Abstract
Aim: To analyse the relationship between two potentially functional single-nucleotide polymorphisms (SNPs) of the miR-146a gene (rs2910164 and rs57095329) and the risk of atherosclerotic cerebral infarction (ACI).
Methods: A total of 297 patients with ACI and 300 matched healthy individuals were enrolled in the study. The miR-146a polymorphism was detected using the polymerase chain reaction–restriction fragment length polymorphism method.
Results: A significant difference in the C allele frequency at rs2910164 (p = 0.028) was noted between patients with ACI and control subjects. In contrast, the genotype and allele frequencies of rs57095329 were not statistically associated with ACI. In addition, the decreased expression of miR-146a was significantly more frequent in ACI patients who were ApoEε4 (+) carriers (p = 0.0233), and rs2910164 G> C was intimately associated with the ApoEε4-containing genotype in patients compared with the ApoEε4 (−) carriers (p = 0.0323).
Conclusions: Our findings indicated that the C allele of rs2910164 miR-146a is an important risk factor for ACI, and ApoEε4 may function through attenuating miR-146a expression to enhance ACI susceptibility. This study provides new information about the possible relationship between miR-146a and ApoEε4 in the development of ACI, with potentially important therapeutic implications.
Keywords: Polymorphism, microRNA-146a, Apolipoprotein E, Atherosclerotic cerebral infarction
Introduction
Atherosclerotic cerebral infarction (ACI) is a complex disease caused by the combination of multiple risk factors, such as atherosclerosis, hypertension, diabetes, and dyslipidaemia, among which atherosclerosis is considered as the main factor involved in the pathogenesis of ACI1). Atherosclerosis is characterized by chronic local inflammation of the vascular wall, resulting in the accumulation of lipids and macrophage-derived foam cells in the subendothelial space2). Although the inflammatory nature of atherosclerosis is widely accepted, the complex mechanisms underlying the atherogenic proinflammatory processes remain controversial. Recent findings have suggested that ACI has a significant genetic component3, 4). Considerable evidence gathered to date has allowed us to identify at least five potential important pathways that may be related to the genetic risk factors of atherothrombosis, including lipoprotein metabolism, inflammation, the renin-angiotensin-aldosterone system (RAAS), platelet biology and function, and blood coagulation and fibrinolysis, contributing to the risk of ACI5).
MicroRNAs (miRNAs) are small noncoding RNAs that are 20–23 nucleotides in length and regulate gene expression in a sequence-specific manner6). Accumulating evidence has implicated miRNAs as essential regulators of atherosclerosis by targeting important factors or key pathways7, 8). Abnormal miRNA expression has also been observed in various diseases that are associated with inflammatory and immune processes9–11). MiR-146a is recognized for its ability to silence multiple inflammatory targets, including IRAK1 and TRAF6, and suppress TLRdriven NF-κB signaling in macrophages and other haematopoietic cells. Reduction of miR-146a levels in these cell lines could enhance the susceptibility to atherosclerosis and sepsis12–14). The current evidence supports the hypothesis that miR-146a operates as an important epigenetic regulator in various pathways involved in atherosclerosis and stroke. Thus, we were prompted to explore the relationship between miR-146a and ACI15, 16). Certain single-nucleotide polymorphisms (SNPs) could influence the function of the target genes and are associated with diseases risk17). Two SNPs in the miR-146a gene, rs2910164 and rs57095329, have functional importance in modifying the expression of mature miR-146a and have been reported to be associated with several inflammation-associated diseases18–21).
Apolipoprotein E (ApoE) plays an important role in lipid metabolism and cholesterol transport, which are considered to be candidate pathways with antiatherogenic functions22, 23). Several studies have reported that ApoE polymorphisms serve as a major genetic risk factor for a wide spectrum of inflammatory metabolic diseases, including atherosclerosis, cerebrovascular disorders, and hyperlipoproteinaemia, and they are also well recognized for their ability to suppress atherosclerosis24–26). Interestingly, it has been recently reported that ApoE regulates the expression of the anti-inflammatory microRNA miR-146a in monocytes and macrophages14). To the best of our knowledge, no study to date has examined the association of miR-146a polymorphisms with the risk of ACI. In this study, we conducted an association analysis to ascertain whether the two functional polymorphisms of miR-146a contribute to the risk of ACI and further studied the distribution of ApoE genotypes in these study populations.
Methods
Study Population
Our study consecutively recruited 297 patients with ACI (120 women and 177 men; mean age = 62.6 ± 8.63 years) from the Department of Cardiovascular Internal Medicine and the Department of Neurosurgery of the Affiliated second Hospital of Guangdong Medical University and 300 healthy controls (130 women and 170 men; mean age = 61.1 ± 9.58 years) from the medical examination center of the Affiliated Hospital of Guangdong Medical University. Patient diagnoses were verified with either computed tomography (CT) or magnetic resonance imaging (MRI), and all the patients with ACI were classified into subtypes according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification27). All subjects enrolled in our study were Chinese Han with similar dietary pattern, and the patients with histories of ischemic cerebrovascular diseases, cardiogenic cerebral infarctions, cerebral hemorrhage, coronary artery diseases (CAD), autoimmune diseases, systemic inflammatory diseases, blood diseases, and malignant tumors were excluded from the study. All experiments on human subjects were conducted in accordance with the Declaration of Helsinki, written informed consent was obtained from all the enrolled participants, and this study was approved by the Ethics Committee of the Guangdong Medical University.
DNA Isolation and Genotyping
Genomic DNA was isolated from peripheral blood samples using a blood genomic DNA extraction kit (Tiangen, China). The DNA was genotyped using the ABI PRISM SNapShot method (Applied Biosystems, Foster, CA). The polymerase chain reaction (PCR) primers used for the rs2910164 polymorphism were 5′-GAACTGAATTCCATGGGTTG-3′ and 5′-CACGATGACAGAGATATCCC-3′. The primers used for rs57095329 polymorphism were 5′-TCATTGGGCAGCCGATAAAG-3′ and 5′-AGGAAGTTCTGGTCAGGCG-3′. In brief, the SNapShot reaction was performed in a 10-ml final volume containing the SNapShot Multiplex Ready Mix (5 ml), primer mix (0.02–0.6 mmol/L), and templates (4 ml) consisting of the multiplex PCR products, which were purified using the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany). The cycling program included 25 cycles of 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 40 s. Extension products were purified by a 15-min incubation with 1 U of shrimp alkaline phosphatase (Promega, Madison, WI) at 37°C and a subsequent 15-min incubation at 80°C to inactivate the enzyme. The purified products (0.5 ml) were mixed with 9 ml of formamide and 0.5 ml of GeneScan-120 LIZ size standard (Applied Biosystems) and separated by capillary electrophoresis (ABI PRISM 310 Genetic Analyzer; Applied Biosystems). The results were analyzed using GeneMapper 3.0 software (Applied Biosystems). For quality control, random duplicate samples (5%) were run for each sequence analysis.
Mononuclear Cell Isolation
In total, 30 ApoEε4 (+) carriers and 30 ApoEε4 (−) carriers from among 297 ACI patients and 30 ApoEε4 (+) carriers and 30 ApoEε4 (−) carriers from 300 healthy subjects were chosen randomly for the isolation of mononuclear cells. Peripheral blood mononuclear cells (PBMCs) were isolated using the density gradient centrifugation method with LymphoprepTM (Axis-Shield PoCAS, Oslo, Norway). In brief, blood samples were mixed with an equal volume of 0.9% NaCl. The diluted blood was then slowly added to tubes containing a Ficoll PREMIUM solution to layer the blood on the Ficoll. Samples were centrifuged at 800 × g for 30 min at room temperature. After centrifugation, the mononuclear cells form a distinct band at the medium interface. The cells were then transferred to other tubes using a Pasteur pipette without removing the upper layer and washed with 0.9% NaCl. The samples were then centrifuged again at 250 × g for 10 min. The mononuclear cells were harvested and stored at −80°C. Isolated PBMCs (2 × 106 cells per well) were seeded in 24-well plates and cultured in RPMI medium 1640 supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 mg/ml streptomycin.
RNA Extraction
Total cellular RNA was extracted from PBMCs using the RNAprep Pure Blood Kit (TianGen Biotech, Beijing, China) according to the manufacturer's instructions. In brief, PBMCs were pelleted and lysed using Trizol. The lysate was centrifuged at 13,400 × g for 2 min. The aqueous phase was transferred to a fresh microcentrifuge tube. Ethanol was added to the sample, and then the sample was transferred to the silica surface of a spin column. All contaminating genomic DNA was eliminated by performing RNase-free DNase I digestion on the silica membrane. Total RNA from all samples was further purified by performing the washing steps and then eluting the RNA from the membrane in water. Next, the integrity of the RNA samples was verified by agarose gel electrophoresis and stored at −80°C.
Real-Time PCR
MiR-146a expression was analyzed using the miScript SYBR Green PCR kit (Qiagen, Germany) according to the manufacturer's instructions (Qiagen, Germany). The assay was performed in triplicate. Human U6 was used as the internal control. The comparative threshold cycle (Ct) method was used to assess the relative changes in expression. In brief, 2ΔDDCt represents the fold change in miR-146a expression between sample groups. A no-template control was run with the positive samples to assess the overall specificity of the reaction10). The expression levels of IRAK-1 were analyzed using the miScript SYBR Green PCR kit. The IRAK-1 expression levels were analyzed in triplicate, and the expression was normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which was used as an internal control. The expression levels of IRAK-1 were also calculated using the 2ΔDDCt method.
Plasma Lipid Measurements
Blood samples for lipid measurements were withdrawn from subjects after an overnight fast. The plasma triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol levels were measured using a Hitachi 7600 automatic analyser (Hitachi Instruments Corporation, Tokyo, Japan).
Statistical Analyses
Statistical analyses were performed using SPSS software, version 19.0 (IBM, Armonk, NY, USA). These data are presented as percentage frequencies or means ± standard deviation (SD). Allele frequencies were calculated from the genotypes of all subjects. The allele and genotype frequencies of miR-146a between the patients and control subjects were compared using Fisher's exact test or chi-squared test. Hardy–Weinberg equilibrium (HWE) was assessed using HWE software. Risk factors were screened using Student's t-test or chi-squared test. Analyses were repeated in subgroups stratified by miR-146a and ApoEε4 carrier status. Associations were expressed as odds ratios (ORs) or risk estimates with 95% confidence intervals (CIs). The relationships between different genotypes of miR-146a and ACI were evaluated using analysis of variance (ANOVA). A p value < 0.05 was considered to be statistically significant. Power analysis was performed using QUANTO 1.2 software.
Results
Clinical Characteristics
The clinical characteristics of all the participants in the study are summarized in Table 1. Of the 597 participants, 297 were ACI patients and 300 were healthy controls. The mean age was 62.6 (± 8.63) years for the ACI patients and 61.1 (± 9.58) years for the control subjects, and the gender (male-to-female) ratio was 1:1.48 in the case group and 1:1.31 in the control group. No statistically significant difference was noted between the patients and controls in terms of sex (p = 0.468) or age (p = 0.064). The risk factors examined (e.g., hypertension and diabetes) were significantly more common in the ACI group compared with the control group. Moreover, compared with the controls, the patients with ACI exhibited increased TC (p = 0.028), HDL (p = 0.015), and LDL (p = 0.043). Individuals with hypertension (p < 0.001) and diabetes (p < 0.001) exhibited increased risk of ACI. However, patients with ACI exhibited a trend of increased total TG compared with controls, but no significant difference was noted (p = 0.075). Moreover, as expected, ApoEε4 allele frequencies were significantly elevated in ACI patients compared with controls (p < 0.001). Power analysis revealed that, with our sample size, we would have 93.2% power for rs2910164 and 78.1% power for rs57095329 to detect a genotype relative risk with an OR of 1.5 at a significance level of 0.05.
Table 1. Characteristic of Controls and ACI Patients.
| Clinic data | ACI patients (297) | Controls (300) | P value |
|---|---|---|---|
| Mean ages (years) | 62.6 ± 8.63 | 61.1 ± 9.58 | 0.064 |
| Male/Female | 177/120 | 170/130 | 0.468 |
| BMI (kg/m2) | 22.6 ± 3.41 | 23.35 ± 2.51 | 0.754 |
| History of hypertension, n (%) | 197 (66.33%) | 66 (22.00%) | < 0.001* |
| History of diabetes, n (%) | 62 (20.86%) | 11 (3.67%) | < 0.001* |
| HDL (mmol/L) | 1.19 ± 0.47 | 1.25 ± 0.31 | 0.015* |
| LDL (mmol/L) | 2.85 ± 1.06 | 2.91 ± 0.86 | 0.043* |
| Total Cholesterol (mmol/L) | 4.91 ± 0.97 | 4.59 ± 0.62 | 0.028* |
| Total Cholesterol (mmol/L) | 1.84 ± 1.04 | 1.62 ± 0.63 | 0.075 |
| ApoEε4, n (%) | 94 (31.65%) | 37 (12.33%) | < 0.001* |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
p < 0.05.
MiR-146a Polymorphisms in ACI Patients and Controls
All the enrolled participants were successfully genotyped for the rs2910164 and rs57095329 SNPs. All genotype distributions were in HWE in the ACI patients and controls (Supplement Table 1). The genotype and allele frequencies of miR-146a polymorphisms in the study are presented in Table 2. Although a trend was noted, no statistical association with the genotype frequencies of the rs2910164 SNP with ACI patients and controls was observed (p = 0.052). In contrast, a significant difference in the C allele frequency of the rs2910164 SNP was noted (p = 0.028). Moreover, a significant difference was observed in the ACI patients compared with the controls in a recessive model (GG+GC. vs. CC) (p = 0.015), which indicated that the rs2910164 polymorphism is a risk factor for ACI (Table 2). Regarding the rs57095329 polymorphism, no statistically significant difference was observed between the ACI patients and healthy controls for either the genotype or allele frequencies of the rs57095329 SNP (genotype frequencies p = 0.620, allele frequencies p = 0.354).
Supplement Table 1. The Hardy-Weinberg equilibrium assay for rs653765 and rs514049 genotypes in the ACI cases and controls.
| Rs2910164 | CC | GC | GG | P-value |
|---|---|---|---|---|
| ACI patients | 141 | 128 | 28 | 0.892 |
| Control | 113 | 152 | 35 | 0.133 |
| Rs57095329 | GG | GA | AA | |
| ACI patients | 200 | 87 | 10 | 0.887 |
| Control | 211 | 82 | 7 | 0.770 |
| ApoEε4(+) | ||||
| Rs2910164 | CC | GC | GG | |
| ACI patients | 59 | 28 | 7 | 0.170 |
| Control | 16 | 16 | 5 | 0.755 |
| Rs57095329 | GG | GA | AA | |
| ACI patients | 66 | 27 | 1 | 0.328 |
| Control | 25 | 10 | 2 | 0.469 |
| ApoEε4(-) | ||||
| Rs2910164 | CC | GC | GG | |
| ACI patients | 82 | 100 | 21 | 0.237 |
| Control | 97 | 136 | 30 | 0.086 |
| Rs57095329 | GG | GA | AA | |
| ACI patients | 134 | 60 | 9 | 0.495 |
| Control | 186 | 72 | 5 | 0.514 |
Table 2. Genotype and allele Distributions of the miR-146a polymorphisms between controls and ACI Patients.
| rs2910164 G > C | ACI patients (297), n (%) | Controls (300) n (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| CC | 141 (47.47%) | 113 (37.67%) | 0.052 | |
| GC | 128 (43.10%) | 152 (50.66%) | ||
| GG | 28 (9.43%) | 35 (11.67%) | ||
| GC/GG | 156 (52.52%) | 187 (62.33%) | 0.667 (0.482–0.927) | 0.015* |
| GC/CC | 269 (90.57%) | 265 (88.33%) | 1.27 (0.750–2.15) | 0.373 |
| C alleles | 410 (69.02%) | 378 (63.00%) | 0.764 (0.601–0.972) | 0.028* |
| G alleles | 184 (30.98%) | 222 (37.00%) | ||
| rs57095329 G > A | ||||
| AA | 200 (67.34%) | 211 (70.33%) | 0.620 | |
| GA | 87 (29.29%) | 82 (27.33%) | ||
| GG | 10 (3.37%) | 7 (2.33%) | ||
| GA/GG | 97 (32.66%) | 89 (29.66%) | 1.15 (0.813–1.63) | 0.480 |
| GA/AA | 287 (95.63%) | 293 (97.67%) | 0.686 (0.257–1.83) | 0.472 |
| A alleles | 487 (81.99%) | 504 (84.00%) | 1.153 (0.852–1.561) | 0.354 |
| G alleles | 107 (18.01%) | 96 (16.00%) | ||
Adjusted for age, sex, hypertension and diabetes.
p < 0.05.
MiR-146a Polymorphisms in ApoE Genotype Groups in ACI Patients and Controls
The prevalence of the ApoEε4-containing phenotypes was significantly increased in individuals with cardiovascular and cerebrovascular ailments, such as myocardial infarction, hypertension, coronary heart disease, and stroke. We examined whether a significant association exists between miR-146a and ApoE4 isoforms in ACI patients. As shown in Table 3, when these data were stratified for the presence or absence of the E4 isoform, the C allele of rs2910164 remained significantly different between ACI patients and controls in the ApoEε4(+) subgroup (p = 0.033) but not in the ApoEε4(−) groups (p = 0.472). In the recessive model (GG+GC. vs. CC), a significant difference was observed in ApoEε4(+) patients compared with ApoEε4(+) controls (p = 0.04). However, no significant difference was detected in the ApoEε4(−) subgroups (p = 0.440). Besides, neither the genotype nor the allele frequencies in rs57095329 exhibited significant differences in the ApoEε4 mutation subgroups [ApoEε4(+) genotype and allele frequencies: p = 0.326 and p = 0.492, respectively; ApoEε4(−) genotype and allele frequencies: p = 0.222 and p = 0.146, respectively] (Table 3).
Table 3. Genotype and allele distributions in ApoE genotype groups with ACI patients and controls.
| rs2910164 (n) | Genotypes n (%) |
||||
| ApoEε4(−) | CC | GC | GG | p-value | |
| ACI (94) | 59 (62.77%) | 28 (29.79%) | 7 (7.45%) | 0.119 | |
| Controls (37) | 16 (43.24%) | 16 (43.24%) | 5 (13.51%) | ||
| ApoEε4(−) | |||||
| ACI (203) | 82 (40.39%) | 100 (49.26%) | 21 (10.34%) | 0.733 | |
| Controls (263) | 97 (36.88%) | 136 (51.71%) | 30 (11.41%) | ||
| Rs57095329 (n) | Genotypes n (%) |
||||
| ApoEε4(−) | AA | GA | GG | p-value | |
| ACI (94) | 66 (70.21%) | 27 (28.72%) | 1 (1.06%) | 0.326 | |
| Controls (37) | 25 (67.57%) | 10 (27.03%) | 2 (5.41%) | ||
| ApoEε4(−) | |||||
| ACI (203) | 134 (66.01%) | 60 (29.56%) | 9 (4.43%) | 0.222 | |
| Controls (263) | 186 (70.72%) | 72 (27.38%) | 5 (1.90%) | ||
| rs2910164 (n) | Alleles n (%) |
||||
| ApoEε4(−) | G | C | p-value | OR (95% CI) | |
| ACI (94) | 42 (22.24%) | 146 (77.66%) | 0.033* | 0.531 (0.295–0.956) | |
| Controls (37) | 26 (35.14%) | 48 (64.87%) | |||
| ApoEε4(−) | |||||
| ACI (203) | 142 (34.98%) | 264 (65.03%) | 0.472 | 0.906 (0.691–1.186) | |
| Controls (263) | 196 (37.26%) | 330 (62.74%) | |||
| Rs57095329 (n) | Alleles n (%) |
||||
| ApoEε4(−) | G | A | p-value | OR (95% CI) | |
| ACI (94) | 29 (15.43%) | 159 (84.57%) | 0.492 | 0.782 (0.387–1.580) | |
| Controls (37) | 14 (18.92%) | 60 (81.08%) | |||
| ApoEε4(−) | |||||
| ACI (203) | 78 (19.21%) | 328 (80.79%) | 0.146 | 1.288 (0.915–1.811) | |
| Controls (263) | 82 (15.59%) | 444 (84.41%) | |||
| rs2910164 (n) | Recessive model |
||||
| ApoEε4(−) | GG−GC .vs. CC | p-value | OR (95% CI) | ||
| ACI (94) | 35 (37.24%) | 0.04* | 0.452 (0.209–0.980) | ||
| Controls (37) | 21 (56.75%) | ||||
| ApoEε4(−) | |||||
| ACI (203) | 121 (59.61%) | 0.440 | 0.862 (0.5920–1.256) | ||
| Controls (263) | 166 (63.12%) | ||||
| Rs57095329 (n) | Recessive model |
||||
| ApoEε4(−) | GG−GA. vs. AA | p-value | OR (95% CI) | ||
| ACI (94) | 28 (29.897%) | 0.767 | 0.884 (0.390–2.00) | ||
| Controls (37) | 12 (32.44%) | ||||
| ApoEε4(−) | |||||
| ACI (203) | 69 (33.99%) | 0.277 | 1.24 (0.8391–1.844) | ||
| Controls (263) | 77 (29.28%) | ||||
Adjusted for age, sex, hypertension and diabetes.
p < 0.05.
The Influence of ApoEε4 on miR-146a Expression in ACI Patients
From the case-control study above, we found that rs2910164 of miR-146a exhibited a high-risk genotype in ApoEε4 patients with ACI. To further explore the influence of ApoEε4 on the expression of mature miR-146a, we examined the expression levels of the rs2910164 miR-146a genotypes in PBMCs that were obtained from the ACI patients who were E4 allele carriers. According to the data obtained, the mean level of miR-146a mRNA expression was significantly reduced in ApoEε4(+) patients with ACI compared with ApoEε4(−) patients (p = 0.0233), and the mean level of miR-146a mRNA was also significantly reduced in ApoEε4(+) healthy control compared with ApoEε4(−) control (p = 0.039) (Fig. 1A). Moreover, regarding ACI patients with ApoEε4(+), individuals with the CC genotype for rs2910164 exhibited significantly lower levels of miR-146a than those with the GG or GC genotype (p = 0.0323) (Fig. 1B). However, the mean level of miR-146a revealed no significant difference between the CC and GG+GC genotypes for rs2910164 in ACI patients who were ApoEε4(−) carriers, suggesting that the CC genotype for rs2910164 has a stronger influence on mature miR-146a expression in ACI patients with ApoEε4(+) but not ApoEε4 (−). Furthermore, patients with the CC genotype expressed significantly higher relative expression of IRAK-1 in ApoEε4(+) patients (p = 0.048; compared with GG/GC, Fig. 1C); however, no statistically significant difference was observed in ApoEε4(−) subjects. The comparison of genotype distributions between the ACI subjects revealed a statistical association between the rs2910164 polymorphism of miR-146a and risk of ACI. Furthermore, ApoEε4 may alter the effect of C allele mutations in rs2910164 on decreasing miR-146a expression, further contributing to ACI susceptibility.
Fig. 1.
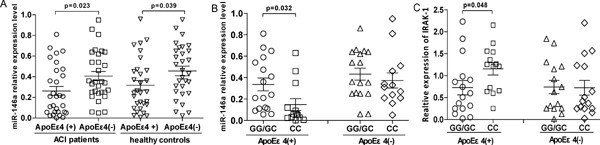
The relative expression level of miR-146a with rs2910164 genotypes in ACI patients and healthy controls. (A) The mature miR-146a expression in PBMCS was analyzed in ACI patients with ApoEε4 (+/−); (B) miR146a expression level with rs2910164 genotypes in ACI patients with ApoEε4 genotypes; (C) the relative mRNA expression of IRAK1 with rs2910164 genotypes in ACI patients with ApoEε4 genotypes. The horizontal line indicates the mean expression level.
To further explore whether rs2910164 SNP may influence lipid metabolism and cholesterol transport that has been proved to be associated with ApoE, we also evaluated the possible association between the rs2910164 SNP of miR-146a and TC/total TG levels in patients with or without ApoEε4(+). However, neither the TC nor the total TG levels exhibited a statistically significant difference between the ApoEε4(+) and ApoEε4(−) groups. In addition, no significant difference was observed between the CC and GG+ GC genotypes for rs2910164 miR-146a (Fig. 2).
Fig. 2.
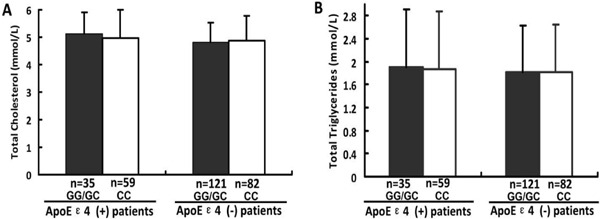
The levels of total cholesterol (A) and total triglycerides (B) with rs2910164 genotypes were analyzed in ACI patients. N presents the numbers of the patients of ACI.
Discussion
MiR-146a is a powerful innate immune and proinflammatory-related regulator of immune and inflammatory responses. MiR-146a drives PBMCs toward Th1 polarization, and the levels of this particular miRNA are increased in patients with diseases such as acute coronary syndrome28). MiR-146a is upregulated in THP-1 cells, macro-phages, Langerhans cells, and monocytes after induction by lipopolysaccharide (LPS), TNF-α, IL-1β, or TGF-β129, 30). Furthermore, miR-146a is a novel regulator of vascular smooth muscle cell (VSMC) fate, which is crucial in the pathogenesis of proliferative cardiovas-cular diseases, including atherosclerosis and post-angioplasty restenosis31). These data suggest that miR-146a is associated with the development of atherosclerosis, which is one of the important risk factors for ACI32, 33). However, few studies to date have examined the relationship between miR-146a and ACI. Here we focused on identifying the possible association between miR-146a functional polymorphisms and other risk factors of ACI in a case-control study.
SNPs present in precursor and mature miRNAs influence the levels of mature miRNAs and are associated with various diseases34–36). In our study, two functional SNPs in miR-146a were chosen to evaluate the association between miR-146a polymorphisms and ACI risk. The two SNPs in miRNA-146a, rs2910164 and rs57095329, have been reported to influence the expression level of mature miR-146a; thus, these SNPs may ultimately affect an individual's susceptibility to disease10, 37, 38). Our results revealed that the rs2910164 polymorphism was associated with an increased risk of ACI; the C allele of rs2910164 was more frequent in patients with ACI compared with the G allele, indicating that the C allele of rs2910164 is associated with an increased risk of ACI susceptibility. The SNP rs2910164 G/C is located in the stem region, and the C allele creates a mismatch in the stem structure of pre-miR-146a. This mismatch results in a decrease in the total levels of mature miR-146a and influences the transcription of target genes, ultimately leading to disease pathogenesis17, 18). MiR-146a is assumed to be protective against atherosclerosis given its ability to suppress NF-κB signaling, the TLR4 signaling pathway, and the apparent activation of endothelial cells. miR-146a levels were elevated in human atherosclerotic plaques as well as in the circulation and plaques of atherosclerotic mice because of the strong activation of NF-κB signaling in plaques, implying that miR-146a is induced as part of a negative feedback loop32, 39, 40). Xiong et al. stated that the GC and CC genotypes of the miRNA-146a rs2910164 polymorphism are associated with an increased risk of CAD41), whose risk subsequently increases with the presence of metabolic syndrome induced by inflammation42, 43). In this study, we confirmed that patients with reduced miR-146a expression exhibit an increased risk of ACI. In this study, significantly reduced miR-146a mRNA levels were observed in ACI subjects. Considering the supporting evidence that the decreased miR-146a levels are also associated with the development of cardiovascular diseases32, 33, 41), we speculate that the C allele of rs2910164 may reduce miR-146a expression and subsequently weaken anti-inflammatory action in the pathogenesis of ACI, thus contributing to the pathological process and increased risk of ACI.
Another novel finding of this study was that ACI patients with the ApoEε4 allele exhibited reduced miR-146a expression compared with controls. More interestingly, CC genotype carriers exhibited reduced miR-146a expression compared with GG or GC genotype carriers. The ApoEε4(+) group contained 2.5-fold more ACI patients than controls, whereas the ApoEε4(−) group included 1.4-fold more controls than ACI patients. It appears that carrying the E4 allele attenuates the level of miR-146a, which increases the risk of ACI. Moreover, the CC genotype of the miRNA-146a rs2910164 polymorphism exhibited a more significant effect in the ApoEε4(+) group compared with the ApoEε4(−) group. ApoE is an important regulator of plasma lipoprotein metabolism and cholesterol homeostasis, and variants of ApoE are major genetic risk factors for a wide spectrum of inflammatory metabolic diseases44, 45). Individuals who carry at least one copy of the ApoEε4 allele have an increased risk of developing atherosclerosis, which is an accumulation of fatty deposits and scar-like tissue in the lining of the arteries that narrow the arteries, thus increasing the risk of ACI46). MiR-146a has been reported to negatively regulate multiple inflammatory targets, including IRAK1 and TRAF6, and to suppress NF-κB-driven TNF-α expression47, 48). As shown in our results, the reduction of miR-146a expression increased IRAK1 levels significantly in CC genotypes in ApoEε4(+) carries. Reduction of miR-146a expression can reverse the effect of NF-κB activity inhibition49). ApoE controls inflammation by suppressing inflammatory NF-κB signaling24). Mechanistically, ApoE increases the expression of the transcription factor PU.1, which increases miR-146 levels to suppress NF-κB signaling. Li et al. demonstrated that ApoE regulates the expression of miR-146a in monocytes and macrophages to repress NF-κB signaling in these cells, and intravascular delivery of miR-146a mimetics can inhibit atherogenesis in mouse models13). However, the ε4 gene polymorphism causes strong dysfunction of ApoE, which suppresses the level of miR-146a13, 38, 50). Based on the finding that ApoEε4 induced decreasing expression of miR-146a in ACI patients, we suggest that the downregulation of NF-κB-dependent pathways by miR-146a through a critical negative feedback regulatory loop was attenuated by the effects of ApoEε4. Furthermore, considering the important roles of ApoE in lipid and cholesterol metabolism, we explored whether rs2910164 influences TC and TT levels. Although no significant association was observed, we speculate that the rs2910164 polymorphism, with its synergistic effect with ApoE, may contribute to the risk of ACI but not through the lipid and cholesterol pathway. Nevertheless, the mechanisms of the interaction of ApoEε4 dysfunction with the CC genotype or C allele of rs2910164 should be further examined.
Conclusions
This is the first study to identify a significant association between the rs2910164 polymorphism of miR-146a and risk of ACI in the south Chinese Han population. The C allele of rs2910164 was associated with the E4-containing phenotype of ApoE, which plays an important role in enhancing ACI susceptibility. This study provides new information about the relationship between miR-146a and ApoE in the development of ACI. However, more work is still required to shed light on the role of miR-146a and ApoE in the pathogenesis of ACI and further clarify its prognostic and therapeutic potential.
Acknowledgment
Supports for this work include funding from the Medical Scientific Research Foundation of Guangdong Province (B2013306) and the Financial science and technology special competitive allocation project of Zhanjiang city (2014A01021).
References
- 1). Barbarash OL, Usol'tseva EN, Kashtalap VV, Kolomytseva IS, Sizova IN, Volykova MA, Shibanova IA: [The role of subclinical inflammation in progression of multifocal atherosclerosis during one year after myocardial infarction]. Kardiologiia, 2014; 54: 19-25 [DOI] [PubMed] [Google Scholar]
- 2). Xia X, Li Y, Su Q, Huang Z, Shen Y, Li W, Yu C: Inhibitory effects of Mycoepoxydiene on macrophage foam cell formation and atherosclerosis in ApoE-deficient mice. CELL BIOSCI, 2015; 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Cunnington MS, Mayosi BM, Hall DH, Avery PJ, Farrall M, Vickers MA, Watkins H, Keavney B: Novel genetic variants linked to coronary artery disease by genome-wide association are not associated with carotid artery intimamedia thickness or intermediate risk phenotypes. Atherosclerosis, 2009; 203: 41-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Li Y, Liao F, Yin XJ, Cui LL, Ma GD, Nong XX, Zhou HH, Chen YF, Zhao B, Li KS. An association study on ADAM10 promoter polymorphisms and atherosclerotic cerebral infarction in a Chinese population. CNS Neurosci Ther, 2013; 19(10): 785-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Montagnana M, Danese E, Lippi G: Genetic risk factors of atherothrombosis. Pol Arch Med Wewn, 2014; 124: 474-482 [DOI] [PubMed] [Google Scholar]
- 6). Bartel DP: MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004; 116: 281-297 [DOI] [PubMed] [Google Scholar]
- 7). Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW: Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ res, 2014; 114: 32-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J clin invest, 2012; 122: 4190-4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Dombkowski AA, Batista CE, Cukovic D, Carruthers NJ, Ranganathan R, Shukla U, Stemmer PM, Chugani HT, Chugani DC: Cortical Tubers: Windows into Dysregulation of Epilepsy Risk and Synaptic Signaling Genes by MicroRNAs. Cereb cortex, 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Li MM, Li XM, Zheng XP, Yu JT, Tan L: MicroRNAs dysregulation in epilepsy. Brain res, 2014; 1584: 94-104 [DOI] [PubMed] [Google Scholar]
- 11). Cui L, Li Y, Ma G, Wang Y, Cai Y, Liu S, Chen Y, Li J, Xie Y, Liu G, Zhao B, Li K: A functional polymorphism in the promoter region of microRNA-146a is associated with the risk of Alzheimer disease and the rate of cognitive decline in patients. Plos one, 2014; 9: e89019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hung PS, Liu CJ, Chou CS, Kao SY, Yang CC, Chang KW, Chiu TH, Lin SC: miR-146a enhances the oncogenicity of oral carcinoma by concomitant targeting of the IRAK1, TRAF6 and NUMB genes. Plos one, 2013; 8: e79926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M: Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin Sci (Lond), 2010; 119: 395-405 [DOI] [PubMed] [Google Scholar]
- 14). Li K, Ching D, Luk FS, Raffai RL: Apolipoprotein E Enhances MicroRNA-146a in Monocytes and Macrophages to Suppress Nuclear Factor-kappaB-Driven Inflammation and Atherosclerosis. Circ res, 2015; 117: e1-e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kahonen M, Karhunen PJ, Laaksonen R, Lehtimaki T: miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis, 2011; 219: 211-217 [DOI] [PubMed] [Google Scholar]
- 16). Zhu R, Liu X, He Z, Li Q: miR-146a and miR-196a2 polymorphisms in patients with ischemic stroke in the northern Chinese Han population. Neurochem res, 2014; 39: 1709-1716 [DOI] [PubMed] [Google Scholar]
- 17). Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, Laville E, Meish F, Milenkovic D, Tobin J, Charlier C, Georges M: A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet, 2006; 38: 813-818 [DOI] [PubMed] [Google Scholar]
- 18). Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A: Common SNP in premiR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. P Natl Acad Sci USA, 2008; 105: 7269-7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Luo X, Yang W, Ye DQ, Cui H, Zhang Y, Hirankarn N, Qian X, Tang Y, Lau YL, de Vries N, Tak PP, Tsao BP, Shen N: A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLos Genet, 2011; 7: e1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Shao Y, Li J, Cai Y, Xie Y, Ma G, Li Y, Chen Y, Liu G, Zhao B, Cui L, Li K: The functional polymorphisms of miR-146a are associated with susceptibility to severe sepsis in the Chinese population. Mediat Inflamm, 2014; 2014: 916202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Sakoguchi A, Jinnin M, Makino T, Kajihara I, Makino K, Honda N, Nakayama W, Inoue K, Fukushima S, Ihn H: The miR-146a rs2910164 C/G polymorphism is associated with telangiectasia in systemic sclerosis. Clin Exp Dermatol, 2013; 38: 99-100 [DOI] [PubMed] [Google Scholar]
- 22). Zende PD, Bankar MP, Kamble PS, Momin AA: Apolipoprotein e gene polymorphism and its effect on plasma lipids in arteriosclerosis. J Clin Diagn Res, 2013; 7: 2149-2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U, Danesh J: Association of apolipoprotein E genotypes with lipid levels and coronary risk. Jama, 2007; 298: 1300-1311 [DOI] [PubMed] [Google Scholar]
- 24). Vaisi-Raygani A, Rahimi Z, Nomani H, Tavilani H, Pourmotabbed T: The presence of apolipoprotein epsilon4 and epsilon2 alleles augments the risk of coronary artery disease in type 2 diabetic patients. Clin biochem, 2007; 40: 1150-1156 [DOI] [PubMed] [Google Scholar]
- 25). Anoop S, Misra A, Meena K, Luthra K: Apolipoprotein E polymorphism in cerebrovascular & coronary heart diseases. Indian med res, 2010; 132: 363-378 [PubMed] [Google Scholar]
- 26). Johnson LA, Arbones-Mainar JM, Fox RG, Pendse AA, Altenburg MK, Kim HS, Maeda N: Apolipoprotein E4 exaggerates diabetic dyslipidemia and atherosclerosis in mice lacking the LDL receptor. Diabetes, 2011; 60: 2285-2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24(1): 35-41 [DOI] [PubMed] [Google Scholar]
- 28). Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, Zhou W, Xiong B, Zeng Q: miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol cell biol, 2010; 88: 555-564 [DOI] [PubMed] [Google Scholar]
- 29). Taganov KD, Boldin MP, Chang KJ, Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. P NATL ACAD SCI USA, 2006; 103: 12481-12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Jurkin J, Schichl YM, Koeffel R, Bauer T, Richter S, Konradi S, Gesslbauer B, Strobl H: miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. J immunol, 2010; 184: 4955-4965 [DOI] [PubMed] [Google Scholar]
- 31). Dong S, Xiong W, Yuan J, Li J, Liu J, Xu X: MiRNA-146a regulates the maturation and differentiation of vascular smooth muscle cells by targeting NF-kappaB expression. Mol Med Rep, 2013; 8: 407-412 [DOI] [PubMed] [Google Scholar]
- 32). He Y, Yang J, Kong D, Lin J, Xu C, Ren H, Ouyang P, Ding Y, Wang K: Association of miR-146a rs2910164 polymorphism with cardio-cerebrovascular diseases: A systematic review and meta-analysis. Gene, 2015; 565: 171-179 [DOI] [PubMed] [Google Scholar]
- 33). Ramkaran P, Khan S, Phulukdaree A, Moodley D, Chuturgoon AA: miR-146a polymorphism influences levels of miR-146a, IRAK-1, and TRAF-6 in young patients with coronary artery disease. Cell biochem biophys, 2014; 68: 259-266 [DOI] [PubMed] [Google Scholar]
- 34). Gong W, Xiao D, Ming G, Yin J, Zhou H, Liu Z: Type 2 diabetes mellitus-related genetic polymorphisms in microRNAs and microRNA target sites. J diabetes, 2014; 6: 279-289 [DOI] [PubMed] [Google Scholar]
- 35). Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, Zeng Y, Miao R, Jin G, Ma H, Chen Y, Shen H: Genetic variants of miRNA sequences and non-small cell lung cancer survival. J clin invest, 2008; 118: 2600-2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Iekushi K, Seeger F, Assmus B, Zeiher AM, Dimmeler S: Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. CIRCULATION, 2012; 125: 1765-1773, S1761-1767 [DOI] [PubMed] [Google Scholar]
- 37). Cui L, Tao H, Wang Y, Liu Z, Xu Z, Zhou H, Cai Y, Yao L, Chen B, Liang W, Liu Y, Cheng W, Liu T, Ma G, Li Y, Zhao B, Li K: A functional polymorphism of the microRNA-146a gene is associated with susceptibility to drug-resistant epilepsy and seizures frequency. Seizure, 2015; 27: 60-65 [DOI] [PubMed] [Google Scholar]
- 38). Zhou Q, Hou S, Liang L, Li X, Tan X, Wei L, Lei B, Kijlstra A, Yang P: MicroRNA-146a and Ets-1 gene polymorphisms in ocular Behcet's disease and Vogt-Koyanagi-Harada syndrome. Ann rheum dis, 2014; 73: 170-176 [DOI] [PubMed] [Google Scholar]
- 39). Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE: MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO mol med, 2013; 5: 949-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Fish JE, Cybulsky MI: ApoE Attenuates Atherosclerosis via miR-146a. Circ res, 2015; 117: 3-6 [DOI] [PubMed] [Google Scholar]
- 41). Xiong XD, Cho M, Cai XP, Cheng J, Jing X, Cen JM, Liu X, Yang XL, Suh Y: A common variant in premiR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutat Res, 2014; 761: 15-20 [DOI] [PubMed] [Google Scholar]
- 42). Haffner SM: The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am j cardiol, 2006; 97: 3A-11A [DOI] [PubMed] [Google Scholar]
- 43). Mehanna ET, Ghattas MH, Mesbah NM, Saleh SM, Abo-Elmatty DM: Association of MicroRNA-146a rs2910164 Gene Polymorphism with Metabolic Syndrome. Folia biol-prague, 2015; 61: 43-48 [DOI] [PubMed] [Google Scholar]
- 44). Patties I, Haagen J, Dorr W, Hildebrandt G, Glasow A: Late inflammatory and thrombotic changes in irradiated hearts of C57BL/6 wild-type and atherosclerosisprone ApoE-deficient mice. Strahlenther Onkol, 2015; 191: 172-179 [DOI] [PubMed] [Google Scholar]
- 45). Pfeuffer M, Auinger A, Bley U, Kraus-Stojanowic I, Laue C, Winkler P, Rufer CE, Frank J, Bosch-Saadatmandi C, Rimbach G, Schrezenmeir J: Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr metab cardiovas, 2013; 23: 403-409 [DOI] [PubMed] [Google Scholar]
- 46). Yousuf FA, Iqbal MP: Review: Apolipoprotein E (Apo E) gene polymorphism and coronary heart disease in Asian populations. Pak j pharm sci, 2015; 28: 1439-1444 [PubMed] [Google Scholar]
- 47). Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L, Kalbfleisch JH, Gao X, Kao RL, Williams DL, Li C: Attenuation of Cardiac Dysfunction in Polymicrobial Sepsis by MicroRNA-146a Is Mediated via Targeting of IRAK1 and TRAF6 Expression. J Immunol, 2015; 195: 672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Saba R, Sorensen DL, Booth SA: MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front Immunol, 2014; 5: 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Sha M, Ye J, Zhang LX, Luan ZY, Chen YB: Celastrol induces apoptosis of gastric cancer cells by miR-146a inhibition of NF-kappaB activity. Cancer cell int, 2013; 13: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Nahid MA, Rivera M, Lucas A, Chan EK, Kesavalu L: Polymicrobial infection with periodontal pathogens specifically enhances microRNA miR-146a in ApoE-/- mice during experimental periodontal disease. Infect immu, 2011; 79: 1597-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]


