Abstract
Acute inflammation is a fundamental, protective response that orchestrates immune system to address harmful stimuli both from within and via invasion. New evidences indicate that the resolution of acute inflammation is not simply passive but active and highly regulated processes coordinated by new families of potent bioactive lipid mediators (LMs), coined specialized proresolving mediators (SPMs). These SPMs are biosynthesized from n-3 polyunsaturated fatty acids. Low concentrations of SPM (nM range) stimulate proresolving cellular processes, such as inhibition of neutrophil infiltration, enhancement of macrophage phagocytosis of bacteria and efferocytosis of cellular debris, and reduction of inflammatory pain through specific G-protein coupled receptors.
Of the many bioactive mediators that regulate inflammation resolution, low-dose carbon monoxide (CO) functions as a tissue-protective gaso-transmitter that is endogenously produced by the heme oxygenase (HO) system. Specific SPMs activate the HO system, which in turn enhances endogenous CO production locally, thus establishing a protective feed-forward circuit between SPMs and CO. In addition, treatment with low-dose CO and SPMs exerts protective effects against ischemia/reperfusion injury by decreasing leukocyte–platelet interaction and proinflammatory LM levels.
Recent studies reviewed herein assessed the impact of SPMs and low-dose inhaled CO on inflammatory diseases. LM metabololipidomics approach allows the assessment of the efficacy of novel treatments with SPMs and low-dose CO. Moreover, this approach indicates the regions where the action of individual LMs may be physiologically relevant and when these LMs are produced in vivo to serve their proresolving mediator functions that may also permit new directions for treating human diseases.
Keywords: Specialized proresolving mediator, Carbon monoxide, Resolution of inflammation
Introduction
Acute inflammation is a host protective response orchestrated by the innate immune system against harmful stimuli, such as infection or tissue damage. Temporally and locally coordinated cellular processes are involved in the resolution mechanisms of inflammation. However, when uncontrolled, inflammation leads to chronic disorders in different organs. It was once believed that simple passive dilution of proinflammatory chemical mediators at the inflammation site could stop ongoing inflammation, with subsequent responses ending with the loss of chemotactic gradients that direct cell traffic1, 2). Recent results from the Serhan laboratory indicate that the resolution of acute inflammation and return to homeostasis is not a passive process but an active biosynthetic process that is highly regulated and programmed at the tissue level by several newly discovered families of bioactive lipid mediators (LMs), which together are coined the specialized proresolving mediators (SPMs)1–3).
Of the many different endogenous small molecules contributing to the resolution of inflammation, carbon monoxide (CO) functions as a tissue-protective gaso-transmitter that is endogenously synthesized by heme oxygenase (HO) system4). Inhalation of low-dose CO exerts anti-inflammatory effects both in vivo and in vitro and protective effects against inflammatory diseases5). For example, inhalation of low-dose CO ameliorates collagen-induced arthritis, prevents bone destruction, and decreases anti-collagen antibody levels and osteoclast number6). In non-human primates, exposure to low-dose CO after LPS inhalation protects against lung inflammation, decreases TNF-α release in bronchoalveolar lavage fluid, and reduces airway neutrophil influx7). A recent study reported that specific SPMs activated the endogenous HO system, which in turn enhanced CO production locally, thus establishing a protective feed-forward circuit between SPMs and CO8). This review focuses on the new evidences indicating specific novel mechanisms of and functional relationships between SPMs and CO in resolving inflammation.
Resolution of Acute Inflammation is an Active Process
After infection or tissue damage, exogenous and endogenous chemical mediators are released at the injury site2). These chemical mediators regulate the permeability of vasculature and enhance the formation of inflammatory exudate and infiltration of polymorphonuclear neutrophils (PMNs) (Fig. 1A). The inflammatory exudate transfers water soluble fractions from circulating plasma and polyunsaturated fatty acids (PUFAs), such as n-3 PUFAs along with albumin9). These n-3 PUFAs are substrates for the biosynthesis of local-acting SPMs by exudate cells (Fig. 1B). SPMs function to temporally limit the further influx of PMNs into the inflammation sites in vivo and regulate the apoptosis of PMNs10). During the resolution of inflammation, SPMs enhance the infiltration and M2-type polarization of macrophages, which have anti-inflammatory and proresolving characteristics. In addition, SPMs promote macrophage-mediated phagocytosis of apoptotic PMNs (efferocytosis) and their trafficking to the lymph nodes and spleen11). These SPM-controlled active cellular processes contribute to the clearance of “inflammatory remnants” and establishment of tissue homeostasis.
Fig. 1.
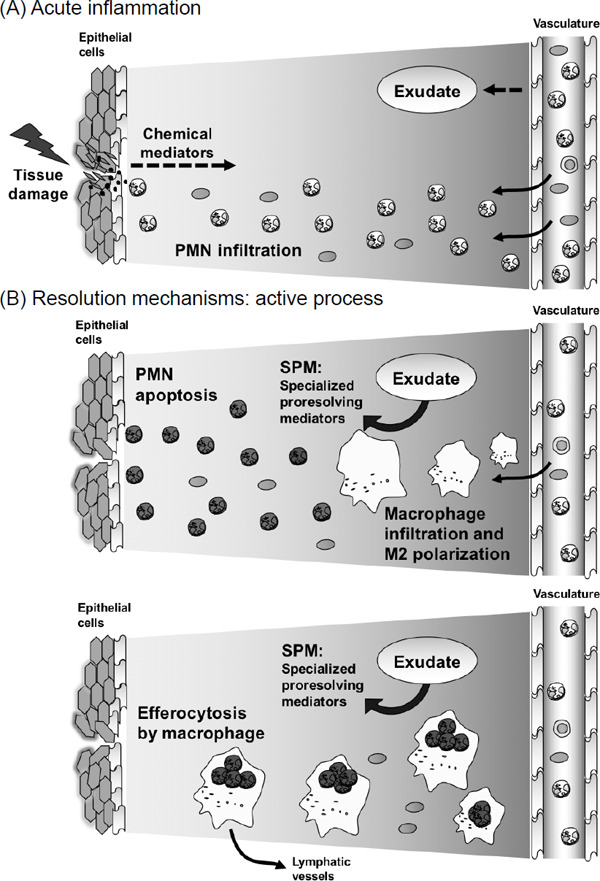
Resolution phase of acute inflammation is a highly coordinated and active process
(A) Acute inflammation. At the tissue damage site, exogenous and endogenous chemical mediators are released. These chemical mediators regulate the permeability of vasculature and enhance the formation of inflammatory exudate and infiltration of polymorphonuclear neutrophil (PMNs). (B) Resolution of acute inflammation. Specialized proresolving mediators, coined SPMs, are synthesized from inflammatory exudate through specific enzymatic conversions. SPMs stimulate resolution processes, such as enhancement of PMN apoptosis, macrophage infiltration and M2-type polarization, and macrophage-mediated efferocytosis. These SPM-controlled active cellular processes contribute to the resolution mechanisms.
Novel SPMs Derived from Arachidonic Acid, Eicosapentaenoic Acid, Docosahexaenoic Acid, and n-3 Docosapentaenoic Acid
The arachidonic acid (AA) bioactive metabolome includes cyclooxygenase (COX) products, such as prostaglandins (PGs) and thromboxanes (Txs), and 5-lipoxygenase (5-LO) products, such as leukotrienes (LTs), that are well-known proinflammatory bioactive LMs12) that function in the initiation phase of the acute inflammatory response1). Emerging evidences indicate that resolution of inflammation is an active process that is accompanied by the stereospecific biosynthesis of potent LMs that stimulate this resolution (Fig. 1), which has been reviewed in previous studies1, 2, 13). Potent LMs identified to date (Fig. 2) include AA-derived lipoxins (LXs)14, 15); n-3 eicosapentaenoic acid (EPA)-derived E-series resolvins (Rvs)16–19); and docosahexaenoic acid (DHA)-derived D-series Rvs20, 21), protectins22), and maresins23, 24). Fatty acids belonging to n-3 docosapentaenoic acid (DPA)-specific bioactive metabolome also serve as SPMs. Dalli et al. identified novel n-3 DPA Rvs, protectins, maresins25), and 13-series Rvs (RvTs)26) in human and mouse tissues. These compounds exert potent tissue-protective effects and induce enhanced resolution of acute inflammation and increased survival of mice during infections. RvTs regulate phagocytosis in humans and mice by stimulating bacterial phagocytosis and regulating inflammasome components. RvT biosynthesis during neutrophil–endothelial cell interaction is initiated by COX-2 and is increased after treatment with atorvastatin because of S-nitrosylation of COX-226). These findings may explain one of the possible mechanisms underlying the pleiotrophic and potentially beneficial effects of statin therapy observed in human studies.
Fig. 2.
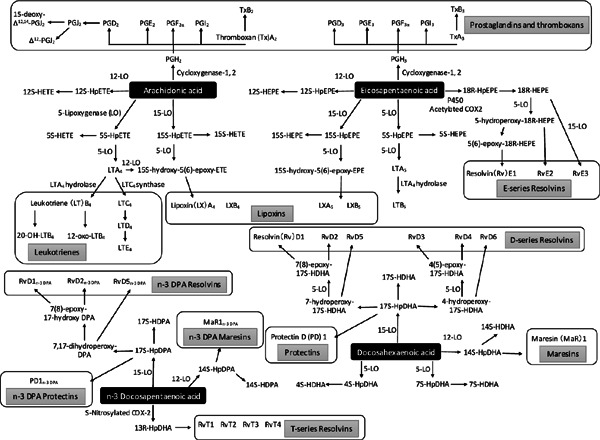
Bioactive LMs and SPM derived from AA, EPA, DHA, and n-3 DPA: essential fatty acids
AA metabolome includes COX products, such as PGs and Txs, and 5-LO products, such as LTs and LO interaction products LXs. EPA metabolome includes PGs, Txs, LTs, and E-series Rvs. DHA metabolome includes D-series Rvs, protectins, and maresins. n-3 DPA metabolome includes n-3 DPA Rvs, protectins, maresins, and recently discovered 13-series Rvs.
SPMs exert proresolving effects at low concentration, suggesting that they interact with specific cell surface receptors. Table 1 shows SPMs and their corresponding receptors. LXA4 and RvD1 act as agonists of ALX/FPR2 and DRV/GPR3227, 28). Recently, Chiang et al. reported a novel proresolving receptor GPR18 of RvD229). RvD3 and RvD5 exert proresolving effects through DRV/GPR3221, 30). E-Series Rvs act as agonists of ERV/ChemR2317, 31) and antagonists of LTB4 receptor BLT132).
Table 1. Proresolving lipid and gaseous mediators and their corresponding mechanism of function.
| Proresolving lipid mediators | Mechanism of function |
|---|---|
| Lipoxins | |
| LXA4 | agonist for ALX/FPR227), DRV/GPR3228) |
| Resolvin D-series | |
| RvD1 | agonist for ALX/FPR2, DRV/GPR3228) |
| RvD2 | agonist for GPR1829) |
| RvD3 | agonist for DRV/GPR3221) |
| RvD5 | agonist for DRV/GPR3230) |
| Resolvin E-series | |
| RvE1 | agonist for ERV/ChemR2317), antagonist for BLT132) |
| RvE2 | partial agonist for ERV/ChemR2331) |
| Proresolving gaseous mediators | Mechanisms of function |
| Carbon monoxide (CO) | mRNA, protein expression of 15-LO ↑8) |
| Enzymatic activity of 15-PGDH/EOR ↓8) | |
| Regulation of heme binding proteins4) | |
SPMs and CO Constitute a Proresolving Circuit
Inhalation of low-dose CO accelerates the resolution of acute inflammation, enhances macrophage-induced phagocytosis and efferocytosis in humans and mice, and regulates local LMs temporally8). The effect of CO on SPM production was demonstrated in human macrophages incubated with serum-treated zymosan (STZ). Treatment of these macrophages with CO (250 ppm, 8 h, 37°C) significantly decreased the levels of proinflammatory PGE2 and TxB2 but increased the levels of proresolving RvD1, RvD2, PD1, RvE1, and RvE3 (Fig. 3A). Moreover, CO treatment increased the mRNA and protein levels of 15-LO (Table 1). Specific Rvs are converted and metabolized through the 15-hydroxy PG dehydrogenase (15-PGDH)/eicosanoid oxidoreductase (EOR) pathway. CO decreases the enzymatic activity of 15-PGDH/EOR, thereby increasing SPM accumulation locally. Conversely, blockade of 15-LO expression in macrophages using specific shRNA decreases CO-induced SPM production8). Together, these results suggest that impacts of CO on the regulation of inflammatory responses are mediated by both decreased levels of proinflammatory LMs and increased levels of proresolving mediators such as specific SPMs.
Fig. 3.
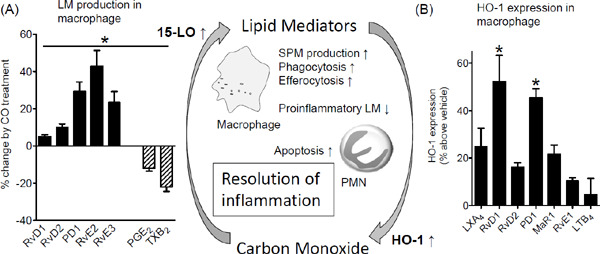
SPMs and CO constitute a novel proresolving circuit
(A) GM–CSF differentiated human macrophages were incubated with or without low-dose CO (250 ppm, 8 h, 37°C) and then incubated with serum-treated zymosan for 30 min. Results of LM metabololipidomics are expressed as percent changes by CO treatment; mean ± SEM, n = 4. *p < 0.05 versus normoxic incubation. (B) SPMs induce HO-1 with human macrophages. Human macrophages were incubated with the selected SPMs at 0.1 nM concentration. Cells were harvested, and HO-1 expressions were determined using FITC-labeled anti-HO-1 antibody. Results are mean ± SEM, n = 4. *p < 0.05 versus vehicle. Reproduced from reference8) with permission. Copyright 2013. The American Association of Immunologist, Inc.
SPMs directly activate the HO-1/CO pathway as a part of endogenous proresolving mechanisms. For example, HO-1 levels in human macrophages were monitored using flow cytometry with an FITC-conjugated anti-HO-1 antibody. A panel of SPMs (LXA4, RvD1, RvD2, PD1, MaR1, and RvE1) was tested to establish their rank order of potency. At 0.1 nM concentration, RvD1 was the most potent SPM because it significantly increased HO-1 levels by approximately 50% compared with vehicle, followed by PD1 (Fig. 3B). Equi-concentration of proinflammatory LTB4 did not significantly increase HO-1 levels. These results indicated that SPMs increased HO-1 expression and contributed to endogenous CO production in human macrophages at the tissue injury site.
These observations indicate that low-dose CO is a proresolving gaseous mediator. Particularly, inhalation of CO promotes the phagocytic ingestion of apoptotic PMNs (efferocytosis) and their exit to the lymphatic system8). In addition, these results highlight the association between the 15-LO/SPM and HO-1/CO pathways that amplify each other and constitute the proresolving circuit (Fig. 3).
CO and RvD1 Protect Against Ischemia/Reperfusion injury by Decreasing PMN–Platelet Interaction
Ischemia/reperfusion (I/R) injury is a major challenge in various clinical settings, such as cardiovascular, surgical, and critical care settings33, 34). During ischemia, inflammatory responses are activated by PMNs and platelets, which first occur locally. Initiation of reperfusion or reflow results in the release of activated PMNs, platelets, and inflammatory mediators. Virtually, all organs are susceptible to remote injury caused by leukocyte-mediated tissue damage. Inhalation of low-dose CO and intravenous administration of SPMs exert protective effects against PMN-mediated acute lung injury after I/R injury in a murine model35).
Histological analysis of samples from a murine model (Fig. 4A) indicated that I/R injury initiated second-organ lung injury through PMN infiltration. Inhalation of low-dose CO (250 ppm) or intravenous administration of RvD1 (500 ng/mouse) markedly decreased PMN infiltration into the lungs. Compared with CO or RvD1 treatment alone, combined treatment with CO (250 ppm) ansd RvD1 (500 ng) further decreased PMN infiltration into the lungs. These results indicated that CO and RvD1 protected the lungs from PMN-induced tissue injury during reflow by decreasing PMN infiltration. In addition, combined treatment with CO and RvD1 exerted additive tissue-protective actions.
Fig. 4.
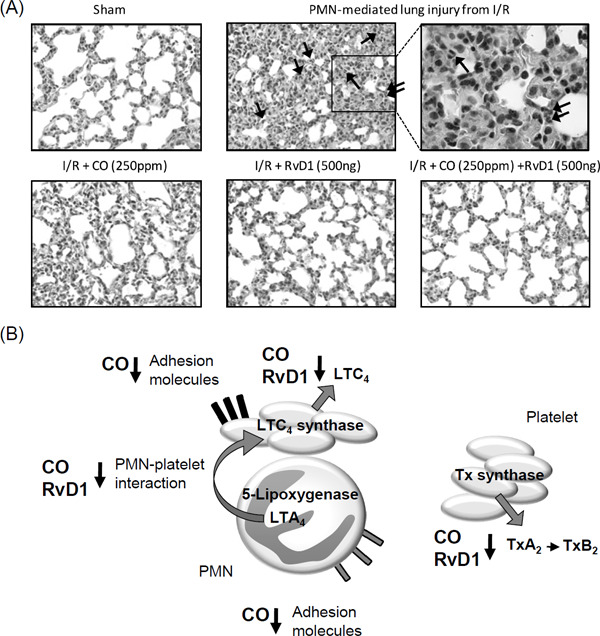
CO and RvD1 protect lungs against I/R injury by decreasing PMN–platelet interaction
Bilateral hindlimb ischemia was initiated using tourniquets placed on each hindlimb. Mice were subjected to hindlimb ischemia for 60 min, after which the tourniquets were removed to initiate reperfusion. Selected mice were kept in the low-dose CO chamber (250 ppm) for 60 min before the induction of hindlimb ischemia. RvD1 (500 ng) was intravenously administered to the tail vein 5 min before the start of reperfusion period. Following the reperfusion period (120 min), their lung damages were investigated. (A) I/R injured lung tissue histology with hematoxylin and eosin staining was shown (magnification: × 40; top-right, × 100). Arrows indicate infiltrated PMNs. (B) Proposed mechanisms of action of low-dose inhaled CO and intravenous administration of RvD1 on PMN–platelet interaction in lung protection after I/R injury. Reproduced from reference28) with permission. Copyright 2014. The American Physiological Society.
Each LM identified in the lung tissue was quantified earlier8, 35). Treatment with low-dose inhaled CO (250 ppm) or RvD1 (500 ng/mouse) alone as well as with the combination of CO and RvD1 significantly decreased the levels of proinflammatory LTs and Txs. Of these mediators, levels of cysteinyl LTs LTC4 and LTE4 were notably decreased by approximately 85%. It is now well appreciated that cellular aggregation and interactions between PMNs with platelets result in the transcellular biosynthesis of cysteinyl LTs (Fig. 4B)36, 37). LTA4 produced in PMNs from AA by the conversion of 5-LO is transferred to platelets interacting with these PMNs. In platelets, LTA4 is converted to LTC4 by LTC4 synthase. The murine I/R injury model showed a 2-fold increase in the number of PMN–platelet aggregates, indicating the transcellular biosynthesis of LTC4. Treatment with low-dose inhaled CO and RvD1 significantly decreased the formation of PMN–platelet aggregates and levels of proinflammatory cysteinyl LTs.
Taken together, these results suggested that the therapeutic potential of low-dose inhaled CO and SPMs in regulating various disease processes involving proinflammatory LMs and PMN–platelet interaction, such as PMN-mediated tissue injury35). These findings may be used for treating single-tissue I/R injury. Recently, Kain et al. reported that RvD1 activated an inflammation resolution response at splenic and ventricular sites in a murine model of myocardial infarction (MI)38). In these studies, RvD1 improved fractional shortening after MI and an early exit of neutrophils from the left ventricle and spleen, with an increased expression of LXA4 receptor (ALX). In addition, RvD1 promoted the resolution of acute inflammation induced by MI and delayed the onset of heart failure.
Metabololipidomics Approach with Human Samples
Potential involvement of SPMs in human tissue has been highlighted by the identification of SPMs in clinical samples. Levels of LXs were elevated in the mucosa during remission in ulcerative colitis individuals39). Decreased levels of LXA4 were detected in the blood of patients with localized aggressive periodontitis compared with those in healthy donors40). Levels of aspirin-triggered LXs were increased in the plasma of patients with type 2 diabetes who were treated with pioglitazone41), but were decreased in patients with symptomatic peripheral artery disease compared with those in healthy volunteers42). For example, RvE1 and RvE2 were identified in the peripheral blood of healthy volunteers17, 18). RvD1 and RvD2 were identified in human blood after supplementation with n-3 fatty acids43) and in the human adipose tissue44). PD1 was identified in exhaled breath condensates of people with asthma45). Decreased PD1 level in eosinophils was observed in patients with severe asthma compared with that in healthy individuals46). PD1 is also produced by embryonic stem cells47). By performing mass spectral identification, MaR1 was identified in the synovial fluid of patients with rheumatoid arthritis48).
To investigate LM–SPM in the human peripheral blood, wide-targeted LC–MS/MS-based LM metabololipidomics was performed using human plasma (Fig. 5A)49). In addition, total/free fatty acid profiling was performed using GC–MS procedure (Fig. 5B). The metabololipidomics approach showed that the plasma of baboons with Streptococcus pneumonia-induced pneumonia had significantly reduced levels of LM and SPMs, including RvE2 and LXs50). Inhalation of low-dose CO increased the levels of RvEs and LXs in the plasma and significantly decreased the levels of proinflammatory Txs relative to pre-exposure levels, indicating a systemic impact of low-dose CO inhalation on infection-initiated inflammation50).
Fig. 5.
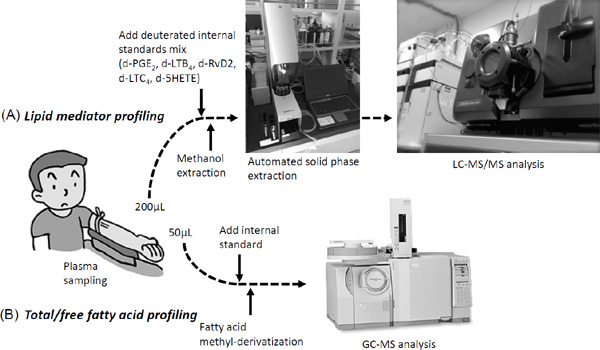
Targeted metabololipidomics approach with human tissues
(A) LM profiling with human plasma. To assess potential losses during processing, deuterated internal standards that marked specific chromatographic regions of interest were used. Internal labeled standards d8-5S-HETE, d4-LTB4, d5-RvD2, and d4-PGE2 (500 pg each) were added. Next, samples were held at −20°C for 60 min to allow protein precipitation and then centrifuged (1,200 g, 4°C, 10 min). Supernatants were collected and then placed into an automated extraction system. Solid-phase C18 cartridges (500 mg) were equilibrated with methanol and H2O. The samples were acidified by adding H2O (pH 3.5, HCl) and then rapidly loaded onto the conditioned C18 column that was washed with H2O to neutralize the acid followed by hexane. The products were eluted with methyl formate and methanol. The products were brought to dryness under gentle nitrogen flow and immediately suspended in methanol–water (50:50 vol/vol) for LC–MS/MS injection. The LC–MS/MS was operated in negative ionization mode using scheduled multiple reaction monitoring mode, followed by information-dependent enhanced product ion scanning for identification of lipid mediators with their fragmentation patterns. (B) Total/free fatty acid profiling with plasma. An internal standard was added to plasma, followed by methyl-derivatization with or without saponification. Total/free fatty acid profile was investigated by GC-MS procedure using selected ion monitoring.
This LC–MS/MS- and GC–MS-based comprehensive targeted metabololipidomics approach35, 49) allows further investigation of “when” and “where” each bioactive LM is biosynthesized at an optimal concentration to serve as a proresolving mediator for improving human health and for controlling various diseases.
Conclusion
SPMs and CO exert proresolving actions in inflammation and protect organ functions. In addition, SPMs and CO each enhance PMN apoptosis and macrophage-induced phagocytosis and efferocytosis. SPMs increase HO-1 expression, which in turn contributes to endogenous CO production. CO increases 15-lipoxygenase expression and decreases enzymatic activity of 15-PGDH that inactivates many of the local SPM, thus enhancing SPM accumulation locally. Together, SPMs and CO form a beneficial feed-forward loop for resolving inflammation. Combined treatment with SPMs and CO exerts several effects on disease processes involving uncontrolled inflammation to resolve the inflammation and to maintain homeostasis.
In a recent phase 2 clinical trial involving patients with dry eye syndrome, an RvE1 analog significantly improved signs and symptoms of corneal inflammation (http://www.resolvyx.com, U.S. Patent 7582785). This is the first trial to show the clinical efficacy of the novel class of Rv therapeutics that stimulate resolution rather than inhibit inflammatory mediators. A phase 3 clinical trial is in progress (Safety and Efficacy Study of RX-10045 on the Signs and Symptoms of Dry Eye; ClinicalTrials.gov. identifier: NCT00799552). Specific impact of low-dose inhaled CO is also under investigation in clinical trials in the field of respiratory medicine. Intubated ICU patients with sepsis-induced ARDS have been enrolled in a clinical trial designed to assess the safety and efficacy of treatment with low-dose (100–200 ppm) inhaled CO (ClinicalTrials.gov. Identifier: NCT02425579). Results of these clinical trials may provide new strategies to control inflammation by using proresolving SPMs and gaseous mediators in treating human diseases.
Acknowledgements
Masakazu Shinohara is partially supported by grants from the Uehara Memorial Foundation, Bayer Grants4Targets, Hyogo Science and Technology Association, Foundation for Total Health Promotion, and JSPS KAKENHI. Charles N. Serhan is supported by grants from the National Institutes of Health USA P01GM095467 and GM38765.
References
- 1). Serhan CN: Pro-resolving lipid mediators are leads for resolution physiology: Nature: 510: 7503 (92-101), 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Buckley CD, Gilroy DW, Serhan CN: Proresolving lipid mediators and mechanisms in the resolution of acute inflammation: Immunity: 40: 3 (315-27), 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Tabas I, Glass CK: Anti-inflammatory therapy in chronic disease: challenges and opportunities: Science: 339: 6116 (166-72), 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Mustafa AK, Gadalla MM, Snyder SH: Signaling by gasotransmitters: Sci Signal: 2: 68 (re2), 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Ryter SW, Choi AM: Gaseous therapeutics in acute lung injury: Compr Physiol: 1: 1 (105-21), 2011 [DOI] [PubMed] [Google Scholar]
- 6). Bonelli M, Savitskaya A, Steiner CW, Rath E, Bilban M, Wagner O, Bach FH, Smolen JS, Scheinecker C: Heme oxygenase-1 end-products carbon monoxide and biliverdin ameliorate murine collagen induced arthritis: Clin Exp Rheumatol: 30: 1 (73-8), 2012 [PubMed] [Google Scholar]
- 7). Mitchell LA, Channell MM, Royer CM, Ryter SW, Choi AM, McDonald JD: Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation: Am J Physiol Lung Cell Mol Physiol: 299: 6 (L891-7), 2010 [DOI] [PubMed] [Google Scholar]
- 8). Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, Serhan CN: Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits: Journal of immunology: 190: 12 (6378-88), 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN: Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution: Journal of immunology: 181: 12 (8677-87), 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). El Kebir D, Gjorstrup P, Filep JG: Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation: Proceedings of the National Academy of Sciences of the United States of America: 109: 37 (14983-8), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Schwab JM, Chiang N, Arita M, Serhan CN: Resolvin E1 and protectin D1 activate inflammation-resolution programmes: Nature: 447: 7146 (869-74), 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Samuelsson B: Role of basic science in the development of new medicines: examples from the eicosanoid field: J Biol Chem: 287: 13 (10070-80), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Serhan CN: Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways: Annual review of immunology: 25: (101-37), 2007 [DOI] [PubMed] [Google Scholar]
- 14). Serhan CN, Hamberg M, Samuelsson B: Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes: Biochemical and biophysical research communications: 118: 3 (943-9), 1984 [DOI] [PubMed] [Google Scholar]
- 15). Serhan CN, Hamberg M, Samuelsson B: Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes: Proceedings of the National Academy of Sciences of the United States of America: 81: 17 (5335-9), 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K: Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing: The Journal of experimental medicine: 192: 8 (1197-204), 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN: Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1: The Journal of experimental medicine: 201: 5 (713-22), 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN: Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation: The Journal of clinical investigation: 121: 2 (569-81), 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H: Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid: J Biol Chem: 287: 13 (10525-34), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL: Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals: The Journal of experimental medicine: 196: 8 (1025-37), 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN: Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents: Chemistry & biology: 20: 2 (188-201), 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN: Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation: J Biol Chem: 278: 17 (14677-87), 2003 [DOI] [PubMed] [Google Scholar]
- 23). Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M: Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions: The Journal of experimental medicine: 206: 1 (15-23), 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA: Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain: FASEB journal : official publication of the Federation of American Societies for Experimental Biology: 26: 4 (1755-65), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Dalli J, Colas RA, Serhan CN: Novel n-3 immunoresolvents: structures and actions: Scientific reports: 3: (1940), 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Dalli J, Chiang N, Serhan CN: Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections: Nat Med: 21: 9 (1071-5), 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Fiore S, Maddox JF, Perez HD, Serhan CN: Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor: The Journal of experimental medicine: 180: 1 (253-60), 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN: Resolvin D1 binds human phagocytes with evidence for proresolving receptors: Proceedings of the National Academy of Sciences of the United States of America: 107: 4 (1660-5), 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Chiang N, Dalli J, Colas RA, Serhan CN: Identification of resolvin D2 receptor mediating resolution of infections and organ protection: The Journal of experimental medicine: 212: 8 (1203-17), 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN: Infection regulates proresolving mediators that lower antibiotic requirements: Nature: 484: 7395 (524-8), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN: Resolvin E2 formation and impact in inflammation resolution: Journal of immunology: 188: 9 (4527-34), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN: Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation: Journal of immunology: 178: 6 (3912-7), 2007 [DOI] [PubMed] [Google Scholar]
- 33). Eltzschig HK, Eckle T: Ischemia and reperfusion--from mechanism to translation: Nature medicine: 17: 11 (1391-401), 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Eltzschig HK, Carmeliet P: Hypoxia and inflammation: N Engl J Med: 364: 7 (656-65), 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Shinohara M, Kibi M, Riley IR, Chiang N, Dalli J, Kraft BD, Piantadosi CA, Choi AM, Serhan CN: Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1: Am J Physiol Lung Cell Mol Physiol: 307: 10 (L746-57), 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Brady HR, Serhan CN: Adhesion promotes transcellular leukotriene biosynthesis during neutrophil-glomerular endothelial cell interactions: inhibition by antibodies against CD18 and L-selectin: Biochemical and biophysical research communications: 186: 3 (1307-14), 1992 [DOI] [PubMed] [Google Scholar]
- 37). Fiore S, Serhan CN: Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils: The Journal of experimental medicine: 172: 5 (1451-7), 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV: Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function: J Mol Cell Cardiol: 84: (24-35), 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Vong L, Ferraz JG, Dufton N, Panaccione R, Beck PL, Sherman PM, Perretti M, Wallace JL: Up-regulation of Annexin-A1 and lipoxin A(4) in individuals with ulcerative colitis may promote mucosal homeostasis: PLoS One: 7: 6 (e39244), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE: Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1: PLoS One: 6: 9 (e24422), 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Gutierrez AD, Sathyanarayana P, Konduru S, Ye Y, Birnbaum Y, Bajaj M: The effect of pioglitazone treat ment on 15-epi-lipoxin A4 levels in patients with type 2 diabetes: Atherosclerosis: 223: 1 (204-8), 2012 [DOI] [PubMed] [Google Scholar]
- 42). Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS: Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis: The American journal of pathology: 177: 4 (2116-23), 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Mas E, Croft KD, Zahra P, Barden A, Mori TA: Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation: Clin Chem: 58: 10 (1476-84), 2012 [DOI] [PubMed] [Google Scholar]
- 44). Claria J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN: Diversity of lipid mediators in human adipose tissue depots: American journal of physiology Cell physiology: 304: 12 (C1141-9), 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN: Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness: Journal of immunology: 178: 1 (496-502), 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K: Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma: J Allergy Clin Immunol: 131: 2 (353-60 e1-2), 2013 [DOI] [PubMed] [Google Scholar]
- 47). Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G: Metabolic oxidation regulates embryonic stem cell differentiation: Nat Chem Biol: 6: 6 (411-7), 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, Serhan CN, Mayboroda OA: Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS: Biochimica et biophysica acta: 1821: 11 (1415-24), 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN: Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue: American journal of physiology Cell physiology: 307: 1 (C39-54), 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Dalli J, Kraft BD, Colas RA, Shinohara M, Fredenburgh LE, Hess DR, Chiang N, Welty-Wolf K, Choi AM, Piantadosi CA, Serhan CN: The Regulation of Proresolving Lipid Mediator Profiles in Baboon Pneumonia by Inhaled Carbon Monoxide: Am J Respir Cell Mol Biol: 53: 3 (314-25), 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]


