Abstract
Aim
Hyperlipidemia and diabetic retinopathy increase the risk of cardiovascular disease (CVD). The standard versus intEnsive statin therapy for hypercholesteroleMic Patients with diAbetic retinopaTHY (EMPATHY) study examines whether intensive lipid-lowering therapy is superior to standard therapy in reducing the incidence of cardiovascular events in patients with hyperlipidemia and diabetic retinopathy, but without a history of coronary artery disease.
Methods
Patients who had elevated low-density lipoprotein cholesterol (LDL-C) and diabetic retinopathy without a history of coronary artery disease were eligible for the study. Patients were randomly assigned in a 1:1 ratio to receive intensive or standard therapy. Patients are being treated with monotherapy with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (statin) for a maximum of 5.5 years to achieve the following LDL-C target: <70 mg/dL for the intensive therapy group or ≥ 100 and <120 mg/dL for the standard therapy group. The primary endpoint is a composite of incidence of CVD and death from CVD.
Results
Between May 2010 and October 2013, 5,995 patients were assessed for eligibility, and 5,144 were assigned to the study treatment (2,571 and 2,573 in the intensive and standard therapy groups, respectively), and baseline data were analyzed from 5,107 (2,550 in the intensive therapy group and 2,557 in the standard therapy group).
Conclusions
This is the first study assessing the benefits of intensive statin therapy in patients with hypercholesterolemia and diabetic retinopathy in a primary prevention setting. Furthermore, this study evaluates the appropriateness of the treat-to-target approach because all patients are treated to achieve specific LDL-C targets by titrating statin therapy.
Clinical Trial Registration Number: UMIN000003486.
Keywords: Dyslipidemia, Diabetic retinopathy, Hydroxymethylglutaryl-CoA reductase inhibitors, Randomized controlled trial, Primary prevention
Introduction
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for cardiovascular disease (CVD) such as myocardial infarction, angina pectoris, stroke, and peripheral arterial disease. Previous results of randomized controlled trials have indicated that lipid-lowering therapy with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) is effective for reducing the event rate of CVD in a wide range of individuals1). On the basis of these findings, a recent clinical guideline has identified elevated LDL-C as the primary target of lipid-lowering therapy and has recommended several therapeutic approaches2).
Hyperglycemia is another major risk factor for CVD3). The results of observational studies suggest that diabetic patients without previous myocardial infarction have as high a risk of myocardial infarction as nondiabetic patients with previous myocardial infarction4, 5). Elevated LDL-C is a powerful risk factor for coronary artery disease in patients with diabetes mellitus (DM)6), and the effectiveness of lipid-lowering therapy in this population has been established by meta-analysis of randomized controlled trials7). These findings provide a rationale for intensive lipid-lowering therapy in patients who have both hypercholesterolemia and DM, and these high-risk patients have been treated to achieve strict target LDL-C goals2, 3). However, previous randomized studies have included only a limited number of patients with DM7), and the effectiveness of lipid-lowering therapy in such subpopulations has not been completely clarified, particularly in those with diabetic complications.
Diabetic retinopathy is a common chronic microvascular complication of DM8). In patients with type 2 DM and retinopathy, the risk of incident coronary heart disease and ischemic stroke is elevated independently of known risk factors9, 10). Diabetic retinopathy is strongly associated with all-cause mortality8). Therefore, this form of microvascular disease may contribute to the development of CVD. This concept is supported by study results showing that approximately 25% of patients with diabetic retinopathy had significant stenotic coronary artery disease11). Based on these findings, diabetic patients with retinopathy may need more intensive therapy than those without retinopathy.
The effectiveness of lipid-lowering therapy in reducing the event rate of CVD has been well established in Japan12–15), and elevated LDL-C is a powerful risk factor for CVD in patients with DM16). However, for primary prevention of CVD in patients with DM, the target level of LDL-C in the Japanese guidelines17) is not as low as in guidelines in the United States2, 3). This suggests that the appropriateness of the Japanese target level should be reconsidered.
Furthermore, the American College of Cardiology (ACC)/American Heart Association (AHA) Task Force on Practice Guideline endorses a paradigm shift in strategies for reducing the events of CVD18). This guideline calls for adjusting the intensity of statin therapy based on individual patient risk rather than lowering lipid levels to prespecified targets18, 19). It also recommends moderate-intensity statin therapy for primary prevention of CVD in patients with DM 40–75 years of age18). These statements in the guideline are derived from the fact that most clinical studies have used fixed-dose regimens, and few studies have investigated the effect of high-intensity statin therapy in a primary prevention setting. However, there is a possibility that more aggressive lipid target levels or high-intensity statin therapy may benefit high-risk patients, particularly those with diabetic retinopathy.
Aim
The standard versus intEnsive statin therapy for hypercholesteroleMic Patients with diAbetic retinopa-THY (EMPATHY) study is currently being conducted to determine whether intensive statin therapy is superior to standard therapy in reducing the incidence of cardiovascular (CV) events in patients with hypercholesterolemia and diabetic retinopathy who have no history of coronary artery disease. In this study, we compare the effectiveness of different target LDL-C levels by titrating statin therapy to achieve specific targets. This article reports the design of the study in detail.
Methods
Study Design and Ethical Considerations
This multicenter, prospective randomized, open-label, blinded-endpoint study is being conducted in Japan in accordance with the Declaration of Helsinki and Japanese ethical guidelines for clinical studies. A total of 769 centers (325 hospitals and 444 clinics) are participating in the study. The protocol was reviewed and approved by the institutional review board of each participating center. All patients provided written informed consent. This study has been underway since May 2010, and patient recruitment ended in October 2013. The study is registered with the University Hospital Medical Information Network clinical trials registry, number UMIN000003486.
Study Population
Table 1 shows the inclusion and exclusion criteria. Patients aged at least 30 years who had no history of coronary artery disease were eligible for the study if they had diabetic retinopathy and elevated LDL-C with or without lipid-lowering therapy.
Table 1. Inclusion and exclusion criteria.
1) Inclusion criteria
|
2) Exclusion criteria
|
LDL-C, low-density lipoprotein cholesterol; CAD, coronary artery disease; PAD, peripheral artery disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; TG, triglyceride.
Values of LDL-C are calculated by the following Friedewald equation; LDL-C=total cholesterol (TC) −[high-density lipoprotein cholesterol (HDL-C) + TG/5] (when TG values are less than 400 mg/dL) or measured by direct homogeneous assay. Values measured within 3 months before obtaining informed consent can be used for assessing eligibility.
If patients are treated with atorvastatin, pitavastatin, or rosuvastatin, they should receive no more than the following doses: atorvastatin 10 mg/day, pitavastatin 2 mg/day, rosuvastatin 2.5 mg/day.
Treatment
Fig. 1 summarizes the study design. The study consists of a run-in period (4–8 weeks) and a treatment period (2–5.5 years). During the run-in period, patients received statin monotherapy to achieve a target LDL-C level of ≥ 100 and <120 mg/dL and were assessed for eligibility. The diagnosis of diabetic retinopathy was confirmed by an ophthalmologist by the end of the run-in period. Thereafter, eligible patients were randomly assigned in a 1:1 ratio to receive intensive or standard therapy. The allocation sequence was computer generated by a data center and stratified by sex, age (<60 or ≥ 60 years), and baseline hemoglobin A1c (HbA1c) level (< 8.4% or ≥ 8.4%). If eligibility of the patient was confirmed at the end of the run-in period, the investigator contacted the data center and was notified of the allocated treatment. The person generating the allocation sequence was not involved in patient enrollment.
Fig. 1.
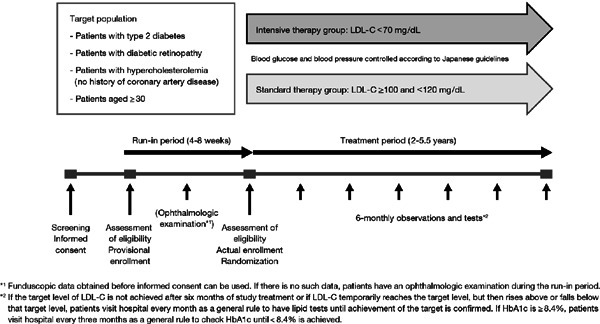
Summary of the study design
LDL-C, low-density lipoprotein cholesterol; HbA1c, hemoglobin A1c
During the treatment period, patients receive statin monotherapy to achieve an LDL-C target of < 70 mg/dL for the intensive therapy group or ≥ 100 and < 120 mg/dL for the standard therapy group. LDL-C levels are calculated from the Friedewald formula. These LDL-C targets are based on guidelines in the United States and Japan, respectively3, 17). Statin dose escalation and switching to another statin are permitted in both groups. Individual investigators are permitted to select the statin of their choice as mono-therapy, during the run-in and treatment periods.
Concomitant treatment with the following lipid-lowering drugs is prohibited: fibrates, ezetimibe, ethyl icosapentate, anion exchange resins, probucol, nicotinic acid derivatives, phytosterols, elastase, dextran sulfate sodium sulfur, pantethine, and polyenephosphatidylcholine.
Patients are treated with antidiabetic drugs to achieve a target HbA1c level of < 6.9% in both groups20). They are also treated with antihypertensive drugs to achieve a blood pressure target of < 130/80 mmHg21).
Outcomes
Medical histories, physical examination findings, and laboratory data were obtained for all patients at the beginning of the run-in period. During the treatment period, body weight, blood pressure, pulse rate, and laboratory data are measured every 6 months. Laboratory data include lipids (total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglyceride, apolipoprotein A1, apolipoprotein B, and small dense LDL); HbA1c; glucose; insulin; hematology, hepatic, and renal function tests; serum electrolytes (Na, K, and Cl); creatine kinase; and urinalysis (albumin, creatinine, protein, and urinary sugar). In addition, pleiotropic effects, including antioxidative and anti-inflammatory effects, have been reported to contribute to the effects of statins in treatment to reduce LDL-C22, 23). For an auxiliary report of the usefulness of the treatment, explored in this research, brain natriuretic peptide (BNP), high-sensitive C-reactive protein (hsCRP), and high-molecular weight adiponectin are measured every 12 months. Lipid levels, BNP, hsCRP, high-molecular weight adiponectin, and serum creatinine are analyzed at a central laboratory (SRL Inc., Tokyo, Japan). Electrocardiograms are recorded every 6 months. Funduscopy is performed every 12 months. Statin treatment compliance, concomitant use of other drugs, and adverse events are periodically investigated throughout the study.
The study endpoints are shown in Table 2. The primary endpoint is defined as a composite of incidence of CVD and death from CVD. Most of the previous large-scale clinical studies of statins have used coronary artery disease and stroke as primary endpoints. However, beginning in the 2000s, there has been increasing recognition of the concept of chronic kidney disease (CKD), and a growing understanding that ischemic heart disease, cerebrovascular disease, peripheral vascular disease, and renal impairment are ischemic conditions stemming from arteriosclerosis, and that these conditions are associated with each other. Based on this growing understanding, we set a wider range of primary endpoints of such pathologies based on arteriosclerosis. This range included renal events, which are rarely used in composite endpoints. The clinical significance of establishing the necessity of lipid control in terms of the onset and progression of renal events is based on the findings of a meta-analysis of randomized control and crossover studies of statins, which showed inhibition of proteinuria and mild inhibition of progression of nephropathy24). Similar findings have also been reported in Japanese patients25). The secondary endpoints are defined as follows: all-cause mortality; occurrence of cardiac, cerebral, renal, and vascular events; occurrence of stroke; prespecified changes in laboratory variables associated with CKD; and safety (adverse events and adverse drug reactions). Primary and secondary endpoints are adjudicated by an event evaluation committee whose members are unaware of the treatment allocation. Definition of events and items assessed for safety are listed in Tables 3 and 4.
Table 2. Primary and secondary endpoints.
| 1) Primary endpoints The primary endpoint is the combined incidence of cardiovascular events or death associated with cardiovascular events. Cardiovascular events are defined as follows.
|
| 2) Secondary endpoints The secondary endpoints are defined as follows.
|
PAD, peripheral artery disease; eGFR, estimated glomerular filtration rate
Table 3. Definitions of endpoints.
|
Table 4. Definitions related to adverse events.
| 1) Definition of an adverse event An adverse event is defined as follows. An “adverse event” is defined as “any unfavorable medical event experienced by a patient or subject receiving a drug. It does not necessarily refer only to those events for which there is a clear causal relationship with the said drug. Briefly, an adverse event means any unfavorable or unintended sign (including an abnormal laboratory finding), symptom, or disease associated with the use of a drug that may or may not be considered related to, or caused by, the drug.” Developments such as a change in blood glucose, etc., aggravation of diabetes mellitus, or a change in serum lipid levels associated with the target disease (diabetic retinopathy complicated by hypercholesterolemia) do not constitute adverse events unless the change is excessive and contraindicates continuation of the study. In addition, if abnormal changes in laboratory findings, vital signs, or ECG results associated with signs, symptoms, or disease are observed, the sign, symptom, or disease will be reported as an adverse event. Laboratory findings outside the range specified in the Study Protocol will be reported as additional information regarding the adverse event. |
| 2) Definition of a serious adverse event Those adverse events that satisfy any of the following definitions will be regarded as “serious adverse events.”
|
| 3) Definition of an adverse reaction All adverse events other than those classified as “not related” to statin therapy will be handled as adverse reactions due to statin therapy. If an adverse event occurs, the investigator will record the name of the adverse event, date of onset, severity, seriousness/non-seriousness, changes (if any) in statin therapy, changes (if any) in treatment with drugs other than statin, outcome and date outcome confirmed, causal relationship with statin, and causal relationship with concomitant drugs. The survey of adverse events will be performed during the observation period and the study treatment period. |
Discontinuation or Suspension of the Entire Study
The principal investigators will examine the feasibility of continuation of the study if any of the following situations are encountered: (1) Important safety or efficacy information associated with the study is obtained. (2) On the basis of the results of the interim analysis, the Independent Data Monitoring Committee deems that the study has achieved its objective(s) prior to the scheduled number of subjects being reached or completion of the scheduled study period. (3) If changes to the protocol, as specified by the Independent Data Monitoring Committee, are difficult to accommodate. If the Independent Data Monitoring Committee recommends or requires that the study be discontinued, the principal investigators will discontinue the study. In the event that the principal investigators decide to discontinue or suspend the study, the principal investigators will promptly communicate that fact and the reason for suspension in writing to the heads of the participating test sites.
Quality Management
During the study, the participating institutions and investigators are periodically monitored by contract research organizations. In addition, a prespecified proportion of the institutions is scheduled to undergo data audit including direct access to source data. This study is conducted under contract among the following organizations: the funder (Shionogi & Co., Ltd. Osaka, Japan), participating institutions, and the contract research organizations.
Statistical Considerations
A sample size of 5,000 patients (2,500 in each group) was selected to detect the superiority of intensive therapy with a power of 80% and a two-sided significance level of 5%. Based on earlier reports in the literature26–29), we estimated that the incidence of CV events during the 3-year treatment period would be 4.1% and 2.7% for the standard and intensive therapy groups, respectively. The sample size was chosen based on these estimates, assuming a withdrawal rate of 15%, and a study period of 4.5 years was selected.
The main analysis set for efficacy will be the full analysis set (FAS). For primary and secondary endpoints, the same analysis will be performed in the per-protocol set (PPS) as in FAS, and the consistency of results for the two sets will be examined. FAS will consist of all randomly allocated subjects except for those for whom efficacy endpoints were not observed. PPS will consist of FAS less nonqualifying cases, untreated cases, protocol violations, and noncompliance cases. The safety analysis set will consist of those subjects who receive the study treatment at least once and from whom any information pertaining to safety is obtained. In all analyses, each subject will be included in the allocation group.
A log-rank test, stratified by sex, age, and baseline HbA1c, will be used to compare the primary endpoint between the treatment groups. The hazard ratio and its 95% confidence interval will be estimated using the stratified Cox proportional hazards model. During the study, an interim analysis is planned once at a prespecified time point adjusted by the Lan-DeMets α spending function with O'Brien-Fleming boundaries. Results of the interim analysis are to be assessed by an Independent Data Monitoring Committee.
Results
Fig. 2 shows a flow chart of the study patients. Between May 2010 and October 2013, 5,995 patients were enrolled into the run-in period, and 5,144 patients were randomized to treatment (2,571 to the intensive therapy group and 2,573 to the standard therapy group). Of that number, 37 were excluded from analysis because of unavailability of baseline information; reasons included data loss due to the major earthquake in eastern Japan in March 2011, major deviations from study ethics, and withdrawal of patient consent. The analysis population was thus 5,107 patients (2,550 in the intensive therapy group and 2,557 in the standard therapy group).
Fig. 2.
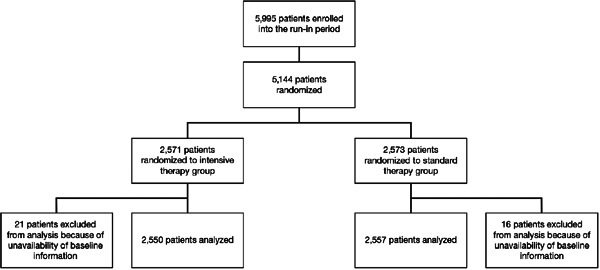
Flow chart of the study patients
The baseline characteristics of the patients enrolled into the treatment period are shown in Table 5. The baseline characteristics were well balanced between the treatment groups. Mean LDL-C levels decreased from the run-in period to the beginning of the treatment period in both groups. Retinopathic complications were present in less than 100% of subjects because 20 subjects had previously undergone laser therapy for retinopathy and 8 subjects were erroneously registered as having retinopathy at the time of enrollment. Those patients were included in the FAS analysis.
Table 5. Baseline characteristics.
| Intensive therapy group (n = 2,550) n (%) | Standard therapy group (n = 2,557) n (%) | |
|---|---|---|
| Sex, Male | 1,213 (47.6%) | 1,219 (47.7%) |
| Age (mean ± SD) | 63.0 ± 10.8 | 63.2 ± 10.4 |
| Body mass index (kg/m2) (mean ± SD) | 25.69 ± 4.25 | 25.59 ± 4.36 |
| Anti-hyperlipidemia medicine§ | ||
| None | 1,122 (44.0%) | 1,053 (41.2%) |
| One drug | 1,421 (55.7%) | 1,501 (58.7%) |
| Two drugs | 7 (0.3%) | 3 (0.1%) |
| Smoking† | 465 (18.2%) | 490 (19.2%) |
| Family history of CAD | 326 (12.8%) | 318 (12.4%) |
| Family history of cerebrovascular disease | 497 (19.5%) | 531 (20.8%) |
| Duration of diabetes (yr, mean ± SD) | 12.8 ± 8.6 | 13.0 ± 9.0 |
| Diabetic complication | ||
| Retinopathy | 2,543 (99.7%) | 2,546 (99.6%) |
| Neuropathy | 756 (29.6%) | 764 (29.9%) |
| Nephropathy | 1,320 (51.8%) | 1,270 (49.7%) |
| Hypertension | 1,769 (69.4%) | 1,791 (70.0%) |
| PAD | 117 (4.6%) | 98 (3.8%) |
| Funduscopy | ||
| Simple retinopathy | 1,706 (66.9%) | 1,692 (66.2%) |
| Preproliferative retinopathy | 424 (16.6%) | 480 (18.8%) |
| Proliferative retinopathy | 396 (15.5%) | 368 (14.4%) |
| Hemoglobin A1c (%)6 [mean ± SD (n)] | 7.77 ± 1.27 (2,550) | 7.77 ± 1.25 (2,557) |
| LDL-C (mg/dL)‖ [mean ± SD (n)] | ||
| Beginning of the run-in period§§ | ||
| Patients who had not received lipid-lowering therapy previously | 143.1 ± 23.5 (714) | 140.4 ± 22.9 (666) |
| Patients who had received previous therapy | 120.5 ± 22.1 (917) | 120.9 ± 23.5 (958) |
| Beginning of the treatment period | ||
| Patients who had not received lipid-lowering therapy previously | 98.4 ± 25.8 (1,101) | 97.7 ± 24.6 (1,033) |
| Patients who had received previous therapy | 112.3 ± 25.8 (1,398) | 112.0 ± 25.0 (1,460) |
| All | 106.2 ± 26.7 (2,499) | 106.0 ± 25.8 (2,493) |
| SBP (mmHg)†† [mean ± SD (n)] | 134.7 ± 16.9 (2,526) | 134.5 ± 16.2 (2,536) |
| DBP (mmHg)†† [mean ± SD (n)] | 74.9 ± 11.6 (2,526) | 74.7 ± 11.1 (2,536) |
CAD, coronary artery disease; PAD, peripheral artery disease; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Values were calculated at the beginning of the run-in period.
The categories of “Past Smoker” and “Non Smoker” were combined as “Not Current Smoker.”
Values were calculated at the time of consent.
Values were calculated using the Friedewald equation; LDL-C=total cholesterol (TC)∓[high-density lipoprotein cholesterol (HDL-C) + triglyceride (TG)/5].
Values were measured at each study center. Only data obtained by the Friedewald equation were used for totaling.
Values were calculated at the beginning of the treatment.
Discussion and Conclusion
We are conducting this study to determine whether intensive statin therapy is superior to standard therapy in reducing CV events in patients who have hypercholesterolemia and diabetic retinopathy in a primary prevention setting.
Although the risk of CV events is as high in diabetic patients with no previous history of CV events as in nondiabetic patients with previous myocardial infarction4, 5), few studies have investigated the benefits of intensive statin therapy for the primary prevention of CVD in these high-risk patients, especially those with diabetic complications. In previous randomized controlled trials that assessed the benefits of intensive therapy, only a limited proportion of patients with DM were included, and the studies assessed the benefits of statins in secondary prevention30–34). Thus, the benefits of intensive therapy for primary prevention in high-risk patients remain poorly elucidated. It may be desirable to reconsider the currently recommended lipid-lowering targets in Japan, in light of international recommendations based on the study results described above.
There is also clear evidence that diabetic patients with retinopathy have a higher risk of CVD than those without retinopathy. In the previous cohort studies in patients with type 2 DM, the presence of diabetic retinopathy was associated with a higher risk of incident coronary heart disease and ischemic stroke9, 10). To our knowledge, however, no studies have assessed the effectiveness of statin therapy in this subpopulation. Our results will add new findings to the existing data, because approximately 5,000 patients with diabetic retinopathy will be followed for 2–5.5 years.
In our study, we treat all patients to achieve specific LDL-C targets by titrating statin therapy instead of using fixed-dose regimens. In other words, we aim to compare the effectiveness of different LDL-C targets. Recently, the 2013 ACC/AHA Task Force Guideline was unable to find evidence to support titrating statin therapy to achieve LDL-C targets, because most clinical studies confirming the effectiveness of statins have used fixed-dose regimens18). However, the treat-to-target approach was recommended in a guideline from the Adult Treatment Panel III2) and a related Japanese guideline also adopted this approach17). Under these circumstances, it is worthwhile to conduct a clinical study to determine the appropriateness of the treat-to-target approach.
To ensure the reliability of study records, a prespecified proportion of the institutions are scheduled to undergo data audit including direct access to source data. Although data audit should be conducted in all interventional clinical trials, it has been omitted in some Japanese clinical trials except for those aimed at new drug applications. Especially in large-scale, longterm trials in patients with chronic disease, data audit has seldom been scheduled. Thus, our results may also give some insights into the quality of clinical trials in Japan.
Within the category of baseline characteristics, mean LDL-C levels decreased during the run-in period in both groups.
In conclusion, this is the first study assessing the benefits of intensive statin therapy in patients with hypercholesterolemia and diabetic retinopathy who do not have a history of coronary artery disease. It also evaluates the appropriateness of the treat-to-target approach by titrating statin therapy to achieve specific LDL-C targets.
Acknowledgements
The study is funded by Shionogi & Co., Ltd. Shionogi & Co., Ltd. does not participate in data collection or event evaluation, does not have data access rights, and does not play a role in statistical analysis. The principal investigators and members of the Protocol Committee designed the study in collaboration with Shionogi & Co., Ltd. EDIT, Inc. (Tokyo, Japan) provided medical writing and editing. The authors had final responsibility for the decision to submit for publication.
Appendix
The following persons participated in this study.
Principal Investigators: Hiroshi Itoh and Issei Komuro.
Supervisors: Ryozo Nagai and Kazuwa Nakao, Kyoto University Graduate School of Medicine.
Steering Committee: Yoshiki Egashira, Sakura Hospital; Jitsuo Higaki, Ehime University Graduate School of Medicine; Shun Ishibashi, Jichi Medical University; Sadayoshi Ito, Tohoku University Graduate School of Medicine; Atsunori Kashiwagi, Kusatsu General Hospital; Satoshi Kato, The University of Tokyo; Masafumi Kitakaze, National Cerebral and Cardiovascular Center; Masahiko Kurabayashi, Gunma University Graduate School of Medicine; Toyoaki Murohara, Nagoya University Graduate School of Medicine; Koichi Node, Department of Cardiovascular Medicine, Saga University; Yoshihiko Saito, Nara Medical University; Masahiro Sugawara, Sugawara Medical Clinic; Yasuo Terauchi, Yokohama City University School of Medicine; Shoei Yo, Yo Clinic; Michihiro Yoshimura, The Jikei University School of Medicine; Nagahisa Yoshimura, Kyoto University Graduate School of Medicine.
Protocol Committee: Hideo Fujita, Saitama Medical Center, Jichi Medical University; Ken-ichi Hirata, Kobe University Graduate School of Medicine; Katsumi Miyauchi, Graduate School of Medicine Juntendo University; Tomoaki Murakami, Kyoto University Graduate School of Medicine; Seigo Sugiyama, Jinnouchi Hospital; Kenji Ueshima*; Kazunori Utsunomiya, The Jikei University School of Medicine; Tsutomu Yamazaki*; Koutaro Yokote, Chiba University Graduate School of Medicine.
Statistical analysis: Masahiro Takeuchi.
Event Evaluation Committee: Takashi Akasaka, Wakayama Medical University; Hiroyuki Daida, Graduate School of Medicine Juntendo University; Takaaki Isshiki, Teikyo University; Kazuo Kitagawa, Tokyo Women's Medical University School of Medicine; Takanari Kitazono, Department of Graduate School of Medical Sciences, Medicine and Clinical Science, Kyushu University; Susumu Ogawa, Tohoku University Graduate School of Medicine; Yoshihiko Seino, Nippon Medical School Chiba Hokuso Hospital; Takashi Shigeeda, Ideta Eye Clinic; Shunya Shindo, Tokyo Medical University Hachioji Medical Center; Masakazu Yamagishi*, Kanazawa University Graduate School of Medicine; Kiyoshi Yoshida, Sakakibara Heart Institute of Okayama.
Independent Data Monitoring Committee: Tatsuro Ishibashi, Department of Ophthalmology, Graduate School of Medical Sciences, Kyushu University; Yasushi Saito*, Chiba University Graduate School of Medicine; Lee-Jen Wei, Harvard School of Public Health; Junichi Yoshikawa, Nishinomiya Watanabe Cardiovascular Center. (*; Chair)
Data Center: Mebix, Inc., Tokyo, Japan.
Investigators: Aya Abe; Toshiyuki Abe; Norio Abiru; Ken-ichi Aihara; Nobuyuki Aizawa; Masaki Akahata; Hiroshi Akahori; Etsuko Akita; Kazumi Akiyama; Kuniki Amano; Jiro Ando; Jiichi Anzai; Hiromi Aoki; Keiko Arai; Masaru Arai; Tadashi Arai; Yoshiyuki Arai; Atsushi Araki; Zenei Arihara; Tetsuro Arimura; Shingo Asahara; Nobuteru Asahi; Takayuki Asahina; Taro Asakura; Akira Asano; Hiroshi Asano; Shogo Asano; Keiko Ashidate; Katsumi Aso; Kazuyoshi Aso; Keita Ato; Hiroshi Awasaki; Nobuyuki Azuma; Hidenori Bando; Yukihiro Bando; Toru Chiba; Rina Chin; Michiko Chosa; Shuji Dodo; Kenji Doi; Kentaro Doi; Masatoshi Domen; Kenichi Doniwa; Kenji Dote; Isao Ebihara; Toyohisa Eguchi; Genshi Egusa; Yoichi Ehara; Mikiko Endo; Hiromitsu Enomoto; Tetsuya Enomoto; Kazuhiro Eto; Masahiro Eto; Hitomi Fujii; Yasuhiro Fujii; Makiko Fujikawa; Hiroshi Fujimoto; Yukari Fujimura; Kazuo Fujisawa; Motohiro Fujita; Nobuhiko Fujita; Hitoshi Fujiwara; Machiko Fukamizu; Gen Fukuda; Ken Fukuda; Naofumi Fukuda; Nobuo Fukuda; Shuichi Fukuda; Masataka Fukue; Takeshi Fukui; Toshiki Fukui; Yoshihide Fukumoto; Takashi Fukushima; Kumiko Furui; Kenji D Furukawa; Toyokazu Furumoto; Nobutoshi Fushimi; Hajime Goichi; Shigeki Gondo; Hiromasa Goto; Shinobu Goto; Takashi Goto; Yoshie Goto; Tatsuya Haga; Shigeru Hagimoto; Tomomi Hakoda; Yutaka Hamano; Masao Hanaki; Hisato Hara; Masumi Hara; Yasuhiko Hara; Hirofumi Harada; Kazuhiro Harada; Atsushi Hasegawa; Hisayoshi Hasegawa; Koichi Hasegawa; Yasuhiro Hashiguchi; Kunihiko Hashimoto; Naotake Hashimoto; Yoshiaki Hashimoto; Sumiko Hasumi; Katsuhiro Hatao; Masahiro Hatazaki; Satomi Hayakawa; Tetsuo Hayakawa; Hitoshi Hayashi; Masayuki Hayashi; Tatsunobu Hayashi; Tsutomu Hayashi; Kazuyuki Hida; Senshu Hifumi; Takayuki Higashi; Hiroshi Higashihara; Yoshiki Hirabayashi; Yoshio Hiraiwa; Kazuhiro Hiramine; Tsutomu Hirano; Kanna Hirasawa; Hiromi Hirata; Tadanori Hirata; Hiroyoshi Hirayama; Yoshihide Hirohata; Kenichi Hirose; Hisayoshi Hirota; Naoko Hisakawa; Toru Hiyoshi; Yasuko Hori; Yuhji Hori; Hiroaki Horie; Shuji Horinouchi; Tetsuo Hoshino; Akiko Hosokawa; Kazuhiro Hosokawa; Takeshi Hosoya; Kaori Hosoyamada; Yoshisuke Hot-chi; Myung Woo Hwang; Toshiki Ichimori; Yumiko Ide; Masahiko Igarashi; Kiyoshi Iha; Junpei Iinuma; Takashi Iizuka; Motoyoshi Ikebuchi; Hiroshi Ikegami; Yasuhide Ikenaka; Kiyomitsu Ikeoka; Hideya Imai; Minoru Imamura; Haruyo Imanari; Shinobu Imoto; Takeshi Inazawa; Ikuo Inoue; Mamoru Inoue; Mari Inoue; Masanori Inoue; Takeshi Inoue; Tatsuhide Inoue; Kenichi Ishibashi; Kazufumi Ishida; Keiichi Ishida; Yasushi Ishigaki; Motoyuki Ishiguro; Hisamitsu Ishihara; Hajime Ishii; Hiroyuki Ishii; Masashi Ishikawa; Naoto Ishikawa; Norikazu Ishikawa; Masahiko Ishimura; Akihiro Isogawa; Yukinori Isomura; Motohide Isono; Naoki Itabashi; Tokushichi Itai; Yasunori Itakura; Midoriko Itano; Chikako Ito; Junko Ito; Shun Ito; Toru Ito; Takahiko Iuchi; Yasushi Iwaita; Gensho Iwami; Suzuko Iwami; Tomoyuki Iwasaki; Fumiko Iwashima; Masatora Iwashina; Michihiro Iwata; Miwa Izaki; Kiyohiro Izumino; Kiyoshi Izumino; Yumi Jimbu; Kenji Kahara; Shoko Kajiya; Hitoshi Kakimoto; Fumitaka Kamada; Tetsuro Kamada; Hiroki Kamata; Nozomu Kamei; Takashi Kamiyama; Reibun Kanbara; Tsugiyasu Kanda; Hirosumi Kaneko; Yoshihito Kaneko; Mizuki Kaneshiro; Hiroyuki Kanno; Jin Kasahara; Yasushi Kasai; Soji Kasayama; Toshiyuki Kashiwagi; Hiromi Kato; Masakazu Kato; Sumio Kato; Taiya Katoh; Yasuhiro Katsura; Ikkyo Kawa; Toshihiro Kawabata; Ichiro Kawada; Kimiko Kawada; Toshio Kawada; Yasuhiko Kawade; Naoki Kawai; Toru Kawai; Shigeru Kawaida; Akitoshi Kawakubo; Hideyasu Kawamura; Mitsunobu Kawamura; Tomonori Kawano; Keiko Kawarabayashi; Satsuki Kawasaki; Yukinori Kawase; Kunihiro Kawashima; Osamu Kawashima; Kazuko Kawata; Hidenori Kido; Hajime Kihara; Noriyuki Kikuchi; Ryo Kikuchi; Takashi Kikuchi; Osamu Kimura; Shiro Kimura; Yuusuke Kimura; Mitsuo Kina; Saori Kinami; Kei Kiribayashi; Kiyohiko Kishi; Shiroshi Kitagawa; Haruko Kitaoka; Kenichi Kobayashi; Kenji Kobayashi; Kunihisa Kobayashi; Kyoko Kobuke; Tetsuya Kogawa; Sawako Koishi; Kuniyoshi Kojima; Hitoshi Komaki; Rieko Komi; Manabu Komiyama; Yoshimi Komizo; Tadamitsu Komori; Eri Kondo; Hiroyasu Konishi; Ichiro Konno; Tadashi Konoshita; Hiroyuki Konya; Keisuke Kosugi; Kei Kotani; Hiroshi Kouno; Teruo Kowatari; Daisuke Koya; Kazunori Koyama; Kunihiko Koyama; Takeshi Kubota; Norishige Kudo; Etsuko Kumagai; Isao Kumagai; Yuji Kumano; Makoto Kunishige; Hisamoto Kuroda; Shigetaka Kuroki; Teruji Kurosawa; Takaaki Kusaka; Masahiko Kushima; Hiroto Kusunose; Masamichi Kuwajima; Hiroyuki Machino; Kazuo Maeda; Shuichi Maeda; Hiroshi Maegawa; Michihiro Maeshima; Takeshi Maki; Shinya Makino; Hideo Manaka; Yasuyuki Maruyama; Shoji Mashiba; Izuru Masuda; Akira Matsuba; Tatsuaki Matsubara; Sunao Matsubayashi; Akira Matsuda; Shigeaki Matsukawa; Takayuki Matsuki; Koji Matsumoto; Izumi Matsumura; Ken-taro Matsumura; Kaneyuki Matsuo; Ko Matsuo; Miyuki Matsuo; Naoki Matsuoka; Kosho Matsuura; Naotaka Matsuura; Yoshifusa Matsuura; Jun Michiura; Masahiro Mimura; Fuyuki Minagawa; Shinya Minagawa; Shirou Minagawa; Daiki Minami; Naoto Minamitani; Toyoaki Miura; Yoshitaka Miura; Munenori Miyake; Nobuyuki Miyake; Takafumi Miyake; Yutaka Miyake; Yoshihiro Miyamoto; Kazunori Miyata; Hiroyuki Miyazaki; Kazuhiro Miyazawa; Ryuichi Mizubayashi; Kenji Mizuno; Yutaka Mizushima; Masahiro Mizutani; Hisaya Mori; Masanori Mori; Masaya Mori; Tsutomu Mori; Akizuki Morikawa; Taro Morimoto; Yuko Morita; Tadashi Mugihara; Yasunari Muramatsu; Koji Murao; Satoshi Murao; Kazuya Murata; Seiji Muro; Shigeo Nagafuchi; Sho Nagai; So Nagai; Shigeru Naganuma; Tadasu Nagaoka; Takao Nagasu; Masayuki Nagata; Koji Nagayama; Kotaro Naito; Satoru Naito; Masahiro Nakada; Kazuaki Nakai; Masahide Nakai; Kouji Nakajima; Masahiro Nakajima; Shigeru Nakajima; Taichiro Nakajima; Masayuki Nakamura; Shuji Nakamura; Takaaki Nakamura; Koji Nakanishi; Toshiaki Nakanishi; Hiroki Nakano; Junko Nakano; Kimisato Nakano; Masayuki Nakano; Masayuki Nakano; Eitaro Nakashima; Misuzu Nakasone; Masaya Nakata; Shiro Nakayama; Toru Nakayama; Fumiaki Nakazawa; Masahiko Namiki; Hiroshi Nariko; Sachiko Narita; Takako Naruo; Tetsuji Niiya; Masamichi Niizuma; Ichiro Ninomiya; Shigeo Nishi; Yusa Nishi; Haruo Nishimura; Masato Nishimura; Keiichiro Nishino; Kiyoshi Nishino; Naonobu Nishino; Yoshihiko Nishio; Mariko Nishioka; Tomoko Nishiumi; Masato Nishiwaki; Osamu Nogi; Kazuko Nomura; Naoki Nomura; Nobuyasu Noritake; Shuichi Nozaki; Hiroyuki Numata; Tatsuya Nunohiro; Kiyoshi Oda; Yoshiaki Oda; Yukinari Odagawa; Masashi Ogawa; Takanori Ogawa; Yoshihiro Ogawa; Yoshiji Ogawa; Masaro Ogimoto; Ichiro Ohara; Hiroshi Ohashi; Makoto Ohashi; Tetsuya Ohishi; Yasuhiro Ohno; Mitsuru Ohsugi; Itsuro Ohta; Kazuyasu Ohta; Masao Ohta; Hiromasa Ohtani; Hiroshi Ohtani; Sumire Ohtani; Takayuki Ohwada; Mariko Oishi; Yutaka Oiso; Susumu Oka; Mizuho Okada; Setsuro Okada; Yosuke Okada; Aki Okamoto; Hideki Okamoto; Yutaka Okamoto; Hiro-oki Okamura; Ken Okano; Yasuhiro Okauchi; Masumi Okawara; Hisashi Okimoto; Kohei Okita; Ken Okubo; Takeshi Okuda; Fuminobu Okuguchi; Shinichiro Okuno; Mari Okuyama; Hiroaki Omori; Takashi Omura; Yukiko Onishi; Akira Ono; Koichi Ono; Masahiro Ono; Shigemitsu Ono; Takuya Ono; Yasuhiro Ono; Yoshiaki Ono; Hikari Ooka; Tadatoshi Oomiya; Katsuya Oshima; Kayo Oshita; Akira Ota; Masayuki Otaki; Morihiro Ozaki; Noriyuki Ozawa; Masahide Sagara; Koumei Sagawa; Jun Saito; Kazuko Saito; Kazuyuki Saito; Shumpei Saito; Setsuya Sakagashira; Daisuke Sakaguchi; Ichiro Sakaguchi; Eiji Sakai; Naoshi Sakai; Noriko Sakamoto; Koichiro Sakota; Ichiro Sakuma; Kenichi Sakurai; Shunichiro Sakurai; Hisako Sameshima; Akira Sanada; Yutaka Sasagawa; Hiromitsu Sasaki; Iwao Sasaki; Takashi Sasaki; Masataka Sata; Atsushi Sato; Kazutoshi Sato; Koichi Sato; Koichiro Sato; Naoichi Sato; Nobuyuki Sato; Takako Sato; Tatsuyuki Sato; Ken Sawada; Tadashi Sawada; Kimikazu Sawai; Hideaki Sawaki; Yoshitaka Sayo; Ikuo Segawa; Tadashi Seguchi; Hiroaki Seino; Naoto Seki; Taiji Sekigami; Naotaka Sekiguchi; Syunji Sekiguchi; Nobuo Sekine; Risa Sekioka; Nobuko Sera; Yasunori Sera; Osamu Seto; Kozo Shaura; Masaaki Shibamoto; Hirotaka Shibata; Toshiro Shibata; Makoto Shibuya; Ryutaro Shigeta; Takao Shimada; Ryuji Shimamura; Ikki Shimizu; Kazuhiko Shimizu; Masashi Shimizu; Mitsuo Shimizu; Satoshi Shimizu; Masashi Shimoda; Shigeto Shimoda; Yoshio Shindo; Kouichiro Shiojima; Toshihiko Shiraiwa; Takuhiro Shirakawa; Nobuhiro Shiroyama; Yoshihito Shoda; Tetsuo Shoji; Hirohisa Shono; Hiroshi Shuto; Satoshi Soda; Kuninori Soejima; Shoichi Suemori; Minoru Suezawa; Muneki Sugata; Tatsushi Sugiura; Toru Sugiyama; Yasuhiro Sumida; Hiroshi Sunagawa; Katsuo Suyama; Hitoshi Suzuki; Susumu Suzuki; Takanori Suzuki; Tsunehito Suzuki; Haruyuki Taguchi; Shigeru Tai; Tsuyoshi Taira; Ichitaro Takada; Yoshihisa Takada; Junko Takagi; Shuichi Takagi; Yusuke Takagi; Kazuko Takahashi; Kazunori Takahashi; Kenro Takahashi; Kiyoshi Takahashi; Nobuo Takahashi; Shunsuke Takahashi; Soichiro Takahashi; Tadayoshi Takahashi; Toru Takahasi; Masato Takaki; Ichiro Takamura; Toshinari Takamura; Noriyuki Takano; Tatsuro Takano; Ken Takao; Taizo Takase; Hiroshi Takeda; Tomoo Takeda; Masanori Takeishi; Kiyoshi Takekawa; Yuji Takemoto; Ken Takenaka; Naohide Takeuchi; Yasuo Takeuchi; Hirofumi Takino; Toru Tamai; Kazuhiro Tamaki; Noboru Tamaki; Toshio Tamaki; Hideki Tamura; Hiroyuki Tamura; Yukihiro Tamura; Akihiko Tanaka; Hideki Tanaka; Hiroaki Tanaka; Kenji Tanaka; Masayuki Tanaka; Toru Tanaka; Toru Tanaka; Tsuyoshi Tanaka; Yasushi Tanaka; Makio Tani; Ken Tanigawa; Masato Taniguchi; Matsuo Taniyama; Toshihiro Tanzawa; Eiji Tatsumi; Noriyasu Taya; Jin Temma; Shouji Terada; Yasushi Terada; Yoshio Terada; Naoki Tezuka; Hisako Toda; Haruhiko Tokuda; Eiichi Tokutake; Kenichi Tokuyama; Takahiko Tokuyama; Katsuyuki Tome; Naruya Tomita; Yukio Tone; Rieko Totani; Jo Toyota; Tetsuo Tsubone; Akihito Tsuchida; Atsushi Tsuchiya; Hiroaki Tsuchiya; Norihiro Tsuchiya; Masahiro Tsuji; Motoyoshi Tsujino; Kazuhisa Tsukamoto; Taku Tsunekawa; Masatoshi Tsuru; Masahiro Tsutsui; Akihito Tsutsumi; Sachie Tsuzura; Daigaku Uchida; Yasuko Uchigata; Kazuaki Uchiyama; Hiroo Ueda; Junichi Ueda; Kazuya Ueda; Naohiko Ueda; Nobuyuki Ueda; Yasuo Ueda; Koichiro Uehara; Hiroaki Ueno; Makoto Ujihara; Fumio Umeda; Nobuo Uno; Satoshi Uramoto; Toshihiko Urushibara; Yoshihide Ushitani; Mikiya Usukura; Satoko Wada; Yutaka Wakasa; Takanobu Wakasugi; Masako Waki; Genichi Watanabe; Hitoshi Watanabe; Ikuo Watanabe; Masayuki Watanabe; Ryouichiro Watanabe; Yoshiyuki Watanabe; Matahiro Yabuta; Ken Yaga; Kunimasa Yagi; Kenji Yaginuma; Ryuichiro Yagyu; Hiroharu Yamada; Kenji Yamada; Masayo Yamada; Mitsutoshi Yamada; Satoru Yamada; Shoichi Yamada; Tetsuhiro Yamada; Tsutomu Yamada; Yoshihiko Yamada; Shigeru Yamaga; Toshiharu Yamagata; Toru Yamaguchi; Kouzaburo Yamaji; Chifumi Yamamoto; Hidefumi Yamamoto; Kenichi Yamamoto; Koji Yamamoto; Manabu Yamamoto; Yoshikazu Yamamoto; Ritsuko Yamamoto-Honda; Hidetoshi Yamashita; Iwao Yamashita; Shigeo Yamashita; Tetsuji Yamashita; Kazuhiko Yamauchi; Kenji Yamauchi; Yuichiro Yamauchi; Seiichi Yamawaki; Jun Yan; Tatsuo Yanagawa; Katsuyuki Yanagisawa; Masatoshi Yanagisawa; Toshihiko Yanase; Harumi Yano; Mayumi Yano; Yutaka Yano; Hideki Yasuda; Koichiro Yasuda; Takahiro Yazu; Mineto Yokoi; Tamotsu Yokota; Akihiro Yokoyama; Kazunori Yokoyama; Hidetada Yoshida; Katsumi Yoshida; Kenichi Yoshida; Masanori Yoshida; Tomoki Yoshida; Toshimi Yoshida; Naomi Yoshimura; Mototaka Yoshinari; Gen Yoshino; Munenori Yoshizumi; Atsuyoshi Yuhara; Masakatsu Yuito; Yasuo Yumori.
Disclosure
Kenji Ueshima reports honoraria from Shionogi & Co., Ltd., Pfizer Japan Inc. and Astellas Pharma Inc., and research funding from Pfizer Japan Inc., Daiichi Sankyo Co., Ltd. and Chugai Pharmaceutical Co., Ltd. Hiroshi Itoh reports advisory role of Nipro Corporation and SBI Pharmaceuticals Co., Ltd., honoraria from Takeda Pharmaceutical Co., Ltd., MSD K.K., Novartis Pharma K.K., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Astellas Pharma Inc. and Shionogi & Co., Ltd., and research funding from Mitsubishi Tanabe Pharma Corporation, MSD K.K., Daiichi Sankyo Co., Ltd., Astellas Pharma Inc., Kyowa Hakko Kirin Co., Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., Sanofi K.K., Teijin Pharma Ltd., Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Pfizer Japan Inc., Johnson & Johnson K.K., Torii Pharmaceutical Co., Ltd. and Bristol-Myers Squibb Company. Nobuaki Kanazawa reports employment of Shionogi & Co., Ltd. Issei Komuro reports honoraria from Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corporation and Daiichi Sankyo Co., Ltd., and research funding from Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Genzyme Japan K.K., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., MSD K.K., GlaxoSmithKline K.K., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Bayer Yakuhin, Ltd., Teijin Pharma Ltd., Chugai Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Actelion Pharmaceuticals Japan Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Bristol-Myers Squibb Company. Ryozo Nagai reports honoraria from Mitsubishi Tanabe Pharma Corporation, Kowa Pharmaceutical Co., Ltd., Shionogi & Co., Ltd. and Nippon Boehringer Ingelheim Co., Ltd. Masahiro Takeuchi reports honoraria from Hisamitsu Pharmaceutical Co., Inc., AstraZeneca K.K., Taiho Pharmaceutical Co., Ltd., Shionogi & Co., Ltd. and Kowa Pharmaceutical Co., Ltd. Tsutomu Yamazaki reports honoraria from Takeda Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., AstraZeneca K.K. and Sumitomo Dainippon Pharma Co., Ltd.
References
- 1). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists' (CTT) Collaborators: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 2). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA, 2001; 285: 2486-2497 [DOI] [PubMed] [Google Scholar]
- 3). Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL: Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care, 2008; 31: 811-822 [DOI] [PubMed] [Google Scholar]
- 4). Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med, 1998; 339: 229-234 [DOI] [PubMed] [Google Scholar]
- 5). Nishimura T, Nakajima K, Kusuoka H, Yamashina A, Nishimura S: Prognostic study of risk stratification among Japanese patients with ischemic heart disease using gated myocardial perfusion SPECT: J-ACCESS study. Eur J Nucl Med Mol Imaging, 2008; 35: 319-328 [DOI] [PubMed] [Google Scholar]
- 6). Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR: Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ, 1998; 316: 823-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C; Cholesterol Treatment Trialists' (CTT) Collaborators: Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet, 2008; 371: 117-125 [DOI] [PubMed] [Google Scholar]
- 8). Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ: Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 Diabetes: meta-analysis of observational studies. Diabetes Care, 2011; 34: 1238-1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY: Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care, 2007; 30: 1742-1746 [DOI] [PubMed] [Google Scholar]
- 10). Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY: Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke, 2007; 38: 398-401 [DOI] [PubMed] [Google Scholar]
- 11). Ohno T, Kinoshita O, Fujita H, Kato S, Hirose A, Sigeeda T, Otomo K, Ando J, Kadowaki T, Araie M, Nagai R, Takamoto S: Detecting occult coronary artery disease followed by early coronary artery bypass surgery in patients with diabetic retinopathy: report from a diabetic retinocoronary clinic. J Thorac Cardiovasc Surg, 2010; 139: 92-97 [DOI] [PubMed] [Google Scholar]
- 12). Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H: Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation, 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 13). Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M: Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302 [DOI] [PubMed] [Google Scholar]
- 14). Takayama T, Hiro T, Yamagishi M, Daida H, Hirayama A, Saito S, Yamaguchi T, Matsuzaki M: Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J, 2009; 73: 2110-2117 [DOI] [PubMed] [Google Scholar]
- 15). Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y: Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 16). Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N: Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab, 2011; 96: 3448-3456 [DOI] [PubMed] [Google Scholar]
- 17). Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, Daida H, Biro S, Hirobe K, Funahashi T, Yokote K, Yokode M: Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb, 2007; 14: 45-50 [DOI] [PubMed] [Google Scholar]
- 18). Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2014; 63: 2889-2934 [DOI] [PubMed] [Google Scholar]
- 19). Smith SC, Jr, Grundy SM: 2013 ACC/AHA guideline recommends fixed-dose strategies instead of targeted goals to lower blood cholesterol. J Am Coll Cardiol, 2014; 64: 601-612 [DOI] [PubMed] [Google Scholar]
- 20). The Japan Diabetes Society: Guideline for the diagnosis of diabetes mellitus. In: Evidence-based practice guidelines for the treatment of diabetes in Japan 3rd Ed, ed by The Japan Diabetes Society, pp13-19, Nankodo Co., Ltd, Tokyo, Japan, 2010. (in Japanese) [Google Scholar]
- 21). Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H: The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res, 2009; 32: 3-107 [PubMed] [Google Scholar]
- 22). Ballantyne CM, Nambi V: Markers of inflammation and their clinical significance. Atheroscler Suppl, 2005; 6: 21-29 [DOI] [PubMed] [Google Scholar]
- 23). Qu HY, Xiao YW, Jiang GH, Wang ZY, Zhang Y, Zhang M: Effect of atorvastatin versus rosuvastatin on levels of serum lipids, inflammatory markers and adiponectin in patients with hypercholesterolemia. Pharm Res, 2009; 26: 958-964 [DOI] [PubMed] [Google Scholar]
- 24). Sandhu S, Wiebe N, Fried LF, Tonelli M: Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol, 2006; 17: 2006-2016 [DOI] [PubMed] [Google Scholar]
- 25). Koya D, Campese VM: Statin use in patients with diabetes and kidney disease: the Japanese experience. J Atheroscler Thromb, 2013; 20: 407-424 [DOI] [PubMed] [Google Scholar]
- 26). Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y: Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA, 2008; 300: 2134-2141 [DOI] [PubMed] [Google Scholar]
- 27). Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH: Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet, 2004; 364: 685-696 [DOI] [PubMed] [Google Scholar]
- 28). Sever PS, Poulter NR, Dahlöf B, Wedel H, Collins R, Beevers G, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J: Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial —lipid-lowering arm (ASCOT-LLA). Diabetes Care, 2005; 28: 1151-1157 [DOI] [PubMed] [Google Scholar]
- 29). Collins R, Armitage J, Parish S, Sleigh P, Peto R: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet, 2003; 361: 2005-2016 [DOI] [PubMed] [Google Scholar]
- 30). Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM: Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med, 2004; 350: 1495-1504 [DOI] [PubMed] [Google Scholar]
- 31). de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E: Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA, 2004; 292: 1307-1316 [DOI] [PubMed] [Google Scholar]
- 32). LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK: Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med, 2005; 352: 1425-1435 [DOI] [PubMed] [Google Scholar]
- 33). Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J: High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA, 2005; 294: 2437-2445 [DOI] [PubMed] [Google Scholar]
- 34). Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group: Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet, 2010; 376: 1658-1669 [DOI] [PMC free article] [PubMed] [Google Scholar]


