 2013; Rummer et al. 2016; Hvas and Oppedal 2019; Zhang et al. 2020) our findings suggest exhaustive exercise rapidly followed by ṀO2 measurement in a static respirometer does underestimate ‘true’ MMR in many species (i.e. based on a comparison with swim tunnel respirometry). That the chase method underestimates MMR may be particularly true in fishes like salmon that use ram ventilation and are willing to swim in a swim tunnel respirometer (but see Little et al. 2020 whose findings with adult coho salmon do not support this conclusion). Indeed, for less ‘athletic’ species that refuse to swim in a swim tunnel, the chase method may be the only option for estimating MMR (Clark et al. 2013), and many fish biologists do not have access to a swim tunnel respirometer. In rare cases, estimates of MMR may even be higher while fish digest large meals than during post-exercise measurements (Steell et al. 2019). An important finding in our study was that the discrepancy between methods was not temperature or body size dependent, nor did it appear to be caused by fish being sealed into respirometers too slowly after the end of the exhaustive chase (Zhang et al. 2020). However, more evidence is needed—replication across a wider range of temperatures and body sizes, more repeat measurements within individual fish—before we can confidently conclude that the chase method provides an unbiased ‘proxy’ for MMR. The additional evidence needed to support the chase method’s use as a proxy for MMR needs to be collected on a species-specific basis because the effect of methodology is unlikely to be consistent among species (Rummer et al. 2016).
Funding
This research was supported by the Canada Foundation for Innovation (T.E.P.), the Natural Sciences and Engineering Research Council of Canada (NSERC; T.E.P. and A.T.F.) and by the Canada Research Chairs program (A.T.F.). G.D.R. was supported by an NSERC Post-Doctoral Fellowship.
Supplementary Material
Acknowledgments
Madison Lucas and Matthew Charron assisted with respirometry trials. Jason Lewis, Madison Dugdale and Katelynn Johnson provided additional logistical support. We thank the staff at the University of Windsor Technical Support Centre, especially Steven Budinsky, Marc St. Pierre, and Gangyong Zhang for help with respirometry design, construction and troubleshooting. Tommy Norin, Tim Clark, Ben Speers-Roesch and Fredrik Jutfelt provided technical advice. Fredrik Jutfelt also provided helpful comments on an early version of the manuscript, and Tim Clark provided some of the respirometry equipment we used.
References
- Barton K. (2018) MuMIn: Multi-Model Inference.
- Brett J. (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Canada 21: 1183–1226. [Google Scholar]
- Chabot D, Steffensen JF, Farrell AP (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88: 81–121. [DOI] [PubMed] [Google Scholar]
- Clark TD, Jeffries KM, Hinch SG, Farrell AP (2011) Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J Exp Biol 214: 3074–3081. [DOI] [PubMed] [Google Scholar]
- Clark TD, Donaldson MR, Pieperhoff S, Drenner SM, Lotto A, Cooke SJ, Hinch SG, Patterson DA, Farrell AP (2012) Physiological benefits of being small in a changing world: responses of coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS One 7: e39079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Cook KV, Lennox RJ, Hinch SG, Cooke SJ (2015) Fish out of water: how much air is too much? Fisheries 40: 452–461. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Farrell AP. (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88: 322–343. [DOI] [PubMed] [Google Scholar]
- Farrell A, Lee C, Tierney K, Hodaly A, Clutterham S, Healey M, Hinch S, Lotto A (2003) Field-based measurements of oxygen uptake and swimming performance with adult Pacific salmon using a mobile respirometer swim tunnel. J Fish Biol 62: 64–84. [Google Scholar]
- Fry F. (1947) Effects of the environment on animal activity. Publ Ontario Fish Res Lab 55: 1–62. [Google Scholar]
- Halsey LG, Curran-Everett D, Vowler SL, Drummond GB (2015) The fickle P value generates irreproducible results. Nat Methods 12: 179–185. [DOI] [PubMed] [Google Scholar]
- Healy TM, Schulte PM (2012) Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic scope in the common killifish (Fundulus heteroclitus). Physiol Biochem Zool 85: 107–119. [DOI] [PubMed] [Google Scholar]
- Hvas M, Oppedal F (2019) Influence of experimental set-up and methodology for measurements of metabolic rates and critical swimming speed in Atlantic salmon Salmo salar. J Fish Biol 95:1–10. [DOI] [PubMed] [Google Scholar]
- Jones D, Kiceniuk J, Bamford O (1974) Evaluation of the swimming performance of several fish species from the Mackenzie River. J Fish Res Board Canada 31: 1641–1647. [Google Scholar]
- Jutfelt F, et al. (2018) Oxygen- and capacity-limited thermal tolerance: blurring ecology and physiology. J Exp Biol 221: 2016–2019. [DOI] [PubMed] [Google Scholar]
- Kelly NI, Burness G, McDermid JL, Wilson CC (2014) Ice age fish in a warming world: minimal variation in thermal acclimation capacity among lake trout (Salvelinus namaycush) populations. Conserv Physiol 2: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Norin T, Halsey LG (2017) Do method and species lifestyle affect measures of maximum metabolic rate in fishes? J Fish Biol 90: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206: 3239–3251. [DOI] [PubMed] [Google Scholar]
- Little AG, et al. (2020) Maxed out: optimizing accuracy, precision, and power for field measures of naximum netabolic rate in fishes. Physiol Biochem Zool 93: 243–254. [DOI] [PubMed] [Google Scholar]
- Montgomery DW, Simpson SD, Engelhard GH, Birchenough SNR, Wilson RW (2019) Rising CO2 enhances hypoxia tolerance in a marine fish. Sci Rep 9: 15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG (2015) To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav Ecol 26: 1083–1090. [Google Scholar]
- Norin T, Clark TD (2016) Measurement and relevance of maximum metabolic rate in fishes. J Fish Biol 88: 122–151. [DOI] [PubMed] [Google Scholar]
- Norin T, Malte H (2011) Repeatability of standard metabolic rate, active metabolic rate and aerobic scope in young brown trout during a period of moderate food availability. J Exp Biol 214: 1668–1675. [DOI] [PubMed] [Google Scholar]
- Norin T, Malte H, Clark TD (2016) Differential plasticity of metabolic rate phenotypes in a tropical fish facing environmental change. Funct Ecol 30: 369–378. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, RCoreTeam (2017) nlme: Linear and Nonlinear Mixed Effects Models.
- Raby GD, Casselman MT, Cooke SJ, Hinch SG, Farrell AP, Clark TD (2016) Aerobic scope increases throughout an ecologically relevant temperature range in coho salmon. J Exp Biol 219: 1922–1931. [DOI] [PubMed] [Google Scholar]
- Reemeyer JE, Harris JC, Hernandez AM, Rees BB (2019) Effects of passive integrated transponder tagging on cortisol release, aerobic metabolism and growth of the Gulf killifish Fundulus grandis. J Fish Biol 94: 422–433. [DOI] [PubMed] [Google Scholar]
- Reidy SP, Nelson JA, Tang Y, Kerr SR (1995) Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J Fish Biol 47: 377–386. [Google Scholar]
- Reidy SP, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203: 347–357. [DOI] [PubMed] [Google Scholar]
- Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216: 2103–2110. [DOI] [PubMed] [Google Scholar]
- RStudio Team (2016). RStudio.
- Rummer JL, Binning SA, Roche DG, Johansen JL (2016) Methods matter: considering locomotory mode and respirometry technique when estimating metabolic rates of fishes. Conserv Physiol 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers-Roesch B, Norin T, Driedzic WR (2018) The benefit of being still: energy savings during winter dormancy in fish come from inactivity and the cold, not from metabolic rate depression. Proc R Soc B Biol Sci 285: 20181593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steell SC, Van, Leeuwen TE, Brownscombe JW, Cooke SJ, Eliason EJ (2019) An appetite for invasion: digestive physiology, thermal performance and food intake in lionfish (Pterois spp.). J Exp Biol 222: jeb209437. [DOI] [PubMed] [Google Scholar]
- Steffensen J. (1985) The transition between branchial pumping and ram ventilation in fishes: energetic consequences and dependence on water oxygen tension. J Exp Biol 114: 141–150. [Google Scholar]
- Steffensen JF, Johansen K, Bushnell P (1984) An automated swimming respirometer. Comp Biochem Physiol A Mol Integr Physiol 79: 437–440. [Google Scholar]
- Team RC (2017) R: A language and environment for statistical computing.
- White CR, Schimpf NG, Cassey P (2013) The repeatability of metabolic rate declines with time. J Exp Biol 216: 1763–1765. [DOI] [PubMed] [Google Scholar]
- Wood SN. (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B Stat Methodol 73: 3–36. [Google Scholar]
- Wood C, Turner J, Graham M (1983) Why do fish die after severe exercise? J Fish Biol 22: 189–201. [Google Scholar]
- Zhang Y, Claireaux G, Takle H, Jørgensen SM, Farrell AP (2018) A three-phase excess post-exercise oxygen consumption in Atlantic salmon Salmo salar and its response to exercise training. J Fish Biol 92: 1385–1403. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilbert MJH, Farrell AP (2019) Finding the peak of dynamic oxygen uptake during fatiguing exercise in fish. J Exp Biol 222: jeb196568. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilbert MJH, Farrell AP (2020) Measuring maximum oxygen uptake with an incremental swimming test and by chasing rainbow trout to exhaustion inside a respirometry chamber yield the same results. J Fish Biol 97: 28–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


