Abstract
Aim: Hepatic effects of estrogen therapy on low-density lipoprotein (LDL) subfraction or oxidative stress have not been previously evaluated. The purpose of the present study was to investigate whether the differential hepatic effects of estrogen affect plasma distribution of small dense LDL and free radical production in postmenopausal women.
Methods: In all, 45 postmenopausal women were given 0.625 mg/day of oral conjugated equine estrogen (CEE) (n = 15), 1.0 mg/day of oral 17β estradiol (E2) (n = 15), or 50 µg/day of transdermal 17βE2 (n = 15) for 3 months. Subjects received either estrogen alone or with dydrogesterone at 5 mg/day. Plasma concentrations of sex hormone-binding globulin (SHBG), lipids, metallic ions, and derivatives of reactive oxygen metabolites (d-ROMs) were measured.
Results: CEE, but not oral 17βE2, increased the plasma concentrations of triglyceride, copper (Cu), and d-ROMs and the ratio of small dense LDL/total LDL cholesterol, a marker for plasma distribution of small dense LDL. Transdermal 17βE2 decreased d-ROMs concentrations but did not significantly change other parameters. Plasma concentrations of SHBG increased in the 3 groups. Estrogen-induced changes in triglyceride correlated positively either with changes in SHBG (R = 0.52, P = 0.0002) or the ratio of small dense LDL/total LDL cholesterol (R = 0.65, P < 0.0001). Changes in Cu also correlated positively either with changes in SHBG (R = 0.85, P < 0.0001) or d-ROMs (R = 0.86, P < 0.0001).
Conclusion: The hepatic effects of different routes or types of estrogen therapy may be associated with plasma distribution of small dense LDL and free radical production in postmenopausal women.
Keywords: Hormone replacement therapy, Small dense low-density lipoprotein, Oxidative stress, Postmenopausal women
Introduction
Although the Women's Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) is associated with an initial increased risk of events related to cardiovascular disease (CVD)1), several potential factors such as age; pre-existing CVD risk; time of HRT initiation; as well as the type, route, and dosage of HRT given2–5) may also contribute to this adverse outcome.
A previous study has demonstrated that oral conjugated equine estrogen (CEE)-induced increase in plasma triglycerides led to a decrease in the low-density lipoprotein (LDL) mean particle diameter6, 7). Smaller and denser LDL particles are associated with an increased risk of CVD because they are more susceptible to oxidative modification, an initial step in the atherosclerotic process8). Accordingly, the CEE-induced reduction of the LDL mean particle diameter may be an underlying factor in the increase of early cardiovascular events by HRT, as demonstrated in the WHI study. Unlike oral CEE, transdermal 17β estradiol (17βE2) decreases plasma triglycerides and produces larger LDL particles that are resistant to oxidation9). Because triglycerides are synthesized in the liver, estrogen-induced hepatic stimulation may affect their production and the LDL mean particle diameter.
Plasma LDL particles infiltrate the intimal space of arteries and are exposed to free radicals. Macrophages readily take up oxidized LDL particles via scavenger receptors and develop into foam cells. Oxidative modification of LDL by free radicals plays a key role in the development of atherosclerosis. Pro- and antioxidant balance may be important in the development of atherosclerosis. Enhanced oxidative stress is reportedly related to cardiovascular risk with endothelial dysfunction and LDL oxidation10–12). Estrogen can act as an antioxidant, and postmenopausal estrogen therapy has been reported to inhibit LDL oxidation13, 14). In contrast, ethinyl estradiol present in combined oral contraceptive increases the plasma levels of free radicals and lipid peroxides15, 16). Therefore, estrogen therapy has both pro- and antioxidant effects. Hydroxyl radicals are formed in biological systems when transition-metal ions such as copper (Cu) or iron (Fe) ions participate in a Fenton reaction. Estrogen stimulates the hepatic synthesis of ceruloplasmin, which may increase plasma Cu17–19), because ceruloplasmin, being a major Cu-carrying protein in the blood, is synthesized in liver microsomes. Hepatic stimulation of estrogen may consequently affect Cu and free radical production.
The carrier protein, sex hormone-binding globulin (SHBG), is synthesized in the liver and binds to plasma estrogen and testosterone for transport. Because oral estrogen administration causes a dose-dependent increase in plasma SHBG, changes in the plasma levels of SHBG may be associated with the hepatic effects of estrogen20). In the present study, we investigated whether differential hepatic stimulation induced by oral CEE, oral 17βE2, and transdermal 17βE2 affects plasma lipids and plasma distribution of small dense LDL or metal ions and free radical production in healthy postmenopausal women.
Methods
Subjects
The subjects were 45 Japanese women recruited from patients visiting the outpatient clinic of the Department of Obstetrics and Gynecology, Aichi Medical University. The subjects fulfilled the following inclusion criteria: healthy women aged 38–58 years (mean age, 49 years), had undergone natural or surgical menopause, had a menopausal interval of < 1 year, and had climacteric symptoms. None of the subjects had menstruated for ≥ 1 year, smoked, nor drank coffee or alcohol. Exclusion criteria included the presence of pre-existing diseases such as thyroid disease, hypertension, dyslipidemia, liver disease, diabetes mellitus, CVD, systemic lupus erythematosus, and any other types of infection. None of the subjects were taking medication known to influence lipoprotein metabolism and HRT, nor did they undergo exercise or dietary therapy before the study. Written informed consent was obtained from each subject before admission to the study. The study design was approved by the Ethics Committee of Aichi Medical University.
Subjects were randomly assigned in an open-label, parallel group fashion to three treatment groups. After signing informed consent forms, the patients were randomized by opening sealed envelopes containing the group assignments, as determined by a random number generator. Neither the subject, the physician, nor the investigator knew in advance whether assignment would be to the CEE, oral 17βE2 or transdermal 17βE2 group. For 3 months, 15 subjects in the CEE group received 0.625 mg of oral CEE alone (n = 13) or oral CEE with dydrogesterone 5 mg daily (n = 2). Fifteen subjects in the oral 17βE2 group received 1.0 mg of oral 17βE2 alone (n = 12) or oral 17 β E2 with dydrogesterone 5 mg daily (n = 3). Another 15 subjects in the transdermal 17βE2 group received a 17βE2 patch (absorption rate, 50 µg/day) alone (n = 11) or a 17βE2 patch with dydrogesterone 5 mg daily (n = 4). Body weight, blood pressure (BP), and heart rate (HR) were measured, and blood samples were collected before and after treatment.
Laboratory Analysis
Venous blood samples were collected from each subject between 8:00 and 11:00 AM after a 12-h fasting period. The concentrations of total cholesterol (TC), triglycerides, and LDL cholesterol were measured enzymatically. HDL cholesterol concentrations were measured using similar methods after precipitation of apolipoprotein B-containing lipoproteins with sodium phosphotungstate in the presence of magnesium chloride21).
Plasma concentrations of small dense LDL cholesterol were measured by the simple homogeneous assay described by Ito et al.22) (sd-LDL-Ex SEIKEN, Denka Seiken Co. Ltd, Tokyo, Japan). The physicochemical principle of the method is as follows. In the first step, surfactants and sphingomyelinase degrade lipoproteins other than the small dense LDL cholesterol (density = 1.044–1.063 g/ml), such as chylomicrons (density < 0.94 g/ml), very LDL (density < 1.006 g/ml), intermediate-density lipoprotein (density = 1.006–1.019 g/ml), large buoyant LDL (density = 1.019–1.044 g/ml), and HDL (density = 1.063–1.21 g/ml) to water and oxygen via enzymatic action. In the next step, cholesterol is released from small dense LDL through the action of another surfactant, leading to color development.
The ratio of small dense LDL cholesterol/total LDL cholesterol was calculated and used as an indicator for plasma distribution of small dense LDL. Plasma concentrations of E2 and follicle-stimulating hormone (FSH) were measured by radioimmunoassay. Plasma concentrations of Cu, Zinc (Zn), and ceruloplasmin were measured, respectively, with a colorimetric method, atomic absorption spectrophotometry, and nephelometry. Plasma concentrations of SHBG were measured by immunoradiometric assay23).
Derivatives of Reactive Oxygen Metabolites and Biological Antioxidant Potential
Derivatives of reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) were analyzed using the free radical analytical system 4 (Diacron, Parma, Italy)24–26). Plasma concentrations of d-ROMs were measured to determine the plasma levels of free radicals. In an acidic buffer, hydroperoxides react with iron, a transition metal that is liberated from proteins and converted to alkoxyl and peroxyl radicals. These radicals oxidize an additive (N,N-diethyl-para-phenylenediamine) to radical cation species. The concentrations of this persistent species were determined using spectrophotometry (505 nm). Plasma concentrations of d-ROMs are expressed as Carratelli units. Plasma BAP was measured to determine plasma antioxidant levels. The BAP test is based on the ability of a colored solution, containing ferric ions adequately bound to a special chromogenic substrate, to lose color when its ferric ions are reduced to ferrous ions upon addition of a reducing/antioxidant source. We estimated the intensity of this chromatic change using spectrophotometry (505 nm).
Statistical Analysis
Data are expressed as mean ± standard deviation. Differences in baseline subject characteristics, hormone levels, SGBG, lipids, metallic ions, d-ROMs, BAP, and treatment-induced changes in these parameters between the three groups were analyzed either by one-way factorial analysis of variance for parameters with a normal distribution or by the Kruskal–Wallis test when the parameters were not normally distributed, and the Bonferroni–Dunn test was used to assess association between parameters. Treatment-induced changes in these parameters were analyzed by the Student's paired t-test when the changes were normally distributed or by the Mann–Whitney U test when they were not. Regression lines were determined by the least squares method. P < 0.05 was considered statistically significant.
Results
The climacteric symptoms were improved comparably after treatment in the three groups. No significant differences were found in age, baseline BMI, systolic BP (SBP), diastolic BP (DBP), HR, concentrations of hormone and lipid, SHBG, metallic ion, d-ROMs, and BAP between the three treatment groups. BMI did not significantly change in any group. HR significantly decreased in the oral 17βE2 group, and SBP and DBP significantly decreased in the transdermal 17βE2 group. In all treatment groups, concentrations of E2 significantly increased and those of FSH significantly decreased (Table 1).
Table 1. Body mass index, Blood pressure, Heart rate, Estradiol and follicle stimulating hormone.
| CEE (n = 15) |
Oral 17βE2 (n = 15) |
Transdermal 17βE2 (n = 15) |
||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| BMI (kg/m2) | 20.7 ± 3.4 | 20.9 ± 3.1 | 22.5 ± 3.1 | 22.6 ± 3.0 | 21.1 ± 3.0 | 21.1 ± 2.7 |
| SBP (mmHg) | 124 ± 11 | 117 ± 13 | 122 ± 19 | 116 ± 22 | 119 ± 14 | 111 ± 12** |
| DBP (mmHg) | 66 ± 10 | 64 ± 10 | 67 ± 9 | 63 ± 14 | 64 ± 6 | 61 ± 8* |
| Heart rate (beats/min) | 64 ± 9 | 61 ± 8 | 65 ± 9 | 60 ± 7** | 61 ± 8 | 60 ± 8 |
| E2 (pg/mL) | 11.7 ± 6.1 | 90.4 ± 58.8** | 11.1 ± 3.2 | 71.9 ± 33.0** | 14.7 ± 10.0 | 125.4 ± 105.4** |
| FSH (mIU/mL) | 102.2 ± 35.3 | 47.9 ± 21.9** | 83.2 ± 40.7 | 62.1 ± 22.7* | 104.2 ± 36.1 | 35.6 ± 30.5** |
Date are expressed as mean ± S.D.; CEE, conjugated equine estrogen; E2, estradiol; BMI, body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FSH, follicle stimulating hormone;
P < 0.05
P < 0.01 vs pretreatment
CEE significantly reduced the plasma concentrations of TC and LDL cholesterol, whereas it significantly increased the plasma concentrations of HDL cholesterol and triglycerides as well as the ratio of small dense LDL cholesterol/total LDL cholesterol. Plasma concentrations of small dense LDL cholesterol did not significantly change in any group. Oral 17βE2 significantly increased the plasma concentrations of HDL cholesterol, whereas transdermal 17βE2 significantly decreased the plasma concentrations of triglycerides. Both oral and transdermal 17βE2 did not affect other lipid parameters. All therapies significantly elevated the plasma concentrations of SHBG (Table 2). Treatment-induced changes in plasma triglyceride correlated positively either with changes in plasma SHBG (R = 0.52, P = 0.0002) (Fig. 1A) or in the ratio of small dense LDL/total LDL cholesterol (R = 0.65, P = 0.0001) (Fig. 1B).
Table 2. Plasma Lipids and SHBG.
| CEE (n = 15) |
Oral 17βE2 (n = 15) |
Transdermal 17βE2 (n = 15) |
||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Total cholesterol (mg/dL) | 222.5 ± 38.4 | 216.9 ± 34.2 | 238.7 ± 49.1 | 233.3 ± 44.7 | 229.7 ± 50.6 | 222.4 ± 50.8 |
| Triglyceride (mg/dL) | 81.1 ± 34.3 | 98.6 ± 48.5** | 84.7 ± 31.3 | 87.5 ± 40.7 | 80.1 ± 33.6 | 66.4 ± 26.9* |
| HDL cholesterol (mg/dL) | 70.3 ± 14.9 | 75.8 ± 16.9* | 69.9 ± 16.4 | 74.8 ± 10.2* | 73.5 ± 12.8 | 70.2 ± 10.8 |
| LDL cholesterol (mg/dL) | 135.6 ± 30.1 | 119.6 ± 30.3* | 147.3 ± 44.2 | 137.9 ± 45.7 | 136.2 ± 48.9 | 133.3 ± 45.4 |
| Sd-LDL cholesterol (mg/dL) | 37.2 ± 14.7 | 37.0 ± 10.7 | 40.3 ± 21.9 | 41.0 ± 21.9 | 33.7 ± 16.0 | 32.1 ± 15.7 |
| Sd-LDL/LDL ratio | 0.27 ± 0.07 | 0.31 ± 0.05* | 0.26 ± 0.06 | 0.29 ± 0.07 | 0.24 ± 0.12 | 0.23 ± 0.10 |
| SHBG (nmol/ml) | 112.4 ± 51.8 | 254.1 ± 112.5** | 79.1 ± 28.8 | 125.1 ± 54.0** | 101.6 ± 43.3 | 110.3 ± 43.8* |
Date are expressed as mean ± S.D.; CEE, conjugated equine estrogen; E2, estradiol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Sd-LDL, small dense LDL; SHBG, sex hormone-binding globulin;
P < 0.05
P < 0.01 vs pretreatment
Fig. 1.
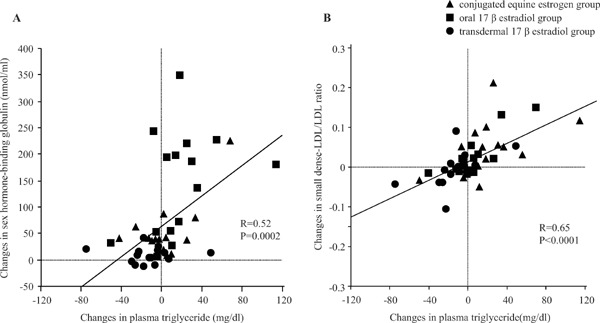
(A) Relationship between changes in plasma triglyceride and sex hormone-binding globulin. Triangles indicate conjugated equine estrogen group, squares indicate oral 17β estradiol (E2) group, and circles indicate transdermal 17βE2 group. (B) Relationship between changes in plasma triglyceride and small dense LDL/LDL ratio.
Plasma concentrations of Cu and the Cu/Zn ratio significantly increased in the CEE group, whereas no significant changes were observed in the oral and transdermal 17βE2 groups. Plasma concentrations of Zn did not change in any group. Plasma concentrations of ceruloplasmin and d-ROMs increased in the CEE group, remained unchanged in the oral 17βE2 group, and significantly decreased in the transdermal 17βE2 group. Plasma BAP did not change in any group (Table 3). Treatment-induced changes in the plasma concentrations of Cu also correlated positively either with changes in SHBG (R = 0.85, P < 0.0001) (Fig. 2A) or in the plasma concentrations of d-ROMs (R = 0.86, P < 0.0001) (Fig. 2A).
Table 3. Copper, Zinc, Ceruloplasmin, d-ROMs and BAP.
| CEE (n = 15) |
Oral 17βE2 (n = 15) |
Transdermal 17βE2 (n = 15) |
||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Copper (µg/dL) | 112.3 ± 14.0 | 152.1 ± 36.6** | 114.9 ± 18.0 | 125.6 ± 30.3 | 106.1 ± 13.5 | 99.7 ± 16.8 |
| Zinc (µg/dL) | 88.1 ± 13.2 | 80.8 ± 15.3 | 88.0 ± 11.3 | 84.7 ± 9.8 | 86.5 ± 10.7 | 81.3 ± 12.7 |
| Cu/Zn ratio | 1.31 ± 0.28 | 1.92 ± 0.52** | 1.32 ± 0.26 | 1.48 ± 0.30 | 1.25 ± 0.25 | 1.25 ± 0.27 |
| Ceruloplasmin (mg/dL) | 32.3 ± 5.2 | 42.5 ± 9.8** | 33.1 ± 6.5 | 35.2 ± 8.9 | 29.9 ± 3.8 | 27.8 ± 4.9* |
| d-ROMs (U.CARR) | 342.3 ± 43.5 | 501.5 ± 122.0** | 372.7 ± 79.5 | 399.3 ± 92.2 | 360.9 ± 73.5 | 308.1 ± 52.9* |
| BAP (µmol/L) | 2735 ± 383 | 2739 ± 193 | 2836 ± 538 | 2634 ± 267 | 2794 ± 352 | 2565 ± 304 |
Date are expressed as mean ± S.D.; CEE, conjugated equine estrogen; E2, estradiol; d-ROMs, derivatives of reactive oxygen metabolites; BAP, biological antioxidant potential;
P < 0.05
P < 0.01 vs pretreatment
Fig. 2.
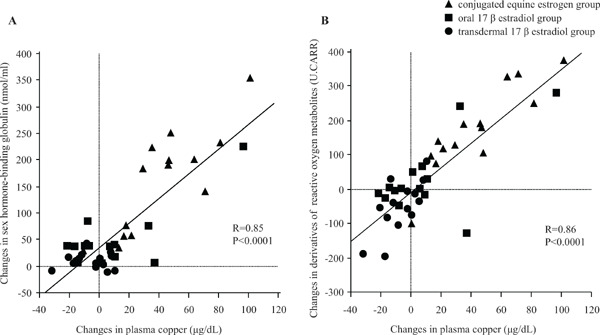
(A) Relationship between changes in plasma copper and sex hormone-binding globulin. Triangles indicate conjugated equine estrogen group, squares indicate oral 17β estradiol (E2) group, and circles indicate transdermal 17βE2 group. (B) Relationship between changes in plasma copper and derivatives of reactive oxygen metabolites.
Treatment-induced changes in the plasma concentrations of SHBG, Cu, and d-ROMs were significantly greater in the CEE group compared with the oral and transdermal 17βE2 groups. Changes in the plasma concentrations of triglycerides were significantly greater in the CEE group compared with the transdermal 17βE2 group (Table 4).
Table 4. Changes in SHBG, triglyceride, Sd-LDL/LDL ratio, Copper, and d-ROMs.
| CEE (n = 15) | Oral 17βE2 (n = 15) | Transdermal 17βE2 (n = 15) | P value | |
|---|---|---|---|---|
| Changes in SHBG (nmol/ml) | 141.7 ± 101*, ** | 46.0 ± 53.5 | 8.7 ± 14.6 | < 0.0001 |
| Changes in Triglyceride (mg/dL) | 17.5 ± 35.5** | 2.8 ± 25.6 | −13.7 ± 25.8 | 0.02 |
| Changes in Sd-LDL/LDL ratio | 0.045 ± 0.067 | 0.029 ± 0.052 | −0.003 ± 0.045 | 0.06 |
| Changes in Copper (µg/dL) | 39.7 ± 31.4*, ** | 9.7 ± 30.8 | −6.4 ± 12.3 | < 0.0001 |
| Changes in d-ROMs (U.CARR) | 162.9 ± 123.4*, ** | 21.6 ± 107.6 | −52.9 ± 77.3 | < 0.0001 |
Date are expressed as mean ± S.D.; CEE, conjugated equine estrogen; E2, estradiol; SHBG, sex hormone-binding globulin; Sd-LDL, small dense LDL; d-ROMs, derivatives of reactive oxygen metabolites
P value was determined with One-way factorial ANOVA
P < 0.01 vs Oral 17βE2 group by Bonferroni-Dunn test
P < 0.01 vs Transdermal 17βE2 group by Bonferroni-Dunn test
Discussion
SHBG is secreted by the liver and binds to plasma estrogen and testosterone. Because estrogen directly enters the hepatic circulation when orally administered, it stimulates the hepatic synthesis of SHBG. Therefore, estrogen-induced changes in plasma SHBG levels can be used as a marker to indicate the hepatic effects of estrogen. In the present study, plasma SHBG levels were found elevated by both oral estrogen therapies. However, increase in SHBG levels induced by CEE was higher than that induced by oral 17βE2 therapy, indicating that hepatic stimulation of CEE may be greater than that of oral 17βE2. Because the estrogenicity of 17βE2 is considerably weaker than that of CEE20), oral 17βE2 may be associated with lesser hepatic stimulation than oral CEE. Unlike oral estrogen, transdermal 17βE2 showed a lower SHBG increase, suggesting that transdermal 17βE2 has lesser hepatic effects. Thus, the hepatic effects of estrogen therapy may be influenced by its estrogenicity or route of estrogen administration. Because oral estrogen increases the plasma levels of SHBG and anti-estrogenic progestogen with androgenic action decreases it, changes in the levels of SHBG may represent the net estrogenic effects of hormone therapy20). Although 9 subjects were given 5 mg of dydrogesterone as a progestogen combined with estrogen in the present study, dydrogesterone has no anti-estrogenic or androgenic action and may not affect hepatic SHBG production20, 27).
Plasma concentrations of triglycerides were regulated by synthesis and degradation of triglyceride-rich lipoproteins. Triglyceride-rich lipoproteins are degraded by lipoprotein lipase (LPL) and hepatic triglyceride lipase (H-TGL). In this study, the activity of LPL and H-TGL was not evaluated; however, previous study demonstrated that estrogen decreases the LPL28) and H-TGL activities13). Thus, estrogen-induced suppression of LPL and H-TGL activities may lead to the elevation of plasma concentration of triglyceride. In addition, because triglyceride is synthesized in the liver, hepatic stimulation of estrogen activates the synthesis of triglyceride. In this study, CEE but not oral or transdermal 17βE2 increased plasma concentrations of triglyceride, and CEE-induced changes in triglyceride concentrations were greater than transdermal 17βE2. In addition, changes in triglyceride concentrations were found to correlate positively with changes in SHBG concentrations. Because triglyceride is synthesized in the liver, our results indicate that CEE-induced hepatic stimulation may increase plasma triglyceride. Small dense LDL particles, with a diameter less than 25.5 nm and density ranging from 1.044 to 1.063 g/ml29), are known to be associated with an increased incidence of CVD8, 30, 31). Long residence time in the plasma, enhanced oxidizing ability, permeability through the endothelial barrier, arterial proteoglycan binding, and lower affinity for LDL receptors have been proposed as factors for the enhanced atherogenicity of these particles32). Plasma concentration of triglycerides has been reported to be the most important factor affecting the LDL mean particle diameter33). Wakatsuki and coworkers have reported that estrogen-induced changes in plasma triglycerides correlated negatively with estrogen-induced changes in the LDL mean particle diameter, as measured by the electrophoretic technique9). In the present study, we measured the plasma concentrations of total and small dense LDL cholesterol, as well as estimated the distribution of small dense LDL in plasma by calculating the ratio of small dense LDL cholesterol/total LDL cholesterol. We found that plasma concentrations of total LDL cholesterol decreased upon CEE therapy, whereas concentrations of small dense LDL cholesterol remained unchanged in the three groups. This is because small dense LDL particles have a low affinity for LDL receptors. In addition, we found that oral CEE, but not oral or transdermal 17βE2, increased the ratio of small dense LDL cholesterol/total LDL cholesterol, and that estrogen-induced changes in plasma triglycerides correlated positively with estrogen-induced changes in the ratio. Collectively, these results suggest that CEE and not oral or transdermal 17βE2 stimulates hepatic triglyceride production that may increase the distribution of small dense LDL in plasma. Ai et al. have reported that the mean percentage of LDL cholesterol as small dense LDL cholesterol was higher in women with coronary heart disease than controls34). Accordingly, CEE-induced increase in the distribution of small dense LDL may be atherogenic, despite the LDL lowering effect of CEE.
We have further evaluated oxidative stress by measuring plasma concentrations of d-ROMs (an index of reactive oxygen species production) and BAP (an index of antioxidant potential). We found that the plasma concentrations of d-ROMs and not BAP increased in the CEE group, and CEE-induced increases in d-ROMs were greater than oral and transdermal 17βE2 groups. This suggests that although estrogen has antioxidant effects, CEE may additionally enhance free radical production. A previous study has demonstrated that LDL oxidation is not inhibited by CEE therapy, despite the antioxidant effects of CEE. Based on all these findings, CEE-induced increase in free radicals and the distribution of small dense LDL particles may offset the antioxidative effects of estrogen against LDL oxidation.
In contrast, the plasma concentrations of d-ROMs remained unchanged in the oral 17βE2 group and decreased in the transdermal 17βE2 group. Plasma BAP did not change in these two groups. Transdermal 17βE2 has been reported to decrease the plasma concentrations of LDL-derived thiobarbituric acid reactive substances, as it increases the size of LDL particles and preserves the antioxidant effects of estrogen9). Apart from these favorable effects of transdermal 17βE2, decreases in free radical production may also be associated with the inhibition of LDL oxidation. Earlier findings demonstrated that low-dose oral CEE does not affect plasma triglyceride and LDL particle size and reduces LDL oxidation35). Similarly, it has been reported here that oral 17βE2 did not alter the plasma concentrations of triglyceride and small dense LDL cholesterol. Accordingly, oral 17βE2 may inhibit LDL oxidation, although we did not measure the susceptibility of LDL oxidation in the present study.
Mezzetti et al.36) demonstrated that plasma levels of lipid peroxide correlate positively with Cu and the Cu/Zn ratio and negatively with Zn. The d-ROMs test used in the present study evaluates oxidative stress by measuring the levels of reactive oxygen metabolites, mainly hydroperoxides. Hydroperoxides are metabolites oxidized by reactive oxygen species, particularly hydroxyl radicals. Hydroxyl radicals are formed in biological systems when transition-metal ions, such as Cu or Fe ions, participate in a Fenton reaction. We found that plasma concentrations of Cu and ceruloplasmin, together with the Cu/Zn ratio but not Zn concentrations, increased in the CEE group while they remained unchanged in the oral and transdermal 17βE2 groups. Moreover, CEE-induced changes in plasma Cu were greater than oral and transdermal 17βE2 groups, and changes in plasma Cu correlated positively either with changes in SHBG or d-ROMs. Several studies reported that estrogen stimulates the hepatic synthesis of ceruloplasmin, which may increase plasma Cu17–19) as ceruloplasmin, a major Cu-carrying protein in the blood, is synthesized in liver microsomes. These findings suggest that CEE may stimulate hepatic Cu production, which in turn may increase free radical production via Fenton reaction. In contrast, oral 17βE2 may not adversely affect the production of Cu and free radicals given its reduced hepatic effects. The decrease in transdermal 17βE2-induced plasma free radical levels can be explained by its antioxidant properties and less hepatic effects.
Conclusion
Our present study demonstrated that different hepatic effects of estrogen therapy may be associated with the risk of CVD by affecting plasma distribution of small dense LDL and free radical production. Lokkegaard et al.37) demonstrated that transdermal estrogen significantly reduces the risk of myocardial infarction by about 40%. In addition, Nicholas et al.38) reported that postmenopausal women taking oral 17β E2 had a lower risk of cardiovascular events compared with those taking CEE. Further studies are needed to evaluate whether postmenopausal women on oral or transdermal 17βE2 having lesser hepatic effects may be at lower risk of CVD relative to CEE.
Disclosure Statement
Akihiko Wakatsuki received honoraria from Bayer Yakuhin Limited and ASKA pharmaceutical Company Limited. Our department received grants from Mochida Pharmaceutical Company Limited, Takeda Pharmaceutical Company Limited and Daiichi Sankyo Healthcare Limited.
References
- 1). Writing Group for the Women's Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA, 2002; 288: 321-333 [DOI] [PubMed] [Google Scholar]
- 2). Hodis HN: Assessing benefits and risks of hormone therapy in 2008: new evidence, especially with regard to the heart. Cleve Clin J Med, 2008; 75: S3-S12 [DOI] [PubMed] [Google Scholar]
- 3). Schnatz PF: Hormonal therapy: does it increase or decrease cardiovascular risk? Obstet Gynecol Surv, 2006; 61: 673-681 [DOI] [PubMed] [Google Scholar]
- 4). Haines CJ, Farrell E: Menopause management: a cardiovascular risk-based approach. Climacteric, 2010; 13: 328-339 [DOI] [PubMed] [Google Scholar]
- 5). Harman SM: Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS. Gend Med, 2006; 3: 254-269 [DOI] [PubMed] [Google Scholar]
- 6). Wakatsuki A, Ikenoue N, Sagara Y: Effect of estrogen on the size of low-density lipoprotein particles in postmenopausal women. Obstet Gynecol, 1997; 90: 22-25 [DOI] [PubMed] [Google Scholar]
- 7). Wakatsuki A, Ikenoue N, Sagara Y: Estrogen-induced small low-density lipoprotein particles in postmenopausal women. Obstet Gynecol, 1998; 91: 234-240 [DOI] [PubMed] [Google Scholar]
- 8). Tribble DL, Holl LG, Wood PD, Krauss RM: Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis, 1992; 93: 189-199 [DOI] [PubMed] [Google Scholar]
- 9). Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T: Different effects of oral conjugated equine estrogen and transdermal estrogen replacement therapy on size and oxidative susceptibility of low-density lipoprotein particles in postmenopausal women. Circulation, 2002; 106: 1771-1776 [DOI] [PubMed] [Google Scholar]
- 10). Steinbrecher UP, Parthasarathy S, Leake DS, Witztum JL, Steinberg D: Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A, 1984; 81: 3883-3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Palinski W, Rosenfeld ME, Ylä-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL: Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A, 1989; 86: 1372-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Wang Y, Tabas I: Emerging roles of mitochondria ROS in atherosclerotic lesions: causation or association? J Atheroscler Thromb, 2014; 21: 381-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Yang XP, Reckelhoff JF: Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens, 2011; 20: 133-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Wakatsuki A, Ikenoue N, Sagara Y: Effects of estrogen on susceptibility to oxidation of low-density and high-density lipoprotein in postmenopausal women. Maturitas, 1998; 28: 229-234 [DOI] [PubMed] [Google Scholar]
- 15). Pincemail J, Vanbelle S, Gaspard U, Collette G, Haleng J, Cheramy-Bien JP, Charlier C, Chapelle JP, Giet D, Albert A, Limet R, Defraigne JO: Effect of different contraceptive methods on the oxidative stress status in women aged 40-48 years from the ELAN study in the province of Liege, Belgium. Hum Reprod, 2007; 22: 2335-2343 [DOI] [PubMed] [Google Scholar]
- 16). De Groote D, Perrier d'Hauterive S, Pintiaux A, Balteau B, Gerday C, Claesen J, Foidart JM: Effects of oral contraception with ethinylestradiol and drospirenone on oxidative stress in women 18-35 years old. Contraception, 2009; 80: 187-193 [DOI] [PubMed] [Google Scholar]
- 17). Akhter S, Shamsuzzaman AK, Banarjee M, Seema SA, Deb K: Serum copper in rural women taking combined oral contraceptive. Mymensingh Med J, 2006; 15: 25-29 [DOI] [PubMed] [Google Scholar]
- 18). Berg G, Kohlmeier L, Brenner H: Effect of oral contraceptive progestins on serum copper concentration. Eur J Clin Nutr, 1998; 52: 711-715 [DOI] [PubMed] [Google Scholar]
- 19). Benes B, Spevácková V, Smíd J, Batáriová A, Cejchanová M, Zítková L: Effects of age, BMI, smoking and contraception on levels of Cu, Se and Zn in the blood of the population in the Czech Republic. Cent Eur J Public Health, 2005; 13: 202-207 [PubMed] [Google Scholar]
- 20). Kuhl H: Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric, 2005; 8: 3-63 [DOI] [PubMed] [Google Scholar]
- 21). Allain CC, Poon LS, Chan CS, Richmond W, Fu PC: Enzymatic determination of total serum cholesterol. Clin Chem, 1974; 20: 470-475 [PubMed] [Google Scholar]
- 22). Ito Y, Fujimura M, Ohta M, Hirano T: Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 23). Hammond GL, Langley MS, Robinson PA: A liquid-phase immunoradiometric assay (IRMA) for human sex hormone binding globulin (SHBG). J Steroid Biochem, 1985; 23: 451-460 [DOI] [PubMed] [Google Scholar]
- 24). Trotti R, Carratelli M, Barbieri M: Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med, 2002; 44: 37-40 [PubMed] [Google Scholar]
- 25). Parmigiani S, Payer C, Massari A, Bussolati G, Bevilacqua G: Normal values of reactive oxygen metabolites on the cord-blood of full-term infants with a colorimetric method. Acta Biomed Ateneo Parmense, 2000; 71: 59-64 [PubMed] [Google Scholar]
- 26). Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A: A simple test to monitor oxidative stress. Int Angiol, 1999; 18: 127-130 [PubMed] [Google Scholar]
- 27). Nugent AG, Leung KC, Sullivan D, Reutens AT, Ho KK: Modulation by progestogens of the effects of oestrogen on hepatic endocrine function in postmenopausal women. Clin Endocrinol (Oxf), 2003; 59: 690-698 [DOI] [PubMed] [Google Scholar]
- 28). Iverius PH, Brunzell JD: Relationship between lipoprotein lipase activity and plasma sex steroid level in obese women. J Clin Invest, 1988; 82: 1106-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Berneis KK, Krauss RM: Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res, 2002; 43: 1363-1379 [DOI] [PubMed] [Google Scholar]
- 30). Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T, Kobayashi Y: Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb, 2014; 21: 755-767 [DOI] [PubMed] [Google Scholar]
- 31). Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y: Small dense lowdensity lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb, 2013; 20: 195-203 [DOI] [PubMed] [Google Scholar]
- 32). Krauss RM: Heterogeneity of plasma low-density lipoproteins and atherosclerosis risk. Curr Opin Lipidol, 1994; 5: 339-349 [DOI] [PubMed] [Google Scholar]
- 33). McNamara JR, Jenner JL, Li Z, Wilson PW, Schaefer EJ: Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb, 1992; 12: 1284-1290 [DOI] [PubMed] [Google Scholar]
- 34). Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, Cupples LA, Wilson PW, Schaefer EJ: Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem, 2010; 56: 967-976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Wakatsuki A, Okatani Y, Ikenoue N, Shinohara K, Watanabe K, Fukaya T: Effect of lower dose of oral conjugated equine estrogen on size and oxidative susceptibility of low-density lipoprotein particles in postmenopausal women. Circulation, 2003; 108: 808-813 [DOI] [PubMed] [Google Scholar]
- 36). Mezzetti A, Pierdomenico SD, Costantini F, Romano F, De Cesare D, Cuccurullo F, Imbastaro T, Riario-Sforza G, Di Giacomo F, Zuliani G, Fellin R: Copper/zinc ratio and systemic oxidant load: effect of aging and aging-related degenerative diseases. Free Radic Biol Med, 1998; 25: 676-681 [DOI] [PubMed] [Google Scholar]
- 37). Løkkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard Ø: Hormone therapy and risk of myocardial infarction: a national register study. Eur Heart J, 2008; 29: 2660-2668 [DOI] [PubMed] [Google Scholar]
- 38). Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, Hwang M, Bis JC, McKnight B, Rice KM, Lumley T, Rosendaal FR, Heckbert SR, Psaty BM: Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med, 2014; 174: 25-31 [DOI] [PMC free article] [PubMed] [Google Scholar]


