Abstract
Aim
Recent studies have suggested that metabolic disorders such as obesity and type 2 diabetes are associated with gut microbiota. The association between atherosclerosis and gut microbiota has also been attracting increased attention. Our aim was to specify a characteristic trend of gut microbiota in coronary artery disease (CAD).
Methods
This study included 39 CAD patients, 30 age- and sex-matched no-CAD controls (Ctrls) with coronary risk factors and 50 healthy volunteers (HVs) without coronary risk factors. Bacterial DNA was extracted from their fecal samples and analyzed by terminal restriction fragment length polymorphism.
Results
A characteristic change of gut microbiota was observed in CAD patients, where the order Lactobacillales was increased (CAD, Ctrl vs. HV; 13.6% ± 12.0%, 6.2% ± 7.7% vs. 4.1% ± 5.9%; p < 0.001) and the phylum Bacteroidetes (Bacteroides + Prevotella) was decreased (CAD, Ctrl vs. HV; 35.5% ± 11.6%, 43.9% ± 11.2% vs. 47.4% ± 11.5%; p < 0.001). The CAD group was over-represented in enterotype “others” (III), compared with the Ctrl or HV group (p < 0.001, chi-squared test), although we could not deny the possibility that some drugs affect the gut flora types.
Conclusions
Although this study had some limitations, we demonstrated that the incidence of CAD was linked with an alteration of gut microbiota. A prospective study is desired to clarify a causal relationship between CAD and gut microbiota.
Keywords: Coronary artery disease, Gut microbiota, Lactobacillales, Bacteroidetes
See editorial vol. 23: 901–902
Introduction
The human gastrointestinal tract is estimated to contain approximately 100 trillion (1014) bacterial cells, comprising 1,000 bacterial species. This large array of gene products performs a diverse range of biochemical and metabolic activities to complement the host physiology. Host diet, lifestyle, hygiene, host genetics, use of antibiotics, and bacterial composition of the environment have all been found to affect the individual composition of gut microbiota1, 2). Despite the diversity among individuals, the unique core of gut micro-biota composition has remained remarkably stable over time, suggesting that intestinal bacteria may be a potential risk factor for human diseases3).
The natural ability of gut microbiota to affect host immunity is an important therapeutic target for many mucosal and nonmucosal immune-related conditions, such as inflammatory bowel diseases, metabolic syndrome, autoimmune diseases, diabetes, and chronic inflammatory diseases4). Gut microbiota actively regulate the host immune system, and recent studies have revealed that specific commensal bacterial species induce the accumulation of specific immune cell populations, for example, Foxp3+ regulatory T cells (Tregs)5, 6). We previously demonstrated that oral CD3 antibody or vitamin D3 can inhibit the progresion of atherosclerosis by increasing Tregs via modulating inetestinal immune system in mice7, 8), and coronary artery disease (CAD) patients have reduced Treg and Treg/Teff ratio compared with healthy controls9). Thus, we supposed that the composition of gut microbiota affects CAD10).
Recent studies have shown that obesity is associated with changes in the relative abundance of the two dominant bacterial phyla, Bacteroidetes and Firmicutes; Firmicutes is predominant in the guts of obese individuals11). Turnbaugh et al. first demonstrated that transplanting fecal microbiota from obese mice into germ-free mice resulted in the efficient transmission of the obese phenotype into the recipients, which suggested that a change of microbiota could be a cause of the obese phenotype12).
The association between atherosclerosis and gut microbiota has been attracting increased attention13, 14). A recent study reported that the gut microbiota-dependent metabolite, trimethylamine N oxide (TMAO), is associated with the incidence of cardiovascular disease. Oral dietary supplementation with choline or L-carnitine, which are metabolized to TMAO by gut micro-biota and liver enzymes, was shown to increase aortic root atherosclerotic plaque levels in apolipoprotein E-null mouse model. This effect was not observed in germ-free mice or with antibiotics-treated conditions, suggesting that gut microbiota are essential for choline metabolism and atherogenesis15, 16).
Aim
Our aim of this study was to investigate a link between gut microbiota and CAD and to specify a characteristic trend of gut microbiota in CAD. Karlsson et al. identified several compositional and functional alterations in the gut microbiota population that may be related to symptomatic atherosclerosis in patients who had undergone carotid endarterectomy17). However, it remains unclear whether CAD is associated with compositional changes of gut microbiota. We analyzed gut microbiota derived from fecal samples of CAD patients, which were compared with those from controls (Ctrls) and healthy volunteers (HVs) using terminal restriction fragment length polymorphism (T-RFLP) analysis. T-RFLP analysis is one of the most well-established and reliable 16S ribosomal RNA-based methods, especially when considering its high throughput and reproducibility18).
Methods
Recruitment of Patients and Volunteers
We compared the composition of gut microbiota among the CAD, Ctrl, and HV groups in this small case-control study. Thirty-nine CAD patients and 30 Ctrls with coronary risk factors were recruited from Kobe University Hospital. Blood samples were collected after an overnight fasting. Fecal samples were collected at Kobe University Hospital under the hospital diet. Fifty HVs without coronary risk factors were recruited from a health medical center, Kenko Life Plaza, Hyogo Health Service Association. Fecal samples were collected at home under the usual diet.
The CAD group was defined as patients with stable angina pectoris and old myocardial infarction, with preserved left ventricular ejection fraction (>40%) who underwent percutaneous coronary intervention or coronary artery bypass graft surgery and for at least 6 months interval; acute coronary syndrome patients were excluded. Single- or multi-vessel disease referred to the number of major coronary vessels demonstrating >75% stenosis on diagnostic coronary angiography.
Thirty patients who had coronary risk factors such as hypertension, diabetes, and/or dyslipidemia, but who did not have coronary or other vascular diseases were recruited as age- and sex-matched Ctrls. The criteria for inclusion in the HV group were no history of vascular disease or treatment for hypertension, diabetes or dyslipidemia. No history of coronary or other vascular disease was defined as no documented vascular disease, symptoms indicating angina pectoris, abnormality in electrocardiogram indicating old myocardial infarction or angina pectoris, or abnormality in the chest X-ray.
Patients with systemic diseases, including hepatic disease, renal disease (serum creatinine levels > 2.0 mg/dl), collagen disease, and malignancy, were excluded from all groups. Patients treated with antibiotics were also excluded. Diabetes was defined as HbA1c > 6.5% (National Glycohemoglobin Standardization Program) use of oral antidiabetic drugs, or insulin therapy. Hypertension was defined as blood pressure > 140/90 mmHg or use of antihypertensive drugs. Dyslipidemia was defined as low-density lipoprotein cholesterol > 140 mg/dl, triglycerides > 150 mg/dl, or use of antidyslipidemic drugs, based on the guideline of Japan Atherosclerosis Society19).
This study was performed in compliance with the Declaration of Helsinki and was approved by the Ethics Committee of Kobe University (No.1318) and Kenko Life Plaza, Hyogo Health Service Association. All subjects provided oral and written informed consent to participate in this study.
T-RFLP
T-RFLP analyses of fecal samples were performed by TechnoSuruga Laboratory (Shizuoka, Japan). DNA was extracted from fecal samples and then amplified by polymerase chain reaction. The resulting 16S rDNA amplicons were treated with BslI (New England BioLabs). The details were shown in Supplemental Methods.
Statistical Analysis
The distances were calculated to determine any similarity among the samples and were represented graphically by constructing a dendrogram. Pearson's correlation analysis and the unweighted pair-group method with arithmetic mean were used to establish the type of dendrogram, which was calculated by Gene Maths (Applied Maths, Belgium) (Fig. 1B). Data represent the mean ± standard deviation. Continuous parametric data were compared using one-way ANOVA test followed by Tukey's post-hoc analysis. Nonparametric data were compared using the Kruskal – Wallis test, followed by Dunn's post-hoc analysis. Categorical variables are presented using frequency counts, and intergroup comparisons were analyzed by the chi-squared tests. Pearson's correlation analysis was used for statistical correlation between two parameters. Factors with univariate p < 0.05 were entered into the multivariate analysis. Age and sex were entered into the multivariate model as an exception. All statistical analyses were two sided; p < 0.05 was considered statistically significant. For statistical analysis, GraphPad Prism version 6.0 (GraphPad Software; San Diego, CA, USA) and Medcalc Software (version 14.12; Mariakerke, Belgium) were used.
Fig. 1.
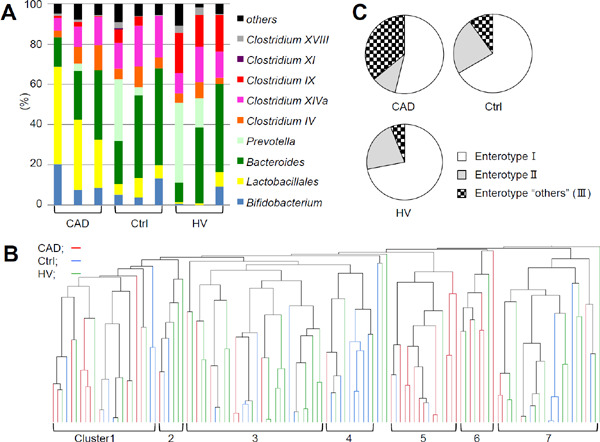
Distribution of gut microbiota and classification of enterotype in the coronary artery disease (CAD), control, and healthy volunteer groups (A) Representative profiles of gut microbiota. (B) Dendrogram of the similarity was shown. (C) Enterotype “others” (III) was enriched in CAD group (p < 0.001, chi-squared test). The terminal restriction fragment length polymorphism patterns were analyzed using Pearson's correlation and the unweighted pair-group method with arithmetic mean algorithm. Enterotypes were defined as follows: I, > 30% Bacteroides; II, > 15% Prevotella; and the remaining as “others” (III).
Results
Baseline Characteristics
The baseline characteristics, medications, and laboratory data of the CAD (multi- and single-vessel disease), Ctrl, and HV groups are presented in Table 1. It was difficult to match the clinical background among CAD, Ctrl, and HV groups as shown in Table 1. Especially, the background medication between the three groups was greatly different, and it was the limitation of this study. There was no significant difference in body mass index (BMI), percentage of hypertension, and diabetes patients between the CAD and Ctrl groups. The HV group received no medications. There was no significant difference in clinical backgrounds between multi- and single-vessel diseases in the CAD group.
Table 1. Baseline characteristics and laboratory data of the study population.
| Variables | CAD Total (n = 39) | Multi-vessel disease (n = 28) | Single-vessel disease (n = 11) | Ctrl (n = 30) | HV (n = 50) |
|---|---|---|---|---|---|
| Characteristics | |||||
| Age (years) | 61.1 ± 9.4 | 59.5 ± 9.2 | 65.1 ± 9.7 | 62.1 ± 6.4 | 58.7 ± 7.3 |
| Sex (male,%) | 85 | 86 | 82 | 77 | 78 |
| BMI (kg/m2) | 25.7 ± 4.1 | 25.9 ± 4.2 | 25.2 ± 4.2 | 25.6 ± 4.1 | 22.4 ± 2.4*** |
| History of smoking (%) | 71* | 76 | 55 | 50 | 56 |
| Current smoking (%) | 17* | 21 | 9 | 7 | 32* |
| Dyslipidemia (%) | 95* | 93 | 100 | 63 | 50 |
| Hypertension (%) | 87 | 86 | 91 | 83 | 20*** |
| Diabetes (%) | 38 | 46 | 18 | 40 | 2*** |
| Medications | |||||
| Anti diabetes drug (%) (including insulin therapy) | 38 | 46 | 18 | 37 | 0 |
| Statin (%) | 92*** | 89*** | 100*** | 40 | 0 |
| ACE-I/ARB (%) | 74* | 75 | 73 | 47 | 0 |
| β-blocker (%) | 54* | 54 | 55 | 23 | 0 |
| Calcium channel blocker (%) | 56 | 54 | 64 | 37 | 0 |
| PPI/H2 blocker (%) | 95*** | 93** | 100** | 53 | 0 |
| Anticoagulant (%) | 10*** | 7*** | 18 | 57 | 0 |
| Antiplatelet (%) | 100*** | 100*** | 100*** | 0 | 0 |
| Laboratory data | |||||
| AST (U/I) | 25.5 ± 13.6 | 26.0 ± 14.1 | 24.3 ± 13.5 | 25.8 ± 11.3 | 20.6 ± 5.0 |
| ALT (U/I) | 26.5 ± 20.4 | 27.8 ± 21.8 | 23.4 ± 18.0 | 25.7 ± 14.5 | 20.3 ± 10.8 |
| BUN (mg/dL) | 16.4 ± 5.1 | 15.8 ± 3.6 | 17.9 ± 8.0 | 16.6 ± 4.0 | 13.8 ± 2.76* |
| Creatinine (mg/dL) | 0.87 ± 0.27 | 0.83 ± 0.16 | 0.98 ± 0.34 | 0.94 ± 0.25 | 0.77 ± 0.14** |
| HDL-C (mg/dL) | 43.4 ± 9.3* | 43.3 ± 9.74 | 43.9 ± 8.8 | 52.3 ± 12.9 | 64.6 ± 15.9*** |
| LDL-C (mg/dL) | 90.4 ± 22.7* | 90.9 ± 23.9 | 89.1 ± 21.4 | 108.5 ± 29.6 | 130.9 ± 27.7** |
| TG (mg/dL) | 186.7 ± 126.5* | 172.9 ± 119.0 | 221.7 ± 149.2* | 124.8 ± 55.7 | 101.6 ± 61.2 |
| HbA1c (NGSP%) | 6.64 ± 1.56 | 6.66 ± 1.53 | 6.59 ± 1.77 | 6.36 ± 1.04 | 5.45 ± 0.40*** |
| CRP (mg/dL) | 0.11 ± 0.12 | 0.12 ± 0.13 | 0.06 ± 0.07 | 0.13 ± 0.22 | 0.10 ± 0.18 |
Results are expressed as the mean ± SD or %. Comparisons were performed using the Kruskal-Wallis test, one-way ANOVA test or chi sqared test followed post-hoc analysis. Natural logarithmic transformation is used for comparison of TG and CRP. Comparisons among all CAD, Ctrl, HV groups and those among multi-disease and single-vessel, Ctrl and HV groups were performed separately. There was no significant difference in each parameter between multi- and single-vessel disease. Single- or multi-vessel disease in the CAD group referred to the number of major coronary vessels demonstrating > 75% stenosis on diagnostic coronary angiography. P values show the result against controls (*p < 0.05, **p < 0.01, ***p < 0.001).
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; Ctrl, controls; CRP, C-reactive protein; H2 blocker, histamine H2-receptor antagonist; HDL-C, high-density lipoprotein cholesterol; HV; healthy volunteers; LDL-C, low-density lipoprotein cholesterol; NGSP, National Glycohemoglobin Standardization Program; TG, triglycerides; PPI; proton pomp inhibitor.
Distribution of Gut Microbiota and Enterotypes
The results of the T-RFLP comparison of gut microbiota distribution among the CAD, Ctrl, and HV groups are shown in Fig. 1A–C. T-RFLP using Bsl I could classify gut microbiota into the following 10 groups: Prevotella, Bacteroides, Lactobacillales, Bifidobacterium, Clostridium cluster IV, Clostridium subcluster XIVa, Clostridium cluster IX, Clostridium cluster XI, Clostridium cluster XVIII, and others, combining the operational taxonomic units that belonged to the same group. Representative distributions of gut microbiota are shown in Fig. 1A. The samples were divided into seven clusters according to a similarity setting. Clusters 5 and 6 consisted mainly of CAD patients (5, 80%; 6, 63%), whereas clusters 2, 3, 4, and 7 predominantly contained Ctrl or HV individuals (2, 83%; 3, 80%; 4, 91%; and 7, 82%; Fig. 1B). Arumugam et al. suggested that the human gut microbiota could be stratified into three enterotypes: enterotypes I, II, and III were predominant in Bacteroides, Prevotella, and Ruminococcus, respectively20). However, different contributors can identify enterotype III, depending on the source of sequences; this cluster can also be characterized by low levels of Bacteroides and Prevotella rather than a dominant genus17). In contrast, Ding et al. demonstrated that human gut microbiota could be divided into four community types; Bacteroides and Prevotella were also key gut microbiota that could be divided into four types21). According to the two reports described above, we defined enterotype I as Bacteroides > 30%, enterotype II as Prevotella > 15%, and the remaining as enterotype “others” (III), using this T-RFLP analysis of gut microbiota20, 21). Data revealed that CAD patients were over-represented in the enterotype “others” (III) compared with the Ctrl or HV group (p < 0.001, chi-squared test; Fig. 1C).
The Order Lactobacillales
We found that the order Lactobacillales was significantly increased in the CAD group, compared with the Ctrl or HV group (CAD, Ctrl vs. HV; 13.6% ± 12.0%, 6.2% ± 7.7% vs. 4.1% ± 5.9%; p < 0.001, Fig. 2A and B). However, there was no significant difference in any other groups of gut microbiota between the Ctrl and HV groups (Fig. 2A). Although the sample size was too small to achieve completely reliable evaluation by logistic regression analysis, we could show that this increase of the order Lactobacillales was an independent factor from the known risk factors like type 2 diabetes, dyslipidemia, history of smoking, and hypertension (Table 2A). However, drugs could be confounding factors for the presence of CAD (Table 2B). The percentage of the order Lactobacillales in CAD patients with multi-vessel disease was significantly higher than Ctrls and tended to be higher than CAD patients with single-vessel disease, although there was no significant difference between CAD patients with single-vessel disease and Ctrls (multi- and single-vessel disease vs. Ctrl; 16.5% ± 12.6%, 6.3% ± 6.1%, 6.2% ± 7.7%; p = 0.002, Fig. 2C and Supplemental Fig. 1).
Fig. 2.
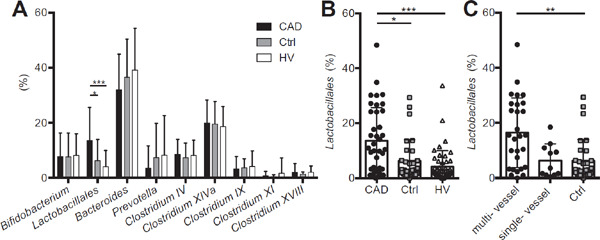
Comparison of the order Lactobacillales. (A) The percentage of each group of gut microbiota was compared among the coronary artery disease (CAD), control (Ctrl), and healthy volunteer (HV) groups. The percentage of the order Lactobacillales was increased (B) in the CAD group (CAD, Ctrl vs. HV) (C) especially in CAD patients with multi-vessel disease (multiand single-vessel disease vs. Ctrl). Closed circles indicate the CAD group; gray squares indicate the Ctrl group, whereas open triangles indicate the HV group. Single- or multi-vessel disease referred to the number of major coronary vessels demonstrating > 75% stenosis on diagnostic coronary angiography. Kruskal–Wallis test followed by Dunn's post-hoc analysis was used to calculate p-values (*p < 0.05, **p < 0.01, and ***p < 0.001).
Table 2. Logistic regression analysis for the presence of coronary artery disease (CAD) in the CAD and control (Ctrl) groups (n = 69).
| (A) Variables | Univariate regression |
Multivariate regression |
||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| Age | 0.99 (0.93–1.05) | 0.63 | 0.97 (0.90–1.04) | 0.43 |
| Sex | 1.67 (0.50–5.63) | 0.41 | 2.50 (0.64–9.73) | 0.19 |
| BMI (kg/m2) | 1.01 (0.90–1.13) | 0.88 | Not selected | |
| DM | 0.94 (0.35–2.48) | 0.90 | Not selected | |
| HT | 1.36 (0.36–5.21) | 0.65 | Not selected | |
| DL | 10.7 (2.15–53.32) | 0.004** | 9.64 (1.76–52.78) | 0.009** |
| History of smoking | 2.55 (0.93–6.91) | 0.067 | Not selected | |
| ln CRP (mg/dL) | 0.90 (0.33–2.39) | 0.83 | Not selected | |
| Lactobacillales | (%) 1.08 (1.02–1.15) | 0.009** | 1.07 (1.00–1.13) | 0.048* |
| (B) Variables | Univariate regression |
Multivariate regression |
||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| PPI/H2 blocker | 16.2 (3.29–79.7) | <0.001*** | 121 (5.89–2480) | 0.002** |
| Statin | 18.0 (4.50–72.0) | <0.001*** | 21.6 (1.48–317) | 0.025* |
| βblocker | 3.83 (1.34–11.0) | 0.013* | 3.79 (0.31–46.9) | 0.30 |
| ACE–I/ARB | 3.31 (1.20–9.15) | 0.021* | 0.86 (0.11–6.55) | 0.89 |
| Anticoagulant | 0.09 (0.03–0.31) | <0.001*** | 0.01 (0.0004–0.13) | <0.001*** |
| Lactobacillales (%) | 1.08 (1.02–1.15) | 0.009** | 1.00 (0.92–1.09) | 0.97 |
Because the sample size was small, we divided this analysis into two models; (A) variables = known risk factors and Lactobacillales (%) (B) variables = medication and Lactobacillales (%). Factors with univariate p < 0.05 were entered into the multivariate analysis (*p < 0.05, **p < 0.01, ***p0.001). (A) Age and sex were entered into the multivariate model as exceptions. Abbreviations are same as Table 1 and 2.
The Phylum Bacteroidetes (Bacteroides and Prevotella)
The prevalence of the phylum Bacteroidetes (Bacteroides + Prevotella) was significantly decreased in the CAD group compared with the Ctrl or HV group (CAD, Ctrl vs. HV; 35.5% ± 11.6%, 43.9% ± 11.2% vs. 47.4% ± 11.5%; p > 0.001, Fig. 3A). The Firmicutes/Bacteroidetes ratio (F/B ratio) was increased in the CAD group compared with the Ctrl group (CAD, Ctrl vs. HV; 1.6 ± 1.0, 1.3 ± 2.0 vs. 1.1 ± 1.4; p < 0.001, Fig. 3B). The phylum Firmicutes included Lactobacillales and Clostridium, whereas the phylum Bacteroidetes included Bacteroides and Prevotella, according to this T-RFLP analysis.
Fig. 3.
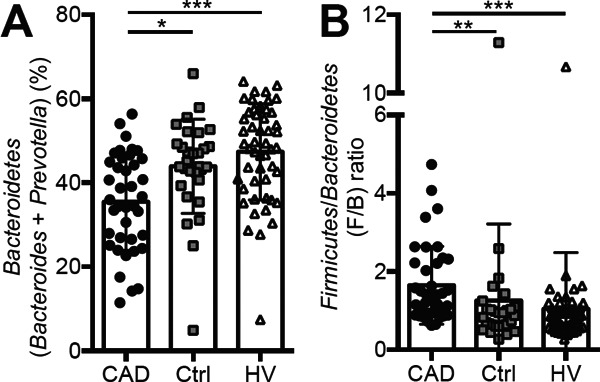
Comparison of the phylum Bacteroidetes and the Firmicutes/Bacteroidetes (F/B) ratio. (A) The phylum Bacteroidetes (Prevotella + Bacteroides) was decreased; (B) the F/B ratio was increased in the coronary artery disease (CAD) group. Kruskal -Wallis test followed by Dunn's post-hoc analysis was used to calculate p-values (*p < 0.05, **p < 0.01, and ***p < 0.001). The phylum Firmicutes = Lactobacillales + Clostridium. The phylum Bacteroidetes = Bacteroides + Prevotella.
Obesity and Type 2 Diabetes
Obesity and type 2 diabetes have been previously reported to be associated with gut microbiota. We found a positive correlation between BMI and the order Lactobacillales or the F/B ratio and a negative correlation between BMI and the phylum Bacteroidetes (Supplemental Fig. 2A–C). We also found a negative correlation between HbA1c and the phylum Bacteroidetes, but no significant correlation between HbA1c and the order Lactobacillales or the F/B ratio was found (Supplemental Fig. 3A–C).
Medication
All patients were treated based on the Japan Atherosclerosis Society guideline19). It was difficult to match the background medication between the CAD and Ctrl group and to analyze the effect of each drug in the CAD group because almost all of CAD patients were treated with essential drugs for secondary prevention. The multivariate logistic regression analysis for the presence of CAD could not show that the percentage of the order Lactobacillales was an independent factor from drugs (Table 2B). Therefore, we evaluated the effect of each unmatched drug (ACE-I/ARB, β-blocker, statin, PPI/H2 blocker, antiplatelet, or anticoagulant) in the Ctrl group by comparing the composition of gut microbiota between patients treated with and without each drug. Because antiplatelet therapy was applied to all CAD patients but not to Ctrl patients at all, we could not evaluate the effect of antiplatelet therapy in this study. The percentage of the order Lactobacillales was higher in Ctrls treated with PPI/H2blocker (treated vs. not treated; 9.23% ± 9.17% vs. 2.83% ± 3.47%; p = 0.008) or anticoagulant (treated vs. not-treated; 7.61% ± 7.95% vs. 4.45% ± 7.28%; p = 0.048). The percentage of Prevotella was higher in Ctrls treated with β-blocker (treated vs. not treated; 15.5% ± 16.1% vs. 4.94% ± 10.3%; p = 0.018). The percentage of Clostridium XI was also higher in Ctrls treated with β-blocker (treated vs. not treated; 0.55% ± 0.26% vs. 0.42% ± 0.83%; p = 0.027). Because these results demonstrated that drugs should be considered as confounding factors, we subsequently analyzed the relationship between gut microbiota, especially the percentage of the order Lactobacillales, and the severity of CAD. The multivariate logistic regression analysis for the presence of multi-vessel disease showed that the percentage of the order Lactobacillales could be an independent factor from drugs (Supplemental Table). We found that the percentage of the order Lactobacillales tended to be higher in CAD patients, especially with multi-vessel disease, than Ctrls even after adjusting the use of PPI/H2blocker (Supplemental Fig. 4A) or statin (Supplemental Fig. 4B). Although the increase of the order Lactobacillales was possibly because of the presence of CAD itself, we could not deny that medication could affect the composition of gut microbiota due to the limitation of the study design.
Discussion
We analyzed the distribution of gut microbiota among the CAD, Ctrl, and HV groups (Fig. 1). Representative data indicated the composition of gut microbiota in the CAD group was, as a whole, different from the Ctrl or HV group (Fig. 1A). A similarity dendrogram revealed that the fecal bacterial communities differed in the CAD group, compared with the Ctrl and HV groups (Fig. 1B). We also found an increased prevalence of enterotype “others” (III) in CAD patients (Fig. 1C), consistent with the previous study in patients who had undergone carotid endarterectomy17).
Comparison of each group of gut microbiota revealed that the order Lactobacillales was increased, whereas the phylum Bacteroidetes (the genera Bacteroides + Prevotella) was decreased in the CAD group (Figs. 2 and 3). The order Lactobacillales is one of the main components of the human gut microbiota and belongs to the phylum Firmicutes. The order Lactobacillales is divided into several genera, including Lactobacillus, Streptococcus, and Enterococcus. Lactobacillus is the main genus within the order Lactobacillales found in human gut microbiota. A previous high-throughput sequencing study also demonstrated increased Lactobacillus species in feces of a European female cohort with type 2 diabetes22). However, we did not find a correlation between diabetes markers and the order Lactobacillales in this study (Supplemental Fig. 3A). In addition, there was still a significant increase in the order Lactobacillales in the CAD group compared with the Ctrl group only in the population of patients with type 2 diabetes (data not shown).
We demonstrated a decrease in the phylum Bacteroidetes in CAD patients. The phylum Bacteroidetes is composed mainly of two genera, Bacteroides and Prevotella, which contribute to the classification of enterotypes. Bacteroides fragilis affects mucosal T-cell homeostasis by promoting regulatory T-cell function23). Other Bacteroides species can also establish mutualistic relationships with the host by flourishing in the plant polysaccharide-enriched gut environment and by providing the biological byproducts necessary for the well-being of the host1). The role of Bacteroidetes in CAD should be assessed in further studies.
Several previous reports demonstrated that the F/B ratio was associated with obesity. Although no previous T-RFLP studies demonstrated an association between the F/B ratio and BMI, we demonstrated a direct correlation (Supplemental Fig. 2C), which was compatible with the results of other sequencing studies. Because there was no difference in the level of BMI between the CAD and Ctrl groups, the significant increase in the F/B ratio in the CAD group was possibly reflected by the fact of suffering from CAD. The increase in the phylum Firmicutes might be followed by the increase in the order Lactobacillales and the decrease in the phylum Bacteroidetes in this study.
There was no significant difference in the comparison of gut microbiota between the Ctrl and HV groups, which indicated that a change of gut microbiota in the CAD group was not associated with the coronary risk factors such as hypertension or type 2 diabetes. Although we could not clearly eliminate the influence of conflicting factors such as medication, we demonstrated that the composition of gut microbiota differed in CAD patients. A prospective cohort study is desired to identify whether gut-microbial alteration precedes development of atherosclerosis or CAD itself or medication cause modulation of gut microbiota. In addition, we hope that the biological significance of gut microbiota in the pathogenesis of CAD will be clarified in the future.
This study had some limitations that should be considered when interpreting the results. First, the number of patients was small; therefore, additional larger trials are needed to validate these observations. Second, we analyzed fecal samples using T-RFLP, but high-throughput DNA sequencing technology would be desirable to find a specific genus or species of gut microbiota involved in CAD. Therefore, we are currently performing a novel project using high-throughput DNA sequencing. Third, as mentioned in the result session, it was difficult to match the background medication between the CAD and Ctrl groups. Finally, it remains unclear whether these differences were a response to atherosclerosis or whether they actively induced atherosclerosis.
Conclusion
We, for the first time, analyzed the composition of gut microbiota in CAD patients and demonstrated a link between the composition of gut microbiota and CAD. A prospective cohort study is desired to identify whether gut-microbial alteration precedes development of atherosclerosis or not.
Acknowledgement
The authors are grateful to Dr. Katsuji Ikekubo and Tsutomu Kamino in Kenko Life Plaza, Hyogo Health Service Association for assistance in collecting control volunteer data, and to Takayoshi Hisada at the TechnoSuruga Laboratory for help in interpreting the T-RFLP data.
Disclosures
The authors declare no conflict of interest.
Sources of Funding
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant No. 24591114 (T. Y.), Suzuken Memorial Foundation (T. Y. and N. S.), Takeda Scientific Foundation (T. Y. and N. S.), Mochida Memorial Foundation (T. Y.), Senshin Medical Research Foundation (T. Y. and N. S.), Yakult Bioscience Research Foundation (T. Y.), Uehara Memorial Foundation (K. H. and N. S.), and The Japanese Circulation Society Translational Research Foundation (K. H.).
Clinical Trial Registration Information
URL: http://www.umin.ac.jp/ctr/. Unique identifier: UMIN000012049
Supplemental Methods
T-RFLP
The fecal samples were suspended in a solution containing 100 mM Tris-HCl (pH 9.0), 40 mM ethylenediaminetetraacetic acid, 4 M guanidine thiocyanate, and 0.001% bromothymol blue. Fecal solids in the suspension were broken down using a FastPrep FP100A Instrument (MP Biomedicals; CA, USA) with zirconia beads at 5 m/s for 2 min. DNA was then extracted from a 200-mL suspension using an automatic nucleic acid extractor (Precision System Science; Chiba, Japan). MagDEA® DNA 200 (Precision System Science) was used as the reagent for the automatic nucleic acid extraction. Polymerase chain reaction (PCR) was performed using total fecal DNA and the following primers: 5′ FAM-labeled 516f (5′-TGCCAGCAGCCGCGGTA-3′; Escherichia coli positions 516–532) and 1510r (5′-GGTTACCTTGTTACGACTT-3′; E. coli positions 1510–1492). The resulting 16S rDNA amplicons were treated with 10 U of BslI (New England BioLabs) for 3 h, fractionated using an automated sequence analyser (ABI PRISM 3130xl Genetic Analyzer; Applied Biosystems), and analysed using the DNA analysis software Gene Mapper. Because the apparent size of identical terminal restriction fragments can vary from 1 – 3 base pairs (bp), major fragments differing in size by 1 – 3 bp were classified into operational taxonomic units (OTUs)1, 2).
Supplemental Fig. 1.
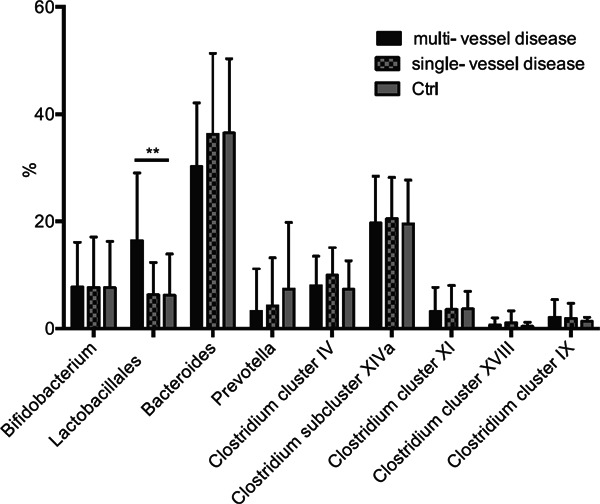
Relationship between the severity of coronary artery disease and each group of gut microbiota.
The percentage of each group of gut microbiota was compared between Ctrl, CAD with single-vessel disease and CAD with multi-vessel disease. Single- or multi-vessel disease referred to the number of major coronary vessels demonstrating > 75% stenosis on diagnostic coronary angiography. Kruskal-Wallis test followed by Dunn's post-hoc analysis was used to calculate p-values (**p < 0.01).
Supplemental Fig. 2.
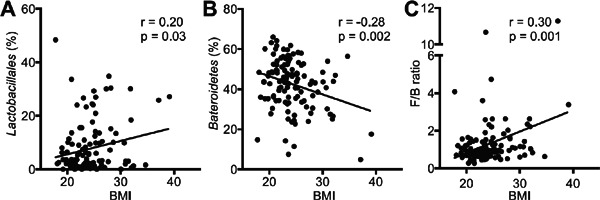
Obesity and distribution of gut microbiota.
Correlations between BMI and (A) the order Lactobacillales, (B) the phylum Bacteroidetes (Prevotella+ Bacteroides) or (C) the Firmicutes/Bacteroidetes (F/B) ratio were shown. Pearson's correlation analysis was used for statistical correlation between two parameters.
Supplemental Fig. 3.
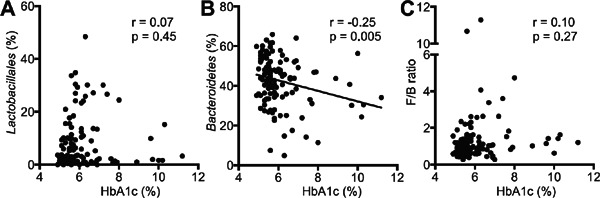
Type 2 diabetes and distribution of gut microbiota.
Correlations between HbA1c (%) and (A) the order Lactobacillales, (B) the phylum Bacteroidetes (Prevotella + Bacteroides) or (C) the Firmicutes/Bacteroidetes (F/B) ratio were shown. Pearson's correlation analysis was used for statistical correlation between two parameters.
Supplemental Table. Logistic regression analysis for the presence of multi-vessel disease in the coronary artery disease (CAD) and control (Ctrl) groups (n = 69).
| (A) Variables | Univariate regression |
Multivariate regression |
||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| Age | 0.95 (0.90–1.01) | 0.11 | 0.93 (0.86–1.00) | 0.066 |
| Sex | 1.69 (0.46–6.14) | 0.43 | 0.73 (0.12–4.53) | 0.74 |
| BMI (kg/m2) | 1.03 (0.91–1.15) | 0.68 | Not selected | |
| DM | 1.67 (0.62–4.47) | 0.31 | Not selected | |
| HT | 1.03 (0.26–4.04) | 0.97 | Not selected | |
| DL | 4.77 (0.97–23.5) | 0.055 | Not selected | |
| History of smoking | 3.49 (1.17–10.4) | 0.025* | 9.85 (1.72–56.5) | 0.010* |
| ln CRP (mg/dL) | 1.54 (0.57–4.16) | 0.4 | Not selected | |
| Lactobacillales (%) | 1.11 (1.05–1.18) | <0.001*** | 1.16 (1.06–1.26) | <0.001*** |
| (B) Variables | Univariate regression |
Multivariate regression |
||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| PPI/H2 blocker | 6.74 (1.39–32.6) | 0.018* | 6.80 (1.05–43.7) | 0.043* |
| Statin | 6.52 (1.70–25.1) | 0.006** | 1.14 (0.17–7.75) | 0.89 |
| βblocker | 2.48 (0.92–6.70) | 0.072 | Not selected | |
| ACE-I/ARB | 2.59 (0.90–7.43) | 0.076 | Not selected | |
| anticoagulant | 0.09 (0.02–0.43) | 0.002**0.02 | (0.003–0.25) | 0.002** |
| Lactobacillales (%) | 1.11 (1.05–1.18) | <0.001*** | 1.11 (1.03–1.20) | 0.007** |
Because the sample size was small, we divided this analysis into two models; (A) variables = known risk factors and Lactobacillales (%) (B) variables = medication and Lactobacillales (%). Factors with univariate p < 0.05 were entered into the multivariate analysis (*p < 0.05, **p < 0.01, ***p < 0.001). (A) Age and sex were entered into the multivariate model as an exception. Abbreviations are same as Table 1 and 2.
Supplemental Fig. 4.
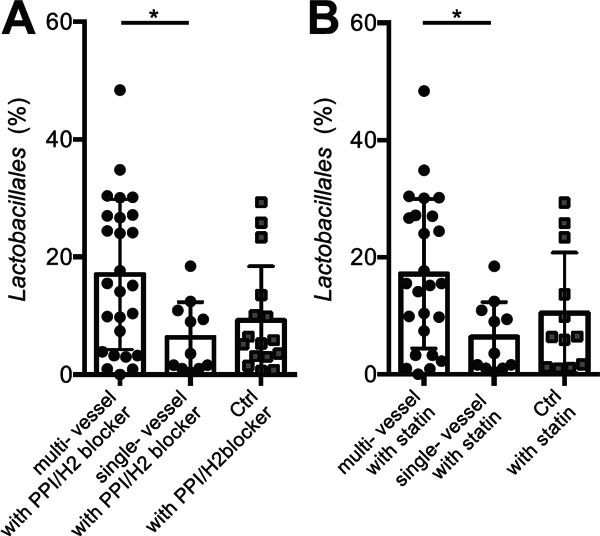
The comparison of Lactobacillales after adjusting medication.
(A) The percentage of the order Lactobacillales tended to be increased in CAD patients with PPI/H2 blocker, compared with Ctrls with PPI/H2 blocker (CAD with PPI/H2 blocker vs. Ctrls with PPI/H2 blocker; 13.8 ± 12.1% vs 9.2 ± 9.2%; p= 0.24, Figure not shown), especially in those with multi-vessel disease (multi-, single-vessel disease vs. Ctrl; 17.0 ± 12.8%, 6.3 ± 6.1% vs. 9.2 ± 9.2%; p= 0.025). (B) The percentage of the order Lactobacillales was also tended to be increased in CAD patients with statin, compared with Ctrls with statin (CAD with statin vs. Ctrls with statin; 13.9 ± 12.1% vs. 10.5 ± 10.3%; p= 0.41, Figure not shown), especially in those with multi-vessel disease with statin (multi-, single-vessel disease vs. Ctrl; 17.2 ± 12.7%, 6.3 ± 6.1% vs. 10.5 ± 10.3%, p= 0.034). Kruskal-Wallis test followed by Dunn's post-hoc analysis or Mann-Whitney U test were used to calculate p-values (*p < 0.05). Single- or multi-vessel disease referred to the number of major coronary vessels demonstrating > 75% stenosis on diagnostic coronary angiography.
References
- 1). Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD: Linking long-term dietary patterns with gut microbial enterotypes. Science, 2011; 334: 105-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P: Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America, 2010; 107: 14691-14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI: The long-term stability of the human gut microbiota. Science, 2013; 341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Ivanov II, Honda K: Intestinal commensal microbes as immune modulators. Cell host & microbe, 2012; 12: 496-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K: Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 2013; 500: 232-236 [DOI] [PubMed] [Google Scholar]
- 6). Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K: Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 2011; 331: 337-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, Ishida T, Hirata K: Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arteriosclerosis, thrombosis, and vascular biology, 2010; 30: 2495-2503 [DOI] [PubMed] [Google Scholar]
- 8). Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, Hirata K: Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation, 2009; 120: 1996-2005 [DOI] [PubMed] [Google Scholar]
- 9). Emoto T, Sasaki N, Yamashita T, Kasahara K, Yodoi K, Sasaki Y, Matsumoto T, Mizoguchi T, Hirata K: Regulatory/effector T-cell ratio is reduced in coronary artery disease. Circulation journal : official journal of the Japanese Circulation Society, 2014; 78: 2935-2941 [DOI] [PubMed] [Google Scholar]
- 10). Yamashita T, Kasahara K, Emoto T, Matsumoto T, Mizoguchi T, Kitano N, Sasaki N, Hirata K: Intestinal Immunity and Gut Microbiota as Therapeutic Targets for Preventing Atherosclerotic Cardiovascular Diseases. Circulation journal : official journal of the Japanese Circulation Society, 2015; 79: 1882-1890 [DOI] [PubMed] [Google Scholar]
- 11). Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Microbial ecology: human gut microbes associated with obesity. Nature, 2006; 444: 1022-1023 [DOI] [PubMed] [Google Scholar]
- 12). Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 2006; 444: 1027-1031 [DOI] [PubMed] [Google Scholar]
- 13). Vinje S, Stroes E, Nieuwdorp M, Hazen SL: The gut microbiome as novel cardio-metabolic target: the time has come! European heart journal, 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Org E, Mehrabian M, Lusis AJ: Unraveling the environmental and genetic interactions in atherosclerosis: Central role of the gut microbiota. Atherosclerosis, 2015; 241: 387-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 2011; 472: 57-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL: Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine, 2013; 19: 576-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Backhed F, Nielsen J: Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature communications, 2012; 3: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Osborn AM, Moore ER, Timmis KN: An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environmental microbiology, 2000; 2: 39-50 [DOI] [PubMed] [Google Scholar]
- 19). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Coronary artery disease. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan--2012 version. Journal of atherosclerosis and thrombosis, 2014; 21: 86-92 [DOI] [PubMed] [Google Scholar]
- 20). Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, Meta HITC, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Merieux A, Melo Minardi R, M'Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P: Enterotypes of the human gut microbiome. Nature, 2011; 473: 174-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Ding T, Schloss PD: Dynamics and associations of microbial community types across the human body. Nature, 2014; 509: 357-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F: Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature, 2013; 498: 99-103 [DOI] [PubMed] [Google Scholar]
- 23). Mazmanian SK, Round JL, Kasper DL: A microbial symbiosis factor prevents intestinal inflammatory disease. Nature, 2008; 453: 620-625 [DOI] [PubMed] [Google Scholar]
References
- 1). Nagashima K, Hisada T, Sato M, Mochizuki J. Application of New Primer-Enzyme Combinations to Terminal Restriction Fragment Length Polymorphism Profiling of Bacterial Populations in Human Feces. Applied and Environmental Microbiology 2003; 69: 1251-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Nagashima K, Mochizuki J, Hisada T, Suzuki S, Shimomura S. Phylogenetic Analysis of 16S Ribosomal RNA Gene Sequences from Human Fecal Microbiota and lmproved Utility of Terminal Restriction Fragment Length Polymorphism Profiling. Bioscience Microflora 2006; 25: 99-107 [Google Scholar]


