Abstract
Whole exome sequencing (WES) technologies have accelerated genetic studies of Mendelian disorders, yielding approximately 30% diagnostic success. We encountered a 13-year-old Japanese female initially diagnosed with familial hypercholesterolemia on the basis of clinical manifestations of severe hypercholesterolemia (initial LDL cholesterol = 609 mg/dl at the age of one) and systemic intertriginous xanthomas with histories of recurrent self-limiting episodes of fever and arthritis. Both her phenotypes seemed to co-segregate in a recessive manner. We performed WES on this patient, who was considered a proband. Among 206,430 variants found in this individual, we found 18,220 nonsense, missense, or splice site variants, of which 3,087 were rare (minor allele frequency ≤ 0.01 or not reported) in 1000 Genome (Asian population). Filtering by assuming a recessive pattern of inheritance with the use of an in silico annotation prediction tool, we successfully narrowed down the candidates to the compound heterozygous mutations in the ABCG5 gene (c.1256G > A or p.Arg419His/c.1763-1G> A [splice acceptor site]) and to the double-compound heterozygous mutations in the MEFV gene (c.329T > C/C or p.Leu110Pro/c.442G > C/C or p.Glu148Val). The patient was genetically diagnosed with sitosterolemia and familial Mediterranean fever using WES for the first time. Such a comprehensive approach is useful for identifying causative mutations for multiple unrelated inheritable diseases.
Keywords: Sitosterolemia, Familial Mediterranean fever, ABCG5, ABCG8, Whole exome sequencing
Introduction
Sitosterolemia (OMIM #210250) caused by mutations in either of two genes ATP-binding cassette (ABC) sub-family G members 5 and 8 (ABCG5 and ABCG8) is an extremely rare autosomal recessive disorder of sterol metabolism characterized by increased absorption and decreased biliary excretion of plant sterols and cholesterol, resulting in prominently elevated serum levels of plant sterols such as sitosterol and campesterol1, 2). Subjects suffering from sitosterolemia primarily present tendinous and tuberous xanthomas and premature coronary atherosclerosis resembling familial hypercholesterolemia (FH)3). Familial Mediterranean fever (FMF, OMIM #249100), which is caused by mutations in the pyrin (MEFV) gene, is an autosomal recessive disorder characterized by recurrent attacks of fever and inflammation in the peritoneum, synovium, or pleura and is accompanied by pain4, 5).
We encountered a Japanese female initially diagnosed with FH on the basis of clinical manifestations of severe hypercholesterolemia and systemic intertriginous xanthomas with histories of recurrent self-limiting episodes of fever and arthritis. Both phenotypes seemed to co-segregate in a recessive manner. We therefore tried to establish a comprehensive molecular diagnosis for this rare condition using whole exome sequencing (WES).
Case Report
Study Subjects
A 13-year-old Japanese female was referred to our lipid clinic due to her extremely high levels of LDL cholesterol, without any apparent secondary causes. Her LDL cholesterol at the age of one was 609 mg/dl when she was under breastfeeding. Both her parents showed no evidence of consanguineous marriage, and her younger sister was also included in this study.
Ethical Considerations
This study was approved by the Ethics Committee of Kanazawa University and conducted in accordance with the Declaration of Helsinki (2008) of the World Medical Association. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consents were obtained from all subjects for being included in the study.
Biochemical Analysis
Blood samples were drawn for assays after overnight fasting. Serum levels of total cholesterol, triglycerides, and HDL cholesterol were determined enzymatically6). Serum levels of sterols, including sitosterol, lathosterol, and campesterol, were determined using gas-liquid chromatography-mass spectrometry as previously described2).
Exome Sequencing and Bioinformatics
Exome capture and sequencing were performed as previously described7). We applied three independent filters after standard variant quality controls in order to successfully discover a causal variant among the proband as previously described7). We filtered out the variants as 1) benign variants predicted by SnpEff, 2) minor allele frequency (MAF) > 1% in an Asian population, and 3) unmatched segregation under the assumption of a recessive form of inheritance.
For the sample, paired-end reads were aligned using the Burrows-Wheeler Aligner on the human reference genome build hg19 using quality score calibration, soft clipping, and adapter trimming. Following the exclusion of PCR duplicate reads using Picard, insertion-deletions and single-nucleotide polymorphisms (SNPs) were identified using GATK8, 9). Variants (SNPs/indels) were filtered out on the basis of the Phred-scaled genotype quality score. Re-alignment was performed, and a calling algorithm was used to merge the output of the GATK UnifiedGenotyper. All samples were annotated using SnpEff version 3.6 to classify variants (e.g., missense, nonsense, splice site, synonymous, intronic, or stop gain/loss)10).
Functional filtering, missense, nonsense, and splice site variants were considered as candidate variants. The frequency filter was uses to quantify the allele frequency estimates from the Asian cohort database in the 1000 Genomes Project, and a MAF > 1% was used as the cut-off. Segregation pattern matching was defined as that the situation where the affected exhibited homozygous or compound heterozygous of alternative alleles in a particular gene. We also filtered out variants with the use of an in silico annotation prediction tool as described previously7). Following those evaluations, the putative variants identified by bioinformatics were confirmed using Sanger sequencing methods as previously described11).
Characteristics of Study Subjects
The clinical characteristics of the study subjects are shown in Table 1. The proband (II.1) initially visited a hospital due to her intertriginous xanthomas in her legs at one year of age. At that time she was diagnosed with FH based on her having extremely high levels of LDL cholesterol as well as on physical findings. She was thought to have heterozygous FH, because her LDL cholesterol dramatically reduced to around 140 mg/dl with a small dose of a standard statin (5 mg pravastatin or 5 mg simvastatin) and because she was weaned from breastfeeding (Fig. 1). At the age of 13 she was referred to Kanazawa University Hospital to undergo a genetic diagnosis, because it was quite unusual to observe such a great reduction in LDL cholesterol in a patient with FH using such therapies.
Table 1. Characteristics of the study subjects.
| Subject (gender) | I.1 (male) | I.2 (female) | II.1 (female) | II.2 (female) |
|---|---|---|---|---|
| ABCG5 genotype | W/M1 | W/M2 | M1/M2 | W/W |
| MEFV genotype | W/M1 | W/M2 | M1/M2 | W/W |
| Age (yr) | 51 | 47 | 1 | 6 |
| Total cholesterol (mg/dl) | 214* | 202 | 681 | 137 |
| Triglyceride (mg/dl) | 123* | 60 | 213 | 89 |
| HDL cholesterol (mg/dl) | 44* | 45 | 29 | 39 |
| LDL cholesterol (mg/dl) | 145* | 143 | 609 | 90 |
| ApoA-I (mg/dl) | 138* | 124 | 96 | 120 |
| ApoB (mg/dl) | 116* | 101 | 340 | 63 |
| ApoE phenotype | 3/3 | 3/3 | 3/3 | 3/3 |
| CETP (µg/ml) | 1.9* | 1.9 | 3.1** | 3.3 |
| Sitosterol (µg/ml) | 6* | 7.1 | 182** | 1.7 |
| Lathosterol (µg/ml) | 1.2* | 1.4 | 0.6** | 0.8 |
| Campesterol (µg/ml) | 13.1* | 13.6 | 125** | 3.5 |
ABCG5: ATP-binding cassette sub-family G member 5, MEFV: Mediterranean fever, CETP: cholesteryl ester transfer protein
ABCG5 genotype: W = wild type, M1 = c.1256G > A, M2 = c.1763-1G > A, MEFV genotype: W = wild type, M1 = c.329 T > C/C, M2 = c.442G > C/C
Values measured when rosuvastatin was 2.5 mg.
CETP, sitosterol, lathosterol, and campesterol were measured at the age of 13 (when rosuvastatin was 5 mg).
Fig. 1.
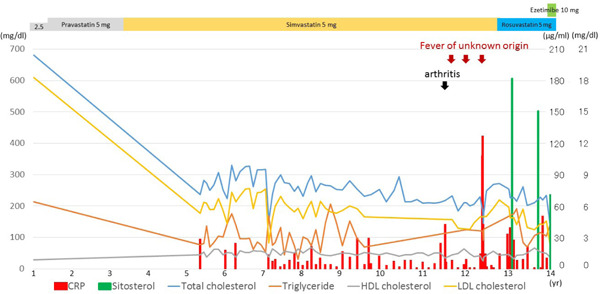
Clinical course of the proband
X-axis indicates age. Line graphs on the Y-axis (left side) indicate total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol in mg/dl. Bar graphs on the Y-axis (right side) indicate CRP in mg/dl, and sitosterol in µg/ml.
At the time of genetic diagnosis, the patient had already exhibited Achilles' tendon thickness (Fig. 2), thickness in her carotid artery (Fig. 3), and moderate aortic regurgitation associated with aortic valve calcification (Fig. 4). Her father was taking statin (2.5 mg rosuvastatin) without any physical xanthomas or any family history of premature coronary artery disease. Her mother exhibited normolipidemia. Based on the recessive pattern of inheritance as well as the great reduction in LDL cholesterol, we suspected her condition to be sitosterolemia, and thus measured her serum sitosterol. Her initial serumal levels of sitosterol at the age of 13 was as high as 182 µg/ml, confirming her clinical diagnosis as sitosterolemia. Her blood counts were within normal range. Her sitosterol levels could be reduced to 70 µg/ml using 10 mg ezetimibe in accordance with a previous report2). We also observed great reduction in her LDL cholesterol from 156 mg/dl to 76 mg/dl using 10 mg of ezetimibe. In addition, she had experienced several occurrences of high grade fever and arthritis associated with elevated C-reactive protein (Fig. 1).
Fig. 2.
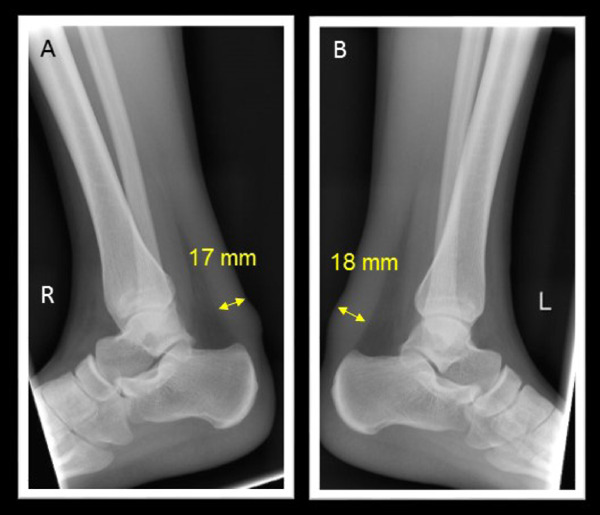
X-ray for Achilles' tendon
A. Right. B. Left
Fig. 3.
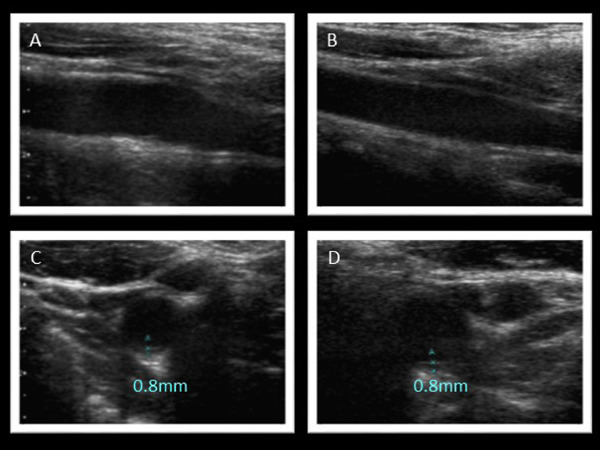
Ultrasound images of carotid arteries
A. Long-axis view (right). B. Long-axis view (left). C. Short-axis view (right). D. Short-axis view (left). Bilateral carotid intima-media thickness was 0.8 mm.
Fig. 4.
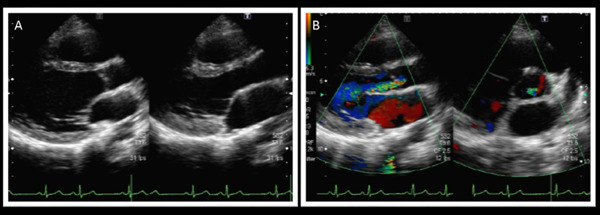
Echocardiography
A. Long-axis view (plain). B. Long- and short-axis view (in color). Echocardiography revealed moderate aortic regurgitation associated with aortic valve calcification.
Exome Sequencing and Bioinformatics Analysis
The proband underwent whole exome sequencing, and subsequent bioinformatics filtering. The mean depth was 99.9 × per base across the whole exome. The percentage of on-target reads was 84.6%. Also, the coverage rate of target coding lesions (10 ×) was 99.0%.
The number of aligned variants in the proband that passed through standard quality control was 206,430. Of those, 18,220 were missense, nonsense, splice site, or frameshift variants. After removing “common” variants with MAF > 1% using the Asian cohort in the 1000 genome project12), 3,087 variants were detected. Subsequently, filtering against the segregation pattern while assuming the recessive form of inheritance with the use of in silico annotation prediction tool reduced the candidate variants to compound heterozygous mutations in the ABCG5 gene (c.1256G > A or p.Arg419His/c.1763-1G > A [splice acceptor site]) on chromosome 2 and double compound heterozygous mutations in the MEFV gene (c.329 T> C/C or p.Leu110Pro/c.442G > C/C or p.Glu148Val) on chromosome 16. The former mutation in the ABCG5 gene is one of the most common mutations among Asians with sitosterolemia13), while the latter mutation in the ABCG5 gene is considered novel. Meanwhile, both mutations in the MEFV gene identified in the proband are two of the most common mutations among Japanese patients with FMF14). The status of mutations in her family members (Fig. 5A) was determined by subsequent Sanger sequencing (Fig. 5B–5E). Finally, we diagnosed her as having sitosterolemia and FMF; this is considered to be a rare condition of sitosterolemia. We consider this to be the first time such a condition has been diagnosed using an exome-wide approach.
Fig. 5.
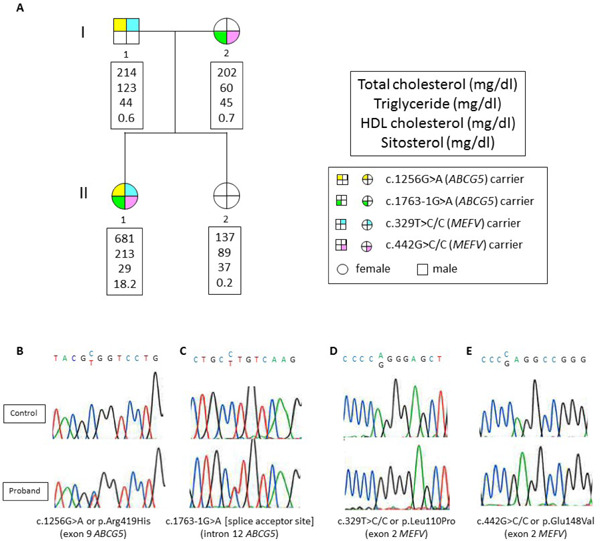
Family tree and mutation validation
A. Yellow indicates the carrier of c.1256G > A or p.Arg419His mutation in the ABCG5 gene. Green indicates the carrier of the c.1763-1G > A mutation in the ABCG5 gene. Blue indicates the carrier of the c.329 T > C/C or p.Leu110Pro mutation in the MEFV gene. Pink indicates the carrier of the c.442G> C/C or p.Glu148Val mutation in the MEFV gene.
B-E. Mutations were validated by Sanger sequencing.
Discussion
Using a WES approach, we identified the first case of a rare coincidence of sitosterolemia and FMF exhibiting compound heterozygous mutations in the ABCG5 gene and double compound heterozygous mutations in the MEFV gene. To the best of our knowledge, this is the first report of this coincidence. These findings allow us to draw several conclusions. First, the WES approach is useful to identify causative mutations in a family with a recessive form of inheritance, even when DNA of only one affected individual is available. Second, we can determine true causative mutations not from target re-sequencing, but from an exome-wide approach. Third, such a comprehensive approach is useful in identifying causative mutations for multiple unrelated inherited diseases.
In terms of mode of inheritance, her father exhibited mildly elevated levels of LDL cholesterol under statin therapy without any evidence of extreme hypercholesterolemia or cutaneous xanthomas. There was no deleterious mutation in the coding lesion of the LDL receptor or in the PCSK9 gene in her father; this led us to the notion of recessive inheritance.
Thus far, only 100 or so patients have been diagnosed with sitosterolemia15). The clinical features of sitosterolemia includes xanthomas, premature atherosclerosis, and increased serum levels of plant sterols1, 2). In addition, they may exhibit extreme hypercholesterolemia induced by breastfeeding in accordance with our previous findings in infantile cases with sitosterolemia2). Separately, FMF, which is characterized by recurrent, self-limiting episodes of fever, affects more than 100,000 patients, primarily in the Mediterranean Basin16). The proband in this study was diagnosed with FH and was treated with statins; however, ezetimibe could be a much more reasonable therapy for her based on her recent diagnosis with sitosterolemia17–19).
Careful monitoring and additional therapeutic approaches are needed to reduce her serum levels of sitosterol because of the unfavorable combination of hypersitosterolemia and systemic inflammatory disease, both of which have been shown to be associated with an increased risk of cardiovascular disease.
We report a case exhibiting a rare coincidence of sitosterolemia and FMF identified using WES. Such a comprehensive approach is useful in identifying causative mutations for multiple unrelated inherited diseases.
Acknowledgments
None.
Source of Finding
A scientific research grant from the Ministry of Education, Science, and Culture of Japan (No. 26893094).
Conflict of Interest Statement
None.
References
- 1). Shulman RS, Bhattacharyya AK, Connor WE, Fredrickson DS: Beta-sitosterolemia and xanthomatosis. N Engl J Med, 1976; 294: 482-483 [DOI] [PubMed] [Google Scholar]
- 2). Tada H, Kawashiri MA, Takata M, Matsunami K, Imamura A, Matsuyama M, Sawada H, Nunoi H, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M: Infantile cases of sitosterolaemia with novel mutations in the ABCG5 gene: Extreme hypercholesterolaemia is exacerbated by breastfeeding. JIMD Rep, 2015; 21: 115-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Salen G, Patel S, Batta AK: Sitosterolemia. Cardiovasc Drug Rev, 2002; 20: 255-270 [DOI] [PubMed] [Google Scholar]
- 4). Sohar E, Gafni J, Pras M, Heller H: Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am J Med, 1967; 43: 227-253 [DOI] [PubMed] [Google Scholar]
- 5). French FMF Consortium: A candidate gene for familial Mediterranean fever. Nat Genet, 1997; 17: 25-31 [DOI] [PubMed] [Google Scholar]
- 6). Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M, Hayashi K: Lipoprotein metabolism in familial hypercholesterolemia: Serial assessment using a one-step ultracentrifugation method. Practical Lab Med, 2015; 1: 22-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Tada H, Kawashiri MA, Nohara A, Saito R, Tanaka Y, Nomura A, Konno T, Sakata K, Fujino N, Takamura T, Inazu A, Mabuchi H, Yamagishi M, Hayashi K: Whole exome sequencing combined with integrated variant annotation prediction identifies asymptomatic Tangier disease with compound heterozygous mutations in ABCA1 gene. Atherosclerosis, 2015; 240: 324-329 [DOI] [PubMed] [Google Scholar]
- 8). McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA: The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res, 2010; 20: 1297-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ: A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet, 2011; 43: 491-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM: A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6 (2012) 80-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Tada H, Kawashiri MA, Ikewaki K, Terao Y, Noguchi T, Nakanishi C, Tsuchida M, Takata M, Miwa K, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M: Altered metabolism of low-density lipoprotein and very-low-density lipoprotein remnant in autosomal recessive hypercholesterolemia: results from stable isotope kinetic study in vivo. Circ Cardiovasc Genet, 2012; 5: 35-41 [DOI] [PubMed] [Google Scholar]
- 12). 1000 Genomes Project Consortium. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA: A map of human genome variation from population-scale sequencing. Nature, 2010; 467: 1061-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Wang G, Wang Z, Liang J, Cao L, Bai X, Ruan C: A phytosterolemia patient presenting exclusively with macrothrombocytopenia and stomatocytic hemolysis. Acta Haematol, 2011; 126: 95-98 [DOI] [PubMed] [Google Scholar]
- 14). Kishida D, Nakamura A, Yazaki M, Tsuchiya-Suzuki A, Matsuda M, Ikeda S: Genotype-phenotype correlation in Japanese patients with familial Mediterranean fever: differences in genotype and clinical features between Japanese and Mediterranean populations. Arthritis Res Ther, 2014; 16: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Escolà-Gil JC, Quesada H, Julve J, Martín-Campos JM, Cedó L, Blanco-Vaca F: Sitosterolemia: diagnosis, investigation, and management. Curr Atheroscler Rep, 2014; 16: 424. [DOI] [PubMed] [Google Scholar]
- 16). Neocleous V, Costi C, Kyriakou C, Kyriakides TC, Shammas C, Skordis N, Toumba M, Kyriakou S, Koliou M, Kousparou M, Onoufriou M, Hadjipanayis A, Iasonides M, Atamyan VN, Pierides A, Christophidou-Anastasiadou V, Tanteles GA, Phylactou LA: Familial Mediterranean fever associated with MEFV mutations in a large cohort of Cypriot patients. Ann Hum Genet, 2015; 79: 20-27 [DOI] [PubMed] [Google Scholar]
- 17). Tsubakio-Yamamoto K, Nishida M, Nakagawa-Toyama Y, Masuda D, Ohama T, Yamashita S: Current therapy for patients with sitosterolemia — effect of ezetimibe on plant sterol metabolism. J Atheroscler Thromb, 2010; 17: 891-900 [DOI] [PubMed] [Google Scholar]
- 18). Niu DM, Chong KW, Hsu JH, Wu TJ, Yu HC, Huang CH, Lo MY, Kwok CF, Kratz LE, Ho LT: Clinical observations, molecular genetic analysis, and treatment of sitosterolemia in infants and children. J Inherit Metab Dis, 2010; 33: 437-443 [DOI] [PubMed] [Google Scholar]
- 19). Hu M, Yuen YP, Kwok JS, Griffith JF, Tomlinson B: Potential effects of NPC1L1 polymorphisms in protecting against clinical disease in a chinese family with sitosterolaemia. J Atheroscler Thromb, 2014; 21: 989-995 [DOI] [PubMed] [Google Scholar]


