Abstract
We investigated changes in oxidative stress markers during the transition period in healthy Holstein cows and those with postpartum diseases. Transition control (TC) Holstein cows (n=9) were evaluated for longitudinal changes during the transition period and postpartum diseased (PD) cows with ketosis (n=10), abomasal displacement (n=9), and acute mastitis (n=10) were evaluated in comparison to control cows (n=10). In the TC group, blood samples were collected at 2 weeks prepartum and at 1, 2, 4, 6, and 8 weeks postpartum. Milk yield and composition were measured at 2 and 4 weeks postpartum. In the PD group, blood samples were collected at the first day of examination during the 60 days postpartum. Peripheral oxidative stress parameters (malondialdehyde, MDA; potential antioxidant capacity, PAO; and glutathione peroxidase) were measured, and biochemical analyses were performed. In the TC group, MDA increased significantly postpartum and was correlated with milk yield, blood glucose (Glu), free fatty acid (FFA), β-hydroxybutyric acid (BHB), and aspartate aminotransferase. Compared to the control cows, PD cows with ketosis had significantly higher MDA and significantly lower PAO. Moreover, MDA was significantly correlated with Glu, FFA, and BHB. Postpartum increase in MDA might interact with milk yield and Glu, FFA, and BHB in the TC cows, and postpartum diseases, especially ketosis, might signify its increase and interaction with Glu, FFA, and BHB.
Keywords: dairy cow, negative energy balance, oxidative stress, postpartum disease, transition period
Significant physiological changes occur during the 3 weeks before and after parturition. A negative energy balance (NEB) is likely due to the increased energy demand of lactation and decreased dry matter intake postpartum [7, 9, 19]. Cows during this transition period are more susceptible to metabolic diseases such as fatty liver and ketosis and to infectious diseases such as mastitis and uteritis [26]. Oxidative stress, which reflects an imbalance between oxidant and antioxidant levels [6], increases the risk of metabolic and infectious diseases by causing dysfunction in inflammatory responses [18, 26]. Furthermore, oxidative stress causes oxidative damage to macromolecules such as lipids, proteins, and DNA [18]. Oxidative stress increases during early lactation in cows [2, 5, 11]. In addition, oxidative stress increases susceptibility to metabolic and infectious diseases such as ketosis, abomasal displacement, mastitis, and uteritis [1, 26].
Oxidative stress can be measured by biological markers including malondialdehyde (MDA), glutathione peroxidase (GPx), and potential antioxidant capacity (PAO). Among these, MDA is the end product of lipid peroxidation caused by the generation of reactive oxygen species [27]. GPx is an antioxidant enzyme involved in the lipid peroxidation that produces MDA, and it converts hydrogen peroxide to water in the presence of glutathione [27]. PAO measures the total antioxidant capacity in serum using the reduction reaction of copper ions [20]. Oxidative stress in cattle is evaluated by combining oxidative stress marker measurements [1, 4, 11].
During the early postpartum period, lactation, lipid mobilization, and lipid β-oxidation in liver tissue are associated with increase in oxidative stress in transition cows [26], and higher oxidative stress is identified in diseased cows such as those with ketosis [8, 15, 21], abomasal displacement [16], or acute mastitis [17]. For example, Bernabucci et al. [2] reported that cows with higher β-hydroxybutyric acid (BHB) and free fatty acids (FFA) showed higher oxidative stress parameters (reactive oxygen metabolites and thiobarbituric acid reactive substance; TBARS) and lower levels of antioxidants. In contrast, Castillo et al. [4] observed no significant correlation between FFA and MDA levels. Therefore, we assumed that till recently, no consensus has been achieved on factors associated with increased postpartum oxidative stress, and there is little information on factors associated with change in oxidative stress. We investigated values of antioxidant defense measures such as MDA, GPx, and PAO, vitamin A and vitamin E, and blood biochemical components that are related to energy metabolism, protein metabolism, and liver function to clarify the relationships among oxidative stress, postpartum transition, and disease in healthy and diseased Holstein cows during the transition period.
MATERIALS AND METHODS
Animals and management
All animal care protocols were approved by the Iwate University Laboratory Animal Care and Use Committee (A201452-2; Morioka, Japan). This study was conducted in two parts. The first experiment was conducted at a commercial dairy farm in Nanyo City, Yamagata Prefecture, from September 2018 to February 2019. Nine transition control (TC) Holstein cows under the tie-stall housing system were selected to investigate oxidative stress during the periparturient period. The age of the cows was 4.7 ± 1.5 years (mean ± standard deviation (SD)), and the parity was 2.2 ± 1.5. The cows were clinically healthy and no symptoms of ketosis, abomasal displacement, or acute mastitis were observed. The feed ingredient, chemical composition, and nutrient sufficiency rate during the dry and lactation periods are shown in Table 1.
Table 1. Ingredients, chemical composition, and nutrient sufficiency rate of the diets.
| Items | Dry period | Lactation period | |
|---|---|---|---|
| Ingredient, % of DM a) | |||
| Concentrate | 22.0 | 55.8 | |
| Timothy hay | 43.2 | 20.5 | |
| Alfalfa hay | 0 | 7.1 | |
| Oats hay | 10.3 | 4.9 | |
| Grass silage | 23.7 | 11.3 | |
| Dicalcium phosphate | 0.8 | 0.4 | |
| Chemical composition b), % of DM | |||
| TDN | 59.2 | 66.6 | |
| CP | 11.7 | 14 | |
| NDF | 53.2 | 36.4 | |
| ADF | 33.1 | 21.6 | |
| Starch | 8.9 | 23.1 | |
| Calcium | 0.6 | 0.6 | |
| Phosphorus | 0.4 | 0.4 | |
| Nutrient sufficiency rate c), % | |||
| DM | 102.4 | 106.9 | |
| TDN | 122.7 | 92.3 | |
| CP | 115.5 | 94.5 | |
a) DM: dry matter. b) TDN: total digestible nutrients, CP: crude protein, NDF: neutral detergent fiber, ADF: acid detergent fiber. c) Calculated to meet the requirement of cows outlined by the Japanese Feeding Standard for Dairy cattle (2006).
The second experiment was conducted at commercial dairy farms (n=6) in Nanyo City, Yamagata Prefecture, from April 2018 to April 2019. The cows were raised under the tie-stall housing system in five of six farms (including a farm used in the first experiment), and the other farm used the free stall style. Postpartum diseased (PD) cows (n=29) and healthy cows (control, n=10) from six herds were studied. The PD cows were diagnosed with ketosis (n=10), abomasal displacement (n=9), and acute mastitis (n=10) during the 60 days after parturition. Ketosis was diagnosed when ≥3 urinary ketone bodies were detected by urinalysis (Uro Paper III, Eiken Chemical, Shimotsuga, Japan) with clinical symptoms such as anorexia and decreased milk yield. Abomasal displacement was diagnosed by a ping sound in the left or right intercostal space with clinical symptoms such as anorexia and decreased milk yield. Acute mastitis was diagnosed by clinical symptoms such as anorexia, a fever of 39.5°C or higher, a positive milk test in one or more quarters (mastitis simple diagnostic solution, P.L. Tester, ZENOAQ, Koriyama, Japan), and udder swelling or hardening. PD and control cow individuals were from the same herds.
Blood sampling
Peripheral blood samples were collected from the TC group at 2 weeks prepartum and at 1, 2, 4, 6, and 8 weeks postpartum (15 ± 4 days before, and 7 ± 2, 14 ± 2, 29 ± 3, 42 ± 1, and 56 ± 1 days after parturition, respectively; means ± SD). In the PD group, peripheral blood samples were collected at the first clinical examination of ketosis, abomasal displacement, or mastitis, and in control cows at 24 ± 18, 14 ± 10, 5 ± 8, and 16 ± 18 days after parturition, respectively; means ± SD. Blood samples were collected from the jugular vein into vacuum tubes coated with serum separation agents, sodium heparin, and sodium fluoride, sodium heparin, and EDTA-2Na (TERUMO, Tokyo, Japan). To separate the serum, the tubes with serum separation agents were centrifuged at 1,400 × g for 15 min. Then, the biochemical component measurement was performed, and remaining samples were stored at −50°C for estimation of PAO values. To separate the heparin plasma, the tubes with sodium heparin were centrifuged at 1,400 × g for 15 min. The samples were stored at −50°C for estimation of MDA values and GPx activities. To separate the sodium fluoride plasma, the tubes with sodium fluoride, sodium heparin, and EDTA-2Na were centrifuged at 1,400 × g for 5 min, and the samples used for glucose (Glu) evaluation.
Oxidative stress measurements
Plasma MDA was measured from heparin plasma samples using a commercial kit (Malondialdehyde Assay, Northwest Life Science Specialties LCC, Vancouver, WA, USA) as described elsewhere [3]. Serum PAO was measured using a commercial kit (PAO antioxidant capacity measurement kit, Nikken Zile Japan Aging Control Laboratory, Shizuoka, Japan). Plasma GPx was measured in heparin plasma samples of TC cows using a commercial kit (Glutathione Peroxidase Assay, Northwest Life Science Specialties LCC).
Blood properties
Blood Glu was measured using sodium fluoride, sodium heparin, and EDTA-2Na plasma. FFA, BHB, total cholesterol, total protein, albumin (ALB), blood urea nitrogen, aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), and Ca were measured in serum with an automatic analyzer (AU-680, Beckman Coulter, Tokyo, Japan). Vitamin A and vitamin E were measured in serum using high-performance liquid chromatography (Nanospace, Osaka Soda, Osaka, Japan).
Body condition scoring, milk yield, and composition
Body condition scoring (BCS) was performed in both groups according to Ferguson et al. [10]. Milk yield and composition were measured in the TC group at 2 and 4 weeks postpartum. The total milk yield was recorded in the morning and evening using a bucket milker or autosampler. To measure milk composition, approximately 30 ml of milk was collected using a bucket milker or autosampler. An automatic analyzer (Milkoscan FT+, FOSS Japan, Tokyo, Japan) was used to measure the proportion of milk fat, non-fat solid content, milk protein, lactose, and milk urea nitrogen.
Statistical analysis
The data obtained are expressed as mean ± SD. Repeated-measures analysis of variance (ANOVA) and Tukey’s method were used for intragroup comparisons of the plasma MDA values and GPx activities over time, serum PAO values, BCS, and blood properties in the TC cows. One-way ANOVA and Tukey’s method were used for comparisons of the plasma MDA, serum PAO values, and blood properties in the PD and control cows. After confirming normal distribution by the Shapiro−Wilk normality test, Pearson’s product-moment correlation coefficient or Spearman’s rank correlation coefficient was calculated. For statistical processing, GraphPad Prism ver. 5.01 (La Jolla, CA, USA) was used. In all cases, P-values <0.05 were considered as indicating significant differences, and P-values <0.1 were considered as indicating significant tendency.
RESULTS
Oxidative stress and blood properties
In the TC group, the plasma MDA values were significantly higher at 2 and 6 weeks postpartum than those at 2 weeks prepartum (P<0.05; Table 2); however, no significant changes in plasma MDA values were observed after parturition. Plasma GPx activities and serum PAO values did not change significantly. Glu was significantly lower at 1 and 2 weeks postpartum (P<0.05), whereas FFA was significantly higher at 1 and 2 weeks postpartum (P<0.05). BHB was significantly higher at 2 weeks postpartum (P<0.05), and AST was significantly higher at 1, 2, and 4 weeks postpartum (P<0.05). GGT was significantly higher at 6 and 8 weeks postpartum (P<0.05; Table2).
Table 2. Changes in body condition score (BCS), blood malondialdehyde (MDA), glutathione peroxidase (GPx), serum potential antioxidant capacity (PAO) values, and other biochemical components in the transition control cows at 2 weeks prepartum and at 1, 2, 4, 6, and 8 weeks postpartum.
| Items a) | (unit) | Weeks after parturition |
|||||
|---|---|---|---|---|---|---|---|
| −2 | 1 | 2 | 4 | 6 | 8 | ||
| BCS | 3.4 ± 0.3 | 3.3 ± 0.3 | 3.1 ± 0.3* | 2.9 ± 0.3* | 2.9 ± 0.3* | 3.0 ± 0.3* | |
| MDA | (µM) | 0.11 ± 0.04 | 0.17 ± 0.03 | 0.18 ± 0.07* | 0.17 ± 0.03 | 0.18 ± 0.06* | 0.13 ± 0.04 |
| GPx | (mU/ml) | 16.1 ± 4.9 | 11.2 ± 4.1 | 22.6 ± 10.9 | 13.1 ± 2.7 | 12.4 ± 3.7 | 14 ± 1.7 |
| PAO | (µM) | 538 ± 121 | 624 ± 172 | 583 ± 91 | 582 ± 74 | 528 ± 85 | 616 ± 135 |
| Glu | (mg/dl) | 53.9 ± 4.6 | 45.6 ± 6.5* | 42.4 ± 6.4* | 48.0 ± 8.7 | 50.1 ± 5.3 | 49.1 ± 5.2 |
| FFA | (µmol/l) | 78 ± 12 | 656 ± 366* | 536 ± 271* | 230 ± 125 | 181 ± 114 | 139 ± 95 |
| BHB | (µmol/l) | 548 ± 113 | 773 ± 249 | 904 ± 299* | 753 ± 300 | 711 ± 298 | 699 ± 160 |
| T-cho | (mg/dl) | 81 ± 10 | 77 ± 16 | 109 ± 25* | 160 ± 31* | 188 ± 36* | 199 ± 37* |
| TP | (g/dl) | 7.9 ± 0.6 | 7.5 ± 0.5 | 7.7 ± 0.3 | 8.0 ± 0.4 | 8.0 ± 0.4 | 7.9 ± 0.3 |
| ALB | (g/dl) | 3.4 ± 0.2 | 3.6 ± 0.1* | 3.6 ± 0.1* | 3.7 ± 0.1* | 3.7 ± 0.1* | 3.7 ± 0.2* |
| BUN | (mg/dl) | 15.6 ± 3.3 | 13.2 ± 3.7 | 14.0 ± 4.4 | 14.4 ± 2.8 | 13.8 ± 2.8 | 14.8 ± 2.0 |
| AST | (IU/l) | 69 ± 11 | 94 ± 19* | 92 ± 14* | 84 ± 16* | 77 ± 8 | 79 ± 12 |
| GGT | (IU/l) | 18 ± 4.7 | 17.6 ± 5.0 | 17.4 ± 4.5 | 19.3 ± 5.7 | 20.6 ± 5.6* | 21.6 ± 6.9* |
| Ca | (mg/dl) | 9.7 ± 0.5 | 9.3 ± 0.3 | 9.5 ± 0.4 | 9.5 ± 0.5 | 9.4 ± 0.3 | 9.5 ± 0.4 |
| VA | (IU/dl) | 105 ± 14 | 79 ± 17* | 89 ± 17 | 113 ± 17 | 112 ± 20 | 113 ± 23 |
| VE | (µg/dl) | 253 ± 88 | 190 ± 48 | 262 ± 47 | 380 ± 69* | 432 ± 92* | 426 ± 167* |
Values are means ± SD (n=9). a) Glu; Glucose, FFA; free fatty acid, BHB; β-hydroxybutyric acid, T-cho; total cholesterol, TP; total protein, ALB; albumin, BUN; blood urea nitrogen, AST; aspartate aminotransferase, GGT; γ-glutamyl transpeptidase, Ca; calcium, VA; vitamin A, VE; vitamin E. *Denotes significant difference (P<0.05) compared with the 2 weeks prepartum.
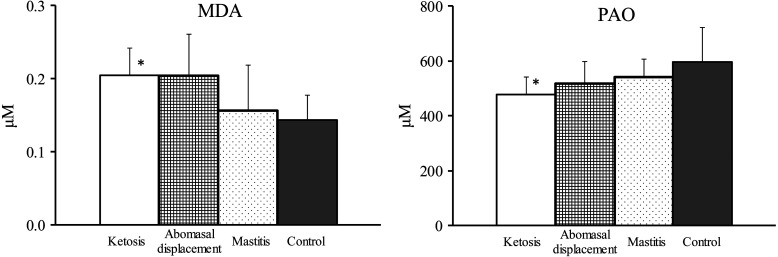
In the PD group, the plasma MDA values were numerically higher in cows with ketosis and abomasal displacement and was significantly higher in cows with ketosis than in the control cows (P<0.05; Fig. 1). Serum PAO values were low in the diseased cows, and it was significantly lower in cows with ketosis than in the control cows (P<0.05; Fig. 1). FFA and BHB were significantly higher in cows with ketosis and abomasal displacement than in the control cows (P<0.05; Table 3). There was no significant difference in Glu concentrations between groups (Table 3).
Fig. 1.
Plasma malondialdehyde (MDA) and serum potential antioxidant capacity (PAO) values in control (n=10) cows and those with ketosis (n=10), abomasal displacement (n=9), or mastitis (n=10). Values are means ± SD. *Denotes significant difference (P<0.05) compared with the control cows.
Table 3. Body condition score (BCS) and peripheral blood biochemical components in the control and postpartum diseased cows.
| Items a) | (unit) | Ketosis | Abomasal displacement | Mastitis | Control |
|---|---|---|---|---|---|
| (n=10) | (n=9) | (n=10) | (n=10) | ||
| BCS | 3.2 ± 0.2 | 3.1 ± 0.4 | 3.0 ± 0.2 | 3.1 ± 0.3 | |
| Glu | (mg/dl) | 40 ± 11 | 40 ± 11 | 66 ± 16 | 52 ± 6 |
| FFA | (µmol/l) | 1,345 ± 501* | 1,525 ± 580* | 864 ± 373 | 435 ± 223 |
| BHB | (µmol/l) | 4,041 ± 2,019* | 3,373 ± 1,918* | 651 ± 212 | 698 ± 315 |
| T-cho | (mg/dl) | 120 ± 40 | 80 ± 19 | 71 ± 24 | 101 ± 41 |
| TP | (g/dl) | 7.0 ± 0.8 | 7.4 ± 0.6 | 7.4 ± 0.9 | 7.4 ± 0.8 |
| ALB | (g/dl) | 3.5 ± 0.3 | 3.4 ± 0.3 | 3.3 ± 0.5 | 3.4 ± 0.2 |
| BUN | (mg/dl) | 10.3 ± 4.2 | 13.2 ± 2.9 | 12.7 ± 5.3 | 9.9 ± 4.0 |
| AST | (IU/l) | 126 ± 59 | 185 ± 112* | 117 ± 42 | 98 ± 28 |
| GGT | (IU/l) | 44 ± 36 | 36 ± 21 | 32 ± 10 | 22 ± 6 |
| Ca | (mg/dl) | 8.8 ± 1.0* | 9.1 ± 0.8 | 8.4 ± 0.8* | 9.9 ± 0.7 |
| VA | (IU/dl) | 73 ± 25 | 51 ± 27* | 29 ± 17* | 87 ± 30 |
| VE | (µg/dl) | 256 ± 106 | 140 ± 79 | 184 ± 112 | 204 ± 114 |
Values are means ± SD. a) Glu; glucose, FFA; free fatty acid, BHB; β-hydroxybutyric acid, T-cho; total cholesterol, TP; total protein, ALB; albumin, BUN; blood urea nitrogen, AST; aspartate aminotransferase, GGT; γ-glutamyl transpeptidase, Ca; calcium, VA; vitamin A, VE; vitamin E. *Denotes significant difference (P<0.05) compared with the control cows.
Body condition in all cows, and milk yield and composition in the TC group cows
The changes in BCS, milk yield, and composition in the TC group are shown in Tables 2 and 4. BCS was significantly lower at 2, 4, 6, and 8 weeks postpartum than at 2 weeks prepartum. Milk yield increased from 2 to 4 weeks postpartum. In the PD group, there were no significant differences in BCS between cows with ketosis, abomasal displacement, or mastitis and control cows (Table 3).
Table 4. Changes in milk yield and milk composition in the transition control cows at 2 weeks and 4 weeks postpartum.
| Items | (unit) | Weeks after parturition |
|
|---|---|---|---|
| 2 | 4 | ||
| Milk yield | (kg/day) | 31.3 ± 5.8 | 35.3 ± 6.4 |
| Milk fat | (%) | 5.8 ± 0.9 | 4.6 ± 1.0 |
| Milk protein | (%) | 3.5 ± 0.3 | 3.1 ± 0.2 |
| Non-fat solid | (%) | 8.7 ± 0.3 | 8.4 ± 0.3 |
| Lactose | (%) | 4.1 ± 0.4 | 4.3 ± 0.2 |
| Milk urea nitrogen | (mg/dl) | 14.0 ± 3.5 | 13.4 ± 2.7 |
Values are means ± SD (n=9).
Correlation between oxidative stress markers and BCS, milk yield, and blood properties
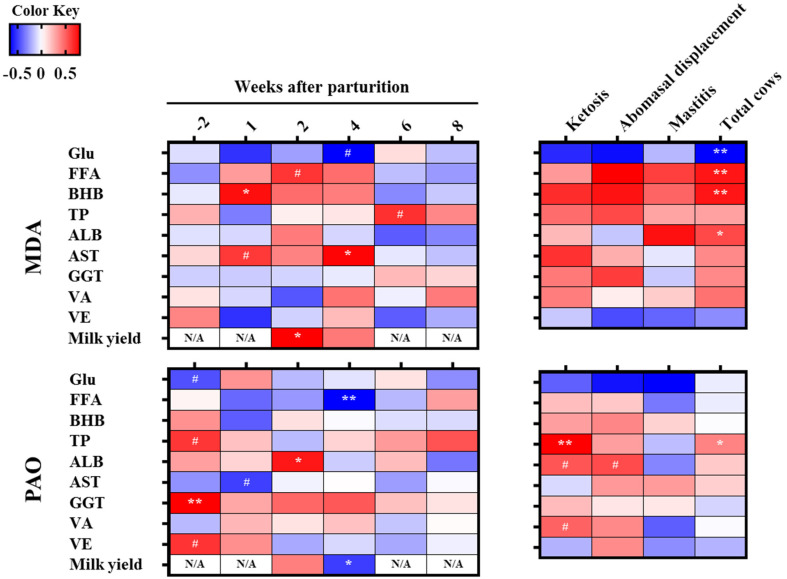
The relationships between the plasma MDA and blood biochemical components and milk yield in the TC and PD cows are shown in Fig. 2. The relationships between the plasma GPx and blood biochemical components and milk yield in the TC cows are shown in Supplementary Fig. 1. In the TC cows, the MDA value was negatively correlated with the Glu value (r= −0.663, P=0.052) at 4 weeks after parturition, and positively correlated with the FFA value (r=0.633, P=0.067) at 2 weeks, BHB value (r=0.751, P<0.05) at 1 week, AST value at 1 (r=0.620, P=0.075) and 4 (r=0.782, P<0.05) weeks, and milk yield (r=0.797, P<0.05) at 2 weeks after parturition. In addition, PAO value was negatively correlated with Glu value (r= −0.641, P=0.063) at 2 weeks before, FFA value (r= −0.934, P<0.01) at 4 weeks after, AST value (r= −0.704, P=0.051) at 1 week after, and milk yield (r= −0.714, P<0.05) at 4 weeks after parturition. Moreover, a positive correlation coefficient was observed between the PAO and total protein value (r=0.656, P=0.059), GGT value (r=0.834, P<0.01), and vitamin E value (r=0.666, P=0.050) at 2 weeks before, and between the PAO and ALB value (r=0.762, P<0.05) at 2 weeks after parturition. In the PD cows with ketosis, abomasal displacement, or mastitis (n=29), there were significant positive correlations between plasma MDA and FFA (r=0.532, P<0.01), BHB (r=0.531, P<0.01), and ALB (r=0.414, P<0.05), and a significant negative correlation between plasma MDA and Glu (r= −0.541, P<0.01). Furthermore, tendencies toward significant correlation were identified between PAO and ALB (r=0.610, P=0.061) in cows with ketosis and those with abomasal displacement (r=0.647, P=0.067).
Fig. 2.
Correlation analysis between the oxidative stress markers (MDA; malondialdehyde, and PAO; potential antioxidant capacity) and blood biochemical components (Glu; glucose, FFA; free fatty acid, BHB; β-hydroxybutyric acid, TP; total protein, ALB; albumin, AST; aspartate aminotransferase, GGT; γ-glutamyl transpeptidase, VA; vitamin A, VE; vitamin E) and milk yield in the transition control (TC) cows and postpartum diseased (PD) cows with ketosis, abomasal displacement, or mastitis. **, *, #Denote significant correlation (P<0.01, 0.05, and 0.1, respectively).
DISCUSSION
Plasma MDA values increased after parturition in the TC cows, which is consistent with previous reports that oxidative stress increases postpartum [2, 5, 11]. Therefore, the TC cows in this study were in a state of high oxidative stress after parturition. However, there were no significant changes in the plasma GPx activities and serum PAO values in the TC cows. Bernabucci et al. [2] measured TBARS, an index of lipid peroxidation, and superoxide dismutase, a type of antioxidant enzyme in periparturient dairy cows to evaluate oxidative stress status in dairy cows during the transition period. In their study, plasma superoxide dismutase and TBARS values increased until parturition, and superoxide dismutase activities gradually decreased in contrast to that of TBARS values which remained elevated after parturition. Therefore, no significant changes in plasma GPx or PAO during the periparturient period in the TC group might suggest that oxidative stress levels were within the compensatory range of antioxidants.
Higher MDA and lower PAO values were observed in cows with ketosis and abomasal displacement. Therefore, oxidative stress increased in cows with ketosis and abomasal displacement after parturition. These results are consistent with previous reports that oxidative stress increases with these ailments [8, 15, 16]. In addition, the cows with ketosis had significantly lower PAO values than the control cows, indicating that their level of oxidative stress exceeded the compensatory effects of antioxidants. Therefore, although the timing of postpartum sample collection was different among diseased and control cows in the PD group, it seems not to affect oxidative stress measurements, because MDA and PAO values were not changed significantly after parturition in the TC group.
Energy demand and metabolism increase with lactation onset [26], increasing free radical production [1]. Postpartum cows experience NEB owing to heightened energy demands and reduced dry matter intake, which causes fat mobilization and higher blood FFA values and ketone bodies [7, 9, 19]. In the TC cows, the BCS declined from 2 weeks prepartum to 6 weeks postpartum. FFA and BHB values increased, wherease Glu values decreased in the early lactation period. Cows with higher milk production have higher MDA values in the milk [12] and may be more susceptible to lipid peroxidation owing to free radicals [13, 22]. Therefore, the TC cows experienced NEB after parturition, and the significant positive correlation between MDA values and milk yield suggests positive relationships between the milk production, metabolism, and peripheral MDA values during the transition period.
Oxidative stress is associated with high FFA values and hyperketonemia [15, 21, 26], and cows with declining BCS and high FFA and BHB values show higher oxidative stress during the periparturient period [2]. FFA is mobilized from adipose tissue and increases β-oxidation in the liver during NEB [7, 19], enhancing the production of reactive oxygen species [24]. In the TC cows, MDA values were negatively correlated to Glu values and positively correlated to FFA and BHB values. In addition, FFA acts as a signaling molecule and regulates liver metabolism in cows during the transition period [14]. High values of FFA increase ROS production in hepatocytes, increasing MDA production and decreasing antioxidant enzyme activity [23]. In this study, AST values increased after parturition in the TC cows. There was also a significant positive correlation between MDA values and AST values. Therefore, oxidative stress in postpartum cows is associated with the NEB status, such as decreased Glu values and increased FFA and BHB values, and with liver function by β-oxidation of FFA.
Cows with ketosis and abomasal displacement had higher FFA and BHB values than the control cows, possibly owing to NEB status. In addition, MDA was negatively correlated with Glu values and positively correlated with FFA and BHB values all PD cows. Similar to that in the TC cows, NEB, low Glu, and high FFA and BHB values were associated with oxidative stress in PD cows. Furthermore, PAO is an overall antioxidant capacity value and ALB is included among non-enzymatic protein antioxidants [6]. PAO was positively correlated with ALB value in the PD cows with ketosis and abomasal displacement. Therefore, it suggests that ALB value is related to overall antioxidant capacity in cows with ketosis and abomasal displacement.
In cows with abomasal displacement, oxidative stress increases with acute phase response and abomasal tissue damage; however, the relationship between NEB status and oxidative stress markers was not examined [16]. In the present study, MDA, PAO, Glu, FFA, and BHB values in cows with abomasal displacement showed almost the same values as those in cows with ketosis. Specifically, increased BHB values in the cows with abomasal displacement and ketosis suggested that abomasal displacement was accompanied by ketosis in the PD cows. Therefore, the increase in oxidative stress in cows with abomasal displacement in the present study was not only induced by tissue damage from abomasal displacement but also by NEB status, such as decreased Glu and increased FFA and BHB.
Oxidative stress in the transition period triggers infectious diseases including mastitis [26], and cytokines and oxidative stress are associated with the pathological condition of acute coliform mastitis [17, 25]. However, no significant change in MDA and PAO values was observed in cows with acute mastitis, and they did not show a decrease in Glu value and increases in FFA and BHB values unlike the cows with ketosis and abomasal displacement in the present study. Therefore, further study is required to clarify the specific etiology of acute mastitis and its correlation to oxidative stress values in postpartum cows.
In conclusion, oxidative stress increased after parturition in Holstein cows. Postpartum oxidative stress was affected by hypermetabolism for increased milk yield during the early lactation period and peripheral blood components (Glu, FFA, and BHB) related to NEB status. Furthermore, NEB status influenced oxidative stress in cows with postpartum diseases; ketosis was the most influential factor for high oxidative stress status. Overall, MDA values increase as a physiological mechanism to increase milk production, but with postpartum diseases, especially ketosis, MDA interacts more intensively with peripheral biochemical components, such as Glu, FFA, and BHB. Therefore, the control of oxidative stress in transition or postpartum cows may improve their productivities, although it is necessary to further examine the relationships among oxidative stress, productivity, and diseases in Holstein cows during the transition period.
Supplementary
REFERENCES
- 1.Abuelo A., Hernández J., Benedito J. L., Castillo C.2015. The importance of the oxidative status of dairy cattle in the periparturient period: revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. (Berl.) 99: 1003–1016. doi: 10.1111/jpn.12273 [DOI] [PubMed] [Google Scholar]
- 2.Bernabucci U., Ronchi B., Lacetera N., Nardone A.2005. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 88: 2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2 [DOI] [PubMed] [Google Scholar]
- 3.Botsoglou N. A., Fletouris D. J., Papageorgiou G. E., Vassilopoulos V. N., Mantis A. J., Trakatellis A. G.1994. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 42: 1931–1937. doi: 10.1021/jf00045a019 [DOI] [Google Scholar]
- 4.Castillo C., Hernandez J., Bravo A., Lopez-Alonso M., Pereira V., Benedito J. L.2005. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 169: 286–292. doi: 10.1016/j.tvjl.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Castillo C., Hernández J., Valverde I., Pereira V., Sotillo J., Alonso M. L., Benedito J. L.2006. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 80: 133–139. doi: 10.1016/j.rvsc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Celi P.2011. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 33: 233–240. doi: 10.3109/08923973.2010.514917 [DOI] [PubMed] [Google Scholar]
- 7.Drackley J. K.1999. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: the final frontier? J. Dairy Sci. 82: 2259–2273. doi: 10.3168/jds.S0022-0302(99)75474-3 [DOI] [PubMed] [Google Scholar]
- 8.Du X., Chen L., Huang D., Peng Z., Zhao C., Zhang Y., Zhu Y., Wang Z., Li X., Liu G.2017. Elevated apoptosis in the liver of dairy cows with ketosis. Cell. Physiol. Biochem. 43: 568–578. doi: 10.1159/000480529 [DOI] [PubMed] [Google Scholar]
- 9.Esposito G., Irons P. C., Webb E. C., Chapwanya A.2014. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 144: 60–71. doi: 10.1016/j.anireprosci.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Ferguson J. D., Galligan D. T., Thomsen N.1994. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 77: 2695–2703. doi: 10.3168/jds.S0022-0302(94)77212-X [DOI] [PubMed] [Google Scholar]
- 11.Gong J., Xiao M.2016. Selenium and antioxidant status in dairy cows at different stages of lactation. Biol. Trace Elem. Res. 171: 89–93. doi: 10.1007/s12011-015-0513-2 [DOI] [PubMed] [Google Scholar]
- 12.Kapusta A., Kuczyńska B., Puppel K.2018. Relationship between the degree of antioxidant protection and the level of malondialdehyde in high-performance Polish Holstein-Friesian cows in peak of lactation. PLoS One 13: e0193512. doi: 10.1371/journal.pone.0193512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löhrke B., Viergutz T. T., Kanitz W., Gollnitz K., Becker F., Hurtienne A., Schweigert F. J.2004. High milk yield of dairy cows is associated with oxidant stress. Online J. Vet. Res. 8: 70–78. [Google Scholar]
- 14.Li X., Li X., Chen H., Lei L., Liu J., Guan Y., Liu Z., Zhang L., Yang W., Zhao C., Fu S., Li P., Liu G., Wang Z.2013. Non-esterified fatty acids activate the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. Cell Biochem. Biophys. 67: 1157–1169. doi: 10.1007/s12013-013-9629-1 [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Ding H. Y., Wang X. C., Feng S. B., Li X. B., Wang Z., Liu G. W., Li X. W.2016. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. (Berl.) 100: 844–851. doi: 10.1111/jpn.12454 [DOI] [PubMed] [Google Scholar]
- 16.Maden M., Ozturk A. S., Bulbul A., Avci G. E., Yazar E.2012. Acute-phase proteins, oxidative stress and enzyme activities of blood serum and peritoneal fluid in cattle with abomasal displacement. J. Vet. Intern. Med. 26: 1470–1475. doi: 10.1111/j.1939-1676.2012.01018.x [DOI] [PubMed] [Google Scholar]
- 17.Mavangira V., Mangual M. J., Gandy J. C., Sordillo L. M.2016. 15-F2t-isoprostane concentrations and oxidant status in lactating dairy cattle with acute coliform mastitis. J. Vet. Intern. Med. 30: 339–347. doi: 10.1111/jvim.13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavangira V., Sordillo L. M.2018. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 116: 4–14. doi: 10.1016/j.rvsc.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 19.McArt J. A., Nydam D. V., Oetzel G. R., Overton T. R., Ospina P. A.2013. Elevated non-esterified fatty acids and β-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 198: 560–570. doi: 10.1016/j.tvjl.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 20.Pregel P., Bollo E., Cannizzo F. T., Biolatti B., Contato E., Biolatti P. G.2005. Antioxidant capacity as a reliable marker of stress in dairy calves transported by road. Vet. Rec. 156: 53–54. doi: 10.1136/vr.156.2.53 [DOI] [PubMed] [Google Scholar]
- 21.Senoh T., Oikawa S., Nakada K., Tagami T., Iwasaki T.2019. Increased serum malondialdehyde concentration in cows with subclinical ketosis. J. Vet. Med. Sci. 81: 817–820. doi: 10.1292/jvms.18-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma N., Singh N. K., Singh O. P., Pandey V., Verma P. K.2011. Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Australas. J. Anim. Sci. 24: 479–484. doi: 10.5713/ajas.2011.10220 [DOI] [Google Scholar]
- 23.Shi X., Li D., Deng Q., Li Y., Sun G., Yuan X., Song Y., Wang Z., Li X., Li X., Liu G.2015. NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes. J. Steroid Biochem. Mol. Biol. 145: 103–112. doi: 10.1016/j.jsbmb.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 24.Schönfeld P., Wojtczak L.2008. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 45: 231–241. doi: 10.1016/j.freeradbiomed.2008.04.029 [DOI] [PubMed] [Google Scholar]
- 25.Sordillo L. M., Peel J. E.1992. Effect of interferon-γ on the production of tumor necrosis factor during acute Escherichia coli mastitis. J. Dairy Sci. 75: 2119–2125. doi: 10.3168/jds.S0022-0302(92)77971-5 [DOI] [PubMed] [Google Scholar]
- 26.Sordillo L. M., Raphael W.2013. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. North Am. Food Anim. Pract. 29: 267–278. doi: 10.1016/j.cvfa.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., Telser J.2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39: 44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.