Abstract
Aim: To investigate the relationship between serum uric acid levels and cardiovascular disease in Asians.
Methods: We examined the above relationship using the data of Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN Study). The data of 36,313 subjects (15,628 men and 20,685 women aged 35–89 years without histories of stroke, coronary heart disease, or cancer at baseline) were used for the analyses.
Sex-specific hazard ratios (HRs) of mortality from cardiovascular disease were estimated according to the quintiles of serum uric acid using Cox hazard models stratified by cohorts.
Results: During 441,771 person-years of follow-up, we documented 1,288 cardiovascular deaths. A J- or U-shaped relationship between serum uric acid level and cardiovascular disease mortality was observed. Compared with the lowest quintile of serum uric acid levels, the highest quintile was associated with an increased cardiovascular disease mortality in men [HR: 1.28; 95% confidence interval (CI): 1.01–1.63] and women (HR: 1.51; 95% CI: 1.14–1.99). However, there was no significant association with mortality from stroke, coronary heart disease or heart failure in both men and women.
Conclusion: This large pooled analysis in Japan suggested a J- or U-shaped relationship between serum uric acid levels and cardiovascular mortality. The highest quintile of serum uric acid levels was associated with increased cardiovascular disease mortality in both Japanese men and women.
Keywords: Serum uric acid, Stroke, Cardiovascular disease, Mortality
See editorial vol. 23: 671–672
Introduction
Although high serum uric acid level has been associated with an increased risk of cardiovascular disease1), there are some findings suggesting its protective effect on oxidative stress. The loss of urate oxidase activity leading to high serum uric acid levels has been hypothesized to protect the body from oxidative damage and the prolonged lifespan of hominoids2, 3). In the last decades, a number of epidemiologic studies showed conflicting results4–15).
The Framingham Study was the first to show an independent association between serum uric acid levels and risk of cardiovascular outcomes in the general population under the careful measurement of known cardiovascular risk factors in a 23-year follow-up period4). However, most of the participants in that study were whites, and we do not know whether the results might apply to non-white populations. A 14-year follow-up study of 8,172 Japanese men and women showed that uric acid levels were not associated with mortality from cardiovascular disease or stroke after adjustment for known cardiovascular risk10). Another 8-year follow-up study of 90,393 Taiwanese men and women indicated that hyperuricemia was an independent risk factor of mortality from cardiovascular disease11). Two recent cross-sectional surveys showed that serum uric acid level was significantly associated with various metabolic indicators and elevated carotid intima-media thickness in the Asian Mongolian as well as middle-aged and elderly Chinese subjects, respectively12, 13). Serum uric acid levels predict the incidence of coronary heart disease but not stroke among atomic bomb survivors in Nagasaki or is a risk factor for ultrasonographically determined carotid arterial intima-media thickness in Japanese elderly persons (≥ 74 years)14, 15).
Accordingly, a large-scale prospective study on the association between serum uric acid levels and risk of mortality from cardiovascular disease in Asian adults was still required. For this purpose, the present study has been conducted.
Study Population
The Evidence for Cardiovascular Prevention from Observation Cohorts in Japan (EPOCH-JAPAN) is the pooling project of a number of wellqualified cohort studies, which investigated the relationship between health examination measures (laboratory measures and lifestyle factors) and mortality in the Japanese population. The EPOCH-JAPAN comprises 13 cohort studies in Japan with an average of 10-year follow-up periods. The year range of baseline survey in the cohort was between 1977 and 1995. The details of these projects have been previously described16–23). A total of 90,528, of which the end-point was death owing to cardiovascular disease were included in the study. Serum uric acid was measured using a colorimetric phosphotungstic acid procedure.
Subjects were excluded if they reported a history of stroke, coronary heart disease, or cancer (n = 4,144) at baseline or if they were unable to provide data for serum uric acid levels; in addition, those who were older than 90 years or younger than 35 years (total: n = 50,071) were also excluded. Data from the remaining 36,313 subjects (15,628 men and 20,685 women) were used for the analyses.
Endpoints
The primary endpoints for this analysis were deaths from cardiovascular disease (CVD) (the International Classification of Disease, 9th revision, codes 390–459 and 10th revision, codes I01–I99), which was further divided into hemorrhagic stroke (430–431 and I60–I61) and ischemic stroke (433–434 and I63) as well as coronary heart disease (CHD, 410–414 and I20–I25) and heart failure (428 and I50).
Statistical Methods
The data of serum uric acid were divided into sex-specified quintiles. The median level of serum uric acid of each quintile was 4.0 mg/dl, 4.9 mg/dl, 5.5 mg/dl, 6.2 mg/dl, and 7.3 mg/dl for men and 3.0 mg/dl, 3.6 mg/dl, 4.1 mg/dl, 4.7 mg/dl, and 5.7 mg/dl for women. Hazard ratios (HRs) for mortalities were estimated in both men and women by Cox hazard models, which were stratified by cohorts. The adjustment variables included age (continuous), smoking status (never, past, 1–20/day, or ≥ 21/day), drinking status (drinkers, ex-drinkers, or never-drinkers), body mass index (quartiles), triglycerides (quartiles), total cholesterol (quartiles), and systolic blood pressure (continuous).
Two fitting curves of association between serum uric acid and cardiovascular mortality in both sexes were plotted to clearly demonstrate the results (Fig. 1 & 2).
Fig. 1.

Fitting curve of association between serum uric acid and cardiovascular mortality in men.
Fig. 2.

Fitting curve of association between serum uric acid and cardiovascular mortality in women.
All statistical analyses for two-tailed tests were conducted using SAS version 9.13 (SAS Institute Inc., Cary). P values of < 0.05 were regarded as statistically significant.
Results
During 441,771 person-years of follow-up, we documented 1,288 deaths from CVD (649 in men and 639 in women) including 301 total strokes, 131 coronary heart diseases, and 116 heart failures in men as well as 293 total strokes and 136 heart failures in women.
Table 1 shows the age-adjusted mean values and prevalence of cardiovascular risk factors at baseline according to the quintiles of serum uric acid levels. Men with higher uric acid were younger, whereas women with higher uric acid levels were older. Compared with men and women in the lowest quintile of uric acid levels, those in the higher quintiles were likely to be overweight and to have higher levels of total cholesterol, systolic and diastolic blood pressures, and triglycerides. Men with higher uric acid levels drank less but the opposite trend was observed in women. In addition, uric acid levels were inversely associated with high density cholesterol levels for both men and women.
Table 1. Baseline characteristics according to quintiles of serum uric acid levels.
| Quintile of serum uric acid levels |
P for trend | |||||
|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 | 5 (high) | ||
| Men | ||||||
| No. at risk | 3042 | 3353 | 2939 | 3052 | 3242 | |
| Median uric acid (mg/dl) | 4.0 | 4.9 | 5.5 | 6.2 | 7.3 | |
| Range of uric acid (mg/dl) | 0.6–4.6 | 4.7–5.2 | 5.3–5.8 | 5.9–6.6 | 6.7–16.0 | |
| Mean age (years) | 54.6 | 53.3 | 52.6 | 52.0 | 52.9 | < 0.001 |
| Mean body mass index (kg/m2) | 21.9 | 22.2 | 22.7 | 23.1 | 23.8 | < 0.001 |
| Mean total cholestrerol (mg/dl) | 187.8 | 189.8 | 192.8 | 196.4 | 201.2 | < 0.001 |
| Mean HDL cholesterol (mg/dl) | 53.3 | 52.7 | 51.3 | 50.5 | 50.0 | < 0.001 |
| Mean systolic blood pressure (mmHg) | 131.0 | 131.2 | 132.1 | 133.3 | 137.2 | < 0.001 |
| Mean diastolic blood pressure (mmHg) | 79.1 | 79.7 | 80.8 | 81.6 | 84.4 | < 0.001 |
| Median triglycerides (mg/dl) | 93.0 | 99.0 | 108.0 | 115.0 | 135.0 | < 0.001 |
| Current smokers (%) | 63.8 | 68.7 | 70.6 | 72.6 | 74.6 | < 0.001 |
| Current drinkers (%) | 59.2 | 58.8 | 56.2 | 54.8 | 50.6 | < 0.001 |
| Women | ||||||
| No. at risk | 4388 | 3933 | 4386 | 3628 | 4350 | |
| Median uric acid (mg/dl) | 3.0 | 3.6 | 4.1 | 4.7 | 5.7 | |
| Range of Uric acid (mg/dl) | 0.4–3.3 | 3.4–3.8 | 3.9–4.3 | 4.4–5.0 | 5.1–10.8 | |
| Mean age (years) | 51.3 | 52.3 | 53.3 | 55.1 | 58.2 | < 0.001 |
| Mean body mass index (kg/m2) | 22.1 | 22.5 | 22.8 | 23.3 | 24.2 | < 0.001 |
| Mean total cholestrerol (mg/dl) | 193.9 | 199.5 | 202.5 | 208.5 | 214.2 | < 0.001 |
| Mean HDL cholesterol (mg/dl) | 58.2 | 57.1 | 56.7 | 55.9 | 51.6 | < 0.001 |
| Mean systolic blood pressure (mmHg) | 126.9 | 128.4 | 129.5 | 133.2 | 138.3 | < 0.001 |
| Mean diastolic blood pressure (mmHg) | 75.8 | 76.9 | 77.7 | 79.5 | 81.6 | < 0.001 |
| Median triglycerides (mg/dl) | 82.0 | 87.0 | 92.0 | 104.0 | 120.0 | < 0.001 |
| Current smokers (%) | 5.2 | 5.4 | 6.1 | 7.4 | 8.2 | < 0.001 |
| Current drinkers (%) | 13.0 | 15.0 | 16.0 | 18.2 | 16.2 | < 0.001 |
Table 2 shows sex-specified HRs of stroke, coronary heart disease, heart failure, and total CVD according to the quintiles of serum uric acid levels. In multivariable models, we observe a J- or U-shaped relationship between serum uric acid level and total or cause-specific cardiovascular mortality. We did not observe a linear increase in mortality from almost all causes of death associated with the increase in serum uric acid levels. In most causes of death, the 5th quintile of uric acid showed highest mortality in both men and women; however, few of them reached to statistical significance. For both men and women, the highest uric acid levels (= > 6.7 mg/dl for men and = > 5.1 mg/dl for women) were associated with increased mortality owing to total CVD; we also observed statistical significance for trend test in the association between uric acid quintile and total cardiovascular mortality and in the relationship between uric acid quintile and mortality because of stroke in women.
Table 2. Sex-specific hazard ratios (95% CI) of mortality from stroke, coronary heart disease, heart failure and total cardiovascular diseases according to quintiles of serum uric acid levels.
| Quintiles of serum uric acid levels |
P for trend | |||||
|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 | 5 (high) | ||
| Men | ||||||
| No. at risk | 3042 | 3353 | 2939 | 3052 | 3242 | |
| Person Year | 34762 | 40456 | 34398 | 38145 | 40323 | |
| Total stroke | ||||||
| No. of death | 63 | 59 | 46 | 48 | 85 | |
| Age-adjusted HR | 1.00 | 0.81 (0.56–1.15) | 0.76 (0.52–1.12) | 0.75 (0.52–1.10) | 1.14 (0.82–1.59) | 0.291 |
| Multivariable HR† | 1.00 | 0.83 (0.58–1.18) | 0.77 (0.52–1.13) | 0.77 (0.52–1.13) | 1.19 (0.84–1.68) | 0.258 |
| Ischemic stroke | ||||||
| No. of death | 35 | 34 | 26 | 31 | 47 | |
| Age-adjusted HR | 1.00 | 0.83 (0.52–1.34) | 0.76 (0.45–1.26) | 0.90 (0.55–1.47) | 1.19 (0.76–1.85) | 0.286 |
| Multivariable HR† | 1.00 | 0.87 (0.54–1.40) | 0.75 (0.45–1.26) | 0.91 (0.55–1.50) | 1.19 (0.75–1.90) | 0.350 |
| Hemorrhagic stroke | ||||||
| No. of death | 17 | 18 | 16 | 15 | 29 | |
| Age-adjusted HR | 1.00 | 0.89 (0.46–1.73) | 1.04 (0.52–2.06) | 0.81 (0.41–1.64) | 1.37 (0.75–2.51) | 0.248 |
| Multivariable HR† | 1.00 | 0.90 (0.46–1.77) | 1.07 (0.54–2.14) | 0.83 (0.41–1.68) | 1.41 (0.75–2.65) | 0.252 |
| Coronary heart disease | ||||||
| No. of death | 24 | 27 | 18 | 27 | 35 | |
| Age-adjusted HR | 1.00 | 0.99 (0.57–1.72) | 0.83 (0.45–1.53) | 1.15 (0.66–2.00) | 1.29 (0.76–2.18) | 0.235 |
| Multivariable HR† | 1.00 | 0.98 (0.57–1.71) | 0.75 (0.40–1.39) | 1.02 (0.58–1.79) | 1.12 (0.65–1.93) | 0.600 |
| Heart failure | ||||||
| No. of death | 19 | 23 | 24 | 18 | 32 | |
| Age-adjusted HR | 1.00 | 1.06 (0.57–1.95) | 1.38 (0.76–2.54) | 0.94 (0.49–1.80) | 1.45 (0.82–2.58) | 0.229 |
| Multivariable HR† | 1.00 | 1.09 (0.59–2.03) | 1.46 (0.79–2.69) | 1.05 (0.54–2.03) | 1.76 (0.97–3.18) | 0.066 |
| Total cardiovascular disease | ||||||
| No. of death | 126 | 127 | 102 | 111 | 183 | |
| Age-adjusted HR | 1.00 | 0.87 (0.68–1.19) | 0.86 (0.66–1.20) | 0.87 (0.67–1.12) | 1.24 (0.98–1.55) | 0.028 |
| Multivariable HR† | 1.00 | 0.89 (0.70–1.14) | 0.86 (0.66–1.12) | 0.88 (0.68–1.14) | 1.28 (1.01–1.63) | 0.022 |
| Women | ||||||
| No. at risk | 4388 | 3933 | 4386 | 3628 | 4350 | |
| Person Year | 51098 | 46069 | 55526 | 44685 | 56309 | |
| Total stroke | ||||||
| No. of death | 33 | 42 | 45 | 51 | 122 | |
| Age-adjusted HR | 1.00 | 1.22 (0.77–1.93) | 0.93 (0.59–1.46) | 1.07 (0.69–1.67) | 1.45 (0.98–2.15) | 0.024 |
| Multivariable HR† | 1.00 | 1.27 (0.90–2.01) | 0.98 (0.62–1.54) | 1.05 (0.67–1.64) | 1.46 (0.98–2.19) | 0.036 |
| Ischemic stroke | ||||||
| No. of death | 15 | 23 | 18 | 30 | 57 | |
| Age-adjusted HR | 1.00 | 1.38 (0.71–2.63) | 0.77 (0.39–1.54) | 1.22 (0.65–2.29) | 1.33 (0.75–2.37) | 0.285 |
| Multivariable HR† | 1.00 | 1.42 (0.74–2.74) | 0.80 (0.40–1.61) | 1.22 (0.65–2.30) | 1.35 (0.75–2.44) | 0.314 |
| Hemorrhagic stroke | ||||||
| No. of death | 11 | 14 | 20 | 14 | 38 | |
| Age-adjusted HR | 1.00 | 1.41 (0.64–3.11) | 1.32 (0.63–2.76) | 1.12 (0.51–2.50) | 1.55 (0.78–3.07) | 0.269 |
| Multivariable HR† | 1.00 | 1.41 (0.64–3.13) | 1.33 (0.63–2.80) | 1.09 (0.48–2.43) | 1.54 (0.76–3.10) | 0.301 |
| Coronary heart disease | ||||||
| No. of death | 10 | 13 | 18 | 23 | 50 | |
| Age-adjusted HR | 1.00 | 1.28 (0.56–2.93) | 1.15 (0.53–2.50) | 1.60 (0.75–3.38) | 1.83 (0.92–3.64) | 0.032 |
| Multivariable HR† | 1.00 | 1.29 (0.56–2.96) | 1.20 (0.55–2.61) | 1.49 (0.70–3.18) | 1.75 (0.87–3.54) | 0.067 |
| Heart failure | ||||||
| No. of death | 18 | 13 | 23 | 26 | 56 | |
| Age-adjusted HR | 1.00 | 0.64 (0.31–1.31) | 0.78 (0.42–1.46) | 0.86 (0.47–1.57) | 1.09 (0.63–1.88) | 0.200 |
| Multivariable HR† | 1.00 | 0.69 (0.34–1.41) | 0.84 (0.45–1.56) | 0.96 (0.52–1.77) | 1.29 (0.74–2.25) | 0.071 |
| Total cardiovascular diseases | ||||||
| No. of death | 68 | 82 | 106 | 119 | 264 | |
| Age-adjusted HR | 1.00 | 1.15 (0.83–1.58) | 1.04 (0.76–1.41) | 1.18 (0.87–1.59) | 1.46 (1.11–1.91) | < 0.001 |
| Multivariable HR† | 1.00 | 1.18 (0.86–1.64) | 1.09 (0.80–1.48) | 1.17 (0.86–1.58) | 1.51 (1.14–1.99) | < 0.001 |
Adjusted further for body mass index, smoking status, ethanol intake, systolic blood pressure and total cholesterol.
To check whether there was any reverse causal bias, a sub-analysis was conducted to estimate the associations censoring the first 3 years. The results were shown in Supplementary Table and Supplementary Figs. In multivariable models, a J- or U-shaped relationship between serum uric acid level and total or cause-specific cardiovascular mortality was also observed. The whole results were quite similar to the ones shown in Table 2. Therefore, we considered that there was no or few reverse causal bias.
Supplementary Table. Sex-specific hazard ratios (95% CI) of mortality from stroke, coronary heart disease, heart failure and total cardiovascular diseases according to quintiles of serum uric acid levels censoring the first 3 years.
| Quintiles of serum uric acid levels |
P for trend | |||||
|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 | 5 (high) | ||
| Men | ||||||
| No. at risk | 2944 | 3270 | 2860 | 2984 | 3149 | |
| Person Year | 34600 | 40315 | 34266 | 38029 | 40172 | |
| Total stroke | ||||||
| No. of death | 56 | 53 | 41 | 44 | 78 | |
| Age-adjusted HR | 1.00 | 0.81 (0.56–1.18) | 0.77 (0.51–1.16) | 0.77 (0.52–1.15) | 1.19 (0.84–1.68) | 0.211 |
| Multivariable HR† | 1.00 | 0.82 (0.51–1.20) | 0.77 (0.51–1.16) | 0.78 (0.52–1.17) | 1.21 (0.84–1.74) | 0.220 |
| Ischemic stroke | ||||||
| No. of death | 29 | 31 | 23 | 29 | 46 | |
| Age-adjusted HR | 1.00 | 0.91 (0.55–1.51) | 0.82 (0.46–1.40) | 1.01 (0.60–1.70) | 1.40 (0.87–2.24) | 0.082 |
| Multivariable HR† | 1.00 | 0.94 (0.56–1.58) | 0.79 (0.45–1.37) | 1.01 (0.59–1.71) | 1.37 (0.83–2.24) | 0.142 |
| Hemorrhagic stroke | ||||||
| No. of death | 16 | 16 | 16 | 14 | 24 | |
| Age-adjusted HR | 1.00 | 0.91 (0.52–1.60) | 0.82 (0.44–1.51) | 1.06 (0.60–1.86) | 1.14 (0.66–1.94) | 0.484 |
| Multivariable HR† | 1.00 | 0.85 (0.42–1.71) | 1.19 (0.59–2.41) | 0.83 (0.40–1.73) | 1.29 (0.66–2.51) | 0.406 |
| Coronary heart disease | ||||||
| No. of death | 24 | 25 | 18 | 25 | 31 | |
| Age-adjusted HR | 1.00 | 0.99 (0.57–1.72) | 0.83 (0.45–1.53) | 1.15 (0.66–2.00) | 1.29 (0.76–2.18) | 0.485 |
| Multivariable HR† | 1.00 | 0.90 (0.51–1.59) | 0.74 (0.40–1.37) | 0.92 (0.52–1.64) | 0.97 (0.55–1.70) | 0.992 |
| Heart failure | ||||||
| No. of death | 15 | 20 | 19 | 16 | 28 | |
| Age-adjusted HR | 1.00 | 1.13 (0.58–2.21) | 1.35 (0.68–2.66) | 1.01 (0.50–2.06) | 1.55 (0.83–2.92) | 0.193 |
| Multivariable HR† | 1.00 | 1.17 (0.60–2.30) | 1.39 (0.70–2.77) | 1.09 (0.53–2.23) | 1.86 (0.97–3.57) | 0.068 |
| Total cardiovascular disease | ||||||
| No. of death | 110 | 114 | 90 | 103 | 164 | |
| Age-adjusted HR | 1.00 | 0.89 (0.68–1.15) | 0.87 (0.66–1.15) | 0.91 (0.70–1.19) | 1.26 (0.99–1.61) | 0.023 |
| Multivariable HR† | 1.00 | 0.90 (0.69–1.17) | 0.86 (0.65–1.14) | 0.91 (0.69–1.19) | 1.28 (0.99–1.65) | 0.029 |
| Women | ||||||
| No. at risk | 4320 | 3867 | 4329 | 3555 | 4247 | |
| Person Year | 50989 | 45970 | 55440 | 44575 | 56142 | |
| Total stroke | ||||||
| No. of death | 30 | 35 | 44 | 47 | 103 | |
| Age-adjusted HR | 1.00 | 1.12 (0.69–1.83) | 0.99 (0.62–1.58) | 1.08 (0.68–1.72) | 1.35 (0.89–2.04) | 0.079 |
| Multivariable HR† | 1.00 | 1.16 (0.71–1.89) | 1.05 (0.65–1.67) | 1.06 (0.67–1.69) | 1.35 (0.88–2.06) | 0.121 |
| Ischemic stroke | ||||||
| No. of death | 15 | 21 | 18 | 29 | 49 | |
| Age-adjusted HR | 1.00 | 1.25 (0.64–2.41) | 0.77 (0.38–1.53) | 1.18 (0.63–2.23) | 1.17 (0.65–2.10) | 0.562 |
| Multivariable HR† | 1.00 | 1.31 (0.67–2.55) | 0.80 (0.40–1.60) | 1.19 (0.63–2.25) | 1.19 (0.65–2.18) | 0.588 |
| Hemorrhagic stroke | ||||||
| No. of death | 10 | 10 | 20 | 11 | 34 | |
| Age-adjusted HR | 1.00 | 1.11 (0.46–2.68) | 1.44 (0.67–3.10) | 0.97 (0.41–2.31) | 1.53 (0.74–3.14) | 0.252 |
| Multivariable HR† | 1.00 | 1.09 (0.45–2.64) | 1.45 (0.67–3.12) | 0.90 (0.37–2.15) | 1.44 (0.69–3.00) | 0.360 |
| Coronary heart disease | ||||||
| No. of death | 9 | 13 | 17 | 21 | 43 | |
| Age-adjusted HR | 1.00 | 1.41 (0.60–3.30) | 1.16 (0.51–2.61) | 1.58 (0.72–3.48) | 1.68 (0.81–3.48) | 0.122 |
| Multivariable HR† | 1.00 | 1.41 (0.60–3.33) | 1.20 (0.53–2.72) | 1.49 (0.67–3.30) | 1.61 (0.76–3.39) | 0.206 |
| Heart failure | ||||||
| No. of death | 17 | 11 | 22 | 24 | 47 | |
| Age-adjusted HR | 1.00 | 0.56 (0.26–1.19) | 0.76 (0.40–1.43) | 0.80 (0.43–1.50) | 0.92 (0.52–1.62) | 0.548 |
| Multivariable HR† | 1.00 | 0.61 (0.28–1.31) | 0.81 (0.43–1.55) | 0.91 (0.48–1.73) | 1.11 (0.62–1.99) | 0.236 |
| Total cardiovascular diseases | ||||||
| No. of death | 62 | 72 | 102 | 108 | 226 | |
| Age-adjusted HR | 1.00 | 1.10 (0.78–1.54) | 1.07 (0.78–1.47) | 1.16 (0.84–1.59) | 1.35 (1.01–1.80) | 0.012 |
| Multivariable HR† | 1.00 | 1.13 (0.81–1.59) | 1.12 (0.82–1.55) | 1.16 (0.84–1.59) | 1.40 (1.04–1.88) | 0.010 |
Adjusted further for body mass index, smoking status, ethanol intake, systolic blood pressure and total cholesterol.
Supplementary Fig. 1.

Fitting curve of association between serum uric acid and cardiovascular mortality in men censoring the first three years
Supplementary Fig. 2.

Fitting curve of association between serum uric acid and cardiovascular mortality in women censoring the first three years
Discussion
In this large pooled analysis of Japanese cohort studies with a median follow-up of 10 years, we found a J- or U-shaped relationship between serum uric acid levels and cardiovascular mortality. Compared with the lowest quintile of serum uric acid levels, the highest quintile was associated with an increased cardiovascular mortality in both men and women; however, none of the cause-specific death was significantly associated with serum uric acid levels.
Elevated serum uric acid has been recognized as an independent risk factor for heart failure13, 24, 25). In the Framingham Offspring Study, a longitudinal observational study of 4,912 children from the original Framingham cohort participants and their spouses showed that the serum uric acid levels were associated with the incidence of heart failure. The hazard ratio (95% CI) for the highest versus lowest quintiles of serum uric acid (> 6.3 mg/dl and < 3.4 mg/dl, respectively) was 2.10 (1.04–4.22) after adjusting for sex, age, smoking, body mass index, renal dysfunction, diuretics, systolic blood pressure, valvular heart disease, diabetes, alcohol, and use of antihypertension medications24). Although the risk ratio did not reach statistical significance level, the magnitude of hazard ratio for both men and women in the highest uric acid quintile in the present study was almost similar to that of the Framingham Offspring Study. The MJ Health Screening Cohort conducted in Taiwan13) failed to show significant positive associations between serum uric acid levels and mortality from heart failure in men and women but showed a weak positive association for both sexes combined; the multivariable hazard ratio (95% CI) for serum uric acid level of > 7.0 mg/dl compared with that of ≤ 7.0 mg/dl was 1.13 (1.07–1.19); p value for trend was < 0.0113).
The associations between hyperuricemia and risk of stroke have been investigated in several studies, but the results also have been controversial8–14). The NIP-PON DATA 80 performed in 1980 at baseline was the first cohort study to examine the association between hyperuricemia and deaths because of CVDs in a representative sample of Japanese adults10). In that study, uric acid levels were not significantly associated with mortality because of CVD or stroke in either age-adjusted or multivariable models, probably because of the limited number of deaths10). The present large-cohort study showed no significant association between serum uric acid levels and stroke mortality, although increased risk with borderline statistical significance was observed in the highest quintile of serum uric acid levels in women. MJ Health Screening Cohort reported increased mortality from ischemic stroke with higher serum uric acid levels in women only13). A meta-analysis comprising 16 cohort studies and 238,449 adults showed that persons with hyperuricemia had higher age-adjusted risk for stroke: stroke incidence (6 studies; risk ratio, 1.41; 95% CI: 1.05–1.76) and mortality (6 studies; risk ratio 1.36, 95% CI: 1.03–1.69)26). The subgroup analysis of this study adjusting for known risk of factors including age, hypertension, diabetes mellitus, and cholesterol still showed an increased risk of stroke in persons with hyperuricemia. However, the abovementioned study was a traditional meta-analysis based on bibliographic information, which is not a pooled analysis based on individual data.
The present study showed an excess risk with borderline statistical significance for mortality because of coronary heart disease in women, but not in men. The NIPPON DATA 8010) and the MJ Health Screening Cohort13) showed no significant association between serum uric acid levels and mortality from coronary heart disease in either sex. A recent metaanalysis including 26 studies and 402,997 adults showed that hyperuricemia was associated with increased risk of coronary heart disease: an adjusted risk ratio for incidence (95% CI) of 1.09 (1.01–1.16) and an adjusted risk ratio for mortality (95% CI) of 1.16 (1.01–1.30)27). However, the sex-specific analysis also showed a significant association of hyperuricemia with the incidence of or mortality from coronary heart disease in women, but not in men. Furthermore, their study is not a pooled analysis based on individual data.
In the present study, the overall mortality rate from CVD in women was lower than that in men, particularly at low serum uric acid levels (Fig. 3). Therefore, the hazard ratio for the highest versus lowest quintiles of serum uric acid was higher in women and was more statistically significant than that in men. Several previous studies also showed that elevated serum uric acid levels in women were associated with a higher cardiovascular hazard ratio than that in men9, 13, 28). This may be because of estrogen that probably plays a cardioprotective role in women8, 13, 29), whereas hyperuricemia in women could possibly be a hallmark of escape from estrogen protection13). In addition, another possible reason could be that both the mean age level and the mean glucose level were higher in the higher uric acid level (data not shown) groups of women than of men. Uric acid is also associated with diabetes or glucose intolerance and is a risk factor that confers greater relative risk for CVD in women8, 30, 31).
Fig. 3.
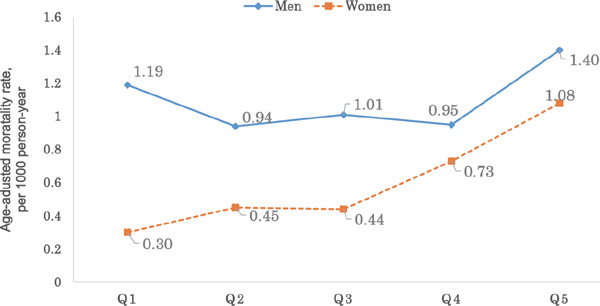
Sex-specific age–adjusted cardiovascular disease (CVD) mortality rates according to quintiles of serum uric acid levels.
Hyperuricemia induces endothelial dysfunction32), which may stimulate glucose assimilation in oxidative process in adipocytes33). In a populationbased study of 783 men, hyperuricemia was associated with increased renal tubular sodium re-absorption, which may provide a link with hyperinsulinemia and hypertension34). In a recent randomized, placebo-controlled, crossover trial of hyperuricemia involving individuals with newly diagnosed hypertension, compared with placebo (n = 15), casual and 24-hour ambulatory blood pressure levels was reduced to a greater extent with allopurinol treatment (n = 15)35). On the other hand, uric acid has been reported to be an antioxidant that may prevent stress-induced cell transformation and oxidant-induced cardiac and renal toxicity36). An in-vivo study showed that uric acid protected cultured rat hippocampal neurons against cell death induced by glutamate and NaCN insults, which are relevant to the pathogenesis of cerebral ischemia37). A clinical study also supported a potential neuroprotective role of the exogenous uric acid administration in stoke patients treated by thrombolysis38). These studies suggested that the effect of hyperuricemia on CVDs was strongly associated with other cardiovascular risk factors such as hypertension and insulin resistance. These findings may explain J- or U-shaped relationship between serum uric acid levels and cardiovascular mortality in the present study. In other words, a certain level of serum uric acid may provide benefit on cardiovascular disease to some extent; however, among the subjects with other cardiovascular risk factors, such as high glucose level or high blood pressure, higher serum uric acid levels would be associated with elevated risk of cardiovascular mortality.
The strengths of the present study include its large population-based individual data from all over Japan as well as its prospective design. To our knowledge, EPOCH-JAPAN is the largest-scale pooled data to examine the associations between uric acid level and risk of CVD in Japan. However, there were several limitations in the present study. First, the pooled data for most of the cohorts were from participants in municipal health examinations, but not the representative samples in communities. Second, we did not adjust for medication use for hypertension or diabetes as well as for glucose or creatinine level because the information of more than 30% participants was missing. However, among the participants who had the information of antihypertensive medication use, we essentially obtained the same estimation for HRs of mortality from CVD according to the quintiles of serum uric acid, adjusting further for medication use for hypertension (data not shown).
In conclusion, the results of our large pooled analysis indicated a J- or U-shaped relationship between serum uric acid level and cardiovascular mortality and also showed that the highest quintile of serum uric acid levels compared with the lowest quintiles were associated with an increased CVD mortality in both Japanese men and women.
Acknowledgments
We are grateful to all of the participants in each cohort study. We thank Mrs. Toshimi Yoshida (Shiga University of Medical Science) and Mrs. Satoko Narikawa (Keio University) for expert clerical assistance.
Appendix
The Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH–JAPAN) Research Group is composed of the following investigators. Chairperson: Hirotsugu Ueshima (Shiga University of Medical Science); Co–Chairperson: Tomonori Okamura (Keio University);
Executive committee: Hirotsugu Ueshima (Shiga University of Medical Science), Yutaka Imai (Tohoku University Graduate School of Pharmaceutical Sciences), Takayoshi Ohkubo (Teikyo University School of Medicine), Fujiko Irie (Ibaraki Prefecture), Hiroyasu Iso, Akihiko Kitamura (Osaka University Graduate School of Medicine), Yutaka Kiyohara (Kyushu University Graduate School of Medicine), Katsuyuki Miura (Shiga University of Medical Science), Yoshitaka Murakami (Toho University), Hideaki Nakagawa (Kanazawa Medical University), Takeo Nakayama (Kyoto University School of Public Health), Tomonori Okamura (Keio University), Akira Okayama (Research Institute of Strategy for Prevention), Toshimi Sairenchi (Dokkyo Medical University), Shigeyuki Saitoh (Sapporo Medical University), Kiyomi Sakata (Iwate Medical University), Akiko Tamakoshi (Hokkaido University Graduate School of Medicine), Ichiro Tsuji (Tohoku University Graduate School of Medicine), Michiko Yamada (Radiation Effects Research Foundation), Masahiko Kiyama (Osaka Center for Cancer and Cardiovascular Disease Prevention), Yoshihiro Miyamoto (National Cerebral and Cardiovascular Center), Shizukiyo Ishikawa (Jichi Medical University) and Hiroshi Yatsuya (Fujita Health University).
Funding Sources
This research was supported by a grant–in–aid from the Ministry of Health, Labour and Welfare, Health and Labor Sciences research grants, Japan (Research on Health Services: H17–Kenkou–007; Comprehensive Research on Cardiovascular Disease and Life–Related Disease: H18–Junkankitou [Seishuu]–Ippan–012; Comprehensive Research on Cardiovascular Disease and Life–Related Disease: H19–Junkankitou [Seishuu]–Ippan–012; Comprehensive Research on Cardiovascular and Life–Style Related Diseases: H20–Junkankitou [Seishuu]–Ippan–013; Comprehensive Research on Cardiovascular and Life–Style Related Diseases: H23–Junkankitou [Seishuu]–Ippan–005), and an Intramural Research Fund (22-4-5) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center; and Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H26–Junkankitou [Seisaku]-Ippan-001.
Disclosures
None.
References
- 1). Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008; 359: 1811-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992; 34: 78-84 [DOI] [PubMed] [Google Scholar]
- 3). Oda M, Satta Y, Takenaka O, Takahata Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002; 19: 640-653 [DOI] [PubMed] [Google Scholar]
- 4). Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol. 1988; 41: 237-242 [DOI] [PubMed] [Google Scholar]
- 5). Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007; 116: 894-900 [DOI] [PubMed] [Google Scholar]
- 6). Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD. Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 2000; 10: 136-143 [DOI] [PubMed] [Google Scholar]
- 7). Liese AD, Hense HW, Löwel H, Döring A, Tietze M, Keil U. Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. World Health Organization Monitoring Trends and Determinants in Cardiovascular Diseases. Epidemiology. 1999; 10: 391-397 [DOI] [PubMed] [Google Scholar]
- 8). Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999; 131: 7-13 [DOI] [PubMed] [Google Scholar]
- 9). Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000; 283: 2404-2410 [DOI] [PubMed] [Google Scholar]
- 10). Sakata K, Hashimoto T, Ueshima H, Okayama A, NIPPON DATA 80 Research Group Absence of an association between serum uric acid and mortality from cardiovascular disease: NIPPON DATA 80, 1980–1994. National Integrated Projects for Prospective Observation of Non-communicable Diseases and its Trend in the Aged. Eur J Epidemiol. 2001; 17: 461-468 [DOI] [PubMed] [Google Scholar]
- 11). You L, Liu A, Wuyun G, Wu H, Wang P. Prevalence of hyperuricemia and the relationship between serum uric acid and metabolic syndrome in the Asian Mongolian area. J Atheroscler Thromb. 2014. 21: 355-365 [DOI] [PubMed] [Google Scholar]
- 12). Chen Y, Xu B, Sun W, Sun J, Wang T, Xu Y, Xu M, Lu J, Li X, Bi Y, Wang W, Ning G. Impact of the Serum Uric Acid Level on Subclinical Atherosclerosis in Middle-aged and Elderly Chinese. J Atheroscler Thromb. 2015; 22: 823-832 [DOI] [PubMed] [Google Scholar]
- 13). Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009; 61: 225-232 [DOI] [PubMed] [Google Scholar]
- 14). Kawamoto R, Tomita H, Oka Y, Kodama A, Ohtsuka N, Kamitani A. Association between uric acid and carotid atherosclerosis in elderly persons. Intern Med. 2005; 44: 87-793 [DOI] [PubMed] [Google Scholar]
- 15). Baba T, Amasaki Y, Soda M, Hida A, Imaizumi M, Ichimaru S, Nakashima E, Seto S, Yano K, Akahoshi M. fatty liver and uric acid levels predict incident coronary heart disease but not stroke among atomic bomb survivors in Nagasaki. Hypertens Res. 2007; 30: 823-829 [DOI] [PubMed] [Google Scholar]
- 16). Murakami Y, Miura K, Okamura T, Ueshima H, EPOCH-JAPAN Research Group Population attributable numbers and fractions of deaths due to smoking: a pooled analysis of 180,000 Japanese. Prev Med. 2011; 52: 60-65 [DOI] [PubMed] [Google Scholar]
- 17). Murakami Y, Hozawa A, Okamura T, Ueshima H, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group (EPOCH-JAPAN) Relation of blood pressure and all-cause mortality in 180,000 Japanese participants: pooled analysis of 13 cohort studies. Hypertension. 2008; 51: 1483-1491 [DOI] [PubMed] [Google Scholar]
- 18). Nakamura K, Nakagawa H, Sakurai M, Murakami Y, Irie F, Fujiyoshi A, Okamura T, Miura K, Ueshima H, EPOCH-JAPAN Research Group Influence of smoking combined with another risk factor on the risk of mortality from coronary heart disease and stroke: pooled analysis of 10 Japanese cohort studies. Cerebrovasc Dis. 2012; 33: 480-491 [DOI] [PubMed] [Google Scholar]
- 19). Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, Ueshima H, Observational Cohorts in Japan (EPOCH-JAPAN) Research Group Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res. 2012; 35: 947-953 [DOI] [PubMed] [Google Scholar]
- 20). Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H, Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN) Research Group Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc;, 2012; 1: 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Satoh M, Ohkubo T, Asayama K, Murakami Y, Sakurai M, Nakagawa H, Iso H, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan (EPOCH-JAPAN) Research Group Combined effect of blood pressure and total cholesterol levels on long-term risks of subtypes of cardiovascular death: evidence for cardiovascular prevention from observational cohorts in Japan. Hypertension. 2015; 65: 517-524 [DOI] [PubMed] [Google Scholar]
- 22). Asayama K, Satoh M, Murakami Y, Ohkubo T, Nagasawa SY, Tsuji I, Nakayama T, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan (EPOCH-JAPAN) Research Group Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: participant-level meta-analysis. Hypertension. 2014; 63: 1189-1197 [DOI] [PubMed] [Google Scholar]
- 23). Nagata M, Ninomiya T, Kiyohara Y, Murakami Y, Irie F, Sairenchi T, Miura K, Okamura T, Ueshima H, EPOCH-JAPAN Research Group Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: pooled analysis of 39,000 individuals from 7 cohort studies in Japan. Am J Epidemiol. 2013; 178: 1-11 [DOI] [PubMed] [Google Scholar]
- 24). Krishnan E. Hyperuricemia and incident heart failure. Circ Heart Fail. 2009; 2: 556-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Wu AH, Ghali JK, Neuberg GW, O'Connor CM, Carson PE, Levy WC. Uric acid level and allopurinol use as risk markers of mortality and morbidity in systolic heart failure. Am Heart J. 2010; 160: 928-933 [DOI] [PubMed] [Google Scholar]
- 26). Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009; 61: 885-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res. 2010; 62: 170-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Freedman DS, Williamson DF, Croft JB, Ballew C, Byers T. Relation of body fat distribution to ischemic heart disease. The National Health and Nutrition Examination Survey I (NHANES I) Epidemiologic Follow-up Study. Am J Epidemiol. 1995; 142: 53-63 [DOI] [PubMed] [Google Scholar]
- 29). Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999; 354: 650. [DOI] [PubMed] [Google Scholar]
- 30). Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991; 265: 627-631 [PubMed] [Google Scholar]
- 31). Sowers JR. Diabetes mellitus and cardiovascular disease in women. Arch Intern Med. 1998; 158: 617-621 [DOI] [PubMed] [Google Scholar]
- 32). Cook S, Hugli O, Egli M, Vollenweider P, Burcelin R, Nicod P, Thorens B, Scherrer U. Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly. 2003; 133: 360-363 [DOI] [PubMed] [Google Scholar]
- 33). Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007; 293: C584-596 [DOI] [PubMed] [Google Scholar]
- 34). Cappuccio FP, Strazzullo P, Farinaro E, Trevisan M. Uric acid metabolism and tubular sodium handling. Results from a population-based study. JAMA. 1993; 21: 354-359 [PubMed] [Google Scholar]
- 35). Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008; 300: 924-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003; 41: 1183-1190 [DOI] [PubMed] [Google Scholar]
- 37). Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998; 53: 613-625 [DOI] [PubMed] [Google Scholar]
- 38). Amaro S, Urra X, Gómez-Choco M, Obach V, Cervera A, Vargas M, Torres F, Rios J, Planas AM, Chamorro A. Uric acid levels are relevant in patients with stroke treated with thrombolysis. Stroke. 2011; 42: S28-32 [DOI] [PubMed] [Google Scholar]


