Abstract
Aim: Atherosclerosis (AS) characterized as a chronic inflammatory disease. Multiple immune cells and inflammatory cytokines, such as high mobility group protein (HMGB1), regulatory T (Treg) cells, T helper (Th17) cells, and inflammation-related cytokines, play a key role in its pathophysiology. A large number of studies report that HMGB1 and Th17 cells may promote atherosclerosis progression, whereas Treg cells may play a protective role in atherosclerosis; thus, alterations in the Treg/Th17 ratio may exist in atherosclerosis diseases. Up till now, the relationships between HMGB1 levels and the Treg/Th17 ratio remain incompletely understood. The major purpose of this study was to investigate the relationship between HMGB1 levels and the Treg/Th17 ratio in patients with coronary artery atherosclerotic plaques.
Methods: We enrolled patients with coronary atherosclerosis and normal coronary artery as the research subjects. Flow cytometry was used to analyze the Treg cells, the Th17 cells frequency, and the Treg/Th17 ratio. Otherwise, real-time polymerase chain reaction was used for assays the mRNA expressions of HMGB1, retinoic acid-related orphan nuclear receptor C (RORC), and forkhead-winged helix transcription factor (Foxp3). Moreover, enzyme-linked immunosorbent assays were used to detect the level of protein and cytokines, such as HMGB1, IL-10, TGF-β1, IL-17A, and IL-23.
Results: Using flow cytometry, we observed a significantly increased of Th17 cell frequency, whereas Treg cell frequency significantly decreased in atherosclerotic patients. Consistently, the levels of RORC mRNA were significantly increased in coronary atherosclerosis (AS) group compared to normal coronary artery (NCA) group (P < 0.01). In contrast, the expression of Foxp3 mRNA was markedly lower in the AS group than in the NCA group (P < 0.01). Furthermore, we observed the serum concentrations of HMGB1, IL-17A, and IL-23 were significantly higher in the AS group than in the NCA group (P < 0.01, respectively), whereas the concentrations of serum IL-10 and TGF-β1 were significantly lower in the AS group than in the NCA group (P < 0.01, respectively). In addition, we also found that HMGB1 levels showed negative correlation with the Treg/Th17 ratio in the two groups (r = −0.6984, P < 0.01).
Conclusions: The data in our study indicated that HMGB1 may promote atherosclerosis progression via modulating the imbalance in the Treg/Th17 ratio.
Keywords: AS, HMGB1, Treg cells, Th17 cells
Introduction
Atherosclerosis(AS) is a chronic inflammatory disease that involves the interaction of several leukocyte subsets and inflammatory cytokines, and it leads to several diseases, such as cardiovascular diseases and cerebrovascular diseases1, 2).
High mobility group box-1 protein (HMGB1), an evolutionarily conserved non-histone DNA-binding protein that functions as a DNA chaperone, is found in most cells and plays a vital role in numerous key DNA events, such as nucleosome stability and sliding, DNA replication and repair, gene transcription and so on3). HMGB1, derived from necrotic and damage cells (passively release) and activated monocytes/macrophages (actively secrete), is involved in the pathological progression of tumor metastasis and invasion, hepatitis B virus infection, atherosclerosis, restenosis of injured vasculature, and angiogenesis after myocardial infarction4–8).
Treg cells and Th17 cells belong to CD4+T cells subset, which can mediate immune responses, contribute to the development of atherosclerotic plaques9). Forkhead family protein 3 (Foxp3) and retinoic acid-related orphan receptor γt (RORγt) are the transcriptional factors of Treg cells and Th17 cells, respectively. Treg cells play a pivotal role in maintaining the immune tolerance and immune homeostasis, whereas Th17 cells repress the function of Treg cells and contribute to the inflammatory diseases.
Studies by other research groups as well as our own previous studies demonstrate that HMGB1 levels are increased in acute myocardial infarction and coronary artery stenosis7, 10). However, how HMGB1 affects atherosclerosis progression remains unclear. Recently, several studies show that Th17 cells and Treg cells can transform into each other during inflammatory environment and autoimmune diseases11, 12). Thus, we hypothesize that HMGB1 may regulate the atherosclerosis plaque formation via modulating Treg cells conversion to Th17 cells in vivo. The major purpose of this study is to investigate the relationship between HMGB1 levels and the Treg/Th17 ratio in atherosclerotic plaques.
Materials and Methods
Patient Population
Commonly, sites with low or oscillatory endothelial shear stress, located near branch points and along inner curvatures, are most susceptible, and the abdominal aorta, coronary arteries, iliofemoral arteries, and carotid bifurcations are typically affected the most1). In our study, we enrolled 66 patients (34 males and 32 females) derived from central people's hospital in Yichang, Hubei Province, China. All of them gave written consent informs to this study and all the experiments were approved by the research Ethics Committee of the central people's hospital in Yichang, Hubei Province, China. Thus, we enrolled the patients with coronary artery diseases diagnosed by coronary angiography as the research subjects. Their ages ranged from 39 to 71 years (mean age = 59.5 ± 8.7 years). Patients were classified into two groups: group 1: coronary atherosclerosis (AS), patients were diagnosed by coronary angiography and displayed one or more coronary arteries with at least 50% stenosis; group 2: normal coronary arteries (NCA), patients were diagnosed by coronary angiography and no vascular diseases were observed in them.
The exclusion criteria13, 14) were as follows: diabetes mellitus and other metabolic diseases; cardiovascular events < 1 year, such as a stroke or myocardial infarction; malignant diseases; renal failure; liver diseases; various chronic and acute infections; connective tissue diseases; surgery; treatment with anti-inflammatory drugs and/or immunosuppressive agents.
Blood Samples
We collected 5–10 mL of peripheral blood from the all participants after an overnight fast. Blood samples were treated with sodium heparin and examined within 4 h. The anti-coagulated blood is for flow cytometry and real-time polymerase chain reaction (qRT-PCR). Otherwise, serum obtained from 2 mL without anticoagulant for enzyme-linked immunosorbent assay (ELISA) stored at −80°C until use.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from 5 mL of sodium heparin-treated venous blood samples by Ficoll–Hypaque gradient centrifugation (1,800 rpm at room temperature for 20 min). Washed and resuspended at a density of 2 × 106 cells/mL in 1640 complete culture medium (RPMI 1640 supplemented with 100 U/mL streptomycin, 100 U/mL penicillin, 2 mM glutamine, and 10% heat-inactivated fetal calf serum) (Lot 31800-022, Gibco, America).
Cell Culture
For Th17 analysis, PBMCs were suspended at a density of 2 × 106 cells/mL in complete culture medium. The cell suspension was transferred to 12-well plates. Then, cell suspension were stimulated with PMA/Ionomycin mixture (Lot LK-CS1001, Liankebio, China) 4 µL/mL for 6 h in the presence of BFA/Monensin mixture (4 µL/mL, Lot LK-CS1002, Liankebio, China) in an incubator with 5% CO2 at 37°C. Then the contents were collected and transferred to 15-mL sterile tubes and washed twice in phosphate-buffered saline (PBS) and prepared for Th17 cells analysis.
Flow Cytometry
FITC-conjugated anti-human CD3 (Lot 11-0039-42), APC-conjugated anti-human CD8 (Lot 17-0088-42), PE-conjugated anti-human IL-17A (Lot 85-12-7178-42), FITC-conjugated anti-human CD4 (Lot 85-11-0047-42), PE-conjugated anti-human CD25 (Lot 85-12-0259-42) and PE-CY7-conjugated antihuman CD127 (Lot 85-25-1278-42) are all purchased from eBioscience, America. For the analysis of Th17, the cells were incubated with anti-human CD3-FITC and anti-human CD8-APC at 4°C for 30 min. After the surface staining, cells were fixed and permeabilized with Fix/Perm buffer (Lot: 88-8824, eBioscience, America) according to the manufacturer's instruction and stained with anti-human IL-17A-PE for intercellular staining. For Treg analysis, the cells were incubated with anti-human CD4-FITC, anti-human CD25-PE, and anti-human CD127-PE-CY7 at 4°C for 30 min. Samples were analyzed using an Accuri 6 BD flow cytometer.
qRT-PCR
The mRNA levels of HMGB1, FOXP3, and RORC were determined by qRT-PCR analysis. Total RNA was extracted from PBMCs with Trizol reagent (Takara) and converted into cDNA using a Prime-Script RT reagent kit (Lot 00171359, Sigma, America) according to the manufacturer's instructions. The mRNA expressions of HMGB1, Foxp3, and RORC were quantified using the SYBRPre mix ExTaq (Lot AK8306, Takara, Japan) on a Agilent SureCycler 8800 system (Agilent, American), with GAPDH expression as a control. Amplification was performed in a total volume of 25 µL for 40 cycles of 15 s at 95°C and 30 s at 60°C alter initial denaturation (95°C, 30 s). The primer sequences were as follows: GAPDH (purchased by Sangon Biotech, China, Lot PHS04); HMGB1 forward: AACCTATATCCCTCCCAAAG; HMGB1 r e ve r s e : ACATCTCTCCCAGTTTCTTC (NM_002128.4); Foxp3 forward: AACAGCACATTCCCAGAGTTCC; Foxp3 reverse: CATTGAGTGTCCGCTGCTTC (NM_014009.3); RORC forward: CCGAGGATGAGATTGCCCTCT; RORC reverse: GGTGGCAGCTTTGCCAGGAT (NM_005060.3). Samples were analyzed in triplicate; 2−ΔΔCt was used to calculate fold change of mRNA expression.
Measurement of Blood Biochemistry
The level of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and fasting plasma glucose (FPG) were measured by enzymatic methods. All of them were measured at a clinical laboratory.
Cytokines in Serum Determined by ELISA
Serum levels of TGF-β1, IL-10, IL-17A, IL-23, and HMGB1 were measured by commercially available ELISA kits according to the manufacturer's instruction. All samples were assessed in triplicate. The human TGF-β1 (Lot EHC107b.96), IL-10 (Lot EHC009.96), IL-17A (Lot EHC170.96), and IL-23 (Lot EHC171.96) ELISA kits were purchased from NeoBioscience China, whereas the HMGB1 ELISA kit was purchased from Westang, China (Lot F01020). The data were recorded at 450 nm in a microplate reader (Thermo, Finland). The sensitivity levels for TGF-β1, IL-10, IL-17A, IL-23, and HMGB1 were 15 pg/mL, 1 pg/mL, 8 pg/mL, 15 pg/mL, and 0.3 ng/mL, respectively.
Statistical Analysis
All data analyses were performed by SPSS statistical software (version 13). Data for continuous variables are presented as the mean ± standard deviation (SD). Group comparison was conducted with Student's t test. The correlation between serum HMGB1 and the Treg/Th17 ratio was described by Pearson correlation coefficients. A two-tailed p-value of < 0.05 was considered to be the significance level.
Results
The Characteristics of Participants
There were no significant differences in age, gender, risk factors, and blood biochemical parameters between patients in the NCA and AS) groups (Table 1).
Table 1. Clinical characteristics of the groups.
| Characteristics | NCA (n = 33) | AS (n = 33) |
|---|---|---|
| Gender (Male/Female) | 16/17 | 19/14 |
| Age, mean ± SD years | 61.4 ± 9.5 | 58.6 ± 7.8 |
| Hypertension, n (%) | 11 (33.3%) | 19 (57.6%) |
| FPG (mmol/L) | 5.3 ± 0.5 | 5.2 ± 0.5 |
| Serum Cr (umol/L) | 71.7 ± 15.4 | 75.0 ± 13.3 |
| TC (mmol/L) | 4.0 ± 1.0 | 4.0 ± 0.9 |
| TG (mmol/L) | 1.4 ± 0.8 | 1.2 ± 0.4 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 1.5 ± 0.2 |
| LDL-C (mmol/L) | 2.0 ± 0.6 | 2.0 ± 0.7 |
Values are expressed as mean ± SD. NCA: normal coronary arteries; AS: coronary atherosclerosis; FPG: fasting plasma glucose; Cr: creatinine; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein-cholesterol; LDL-C: low-density lipoprotein-cholesterol.
Increased Th17 Frequency and Decreased Treg Frequency in PBMC of Coronary Atherosclerosis Patients
As shown in Fig. 1, the frequencies of Th17 (CD3+CD8−IL17+/CD3+CD8− T cells) was significantly higher in AS group (1.6% ± 0.4%) than in the normal coronary arteries (NCA) group (0.9% ± 0.2%) (P < 0.01). The frequency of Treg cells (CD4+CD25+ CD127−/CD4+T cells) was markedly decreased in the AS group (4.3% ± 0.7%) compared to the NCA group (6.2% ± 0.8%) (P < 0.01). As shown in Fig. 1, we found that the Treg to Th17 ratio cells was lower in the AS group (2.8 ± 1.0) than in the NCA group (7.1 ± 1.9). Therefore, the Treg/Th17 ratio was significantly decreased in patients in the AS group as than in those in the NAC group (P < 0.01).
Fig. 1.
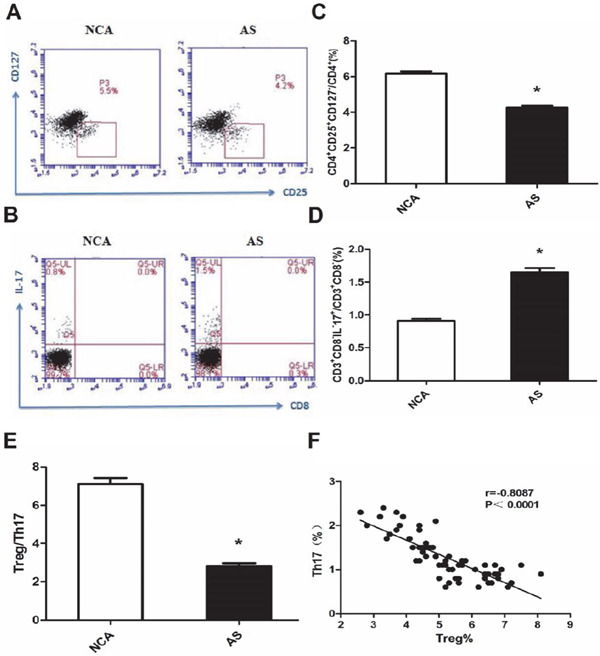
Imbalanced frequencies of Th17 and Treg cells in patients with coronary atherosclerosis (AS). (A) Representative flow cytometric (FCM) dot plots of CD4+CD25+ CD127−Treg cells staining; (B) Representative FCM dot plots of CD3+CD8−IL-17+ Th17 cell quantification; (C) A summary of the percentages of CD4+CD25+CD127− Treg cells in AS patients is shown; p < 0.01 compared to normal coronary arteries (NCA) (NCA, n = 33; AS, n = 33); (D) A summary of the percentage of CD3+CD8− IL-17+T cells in AS patients is shown; p < 0.01 compared to NCA patients (NCA, n = 33; AS, n = 33); (E) The ratio of Treg to Th17 cells was significantly decreased in AS patients; (F) Increased frequencies of Th17 cells in all patients were inversely correlated with the percentages of Treg cells. Pearson's correlation coefficient (normal distributed data) was used to assess interrelationships; *: P < 0.01 is considered statistically significant.
The Expression of HMGB1, RORC, and Foxp3 mRNA in PBMC
HMGB1 is the important inflammatory cytokine in atherosclerotic diseases. RORC and Foxp3 are the specific transcription factors of Th17 and Treg cells, respectively. We thus measured the expressions of HMGB1, RORC, and Foxp3 mRNA in PBMCs from the all participants. As shown in Fig. 2, the levels of HMGB1 and RORC mRNA were significantly increased in the AS group than in the NCA group (both P < 0.01). In contrast, the expression of Foxp3 mRNA was markedly lower in the AS group than in the NCA group (P < 0.01).
Fig. 2.
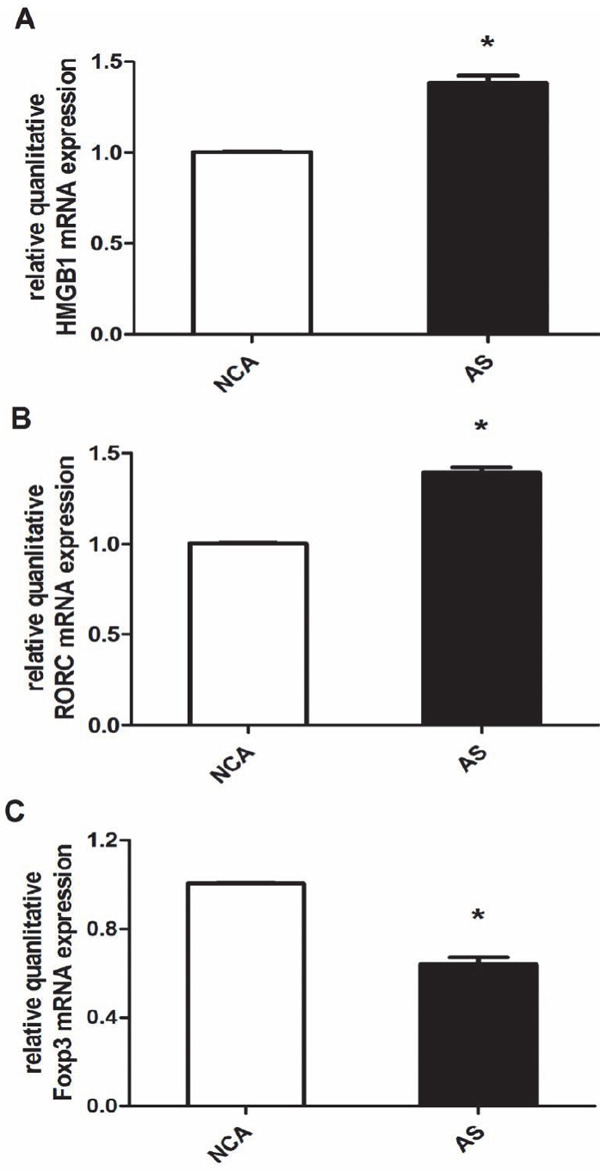
The expression of HMGB1, RORC, and Foxp3 mRNA in PBMC. (A) The relative quanlitative HMGB1 mRNA expression were compared between two groups [coronary atherosclerosis (AS); normal coronary arteries (NCA)]; (B) The relative quanlitative RORC mRNA expressions were compared between the two groups; (C) The relative quanlitative Foxp3 mRNA expressions were compared between the two groups; *P < 0.01 is considered statistically significant.
The Level of Cytokines in the Serum from Patients
Serum levels of TGF-β1, IL-10, IL-17A, IL-23, and HMGB1 were detected in the all participants by means of ELISA tests (Fig. 3). The HMGB1, IL-17A, and IL-23 concentrations in the AS group (HMGB1: 5.45 ± 1.36 ng/mL; IL-17A: 50.78 ± 8.52 pg/mL; IL-23: 25.52 ± 3.93 pg/mL) were significantly higher than those in in the NCA group (HMGB1: 2.33 ± 0.66 ng/mL; IL-17A: 16.45 ± 3.00 pg/mL; IL-23: 14.85 ± 1.74 pg/mL; P < 0.01), whereas serum IL-10 and TGF-β1 concentrations in the AS group (IL-10: 1.12 ± 0.06 pg/mL; TGF-β1: 3013.78 ± 567.54 pg/mL) were significantly lower than those in the NCA group (IL-10: 1.48 ± 0.17 pg/mL; TGF-β1: 6678.04 ± 1178.09 pg/mL; P % 0.01).
Fig. 3.
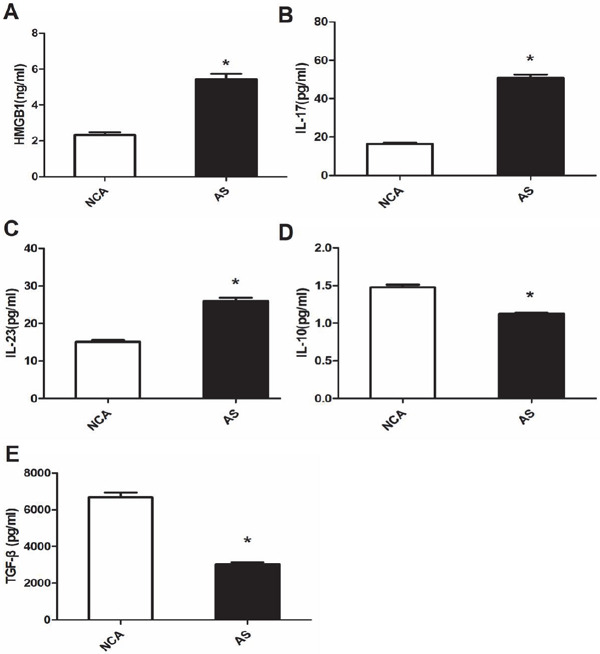
The levels of serum HMGB1, IL-17A, IL-23, IL-10, and TGF-β1 in coronary atherosclerosis (AS) patients. (A) Serum HMGB1 level significantly increased in the AS group compared to the normal coronary arteries (NCA) group (P < 0.01); (B) Serum IL-17A level significantly increased in the AS group compared to the NCA group (P < 0.01); (C) Serum IL-23 level significantly increased in the AS group than in the NCA group (P < 0.01); (D) Serum IL-10 level significantly decreased in the AS group compared to the NCA group (P < 0.01); (E) Serum TGF-β1 level significantly decreased in the AS group compared to the NCA group (P < 0.01); *: P < 0.01 is considered statistically significant.
Correlations between HMGB1 and the Treg/Th17 Ratio and Their Related Cytokines
As shown in Fig. 4, HMGB1 concentration showed negative correlation with Treg cells in the two groups (r = −0.6362, P < 0.01, Fig. 4A) and positive correlation with the frequencies of Th17 cells (r = 0.6057, P < 0.01, Fig. 4B). Consistently, HMGB1 concentrations showed negative correlation with the Treg/Th17 ratio in the two groups (r = −0.6984, P < 0.01, Fig. 4C).
Fig. 4.
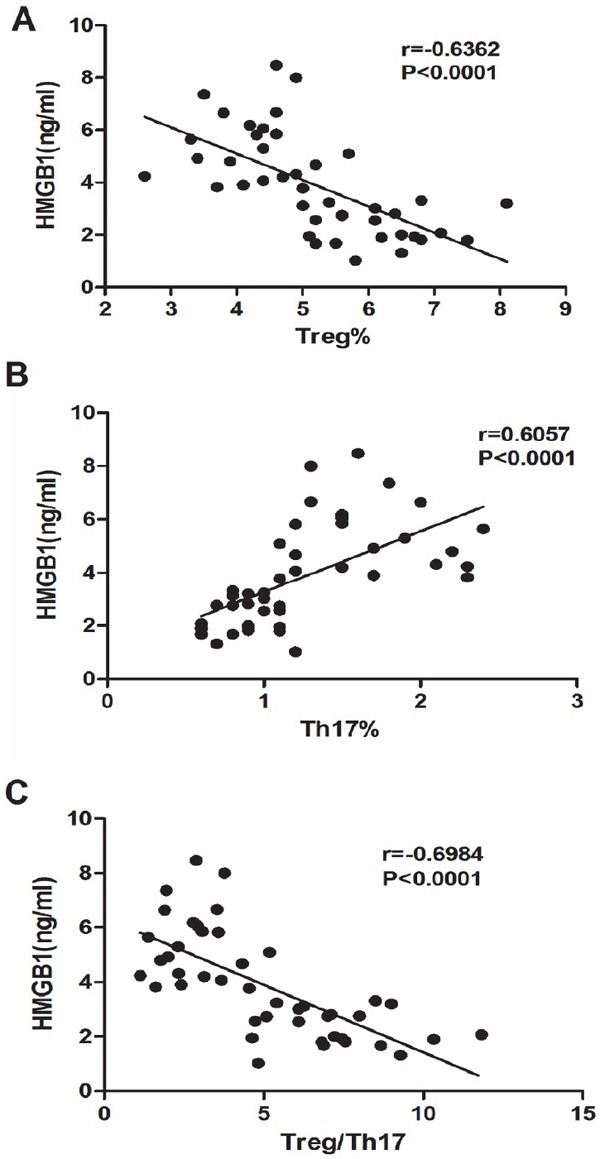
Spearman correlation plot of serum HMGB1 levels and Treg frequency, Th17 frequency and the Treg/Th17 ratio. (A) HMGB1 concentration negatively correlated with Treg frequency (r = −0.6362, P < 0.0001); (B) HMGB1 concentration positively correlates with Th17 frequency (r = 6057, P < 0.0001); (C) HMGB1 concentration negatively correlated with Treg frequency (r = −0.6984, P < 0.0001).
Discussion
The present data demonstrate that AS patients exhibited significantly increasing serum level of HMGB1, peripheral Th17 frequency, Th17-related cytokines (IL-17 and IL-10), and transcription factor (RORC) levels; in addition, AS patients also showed dramatically decreases in the Treg frequency, Tregrelated cytokine (TGF-β1 and IL-10) and transcription factor (Foxp3) levels than the NCA group. Otherwise, the data also provides that the balance between circulating Treg/Th17 cells is impaired in these patients. More importantly, the level of serum HMGB1 negatively correlated with the Treg/Th17 ratio. These results suggest that HMGB1 may promote the process of atherosclerosis by disturbing the Treg/Th17 ratio, which may be the new pathogenesis of atherosclerosis and new target of atherosclerosis treatment.
HMGB1 is found in most cells and plays a vital role in numerous key DNA events, such as nucleosome stability and sliding, DNA replication and repair, and gene transcription3). In addition to the nuclear role, cytoplasmic HMGB1 released by activated immune and non-immune cells, functions as a later inflammatory mediator. In addition, it functions as a pro-inflammatory molecule in several diseases, such as diabetic cardiomyopathy, various liver diseases, active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV), and cardiovascular diseases and so on6, 15–17). Atherosclerosis, a disease of the large/medium arteries, is a chronic inflammatory disease involved in endothelial cells (ECs), vascular smooth muscle cells (VSMCs), monocytes/macrophage, platelets, and other molecules18). HMGB1 mediates chronic inflammatory responses in the above mentioned cells, and it plays a vital role in atherosclerosis progression19–21). In this study, our data confirmed that the level of HMGB1 mRNA and the expression of HMGB1 increased in AS patients, in comparison with the NCA patients. Our results are consistent with the conclusion of other investigators22, 23). Hence, HMGB1 may promote atherosclerosis progression although the mechanisms remain unclear.
In human beings, interleukin 6 (IL-6) and transforming growth factor-β (TGF-β) have been shown to promote the differentiation of Th17 cells, IL-23 is reported to maintain the development of Th17, whereas CD4+ T cells have been shown to be transformed to Treg cells in the presence of TGF-β24). Th17 cells function as pro-inflammatory cells via the production of IL-17, tumor necrosis factor (TNFα), and IL-625). On the other hand, Treg cells play the anti-inflammatory role by achieving direct contact with cells or via releasing anti-inflammatory cytokines, such as IL-10 and TGF-β25). Several studies have demonstrated that Treg cells and Th17 cells are involved in atherosclerosis progression26, 27). In our study, we found that the level of Treg cells frequency decreased significantly in AS patients compared to NCA patients, whereas the frequency of Th17 cells increased in AS patients compared to NCA patients. On the other hand, the Treg/Th17 ratio decreased in the AS patients than in the NCA patients. Moreover, RORC and Foxp3 are the transcription factors of Th17 cells and Treg cells respectively. In our study, the level of RORC mRNA was upregulated but the level of Foxp3 mRNA was downregulated in the AS patients. Consistently, Th17-cell-related cytokine, such as IL-17, increased in AS patients, whereas Treg-cell–related cytokines, such as IL-10 and TGF-β, decreased in AS patients. The results we observed in this study were consistent with those of many other studies13, 27, 28). It was suggested that Treg cells and Th17 cells play a vital role in the development of atherosclerosis, and the balance between Treg cells and Th17 cells was disturbed in patients AS patients. The underlying mechanism needs to be further investigated in the future.
Recently, a great deal of evidence shows that HMGB1, via regulating the balance between Treg and Th17 cells, may modulate the progression of several diseases, such as experimental autoimmune myocarditis, rheumatoid arthritis, and chronic hepatitis B29–31). It is suggested that HMGB1 may promote the disequilibrium between Treg and Th17 cells in these diseases. Consistently, we found that serum HMGB1 levels were positively correlated to Th17 frequency and RORC mRNA levels, whereas they were correlated to Treg frequency and Foxp3 mRNA levels in AS patients. Moreover, serum HMGB1 levels were also negatively correlated to the Treg/Th17 ratio. On the basis of our results, we can conclude that HMGB1 may promote atherosclerosis progression by modulating the imbalance between Treg and Th17 cells. Recently, we find that several researchers have clarified the related underlying mechanisms among other diseases. Li31) et.al has found that in patients with chronic hepatitis B, HMGB1 may promote Th17 differentiation via the TLR4-IL-6 pathway. Meanwhile, He30) et.al has found that HMGB1 facilitates the differentiation of Th17 by enhancing the TLR2 pathway in patients with rheumatoid arthritis. In addition, Zhu32) et al has found that HMGB1 modulates the suppressive capacity of CD4+CD25+Tregs via the TLR4 pathway. However, the mechanism as to how HMGB1 affects the differentiation of Th17 in atherosclerosis diseases remains unclear; this needs to be more investigated in the future.
Although we observed relationships between serum HMGB1 levels and the Treg/Th17 ratio balance in atherosclerotic diseases, the underlying mechanism as to how HMGB1 modulates the differentiation and quantity of Treg cells and Th17 cells needs to be further investigated. We'll focus on the study of mechanism to find new targets for the diagnosis and treatment of atherosclerotic diseases.
Acknowledgments
This work was supported by the Natural Science Foundation of Hubei Province, China (2014CFC1035), and the National Natural Science Foundation of China (81400794).
Conflict of Interest
The authors have no financial conflicts of interest.
References
- 1). Bentzon JF, Otsuka F, Virmani R, Falk E: Mechanisms of plaque formation and rupture. Circ Res, 2014; 114: 1852-1866 [DOI] [PubMed] [Google Scholar]
- 2). Garrido-Urbani S, Meguenani M, Montecucco F, Imhof BA: Immunological aspects of atherosclerosis. Semin Immunopathol, 2014; 36: 73-91 [DOI] [PubMed] [Google Scholar]
- 3). Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, Sun X, Wang H, Wang Q, Tsung A, Billiar TR, Zeh HJ, 3rd, Lotze MT, Tang D: HMGB1 in health and disease. Mol Aspects Med, 2014; 40: 1-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu X, Zheng M: HMGB1 promotes cellular proliferation and invasion, suppresses cellular apoptosis in osteosarcoma. Tumour Biol, 2014; 35: 12265-12274 [DOI] [PubMed] [Google Scholar]
- 5). Deng CQ, Deng GH, Wang YM: HMGB1 gene polymorphisms in patients with chronic hepatitis B virus infection. World J Gastroenterol, 2013; 19: 5144-5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Andrassy M, Volz HC, Maack B, Schuessler A, Gitsioudis G, Hofmann N, Laohachewin D, Wienbrandt AR, Kaya Z, Bierhaus A, Giannitsis E, Katus HA, Korosoglou G: HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PLoS One, 2012; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z, Cui B, Wen H: Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin Chim Acta, 2009; 406: 139-142 [DOI] [PubMed] [Google Scholar]
- 8). Nakamura Y, Suzuki S, Shimizu T, Miyata M, Shishido T, Ikeda K, Saitoh S, Kubota I, Takeishi Y: High Mobility Group Box 1 Promotes Angiogenesis from Bone Marrow-derived Endothelial Progenitor Cells after Myocardial Infarction. J Atheroscler Thromb, 2015; 22: 570-581 [DOI] [PubMed] [Google Scholar]
- 9). Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L: A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol, 2010; 185: 5820-5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Ding HS, Yang J, Chen P, Bo SQ, Ding JW, Yu QQ: The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene, 2013; 527: 389-393 [DOI] [PubMed] [Google Scholar]
- 11). Singh K, Gatzka M, Peters T, Borkner L, Hainzl A, Wang H, Sindrilaru A, Scharffetter-Kochanek K: Reduced CD18 levels drive regulatory T cell conversion into Th17 cells in the CD18hypo PL/J mouse model of psoriasis. J Immunol, 2013; 190: 2544-2553 [DOI] [PubMed] [Google Scholar]
- 12). Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH: Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood, 2009; 113: 6102-6111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Li Q, Wang Y, Yu F, Wang YM, Zhang C, Hu C, Wu Z, Xu X, Hu S: Peripheral Th17/Treg imbalance in patients with atherosclerotic cerebral infarction. Int J Clin Exp Pathol, 2013; 6: 1015-1027 [PMC free article] [PubMed] [Google Scholar]
- 14). Li Q, Wang Y, Zhou Q, Chen K, Wang YM, Wei W, Wang Y: Distinct different sensitivity of Treg and Th17 cells to Fas-mediated apoptosis signaling in patients with acute coronary syndrome. Int J Clin Exp Pathol, 2013; 6: 297-307 [PMC free article] [PubMed] [Google Scholar]
- 15). Wang WK, Wang B, Lu QH, Zhang W, Qin WD, Liu XJ, Liu XQ, An FS, Zhang Y, Zhang MX: Inhibition of high-mobility group box 1 improves myocardial fibrosis and dysfunction in diabetic cardiomyopathy. Int J Cardiol, 2014; 172: 202-212 [DOI] [PubMed] [Google Scholar]
- 16). Chen R, Hou W, Zhang Q, Kang R, Fan XG, Tang D: Emerging role of high-mobility group box 1 (HMGB1) in liver diseases. Mol Med, 2013; 19: 357-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Wang C, Gou SJ, Chang DY, Yu F, Zhao MH, Chen M: Association of circulating level of high mobility group box 1 with disease activity in antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Care Res (Hoboken), 2013; 65: 1828-1834 [DOI] [PubMed] [Google Scholar]
- 18). Lusis AJ: Atherosclerosis. NATURE, 2000; 407: 233-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Luo Y, Li SJ, Yang J, Qiu YZ, Chen FP: HMGB1 induces an inflammatory response in endothelial cells via the RAGE-dependent endoplasmic reticulum stress pathway. Biochem Biophys Res Commun, 2013; 438: 732-738 [DOI] [PubMed] [Google Scholar]
- 20). Yang J, Chen L, Ding J, Rong H, Dong W, Li X: High mobility group box-1 induces migration of vascular smooth muscle cells via TLR4-dependent PI3K/Akt pathway activation. Mol Biol Rep, 2012; 39: 3361-3367 [DOI] [PubMed] [Google Scholar]
- 21). Chen J, Zhang J, Xu L, Xu C, Chen S, Yang J, Jiang H: Inhibition of neointimal hyperplasia in the rat carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis, 2012; 224: 332-339 [DOI] [PubMed] [Google Scholar]
- 22). Andrassy M, Volz HC, Maack B, Schuessler A, Gitsioudis G, Hofmann N, Laohachewin D, Wienbrandt AR, Kaya Z, Bierhaus A, Giannitsis E, Katus HA, Korosoglou G: HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PLoS One, 2012; 7: e52081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Zhao D, Wang Y, Tang K, Xu Y: Increased serum HMGB1 related with HbA1c in coronary artery disease with type 2 diabetes mellitus. Int J Cardiol, 2013; 168: 1559-1560 [DOI] [PubMed] [Google Scholar]
- 24). Dong C: Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect, 2009; 11: 584-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Olson NC, Sallam R, Doyle MF, Tracy RP, Huber SA: Thelper cell polarization in healthy people: implications for cardiovascular disease. J Cardiovasc Transl Res, 2013; 6: 772-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Sasaki N, Yamashita T, Takeda M, Hirata K: Regulatory T cells in atherogenesis. J Atheroscler Thromb, 2012; 19: 503-515 [DOI] [PubMed] [Google Scholar]
- 27). Li Q, Wang Y, Chen K, Zhou Q, Wei W: Treg/Th17 ratio acts as a novel indicator for acute coronary syndrome. Cell Biochem Biophys, 2014; 70: 1489-1498 [DOI] [PubMed] [Google Scholar]
- 28). Ma Y, Yuan X, Deng L, Xu W, Zheng Y, Yue C, Zhang G, Xie F, Yang YH, Gantier MP, Liu J, Xu D, Shen L: Imbalanced frequencies of Th17 and Treg cells in acute coronary syndromes are mediated by IL-6-STAT3 signaling. PLoS One, 2013; 8: e72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Su Z, Sun C, Zhou C, Liu Y, Zhu H, Sandoghchian S, Zheng D, Peng T, Zhang Y, Jiao Z, Wang S, Xu H: HMGB1 blockade attenuates experimental autoimmune myocarditis and suppresses Th17-cell expansion. Eur J Immunol, 2011; 41: 3586-3595 [DOI] [PubMed] [Google Scholar]
- 30). He Z, Shotorbani SS, Jiao Z, Su Z, Tong J, Liu Y, Shen P, Ma J, Gao J, Wang T, Xia S, Shao Q, Wang S, Xu H: HMGB1 promotes the differentiation of Th17 via upregulating TLR2 and IL-23 of CD14+ monocytes from patients with rheumatoid arthritis. Scand J Immunol, 2012; 76: 483-490 [DOI] [PubMed] [Google Scholar]
- 31). Li J, Wang FP, She WM, Yang CQ, Li L, Tu CT, Wang JY, Jiang W: Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat, 2014; 21: 129-140 [DOI] [PubMed] [Google Scholar]
- 32). Zhu XM, Yao YM, Liang HP, Xu CT, Dong N, Yu Y, Sheng ZY: High mobility group box-1 protein regulate immunosuppression of regulatory T cells through toll-like receptor 4. Cytokine, 2011; 54: 296-304 [DOI] [PubMed] [Google Scholar]


