Abstract
Familial hypercholesterolemia (FH) is the most common and serious form of inherited hyperlipidaemia. Dominantly inherited with high penetrance, untreated FH leads to premature death from coronary artery disease due to accelerated atherosclerosis from birth. Despite its importance, there is still a major shortfall in awareness, detection and treatment of FH worldwide. International models of care for FH have recently been published, but their effective implementation requires the garnering of more knowledge about the condition. The “Ten Countries Study” aims to investigate diagnostic, epidemiological and service aspects, as well as physician practices and patient experiences of FH in several countries in the Asia-Pacific Region and the Southern Hemisphere. Five observational studies are being undertaken that will systematically investigate the following aspects of FH: the phenotypic predictors of low-density lipoprotein receptor mutations, the point prevalence in available community populations, current knowledge and clinical practices among primary care physicians, availability and utilisation of services and facilities, and patient perceptions and personal experiences of the condition. The information gathered will inform better clinical practice and will enable the development of country-specific models of care for FH.
Keywords: Familial hypercholesterolaemia, Asia-Pacific, Translational research
Introduction
Familial hypercholesterolemia (FH) is the most common and serious form of inherited hyperlipidemia1). It is dominantly inherited and has a high phenotypic penetrance. If untreated, FH leads to premature death from coronary heart disease (CHD) in many families2). FH accelerates atherosclerotic cardiovascular disease (ACVD), particularly coronary artery disease. The pathogenesis of FH results from mutations in several genes that impair the catabolism of low-density lipoprotein (LDL) particles; the most common of these are in the LDL-receptor (LDLR) gene, which can affect receptor synthesis, transport, binding, internalization, and recycling. Mutations in the genes encoding apolipoprotein B (APOB), the receptor ligand, and proprotein convertase subtilisin/kexin type 9 (PCSK9), a binding protein that degrades LDLR, are less frequently implicated.
The prevalence of heterozygous FH is commonly estimated to be 1:5003) in unselected community populations. However, the prevalence is high in populations subject to a “founder gene effect” such as the Afrikaners, Lithuanian Jews, Christian Lebanese, French Canadian, and the Finns4). The prevalence of true homozygous FH is considered to be 1 in 1,000,0005). The forgoing population prevalence data have been recently questioned and updated6). The frequency of FH is also higher in clinical populations with premature CHD, such as among patients in coronary care units7). Under-detection of FH is a global problem, and there are estimated to be at least 15 million people with FH worldwide8). Screening enables early evidence-based interventions, such as lifestyle measures and cholesterol-lowering medications, which decrease the risk of ACVD, improves the health of families, and saves lives and health expenditure9, 10).
Impetus for the Investigation
The value of the detection and treatment of FH is abundantly supported by the outcome of several international cohort studies11–14). However, there are significant gaps in knowledge that impede the effective implementation of screening services and care pathways worldwide.
Owing to the very high population density in some Asian countries, Asia is estimated to have the largest number of individuals with FH in the world (Fig. 1). In particular, China and India may have at least 5.2 million affected individuals. Despite this, there are several gaps in knowledge of the phenotypic predictors of mutations, the community prevalence, physician awareness, patients' perceptions, and the availability of health services9, 15).
Fig. 1.
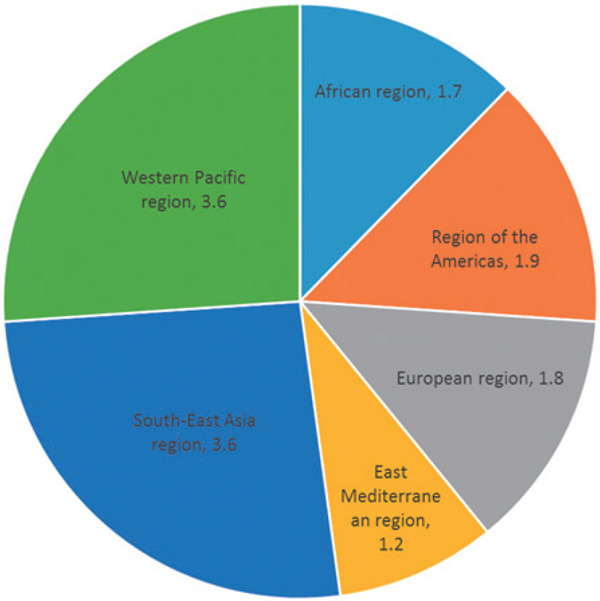
Estimated number (millions) of individuals with FH in WHO-defined regions based on the theoretical prevalence of 1:5003) for heterozygous FH (Adapted from Pang et al42). At least 50% of FH patients in the world are likely to come from Asian countries (included in the Western Pacific region and South-East Asia region).
Guidelines on treatment and management of FH have been published by the International FH Foundation9) and endorsed by the Asian-Pacific Society of Atherosclerosis and Vascular Disease16). This provides an early foundation for the development of expert guidelines, essential services, and new models of care in Asian countries. Guidelines and diagnostic criteria for FH have hitherto been produced by Japan17) and Australasia18); Hong Kong19) and South Korea20) also recently communicated their experiences in managing FH. This is a good start to a long journey.
Core Group and Networks
The “Ten Countries Study” is being undertaken under the auspices of the FH Australasia Network. The study group was assembled to tackle several country-specific questions in the Asia-Pacific region and the southern hemisphere. However, the increasing communication among centers and the development of new networks have resulted in the recruitment of other centers. Hence, the study group and network currently includes at least 15 countries: Australia, Japan, Hong Kong, China, Taiwan, South Korea, Malaysia, Philippines, Vietnam, Singapore, India, New Zealand, South Africa, Brazil, and the United Kingdom (Fig. 2). The United Kingdom, a country with a highly developed healthcare system and a sophisticated guideline for the care of FH developed by the National Institute for Health and Care Excellence (NICE), will provide benchmark data for international comparison. Further networking has spawned a new association with the FH Studies Collaboration (FHSC)21).
Fig. 2.
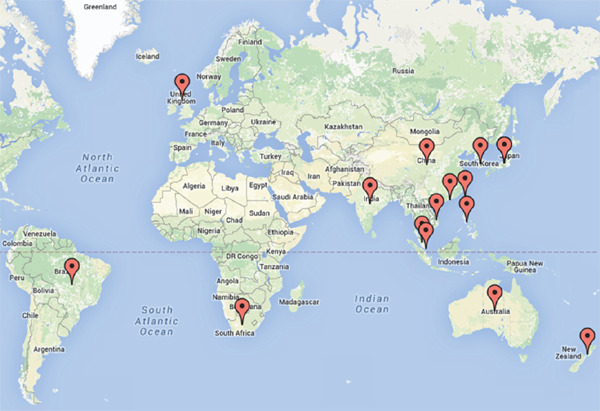
Map showing the countries currently participating in the “Ten Countries Study.”
Overarching Aim and Objectives
The overarching aim of the study is to ultimately improve the care of patients and families with FH via the following projects that address: (1) the phenotypic predictors of FH mutations, (2) the point prevalence of FH in unselected community populations, (3) knowledge and practices of primary care physicians concerning FH, (4) availability and utilization of services and facilities for the care of FH, and (5) patient perceptions and personal experiences of FH (Table 1). These projects are aligned to the FH research agenda recently proposed in a scientific statement from the American Heart Association (Table 2)22).
Table 1. Summary of the five principal projects comprising in the “Ten Countries Study”.
| Project Number | Title | Aim | Translational Value |
|---|---|---|---|
| 1 | Plasma LDL-cholesterol as a predictor of FH mutations | To select a level of plasma LDL-cholesterol that has the highest sensitivity and specificity in predicting a mutation in different countries. | The data will inform a simple screening test for FH, based on LDL-cholesterol measurement, for use in primary care and where genetic testing is not available. |
| 2 | Prevalence of FH in community populations | To assess the prevalence of FH in adult and childhood populations. | The data will emphasize the public health problem presented FH, including the shortfalls in detection and treatment. This will inform screening programs for FH in the community. |
| 3 | Knowledge and practices of Primary Care Physicians (PCPs) concerning FH | To determine awareness, knowledge and practices regarding FH in PCPs; and conduct a comparison across the centres in the region. | Defining the role of PCPs in the care of FH is essential for developing multi-disciplinary and integrated total quality management. Assessing current knowledge and practices is the starting point. The information also will be employed to design effective teaching and training modules for PCPs in the detection and management of FH. |
| 4 | Comparison of services and facilities for the care of FH | To describe and compare existing health services, facilities and resources for the care in FH in the region. | The study will provide international benchmarking of performance in health care for FH. It will identify and promote successful strategies within a context that takes into account cultural, economic and logistic differences and create opportunities for implementing country-specific or region-specific models of care for FH. |
| 5 | Patient perceptions and personal experiences of FH | To investigate the association between patients' psychological factors and key behavioural and clinical outcome variables salient to the management of FH; and conduct a comparison across the centres in the region. | The study will identify the key psychological factors associated with compliance and patient decisions. The factors can then be used as a basis for behavioural interventions to promote better treatment compliance and patient decisions. |
Table 2. The research agenda for FH (adapted and modified from Gidding et al22)). Highlighted are research items that are being addressed in the “Ten Countries Study”.
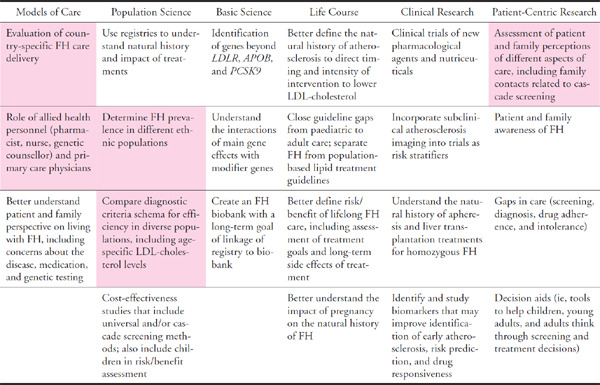 |
A related objective of the “Ten Countries Study” is to close gaps in knowledge and awareness of FH through an educational program utilizing the collective data obtained from the individual studies. This will also more widely facilitate best practices in the care of FH in the region. Education will be provided by country-specific societies that are members of the International Atherosclerosis Society, with the ultimate objective of creating a network of local health-care providers with the expertise in clinical lipidology.
Individual Projects
(1) Screening and Diagnostic Testing: LDL-cholesterol as a Predictor of FH Mutations
FH specifically elevates plasma LDL-cholesterol concentration owing to the decreased uptake of LDL, mediated by the ligand apoB-100 for the LDL receptor. Diagnostic accuracy relies on identifying a causative mutation1, 9, 15). Most mutations causing FH occur in the LDLR (encoding low-density lipoprotein receptor) and fewer in APOB (encoding apolipoprotein B) and PCSK9 (encoding proprotein convertase subtilisin/kexin type 9)1, 23, 24). The prognostic importance of identifying a genetic mutation causative of FH in patients with profound hypercholesterolemia in the community has recently been underscored25). However, genetic testing is often prohibitively expensive and not commonly available, particularly in primary care and in Asian centers. The diagnostic thresholds of untreated LDL-cholesterol have not been defined in relation to their ability to predict a pathogenic FH mutation. Phenotypic criteria also require detailed family history and detection of occasionally subtle physical signs, such as arcus cornealis or xanthomata; these may not be easily detectable, and their age-dependence invalidates them as criteria for the early detection of FH9, 15, 26). A recent study from South Korea demonstrated that traditional criteria have limited detection power and low specificity for mutations in their population27). This underscores the need for region- or country-specific criteria and guidelines. We aim to inform a first-step screening test for FH, based on LDL-cholesterol measurement, for use in primary care.
The study has involved countries where genetic testing is available for clinical or research purposes (Australia, Hong Kong, Brazil, and New Zealand). The cross-sectional investigation aims to select a level of plasma LDL-cholesterol that has the highest sensitivity and specificity in predicting a pathogenic mutation. Adults who have provided informed consent for genetic testing and collection of phenotypic data will mainly be studied. LDL-cholesterol levels from a fasting blood sample at screening will be adjusted if required for the type and dose of cholesterol-lowering drugs28). Mutational analyses for defects in the LDLR, APOB, and PCSK9 genes and assessment of pathogenicity have been uniformly performed as described elsewhere18, 24, 29, 30).
The ability of plasma LDL-cholesterol concentrations to predict a genetic variant will be investigated using receiver operator characteristic curves. The best value of LDL-cholesterol for predicting a mutation will be defined as a having a sensitivity > 90%, with the highest corresponding level of specificity and/or the highest sum of sensitivity and specificity. The effect of other variables (such as family and personal history of high cholesterol or coronary heart disease, physical signs, and ethnicity) on the sensitivity and specificity of the LDL-cholesterol threshold will also be tested by multiple logistic regression analysis.
(2) Epidemiology: Prevalence of FH in Community and High-risk Populations
Excluding rare populations subject to a gene founder effect in whom FH is particularly common, the community prevalence of FH is estimated to be 1 in 500, with reports varying from 1 in 200 to 1 in 20002). Prevalence data enable the design of screening programs for FH in the community31). We have completed studies in China and Australia, where the prevalence of heterozygous FH was found to be 1:211 – 35932) and 1:229 – 35333), respectively, consistent with recent findings from the US34) and Europe35, 36). In children from Australia, we also found a frequency of FH of 1 in 267 by LDL-cholesterol and family history criteria37). The apparent higher frequency of FH in community populations has been well evidenced by two recent studies employing genetic testing25, 35). Data from all countries appear to consistently indicate under-diagnosis and under-treatment of FH across all ages and ethnic groups. The prevalence of FH in coronary care units is also informative for targeted screening for index cases. In Australia7), 14.3% of coronary patients aged less than 60 years were found to have phenotypic FH. These data concur with reports from Europe38).
(3) Education and Training: Knowledge and Practices of FH Among Physicians
The majority of people in the community will have contact with their primary care physician (PCP) or family doctor. PCPs can perform absolute cardiovascular risk assessments and are well placed to opportunistically detect FH39, 40). Well controlled and low complexity patients, initially identified in specialist centers, should be transitioned if feasible to primary care for long-term management or for shared care, whereas high complexity patients should be followed up by the specialist service9). The role of primary care in the care of FH has not been adequately defined. A preliminary study suggested a significant shortfall in awareness, knowledge, and practices among family doctors in the Asia-Pacific region41, 42). Defining the role of PCPs in the care of FH is essential for developing multidiscplinary and integrated total quality management. Assessing current knowledge and practices is considered to be the starting point.
In the investigation, a formal questionnaire is being offered to PCPs via cardiovascular education sessions and/or mail lists from the royal colleges (or country equivalent). Completion of the survey is voluntary and anonymous. The survey enquires about the following elements: general familiarity with FH; awareness of national and international guidelines for FH; the clinical description of FH; identification of the typical lipid profile; prevalence and inheritance of FH; extent of elevation in risk of CVD, definition of premature CVD, and physical features in FH; whether the diagnosis requires genetic confirmation; methods for alerting PCPs about the possibility of FH; type of health professional best placed to detect FH; number of patients with FH currently being treated; specific treatments; knowledge and practices concerning family screening; and treatment and referral practices regarding patients with severely elevated cholesterol. In addition, demographic data, including gender, qualifications and training status, years of experience, and size and location of practice, are recorded. The overall information generated by this project will ultimately be employed to design effective teaching and training modules for PCPs in the care of FH.
(4) Health Service Research: Comparison of Services and Facilities for the Care of FH
Despite the increasing recognition of the importance of FH, the care of patients and families remains suboptimal9, 15). Services need improvement and standardization at several levels18, 43). This includes pediatric services, cascade screening, and multidisciplinary care that involves laboratory medicine, cardiology, and transfusion medicine. Close collaboration between healthcare systems, patient support groups, and non-government organizations is essential9). A clinical registry can also provide invaluable information for research and audit as well as for improving the quality of care. There are no published data describing or comparing healthcare resources for the detection and management of FH across different countries with diverse healthcare systems. This project will provide knowledge that could form an international benchmark for future performance in the care of FH. The knowledge will be generated within a context that takes an account of cultural, economic, and logistic differences and will create opportunities for implementing country-specific or region-specific models of care for FH. It will promote regional and international collaboration that could greatly enhance the development of new services for FH where gaps are identified.
The project is based on an online questionnaire that specifically investigates the key dimensions of a desirable model of care9, 44, 45). This will be completed voluntarily by the main key opinion leaders or experts in FH in the region. The enquiry relates to the following elements: national guidelines and protocols, medical specialties involved in care, role of primary care, screening, diagnostic and assessment protocols, DNA testing facilities, pediatric services, therapeutic strategies, apheresis and liver transplantation; clinical support (nurses, dieticians, counsellors, information technology, and registry), biochemistry laboratory services, cardiology services, funding (public, private, health insurance), drug re-imbursement, education and training programs, research programs, links with and support from government and non-government organizations, and existence of a family support group9, 43, 45).
(5) Health Psychology: Patient Perceptions and Personal Experiences of FH
Numerous psychological factors have been found to be associated with salient adaptive outcomes and individual patient-related behaviors linked with successful treatment and management of FH46). Current research suggests that attitudes and beliefs about the severity of FH predict intentions and motivation to engage in treatment, particularly adherence to lipid-lowering drug regimens and self-management behaviors such as physical activity and diet47). However, much of the research has been conducted in relatively small samples using qualitative methods48). Other additional psychological factors that may be related to important outcomes related to the management of illness should be investigated in FH patients. These include facilitating factors and barriers in compliance with behavioral therapy and lifestyle changes, particularly among those who do not have any clinical manifestation of the illness and are asymptomatic49). In addition, factors that may affect adherence to treatment, including beliefs about the controllability of the illness and efficacy of medication, should be identified and investigated. This project will identify key psychological factors associated with adherence and patient decisions to consent to refer relatives for cascade testing for FH. The factors can then be used as a basis for behavioral interventions to promote better care of families with FH.
Participants will be recruited from regional clinics managing FH patients in different countries. Volunteer patients with FH will be recruited via clinic staff who will offer them the opportunity to participate. The study will adopt a correlational, quantitative design, in which psychometric measures of key psychological and behavioral variables will be elicited from samples of FH patients. The factors include attitudes, motives, and beliefs toward treatment, including drug and self-management behaviors, beliefs in medication, FH illness perceptions, and health literacy. Measures will be based on previous research and will be informed by a preliminary qualitative study50). Key clinical and behavioral outcomes will also be collected from patient records in the collaborating clinics. Language-specific versions of the questionnaire will be developed from the English-language version using standardized back-translation techniques with the aid of bilingual translators. Comparisons of the key psychological correlates of treatment and management behaviors and outcomes across the different countries is of particular interest because it will facilitate understanding of the cultural influences on living with, treating, and managing FH.
A pilot study in Australia51) has demonstrated that patient attitudes and beliefs toward treatment and behaviors rather than beliefs about the illness are the key determinants of intentions to engage in treatment and self-management behaviors, suggesting that changing attitudes and beliefs toward the behaviors is a priority for promoting better management of FH. We plan to investigate and compare the effects of these psychological factors on behaviors and outcomes among the participating countries.
Conclusion and Future Perspective
The “Ten Countries Study” is the first collaborative effort relating to FH in countries in Asia and the southern hemisphere. A series of five studies will garner new knowledge that is likely to enhance the care of patients and families with FH in a region that has the highest density of people with the condition. The projects are aligned with the research agenda recently proposed in a Scientific Statement by the American Heart Association (AHA)22). The complete collection of data and analyses will be completed in the second half of 2016; some findings have already been published32, 33, 37, 51). The future will hopefully see the extension of the projects to other countries, piloting of a regional web-based registry (including a pediatric registry52)), updating international guidelines, and developing a wider research agenda within the remit proposed by the AHA22). Transfer of the research findings to enhance the awareness and knowledge of FH among healthcare professionals is an essential future objective, including the incorporation and utilization of the findings into routine clinical practice. The wider impact and effectiveness of future studies relies on the development of closer collaborative efforts with other international initiatives, such as the FH Studies Collaboration (FHSC)21) and the ScreenPro FH Project53). At country, region, and local levels, the instigation of partnership between clinical and political stakeholders and patient support groups is essential to effectively translate current and future evidence into better healthcare for all patients and families with FH54).
Acknowledgment
We acknowledge the team members for the “Ten Countries Study”: Devi Arikketh (India), Tester F Ashavaid (India), Nurul Atiqah (Malaysia), Shanthi Balasubramaniam (India), Dick C Chan (Australia), Nien-Tzu Chang (Taiwan), Thuy Can Do (Vietnam), Dong Zhao (China), Katrina L Ellis (Australia), Ki Hoon Han (South Korea), Sarah J Hardcastle (Australia), Cinthia E Jannes (Brazil), Alicezah Mohd Kassim (Malaysia), Zaliha Mohd Ismail (Malaysia), See Kwok (United Kingdom), Carolyn SP Lam (Singapore), Peter J Lansberg (the Netherlands), Michael Livingston (International FH Foundation), Manjeet Mehta (India), Lauretta Muir (New Zealand), Hoh Boon Peng (Malaysia), Alexandre C Pereira (Brazil), Thuhairah Hasrah Abdul Rahman (Malaysia), Anis Safura Ramli (Malaysia), Nandhini Rangarajan (India), Suraya Abdul Razak (Malaysia), Eric JG Sijbrands (the Netherlands), David R Sullivan (Australia), E Shyong Tai (Singapore), Hong Chan Tan (Singapore), Ishwar C Verma (India), Xue Wu (China).
Disclosure
Prof Santos has received honoraria and consulting fees from Amgen, Astra Zeneca, Biolab, Boehringer-Ingelheim, Eli-Lilly, Merck, Genzyme, Kowa, Sanofi/Regeneron, Torrent and Pfizer. Prof Santos has also received research grants from Amgen, Sanofi/Regeneron and Genzyme. Prof Yamashita has received honoraria and consulting fees from Bayer, Kowa, Medicar Review, MSD, Sanwa-Kagaku, Skylight Biotec and Shionogi. Prof Yamashita has also received research grants from Japan Boehringer-Ingelheim, Kyowa Medex, Otsuka and scholarship grants from Astra Zeneca, Bayer, Japan Boehringer-Ingelheim, Kowa, Mochida, MSD, Ono, Sanwa-Kagaku and Takeda. All other authors declare no financial interests or potential conflicts of interest.
Funding
The “Ten Countries Study” was funded by the International Atherosclerosis Society (IAS) and Pfizer Independent Grants for Learning & Change (Grant ID: 10839501), and is being undertaken under the aegis of the FH Australasia Network (FHAN) and the Australian Atherosclerosis Society (AAS) Inc. We thank Ms Natasha Whitwell and Ms Jennifer Seabrook for their excellent assistance.
References
- 1). Austin MA, Hutter CM, Zimmern RL, Humphries SE: Genetic causes of monogenic heterozygous familial hypercholesterolaemia: A HuGE prevalence review. Am J Epidemiol, 2004; 160: 407-420 [DOI] [PubMed] [Google Scholar]
- 2). Marks D, Thorogood M, Neil HAW, Humphries SE: A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis, 2003; 168: 1-14 [DOI] [PubMed] [Google Scholar]
- 3). Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG: Hyperlipidemia in coronary heart disease II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest, 1973; 52:1544-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Goldstein JL, Hobbs HH, Brown MS: The Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill Information Services Company, New York, 2001 [Google Scholar]
- 5). Raal FJ, Santos RD: Homozygous familial hypercholesterolemia: Current perspectives on diagnosis and treatment. Atherosclerosis, 2012; 223: 262-268 [DOI] [PubMed] [Google Scholar]
- 6). Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche JC, Sijbrands EJG, Roeters van Lennep JE, Stalenhoef AFH, Wiegman A, de Graaf J, Fouchier SW, Kastelein JJP, Hovingh GK: Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype–phenotype relationship, and clinical outcome. Eur Heart J, 2014; ehu058. [DOI] [PubMed] [Google Scholar]
- 7). Pang J, Poulter EB, Bell DA, Bates TR, Jefferson V-L, Hillis GS, Schultz CJ, Watts GF: Frequency of familial hypercholesterolemia in patients with early-onset coronary artery disease admitted to a coronary care unit. J Clin Lipidol, 2015; 9: 703-708 [DOI] [PubMed] [Google Scholar]
- 8). Watts GF, Juniper A, van Bockxmeer F, Ademi Z, Liew D, O'Leary P: Familial hypercholesterolaemia: a review with emphasis on evidence for treatment, new models of care and health economic evaluations. Int J Evid Based Healthc, 2012; 10: 211-221 [DOI] [PubMed] [Google Scholar]
- 9). Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, Livingston M: Integrated Guidance on the Care of Familial Hypercholesterolaemia from the International FH Foundation. Int J Cardiol, 2014; 171: 309-325 [DOI] [PubMed] [Google Scholar]
- 10). Ademi Z, Watts GF, Juniper A, Liew D: A systematic review of economic evaluations of the detection and treatment of familial hypercholesterolemia. Int J Cardiol, 2013; 167: 2391-2396 [DOI] [PubMed] [Google Scholar]
- 11). Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DCG, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJP, Sijbrands EJG: Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. Br Med J, 2008; 337: a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE. on behalf of the Simon Broome Familial Hyperlipidaemia Register Group: Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J, 2008; 29: 2625-2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Harada-Shiba M, Sugisawa T, Makino H, Abe M, Tsushima M, Yoshimasa Y, Yamashita T, Miyamoto Y, Yamamoto A, Tomoike H, Yokoyama S: Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2010; 17: 667-674 [DOI] [PubMed] [Google Scholar]
- 14). Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, Marais AD: Reduction in Mortality in Subjects With Homozygous Familial Hypercholesterolemia Associated With Advances in Lipid-Lowering Therapy. Circulation, 2011; 124: 2202-2207 [DOI] [PubMed] [Google Scholar]
- 15). Nordestgaard BG, Chapman MJ, Humphries SE, Gins-berg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease Consensus Statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Arai H, Ding Y-A, Yamashita S: Impact of the Integrated Guidance on the Care of Familial Hypercholesterolaemia. J Atheroscler Thromb, 2014; 21: 366-374 [DOI] [PubMed] [Google Scholar]
- 17). Harada-Shiba M, Arai H, Oikawa S, Ohta T, Okada T, Okamura T, Nohara A, Bujo H, Yokote K, Wakatsuki A: Guidelines for the management of familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 1043-1060 [DOI] [PubMed] [Google Scholar]
- 18). Watts GF, Sullivan DR, Poplawski N, van Bockxmeer F, Hamilton-Craig I, Clifton PM, O'Brien R, Bishop W, George P, Barter PJ, Bates T, Burnett JR, Coakley J, Davidson P, Emery J, Martin A, Farid W, Freeman L, Geelhoed E, Juniper A, Kidd A, Kostner K, Krass I, Livingston M, Maxwell S, O'Leary P, Owaimrin A, Redgrave TG, Reid N, Southwell L, Suthers G, Tonkin A, Towler S, Trent R: Familial hypercholesterolaemia: A model of care for Australasia. Atherosclerosis Supplements, 2011; 12: 221-263 [DOI] [PubMed] [Google Scholar]
- 19). Hu M, Hooper AJ, Bockxmeer FMv, Watts GF, Chan JC, Tomlinson B: Management of Familial Hypercholesterolemia in Hong Kong. J Atheroscler Thromb, 2016; 23: 520-531 [DOI] [PubMed] [Google Scholar]
- 20). Lee SH: Characteristics and Vascular Complications of Familial Hypercholesterolemia in Korea. J Atheroscler Thromb, 2016; 23: 532-538 [DOI] [PubMed] [Google Scholar]
- 21). Vallejo-Vaz AJ, Kondapally Seshasai SR, Cole D, Hovingh GK, Kastelein JJP, Mata P, Raal FJ, Santos RD, Soran H, Watts GF, Abifadel M, Aguilar-Salinas CA, Akram A, Alnouri F, Alonso R, Al-Rasadi K, Banach M, Bogsrud MP, Bourbon M, Bruckert E, Car J, Corral P, Descamps O, Dieplinger H, Durst R, Freiberger T, Gaspar IM, Genest J, Harada-Shiba M, Jiang L, Kayikcioglu M, Lam CSP, Latkovskis G, Laufs U, Liberopoulos E, Nilsson L, Nordestgaard BG, O'Donoghue JM, Sahebkar A, Schunkert H, Shehab A, Stoll M, Su T-C, Susekov A, Widén E, Catapano AL, Ray KK: Familial hypercholesterolaemia: A global call to arms. Atherosclerosis, 2015; 243: 257-259 [DOI] [PubMed] [Google Scholar]
- 22). Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes-Virella M, Watts GF, Wierzbicki AS: The Agenda for Familial Hypercholesterolemia - A Scientific Statement From the American Heart Association. Circulation, 2015; 132: DOI: 10.1161/CIR.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 23). Soutar AK, Naoumova RP: Mechanisms of Disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med, 2007; 4: 214-225 [DOI] [PubMed] [Google Scholar]
- 24). Hooper AJ, Nguyen LT, Burnett JR, Bates TR, Bell DA, Redgrave TG, Watts GF, van Bockxmeer FM: Genetic analysis of familial hypercholesterolaemia in Western Australia. Atherosclerosis, 2012; 224: 430-434 [DOI] [PubMed] [Google Scholar]
- 25). Khera AV, Won H-H, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, Morrison AC, Brody JA, Gupta N, Nomura A, Kessler T, Duga S, Bis JC, van Duijn CM, Cupples LA, Psaty B, Rader DJ, Danesh J, Schunkert H, McPherson R, Farrall M, Watkins H, Lander E, Wilson JG, Correa A, Boer-winkle E, Merlini PA, Ardissino D, Saleheen D, Gabriel S, Kathiresan S: Diagnostic Yield of Sequencing Familial Hypercholesterolemia Genes in Patients with Severe Hypercholesterolemia. J Am Coll Cardiol, 2016; 10.1016/j.jacc.2016.1003.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, Daniels SR, Gidding SS, de Ferranti SD, Ito MK, McGowan MP, Moriarty PM, Cromwell WC, Ross JL, Ziajka PE: Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol, 2011; 5: 133-140 [DOI] [PubMed] [Google Scholar]
- 27). Shin DG, Han SM, Kim DI, Rhee M-Y, Lee B-K, Ahn YK, Cho BR, Woo J-T, Hur S-H, Jeong J-O, Jang Y, Lee JH, Lee S-H: Clinical features of familial hypercholesterolemia in Korea: Predictors of pathogenic mutations and coronary artery disease – A study supported by the Korean Society of Lipidology and Atherosclerosis. Atherosclerosis, 2015; 243: 53-58 [DOI] [PubMed] [Google Scholar]
- 28). Haralambos K, Whatley SD, Edwards R, Gingell R, Townsend D, Ashfield-Watt P, Lansberg P, Datta DBN, McDowell IFW: Clinical experience of scoring criteria for Familial Hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis, 2015; 240: 190-196 [DOI] [PubMed] [Google Scholar]
- 29). Muir LA, George PM, Laurie AD, Reid N, Whitehead L: Preventing cardiovascular disease: a review of the effectiveness of identifying the people with familial hypercholesterolaemia in New Zealand. N Z Med J, 2010; 123: 97-102 [PubMed] [Google Scholar]
- 30). Laurie AD, Scott RS, George PM: Genetic screening of patients with familial hypercholesterolaemia (FH): a New Zealand perspective. Atherosclerosis Supplements, 2004; 5: 13-15 [DOI] [PubMed] [Google Scholar]
- 31). Goldberg AC, Gidding SS: Knowing the Prevalence of Familial Hypercholesterolemia Matters. Circulation, 2016; 133: 1054-1057 [DOI] [PubMed] [Google Scholar]
- 32). Shi Z, Yuan B, Zhao D, Taylor AW, Lin J, Watts GF: Familial hypercholesterolemia in China: Prevalence and evidence of underdetection and undertreatment in a community population. Int J Cardiol, 2014; 174: 834-836 [DOI] [PubMed] [Google Scholar]
- 33). Watts GF, Shaw JE, Pang J, Magliano DJ, Jennings GLR, Carrington MJ: Prevalence and treatment of familial hypercholesterolemia in Australian communities. Int J Cardiol, 2015; 185: 69-71 [DOI] [PubMed] [Google Scholar]
- 34). de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC: Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation, 2016; 133: 1067-1072 [DOI] [PubMed] [Google Scholar]
- 35). Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG: Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J, 2016; 10.1093/eurheartj/ehw1028 [DOI] [PubMed] [Google Scholar]
- 36). Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG: Familial Hypercholesterolemia in the Danish General Population: Prevalence, Coronary Artery Disease, and Cholesterol-Lowering Medication. J Clin Endocrinol Metab, 2012; 97: 3956-3964 [DOI] [PubMed] [Google Scholar]
- 37). Pang J, Martin AC, Mori TA, Beilin LJ, Watts GF: Prevalence of familial hypercholesterolaemia in adolescents: potential value of universal screening? The Journal of Pediatrics, 2016; 170: 315-316 [DOI] [PubMed] [Google Scholar]
- 38). De Backer G, Besseling J, Chapman J, Hovingh GK, JJP K, Ray K, Reiner Z, Wood D, De Bacquer D: Prevalence and management of familial hypercholesterolaemia in coronary patients: an analysis of EUROASPIRE IV. Atherosclerosis, 2015; 241: 169-175 [DOI] [PubMed] [Google Scholar]
- 39). Kirke A, Watts GF, Emery J: Detecting familial hypercholesterolaemia in general practice. Aust Fam Physician, 2012; 41: 965-968 [PubMed] [Google Scholar]
- 40). Qureshi N, Humphries SE, Seed M, Rowlands P, Minhas R. NICE Guideline Development Group: Identification and management of familial hypercholesterolaemia: what does it mean to primary care? Br J Gen Pract, 2009; 59: 773-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Bell DA, Garton-Smith J, Vickery A, Kirke A, Pang J, Bates TR, Watts GF: Familial Hypercholesterolaemia in Primary Care: Knowledge and Practices Among General Practitioners in Western Australia. Heart, Lung and Circulation, 2014; 23: 309-313 [DOI] [PubMed] [Google Scholar]
- 42). Pang J, Sullivan DR, Harada-Shiba M, Ding PY, Selvey S, Ali S, Watts GF: Significant gaps in awareness of familial hypercholesterolemia among physicians in selected Asia-Pacific countries: A pilot study. J Clin Lipidol, 2015; 9: 42-48 [DOI] [PubMed] [Google Scholar]
- 43). Datta BN, McDowell IF, Rees A: Integrating provision of specialist lipid services with cascade testing for familial hypercholesterolaemia. Curr Opin Lipidol, 2010; 21: 366-371 [DOI] [PubMed] [Google Scholar]
- 44). National Institute for Health and Clinical Excellence and The National Collaborating Centre for Primary Care: NICE Clinical Guideline 71: Identification and management of familial hypercholesterolaemia. 2008; [Google Scholar]
- 45). Pedersen KMV, Humphries SE, Roughton M, Besford JS: The National Audit of the Management of Familial Hypercholesterolaemia 2010: Full report. 2010; [Google Scholar]
- 46). Claassen L, Henneman L, Kindt I, Marteau TM, Timmermans DRM: Perceived Risk and Representations of Cardiovascular Disease and Preventive Behaviour in People Diagnosed with Familial Hypercholesterolemia. J Health Psychol, 2010; 15: 33-43 [DOI] [PubMed] [Google Scholar]
- 47). Hollman G, Olsson AG, Ek A-C: Disease knowledge and adherence to treatment in patients with familial hypercholesterolemia. J Cardiovasc Nurs, 2006; 21: 103-108 [DOI] [PubMed] [Google Scholar]
- 48). Weiner K, Durrington PN: Patients' understandings and experiences of familial hypercholesterolemia. Public Health Genomics, 2008; 11: 273-282 [DOI] [PubMed] [Google Scholar]
- 49). Muir LA, George PM, Whitehead L: Using the experiences of people with familial hypercholesterolaemia to help reduce the risk of cardiovascular disease: a qualitative systematic review. J Adv Nurs, 2012; 68: 1920-1932 [DOI] [PubMed] [Google Scholar]
- 50). Hardcastle SJ, Legge E, Laundy CS, Egan SJ, French R, Watts GF, Hagger MS: Patients' Perceptions and Experiences of Familial Hypercholesterolemia, Cascade Genetic Screening and Treatment. International Journal of Behavioral Medicine, 2015; 22: 92-100 [DOI] [PubMed] [Google Scholar]
- 51). Hagger MS, Hardcastle SJ, Hingley C, Strickland E, Pang J, Watts GF: Predicting Self-Management Behaviors in Familial Hypercholesterolemia Using an Integrated Theoretical Model: the Impact of Beliefs About Illnesses and Beliefs About Behaviors. International Journal of Behavioral Medicine, 2016; 23: 282-294 [DOI] [PubMed] [Google Scholar]
- 52). Ramaswami U, Cooper J, Humphries SE: The UK Paediatric Familial Hypercholesterolaemia Register: preliminary data. Arch Dis Child, 2016; 10.1136/archdischild-2015-308570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Screening Project for Familial Hypercholesterolemia in Central, Southern and Eastern Europe. 2015; http://screenprofh.com/
- 54). Pang J, Lansberg PJ, Watts GF: International Developments in the Care of Familial Hypercholesterolemia: Where Now and Where to Next? J Atheroscler Thromb, 2016; 23: 505-519 [DOI] [PubMed] [Google Scholar]


