Abstract
Aim: Recent studies reported that low high-density lipoprotein (HDL)-mediated cholesterol efflux capacity rather than low HDL cholesterol (HDL-C) is strongly associated with the increased risk for coronary artery disease. It remains unclear whether exercised-based cardiac rehabilitation (CR) can increase HDL cholesterol efflux capacity.
Method: This study is a retrospective analysis of stored serum from patients with acute coronary syndrome (ACS) who participated in outpatient CR program following successful percutaneous coronary intervention. We employed a cell-based cholesterol efflux system including the incubation of 3H-cholesterol labeled macrophages with apolipoprotein B-depleted serum at the onset or early phase of ACS and at 6-month follow-up periods in 57 male and 11 female patients with ACS. Cardiopulmonary exercise tests were performed at the beginning and end of CR program.
Result: Fifty-seven patients completed the CR program. Compared with patients who dropped out from CR program (non-CR group), CR participants showed marked amelioration in serum lipid levels, increased efflux capacity, and improved exercise capacity. Spearman's rank correlation coefficient analysis revealed that the percent increases of efflux capacity were significantly associated with the percent increases in HDL-C (ρ = 0.598, p < 0.0001) and apolipoprotein A1 (ρ = 0.508, p < 0.0001), whereas no association between increases in efflux capacity and increases in cardiopulmonary fitness was observed. Increases in cholesterol efflux capacity were not seen in patients who continued smoking and those who did not achieve all risk factor targets and higher exercise tolerance.
Conclusion: CR can markedly increase both HDL-C and HDL cholesterol efflux capacity. These results suggest that CR is a very useful therapy for reverse cholesterol transport and secondary prevention.
Keywords: High-density lipoprotein, Cholesterol efflux capacity, Apolipoprotein A1, Cardiac rehabilitation, Acute coronary syndrome
Introduction
Exercise-based cardiac rehabilitation (CR) has been shown to be beneficial in improving exercise capacity and quality of life and/or prolonging survival in patients with coronary artery disease (CAD) including acute coronary syndrome (ACS)1–4). The components of CR include smoking cessation, nutritional counseling, weight management, and lipid management such as decreases in low-density lipoprotein (LDL) cholesterol (LDL-C) and triglycerides and increases in high-density lipoprotein (HDL) cholesterol (HDL-C)1). Aerobic exercise has been shown to increase HDL-C levels in healthy subjects, particularly sedentary or obese subjects, and/or subjects with risk factors for CAD such as metabolic syndrome, hypertension, diabetes mellitus (DM), and dyslipidemia5, 6); however, a previous meta-analysis of 12 randomized controlled trials published till March 2003 with a total of 1,165 patients with CAD failed to show that CR significantly increased HDL-C levels compared with usual care4).
Recent clinical studies of HDL-C-raising therapy using nicotinic acid7) or cholesteryl ester transfer protein inhibitors8) and genome-wide association studies to examine genetic increases in HDL-C9, 10) have failed to demonstrate the relationship between elevated HDL-C and reduced risk for CAD, although numerous population studies have shown inverse relationship between HDL-C and the risk of CAD9, 11). HDL has been proposed to have several antiatherogenic properties, including reverse cholesterol transport, antioxidant capacity, anti-inflammatory properties, nitric oxide-promoting activity, and ability to transport proteins with their own intrinsic biological activities12). Among them, cholesterol efflux from peripheral tissues is a key function of HDL particles and the first step of reverse cholesterol transport to the liver12, 13). Khera et al. reported that HDL-mediated cholesterol efflux capacity from macrophage, measured by a cell-based ex vivo assay that involved incubation of macrophages with apolipoprotein B-depleted serum from the study participants, was a strong inverse predictor of CAD independently of HDL-C levels or apolipoprotein A-1 in case-control studies in two independent cohorts14). Rohatgi et al. used fluorescently labeled cholesterol method to measure cholesterol efflux capacity in the Dallas Heart Study, a population-based cohort study, and demonstrated that lower levels of cholesterol efflux capacity at baseline was a significant predictor for the incidence of atherosclerotic cardiovascular disease (CVD) during a 9.4-year follow-up period, independently of traditional risk factors including HDL-C, whereas HDL-C was not associated with the incidence of CVD15). Saleheen et al. used radiolabeled cholesterol method to measure cholesterol efflux capacity in the EPIC-Norfolk study, a population-based prospective case-control study, and demonstrated that cholesterol efflux capacity was significantly inversely associated with incident CAD independent of established cardiovascular risk factors even after adjusting for HDL-C or apolipoprotein A116). These results, therefore indicate the not level of HDL-C but the HDL function would attract much attention as an important therapeutic target for CAD. Previous studies failed to show that treatment with statins and niacin increase HDL cholesterol efflux capacity14, 17). In contrast, studies including ours reported that treatment with pioglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist, resulted in a significant increases in HDL cholesterol efflux capacity in patients with metabolic syndrome and/or DM14, 18). It was shown that PPARγ agonist increased ATP-binding cassette A1 (ABC A1) in liver X receptor (LXR)-dependent manner, which resulted in enhanced cholesterol efflux capacity18). In addition, our group reported that addition of telmisartan, a PPARγ-activating angiotensin II type 1 receptor blocker to cell cultures also increased ABC A1 and LXR in macrophage gene expression and enhanced cholesterol efflux capacity from THP-1 macrophages19). However, little is known about the effects of CR with cardiovascular medication on HDL cholesterol efflux capacity12, 20). The aim of the present preliminary study was to determine whether CR can improve HDL cholesterol efflux capacity in patients with ACS.
Methods
Subjects
The present study enrolled patients who were selected from the cohort of consecutive patients with following criteria: (1) Patients were admitted to Showa university hospital because of ACS and received successful percutaneous coronary intervention (PCI) on admission between April 2005 and October 2011. The diagnoses of ACS were based on clinical symptoms, electrocardiographic changes, blood examinations, and emergency coronary arteriograms. (2) Patients were encouraged to participate in outpatient CR program. (3) Serum samples were collected immediately before the emergency coronary angiography on admission for ACS and/or in fasting state at the beginning of CR program during the early-phase hospitalization, and unused samples were stored for long-term storage at −80°C. (4) Unused serum samples in fasting sate between 5 and 18 months after ACS were also stored at −80°C. Exclusion criteria included severe hepatic disease, end-stage renal diseases such as hemodialysis, current treatment for malignancy, any other serious condition, patients who took drugs for thyroid dysfunction, and patients with missed blood samples. After applying these criteria, 57 men and 11 women were included in the present analysis. Serum samples were kept frozen at −80°C until the assay for cholesterol efflux capacity was performed. The patients were divided into two groups based on whether they completed the 6-month outpatient CR program. The institutional review board of Showa University approved this protocol. The investigation conformed to the principles of the Declaration of Helsinki.
Baseline Examination
The clinical characteristics of the patients studied at baseline are given in Table 1. Nine men had previously undergone PCI and three had previously suffered from myocardial infarction (MI). In five patients with missed blood samples on admission, the fasting blood samples at the beginning of CR were used. Forty-nine men and eight women completed the 6-month outpatient CR program (CR group), and nine men and two women dropped out from CR program (non-CR group).
Table 1. Clinical characteristics at baseline.
| Whole (N = 68) | Non-CR (N = 11) | CR (N = 57) | |
|---|---|---|---|
| Men/Women | 57/11 | 9/2 | 49/8 |
| Age, years | 63.5 ± 11.7 | 62.3 ± 12.7 | 63.7 ± 11.6 |
| Prior MI | 3 (4) | 0 (0) | 3 (5) |
| Prior PCI | 9 (13) | 1 (11) | 8 (14) |
| Family history of CAD | 12 (18) | 2 (22) | 10 (18) |
| UAP | 3 | 1 | 2 |
| Anterior MI | 32 | 6 | 26 |
| Lateral MI | 11 | 1 | 10 |
| Inferior MI | 22 | 3 | 19 |
| Peak CK, IU/ml | 2895.8 ± 2581.0 | 3064.4 ± 3277.4 | 2863.2 ± 2458.7 |
| Peak CK-MB, IU/ml | 281.8 ± 230.9 | 321.0 ± 349.2 | 274.3 ± 204.1 |
| LV EF, % | 50.6 ± 10.9 | 48.7 ± 9.1 | 51.0 ± 11.3 |
| CR sessions | 34.8 ± 18.4 | 13.5 ± 12.7 | 38.9 ± 16.4* |
| Risk factor | |||
| Smoking | |||
| None | 11 | 1 | 10 |
| Former | 22 | 3 | 19 |
| Current | 35 | 7 | 28 |
| Hypertension | 53 | 10 | 43 |
| Dyslipidemia | 57 | 9 | 48 |
| Glucose tolerance | |||
| NGT | 22 | 4 | 18 |
| IGT | 20 | 3 | 17 |
| DM by 75g OGTT | 7 | 2 | 5 |
| DM | 19 | 2 | 17 |
| Prior lipid-lowering medication | |||
| Statin | 22 | 3 | 19 |
| Others | 2 | 0 | 2 |
Data are expressed as mean ± SD, or number (%).
p < 0.001 vs non-CR group
CAD = coronary artery disease; CK = creatine kinase; CR = cardiac rehabilitation; DM = diabetes mellitus; IGT = impaired glucose tolerance; LVEF = left ventricular ejection fraction by ultrasound Simpson method; MI = myocardial infarction; NGT = normal glucose tolerance; PCI = percutaneous coronary intervention; UAP = unstable angina pectoris; 75gOGTT = 75g oral glucose tolerance test
The diagnosis of hypertension was based on a history of hypertension or blood pressure (BP) above 140 mmHg (systolic) or 90 mmHg (diastolic). DM was defined as a fasting serum glucose value greater than 126 mg/dL, glycated hemoglobin (HbA1c) values estimated by (= 1.019 × HbA1c(JDS) + 0.3) greater than 6.5%, and/or current use of medication for DM21). Dyslipidemia was defined as the current use of lipid-lowering medication and/or meeting the criteria of the Japan Atherosclerosis Society for fasting serum lipid levels as follows: LDL-C ≥ 140 mg/dL, HDL-C < 40 mg/dL, or triglyceride ≥ 150 mg/dL22). A serum creatinine (Cr)–based estimate of glomerular filtration rate (eGFR) was calculated as follows: eGFR = 194 x Cr−1.094 x Age−0.287 (x 0.739 for women)23). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Patients with a reported smoking habit of at least one cigarette per day on admission were classified as current smokers. Former smokers were defined as having previously smoked but ceased before the onset of ACS. Brachialankle pulse wave velocity (baPWV) and BP were measured using an oscillometric device (Form/ABI, Colin Company, Ltd., Komaki, Japan) at the beginning of CR program and at the follow-up period24). Four pneumatic pressure cuffs, two electrocardiogram electrodes, and one microphone for detecting heart sounds were attached on both arms and ankles, wrists, and the left edge of the sternum, respectively, to record the volume waveform for the brachial and ankle arteries. The subjects were kept at rest in supine position for at least 5–10 min. The examination room was maintained at a standardized temperature. baPWV was automatically calculated as the length of an arterial segment between the brachium and ankle (which was automatically calculated from the body height) divided by the transit time of the pulse wave. Heart rate (HR) was also simultaneously recorded during the measurement of baPWV.
Total-cholesterol, triglycerides, HDL-C, HbA1c (JDS), and lipoprotein(a) were measured using standard laboratory procedures. Non-HDL-C level was estimated by subtracting the HDL-C concentration from the total-cholesterol concentration. The LDL-C levels were measured with a direct homogenous assay (Sekisui Medical Co. Ltd. Tokyo, Japan). Serum apolipoprotein levels were determined by an immunoturbidometric assay (Daiichi Chemicals Co. Ltd. Tokyo, Japan). Remnant lipoproteins (RLPs) were isolated from the serum to an immunoaffinity mixed gel containing anti-apolipoprotein A1 and anti-apolipoprotein B100 monoclonal antibodies (Japan Immunoresearch Laboratories, Takasaki, Japan), and the cholesterol concentrations of the unbound fraction were measured as RLP cholesterol (RLP-C)25). The highsensitivity C-reactive protein (hsCRP) level was measured by the Dade Behring BN assay26). Plasma brain natriuretic peptide (BNP) was measured by chemiluminescence-enzyme immunoassay.
Measurement of Cholesterol Efflux Capacity From Macrophages
Cholesterol efflux capacity was performed by experienced analysts at National Defense Medical College who were blinded to the clinical characteristics of the patients according to the methods used by Khera et al.12, 14, 16–20). J774 macrophages were purchased from RIKEN (Tsukuba, Japan), cultured in RPMI1640 medium containing 10% fetal bovine serum, and kept under constant conditions of 5% carbon dioxide and a temperature of 37°C. J774 cells were plated in 24-well plates, grown to 80% confluence, and radiolabeled with 2 µCi/mL of 3H-cholesterol. Apolipoprotein B-depleted serum was prepared by incubating with 13% polyethylene glycol 6000 solution (Wako Pure Chemicals). Subsequently, an efflux medium containing 2.8% apolipoprotein B-depleted serum was added and incubated for 4 h. All procedures were performed in the presence of the acyl-coenzyme A: cholesterol acyltransferase inhibitor Sandoz 58–035 (2 µg/mL; Sigma, St. Louis, MO, USA) and 8-bromoadenosine 3′5′-cyclic monophosphate (0.3 mmol/L; Sigma). A liquid scintillation counter was used to quantify the efflux of radioactive cholesterol from the cells. The quantity of radioactive cholesterol incorporated into cellular lipids was calculated through hexane:isopropanol (v:v, 1:1) extraction in control wells not exposed to the serum. Percent efflux was calculated using the following formula: (cpm of 3H-cholesterol in media containing 2.8% apolipoprotein B-depleted serum − cpm of 3H-cholesterol in serum-free mediums)/(cpm of 3H-cholesterol in cells extracted before the efflux step) × 100. All assays were performed in duplicate. The cholesterol efflux capacities of patients' sera were expressed as the relative values to those of the pooled sera.
CR Program and Cardiopulmonary Exercise Test
Comprehensive CR programs include exercise training, counseling on lifestyle modification including diet therapy, smoking cessation and physical activity, and encouragement to adherence to medical treatment by physicians, nurses, physical therapists, and dieticians. Educational sessions were performed every month. The CR program began in early phase and continued after hospital discharge a few times a week for 6 months. The exercise training is consisted with supervised exercise sessions of gymnastics and a 30-min-supervised aerobic exercise using a bicycle ergometer. Trained patients were encouraged to home exercise consisted mainly of brisk walking for 30–60 min, three to five times a week. The prescribed intensity was determined individually at 40%–60% of HR reserve (Karvonen's equation, k = 0.4–0.6), at an anaerobic threshold (AT) level obtained by cardiopulmonary exercise test (CPX) or at level 12–13 of Borg Scale for Ratings of Perceived Exertion according to the Japanese Circulation Society guidelines1). Exercise tolerance was measured by CPX using a ramp protocol at the beginning and the end of the 6-month CR program. After a 3-min rest on the bicycle ergometer in the upright position, the patients started pedaling at an intensity of 10 W for 4 min (warm-up), followed by an incremental exercise at 10 or 20 W/min until exhaustion. A 12-lead electrocardiogram (ECG) was continuously monitored, and BP was measured once a minute with a sphygmomanometer. Respiratory flow was measured by breath-by-breath method using gas analyzer (MINATO AE-300s; Minato Medical Science Co. Ltd., Japan). AT was determined by V-slope method27). Oxygen consumption at peak exercise was regarded as peak VO2 and as indices of exercise tolerance. HR recovery (HRR) immediately after the exercise test was obtained as peak HR minus HR at 60 s and at 3 min after exercise.
Lipid-Lowering Treatment and Risk Factor Control
Targets of comprehensive CR are smoking cessation, and achievement of risk factor control including BMI < 25 kg/m28), systolic BP < 140 mmHg29), diastolic BP < 90 mmHg29), LDL-C < 100 mg/dl30), non-HDL-C < 130 mg/dl30), HDL-C ≥ 40 mg/dl30), triglyceride < 150 mg/dl30), and HbA1c < 7.0%28). Categories of intensities of the treatment with statins were classified into four groups: no treatment or discontinuation of statin, low-intensity (Pravastatin ≤ 10 mg, Simvastatin 5 mg, Fluvastatin ≤ 30 mg, Atorvastatin 5 mg, Rosuvastatin 2.5 mg, and Pitavastatin 1 mg), moderate intensity (Pravastatin 20 mg, Simvastatin 10–20 mg, Fluvastatin 40–60 mg, Atorvastatin 10–15 mg, Rosuvastatin 5–7.5 mg, and Pitavastatin 2–3 mg), and high intensity (Atorvastatin ≥ 20 mg, Rosuvastatin ≥ 10 mg, and Pitavastatin 4 mg) according to the 2013 The American College of Cardiology/American Heart Association guidelines31) with minor modification. Strengthening of statin treatment was defined as increased category of statin intensity.
Statistics
All statistical analyses were performed using the SPSS 20 software package (SAS Institute, Cavy, NC, USA). Baseline characteristics were compared between CR group and non-CR group using unpaired t-test for parametric variables and Mann-Whitney U test for nonparametric variables. Categorical variables were compared with chi-square tests. For within-group comparisons, changes in blood biomarkers and in CPX data between the baseline and the follow-up were analyzed using paired t-test for parametric variables and Wilcoxon signed rank test for non-parametric variables. To test whether changes between baseline and follow-up levels in each variable were different between CR and non-CR groups, analysis of covariance (ANCOVA) was performed with each baseline level as covariates. Correlation coefficients between changes in HDL cholesterol efflux capacity and changes in HDL-C, apolipoprotein A1, and other variables including CPX findings or between HDL cholesterol efflux capacity and HDL-C, apolipoprotein A1, and other variables were determined by Spearman's rank analyses. All the statistical analyses were two tailed. P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
The general characteristics at baseline were similar between the two groups (Table 1). The mean number of CR sessions in CR group was 38.9, whereas that in non-CR group was 13.5. Twenty-two patients took statins, and two took other lipid-lowering drugs at the baseline blood sampling.
Laboratory Findings
The follow-up periods for measurement of blood biomarkers were similar between the two groups (6.9 ± 2.7 months in CR group and 7.4 ± 2.8 months in non-CR group). Table 2 compares the BMI and the laboratory findings at the baseline and at the follow-up periods. eGFR, non-HDL-C, LDL-C, apolipoprotein B, hsCRP, and BNP were significantly decreased, and HDL-C and apolipoprotein A1 were significantly increased in CR group; in contrast, those were similar between the baseline and the follow-up in non-CR group. Fig. 1 compares HDL cholesterol efflux capacity, HDL-C, and apolipoprotein A1 levels at baseline and at follow-up in CR and non-CR groups in whole patients and male patients. The cholesterol efflux capacity, HDL-C, and apolipoprotein A1 at follow-up significantly increased compared with the baseline levels in CR group alone and those changes were somewhat potentiated when female patients were excluded from the analysis. According to ANCOVA, however, difference in changes in cholesterol efflux capacity, HDL-C, or apolipoprotein A1 compared with its baseline level was not statistically significant between CR and non-CR groups, adjusted for each baseline level.
Table 2. Comparison of BMI and blood lipids and other biomarkers at baseline and follow-up period.
| Non-CR (N = 11) |
CR (N = 57) |
|||
|---|---|---|---|---|
| Baseline§ | FU | Baseline# | FU | |
| BMI | 24.7 ± 4.1 | 25.0 ± 4.4 | 24.0 ± 3.9 | 23.8 ± 4.2 |
| eGFR, ml/min/1.73 m2 | 79.8 ± 30.3 | 70.9 ± 16.5 | 71.4 ± 24.6 | 64.1 ± 19.8*** |
| HbA1c, % | 6.40 ± 1.85 | 6.01 ± 0.53 | 6.41 ± 1.19 | 6.33 ± 1.06 |
| Non-HDL-C, mg/dl | 146.0 ± 29.5 | 133.5 ± 42.6 | 154.4 ± 49.7 | 120.0 ± 22.1*** |
| LDL-C, mg/dl | 122.0 ± 26.0 | 107.9 ± 33.3 | 127.4 ± 48.9 | 92.5 ± 26.4*** |
| HDL-C, mg/dl | 44.5 ± 11.2 | 46.5 ± 7.43 | 45.8 ± 12.9 | 51.9 ± 14.8*** |
| ApoA1, mg/dl | 120.2 ± 23.2 | 127.3 ± 12.9 | 123.8 ± 22.1 | 143.1 ± 25.6*** |
| Apo B, mg/dl | 96.1 ± 16.5 | 90.5 ± 27.6 | 103.0 ± 31.5 | 82.3 ± 19.7*** |
| RLP-C, mg/dl | 5.7 ± 6.0 | 4.4 ± 2.6 | 6.4 ± 5.3 | 5.3 ± 5.1 (56) |
| Lp (a) , mg/dl | 16.3 ± 14.9 | 17.5 ± 20.1 | 21.4 ± 20.5 | 22.3 ± 20.5 (56) |
| CRP, g/dl | 18.13* 52.44 | 0.85 ± 0.66 | 6.51 ± 15.43 | 1.18 ± 2.66*** |
| BNP, pg/ml | 275.8 ± 362.4 | 206.7 ± 344.0 (8) | 124.6 ± 198.3 | 80.8 ± 148.8 (55)** |
Data are expressed as mean ± SD.
P, 0.05
p < 0.01
p < 0.001 vs baseline by paired t-test or Wilcoxon signed rank test (CRP and BNP).
One sample was collected in fasting state at the beginning of CR.
4 Samples were collected in fasting-state at the beginning of CR.
The others were collected immediate before the emergency coronary angiography on admission. The number in parenthesis indicates paired number of analyzed cases. ApoA1= apolipoprotein A1; ApoB = apolipoprotein B; FU = follow-up; Other abbreviations were described in the text.
Fig. 1.
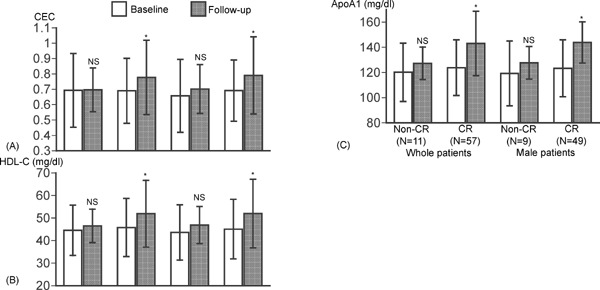
Comparisons of HDL cholesterol efflux capacity, HDL-C, and apolipoprotein A1 at baseline and follow-up period between CR and non-CR group
HDL cholesterol efflux capacity (CEC) (A), HDL-C (B), and apolipoprotein A1 (C) at baseline and follow-up period was compared between CR and non-CR groups in whole patients and male patients. Data are expressed as mean ± SD. Error bars indicate SD. *p < 0.0001 vs baseline by paired t-test. ApoA1= apolipoprotein A1, NS = no statistical significance.
Hemodyanmics
Table 3 compares the HR, BP, baPWV, and CPX findings between the two groups. Two patients in non-CR group did not perform CPX, and one patient in CR group and three patients in non-CR group did not perform the follow-up CPX. Thus, CPX data were analyzed in 56 pairs in CR group and 6 pairs in non-CR group. The VO2 at AT, peak VO2, peak load, double products defined as systolic BP at peak multiplied HR at peak, and HRR at 3 min significantly increased and HR at rest significantly decreased in only CR group. In contrast, all parameters did not change significantly in non-CR group.
Table 3. Comparison of hemodynamic variables at baseline and follow-up period.
| Non-CR (N = 11) |
CR (N = 57) |
|||
|---|---|---|---|---|
| Baseline | FU | Baseline | FU | |
| HR at rest, bpm | 74.5 ± 13.4 | 69.8 ± 11.8 | 74.9 ± 15.4 | 70.0 ± 11.6* |
| SBP at rest, mmHg | 115.6 ± 21.4 | 125.4 ± 13.5 | 115.6 ± 16.3 | 124.6 ± 19.1** |
| DBP at rest, mmHg | 71.4 ± 13.1 | 75.6 ± 10.6 | 71.1 ± 9.0 | 76.4 ± 10.2** |
| R-baPWV, cm/sec | 1534.2 ± 411.0 | 1535.1 ± 345.6 | 1540.3 ± 319.6 | 1612.6 ± 367.2 |
| L-baPWV, cm/sec | 1554.5 ± 362.1 | 1572.5 ± 348.1 | 1570.1 ± 357.4 | 1642.0 ± 397.1 |
| CPX | N = 6 pairs | N = 56 pairs | ||
| HR at rest, bpm | 77.0 ± 14.9 | 82.0 ± 16.2 | 78.7 ± 13.7 | 74.6 ± 12.1** |
| SBP at rest, mmHg | 101.8 ± 19.5 | 129.6 ± 11.7* | 118.9 ± 22.2 | 125.6 ± 19.7** |
| DBP at rest, mmHg | 65.0 ± 7.6 | 82.0 ± 6.9* | 73.3 ± 12.5 | 76.2 ± 11.7 |
| AT VO2, ml/min/kg | 10.5 ± 0.9 | 11.6 ± 1.27 | 10.8 ± 2.0 | 12.3 ± 2.1*** |
| Peak VO2, ml/min/kg | 17.4 ± 0.9 | 17.9 ± 0.9 | 16.5 ± 3.9 | 18.9 ± 4.1*** |
| Peak Load, W | 99.6 ± 31.7 | 108.6 ± 34.3 | 80.2 ± 25.4 | 108.0 ± 32.9*** |
| Peak DPD | 17896 ± 2538 | 22687 ± 3346 | 21531 ± 5890 | 24247 ± 5961*** |
| HRR at 1 min, bpm | 12.8 ± 14.8 | 16.6 ± 7.4 | 16.7 ± 8.1 | 20.0 ± 9.8 |
| HRR at 3 min, bpm | 30.8 ± 14.3 | 36.0 ± 7.6 | 32.6 ± 12.5 | 40.7 ± 12.7*** |
Data are expressed as mean ± SD.
p < 0.05
p < 0.01
p < 0.001 vs baseline by paired t-test
DPD = double product; HRR = heart rate recovery.
Effects of Medical Treatment on HDL Cholesterol Efflux Capacity in Patients who Participated CR
Table 4 compares medical treatment at baseline and at follow-up in both groups. Fig. 2 shows how medical treatment affects CR-mediated increases in HDL cholesterol efflux capacity. Seven patients took telmisartan and five patients took pioglitazone. Patients were divided into two groups based on the consumption of telmisartan and/or pioglitazone. The cholesterol efflux capacity significantly increased irrespective of the taking PPARγ agonist (Fig. 2). Thirty-four of 38 patients untreated with statins at baseline blood sampling started to take statins. Five patients treated with mild-intensity statins and one patient with moderate-intensity statins changed to moderate-intensity statins and high-intensity statins, respectively. Those patients were classified as strengthening of statin treatment. The significant increases in cholesterol efflux capacity were not observed in patients without strengthening statin treatment and those with the high-intensity statin treatment (Fig. 2).
Table 4. Comparison of medical treatment at baseline and follow-up period.
| Non-CR (N = 11) |
CR (N = 57) |
|||
|---|---|---|---|---|
| Baseline§ | FU | Baseline# | FU | |
| Cardiovascular treatment | ||||
| CCB | 4 | 9 | 14 | 17 |
| ACE-I | 1 | 3 | 3 | 9 |
| Telmisartan | 0 | 0 | 5 | 7 |
| Other ARB | 3 | 8 | 13 | 26 |
| Beta blocker | 1 | 4 | 6 | 16 |
| Single antiplatelet thrapy | 1 | 3 | 7 | 8 |
| Dual antiplatelet therapy | 1 | 8 | 7 | 49 |
| Anticoagulants | 0 | 0 | 0 | 3 |
| Glucose-lowering treatment | ||||
| Insulin | 1 | 1 | 3 | 4 |
| SU or glinide | 0 | 1 | 6 | 8 |
| DPP-4 inhibitor | 0 | 0 | 0 | 3 |
| Alfa-GI | 0 | 0 | 5 | 8 |
| Pioglitazone | 0 | 0 | 2 | 5 |
| Metformin | 0 | 1 | 2 | 1 |
| Lipid-lowering treatment, statin | ||||
| Pravastatin 5 mg | 1 | 1 | 1 | 0 |
| Pravastatin 10 mg | 1 | 5 | 2 | 4 |
| Pravastatin 20 mg | 0 | 1 | 0 | 0 |
| Simvastatin 5 mg | 0 | 0 | 1 | 3 |
| Simvastatin 10–20 mg | 0 | 0 | 1 | 0 |
| Atorvastatin 5 mg | 0 | 0 | 0 | 1 |
| Atorvastatin 10 mg | 0 | 0 | 7 | 21 |
| Atorvastatin 20 mg | 0 | 0 | 0 | 3 |
| Rosuvastatin 2.5 mg | 0 | 2 | 2 | 1 |
| Rosuvastatin 5 mg | 0 | 0 | 1 | 11 |
| Rosuvastatin 10–15 mg | 0 | 0 | 1 | 2 |
| Pitavastatin 1 mg | 1 | 0 | 0 | 2 |
| Pitavastatin 2 mg | 0 | 0 | 1 | 3 |
| Fluvastatin 20–30 mg | 0 | 0 | 2 | 1 |
| None or discontinuation | 8 | 2 | 38 | 5 |
| Low-intensity | 3 | 6 | 8 | 12 |
| Moderate-intensity | 0 | 3 | 10 | 35 |
| High-intensity | 0 | 0 | 1 | 5 |
| Lipid-lowering treatment, others | ||||
| Fibrate | 0 | 1 | 0 | 0 |
| Omega-3 fatty acids | 0 | 0 | 2 | 0 |
| Ezetimibe | 0 | 0 | 0 | 3 |
Data are expressed as number. one case in non-CR (§) and 4 cases in CR (#) were evaluated at the beginning of CR. ACE-I = angiotensin-converting enzyme inhibitor; Alfa-GI = α-glucosidase inhibitor; ARB = angiotensin II type 1 receptor blocker; CCB=calcium channel blocker; DPP4= dipeptidyl peptidase-4 inhibitor; SU = sulfonylurea
Fig. 2.
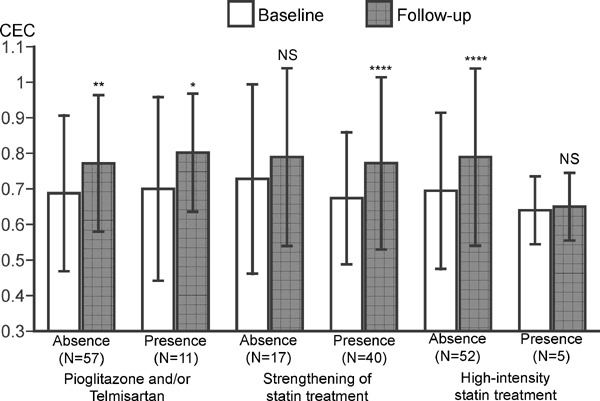
Effects of medical treatment on HDL cholesterol efflux capacity in patients who completed CR program
HDL cholesterol efflux capacity (CEC) was compared in the presence or absence of PPARγ agonist, strengthening of statin treatment and/or high-intensity statin treatment. Data are expressed as mean ± SD. Error bars indicate SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs baseline by paired t-test. NS = no statistical significance.
Effects of Risk Factor Control on HDL Cholesterol Efflux Capacity in Patients who Participated CR
Fig. 3 shows how risk factor control affects CR-mediated increases in HDL cholesterol efflux capacity. Among CR group, 25 patients could stop smoking habit, whereas three patients continued. No patient resumed smoking after ACS. Patients were classified into three groups based on smoking status, and cholesterol efflux capacities were compared. The significant increases in cholesterol efflux capacity were not observed in continuing smokers although the number is 3 (Fig. 3A). The mean level of peak VO2 at the follow-up CPX in 62 patients was 19 mL/min/kg, and this level was considered as the cutoff for higher exercise capacity in the present study. One patient with missing data of follow-up CPX had 15.3 mL/min/kg of peak VO2 at baseline CPX and was classified as lower exercise capacity. Patients were classified into four groups based on the presence or absence of higher exercise tolerance and complete risk factor control of five targets including smoking cessation; weight control; control of both systolic and diastolic BP; lipid control of LDL-C, non-HDL-C, triglyceride, and HDL-C; and glycemic control. The significant increases in cholesterol efflux capacities were observed in only patients classified as higher exercise capacity and complete control of five risk factors.
Fig. 3.
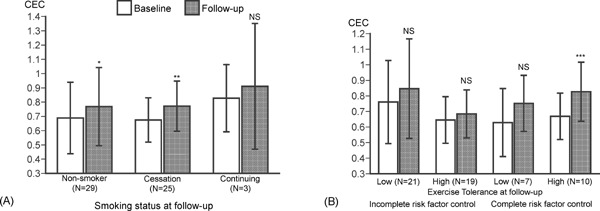
Effects of risk factor control on HDL cholesterol efflux capacity in patients who completed CR program
HDL cholesterol efflux capacity (CEC) was compared in patients divided into three groups based on the smoking status (A) and patients divided into four groups based on complete risk factor control and exercise capacity at follow-up period (B). Data are expressed as mean ± SD. Error bars indicate SD. *p < 0.05, **p < 0.01, ***p < 0.001 vs baseline by paired t-test. NS = no statistical significance.
Correlation Coefficients between Cholesterol Efflux Capacity and Various Parameters
In correlation coefficients between changes in cholesterol efflux capacity and various parameters within the whole study population, percent increases in cholesterol efflux capacity were significantly positively correlated with percent changes in HDL-C (ρ = 0.598, p < 0.0001) and in apolipoprotein A1 (ρ = 0.508, p < 0.0001), and was weakly but significantly negatively correlated with HDL-C at baseline (ρ = −0.246, p < 0.05). At baseline, the cholesterol efflux capacity was significantly positively correlated with HDL-C (ρ = 0.445, p < 0.0001) and apolipoprotein A1 (ρ = 0.512, p < 0.0001). Similarly, the cholesterol efflux capacity at the follow-up was significantly positively correlated with HDL-C (ρ = 0.433, p < 0.0001) and apolipoprotein A1 (ρ = 0.528, p < 0.0001). However, there was no correlation between percent increases in cholesterol efflux capacity or HDL-C and exercise-related factors such as exercise tolerance, CR-related factors such as number of sessions of CR program, risk factor modification such as number of achievement of risk factors, or other blood biomarkers.
Discussion
To the best of our knowledge, this study is the first to show that CR increases HDL-mediated cholesterol efflux capacity in patients with ACS, and those changes were not observed in patients who dropped out from outpatient CR program. Ishikawa et al.32) using the same cholesterol efflux assay have recently reported that lower cholesterol efflux capacity is a significant clinically relevant predictor for CAD among the conventional coronary risk factors and dyslipidemia-related variables in cross-sectional study of 182 patients with CAD and 72 patients without CAD determined by coronary angiography or multislice coronary computed tomography. Similarly, Hafiane et al.33) reported that HDL cholesterol efflux capacity was significantly lower in patients with ACS of both acute phase (< 72 h from the onset) and late phase (12 weeks after onset of ACS), and stable CAD compared with healthy controls, whereas HDL-C and apolipoprotein A-1 were similar among the four groups. In their study, HDL cholesterol efflux capacity remained reduced during acute and late phase of ACS, and a weak correlation between cholesterol efflux capacity and HDL-C was observed. In the present study, HDL cholesterol efflux capacity was measured on two occasions: onset of ACS and 6-month treated period. Compared with the results from Ishikawa et al.32), the cholesterol efflux capacity at the follow-up period was 0.764 ± 0.230, which was similar to those of their CAD patients (0.86 ± 0.26) and remained lower than those in non-CAD controls. In previous cross-sectional studies and the prospective cohort studies, HDL cholesterol efflux was measured once, and in all studied subjects, cholesterol efflux capacity was significantly lower in patients with CAD14–16). Contrast to previous studies, HDL cholesterol efflux capacity significantly increased during the follow-up period in only ACS patients who participated CR program, but those levels were still lower than healthy controls. Overall, the present study is consistent with impaired HDL cholesterol efflux capacity in CAD patients and provides a novel therapeutic strategy using CR to improve the HDL function in ACS patients.
The CR program consists of various therapeutic components. The previous meta-analysis of 13 randomized controlled trials published till March 2003 with a total of 1,734 patients with CAD demonstrated that the proportion of smoker in the follow-up period was significantly reduced with CR compared with usual care4). A meta-analysis of 24 intervention studies of smoking cessation showed significant increases in HDL-C34). Three of 25 patients failed smoking cessation in the present CR program, and the HDL cholesterol efflux capacity did not change in these three patients. Further studies with large number of patients were needed to clarify whether continued smoking abolish the effects of CR on HDL cholesterol efflux capacity or not. Including smoking cessation, 17 of 57 patients completed CR program showed complete risk factor control of five targets such as weight control, BP control, four lipid targets, and glycemic control as well as smoking cessation. In addition, improvement in exercise capacity is one of the most reliable effects of exercise training in CR program. Similar to previous studies35, 36), peak VO2 significantly increased in only patients who completed CR program. Higher exercise capacity was defined as ≥ 19 mL/min/kg of peak VO2 based on the mean values of the peak VO2 at the follow-up CPX. The HDL cholesterol efflux capacity significantly increased in the patients with complete risk factor control and higher levels of exercise capacity. In contrast, increases in HDL cholesterol efflux capacity were not associated with individual's exercise capacity, number of participated sessions of CR program, and other exercise-related factors. Exercise training increases the capillary density of skeletal muscle, promotes a conversion from type II to type I muscle fiber, and increases the number and oxidative enzyme activities of mitochondria'1). These peripheral mechanisms such as peripheral circulation and skeletal muscle function play important roles in the increase in peak VO2 by exercise training. Therefore, not only exercise training, but comprehensive CR might affect the HDL cholesterol efflux capacity.
Previous studies reported that PPARγ agonists significantly increased HDL cholesterol efflux capacity14, 18). In contrast, significant increases in HDL cholesterol efflux capacities were seen, irrespective of the taking pioglitazone and/or telmisartan in the CR patients. Greene et al. reported that 12-week aerobic exercise training increased skeletal muscle PPARα, PPARδ, PPARγ coactivator-1α (PGC-1α), and AMP-activated protein kinase (AMPK)α in muscle biopsy samples from the vastus lateralis in overweight and obese men and women37). In addition, they showed that muscle AMPKα protein content was significantly positively correlated to serum HDL-C37). PGC-1α has been shown to mediate the beneficial effects of exercise38). Butcher et al. reported that 8-week low-intensity exercise significantly increased leukocyte mRNA expression for PPARγ, LXR, and ABCA1, and plasma HDL-C in sedentary adults39). These suggest that aerobic exercise training might play an important role for affect HDL transport and/or metabolism through PPARs, LXR, and ABC A1. Further studies are required to investigate pathways and gene expressions on leukocytes relating to HDL-mediated cholesterol efflux capacity.
Miyamoto-Sasaki et al. reported that 4-week treatment with 2 mg of pitavastatin significantly increased HDL-C by 9% and HDL cholesterol efflux capacity by 8.6% in 30 patients with dyslipidemia40). In contrast, Nicholls et al. have very recently reported that 12-week treatment with 10 mg of rosuvastatin or 40 mg of simvastatin significantly decreased and 12-week treatment with 20 mg of atorvastatin did not affect HDL cholesterol efflux capacity compared with placebo in patients with dyslipidemia41). In the present study, high-intensity statin treatment such as 20 mg of atorvastatin or 10–15 mg of rosuvastatin attenuated CR-induced enhanced HDL cholesterol efflux capacity. In contrast, continuing the same intensity statin treatment also reduced the CR-mediated increases in HDL cholesterol efflux capacity. Further studies are required to assess how lipid-lowering therapy affects cholesterol efflux capacity.
The major limitation of the present study is a retrospective analysis with small sample size. The aim of the study was preliminary evaluation of effects of CR on HDL-C and HDL cholesterol efflux capacity in pooled cohort samples. Therefore, our results should be confirmed by prospective studies with more sample size in future. This study is associated with several other limitations. First, baseline blood samples were taken in early phase hospitalization in five patients. Hafiane et al.33) reported no difference in HDL cholesterol efflux capacity between acute phase (< 72 h from the onset) and late phase (12 weeks after onset) in patients with ACS. Therefore, we included those data at baseline. Second, heterogeneity of HDL was not examined. Recent studies suggest that there are great differences in composition of proteins and lipids of HDL particles and vasoprotective functions of HDL between CAD patients and healthy subjects42). Third, higher exercise capacity was defined as the mean levels at follow-up CPX and was not accepted in general situation. Fourth, BP control was determined using an oscillometric device in supine position. BP variability and home BP were not evaluated. Fifth, regular physical activity was not measured in all patients. Again, future prospective studies should be conducted to evaluate these issues in larger number of sample size.
Conclusion
The present study of ACS patients demonstrated three findings. First, 6-month outpatient CR program significantly improved antiatherogenic function of HDL (HDL-mediated cholesterol efflux capacity) as well as quantity of HDL (HDL-C). Second, an increase in HDL cholesterol efflux capacity was significantly correlated to increases in HDL-C and apolipoprotein A1. Third, continuing smoking, incomplete risk factor control, and less exercise capacity might attenuate increases in HDL-mediated cholesterol efflux capacity by CR. These results suggest that the comprehensive CR can enhance reverse cholesterol transport and is a beneficial treatment in patients with ACS.
Acknowledgments
We would like to thank the nursing stuff of the catheterization laboratory and all of the cardiologists at the Department of Cardiology of Showa University Hospital, for their valuable help with this study. We are also grateful for the statistical advice of Prof. Akatsuki Kokaze from the Department of Public Health, Showa University School of Medicine.
Disclosures
The authors have no conflicts to declare.
References
- 1). The Japanese Circulation Society Joint Working Group: Guidelines for Rehabilitation in Patients With Cardiovascular Disease (JCS 2012) – Digest Version –. Circ J, 2014; 78: 2022-2093 [DOI] [PubMed] [Google Scholar]
- 2). Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS: Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev, 2011. July 6;(7):CD001800. 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N: Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med, 2004; 116: 682-692 [DOI] [PubMed] [Google Scholar]
- 4). Clark AM, Hartling L, Vandermeer B, McAlister FA: Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med, 2005; 143: 659-672 [DOI] [PubMed] [Google Scholar]
- 5). Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H: Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA, 2009; 301: 2024-2035 [DOI] [PubMed] [Google Scholar]
- 6). Koba S, Tanaka H, Maruyama C, Tada N, Birou S, Teramoto T, Sasaki J: Physical activity in the Japan population: association with blood lipid levels and effects in reducing cardiovascular and all-cause mortality. J Atheroscler Thromb, 2011; 18: 833-845 [DOI] [PubMed] [Google Scholar]
- 7). The AIM-HIGH investigators: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 8). Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJV, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, for the dal-OUTCOMES Investigators : Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 9). Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S: Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet, 2012; 380: 572-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Jansen H, Samani NJ, Schunkert H: Mendelian randomization studies in coronary artery disease. Eur Heart J, 2014; 35: 1917-1924 [DOI] [PubMed] [Google Scholar]
- 11). Prospective Studies Collaboration: Blood cholesterol and vascular mortality by age, sex, and blood pressure: a metaanalysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet, 2007; 370: 1829-1839 [DOI] [PubMed] [Google Scholar]
- 12). Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L: Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation, 2012; 125: 1905-1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Brewer HB, Jr: The evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J Clin Endocrinol Metab, 2011; 96: 1246-1257 [DOI] [PubMed] [Google Scholar]
- 14). Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med, 2011; 364: 127-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW: HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med, 2014; 371: 2383-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ: Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol, 2015; 3: 507-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Khera AV, Patel PJ, Reilly MP, Rader DJ: The addition of Niacin to statin therapy improves high-density lipoprotein cholesterol levels but not metrics of functionality. J Am Coll Cardiol, 2013; 62: 1909-1910 [DOI] [PubMed] [Google Scholar]
- 18). Ozasa H, Ayaori M, Iizuka M, Terao Y, Uto-Kondo H, Yakushiji E, Takiguchi S, Nakaya K, Hisada T, Uehara Y, Ogura M, Sasaki M, Komatsu T, Horii S, Mochizuki S, Yoshimura M, Ikewaki K: Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARγ/LXRα pathway: findings from in vitro and ex vivo studies. Atherosclerosis, 2011; 219: 141-150 [DOI] [PubMed] [Google Scholar]
- 19). Nakaya K, Ayaori M, Hisada T, Sawada S, Tanaka N, Iwamoto N, Ogura M, Yakushiji E, Kusuhara M, Nakamura H, Ohsuzu F: Telmisartan enhances cholesterol efflux from THP-1 macrophages by activating PPARgamma. J Atheroscler Thromb, 2007; 14: 133-141 [DOI] [PubMed] [Google Scholar]
- 20). Khera AV, Rader DJ: Cholesterol efflux capacity: Full steam ahead or a bump in the road? Arterioscler Thromb Vasc Biol, 2013; 33: 1449-1451 [DOI] [PubMed] [Google Scholar]
- 21). Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society (JDS) : International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int, 2012; 3: 8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Executive Summary of the Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases in Japan -2012 Version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 23). Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S: Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol, 2009; 13: 621-630 [DOI] [PubMed] [Google Scholar]
- 24). Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res, 2002; 25: 359-364 [DOI] [PubMed] [Google Scholar]
- 25). Nakajima K, Saito K, Tamura A, Suzuki M, Nakano T, Adachi M, Tanaka A, Tada N, Nakamura H, Campos E, Havel RL: Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apoB-100 and anti apoA-1 immunoaffinity mixed gels. Clin Chim Acta, 1993; 223: 53-71 [DOI] [PubMed] [Google Scholar]
- 26). Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N: Evaluation of nine automated high-sensitivity C-reactive protein methods: Implications for clinical and epidemiological applications. Part 2. Clin Chem, 2001; 47: 418-425 [PubMed] [Google Scholar]
- 27). Beaver WL, Wasserman K, Whipp BJ: A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol, 1986; 60: 2020-2027 [DOI] [PubMed] [Google Scholar]
- 28). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Comprehensive Risk Management for the Prevention of Cardiovascular Disease Executive Summary of the Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases in Japan — 2012. J Atheroscler Thromb, 2013; 20: 603-615 [DOI] [PubMed] [Google Scholar]
- 29). The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253-392 [DOI] [PubMed] [Google Scholar]
- 30). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Absolute Risk of Cardiovascular Disease and Lipid Management Targets. Executive Summary of the Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases in Japan — 2012 Version. J Atheroscler Thromb, 2013; 20: 689-69723892530 [Google Scholar]
- 31). Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF: American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014; 129(25 Suppl 2): S1-S45 [DOI] [PubMed] [Google Scholar]
- 32). Ishikawa T, Ayaori M, Uto-Kondo H, Nakajima T, Mutoh M, Ikewaki K: High-density lipoprotein cholesterol efflux capacity as a relevant predictor of atherosclerotic coronary disease. Atherosclerosis, 2015; 242: 318-322 [DOI] [PubMed] [Google Scholar]
- 33). Hafiane A, Jabor B, Ruel I, Ling J, Genest J: High-density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am J Cradiol, 2014; 113: 249-255 [DOI] [PubMed] [Google Scholar]
- 34). Maeda K, Noguchi Y, Fukui T: The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med, 2003; 37: 283-290 [DOI] [PubMed] [Google Scholar]
- 35). Hambrecht R, Walther C, Möbius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G: Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation, 2004; 109: 1371-1378 [DOI] [PubMed] [Google Scholar]
- 36). Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A: Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol, 2001; 37: 1891-1900 [DOI] [PubMed] [Google Scholar]
- 37). Greene NP, Fluckey JD, Lambert BS, Greene ES, Riechman SE, Crouse SF: Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am J Physiol Endocrinol Metab, 2012; 303: E1212-E1221 [DOI] [PubMed] [Google Scholar]
- 38). Handschin C, Spiegelman BM: The role of exercise and PGC-1α in inflammtion and chronic disease. Nature, 2008; 454: 463-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K: Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc, 2008; 40: 1263-1270 [DOI] [PubMed] [Google Scholar]
- 40). Miyamoto-Sasaki M, Yasuda T, Monguchi T, Nakajima H, Mori K, Toh R, Ishida T, Hirata K: Pitavastatin increases HDL particles functionally preserved with cholesterol efflux capacity and antioxidative actions in dyslipidemic patients. J Atheroscle Thromb, 2013; 20: 708-716 [DOI] [PubMed] [Google Scholar]
- 41). Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang, Krueger KA, Adelman SJ, Nissen SE, Rader DJ: Cholesterol efflux capacity and pre-beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. J Am Coll Cardiol, 2015; 66: 2201-2210 [DOI] [PubMed] [Google Scholar]
- 42). Lüscher TF, Landmesser U, von Eckardstein A, Fogelman AM: High-Density Lipoprotein. Vascular Protective Effects, Dysfunction, and Potential as Therapeutic Target. Circ Res, 2014; 114: 171-118 [DOI] [PubMed] [Google Scholar]


