Abstract
Aim: Asymptomatic visceral artery aneurysms (VAAs) have increasingly been found, with most being either atherosclerotic VAAs or fibromuscular dysplasia (FMD)-associated VAAs. However, little is known about the pathogenesis of both diseases. We aimed to identify the differences in the distribution pattern of lipid molecules between atherosclerotic VAAs and FMD-associated VAAs.
Methods: We conducted a histological study of VAAs using imaging mass spectrometry (IMS) to assess the accumulation of lipid molecules in both the aneurysmal sac and the adjacent arteries without aneurysmal changes in 17 VAA samples, which were resected during the surgery.
Results: IMS revealed characteristic distributions of cholesterol ester in intima and media in the atherosclerotic VAAs, which was hardly detected in FMD-associated VAAs. However, lysophosphatidylcholine (lysoPC), a proinflammatory and proapoptotic lipid mediator, was accumulated in the medial ridge of the adventitia of FMD-associated in the aneurysmal sac, and it was also diffusely accumulated in the adjacent arteries. In contrast, lysoPC was accumulated in the area of intimal hyperplasia in atherosclerotic VAAs and the adjacent arteries.
Conclusion: The distribution patterns of lipid molecules were different between the FMD-associated and atherosclerotic VAAs. The diffuse accumulation of lysoPCs in the visceral arteries may be a predisposition for the formation of FMD-associated VAAs.
Keywords: Visceral artery aneurysm, Fibromuscular dysplasia, Lysophosphatidylcholine, Atherosclerosis, Imaging mass spectrometry
See editorial vol. 23: 665–667
Introduction
Recently, asymptomatic visceral artery aneurysms (VAAs) have been increasingly detected incidentally because of the frequent use of computed tomography (CT). This is clinically important because of the high incidence of rupture and life-threatening hemorrhage, with mortality rates ranging from 20% to 75% depending on the location of the aneurysm1, 2). However, the pathogenesis remains to be elucidated. Among VAAs, atherosclerotic VAAs and fibromuscular dysplasia (FMD)-associated VAAs are the two major causes of VAAs2). Clinically, it is difficult to differentiate between the two types of VAAs because their morphological features are similar in preoperative imaging modalities. Lately, an emerging technique called matrix-assisted desorption/ionization imaging mass spectrometry (MALDI-IMS), which can clarify the distribution of lipid molecules at the molecular species level in each layer of arteries, demonstrated regional distribution of lipid molecules, such as cholesterol ester and lysophosphatidylcholime (lysoPC), in atherosclerotic arteries, suggesting that these molecules may play important roles in atherogenesis3, 4). Using this technique, we investigated the accumulation and distribution patterns of the lipid molecules in the atherosclerotic and FMA-associated VAAs and determined the differences in the accumulation patterns between the two types of VAAs.
Methods
This study was performed in accordance with the declaration of Helsinki.
Chemicals and Reagents
2,5-Dihydroxybenzoic acid (DHB) was purchased from Bruker Daltonics (Bremen, Germany). All chemicals used in this study were of the highest purity available.
Sample Collection
We included 17 patients who had undergone resection of their VAAs and revascularization surgery between April 2007 and April 2014 at the Division of Vascular Surgery, Hamamatsu University School of Medicine. All the patients provided informed consent (Table 1), and the study protocol was approved by the university's Ethics Committee of Clinical Research.
Table 1. Demographic and clinical data for patients with visceral artery aneurysm.
| Atherosclerotic | Fibromuscular dysplasia | |
|---|---|---|
| Sex (n) (male/female) | 6/6 | 1/4 |
| Age | 63.3 ± 11.3 | 57.8 ± 12.0 |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Weight (kg) | 55.1 ± 8.4 | 62.8 ± 10.9 |
| BMI (kg/m2) | 21.6 ± 1.6 | 22.8 ± 2.8 |
| Serum TC (mg/dl) | 185.4 ± 27.8 | 190.8 ± 45.3 |
| Serum TG (mg/dl) | 118.0 ± 64.1 | 114.8 ± 57.1 |
| CRP (mg/dl) | 0.30 ± 0.53 | 0.13 ± 0.08 |
| HbA1c (%) | 6.17 ± 1.28 | 5.76 ± 1.23 |
| Maximum diameter (mm) of VAA | 24 ± 0.6 | 22 ± 0.7 |
| Hypertension (n) | 6 | 5 |
| Ever smoked (n) | 8 | 3 |
| Aneurysm localization | ||
| Hepatic Artery | 3 | 1 |
| Renal Artery | 5 | 1 |
| Splenic Artery | 1 | 0 |
| Gastroduodenal Artery | 0 | 3 |
| Pancreaticoduodenal Artery | 3 | 0 |
Values are mean ± SD unless stated otherwise.
Normal ranges: TC 160–220 mg/dl; TG 80–150 mg/dl; HbA1c 4.3–5.8%; CRP ≦0.1 mg/dl. BMI, body mass index; TC, total cholesterol, TG, triglyceride.
The VAA sac tissue and adjacent arteries were intraoperatively obtained from patients who underwent resection of the aneurysms and revascularization (Fig. 1A). The VAAs were diagnosed, and the indication for surgery was determined according to the CT images (Fig. 1B). For control samples, we used the right gastroepiploic arteries, which were obtained from three patients who underwent gastrectomy due to gastric cancer.
Fig. 1.
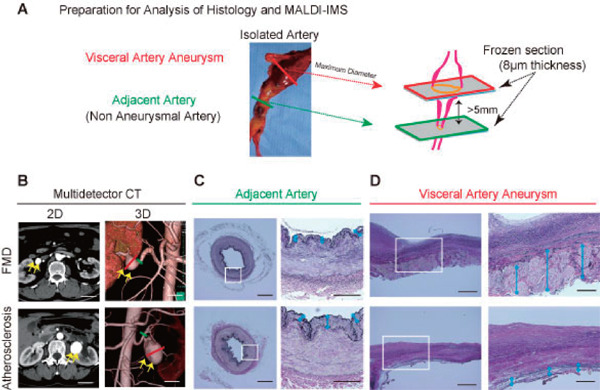
(A) Preparation for histological analysis and matrix-assisted desorption/ionization imaging mass spectrometry (MALDI-IMS). The arterial tissue was obtained from a patient who underwent aneurysmal resection and revascularization. We analyzed both the aneurysmal sac and adjacent artery, which is 5 mm from the aneurysm. (B) Computed Tomography (CT) revealed visceral artery aneurysms (VAAs), which cannot be distinguished from the fibromuscular dysplasia (FMD)-associated VAAs and atherosclerotic-VAAs. Two-dimensional (2D) (left) image and three-dimensional (3D) images of CT angiography (right). The yellow arrows indicate the aneurysm. Scale bar = 30 mm. (C)–(D). Elastica van Gieson staining of the adjacent artery (C) and VAA sac (D). Scale bar = 400 µm in each left panel. The area in the white box of each left panel is magnified in the corresponding panel on the right. Scale bar = 200 µm in each right panel.
Histopathological Analysis of VAAs and Adjacent Arteries
Aneurysmal wall sections were consistent with maximum diameter in VAAs (Fig. 1A, red color). We defined arterial wall from a distance of at least 5 mm at the aneurysm edge as adjacent artery, which was not dilated (Fig. 1A, green color). Comparison of histopathological changes was examined using these cross sections.
MALDI-IMS
The samples were prepared as previously described3). MALDI-IMS was performed using a time-of-flight type instrument (Ultraflex II; Bruker Daltonics Inc., Billerica, MA, USA). Data were acquired using a step size of 100 or 10 µm in the positive ion mode. A total of 500 µL of dihydroxybenzoic acid solution in methanol/water (7/3, v/v) was used as the matrix. Specific fragment patterns of phosphatidylcholines (PCs), cholesterol esters (CEs), and lysophosphatidylcholine were annotated according to previous reports3, 5). The accumulation of each molecule was compared as a ratio of the molecule's signal intensity divided by that of PC(16:0/18:1), which was therefore detected ubiquitously and used as the internal standard molecule.
Statistical Analysis
The data were analyzed using StatView software (version 5.0; SAS Institute, Cary, NC, USA). All the data are expressed as mean ± standard of error. Statistical analysis was performed using analysis of variance for comparison among the three groups (intima, media, and advent). Post-hoc comparison was performed using the Tukey–Kramer test.
Results
Preparation for Histological Analysis and MALDI-IMS
Among 17 cases of VAAs, five were FMD-associated VAAs and the other 12 were atherosclerotic VAAs (Table 1).
Fig. 1B shows the representative multidetector CT images for FMD-associated and atherosclerotic VAAs, which cannot be distinguished from one another using the preoperative three-dimensional images (Fig. 1B). Elastica van Gieson (EVG) staining showed that the FMD-associated VAA had thinned media and thickened collagen-containing medial ridges in the adventitia and periarterial tissue. (Fig. 1D, blue arrows) All the cases in the FMD-associated VAA exhibited similar findings. Alternatively, the atherosclerotic VAAs had thick intimal hyperplasia and thin medial degeneration, but adventitia were preserved (Fig. 1D, blue arrows).
Regarding the adjacent arteries of VAAs, which are non-aneurysmal arteries, the adjacent arteries of the FMD-associated VAAs appeared to be normal in EVG or hematoxylin and eosin (data not shown) staining. However, the adjacent arteries of atherosclerotic VAAs showed intimal hyperplasia (Fig. 1C, blue arrows).
Analysis of the VAAs Using MALDI-IMS
MALDI-IMS revealed the distributions of the lipid molecules, including phosphatidylcholine (PC) (16:0/18:1), CE(18:1), and lysoPC(1-acyl 16:0), in each VAA (Fig. 2A). CE, which is an atherogenic molecule, was accumulated in the intima and media of the atherosclerotic VAAs; conversely, it was hardly detected in the FMD associated-VAAs (Fig. 2A and B). However, lysoPC was markedly accumulated in the medial ridges of the adventitia in the FMD associated-VAAs, which was also detected in the intima and media of the atherosclerotic VAAs (Fig. 2A and C).
Fig. 2.
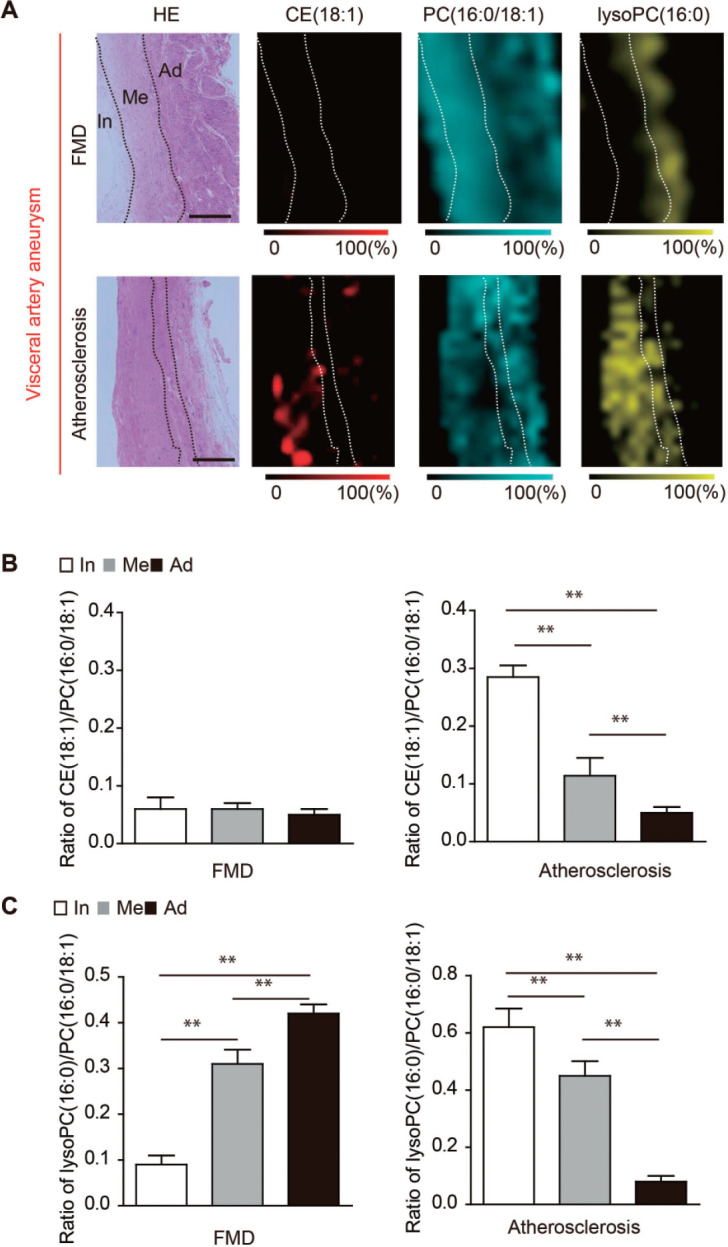
Analysis of the visceral artery aneurysm (VAA) using matrix-assisted desorption/ionization imaging mass spectrometry (MALDI-IMS).
(A) The distributions of choleterol ester (CE) (18:1), phosphatidylcholine (PC) (16:0/18:1), and lysophosphatidylcholine (lysoPC) (1-acyl16:0) in fibromuscular dysplasia (FMD)-associated VAA and atherosclerotic VAA are shown. Scale bar = 100 µm. HE, hematoxylin-eosin staining; In, intima; Me, media; Ad, adventitia (B) The ratios of CE (18:1) to PC (16:0/18:1) in the intima, media, and adventitia in FMD-associated VAAs and atherosclerotic VAAs. In, intima; Me, media; Ad, adventitia. **P < 0.01 indicates a statistically significant difference. (C) The ratios of lysoPC (1-acyl 16:0) to PC (16:0/18:1) in the intima, media, and adventitia in FMD-associated VAAs and atherosclerotic VAAs. In, intima; Me, media; Ad, adventitia. **P < 0.01 indicates a statistically significant difference.
Analysis of the Adjacent Arteries of VAAs Using MALDI-IMS
Regarding the adjacent arteries of VAAs, lysoPC was diffusely accumulated in the intima, media, and adventitia of FMD associated-VAAs group (Fig. 3A and C); alternatively, lysoPC was only observed in the intimal hyperplasia of atherosclerotic VAAs group. In control arteries, neither CE nor lysoPC was observed (Fig. 3A–C).
Fig. 3.
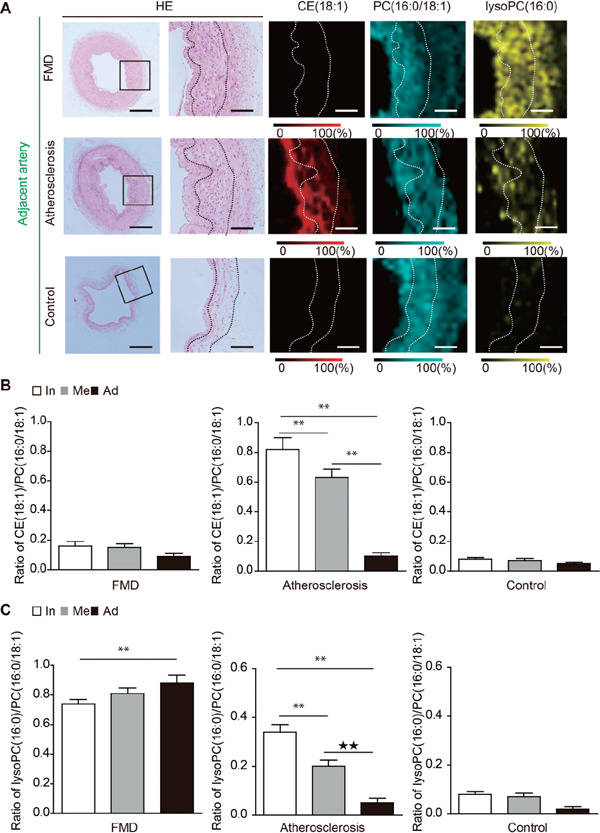
Analysis of the adjacent arteries of visceral artery aneurysm (VAAs) using matrix-assisted desorption/ionization imaging mass spectrometry (MALDI-IMS). (A) The distributions of cholesterol ester (CE) (18:1), phosphatidylcholine (PC) (16:0/18:1), and lysophosphatidylcholine (lysoPC) (1-acyl16:0) are shown. (B) The ratios of CE (18:1) to PC (16:0/18:1) in the intima, media, and adventitia in the adjacent arteries of FMD-associated VAAs, atherosclerotic VAAs, and control arteries are shown. In, intima; Me, media; Ad, adventitia. **P < 0.01 indicates a statistically significant difference. (C) The ratios of lysoPC (1-acyl16:0) to PC (16:0/18:1) in the intima, media, and adventitia in the adjacent arteries of FMD-associated VAAs, atherosclerotic VAAs, and control arteries are shown. In, intima; Me, media; Ad, adventitia. **P < 0.01 indicates a statistically significant difference.
Discussion
FMD is a non-atherosclerotic disease of medium-sized arteries that can cause arterial stenosis, occlusion, dissection, and aneurysm5). Unlike atherosclerotic VAAs, the accumulation of CE was hardly detected in FMD-associated aneurysms and the adjacent arteries by MALDI-IMS assessment, which is the only method that shows the positioning of accumulated lipid molecules at the molecular species level6). Although the cause of FMD is unknown, recent reports identified that serum levels of tumor growth factor (TGF)-β1 and TGF-β2 were elevated in FMD patients7). Considering that the secretion of TGF-β1 and TGF-β2 by fibroblasts derived from FMD patients was elevated, TGF-β signaling pathway appears to be involved in the disease7). In this study, we determined a characteristic distribution of lysoPC in FMD-associated VAAs in the aneurysmal sac and also in the adjacent arteries. LysoPC comprises a long hydrophobic fatty acyl chain, large hydrophilic polar choline head group, and potent proinflammatory lipid mediator, which is attached to a glycerol backbone7). LysoPC is a mediator that regulates TGF-β activation8, 9). Therefore, the accumulation of lysoPC may be associated with the increased secretion of TGF-β by fibroblasts in FMD. Interestingly, lysoPC was accumulated in the aneurysmal sac and also in the adjacent arteries with no dilatation, and it was diffusely accumulated in all layers of the arteries. However, lysoPC was focally accumulated in the media and medial ridge of the adventitia in the aneurysmal sac of the FMD-associated VAAs. This suggests that the diffuse accumulation of lysoPC in visceral arteries is a predisposition for developing aneurysms in FMD patients, although the mechanisms of the changes in the accumulated area of lysoPC during aneurysmal development are unknown.
In comparison with FMD-associated VAAs, the distribution of lysoPC in atherosclerotic VAAs was quite different. The accumulation of lysoPC was mainly found in the region of intimal hyperplasia in the aneurysmal sac and adjacent arteries. These findings appear to be consistent with previous studies10, 11). In intimal hyperplasia, lysoPC is considered to be produced in the process of low density lipoprotein oxidation mediated by lipoprotein-associated phospholipase A2. A study proposed that lysoPC plays an important role in homing the inflammatory cells into the area and augmenting the inflammatory mediators12). In this study, MALDI-IMS clearly demonstrated the accumulation of lysoPC in the area of intimal hyperplasia in both the aneurysmal sac and the adjacent arteries of atherosclerotic VAA. Interestingly, lysoPC was also accumulated in the media of the atherosclerotic VAAs, suggesting that lysoPC accumulation was extended from the intimal hyperplasia to the medial layers, which may damage the medial structures following aneurysmal changes, because lysoPC causes oxidative stress13), cytotoxicity to smooth muscle cells14), and macrophage infiltration15). Thus, the inhibition of lysoPC accumulation could be targets for pharmacological intervention to prevent aneurysmal development.
Further studies are required to clarify the function of lysoPC by which the development of VAAs formation is promoted. This study identifies a key molecule in the pathogenesis of FMD-associated VAAs. Currently, there are many unexplained vascular diseases. Although the pathophysiology of FMD-associated VAAs is not completely understood, our result may urge clinicians and researchers to consider lysoPC as one of the key molecules for unexplained vascular diseases. Our study has limitations. First, the small sample size is limited. Second, our samples were obtained at the time of surgical resection; thus, the obtained results reflect pathological changes at a single time point. However, it is difficult to follow the time course of the aneurysmal development in this type of study.
Conclusion
This study demonstrated the differences in the distribution patterns of lipid molecules, such as CE and lysoPC, between FMD-associated VAAs and atherosclerotic VAAs. The adjacent arteries without aneurysmal changes also showed the differences in the distribution of lysoPC. In FMD-associated VAAs, the accumulation of lysoPC in the visceral arteries may be a predisposition for the development of aneurysms.
Acknowledgements
We would like to thank all the staff of the Second Department of Surgery of the Hamamatsu University School of Medicine who helped with our patients' surgery and postoperative care. This work was supported by a Grants-in-Aid for Scientific Research (B) (20291958) to N.U., (C) (26462103) to N.U., and C) and (C) (00397415) to K.I.; Grants-in-Aid for Young Scientists (A) (25713024) to N.Z.; a Grant-in-Aid for SENTAN from the Japan Science and Technology Agency to JT; and Tokutei Lipid Machinery and Young Scientists S (2067004) to M.Setou.
COI
No financial assistance was received for this study. The authors have no conflicts of interest.
Non-Standard Abbreviations and Acronyms
- VAA
visceral artery aneurysm
- FMD
fibromuscular dysplasia
- IMS
imaging mass spectrometry
- MALDI-IMS
matrix-assisted desorption/ionization imaging mass spectrometry
- CT
computed tomography
- lysoPC
lysophosphatidylcholine
- PC
phosphatidylcholine
- CE
cholesterol ester
References
- 1). Wagner WH, Allins AD, Treiman RL, Cohen JL, Foran RF, Levin PM, Cossman DV: Ruptured visceral artery aneurysms. Ann Vasc Surg, 1997; 11: 342-347 [DOI] [PubMed] [Google Scholar]
- 2). Pasha SF, Gloviczki P, Stanson AW, Kamath PS: Splanchnic artery aneurysms. Mayo Clin Proc, 2007; 82: 472-479 [DOI] [PubMed] [Google Scholar]
- 3). Zaima N, Sasaki T, Tanaka H, Cheng XW, Onoue K, Hayasaka T, Goto-Inoue N, Enomoto H, Unno N, Kuzuya M, Setou M: Imaging mass spectrometry-based histopathologic examination of atherosclerotic lesions. Atherosclerosis, 2011; 217: 427-432 [DOI] [PubMed] [Google Scholar]
- 4). Tada H, Kawashiri MA, Konno T, Yamagishi M, Hayashi K: Common and Rare Variant Association Study for Plasma Lipids and Coronary Artery Disease. J Atheroscler Thromb, 2015; [DOI] [PubMed] [Google Scholar]
- 5). O'Connor SC, Gornik HL: Recent developments in the understanding and management of fibromuscular dysplasia. J Am Heart Assoc, 2014; 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Hayasaka T, Goto-Inoue N., Sugiura Y., Zaima N., Nakanishi H., Ohishi K., Nakanishi S., Naito T., Taguchi R., Setou M.: Matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight (MALDI-QIT-TOF)-based imaging mass spectrometry reveals a layered distribution of phospholipid molecular species in the mouse retina. Rapid Commun Mass Spectrom, 2008; 22: 3415-3426 [DOI] [PubMed] [Google Scholar]
- 7). Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, Tong L, Yang ML, Hunker K, Sloper L, Kuo S, Raza R, Milewicz DM, Francomano CA, Dietz HC, Van Eyk J, McDonnell NB: Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-beta expression and connective tissue features. FASEB J, 2014; 28: 3313-3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Gay I, Schwartz Z, Sylvia VL, Boyan BD: Lysophospholipid regulates release and activation of latent TGF-beta1 from chondrocyte extracellular matrix. Biochim Biophys Acta, 2004; 1684: 18-28 [DOI] [PubMed] [Google Scholar]
- 9). Hasegawa H, Lei J, Matsumoto T, Onishi S, Suemori K, Yasukawa M: Lysophosphatidylcholine enhances the suppressive function of human naturally occurring regulatory T cells through TGF-beta production. Biochem Biophys Res Commun, 2011; 415: 526-531 [DOI] [PubMed] [Google Scholar]
- 10). Zalewski A, Macphee C: Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arteriosclerosis, thrombosis, and vascular biology, 2005; 25: 923-931 [DOI] [PubMed] [Google Scholar]
- 11). Goncalves I, Edsfeldt A, Ko NY, Grufman H, Berg K, Bjorkbacka H, Nitulescu M, Persson A, Nilsson M, Prehn C, Adamski J, Nilsson J: Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol, 2012; 32: 1505-1512 [DOI] [PubMed] [Google Scholar]
- 12). Rong JX, Berman JW, Taubman MB, Fisher EA: Lysophosphatidylcholine stimulates monocyte chemoattractant protein-1 gene expression in rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol, 2002; 22: 1617-1623 [DOI] [PubMed] [Google Scholar]
- 13). McMurray HF, Parthasarathy S, Steinberg D: Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. The Journal of clinical investigation, 1993; 92: 1004-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Hsieh CC, Yen MH, Liu HW, Lau YT: Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL. Atherosclerosis, 2000; 151: 481-491 [DOI] [PubMed] [Google Scholar]
- 15). Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y: Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science, 2001; 293: 702-705 [DOI] [PubMed] [Google Scholar]


