Abstract
The endocannabinoid (eCB) system is a lipid-based neurotransmitter complex that plays crucial roles in the neural control of learning and memory. The current model of eCB-mediated retrograde signaling is that eCBs released from postsynaptic elements travel retrogradely to presynaptic axon terminals, where they activate cannabinoid type-1 receptors (CB1Rs) and ultimately decrease neurotransmitter release on a short- or long-term scale. An increasing body of evidence has enlarged this view and shows that eCBs, besides depressing synaptic transmission, are also able to increase neurotransmitter release at multiple synapses of the brain. This indicates that eCBs act as bidirectional regulators of synaptic transmission and plasticity. Recently, studies unveiled links between the expression of eCB-mediated long-term potentiation (eCB-LTP) and learning, and between its dysregulation and several pathologies. In this review article, we first distinguish the various forms of eCB-LTP based on their mechanisms, resulting from homosynaptically or heterosynaptically-mediated processes. Next, we consider the neuromodulation of eCB-LTP, its behavioral impact on learning and memory, and finally, eCB-LTP disruptions in various pathologies and its potential as a therapeutic target in disorders such as stress coping, addiction, Alzheimer’s and Parkinson’s disease, and pain. Cannabis is gaining popularity as a recreational substance as well as a medicine, and multiple eCB-based drugs are under development. In this context, it is critical to understand eCB-mediated signaling in its multi-faceted complexity. Indeed, the bidirectional nature of eCB-based neuromodulation may offer an important key to interpret the functions of the eCB system and how it is impacted by cannabis and other drugs.
Keywords: endocannabinoids, synaptic plasticity, long-term potentiation, neuromodulation, GABAergic interneurons, cannabinoid receptor type-1, learning and memory, excitation-inhibition balance
Background
Endocannabinoids (eCBs) are a family molecule of biolipids, mainly composed by 2-arachidonoylglycerol (2-AG) and anandamide, synthesized and released on-demand, which mostly act on presynaptic cannabinoid type-1 receptors (CB1R) and postsynaptic transient receptor potential vanilloid type-1 (TRPV1; Piomelli et al., 2007; Castillo et al., 2012; Katona and Freund, 2012; Araque et al., 2017). eCBs have emerged as a major signaling system in learning and memory (Marsicano and Lafenêtre, 2009; Mechoulam and Parker, 2013; Kruk-Slomka et al., 2017) because of their powerful influence on synaptic plasticity, mainly as a depressing synaptic function (Castillo et al., 2012; Araque et al., 2017; Augustin and Lovinger, 2018). eCB signaling has been widely described to decrease the neurotransmitter release probability via diverse presynaptic mechanisms, including inhibition of voltage-gated calcium channels, activation of potassium channels, and protein kinase-A (pkA) signaling. In light of recent studies, this review aims at highlighting evidence for short and long-term eCB-mediated synaptic potentiation (eCB-LTP).
eCB-Mediated Synaptic Potentiation
We have distinguished here the homosynaptic from heterosynaptic eCB-mediated potentiation such that homosynaptic plasticity refers to input-specific plasticity, in which only the neurons belonging to a given stimulated synapse undergo plasticity, whereas heterosynaptic plasticity refers to changes at a synapse resulting from activities of distinct synapses/pathways.
Homosynaptic eCB-Mediated LTP
Using spike-timing-dependent plasticity (STDP), a Hebbian synaptic learning rule relying on paired activity on either side of the synapses (Feldman, 2012), a few numbers of pairings induce eCB-LTP at corticostriatal synapses, which is CB1R- and TRPV1-mediated (Cui et al., 2015, 2016, 2018a; Xu et al., 2018; Figure 1A). 2-AG levels and subsequent CB1R activation have a dual effect on eCB-plasticity: high levels of eCBs synthesis and CB1R activation (reached with ~10–15 post-pre pairings) induce eCB-LTP, while low levels (reached with ~50–100 pre-post pairings) induce eCB-LTD (Cui et al., 2015, 2016). Indeed, few pairings promote efficient eCB synthesis (via maximal calcium influx and efflux from voltage-gated calcium channels and TRPV1, and endoplasmic reticulum, respectively) and thus maximal CB1R activation, combined with minimal CB1R desensitization (Cui et al., 2016). Corticostriatal eCB-plasticity relies on presynaptic pkA/calcineurin balance, such that eCB-LTP requires active pkA, whereas eCB-LTD depends on calcineurin activation (Cui et al., 2016; Figure 1A). Therefore, at corticostriatal synapses, eCB-mediated plasticity is bidirectional, and eCB-LTP or eCB-LTD expression is determined by pre- and postsynaptic activity patterns. A similar form of homosynaptic and bidirectional eCB-plasticity occurs between neocortical pyramidal cells following a limited number of coincident activity (Cui et al., 2018b). Interestingly, eCB-LTP is robust to spike-time jittering, contrarily to NMDAR-LTP, and can thus arise in noisy neural network activity (Cui et al., 2018a).
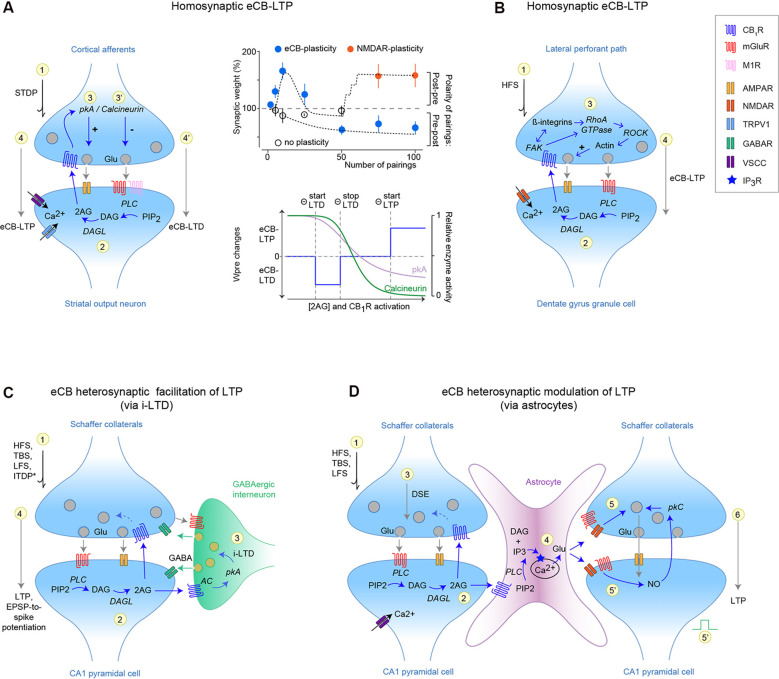
Figure 1.
Homosynaptic and heterosynaptic endocannabinoid (eCB)-mediated long-term potentiation (LTP). (A,B) Homosynaptic eCB-LTP in the striatum (A) and hippocampus (B). (A) eCB-LTP is induced by few spike-timing-dependent plasticity (STDP) pairings (~10–15 post-pre pairings), whereas eCB-LTD is induced by a larger number of pairings (~50–100 pre-post pairings) at the same corticostriatal synapses (Cui et al., 2015, 2016, 2018a; Xu et al., 2018; Gangarossa et al., 2020). Right: top panel illustrates the domains of expression of eCB-LTP, eCB-LTD, and NMDAR-LTP related to the polarity and number of STDP pairings. Bottom panel: eCB-LTP and eCB-LTD are expressed depending on eCB levels [and cannabinoid type-1 receptor (CB1R) activation], such that low and high levels induced eCB-LTD and eCB-LTP, respectively. CB1R activation is expected to decrease pkA and calcineurin activity, via reduced calcium influx through voltage-sensitive calcium channels (VSCC). This effect is schematized by the relative protein kinase-A (pkA) and calcineurin activity changes upon increased CB1R activation, such that eCB-LTD occurs when calcineurin/pkA >1, whereas calcineurin/pkA <1 leads to eCB-LTP. (B) eCB-LTP at synapses between the lateral perforant path (LPP) and the hippocampal granular cells of the dentate gyrus, requires co-operative CB1R/ROCK signaling, which favors glutamate release via a presynaptic actin regulatory mechanism (Wang et al., 2016, 2018b). eCB-LTP illustrated in (A,B) are independent of the GABAergic transmission and astrocytic calcium transients, present a presynaptic locus of expression and hence rely on a homosynaptic mechanism. (C,D) Heterosynaptic eCB-LTP involving intermediary elements such as GABAergic interneurons (C) and astrocytes (D). (C) Heterosynaptic eCB-LTP at CA3–CA1 hippocampal synapses. Activation of mGluR1/5 promotes 2-AG synthesis and release; 2-AG activates CB1R located on neighboring GABAergic interneurons and induces LTD via a pkA-dependent mechanism. This LTD of inhibitory transmission (i-LTD) then facilitates the release of glutamate at CA3–CA1 synapses promoting a local LTP. This eCB-mediated metaplasticity can be induced with various cell conditioning paradigms, and most notably subthreshold stimulations (HFS, LFS, TBS or ITDP; Chevaleyre and Castillo, 2003, 2004; Zhu and Lovinger, 2007; Lin et al., 2011; Pan et al., 2011; Xu et al., 2012; Basu et al., 2013; Orr et al., 2014; Monory et al., 2015; Silva-Cruz et al., 2017; Kim et al., 2019). (D) Heterosynaptic eCB-LTP involving astrocytes at CA3–CA1 synapses. Neuronal activity (HFS, TBS, LFS) induces synthesis of eCBs at synapse-1 which activate astrocytic CB1R. Then, astrocytic glutamate release (via IP3-induced calcium-release mechanism) induces an NMDAR-facilitation or NO-mediated LTP on neighboring CA1 synapse-2. This eCB-mediated lateral synaptic regulation has been observed in the hippocampus (Navarrete and Araque, 2008, 2010; Gómez-Gonzalo et al., 2015; Covelo and Araque, 2016) and striatum (Martín et al., 2015).
A homosynaptic CB1R-dependent eCB-LTP was also characterized in hippocampal granular cells of the dentate gyrus resulting from postsynaptic 2-AG synthesis upon high-frequency stimulation of the lateral perforant path (LPP; Wang et al., 2016, 2018a; Figure 1B). When activated, CB1R, detected presynaptically at LPP terminals using STORM microscopy, engage the presynaptic FAK/ROCK signaling pathway favoring glutamate release. Interestingly, at CA3-CA1 synapses CB1R is preferentially linked to ERK/Munc18–1, whose activation depresses glutamate release (Wang et al., 2018a).
In both cases, the eCB-LTP magnitude did not reach saturating levels and could be increased under monoacylglycerol lipase (MAGL) inhibition, the 2-AG degrading enzyme, suggesting that eCB-LTP might serve as a priming plasticity accounting for fast learning and episodic memory. Finally, homosynaptic CB1R-mediated eCB-LTP was also observed in stratum oriens interneurons (Friend et al., 2019).
eCB-Mediated Heterosynaptic Facilitation of LTP
Via Depression of Inhibitory Transmission
By reducing inhibition from GABAergic synapses through a CB1R-dependent short-term depolarization-induced suppression of inhibition (DSI), eCBs were first shown to facilitate NMDAR-LTP induction at hippocampal CA3-CA1 synapses (Carlson et al., 2002), exclusively in the cell subjected to the subthreshold LTP inducing protocol. eCB-mediated facilitation through long-term disinhibition was then observed at various synapses, cell types, and brain regions. Indeed, in the hippocampus, high or low-frequency stimulations or theta-burst stimulations of Schaffer collaterals induce LTD of local GABAergic interneurons (i-LTD), which in turn facilitates LTP at excitatory CA3-CA1 synapses (Chevaleyre and Castillo, 2003, 2004; Zhu and Lovinger, 2007; Lin et al., 2011; Pan et al., 2011; Xu et al., 2012; Monory et al., 2015; Silva-Cruz et al., 2017; Figure 1C). i-LTD, originating from metabotropic glutamatergic receptor (mGluR) activation and subsequent 2-AG release from CA1 pyramidal cells that leads to the activation of CB1R located on GABAergic terminals, causes relief of the GABAergic brake in a restricted dendritic area (~10 μm) when synaptically-induced or on a cell-wide extent following endogenous CA1 pyramidal cell activity (Younts et al., 2013). In contrast to the transient LTP facilitation induced by DSI in single active cell and up to neighboring naïve cells (Wilson and Nicoll, 2001), i-LTD provides long-lasting priming of at most a single cell (Chevaleyre and Castillo, 2004; Younts et al., 2013). The modulation of CA1-LTP by i-LTD is an example of metaplasticity, i.e. long-lasting neural changes induced by activity at a given time, and that modulate subsequently induced plasticity (Abraham, 2008), orchestrated by eCBs. This i-LTD is finely tuned by the parallel activation of CB1R on GABAergic or glutamatergic cells (Monory et al., 2015) and is also accompanied by changes in excitability enhancing the spiking probability in response to a given EPSP, i.e., EPSP-to-spike potentiation (Chevaleyre and Castillo, 2003; Orr et al., 2014; Kim et al., 2019), and by structural changes (Monory et al., 2015; Hu et al., 2019) both eCB-mediated. Interestingly, a circuit-based synaptic learning rule, consisting of paired stimulation of the perforant path and Schaffer collaterals, induced an input-timing-dependent heterosynaptic LTP at CA3-CA1 but not at cortical-CA1 synapses (Xu et al., 2012; Basu et al., 2013). Input-timing-dependent-LTP depends on CB1R-mediated i-LTD occurring at GABAergic synapses (here cholecystokinin interneurons). Activation of the cortical-CA1 pathway triggers heterosynaptic calcium transients, boosting eCB signaling originating from the CA3-CA1 pathway, which leads ultimately to i-LTD. Similar metaplasticities involving eCB-mediated i-LTD have been reported in the striatum (Adermark, 2011; Mathur et al., 2013), ventral tegmental area (Szabo et al., 2002), basolateral amygdala (BLA; Azad et al., 2004) and spinal cord (Kyriakatos and El Manira, 2007).
Via Astrocytes
eCBs, released from a given stimulated CA3–CA1 synapse, activate astrocytic CB1R and via an IP3-induced calcium-release mechanism promote astrocytic glutamate release, which in turn induces an NMDAR-mediated short- (Navarrete and Araque, 2008, 2010) or nitric oxide(NO)-mediated long-term (Gómez-Gonzalo et al., 2015) potentiation on neighboring CA1 synapses (Figure 1B). This lateral synaptic regulation achieved by astrocytes and eCBs (Covelo and Araque, 2016), also reported in the dorsal striatum (Martín et al., 2015), appears as a means of controlling distant synapses by activated ones. Since, astrocytes are interconnected by gap junctions, permeable to calcium and IP3, both involved in the propagation of intercellular calcium waves (Giaume and Venance, 1998), the role of astrocytic gap junctions in regulating the extent of this lateral synaptic regulation remains to be determined.
Via Dopaminergic Signaling
At the goldfish Mauthner cell, sustained activity at excitatory synapses triggers 2-AG release, which activates CB1R on nearby dopaminergic fibers and promotes an increased release of dopamine (Cachope et al., 2007). In turn, dopamine acts back via a D1/5R-mediated pkA signaling, which induces LTP at electrical and glutamatergic chemical synapses.
Non-CB1R-Mediated eCB-Potentiation of Synaptic Transmission
In the hippocampus, anandamide induces an increase of miniature excitatory (Sang et al., 2010) and inhibitory (Hofmann et al., 2011) postsynaptic currents. Anandamide and 2-AG potentiate NMDAR-mediated currents via respectively TRPV1-dependent and -independent mechanisms (Hampson et al., 1998; Yang et al., 2014). Although, this latter anandamide/2-AG NMDAR-mediated metaplasticity favors hippocampal LTD (Yang et al., 2014), it remains to investigate whether this eCB-NMDAR cross-talk exists in other brain areas and, considering the crucial role of NMDAR in synaptic potentiation, could constitute a metaplasticity promoting LTP.
Neuromodulation of eCB-LTP
eCB-LTP expression or magnitude can be regulated by neuromodulators through a variety of mechanisms targeting eCB synthesis and/or release, or the signaling downstream of CB1R.
Dopamine
The relationship between dopamine and eCB-signaling has been extensively documented for eCB-LTD (Covey et al., 2017). Recent evidence also shows a tight link between dopamine and eCB-LTP. In the globus pallidus, eCB-mediated i-LTD is switched to i-LTP upon D2R activation (Caballero-Florán et al., 2016). Striatal homosynaptic eCB-LTP is prevented when STDP pairings are applied simultaneously to opto-inhibition of nigrostriatal dopaminergic neurons and depends on presynaptic D2R located on cortical afferents, whose activity level shapes the expression domain of eCB-LTP and eCB-LTD (Xu et al., 2018). Interestingly, restricting Gi/o protein availability in presynaptic terminals switches the coupling of CB1R to Gs and stimulates pkA pathway (Glass and Felder, 1997; Gonzalez et al., 2009): this competition for Gi/o availability between CB1R and D2R could favor presynaptic pkA activation and thus promote corticostriatal eCB-LTP (Cui et al., 2016).
GABA
GABA acts as a Hebbian/anti-Hebbian switch, which orientates the polarity of corticostriatal homosynaptic eCB-LTP: eCB-LTP is induced by post-pre pairings in native conditions, but by pre-post pairings under GABAergic transmission blockade (Cui et al., 2015).
NO
Biological actions of eCBs partly rely on their ability to regulate NO signaling (Lipina and Hundal, 2017). At cerebellar parallel fiber-Purkinje cell synapses, low and high-frequency stimulations induce differential CB1R activation leading to low and high amount of NO production, which orientates the plasticity, respectively, towards eCB-LTP and eCB-LTD (Wang et al., 2014). Therefore, NO levels may act as a threshold in the modulation of synaptic strength (Song et al., 2012; Wang et al., 2014).
Brain-Derived Neurotrophic Factor (BDNF)
BDNF modulates not only eCB-LTD (Heifets and Castillo, 2009) but also eCB-LTP. For heterosynaptic eCB-LTP in the hippocampus, neocortex, ventral tegmental area, and striatum, activation of the postsynaptic tropomyosin receptor kinase-B (TrkB) by BDNF increases 2-AG mobilization and consequently CB1R activation, which allows an eCB-mediated depression of IPSCs (Lemtiri-Chlieh and Levine, 2010; Selvam et al., 2018) and i-LTD (Zhao et al., 2015; Zhong et al., 2015), tuning the magnitude of glutamatergic LTP. In the neocortex, eCBs released by dendritic calcium spikes reduce inhibitory transmission, which facilitates postsynaptic calcium spike generation, the calcium-dependent release of BDNF, and ultimately the induction of eCB-LTP (Maglio et al., 2018). For homosynaptic eCB-LTP, TrkB activation facilitates 2-AG synthesis and shapes the expression domain of corticostriatal eCB-LTP (Gangarossa et al., 2020).
eCB-Mediated LTP in Learning
While several links between eCB-LTD and various forms of memories have been woven, such as in habit learning or during critical periods of sensory processing (Augustin and Lovinger, 2018), we focus here on the recent starting evidence of the involvement of homosynaptic eCB-LTP and eCB-mediated heterosynaptic facilitation of LTP in learning, based on studies using electrophysiological recordings, and pharmacological or genetic tools modifying eCB-LTP.
Homosynaptic eCB-LTP
At the LPP-dentate gyrus synapses, conveying cue identity to the hippocampus, eCB-LTP is implicated in memory of both simultaneous and serial two-odor discriminations, acquired after a small number of trials in rats (Wang et al., 2016, 2018a,b). Systemic injections of CB1R or MAGL antagonist, preventing or enhancing, respectively, eCB-LTP, had opposite effects on learning of the simultaneous two-odor discrimination task. Importantly, MAGL inhibition led to long-term memory 24 h after six training trials, a protocol which failed to induce efficient learning in controls (Wang et al., 2016). Moreover, learning performance was correlated with greater expression of pROCK in LPP of trained rats and reduced expression with CB1R inhibitor (Wang et al., 2018b). The serial odor discrimination task, testing the encoding of cues embedded in a sequence, is thought to reflect the constant flow of experience characteristic of episodic memory (Wang et al., 2018a). Frm1-KO mice, characterized by impaired 2-AG signaling, reduced NMDAR-mediated transmission, and a strong impairment of eCB-LTP at LPP-dentate gyrus synapses, show learning deficits in this task. Also, systemic injection of MAGL inhibitor or chemogenetic activation of Gq in the entorhinal cortex was sufficient to rescue in vitro homosynaptic eCB-LTP and learning.
Heterosynaptic eCB-Mediated Facilitation of LTP
Several studies highlight the importance of eCB-mediated i-LTD and the regulation by eCBs of the excitation/inhibition balance in memory formation and maintenance (Figure 2), unraveling a novel role of eCBs in disinhibitory mechanisms during learning (Letzkus et al., 2015). Mice in which only sub-saturating forms of LTP requiring i-LTD expression in vitro were impaired in CA1 pyramidal cells (by a targeted mGluR5 knock-out), showed no deficits in spatial memory but performed poorly in trace-conditioning tasks when a long 30 s interval separated the two cues (Xu et al., 2014). Although, partial occlusion of i-LTD in wild-type mice could be observed ex vivo, LTP could still be induced in CA1 after learning, probably because only a few active synapses had been saturated during the task. The acquisition and retention of this temporally-based associative learning were enhanced by systematic MAGL inhibition, shown to promote i-LTD-mediated LTP. This echoed a previous study (Pan et al., 2011), in which MAGL knock-out mice showed improved learning in the water maze and object recognition tasks (but see Griebel et al., 2015). In the same vein, a subpopulation of hippocampal interneurons appears critical in controlling the level of inhibition and CB1R- dependent LTP expression in pyramidal neurons during incidental learning: GABA-CB1R-KO mice have impaired learning and in vivo LTP, which can both be fully rescued by reducing GABAergic transmission. Furthermore, enhancement of ex vivo i-LTD amplitude in trained mice suggests its involvement in learning (Busquets-Garcia et al., 2018). CB1R activation is also required for encoding emotionally salient stimuli at the BLA-medial prefrontal cortex pathway (Laviolette and Grace, 2006; Tan et al., 2010, 2011): notably BLA pharmacological CB1R activation or anandamide reuptake inhibitor potentiates the formation of associative memories with normally subthreshold footshock, putatively through CB1R-dependent heterosynaptic facilitation of BLA output (Azad et al., 2004), and leads to enhanced cortical activity and bursting in response to olfactory cues previously paired with footshock. Systemic treatment with CB1R antagonist prevents in vivo LTP expression and learning. Finally, astroglial CB1R-knock-out mice do not express NMDAR-LTP at CA3–CA1 synapses in vivo and show impaired performance in the novel object recognition task (Robin et al., 2018), which could be rescued by elevating D-serine levels, gating NMDAR activation.
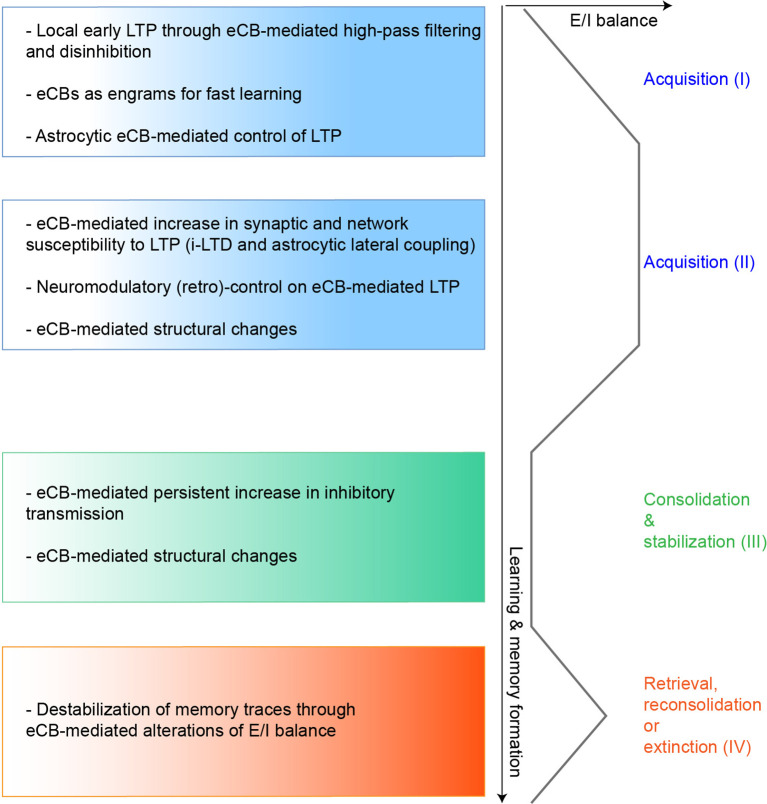
Figure 2.
Hypothetical model of eCBs functions in regulating LTP during learning. We propose here a speculative model of eCBs contribution in controlling LTP expression and the excitation/inhibition (E/I) balance during the different learning stages, based on the mechanisms described in several in vitro and in vivo studies. During the initial phases of memory acquisition (I) eCBs could initially operate as high-pass filters, favoring LTP at strongly active synapses (Silva-Cruz et al., 2017). Also, eCBs-mediated disinhibitory mechanisms (such as DSI or i-LTD operating at different scales) could induce LTP at specific excitatory synapses and fine-tune the E/I balance during learning (Chevaleyre and Castillo, 2003; Xu et al., 2014; Busquets-Garcia et al., 2018). In parallel, eCBs, whose main modus operandi is on-demand biosynthesis and release, behave as highly sensitive and robust detectors of synaptic activity, allowing LTP induction even after a few jittered coincident activity patterns (Cui et al., 2015, 2018a). This feature may thus be used during fast learning and could contribute to episodic memory (Wang et al., 2016). Finally, eCBs can control the astrocytic-dependent release of co-factors necessary for LTP induction (Robin et al., 2018). In the later phases of memory acquisition (II), eCBs could increase the network susceptibility to synaptic modifications, near LTP induction focal points, with more or less spatial extent through heterosynaptic plasticity mechanisms (Chevaleyre and Castillo, 2003; Gómez-Gonzalo et al., 2015; Martín et al., 2015). Importantly, since eCBs can act as bimodal regulators of synaptic plasticity and interact with several neuromodulators, eCB-mediated LTP could also be switched back to normal, or turned to depression (Wang et al., 2014; Caballero-Florán et al., 2016; Xu et al., 2018), depending for instance on late behavioral outcomes, such as in reinforcement learning. During consolidation (III) eCB-mediated potentiation of inhibitory transmission and structural synaptic changes can stabilize acquired memory engrams (Monory et al., 2015; Ghosh et al., 2018; Hu et al., 2019). Finally, reactivation of memories (IV) especially emotional ones, increases eCB signaling, which can operate in feedback and control the lability of these memory traces by modifying the E/I balance (Li et al., 2008; Segev et al., 2018).
Interestingly, a long-lasting enhancement of inhibitory transmission observed in cortical neurons ex vivo after training on a difficult olfactory discrimination task relies on an unusual eCB-mediated mechanism: post-synaptic persistent CB1R activation in pyramidal cells leads to an inhibition of pkA, which induces an increase in postsynaptic GABAA channel conductance (Ghosh et al., 2018).
Overall, the learning of several tasks might initially be associated with an eCB-mediated relief of GABAergic transmission, hence reducing the threshold of LTP induction and could be followed by an elevation of the inhibitory tone that could participate to long-term memory stabilization (Figure 2).
Therapeutic Perspectives
eCBs have long been involved in several brain disorders, in particular drug addiction and pain (Araque et al., 2017). Most of the dysregulation of eCB-mediated LTP described below involve heterosynaptic facilitation of LTP through disinhibitory mechanisms.
Stress Coping
Acute and chronic stress reduce anandamide levels and modify 2-AG signaling and CB1R expression (Ruehle et al., 2012; Morena et al., 2016), and lead to persistent changes in eCB-mediated plasticity expression and polarity ex vivo (Glangetas et al., 2013; Bosch-Bouju et al., 2016) and in vivo (Segev et al., 2018). Mimicking anandamide reduction by selectively overexpressing FAAH at hippocampal CA3-CA1 synapses led to increased anxiety along with an enhancement of LTP expression in vitro, while i-LTD and DSI remained unchanged (Zimmermann et al., 2019). Yet, FAAH overexpression in BLA pyramidal neurons can also attenuate stress and anxiety-like behaviors (Morena et al., 2019): as an explanation, FAAH overexpression could dry out tonic anandamide signaling at GABAergic synapses and shift the excitation/inhibition balance towards inhibition of BLA output neurons. These results highlight the need for considering the excitatory/inhibitory nature of neurons where CB1R is activated to understand the impact of plasticity changes at the network output level.
Conversely, evidence for elevated anandamide during extinction training corroborates with the persistent facilitation of fear extinction induced by pharmacologically increasing anandamide levels in BLA, hippocampus or mPFC (Lin et al., 2008; Gunduz-Cinar et al., 2012; Shoshan et al., 2017; Segev et al., 2018) and with its impairment by CB1R antagonists or FAAH overexpression (De Oliveira Alvares et al., 2008; Lin et al., 2008; Abush and Akirav, 2009; Gunduz-Cinar et al., 2012; Zimmermann et al., 2019). In particular, while increased FAAH activity is observed in BLA and hippocampus following shock exposure, local application of FAAH inhibitor renormalizes stress-induced plasticity changes, re-allowing CA1-CA3 LTP expression while causing a decrease of BLA LTP in vivo (Segev et al., 2018). These manipulations operated immediately or 24 h after a situational reminder of fear-conditioning, persistently attenuated fear expression in mice. Yet, enhanced BLA i-LTD was also reported after local administration of FAAH inhibitor under stressed conditions, and could selectively enhance neuronal excitability in specific BLA glutamatergic networks (Azad et al., 2004; Gunduz-Cinar et al., 2012). Activation of hippocampal TRPV1, which was shown to enhance CA1 LTP via the GABAergic system in vitro (Bennion et al., 2011), could prevent the stress-induced switch from LTP to LTD and stress-induced impairment of spatial memory retrieval (Li et al., 2008). Overall, a targeted elevation of eCBs appears as a strategy for coping with stress, with preliminary clinical applications (Papagianni and Stevenson, 2019; Mayo et al., 2020).
Drug Addiction
The facilitation of LTP in dopaminergic neurons is reported after exposure to cocaine, ethanol, or nicotine (Parsons and Hurd, 2015). For prolonged cocaine exposure, such facilitation, accompanied by increased bursting of dopaminergic neurons, is likely mediated by an eCB-dependent disinhibitory feedback loop (Liu et al., 2005; Pan et al., 2008a,b). Indeed, cocaine intake occludes ex vivo eCB-mediated i-LTD while manipulating eCBs signaling (by local application of CB1R or mGluR5 antagonists, or by blocking 2-AG synthesis) alleviates cocaine-induced reduction of inhibitory transmission (Pan et al., 2008b; Wang et al., 2015; Zhong et al., 2015). Chronic nicotine self-administration facilitates the induction of CB1R-mediated LTP in the bed nucleus of the stria terminalis, and this facilitation resists to a long period of forced abstinence (Reisiger et al., 2014). As this area is involved in cue-induced drug-seeking, these persistent changes could be responsible for increased vulnerability to relapse.
Alzheimer and Parkinson’s Diseases
In Alzheimer’s disease, β-amyloid accumulation results in a reduction of hippocampal LTP, and notably prevents eCB-mediated disinhibition and EPSP-to-spike potentiation in vitro (Orr et al., 2014), a phenomenon that could contribute to memory deficits. Knocking-out CB1R in an Alzheimer mouse model worsens learning impairments, while treatments with an eCB-reuptake inhibitor or exogenous cannabinoids improve memory (Bedse et al., 2014). In parkinsonian rodents, striatal homosynaptic eCB-LTP is prevented ex vivo and can be rescued by Levodopa (Xu et al., 2018), and the globus pallidus exhibits a reduced GABAergic transmission, which is reversed by the co-activation of D2R and CB1R (Muñoz-Arenas et al., 2015).
Pain
In the spinal cord, eCBs have both anti- and pro-nociceptive effects through inversed plasticity mechanisms, respectively by depressing nociceptive and disinhibiting non-nociceptive afferents (Pernia-Andrade et al., 2009; Kato et al., 2012). The underlying mechanism was evidenced in the semi-intact preparation of the nervous ganglia of the medicinal leech, in which eCB-mediated heterosynaptic potentiation of non-nociceptive synapses is critical to producing behavioral sensitization in response to noxious stimuli (Higgins et al., 2013; Wang and Burrell, 2018).
Conclusions
Although, it has encountered some skepticism at times, various forms of eCB-mediated LTP have been characterized in different brain areas. If their involvement in memory has been proposed by a substantial body of experimental evidence, further work is necessary to make a direct link between the two in several paradigms, using targeted in vivo recordings and pharmacological or genetic manipulations. eCB-LTP should not be viewed as an unconventional or atypical form of eCB-plasticity, but as the other side of the eCB-mediated engram, making eCBs bidirectional regulators of synaptic plasticity, similarly to most neurotransmitters. With the increasing promising applications for cannabis and eCB-based drugs in medicine, we need to consider eCBs bidirectional effects, which also expand considerably their potential field of therapeutic applications.
Author Contributions
CP wrote the “eCB-Mediated LTP in Learning,” “Therapeutic Perspectives,” and “eCB-Mediated Synaptic Potentiation” sections, and designed the Figures 1, 2. YC wrote the “eCB-Mediated Synaptic Potentiation” section. NG wrote the “Neuromodulation of eCB-LTP” section. LV wrote the “eCB-Mediated Synaptic Potentiation,” “Non-CB1R-Mediated eCB-Potentiation of Synaptic Transmission,” and “Conclusions” sections, and designed the Figure 1. All authors have edited and corrected the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdala
- CB1R
cannabinoid type-1 receptor
- DSI
depolarization-induced suppression of inhibition
- DXR
dopaminergic type-X receptor
- E/I balance
excitation/inhibition balance
- FAAH
fatty acid amide hydrolase
- eCB
endocannabinoid
- GABA
gamma-aminobutyric acid
- i-LTD
long-term depression of inhibitory transmission
- LPP
lateral perforant path
- LTP
long-term potentiation
- LTD
long-term depression
- MAGL
monoacylglycerol lipase
- mGluR
metabotropic glutamatergic receptor
- mPFC
medial prefrontal cortex
- NMDAR
N-methyl-D-aspartate receptor
- NO
nitric oxide
- pkA
protein kinase-A
- STDP
spike-timing-dependent plasticity
- TrkB
tropomyosin receptor kinase-B
- TRPV1
transient receptor potential vanilloid type-1.
Footnotes
Funding. This work was supported by grants from the Agence Nationale pour la Recherche (grants ANR-CRCNS Dopaciumcity and ANR Mopla) and the LabEx Paris-Sciences et Lettres (PSL). CP was supported by Ecole Normale Supérieure PhD fellowship.
References
- Abraham W.C. (2008). Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9:387. 10.1038/nrn2356 [DOI] [PubMed] [Google Scholar]
- Abush H., Akirav I. (2009). Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 20, 1126–1138. 10.1002/hipo.20711 [DOI] [PubMed] [Google Scholar]
- Adermark L. (2011). Modulation of endocannabinoid-mediated long-lasting disinhibition of striatal output by cholinergic interneurons. Neuropharmacology 61, 1314–1320. 10.1016/j.neuropharm.2011.07.039 [DOI] [PubMed] [Google Scholar]
- Araque A., Castillo P. E., Manzoni O. J., Tonini R. (2017). Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124, 13–24. 10.1016/j.neuropharm.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin S., Lovinger D. (2018). Functional relevance of endocannabinoid-dependent synaptic plasticity in the central nervous system. ACS Chem. Neurosci. 9, 2146–2161. 10.1021/acschemneuro.7b00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad S., Monory K., Marsicano G., Cravatt B., Lutz B., Zieglgänsberger W., et al. (2004). Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J. Neurosci. 24, 9953–9961. 10.1523/JNEUROSCI.2134-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J., Srinivas K. V., Cheung S. K., Taniguchi H., Huang Z. J., Siegelbaum S. A. (2013). A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron 79, 1208–1221. 10.1016/j.neuron.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G., Romano A., Lavecchia A., Cassano T., Gaetani S. (2014). The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 43, 1115–1136. 10.3233/JAD-141635 [DOI] [PubMed] [Google Scholar]
- Bennion D., Jensen T., Walther C., Hamblin J., Wallmann A., Couch J., et al. (2011). Transient receptor potential vanilloid 1 agonists modulate hippocampal CA1 LTP via the GABAergic system. Neuropharmacology 61, 730–738. 10.1016/j.neuropharm.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Bosch-Bouju C., Larrieu T., Linders L., Manzoni O., Layé S. (2016). Endocannabinoid-mediated plasticity in nucleus accumbens controls vulnerability to anxiety after social defeat stress. Cell Rep. 16, 1237–1242. 10.1016/j.celrep.2016.06.082 [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A., Oliveira da Cruz J., Terral G., Pagano Zottola A., Soria-Gómez E., Contini A., et al. (2018). Hippocampal CB1 receptors control incidental associations. Neuron 99, 1247.e7–1259.e7. 10.1016/j.neuron.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Caballero-Florán R. N., Conde-Rojas I., Oviedo Chávez A., Cortes-Calleja H., Lopez-Santiago L. F., Isom L. L., et al. (2016). Cannabinoid-induced depression of synaptic transmission is switched to stimulation when dopaminergic tone is increased in the globus pallidus of the rodent. Neuropharmacology 110, 407–418. 10.1016/j.neuropharm.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Cachope R., Mackie K., Triller A., O’Brien J., Pereda A. E. (2007). Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron 56, 1034–1047. 10.1016/j.neuron.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G., Wang Y., Alger B. E. (2002). Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat. Neurosci. 5, 723–724. 10.1038/nn879 [DOI] [PubMed] [Google Scholar]
- Castillo P. E., Younts T. J., Chávez A. E., Hashimotodani Y. (2012). Endocannabinoid signaling and synaptic function. Neuron 76, 70–81. 10.1016/j.neuron.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V., Castillo P. E. (2003). Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38, 461–472. 10.1016/s0896-6273(03)00235-6 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V., Castillo P. E. (2004). Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43, 871–881. 10.1016/j.neuron.2004.08.036 [DOI] [PubMed] [Google Scholar]
- Covelo A., Araque A. (2016). Lateral regulation of synaptic transmission by astrocytes. Neuroscience 323, 62–66. 10.1016/j.neuroscience.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Covey D. P., Mateo Y., Sulzer D., Cheer J. F., Lovinger D. M. (2017). Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 124, 52–61. 10.1016/j.neuropharm.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Paillé V., Xu H., Genet S., Delord B., Fino E., et al. (2015). Endocannabinoids mediate bidirectional striatal spike-timing-dependent plasticity. J. Physiol. 593, 2833–2849. 10.1113/JP270324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Prokin I., Xu H., Delord B., Genet S., Venance L., et al. (2016). Endocannabinoid dynamics gate spike-timing dependent depression and potentiation. Elife 5:e13185. 10.7554/eLife.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Prokin I., Mendes A., Berry H., Venance L. (2018a). Robustness of STDP to spike timing jitter. Sci. Rep. 8:8139. 10.1038/s41598-018-26436-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Perez S., Venance L. (2018b). Endocannabinoid-LTP mediated by CB1 and TRPV1 receptors encodes for limited occurrences of coincident activity in neocortex. Front. Cell. Neurosci. 12:182. 10.3389/fncel.2018.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira Alvares L., Genro B. P., Diehl F., Quillfeldt J. A. (2008). Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol. Learn. Mem. 90, 1–9. 10.1016/j.nlm.2008.01.009 [DOI] [PubMed] [Google Scholar]
- Feldman D. E. (2012). The spike-timing dependence of plasticity. Neuron 75, 556–571. 10.1016/j.neuron.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend L. N., Williamson R. C., Merrill C. B., Newton S. T., Christensen M. T., Petersen J., et al. (2019). Hippocampal stratum oriens somatostatin-positive cells undergo CB1-dependent long-term potentiation and express endocannabinoid biosynthetic enzymes. Molecules 24:1306. 10.3390/molecules24071306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G., Perez S., Dembitskaya Y., Prokin I., Berry H., Venance L. (2020). BDNF controls bidirectional endocannabinoid plasticity at corticostriatal synapses. Cereb. Cortex 30, 197–214. 10.1093/cercor/bhz081 [DOI] [PubMed] [Google Scholar]
- Ghosh S., Reuveni I., Zidan S., Lamprecht R., Barkai E. (2018). Learning-induced modulation of the effect of endocannabinoids on inhibitory synaptic transmission. J. Neurophysiol. 119, 752–760. 10.1152/jn.00623.2017 [DOI] [PubMed] [Google Scholar]
- Giaume C., Venance L. (1998). Intercellular calcium signaling and gap junctional communication in astrocytes. Glia 24, 50–64. [DOI] [PubMed] [Google Scholar]
- Glangetas C., Girard D., Groc L., Marsicano G., Chaouloff F., Georges F. (2013). Stress switches cannabinoid type-1 (CB1) receptor-dependent plasticity from LTD to LTP in the bed nucleus of the stria terminalis. J. Neurosci. 33, 19657–19663. 10.1523/JNEUROSCI.3175-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M., Felder C. C. (1997). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a gs linkage to the CB1 receptor. J. Neurosci. 17, 5327–5333. 10.1523/JNEUROSCI.17-14-05327.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gonzalo M., Navarrete M., Perea G., Covelo A., Martín-Fernández M., Shigemoto R., et al. (2015). Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex 25, 3699–3712. 10.1093/cercor/bhu231 [DOI] [PubMed] [Google Scholar]
- Gonzalez B., Paz F., Florán L., Aceves J., Erlij D., Florán B. (2009). Cannabinoid agonists stimulate [3H]GABA release in the globus pallidus of the rat when Gi protein-receptor coupling is restricted: role of dopamine D2 receptors. J. Pharmacol. Exp. Ther. 328, 822–828. 10.1124/jpet.108.145425 [DOI] [PubMed] [Google Scholar]
- Griebel G., Pichat P., Beeské S., Leroy T., Redon N., Jacquet A., et al. (2015). Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci. Rep. 5:7642. 10.1038/srep07642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., MacPherson K., Cinar R., Gamble-George J., Sugden K., Williams B., et al. (2012). Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry 18, 813–823. 10.1038/mp.2012.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson A. J., Bornheim L. M., Scanziani M., Yost C. S., Gray A. T., Hansen B. M., et al. (1998). Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J. Neurochem. 70, 671–676. 10.1046/j.1471-4159.1998.70020671.x [DOI] [PubMed] [Google Scholar]
- Heifets B. D., Castillo P. E. (2009). Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306. 10.1146/annurev.physiol.010908.163149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins A., Yuan S., Wang Y., Burrell B. D. (2013). Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids. Mol. Pain 9:26. 10.1186/1744-8069-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M. E., Bhatia C., Frazier C. J. (2011). Cannabinoid receptor agonists potentiate action potential-independent release of GABA in the dentate gyrus through a CB1 receptor-independent mechanism. J. Physiol. 589, 3801–3821. 10.1113/jphysiol.2011.211482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. Y., Kruijssen D. L. H., Frias C. P., Rózsa B., Hoogenraad C. C., Wierenga C. J. (2019). Endocannabinoid signaling mediates local dendritic coordination between excitatory and inhibitory synapses. Cell Rep. 27, 666.e5–675.e5. 10.1016/j.celrep.2019.03.078 [DOI] [PubMed] [Google Scholar]
- Kato A., Punnakkal P., Pernía-Andrade A., von Schoultz C., Sharopov S., Nyilas R., et al. (2012). Endocannabinoid-dependent plasticity at spinal nociceptor synapses. J. Physiol. 590, 4717–4733. 10.1113/jphysiol.2012.234229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I., Freund T. F. (2012). Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 35, 529–558. 10.1146/annurev-neuro-062111-150420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-H., Park J. M., Lee S.-H., Ho W.-K. (2019). Association of mGluR-dependent LTD of excitatory synapses with endocannabinoid-dependent LTD of inhibitory synapses leads to EPSP to spike potentiation in CA1 pyramidal neurons. J. Neurosci. 39, 224–237. 10.1523/JNEUROSCI.2935-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk-Slomka M., Dzik A., Budzynska B., Biala G. (2017). Endocannabinoid system: the direct and indirect involvement in the memory and learning processes—a short review. Mol. Neurobiol. 54, 8332–8347. 10.1007/s12035-016-0313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakatos A., El Manira A. (2007). Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J. Neurosci. 27, 12664–12674. 10.1523/JNEUROSCI.3174-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette S. R., Grace A. A. (2006). Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J. Neurosci. 26, 6458–6468. 10.1523/jneurosci.0707-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F., Levine E. S. (2010). BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J. Neurophysiol. 104, 1923–1932. 10.1152/jn.00472.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus J. J., Wolff S. B. E., Lüthi A. (2015). Disinhibition, a circuit mechanism for associative learning and memory. Neuron 88, 264–276. 10.1016/j.neuron.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Li H.-B., Mao R.-R., Zhang J.-C., Yang Y., Cao J., Xu L. (2008). Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol. Psychiatry 64, 286–292. 10.1016/j.biopsych.2008.02.020 [DOI] [PubMed] [Google Scholar]
- Lin H.-C., Mao S.-C., Su C.-L., Gean P.-W. (2008). The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb. Cortex 19, 165–175. 10.1093/cercor/bhn075 [DOI] [PubMed] [Google Scholar]
- Lin Q.-S., Yang Q., Liu D.-D., Sun Z., Dang H., Liang J., et al. (2011). Hippocampal endocannabinoids play an important role in induction of long-term potentiation and regulation of contextual fear memory formation. Brain Res. Bull. 86, 139–145. 10.1016/j.brainresbull.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Lipina C., Hundal H. S. (2017). The endocannabinoid system: “no” longer anonymous in the control of nitrergic signalling? J. Mol. Cell Biol. 9, 91–103. 10.1093/jmcb/mjx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.-S., Pu L., Poo M.-M. (2005). Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437, 1027–1031. 10.1038/nature04050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglio L. E., Noriega-Prieto J. A., Maraver M. J., Fernández de Sevilla D. (2018). Endocannabinoid-dependent long-term potentiation of synaptic transmission at rat barrel cortex. Cereb. Cortex 28, 1568–1581. 10.1093/cercor/bhx053 [DOI] [PubMed] [Google Scholar]
- Marsicano G., Lafenêtre P. (2009). Roles of the endocannabinoid system in learning and memory. Curr. Top. Behav. Neurosci. 1, 201–230. 10.1007/978-3-540-88955-7_8 [DOI] [PubMed] [Google Scholar]
- Martín R., Bajo-Grañeras R., Moratalla R., Perea G., Araque A. (2015). Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- Mathur B. N., Tanahira C., Tamamaki N., Lovinger D. M. (2013). Voltage drives diverse endocannabinoid signals to mediate striatal microcircuit-specific plasticity. Nat. Neurosci. 16, 1275–1283. 10.1038/nn.3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L. M., Asratian A., Lindé J., Morena M., Haataja R., Hammar V., et al. (2020). Elevated anandamide, enhanced recall of fear extinction and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol. Psychiatry 87, 538–547. 10.1016/j.biopsych.2019.07.034 [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Parker L. A. (2013). The endocannabinoid system and the brain. Annu. Rev. Psychol. 64, 21–47. 10.1146/annurev-psych-113011-143739 [DOI] [PubMed] [Google Scholar]
- Monory K., Polack M., Remus A., Lutz B., Korte M. (2015). Cannabinoid CB1 receptor calibrates excitatory synaptic balance in the mouse hippocampus. J. Neurosci. 35, 3842–3850. 10.1523/jneurosci.3167-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Aukema R. J., Leitl K. D., Rashid A. J., Vecchiarelli H. A., Josselyn S. A., et al. (2019). Upregulation of anandamide hydrolysis in the basolateral complex of amygdala reduces fear memory expression and indices of stress and anxiety. J. Neurosci. 39, 1275–1292. 10.1523/JNEUROSCI.2251-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Patel S., Bains J., Hill M. (2016). Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41, 80–102. 10.1038/npp.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Arenas G., Paz-Bermúdez F., Báez-Cordero A., Caballero-Florán R., González-Hernández B., Florán B., et al. (2015). Cannabinoid CB1 receptors activation and coactivation with D2 receptors modulate GABAergic neurotransmission in the globus pallidus and increase motor asymmetry. Synapse 69, 103–114. 10.1002/syn.21796 [DOI] [PubMed] [Google Scholar]
- Navarrete M., Araque A. (2008). Endocannabinoids mediate neuron-astrocyte communication. Neuron 57, 883–893. 10.1016/j.neuron.2008.01.029 [DOI] [PubMed] [Google Scholar]
- Navarrete M., Araque A. (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126. 10.1016/j.neuron.2010.08.043 [DOI] [PubMed] [Google Scholar]
- Orr A. L., Hanson J. E., Li D., Klotz A., Wright S., Schenk D., et al. (2014). β-amyloid inhibits E-S potentiation through suppression of cannabinoid receptor 1-dependent synaptic disinhibition. Neuron 82, 1334–1345. 10.1016/j.neuron.2014.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Hillard C. J., Liu Q.-S. (2008a). D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase a signaling. J. Neurosci. 28, 14018–14030. 10.1523/jneurosci.4035-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Hillard C., Liu Q. (2008b). Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J. Neurosci. 28, 1385–1397. 10.1523/JNEUROSCI.4033-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Wang W., Zhong P., Blankman J. L., Cravatt B. F., Liu Q.-S. (2011). Alterations of endocannabinoid signaling, synaptic plasticity, learning and memory in monoacylglycerol lipase knock-out mice. J. Neurosci. 31, 13420–13430. 10.1523/jneurosci.2075-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagianni E. P., Stevenson C. W. (2019). Cannabinoid regulation of fear and anxiety: an update. Curr. Psychiatry Rep. 21:38. 10.1007/s11920-019-1026-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L. H., Hurd Y. L. (2015). Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 16, 579–594. 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernia-Andrade A. J., Kato A., Witschi R., Nyilas R., Katona I., Freund T. F., et al. (2009). Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325, 760–764. 10.3410.1126/science.1171870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Astarita G., Rapaka R. (2007). A neuroscientist’s guide to lipidomics. Nat. Rev. Neurosci. 8, 743–754. 10.1038/nrn2233 [DOI] [PubMed] [Google Scholar]
- Reisiger A., Kaufling J., Manzoni O., Cador M., Georges F., Caille S. (2014). Nicotine Self-administration induces CB1-dependent LTP in the bed nucleus of the stria terminalis. J. Neurosci. 34, 4285–4292. 10.1523/jneurosci.3149-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin L. M., Oliveira da Cruz J. F., Langlais V. C., Martin-Fernandez M., Metna-Laurent M., Busquets-Garcia A., et al. (2018). Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron 98, 935.e5–944.e5. 10.1016/j.neuron.2018.04.034 [DOI] [PubMed] [Google Scholar]
- Ruehle S., Rey A. A., Remmers F., Lutz B. (2012). The endocannabinoid system in anxiety, fear memory and habituation. J. Psychopharmacol. 26, 23–39. 10.1177/0269881111408958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N., Zhang J., Chen C. (2010). Anandamide potentiation of miniature spontaneous excitatory synaptic transmission is mediated via IP3 pathway. Neurochem. Int. 56, 590–596. 10.1016/j.neuint.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev A., Korem N., Mizrachi Zer-Aviv T., Abush H., Lange R., Sauber G., et al. (2018). Role of endocannabinoids in the hippocampus and amygdala in emotional memory and plasticity. Neuropsychopharmacology 43, 2017–2027. 10.1038/s41386-018-0135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam R., Yeh M. L., Levine E. S. (2018). Endogenous cannabinoids mediate the effect of BDNF at CA1 inhibitory synapses in the hippocampus. Synapse 73(2):e22075. 10.1002/syn.22075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan N., Segev A., Abush H., Mizrachi Zer-Aviv T., Akirav I. (2017). Cannabinoids prevent the differential long-term effects of exposure to severe stress on hippocampal- and amygdala-dependent memory and plasticity. Hippocampus 27, 1093–1109. 10.1002/hipo.22755 [DOI] [PubMed] [Google Scholar]
- Silva-Cruz A., Carlström M., Ribeiro J. A., Sebastião A. M. (2017). Dual influence of endocannabinoids on long-term potentiation of synaptic transmission. Front. Pharmacol. 8:921. 10.3389/fphar.2017.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Kyriakatos A., El Manira A. (2012). Gating the polarity of endocannabinoid-mediated synaptic plasticity by nitric oxide in the spinal locomotor network. J. Neurosci. 32, 5097–5105. 10.1523/jneurosci.5850-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B., Siemes S., Wallmichrath I. (2002). Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 15, 2057–2061. 10.1046/j.1460-9568.2002.02041.x [DOI] [PubMed] [Google Scholar]
- Tan H., Lauzon N. M., Bishop S. F., Bechard M. A., Laviolette S. R. (2010). Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb. Cortex 20, 1486–1496. 10.1093/cercor/bhp210 [DOI] [PubMed] [Google Scholar]
- Tan H., Lauzon N. M., Bishop S. F., Chi N., Bechard M., Laviolette S. R. (2011). Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J. Neurosci. 31, 5300–5312. 10.1523/jneurosci.4718-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.-J., Su L.-D., Wang Y.-N., Yang D., Sun C.-L., Zhou L., et al. (2014). Long-term potentiation at cerebellar parallel fiber-purkinje cell synapses requires presynaptic and postsynaptic signaling cascades. J. Neurosci. 34, 2355–2364. 10.1523/jneurosci.4064-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. I., Nicoll R. A. (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592. 10.1038/35069076 [DOI] [PubMed] [Google Scholar]
- Wang H., Treadway T., Covey D. P., Cheer J. F., Lupica C. R. (2015). Cocaine-induced endocannabinoid mobilization in the ventral tegmental area. Cell Rep. 12, 1997–2008. 10.1016/j.celrep.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Cox B. M., Jia Y., Le A. A., Cox C. D., Jung K. M., et al. (2018a). Treating a novel plasticity defect rescues episodic memory in fragile x model mice. Mol. Psychiatry 23, 1798–1806. 10.1038/mp.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Jia Y., Pham D. T., Palmer L. C., Jung K.-M., Cox C. D., et al. (2018b). Atypical endocannabinoid signaling initiates a new form of memory-related plasticity at a cortical input to hippocampus. Cereb. Cortex 28, 2253–2266. 10.1093/cercor/bhx126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Trieu B., Palmer L., Jia Y., Pham D., Jung K., et al. (2016). A primary cortical input to hippocampus expresses a pathway-specific and endocannabinoid-dependent form of long-term potentiation. eNeuro 3:ENEURO.0160–16.2016. 10.1523/eneuro.0160-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Burrell B. D. (2018). Endocannabinoid-mediated potentiation of nonnociceptive synapses contributes to behavioral sensitization. J. Neurophysiol. 119, 641–651. 10.1152/jn.00092.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Perez S., Cornil A., Detraux B., Prokin I., Cui Y., et al. (2018). Dopamine-endocannabinoid interactions mediate spike-timing-dependent potentiation in the striatum. Nat. Commun. 9:4118. 10.1038/s41467-018-06409-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Antion M., Nomura T., Kraniotis S., Zhu Y., Contractor A. (2014). Hippocampal metaplasticity is required for the formation of temporal associative memories. J. Neurosci. 34, 16762–16773. 10.1523/jneurosci.2869-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.-Y., Zhang J., Chen C. (2012). Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. J. Physiol. 590, 2305–2315. 10.1113/jphysiol.2011.223511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Lei G., Xie Y.-F., MacDonald J. F., Jackson M. F. (2014). Differential regulation of NMDAR and NMDAR-mediated metaplasticity by anandamide and 2-AG in the hippocampus. Hippocampus 24, 1601–1614. 10.1002/hipo.22339 [DOI] [PubMed] [Google Scholar]
- Younts T. J., Chevaleyre V., Castillo P. E. (2013). CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J. Neurosci. 33, 13743–13757. 10.1523/jneurosci.0817-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Yeh M. L.-W., Levine E. S. (2015). Role for endogenous BDNF in endocannabinoid-mediated long-term depression at neocortical inhibitory synapses. eNeuro 2:ENEURO.0029–14.2015. 10.1523/eneuro.0029-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P., Liu Y., Hu Y., Wang T., Zhao Y.-P., Liu Q.-S. (2015). BDNF interacts with endocannabinoids to regulate cocaine-induced synaptic plasticity in mouse midbrain dopamine neurons. J. Neurosci. 35, 4469–4481. 10.1523/jneurosci.2924-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P. J., Lovinger D. M. (2007). Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J. Neurophysiol. 97, 4386–4389. 10.1152/jn.01228.2006 [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Bartsch J., Beer A., Lomazzo E., Guggenhuber S., Lange M., et al. (2019). Impaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning and emotional responses. Neuropsychopharmacology 44, 1377–1388. 10.1038/s41386-018-0274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]