Abstract
Aim and Methods: The high-density lipoprotein 2 cholesterol (HDL2-C) to HDL3-C ratio is associated with insulin resistance, high-molecular-weight adiponectin (HMW-Ad), and metabolic syndrome (MetS) components and is useful for evaluating MetS in Japanese individuals. We investigated potential associations between changes in HDL2-C/HDL3-C and changes in MetS components, insulin resistance, adipocytokine, lipids, and lifestyle habits in 892 Japanese subjects who underwent annual health examinations twice at a mean interval of 1.1 years. Study subjects were divided into three groups on the basis of HDL2-C/HDL3-C changes.
Results: Average changes in waist circumference (WC) and HDL-C were significantly lower and higher, respectively, in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C change groups compared with those in the reference group (< 0 HDL2-C/HDL3-C change). Among MetS components, average changes in HDL2-C/HDL3-C were associated with changes in WC and HDL-C. Average changes in HMW-Ad and the homeostasis model assessment of insulin resistance were significantly higher and lower, respectively, in the ≥ 0.5 HDL2-C/HDL3-C change group compared with those in the reference group. In addition, the average low-density lipoprotein cholesterol (LDL-C) gradually decreased as HDL2-C/HDL3-C increased. The average change in LDL-C was significantly lower in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C change groups than in the reference group. Moreover, a ≥ 0.5 HDL2-C/HDL3-C change positively correlated with good lifestyle statuses in terms of smoking, physical activity, and alcohol consumption.
Conclusion: Changes in HDL2-C/HDL3-C inversely correlated with changes in WC, insulin resistance, and LDL-C and positively correlated with HMW-Ad and good lifestyle habits. Therefore, HDL2-C/HDL3-C changes comprise a useful marker for both MetS and atherogenic conditions in Japanese population.
Keywords: HDL subtype, Metabolic syndrome, Insulin resistance, Lifestyle habit, Annual health examination
Introduction
High-density lipoprotein (HDL) particles are heterogeneous with respect to density, size, composition, and surface charge. These particles can be separated into two main subfractions, HDL2 and HDL3, on the basis of their density after ultracentrifugation1). The inverse association between the HDL cholesterol (HDL-C) concentration and coronary heart disease (CHD) is well known; however, the associations between HDL subclasses and CHD remain controversial2).
Visceral obesity is part of a phenotype that includes dysfunctional subcutaneous adipose tissue expansion and ectopic triglyceride storage and is closely related to the clustering of cardiometabolic risk factors. The various metabolic alterations that closely associate with this condition include hypertriglyceridemia, increased free fatty acid availability, release of proinflammatory cytokines from adipose tissue, liver insulin resistance and inflammation, increased very low-density lipoprotein synthesis and secretion in the liver, reduced clearance of triglyceride-rich lipoproteins; the presence of small, dense low-density lipoprotein (LDL) particles; and reduced HDL-C levels3). Clinicians often face the burden of educating overweight and obese people regarding the health risks associated with obesity and the appropriate strategies for weight reduction4). The waist circumference (WC) is a simple marker of abdominal obesity and a strong predictor of morbidity and mortality that is independent of the body mass index (BMI)5–7). WC is advantageous because it allows a crude estimation of the absolute amount of visceral adipose tissue; in other words, a reduction in the waistline with weight loss is a clear sign of abdominal fat loss, which is almost always associated with increased insulin activity8, 9). The improved insulin resistance resulting from chronic physical activity, which is independent of weight change, likely involves changes in body composition, particularly a reduction in visceral fat10).
Although HDL-C levels have been shown to be cardioprotective11), recent data have questioned the cardioprotective nature of HDL. In patients at high cardiovascular risk, the inhibition of cholesteryl ester transfer protein, which increases HDL-C levels, has been shown to increase the risk of cardiovascular disease and death from cardiovascular causes12). Although patients receiving niacin treatment had significantly higher HDL-C levels at 2-year follow-up, the rate of adverse cardiovascular events in that group did not significantly differ from that in the placebo group13). Another study revealed that the carriers of a single nucleotide polymorphism in the endothelial lipase gene (LIPG Asn396Ser) had significantly higher levels of HDL-C; however, this polymorphism was not associated with a risk of myocardial infarction14).
Rohatgi et al. recently reported that the HDL-C efflux capacity is a relevant predictive biomarker of cardiovascular events15). Several reports indicated that elevated levels of prebeta-1 HDL, the sole acceptor for cholesterol effluxed from macrophages through the ABCA1 transporter, exhibited a strong positive correlation with CHD risk16–19). These results suggested that all HDL species might not equally contribute to cholesterol efflux. However, cholesterol efflux assays that require special equipment, techniques, and kits with limited availabilities are not widely accepted. Cholesterol efflux capacity will need to be compared with HDL particle concentration and other biomarker assays before becoming widely accepted as a risk-predicting method. For instance, HDL subclass measurements indicated that HDL3 levels were more predictive of carotid artery disease status than were HDL2, HDL-C, or apolipoprotein A1 levels20).
Recently, we demonstrated the importance of analyzing the levels of HDL-C subclasses, as well as the overall HDL-C levels, when evaluating an individual's lifestyle habits21–23). In Japanese male subjects with ≤ 2 risk factors for metabolic syndrome (MetS), HDL-C increased as homeostasis model assessment of insulin resistance (HOMA-IR) decreased. However, in subjects with ≥ 3 risk factors for MetS, HOMA-IR increased when HDL-C was ≥ 90 mg/dL21). Female subjects who were not obese, did not smoke, and drank < 75 g alcohol/day had elevated HDL-C levels, which were associated with improved insulin sensitivity. Alcohol intake of > 75 g/day appeared to provide no advantages in terms of changes in HDL-C or HOMA-IR levels22). HDL2-C levels were associated with alcohol consumption, WC, smoking, exercise, and gender. However, HDL3-C levels were only associated with alcohol consumption23). Both HDL2-C and HDL3-C have been reported to exhibit inverse associations with the incidence of CHD. However, the relative values of these two HDL subclasses as risk predictors remain unknown2). Lagos et al. recently reported that lower HDL-C levels in patients with MetS resulted from reductions in both the large and small HDL subclasses24). As the number of MetS components increases, the HDL phenotype includes a greater percentage of small HDL3 and a lesser percentage of large HDL2 molecules, resulting in a lower HDL2/HDL3 ratio. Furthermore, we reported that the HDL2-C/HDL3-C ratio was associated with MetS components, insulin resistance, and high-molecularweight adiponectin (HMW-Ad) levels and was thus useful for evaluating MetS in Japanese individuals25). Given our21–23, 25) and other reports24) and noting that HDL-C measurement alone is not satisfactory for risk assessment11–15, 20), it becomes apparent that not only HDL-C but also HDL subclasses and ratios should be investigated with respect to CHD risk, obesity, MetS, and lifestyle habits. Because it is unknown whether the HDL subclass ratio varies with obesity and MetS, the present study examined whether changes in the HDL2-C/HDL3-C ratio after a mean 1.1-year follow-up period were associated with changes in MetS components, insulin resistance, adipocytokine, lipids, and lifestyle habits in Japanese adults who underwent annual health examinations.
Methods
Subjects
A total of 937 subjects underwent baseline annual health examinations at the Health Evaluation and Promotion Center at Tokai University Hachioji Hospital between April 2011 and March 2014; follow-up health examinations were performed at the same institute between January 2012 and March 2015 at a mean follow-up interval of 1.1 years. These examinations included HDL subclass analyses. Medical history information was obtained through self-administered questionnaires and interviews conducted by nurses. After excluding 45 subjects who were undergoing treatment for diabetes mellitus, 892 subjects were included in this study. Among these 892 subjects, 134 were under medication for dyslipidemia, and 195 were using antihypertensive drugs at the time of follow-up.
At the first annual health examination, the results of the examinations were explained to all subjects, and well-trained medical doctors and nurses provided instructions to all patients regarding lifestyle improvements.
It should be noted that no fatal atherosclerotic vascular events were recorded during the 1.1-year study period.
Measurements
The WC was measured at the level of the umbilicus while the subject was standing and during slight expiration. Blood pressure (BP) was measured on the upper right arm using an automatic blood pressure monitor (TM-2655P; A&D, Tokyo, Japan) while the subject was seated. Blood samples were collected early in the morning after an overnight fast. Fasting immunoreactive insulin (FIRI) was measured using a fluorescence enzyme immunoassay (ST AIA-PACK IRI; Toso, Tokyo, Japan). The HOMA-IR was calculated as follows: fasting plasma glucose (FPG) (mg/dL) × FIRI (mU/mL)/40526). LDL-C, HDL-C, and triglyceride (TG) levels were measured using visible spectrophotometry (Determiner L LDL-C, Determiner L HDL-C, and Determiner L TG II, respectively; Kyowa Medex, Tokyo, Japan). The HDL2-C and HDL3-C levels were determined via ultracentrifugation. Briefly, after plasma was centrifuged using L-60 (Beckman Coulter, Brea, USA) at 223,00 × g for 4 hours at plasma density (d = 1.063 kg/L) and at a solvent density of 1.125 kg/L, adjusted by adding solid KBr, 40% volume from the top was aspirated, yielding of HDL (a) and HDL3 (b) fractions. The cholesterol concentration of each fraction was measured, and HDL2-C was calculated as follows; [(a) − (b) × 1.54] × 0.627). HMW-Ad was measured using a chemiluminescent enzyme immunoassay based on a monoclonal antibody to human HMW-Ad (Fujirebio, Tokyo, Japan). Smoking, habitual exercise, physical activity, and alcohol drinking were recorded as the self-reported current smoking status (continued smoking and initiated smoking after the first annual examination), current exercise activity (continued exercise < 2 times/week and initiated exercise > 2 times/week after the first annual examination), current physical activity (continued activity < 1 hour/day and initiated activity > 1 hour/day after the first annual examination), and current alcohol consumption (drank alcohol every day at both the baseline and follow-up), respectively. All subjects provided verbal consent for the use of anonymized health records in the study analysis. The study protocol was approved by the institutional ethics committee of the Tokai University School of Medicine.
Statistical Analysis
The significance of pairwise comparisons was determined using a paired t-test. Associations between changes in the HDL2-C/HDL3-C ratio and mean changes in various parameters and comparisons of the mean values among more than two groups were performed using the Dunnett's multiple comparisons test; subjects with HDL2-C/HDL3-C ratio changes < 0 were used as the reference group. Statistical analyses were performed using the SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA). All P values were two-tailed, and a P-value of < 0.05 was considered statistically significant.
Results
Table 1 lists the baseline and follow-up characteristics of the subjects. The mean ages at baseline were 59.1 years for men and 58.3 years for women. Among men, the HDL-C, HDL2-C, and HDL2-C/HDL3-C ratio were significantly higher, and BMI and WC were significantly lower at follow-up than those at baseline. Among women, the HDL-C was significantly higher and BMI was significantly lower at follow-up than that at baseline.
Table 1. Characteristics of study subjects at baseline and follow-up.
| Men (n = 524) |
Women (n = 368) |
|||||
|---|---|---|---|---|---|---|
| ①Baseline | ②Follow-up | Change (②-①) | ①Baseline | ②Follow-up | Change (②-①) | |
| BMI (kg/m2) | 24.2 ± 3.0 | 24.1 ± 3.0 | −0.1 ± 0.8* | 22.5 ± 3.3 | 22.3 ± 3.1 | −0.2 ± 1.0** |
| Waist circumference (cm) | 85.8 ± 8.3 | 85.1 ± 8.2 | −0.7 ± 3.2** | 80.6 ± 9.4 | 80.3 ± 9.0 | −0.4 ± 4.0 |
| Systolic BP (mmHg) | 124.0 ± 15.9 | 124.1 ± 16.1 | 0.1 ± 12.8 | 117.0 ± 18.3 | 118.1 ± 18.2 | 1 ± 12.7 |
| Diastolic BP (mmHg) | 78.2 ± 12.1 | 78.8 ± 11.6 | 0.6 ± 9.7 | 71.4 ± 12.1 | 71.7 ± 12.1 | 0.3 ± 9.9 |
| FPG (mg/dL) | 102.7 ± 11.8 | 103.0 ± 11.0 | 0.3 ± 8.0 | 98.7 ± 10.5 | 98.9 ± 14.7 | 0.2 ± 10.9 |
| FIRI (µIU/mL) | 6.56 ± 4.62 | 6.52 ± 4.60 | −0.04 ± 3.01 | 5.68 ± 3.33 | 5.56 ± 3.08 | −0.12 ± 2.63 |
| HOMA-IR | 1.69 ± 1.24 | 1.70 ± 1.32 | 0.01 ± 0.90 | 1.42 ± 0.93 | 1.40 ± 0.94 | −0.02 ± 0.80 |
| LDL-C (mg/dL) | 122.0 ± 27.8 | 121.8 ± 27.0 | −0.2 ± 21.6 | 129.4 ± 31.4 | 129.1 ± 30.8 | −0.3 ± 21.8 |
| TG (mg/dL) | 129.4 ± 166.4 | 124.8 ± 100.0 | −4.6 ± 110.3 | 96.3 ± 45.8 | 96.3 ± 65.7 | 0.0 ± 59.0 |
| HDL-C (mg/dL) | 59.0 ± 13.9 | 60.6 ± 14.4 | 1.58 ± 7.42** | 72.9 ± 16.2 | 74.3 ± 16.9 | 1.4 ± 7.4** |
| HDL2-C (mg/dL) | 37.0 ± 11.8 | 38.4 ± 11.9 | 1.35 ± 7.41** | 50.3 ± 15.5 | 50.2 ± 15.6 | −0.1 ± 7.6 |
| HDL3-C (mg/dL) | 21.8 ± 3.6 | 21.6 ± 3.5 | −0.13 ± 2.80 | 21.5 ± 3.1 | 21.6 ± 3.4 | 0.1 ± 3.1 |
| HDL2-C/HDL3-C | 1.72 ± 0.60 | 1.79 ± 0.58 | 0.07 ± 0.41* | 2.38 ± 0.79 | 2.37 ± 0.80 | −0.01 ± 0.52 |
| HMW-Ad (µg/mL) | 2.87 ± 1.78 | 2.84 ± 1.83 | −0.03 ± 0.69 | 5.53 ± 3.30 | 5.51 ± 3.15 | −0.02 ± 1.31 |
Variables are given as means ± standard deviations.
BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; FIRI, fasting immunoreactive insulin; HOMA-IR, homeostasis model assessment-insulin resistance; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol; HMW-Ad, high-molecular-weight adiponectin
p < .001
p < .05 (① vs. ②) by paired t-test.
Table 2 shows the changes in parameters stratified according to changes in the HDL2-C/HDL3-C ratio. Study subjects were divided into three groups according to the changes in this ratio. The numbers of subjects with HDL2-C/HDL3-C ratio changes of < 0, 0 to < 0.5, and ≥ 0.5 were 404 (female: 186, 46.0%), 370 (female: 127, 34.3%), and 118 (female: 53, 44.9%), respectively. The mean average HDL2-C/HDL3-C ratio changes among subjects in the < 0, 0 to < 0.5, and ≥ 0.5 groups were −0.34 ± 0.34, 0.22 ± 0.14, and 0.75 ± 0.23 (mean ± SD), respectively.
Table 2. Changes in parameters stratified by changes in HDL2-C/HDL3-C.
| Changes in HDL2-C/HDL3-C |
Total (n = 892) |
|||
|---|---|---|---|---|
| < 0 (n = 404) |
0–< 0.5 (n = 370) |
≥ 0.5 (n = 118) |
||
| BMI (kg/m2) | 0.0 ± 0.8 | −0.2 ± 0.8* | −0.5 ± 1.2** | −0.1 ± 0.9 |
| Waist circumference (cm) | 0.0 ± 3.3 | −0.7 ± 3.6** | −1.9 ± 4.0** | −0.5 ± 3.5 |
| Systolic BP (mmHg) | 0.8 ± 12.3 | 0.4 ± 13.4 | −0.8 ± 12.4 | 0.5 ± 12.9 |
| Diastolic BP (mmHg) | −0.3 ± 9.8 | 1.5 ± 9.8** | −0.1 ± 9.8 | 0.5 ± 9.8 |
| FPG (mg/dL) | 0.9 ± 8.9 | −0.1 ± 10.9 | −0.6 ± 7.9 | 0.3 ± 9.3 |
| TG (mg/dL) | 2.1 ± 48.9 | −6.5 ± 132.4 | −7.2 ± 40.8 | −2.7 ± 92.6 |
| HDL-C (mg/dL) | −0.7 ± 6.8 | 2.4 ± 6.9* | 6.5 ± 7.9* | 1.5 ± 7.4 |
Variables are given as means ± standard deviations.
HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol
p < .01
p < .05 by Dunnett's multiple comparison test, change in HDL2-C/HDL3-C < 0 used as a reference.
The changes in BMI and WC were significantly smaller in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C ratio change groups than those in the reference group (< 0 HDL2-C/HDL3-C ratio change group). Changes in HDL-C were significantly higher in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C ratio change groups than those in the reference group.
Table 3 presents lifestyle status data stratified by changes in the HDL2-C/HDL3-C. Differences were observed in the ≥ 0.5 HDL2-C/HDL3-C ratio change group when compared with < 0.5 and 0 to < 0.5 HDL2-C/HDL3-C ratio change groups, particularly a lower percentage of current smokers, lower percentage of subjects who exercised < 2 times/week, higher percentage of subjects who initiated exercise, lower percentage of subjects with < 1 hour/day of physical activity, higher percentage of subjects who initiated > 1 hour/day of physical activity, and lower percentages of subjects who drank alcohol everyday both at the baseline and follow-up. Overall, higher changes in the HDL2-C/HDL3-C ratio positively correlated with good lifestyle habits.
Table 3. Lifestyle status stratified by changes in HDL2-C/HDL3-C.
| Changes in HDL2-C/HDL3-C |
Total (n = 892) |
||||
|---|---|---|---|---|---|
| < 0 (n = 404) |
0–< 0.5 (n = 370) |
≥ 0.5 (n = 118) |
|||
| Smoking | Current smoker Stop smoking |
51 (13%) 7 (2%) |
51 (14%) 4 (1%) |
8 (7%) 2 (2%) |
110 (12%) 13 (1%) |
| Exercise | No exercise Initiated exercise |
243 (60%) 33 (8%) |
200 (54%) 26 (7%) |
62 (53%) 16 (14%) |
505 (57%) 75 (8%) |
| Physical activity | Inactive Became active |
204 (50%) 38 (9%) |
159 (43%) 46 (12%) |
44 (37%) 18 (15%) |
407 (46%) 102 (11%) |
| Alcohol consumption | Drank alcohol everyday both at the baseline and follow-up | 102 (25%) | 96 (26%) | 17 (14%) | 215 (24%) |
Variables are given as number of subjects (percentage of total).
HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol
Fig. 1 shows the stratification of average changes in HDL2-C and HDL3-C according to the changes in the HDL2-C/HDL3-C ratio. A gradual average increase in HDL2-C was observed as the HDL2-C/HDL3-C ratio increased. This average change in HDL2-C was significantly higher in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C ratio change groups than in the reference group. In contrast, a gradual average decrease in HDL3-C was observed as the HDL2-C/HDL3-C ratio increased. The average change in HDL3-C was significantly lower in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C ratio change groups than in the reference group.
Fig. 1.
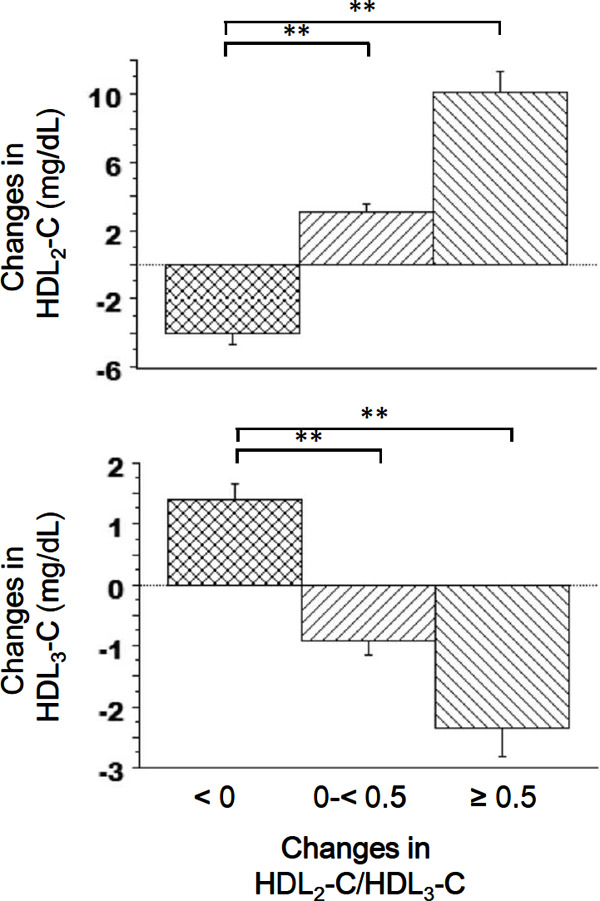
Changes in the mean HDL2-C and HDL3-C values stratified by changes in the HDL2-C/HDL3-C ratio. Error bars represent 95% confidence intervals. **P < 0.01 (Dunnett's multiple comparisons test).
HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol.
Fig. 2 presents a stratification of the average changes in HWM-Ad according to the changes in the HDL2-C/HDL3-C ratio. HMW-Ad gradually increased as the HDL2-C/HDL3-C ratio increased. The average change in HMW-Ad was significantly higher in the ≥ 0.5 HDL2-C/HDL3-C ratio change group than in the reference group.
Fig. 2.
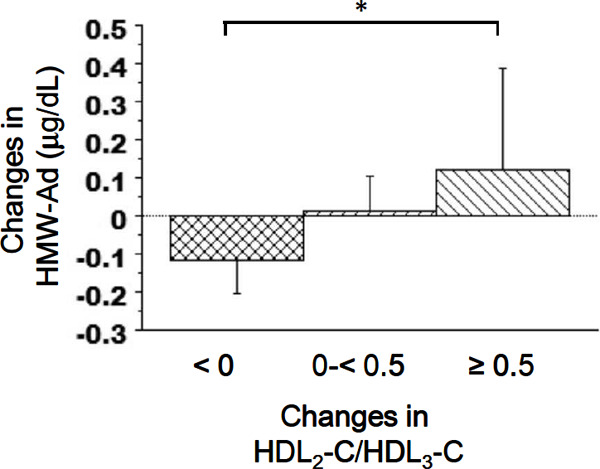
Changes in the mean HMW-Ad values stratified by changes in the HDL2-C/HDL3-C ratio. Error bars represent 95% confidence intervals. *P < 0.05 (Dunnett's multiple comparisons test).
HMW-Ad, high-molecular-weight adiponectin; HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol.
Fig. 3 presents a stratification of the average changes in HOMA-IR according to the changes in the HDL2-C/HDL3-C ratio. The average change in HOMA-IR was significantly lower in the ≥ 0.5 HDL2-C/HDL3-C ratio change group than in the reference group.
Fig. 3.
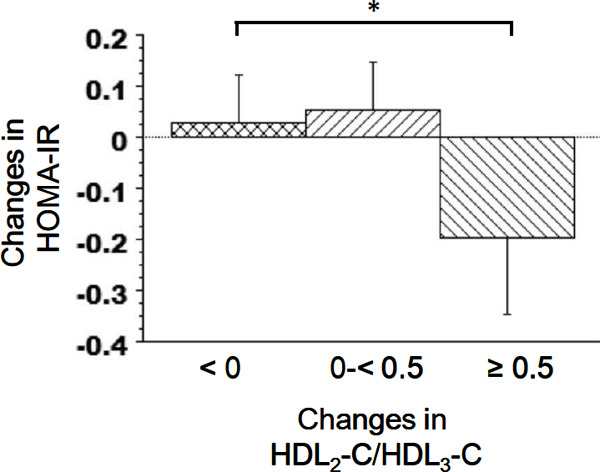
Changes in the mean HOMA-IR values stratified by changes in the HDL2-C/HDL3-C ratio. Error bars represent 95% confidence intervals. *P < 0.05 (Dunnett's multiple comparisons test).
HOMA-IR, homeostasis model assessment of insulin resistance; HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol.
Fig. 4 presents a stratification of the average changes in LDL-C according to the changes in the HDL2-C/HDL3-C ratio. LDL-C gradually decreased as the HDL2-C/HDL3-C ratio increased. The average change in LDL-C was significantly lower in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C ratio change groups than in the reference group.
Fig. 4.
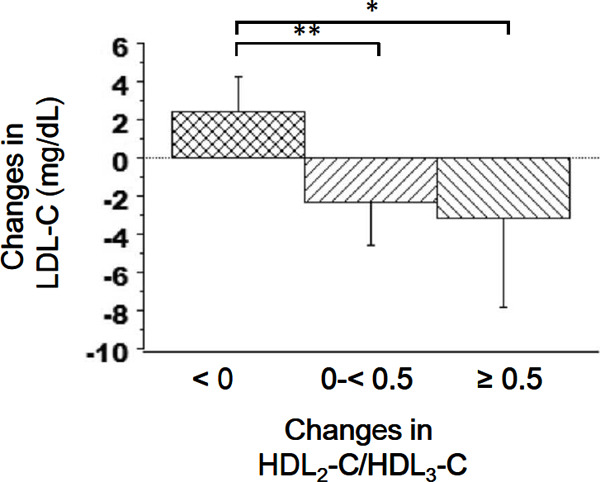
Changes in the mean LDL-C values stratified by changes in the HDL2-C/HDL3-C ratio. Error bars represent 95% confidence intervals. **P < 0.01, *P < 0.05 (Dunnett's multiple comparisons test).
LDL-C, low-density lipoprotein cholesterol; HDL2-C, high-density lipoprotein 2 cholesterol; HDL3-C, high-density lipoprotein 3 cholesterol.
Discussion
In this study, we found that changes in HDL2-C/HDL3-C inversely correlated with changes in WC, insulin resistance, and LDL-C and positively correlated with HMW-Ad and good lifestyle habits. These results suggest that an evaluation of HDL-C alone is not sufficient and that changes in the HDL2-C/HDL3-C ratio serve as a useful marker of not only MetS but also atherogenic conditions in Japanese individuals. To the best of our knowledge, this is the first study to describe associations between changes in the HDL2-C/HDL3-C ratio and changes in MetS components, HMW-Ad, insulin resistance, LDL-C, and lifestyle habits.
Insulin resistance is considered an underlying abnormality in most cases of type 2 diabetes; because diabetes treatment leads to improved insulin resistance28), we excluded subjects who were undergoing treatment for diabetes mellitus.
Subjects in the ≥ 0.5 HDL2-C/HDL3-C ratio change group exhibited improvements in obesity and increased HDL-C and HMW-Ad levels. In contrast, changes in BP and FPG were not associated with changes in the HDL2-C/HDL3-C ratio. A gradual decrease in the average TG was observed as the HDL2- C/HDL3-C ratio increased, but this difference was not significant between the reference and other groups. However, further obesity amelioration might improve the BP, FPG, and TG values. Further studies will be needed to clarify this speculation.
Although insulin resistance drives the overproduction of very low-density lipoproteins, and subsequently LDL, an increased number of LDL particles is not currently a part of the definition of MetS29). Therefore, it is important to note that subjects with MetS do not always have elevated LDL-C levels. In this study, we observed a gradual average decrease in LDL-C as the HDL2-C/HDL3-C ratio increased, suggesting that an improvement in insulin resistance correlated with a decrease in the LDL-C level. Subjects with MetS have been described to exhibit a qualitative LDL abnormality30). Moreover, a 4%–9% reduction in body weight has been shown to decrease the small-dense LDL level by 4.7%31). Our results indicated significantly greater decreases in BMI and WC in the 0 to < 0.5 and ≥ 0.5 HDL2-C/HDL3-C ratio change groups than in the reference group. Therefore, the gradual average decrease in LDL-C, which occurred as the HDL2-C/HDL3-C ratio increased, was likely due to decreased levels of LDL subclasses (e.g., small-dense and/or oxidized LDL). It would be interesting to determine whether this is true in future studies.
When subjects were stratified according to changes in the HDL2-C/HDL3-C ratio, the ≥ 0.5 HDL2-C/HDL3-C ratio change group had a lower smoking rate and a lower percentage of subjects who drank alcohol every day, compared with the < 0.5 and 0 to < 0.5 HDL2-C/HDL3-C ratio change groups. The same group contained fewer inactive subjects and fewer subjects who did not exercise, as well as a higher percentage of subjects who initiated exercise after the first annual examination. Collectively, these findings indicated that subjects with good lifestyle habits had increased HDL2-C/HDL3-C ratios. Further studies are needed to investigate the relationship between improvements in lifestyle habits and changes in the HDL2-C/HDL3-C ratio.
We observed similar results after the exclusion of subjects who were under treatment for dyslipidemia (n = 134) (data not shown).
Limitations of our study were the lack of detailed information regarding treatments for hypertension and dyslipidemia. The possibility of confounding by mutations in lipid-related genes also exists, although these data were not available in the present study. The subjects of this study were middle-aged Japanese individuals, and it is possible that the associations between HDL2-C/HDL3-C ratio changes and MetS components, HMW-Ad, insulin resistance, and lipids will be affected by age and ethnicity.
Conclusion
We found that changes in HDL2-C/HDL3-C inversely correlated with changes in WC, insulin resistance, and LDL-C and positively correlated with HMW-Ad and good lifestyle habits. Our data suggest that monitoring changes in the HDL subclass ratio might help to identify changes in MetS, HMW-Ad, insulin resistance, LDL-C, and lifestyle habits, at least in Japanese populations.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- 1). Glomset JA: The plasma lecithin: cholesterol acyltransferase reaction. J Lipid Res, 1968; 9: 155-167 [PubMed] [Google Scholar]
- 2). Sweetnam PM, Bolton CH, Yarnell JW, Bainton D, Baker IA, Elwood PC, Miller NE: Associations of the HDL2 and HDL3 cholesterol subfractions with the development of ischemic heart disease in British men: the Caerphilly and Speedwell Collaborative Heart Disease Studies. Circulation, 1994; 90: 769-774 [DOI] [PubMed] [Google Scholar]
- 3). Tchernof A, Després JP: Pathophysiology of human visceral obesity: an update. Physiol Rev, 2013; 93: 359-404 [DOI] [PubMed] [Google Scholar]
- 4). Ross R, Janiszewski PM: Is weight loss the optimal target for obesity-related cardiovascular disease risk reduction? Can J Cardiol, 2008; 24: 25D-31D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. INTERHEART Study Investigators: Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet, 2005; 366: 1640-1649 [DOI] [PubMed] [Google Scholar]
- 6). Janiszewski PM, Janssen I, Ross R: Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care, 2007; 30: 3105-3109 [DOI] [PubMed] [Google Scholar]
- 7). Bigaard J, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI: Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res, 2003; 11: 895-903 [DOI] [PubMed] [Google Scholar]
- 8). Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ: Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol, 1994; 73: 460-468 [DOI] [PubMed] [Google Scholar]
- 9). McAuley KA, Smith KJ, Taylor RW, McLay RT, Williams SM, Mann JI: Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obesity, 2006; 30, 342-349 [DOI] [PubMed] [Google Scholar]
- 10). Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I: Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med, 2000; 133: 92-103 [DOI] [PubMed] [Google Scholar]
- 11). Castelli WP: Cardiovascular disease and multifactorial risk: challenge of the 1980s. Am Heart J, 1983; 106: 1191-1200 [DOI] [PubMed] [Google Scholar]
- 12). Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, ILLUMINATE Investigators : Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 13). AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 14). Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, H_olm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AF, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki M-L, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PIW, Klungel OH, Maitland van der Zee A-H, Peters BJM, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VHM, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, VandeWerf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S: Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet, 2012; 380: 572-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW: HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med, 2014; 371: 2383-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Guey LT, Pullinger CR, Ishida BY, O'Connor PM, Zellner C, Francone OL, Laramie JM, Naya-Vigne JM, Siradze KA, Deedwania P, Redberg RF, Frost PH, Seymour AB, Kane JP, Malloy MJ: Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol, 2011; 108: 360-366 [DOI] [PubMed] [Google Scholar]
- 17). Miida T, Nakamura Y, Inano K, Matsuto T, Yamaguchi T, Tsuda T, Okada M: Pre beta 1-high-density lipoprotein increases in coronary artery disease. Clin Chem, 1996; 42: 1992-1995 [PubMed] [Google Scholar]
- 18). Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ: Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol, 2000; 20: 2670-2676 [DOI] [PubMed] [Google Scholar]
- 19). Sethi AA, Sampson M, Warnick R, Muniz N, Vaisman B, Nordestgaard BG, Tybjaerg-Hansen A, Remaley AT: High pre-beta1 HDL concentrations and low lecithin: cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of HDL-cholesterol. Clin Chem, 2010; 56: 1128-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ, Jarvik GP: HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J Am Heart Assoc, 2014; 3: e000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Hiratsuka N, Yamada C, Mitsuhashi T, Inabe F, Araida N, Takahashi E. Significance of high HDL cholesterol levels in Japanese men with metabolic syndrome. Intern Med, 2011; 50: 2113-2120 [DOI] [PubMed] [Google Scholar]
- 22). Moriyama K, Takahashi E, Negami M, Otsuka H, Mitsuhashi T, Tsurugano S, Inabe F, Hiratsuka N. Evaluation of high-density lipoprotein cholesterol levels in Japanese women. Tokai J Exp Clin Med, 2012; 37: 77-83 [PubMed] [Google Scholar]
- 23). Moriyama K, Takahashi E: Relationships of high-density lipoprotein 2 and 3 cholesterols with lifestyle habit factors in Japanese adults. Ningen Dock International 2014; 1: 54-62 [Google Scholar]
- 24). Lagos KG, Filippatos TD, Tsimihodimos V, Gazi IF, Rizos C, Tselepis AD, Mikhailidis DP, Elisaf MS: Alterations in the high density lipoprotein phenotype and HDL-associated enzymes in subjects with metabolic syndrome. Lipids, 2009; 44: 9-16 [DOI] [PubMed] [Google Scholar]
- 25). Moriyama K, Negami M, Takahashi E. HDL2-cholesterol/HDL3-cholesterol ratio was associated with insulin resistance, high-molecularweight adiponectin, and components for metabolic syndrome in Japanese. Diabetes Res Clin Pr, 2014; 106: 360-365 [DOI] [PubMed] [Google Scholar]
- 26). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 27). Bronzert TJ, Brewer HB., Jr. New micromethod for measuring cholesterol in plasma lipoprotein fractions. Clin Chem, 1977; 23: 2089-2098 [PubMed] [Google Scholar]
- 28). Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, Troupin B, Day WW: Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care, 2014; 37: 912-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Krauss RM, Siri PW: Dyslipidemia in type 2 diabetes. Med Clin North Am, 2004; 88: 897-909 [DOI] [PubMed] [Google Scholar]
- 30). Nozue T, Michishita I, Ishibashi Y, Ito S, Iwaki T, Mizuguchi I, Miura M, Ito Y, Hirano T: Small dense low-density lipoprotein cholesterol is a useful marker of metabolic syndrome in patients with coronary artery disease. J Atheroscler Thromb, 2007; 14: 202-207 [DOI] [PubMed] [Google Scholar]
- 31). Despres JP, Golay A, Sjostrom L: Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med, 2005; 353: 2121-2134 [DOI] [PubMed] [Google Scholar]


