Abstract
Cardiac small conductance Ca2+-activated K+ (SK) channels are activated solely by Ca2+, but the SK current (ISK) is inwardly rectified. However, the impact of inward rectification in shaping action potentials (APs) in ventricular cardiomyocytes under β-adrenergic stimulation or in disease states remains undefined. Two processes underlie this inward rectification: an intrinsic rectification caused by an electrostatic energy barrier from positively charged amino acids at the inner pore and a voltage-dependent Ca2+/Mg2+ block. Thus, Ca2+ has a biphasic effect on ISK, activating at low [Ca2+] yet inhibiting ISK at high [Ca2+]. We examined the effect of ISK rectification on APs in rat cardiomyocytes by simultaneously recording whole-cell apamin-sensitive currents and Ca2+ transients during an AP waveform and developed a computer model of SK channels with rectification features. The typical profile of ISK during AP clamp included an initial peak (mean 1.6 pA/pF) followed by decay to the point that submembrane [Ca2+] reached ∼10 μM. During the rest of the AP stimulus, ISK either plateaued or gradually increased as the cell repolarized and submembrane [Ca2+] decreased further. We used a six-state gating model combined with intrinsic and Ca2+/Mg2+-dependent rectification to simulate ISK and investigated the relative contributions of each type of rectification to AP shape. This SK channel model replicates key features of ISK recording during AP clamp showing that intrinsic rectification limits ISK at high Vm during the early and plateau phase of APs. Furthermore, the initial rise of Ca2+ transients activates, but higher [Ca2+] blocks SK channels, yielding a transient outward-like ISK trajectory. During the decay phase of Ca2+, the Ca2+-dependent block is released, causing ISK to rise again and contribute to repolarization. Therefore, ISK is an important repolarizing current, and the rectification characteristics of an SK channel determine its impact on early, plateau, and repolarization phases of APs.
Significance
Ca2+-activated K+ (SK) channels are expressed in ventricular myocytes, and their influence on action potentials (APs) becomes significant under β-adrenergic stimulation or in cardiac diseases. SK channels are voltage insensitive but show inward rectification caused by local electrostatic influence from positively charged amino acids near the pore and voltage-dependent blockade by Ca2+ and Mg2+. Using AP clamp data and computer simulation, we found that the rectification kinetics modulate the temporal trajectory of SK currents during an AP to influence both phase 1 and 3 repolarization depending on the degree of intrinsic and Ca2+/Mg2+-dependent rectification. This study suggests that modulation of the inward rectification properties of SK channels may have significant implications for arrhythmogenesis in various pathophysiological conditions.
Introduction
Small conductance Ca2+-activated K+ (SK) channels are solely activated by changes in intracellular Ca2+ via constitutively bound calmodulin, making the activation rapid within the 5–15-ms timescale (1). This unique characteristic of Ca2+-dependent opening determines its important role in integrating intracellular Ca2+ handling to membrane voltage. SK channels have been identified in many excitable cells, but the initial confirmation of SK channels in cardiac myocytes was relatively recent (2). Three SK channel isoforms (SK1-3) encoded by the genes KCNN1-3 have been identified (3,4). Expression of SK channels has been shown in various species, including mice, rats, rabbits, dogs, horses, and humans, both in ventricular (2,5, 6, 7, 8, 9, 10, 11, 12) and atrial (2,7,10,11,13) tissue. Recent studies suggest critical roles of SK channels in human atrial and ventricular arrhythmias, making them a potential therapeutic target (14, 15, 16, 17). However, the exact roles of SK channels in different types of arrhythmias are yet to be determined (18, 19, 20, 21, 22, 23, 24, 25, 26, 27).
The single channel conductance of SK channels is ∼10–20 pS (28,29), and it is well characterized that SK channels lack a voltage sensor, which makes their gating voltage insensitive. However, detailed biophysical studies of SK channel kinetics revealed two distinct properties that limit the SK current (ISK) predominantly at positive membrane voltages, causing inward rectification, and that may significantly modulate ISK influence on ventricular repolarization. First, an intrinsic rectification (IntR) is caused by two positively charged amino acids (R396 and K397 in rat SK2 (rSK2)) at the inner mouth of the SK channel pore. These residues provide an energy barrier to the entry and exit of K+ ions by a local electrostatic mechanism (30). Second, intracellular divalent ions bind near the selectivity filter of SK channels (S359 in rSK2) and block the flow of K+ ions. Ca2+ and Mg2+ ions bind in SK channels with a higher affinity at positive membrane potentials, contributing to Ca2+/Mg2+-dependent rectification (31,32) under physiological conditions. The amino acids associated with both forms of rectification are conserved in all three mammalian SK channel isoforms (SK1, SK2, and SK3) by PubMed BLAST analysis (33,34). Although most of the studies of SK channel rectification in heterologous expression systems used the rSK2 isoform (30, 31, 32), homomeric human SK1 (35) and SK3 (36,37) also show inward rectification.
Both intrinsic and Ca2+/Mg2+-dependent rectification may play significant roles in modulating cardiac repolarizations. The current amplitude will be smaller at positive voltages because of intrinsic rectification, particularly during action potential (AP) upstrokes and the phase 1 repolarization period. The rise of Ca2+ during the Ca2+ transient may have biphasic effects on ISK. Recent studies showed that SK channels are closely linked to L-type Ca2+ channels (LTCCs) (38,39) and also near to ryanodine receptors (RyR2) (39). Because submembrane Ca2+ ([Ca2+]SM) in ventricular cardiomyocytes (VCMs) can rise up to ∼100 μM (40,41), SK channels close to LTCCs may also see large influxes of Ca2+ that can inhibit the channels via a Ca2+/Mg2+-dependent block. The available data indicate that ventricular SK channels do not contribute to AP repolarization in normal conditions but become significantly augmented to impact AP repolarization in cardiac diseases or under a β-adrenergic stimulation (5,16,19,20,42,43). We recently showed that phosphorylation of SK channels by protein kinase A (PKA) increases ISK in the VCMs of healthy animals. Furthermore, this phenomenon underlies the functional recruitment of SK channels in VCMs from a rat model of hypertrophy induced by pressure overload. We proposed that the PKA phosphorylation of the SK channel attenuates ISK rectification by reducing the Ca2+/voltage-dependent inhibition of SK channels without changing their sensitivity to Ca2+, resulting in an increase of ISK (38). In this study, we aim to determine whether changes in intrinsic rectification also play an important role in this process. Understanding the rectification properties and their modulations are important factors in defining the impact of ISK on cardiac repolarization in addition to the expression level of SK channels.
Our goal in this study is to characterize the ISK trajectory during an AP under β-adrenergic stimulation and determine how intrinsic and Ca2+/Mg2+-dependent rectification of ISK influences this trajectory, thereby modulating AP dynamics. To address the complex role of SK channels in cardiac repolarization, it is crucial to study SK channel physiology under conditions of physiological Ca2+ dynamics, yet most isolated cell physiology experiments have been done with [Ca2+] clamped to one concentration. We simultaneously recorded apamin-sensitive currents and Ca2+ signals in rat VCMs while voltage clamping the cells with an AP stimulus waveform (AP clamp). In addition, we developed a model with rectification properties and incorporated the data from AP clamp to investigate how intrinsic and Ca2+/Mg2+-dependent rectification of ISK affects an AP. The model expands upon a previous SK channel model without rectification characteristics (23). Our results indicate that ISK does not solely follow the rise and decay of intracellular Ca2+ transients but shows an initial peak with a rapid decay, followed by a second rise of ISK during the phase 3 repolarization. This suggests that the rectification properties of SK channels greatly influence their impact on cardiac repolarization over the full range of an AP (phases 1, 2, and 3) and thereby AP dynamics.
Materials and Methods
Ethical approval
All procedures involving animals were approved by The Rhode Island Hospital Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 2011).
VCM isolation, cell culture, and viral infection
Whole hearts and VCMs were isolated from male Sprague-Dawley rats purchased either from Charles River Laboratories (Wilmington, MA) as sham-operated rats for thoracic aortic banding or from Harlan Laboratories (Indianapolis, IN). Animals were acclimatized for 3–4 weeks in the Rhode Island Hospital animal facility. Experiments were performed 4–5 weeks after shipping. Rats were injected with 120 mg/kg pentobarbital intraperitoneally. The heart was removed from euthanized rats via bilateral thoracotomy, mounted on a Langendorff apparatus, and retrogradely perfused with Tyrode solution containing collagenase II (Worthington Biochemical, Lakewood, NJ) at 37°C. VCMs were isolated as previously described (44) before plating onto laminin-coated coverslips for AP clamp experiments. VCMs were recorded under a whole-cell patch clamp within 8 h of isolation. For experiments with rSK2 channel overexpression by viral infection, adult rat VCMs were isolated from 9–12-week-old Sprague-Dawley male rats (Rat Genome Database catalog no. 70508, RRID: RGD_70508) from Harlan Laboratories (Indianapolis, IN), as described previously (44). Myocytes were plated in 24-well plates on laminin-coated glass coverslips, cultured in serum-free medium 199 (Thermo Fisher Scientific, Waltham, MA), and supplemented with 25 mmol/L NaHCO3, 10 mmol/L HEPES, 5 mmol/L creatine, and 5 mmol/L taurine and 10 U/mL penicillin, 10 μg/mL streptomycin, and 10 μg/mL gentamycin (pH 7.3). Unattached cells were removed after 1 h, and the remaining VCMs were infected with adenoviruses at multiplicity of infection (MOI) of 10 for the rSK2 channel. Myocytes were cultured at 37°C in 95% air, 5% CO2 for 36–48 h before analysis.
Construction of wild-type and mutant SK2 adenoviruses
Adenovirus-carrying recombinant wild-type rSK2 sequence was constructed as described previously (8,44), utilizing the ViraPower Gateway Expression System (Thermo Fisher Scientific). Briefly, the coding region of rSK2 sequence was cloned into pENTR 1A vector, and then recombined into pAd/CMV/V5-DEST with LR recombination reaction. The R396E/K397E mutant viral construct was made the same way using a mutant coding region of rSK2 made as described (30), which was the kind gift of Dr. Weiyan Li. Sequence-verified plasmid was digested with restriction enzyme PacI before transfection into HEK293A cells (RRID: CVCL_6910) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The titer of amplified adenoviral stocks was determined using the Adeno-X qPCR Titration Kit (Takara Bio, Mountain View, CA).
Whole-cell patch clamp of VCMs
Whole-cell patch-clamp recordings of currents and membrane potential were carried out using an Axopatch 200B amplifier (Axon Instruments; Molecular Devices, Sunnyvale, CA) as described previously (44). Briefly, to record SK channel currents from rat VCMs, depolarizing voltage steps were applied at 2-s intervals under a voltage clamp at room temperature. The bath solution (pH 7.3) contained 140 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM HEPES, and 5.6 mM glucose. The nominally Ca2+-free pipette solution (pH 7.3) contained 90 mmol/L K-aspartate, 50 mmol/L KCl, 5 mmol/L Mg-ATP, 5 mmol/L NaCl, 1 mmol/L MgCl2, 0.1 mmol/L Tris-GTP, 10 mmol/L HEPES, and 0.1 mmol/L Rhod-2 K+-salt (Thermo Fisher Scientific). Free [Mg2+] was 1.37 mM (Maxchelator (45)). Apamin, a selective SK1, 2, and 3 polypeptide inhibitor (IC50 < 10 nM, Alomone Labs, Jerusalem, Israel) was used to identify ISK. For β-adrenergic stimulation, VCMs were treated with isoproterenol (100 nM; MilliporeSigma, Burlington, MA). Whole-cell capacitance and series resistance were compensated, and a mostly 60–70% series resistance compensation correction was applied. For AP clamp experiments, a linear extrapolated leak conductance was calculated using the recorded current at −86 mV (adjusted offline for liquid junctional potential) and assuming zero current at 0 mV. Leak currents calculated from this conductance were subtracted from the difference currents (total current before apamin − current after apamin). A measured liquid junctional potential of 6 mV was used to adjust voltages in I/V plots and calculation of SK conductance.
Confocal imaging and estimation of [Ca2+]i and [Ca2+]SM
During whole-cell voltage clamp experiments, intracellular Ca2+ imaging was simultaneously performed at room temperature using a Leica SP5 II confocal microscope (Leica Microsystems, Wetzler, Germany) equipped with 63 × 1.4 numerical aperture oil objective in line-scan mode at a rate of 2.5 ms per line, synchronized with the electrophysiological setup. Rhod-2 was excited using a 543-nm line of HeNe laser, and fluorescence emission was collected at 560–660-nm wavelengths. Dynamical Rhod-2 fluorescence signal during Ca2+ transient was converted to [Ca2+]i (46) using the following equation: [Ca2+]i = Kd(F − Fmin)/(Fmax − F), where Kd Rhod-2 = 1.58 μmol/L (47,48) and Fmin = Fmax/15. Fmax was determined by breaking the patch pipette and measuring the Rhod-2 fluorescence as the dye was exposed to the 1 mM Ca2+ bath solution. When possible, F and Fmax were normalized to their respective F0-values before calculation. [Ca2+]SM was estimated based on previous calculations (48,49). Briefly, the [Ca2+]i calculated as above was smoothed by the Savitzky-Golay method, with five points of window. The rise of [Ca2+]SM = [Ca2+]i + γd[Ca2+]i/dt used the smoothed [Ca2+]i and γ = 110 ms. The decay of [Ca2+]SM = [Ca2+]i + Ae(-(t-t0/τ)) (49), where the A = the peak of [Ca2+]i + γd[Ca2+]i/dt and a single exponential decay was fitted to decay of [Ca2+]i.
Rat and human VCM AP and Ca2+ handling model
We used the rat ventricular myocyte AP model of Pandit et al. (50) with adjusted parameters of ICaL and Ito and Iss to better represent our experimental results from AP clamp. The details of the model formulation are given in Appendix S1. Numerical calculation of different equations were performed by the Euler method with an adaptive time step of t = 0.1 ∼ 10 μs for the Markovian chain formulations and the first-order exponential integrator method for Hodgkin-Huxley formulism with a time step of t = 100 μs. The simulations to investigate the role ISK rectification properties in different parameter sets were carried out on a multicore graphic processing unit (Quadro P4000; NVIDIA, Santa Clara, CA) with double precision using the CUDA toolkit (https://developer.nvidia.com/cuda-zone). The data visualization was done using Interactive Data Language (L3Harris Geospatial, Broomfield, CO). For the human ventricular AP model, for which we used the model of O’Hara et al. (51), the parameters were left as in the original except for the integration of our simulated ISK with rectification, as was done for the rat AP model.
Statistics
Statistical analysis of electrophysiological and Ca2+ imaging data was performed using OriginPro 8.1 (OriginLab, Northampton, MA). Data are presented as mean ± standard error (SE). The statistical significance between groups was performed using one-way analysis of variance with Bonferroni post hoc test when appropriate. For all analyses, a p-value of less than 0.05 was considered significant.
Results
Formulation of SK channel kinetics and fitting to the experimental data of overexpressed rSK2
For the development of a mathematical ISK model, we considered ISK to be the product of three independent processes: Ca2+-dependent activation, intrinsic rectification, and Ca2+/Mg2+-dependent rectification. ISK is governed by the Goldman-Hodgkin-Katz current equation:
where pSK is the total permeability (s−1) of SK channels, SKO is the open probability of SK channels, SKIntR is intrinsic rectification by electrostatic permeability reduction, SKFree is the probability that SK channels are not bound to Ca2+/Mg2+, V is membrane potential, F is the Faraday constant, R is the universal gas constant, and T is the temperature. [K]i and [K]o are the internal and external potassium ion concentrations, respectively (see Table S1 for details and units).
The activation scheme of the SK channel is based on previous studies of the single SK channel physiology of rSK2 channels, showing that activation depends on the level of Ca2+ at the SK channel, and the activation scheme was formulated as a Markovian multistate model with four closed states and two independent open states. The first open state, activated by lower levels of Ca2+, has a short duration, and the second open state, activated at higher levels of Ca2+, has a longer duration at higher levels of Ca2+ (Fig. 1 A; (29)). A list of all the corresponding parameters is provided in Table S1.
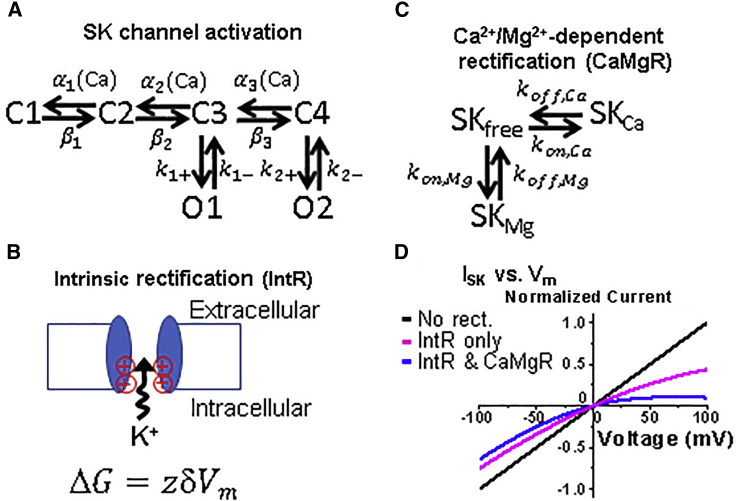
Figure 1.
Six-state SK channel gating scheme and sources of SK channel rectification. (A) SK channel gating scheme modified from Hirschberg et al. (29). (B) Cartoon of the source of intrinsic rectification (IntR) of the SK current showing two subunits of the SK channel. Two positively charged amino acids in each subunit near the mouth of SK channel pore electrostatically interfere with the K+ ion passage. z = charge number; δ = fraction of membrane voltage (Vm) influencing ion; Vm = membrane potential. (C) Scheme to simulate the block of SK channels by Ca2+ and Mg2+. (D) Predicted I/V plot of the SK current with no rectification (black), IntR only (magenta), or combined IntR and Ca2+/Mg2+-dependent rectification (CaMgR) (blue). Equimolar concentrations of K+ inside and outside are given.
The electrostatic influence on SK conductance was modeled based on studies by Li and Aldrich (30) showing that two positively charged amino acids at the mouth of the inner pore modulate K+ conductance of SK channels. In rSK2, R396, K397, and E399 were identified based on comparison with large-conductance, SK channels, which do not show rectification (30). All three amino acids are also conserved in rSK1 and rSK3 by PubMed BLAST analysis (33,34). The positive charges of R396 and K397 are thought to create an energy barrier to K+ at the intracellular mouth of the channel pore that is tempered by negative charge E399. Mutating E399 to positive R399 or neutral N399 leads to increased rectification (30). The change in free energy for ions to pass through the mouth of the pore is dependent on membrane voltage (Vm), the fraction of membrane potential acting at the site of these amino acids (δ), and the valence of ion (z) (Fig. 1 B). Because the intrinsic electrostatic mechanism reduces the single channel conductance (30), we modeled it with the following equation:
Because Ca2+ and Mg2+ bind to the pore of SK channels regardless of open or closed states and share the same binding site in the SK channel (32), we developed a Markov chain model of three states: free SK channel without Ca2+/Mg2+ binding (SKfree), Mg2+-bound SK channel (SKMg), and Ca2+-bound SK channel (SKCa), with respective association and dissociation constants (Fig. 1 C). The equations and parameters of the Ca2+/Mg2+-dependent rectification scheme are provided in Appendix S1 and Table S1. Fig. 1 D shows a predicted I/V plot of ISK during a voltage ramp from −100 to 100 mV without rectification, intrinsic rectification only, or with both intrinsic and Ca2+/Mg2+-dependent rectification.
To establish a parameter set for our model, we reanalyzed previously published data (38) from the overexpression of rSK2 in rat VCMs and optimized the modeling parameters for activation and rectification. The ISK recordings from the overexpressed rSK2 is robust (mean peak current > 19 pA/pF) and does not have to be isolated from other currents, unlike the ISK measured from native cells, which peaks at only 1–2 pA/pF (38). SK2 and SK3 are the dominant isoforms detected in rat VCM plasma membrane fraction (8).
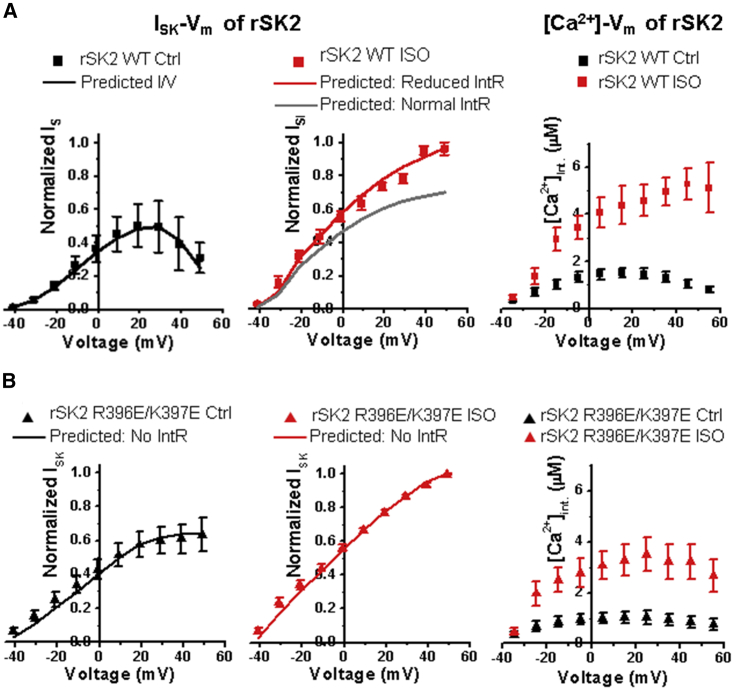
The overexpression system also allowed us to use data from the rSK2 double mutant R396E/K397E that has reduced intrinsic rectification (30). Previous studies reported that SK channels are expressed in close proximity to LTCCs and RyR2s in native cells (38,39), indicating a Ca2+SM concentration that is much higher than the bulk cytosolic Ca2+ concentration. With the overexpression of rSK2, the amplitude of ISK was ∼13 times greater than that of peak ISK in native cells at −10 mV (38). Therefore, we assumed that the number of the SK channels at the surface membrane (non-T-tubule) far outnumbers the existing surface membrane LTCCs in rat VCMs. We estimated there were 4500 native and overexpressed SK channels using a single channel current of 0.5 pA (3,52,53), and the current under ISO conditions at −10 mV (38) vs. 750 native LTCCs or a ratio of 6 using the maximal Ca2+ current and membrane capacitance of cells treated with formamide to remove T-tubules (54) and the unitary LTCC current of 0.2 pA (55), so the majority of the SK channels may not be close to LTCCs and are exposed to cytosolic bulk Ca2+ concentrations rather than the subcellular Ca2+ concentration in close proximity to LTCCs. Therefore, for testing the modeling parameters only, we used the cytosolic bulk Ca2+ recordings plotted in Fig. 2, A and B (right) rather than [Ca2+]SM near LTCCs as in native cells to calculate and fit the peak ISK from overexpressed rSK2. Later, all the modeling of SK channels was performed using the Ca2+SM concentrations. The time to peak of ISK and the Ca2+ transient is slower in the overexpression system (ISK baseline: 29.1 ± 4.6 ms; ISK ISO: 39.0 ± 4.3 ms; Ca2+ baseline: 33.4 ± 1.6 ms; Ca2+ ISO: 28.3 ± 2.7 ms; Fig. S1 B). The mean ISK and Ca2+ time to peak in native rat VCMs is 10.3 ± 1.5 and 18.5 ± 1.0 ms, respectively (38). As reported previously, treatment with isoproterenol (ISO) reduces rectification of ISK (38), similar to the R396E/K397E mutant (30) (Fig. 2, A and B) despite the increase in bulk [Ca2+] (Fig. 2, A and B). Using I/V ISK data and the recorded bulk [Ca2+] (shown in Fig. 2, A and B) from cells overexpressing rSK2 for a parameter set in our model, we were able to generate predicted I/V plots that fit our data for four conditions: rSK2 wild-type baseline, rSK2 wild-type treated with ISO (Fig. 2 A), rSK2 R396E/K397E mutant, and rSK2 R396E/K397E mutant with ISO (Fig. 2 B). Note that for the rSK2 wild-type ISO condition, the parameter set with full rectification properties deviates from the experimental data, particularly at higher Vm, whereas the parameter set with reduced intrinsic rectification can fit the data in the full range of Vm (Fig. 2 A, middle panel). For these simulations, the contribution of the Ca2+/Mg2+-dependent rectification was minimal because the ISK experimentally measured from the overexpressed SK channels is mostly dependent on cytosolic bulk Ca2+ that does not rise sufficiently to block SK channels (IC50 ∼3 μM at 0 mV (56)).
Figure 2.
Fit of ISK I/V plots from rSK2 overexpressed in rat VCMs with I/V plots predicted from the model. (A) Normalized ISK/voltage and bulk [Ca2+]int./voltage (right) for VCMs expressing wild-type rSK2 under control conditions (left) or treated with isoproterenol (ISO; 100 nM, middle). ISK was normalized to the maximal current amplitude for the same cell under ISO conditions. The solid line indicates the I/V plot predicted by the model for the baseline (black) and ISO (red) conditions. ISK recordings from wild-type rSK2 control show a biphasic relation dependent on Vm reaching a peak at 25–30 mV because of the SK channel’s rectification characteristics. ISO stimulation alleviated the inhibition of ISK at higher voltages, showing a continuous increase of ISK with Vm. Note different levels of IntR for the wild-type ISO plot. n = 4–7; N = 4–5; mean ± SE. rSK2 wild-type plots were replotted from raw data published in Hamilton et al. (38). (B) Normalized ISK/voltage and bulk [Ca2+]int/voltage (right) for VCMs expressing mutant rSK2 (R396E-K397E), which has no IntR (Li and Aldrich (30)). The solid lines indicate predicted the I/V plot for the baseline and ISO conditions as in (A). n = 11; N = 5; mean ± SE.
ISK rectification influences an ISK trajectory during a cardiac ventricular AP in native cardiomyocytes under ISO stimulation with Ca2+SM concentration
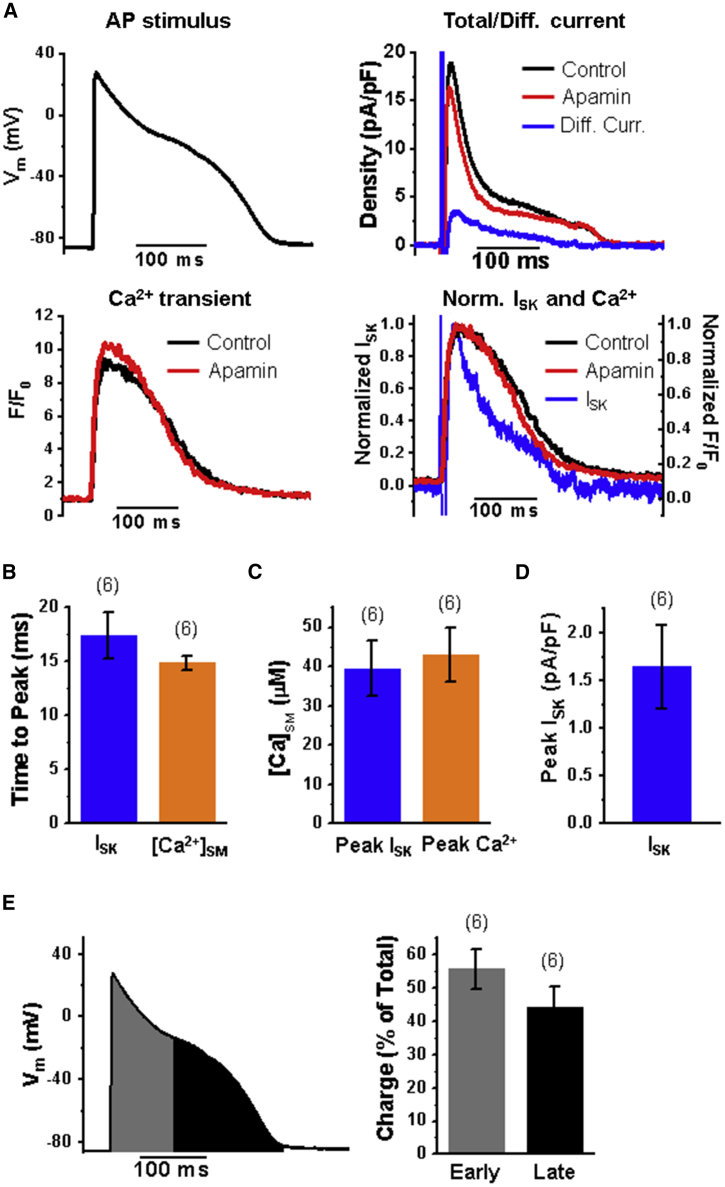
After confirming that our parameter set for the model yielded I/V predictions that fit the data from the overexpression of rSK2, we analyzed native rat VCM AP clamp. The ISK in healthy rat VCMs is barely detectable (38), so we recorded in the presence of ISO for all experiments. Accordingly, for the stimulus AP waveform, we used a typical rat VCM AP recording under ISO conditions, which is characteristically longer than a rat VCM AP under basal conditions (38). Total current was recorded before and after apamin, and the traces were subtracted to generate a difference current (Fig. 3 A). To avoid a potential error from beat-to-beat variation of Ca2+ transients, only the pair of traces (before and after apamin) with similar evoked Ca2+ transients were subtracted (Fig. 3 A, bottom left). The time to peak for ISK (Fig. 3 B) is faster than for the overexpression system, most likely because of the tighter link between SK channels and LTCCs with normal SK channel expression, as reported previously (38,39). The faster rise of [Ca2+]SM is expected to activate SK channels more rapidly and also reach levels sufficiently high to subsequently block them. We estimated [Ca2+]SM changes based on the diffusion rate of [Ca2+]i as described in the Materials and Methods and used for further analysis of ISK. Fig. 3 C shows that the mean [Ca2+]SM at peak ISK and peak [Ca2+] is well above the Ca2+ IC50 for SK channels (32,56), associated with a rapid decrease of ISK during phase 1 of the AP. The peak ISK during AP clamp is in the range of values obtained in rat VCMs with square voltage pulse stimulation (Fig. 3 D; (38)). The calculation of the SK channel charge transferred during the AP reveals that there is a significant amount of ISK during late phases of the AP compared with the early phase of ISK despite significantly lower [Ca2+]i during the late phase of the AP (Fig. 3 E).
Figure 3.
A significant portion of ISK occurs during later stages of AP. (A) Sample AP clamp traces showing voltage stimulus (left), total current before and after apamin with difference current (black minus red trace, blue), Ca2+ transient before and after apamin (bottom left), and normalized ISK and normalized Ca2+ transient (F/F0) showing the correlation of ISK with Ca2+ transient (bottom right). The Ca2+ transients were compared before and after apamin to find recordings with similar evoked Ca2+ transients to properly isolate the apamin-sensitive current. The corresponding current traces were used to find the difference current. All recordings were obtained in the presence of 100 nM ISO. (B) Time to peak plot for peak ISK and peak submembrane Ca2+ concentration ([Ca2+]SM) during AP clamp. (C) [Ca2+]SM at peak ISK and peak Ca2+ transient. (D) Mean peak ISK amplitude during AP clamp. (E) Plot of the stimulus voltage showing the division of the AP stimulus into two regions corresponding to early and late stages (left). Plot of percentage of total SK channel charge in the early and late phases of the AP stimulus (right). n = 6; N = 6; mean ± SE for all plots.
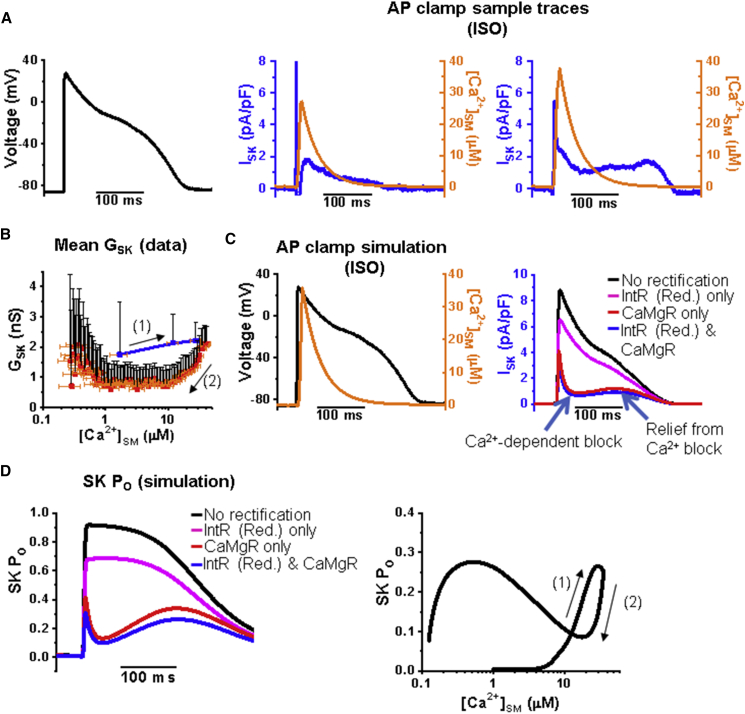
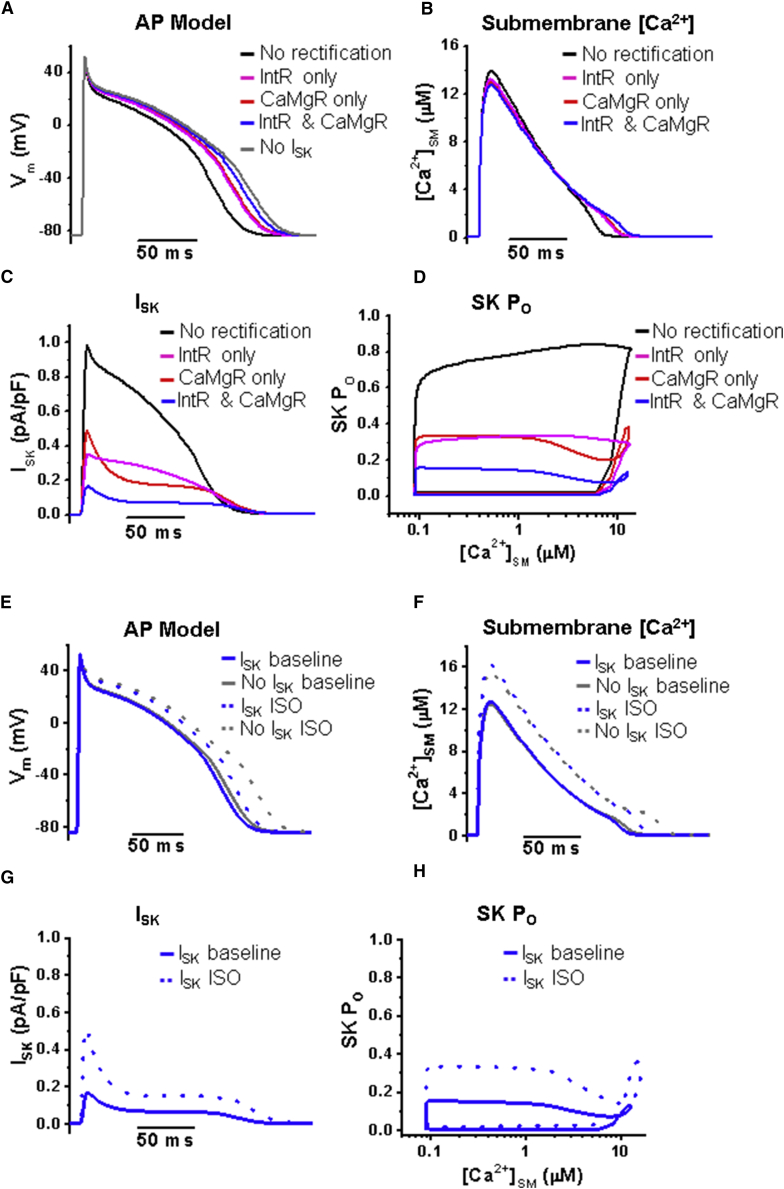
The ISK trajectory during AP clamp typically has an initial peak followed by a decay profile that varies from cell to cell (Fig. 4 A). We used the same AP clamp stimulus for different cells that showed different Ca2+ cycling kinetics and found that the ISK trajectory of decay can be categorized into two prominent groups: a slow decrease of ISK during AP plateau (Fig. 4 A, middle) or a rapid decrease followed by a sustained level of ISK (Fig. 4 A, right). A phase-plane plot of mean SK channel conductance (GSK) versus [Ca2+]SM shows the highest conductance at the first peak during the initial phase of Ca2+ upstroke, but there is a secondary rise of SK channel conductance during the later plateau phase as [Ca2+]SM drops to sub-micromolar concentrations (Fig. 4 B). We incorporated the [Ca2+]SM-values into our model to test the effect of various degrees of intrinsic and Ca2+/Mg2+-dependent rectification on the ISK profile during the same AP stimulus used in the experiments. The right panel of Fig. 4 C shows a comparison of ISK for four different degrees of rectification: 1) no rectification, 2) reduced intrinsic rectification only, 3) normal Ca2+/Mg2+-dependent rectification only, and 4) both reduced intrinsic and Ca2+/Mg2+-dependent rectification. The simulation demonstrates an ISK profile similar to the data, with an initial peak rapidly inhibited by Ca2+ and then a relief from Ca2+-dependent blocking (arrows, Fig. 4 C, right). Ca2+/Mg2+-dependent rectification has a more profound effect on the shape of ISK than intrinsic rectification, which mostly just modifies the amplitude of the first peak (Fig. 4 C, right). The plot of simulated SK channel open probability versus [Ca2+]SM shows a shape like the data (Fig. 4 D, right). Ca2+/Mg2+-dependent rectification dominates the reduction of ISK as seen in the plot of SK channel open probability through time during the AP (Fig. 4 D, left).
Figure 4.
Ca2+/Mg2+ inhibition of SK channels has a dominant effect on SK current profile during AP stimulation. (A) Sample traces from two rat VCMs during AP clamp treated with 100 nM ISO. Given are the AP stimulus waveform (left) and apamin-sensitive SK current density (blue) superimposed on [Ca2+]SM transient (orange) (middle and right). (B) Phase-plane plot of mean SK channel conductance (GSK) versus mean [Ca2+]SM for AP clamp data. The arrows indicate the direction of time through AP. The blue line indicates the phase rising to peak conductance. The SK channel conductance initially increases ((1)) during the AP upstroke and then decreases ((2)) despite high [Ca2+]SM during AP plateau phase, followed by increase again during the early repolarization phase. n = 6; N = 6 mean ± SE. (C) Model simulation of the SK current using the same voltage stimulus as in (A) and [Ca2+]SM from (A) (left trace, orange). The right plot shows SK current density with no IntR or CaMgR (black), with reduced IntR only (ISO condition, magenta), normal CaMgR only (red), and reduced IntR and normal CaMgR (ISO condition, blue). The arrows indicate Ca2+-dependent block and unblock. (D) Simulation plots of SK channel open probability (PO) versus time (left) or [Ca2+]SM (right) comparing different degrees of rectification, suggesting that SK channel block by high [Ca2+] underlies the decrease in conductance during AP plateau followed once again by an increase of SK channel conductance near the repolarization phase of APs. The arrows indicate the direction of time during AP stimulus as in (B).
Intrinsic and Ca2+/Mg2+-dependent rectification of SK channels modulate ISK trajectory during AP
To determine how fully rectified ISK influences APD, we used the Pandit model of rat ventricular APs (50). The effect of rectification on APD is evident in comparing no rectification (black line) with the combined intrinsic and Ca2+/Mg2+-dependent rectification (blue line) stressing the important role of rectification in shaping the AP (Fig. 5 A). Under the combined influence of intrinsic and Ca2+/Mg2+-dependent rectification, the modeled [Ca2+]SM has a lower amplitude and is wider than with no rectification (Fig. 5 B, blue line). Full rectification of SK channels caused an ∼7-fold reduction in ISK (Fig. 5 C, blue line), explaining why ISK is difficult to detect under baseline conditions. Using the rat ventricular AP model, the SK channel open probability dependence on Ca2+ was closest to the data simulation profile in Fig. 4 D when both intrinsic and Ca2+/Mg2+-dependent rectification were incorporated into the model (Fig. 5 D).
Figure 5.
IntR and CaMgR have a significant effect on APD in a model of rat ventricular APs. (A) Simulated baseline AP waveforms using a modified rat ventricular AP model by Pandit et al. (50). The traces compare the AP duration for conditions of no IntR or CaMgR (black) to combined IntR and CaMgR (blue), along with the effect of each individual source of rectification independently, CaMgR (red) or IntR (magenta). (B) Simulated [Ca2+]SM in the rat ventricular AP model (50). (C) Simulated SK currents under conditions in (B). (D) SK channel open probability versus [Ca2+]SM with different combinations of IntR and CaMgR. (E) Simulated AP waveforms a modified rat ventricular AP model by Pandit et al. (50) for baseline as in (A) and ISO conditions in the presence or absence of ISK. (F) Simulated [Ca2+]SM in the rat ventricular AP model (50) for baseline and ISO conditions with and without ISK. (G) Simulated ISK under baseline or ISO conditions. (H) SK channel open probability versus [Ca2+]SM for baseline and ISO conditions.
We previously reported that apamin, a selective SK channel blocker, significantly prolonged APD under β-adrenergic stimulation using ISO in rat hearts (38). We hypothesized that ISO alleviates intrinsic rectification in addition to increasing intracellular Ca2+, which causes changes in ISK to have a greater impact on APDs under β-adrenergic stimulation. The ISO effect on intracellular Ca2+ was modeled by increasing LTCC conductance by 61% (GCa from 0.031 to 0.05), RyR2 sensitivity for luminal Ca2+ by 50% (ka,plus and kb,plus to 1.5 times), Ca2+ sensitivity of SERCA by 25% (reducing Km-value from 0.000168 to 0.000126), and steady-state K+ current by 40% (Gss from 0.007 to 0.0098) in the Pandit rat AP model (50). This modification of parameters resulted in APD prolongation comparable with our previous experimental results from rat hearts (38). Fig. 5, E and F shows AP prolongation and increase of Ca2+SM under ISO conditions. Under baseline condition, APD was slightly prolonged by apamin (blocking SK channels by setting pSK = 0), whereas APD prolongation by apamin is significantly larger under ISO conditions, in line with our previous experimental data (38). Fig. 5, E and G shows that an increase of ISK during AP peak and plateau through alleviated rectification under ISO conditions, resulting in greater APD shortening. Note that the SK channel PO dependence on Ca2+ under ISO conditions (Fig. 5 H) is similar to the baseline condition with Ca2+/Mg2+-dependent rectification only (Fig. 5 D, red line).
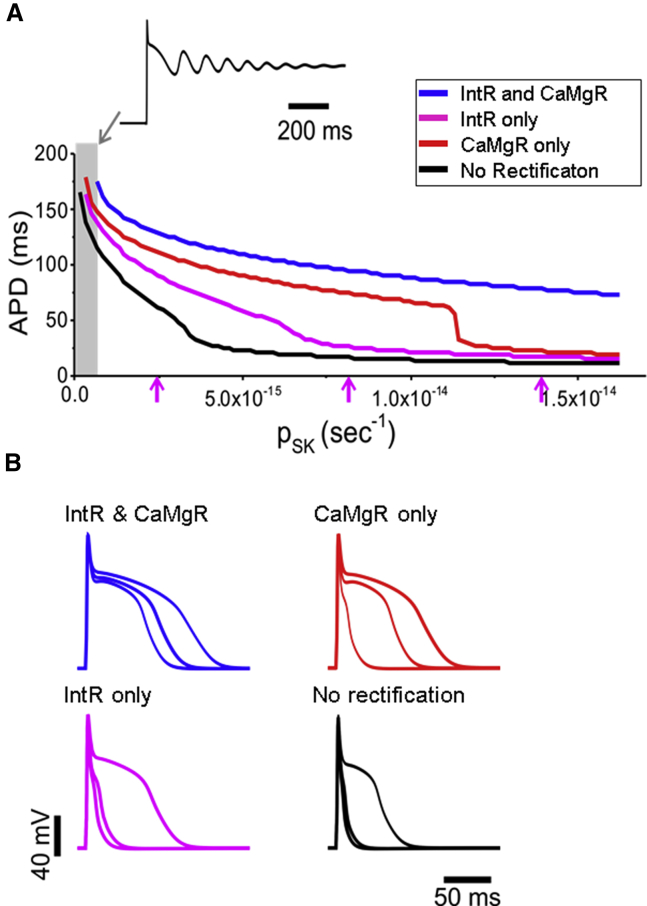
We next investigated the contribution of each type of rectification on APD over a range of total SK channel permeability (pSK). Fig. 6 A shows APD at 10% of peak amplitude (APD90) plotted for different pSK. Under conditions of low or zero psk (gray rectangle), the APD90 is prolonged, leading to early afterdepolarizations (EADs) and repolarization failure (see arrow and inset trace, Fig. 6 A). With no rectification, APD90 shortens rapidly because of a full contribution of ISK. Introducing intrinsic rectification alone prevented APD shortening over a medium range of pSK, followed by steeper APD shortening above ∼6 × 10−15 s−1 (magenta line, Fig. 6 A). Ca2+/Mg2+-dependent rectification alone reduced APD shortening by ISK over a wider range of pSK, but abrupt APD shortening occurs at pSK above ∼1.2 × 10−14 s−1 (red line, Fig. 6 A). The incorporation of both intrinsic and Ca2+/Mg2+-dependent rectification produced a gradual APD shortening over the widest range of pSK (blue line, Fig. 6 A). Under conditions of both types of rectification, the shape of the modeled AP is most consistent (Fig. 6 B, top left) in traces for the pSK indicated by arrows in Fig. 6 A. Note the extremely short APD with no rectification at all (Fig. 6 B, bottom right).
Figure 6.
Both IntR and CaMgR significantly influence the efficacy of APD shortening across a wide range of total SK channel permeabilities. (A) Plot of simulated APD at 10% of peak amplitude versus total SK channel permeability (pSK) for different conditions of IntR or CaMgR. No rectification leads to a steep APD shortening with an increase of pSK (black). IntR only has some improvement (magenta), and CaMgR only (red) maintains a more gradual shortening of APD until a sudden drop. Both IntR and CaMgR together allow a gradual APD shortening over the full range of pSK (blue). With little or no SK conductance (gray rectangle), the AP does not repolarize, and EAD-type events occur (arrow and inset trace). (B) Simulated AP traces for different conditions as in (A). For each condition, the three AP traces are simulated at the permeabilities indicated by the magenta arrows in (A).
Intrinsic and Ca2+/Mg2+-dependent rectification influences different phases of the AP
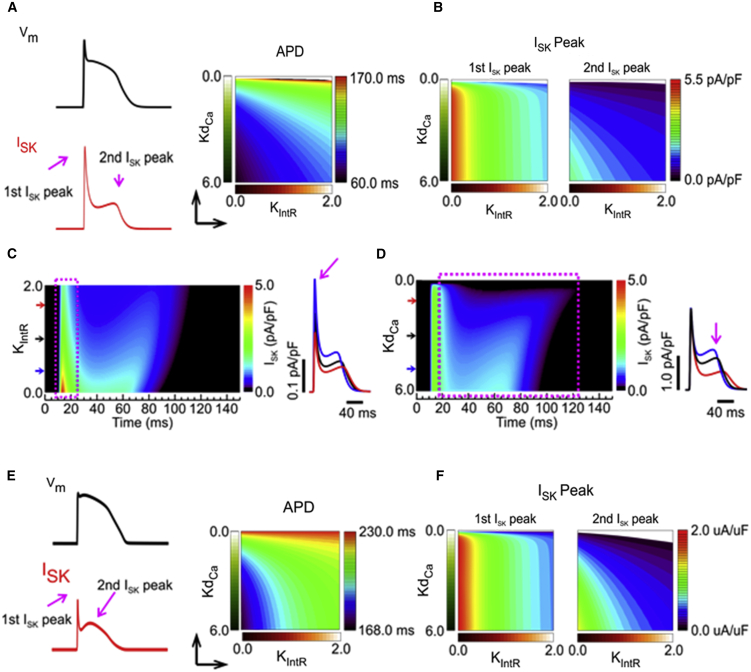
We further investigated the effects of intrinsic and Ca2+/Mg2+-dependent rectification of ISK on APD in the two-dimensional (2D) parameter space of KIntR and KdCa for intrinsic and Ca2+/Mg2+-dependent rectification, respectively. Plotting a 2D color map of APD resulting from increasing levels of intrinsic and Ca2+/Mg2+-dependent rectification shows, overall, the shallowest gradient when both types of rectification are increasing together (Fig. 7 A). Plotting ISK amplitude in a similar way for the first and second ISK peaks shows intrinsic rectification has a dominant effect on the first ISK peak because the gradient is strongest along the x axis, whereas Ca2+/Mg2+-dependent rectification has a greater influence on the second ISK peak (Fig. 7 B). Looking at the ISK amplitude through time for increasing intrinsic rectification (Fig. 7 C), we see the steepest current gradient at the time of the first ISK peak (dashed rectangle), and it transitions to a shallow gradient at later stages of the AP. In contrast, increasing Ca2+/Mg2+-dependent rectification has a limited effect on ISK amplitude at the first peak but generates a steeper current gradient for the second peak (Fig. 7 D, dashed rectangle), suggesting that Ca2+/Mg2+-dependent rectification mainly influences the impact of ISK on the later phases of the AP.
Figure 7.
IntR and CaMgR have distinct contributions to AP shape. (A) Illustration of membrane voltage (Vm) and ISK for reference showing the first and second peaks of ISK. Shown is a two-dimensional (2D) color map of APD showing the effect of varying IntR (KIntR) and CaMgR (KdCa) on APD in the Pandit et al. AP model (50). IntR and CaMgR are shown increasing in the direction of the arrows along the x and y axes, respectively. (B) 2D color map of the amplitude of the first and second peaks of ISK with increasing levels of KdCa and KIntR as in (A). The first peak of ISK is mostly dependent on an x axis KIntR (left). The second peak of ISK is influenced by both KIntR and KdCa. (C) 2D color map of ISK amplitude (right y axis) varying KIntR (left y axis) through time during a simulated rat ventricular AP. The first ISK peak is indicated by a dashed rectangle. Traces at the right are full ISK traces at the indicated KIntR-values (arrows). The first peak is indicated by a purple arrow. (D) 2D color map of ISK amplitude (right y axis) with varying KdCa (left y axis) through time during a simulated rat ventricular AP. Plateau phase and second ISK peak are indicated by a dashed rectangle. Traces at the right are full ISK traces at the indicated KdCa-values (arrows). The second peak is indicated by the purple arrow. (E) Illustration of membrane voltage (Vm) and ISK from the O’Hara et al. human ventricular AP model (51) incorporating ISK with rectification. Given is a 2D color map of modeled human APD showing the effect of varying IntR (KIntR) and CaMgR (KdCa) on APD as in (A). (F) 2D color map of the amplitude of the first and second peaks of modeled human ISK with increasing levels of KdCa and KIntR as in (B). The first peak of ISK is mostly dependent on the x axis KIntR (left). The second peak of ISK is influenced by both KIntR and KdCa.
It is well recognized that the repolarization of murine cardiac AP is mainly caused by transient outward K+ current (Ito), whereas that of large animals, including humans, is composed of slowly activating inward rectifier K+ currents (IKs) and rapidly activating inward rectifier K+ currents (IKr). To investigate the impact of ISK on human cardiac APs, we integrated our model of SK current into the O’Hara et al. model of human ventricular AP (51). Fig. 7 E shows an example of a simulated human AP and ISK traces. We see an ISK trajectory similar to that of Pandit’s rat AP model with the first and second peaks of ISK. The impact of intrinsic rectification of ISK on APD is much greater in the human ventricular AP (Fig. 7 E, steep APD changes in x direction compared with the rat ventricular AP in Fig. 7 A). This is mainly because the human ventricular AP has an elevated plateau Vm so the second peak of ISK becomes larger when the intrinsic rectification is reduced (Fig. 7 F, the map of the second peak), causing an acceleration of repolarization. Ca2+/Mg2+-dependent rectification characteristics affect APD (Fig. 7 E, y direction) in similar way as seen in the rat model (Fig. 7 A).
Discussion
SK channels, because of their activation by intracellular Ca2+, have received recent attention as possible targets to prevent cardiac arrhythmias (17,20,57). However, the effect of SK channels on cardiac ventricular repolarization is complex potentially because of their unique inward rectification properties. In this study, we investigated how the SK channel rectification influences ventricular repolarization and APDs using an AP clamp of native VCMs under ISO conditions and computer simulation. We found that modulation of ISK rectification kinetics determines its temporal impact on repolarization to modulate APDs over a wide range of AP phases (phase 1–3).
SK channels are expressed in various cell types and critically involved in regulation of many physiological functions through Ca2+-dependent repolarization and afterhyperpolarization of membrane potentials, such as vasoregulation (58) and neuronal excitability (28), and also protection from neurodegeneration (59). Although SK channel’s inward rectifying characteristics have been reported in rSK2 overexpression studies (30, 31, 32,38), rat hippocampal neurons (60), and native rat VCMs under physiological conditions (38), other studies have shown less prominent or no rectification of the SK current in cardiac myocytes (19,42,61). These studies kept intracellular [Ca2+] fixed at levels that would not block SK channels, so rectification would be limited to intrinsic rectification and block by Mg2+. The studies were also done in heart disease models in which phosphorylation of SK channels could be reducing rectification as reported previously (38). Importantly, intracellular Ca2+ cycling in cardiac myocytes yields a broad dynamic range of [Ca2+], making it especially important to allow intracellular Ca2+ to change over its physiological range as we have done in this study. In addition, because VCM SK channels are in close proximity to LTCCs, SK channels can experience fluctuations of Ca2+ ranging from sub-micromolar to 10 s of micromolar Ca2+ (40,41,62). This concentration of Ca2+ can sufficiently block the channel and limit its contribution to repolarization during the cardiac cycle (32,56). Our AP clamp simulations have shown that Ca2+/Mg2+-dependent rectification has a dominant effect on the trajectory of ISK (Fig. 4 C). This ISK profile is not limited to VCMs because a recent study of ISK in human atrial myocytes under AP clamp demonstrates complex current dynamics with two peaks (63), similar to our data in rat VCMs (Fig. 4 A), further confirming biphasic regulation of SK channels by [Ca2+] during the Ca2+ transient. To determine how inward rectification of SK currents influences the human ventricular AP, we introduced our simulated SK current into the O’Hara et al. AP model (51). In the human model, the ISK trajectory during the AP is similar to our results from rat with some key differences. The human APD is more dependent on intrinsic rectification than in the rat model, most likely because of the high plateau of the AP (Fig. 7, E and F). Also, higher levels of Ca2+SM in the human model (51) cause a more rapid decay of the SK current after the initial peak.
In rat VCMs, the peak of ISK precedes the peak of Ca2+ transients during voltage clamp steps, suggesting that ISK does not merely follow Ca2+ levels but is inhibited while Ca2+ continues to rise, and its current versus voltage relationship shows diminished amplitude at a high Vm indicative of inward rectification (38). PKA activation by the β-adrenergic agonist, ISO, reduces rectification, causing an increase in channel conductance at high Vm (Fig. 2) (38). Importantly, the phosphorylation of SK channels in VCMs can reduce rectification in pathological conditions such as hypertrophy, increasing ISK contribution to APD. Our modeling results show that the two independent rectification mechanisms contribute to the ISK trajectory differently. Intrinsic rectification has a dominant influence on the first ISK peak amplitude during early repolarization of the AP (phase 1) as well as ISK during the plateau phase (phase 2) (Fig. 7, B and C). This is mainly because the intrinsic rectification is predominantly voltage dependent, so it would dominate at positive voltages. Because Ca2+/Mg2+-dependent rectification is dependent on voltage and Ca2+ concentration (31,32), which is slower to rise than voltage, it limits ISK during the plateau phase (phase 2) until Ca2+SM recovers (Figs. 4, C and D and 7 D). These data strongly indicate that the modulation of SK channel rectification can significantly change its impact on cardiac repolarization and alter arrhythmogenesis, which may explain the puzzling fact that SK channels have very limited roles in shortening APD in normal condition, but their roles become significant in pathological conditions (5,16,19,20,42,43). It is possible that, in heart disease, reduced intrinsic rectification because of PKA phosphorylation of SK channels would increase the initial peak of ISK to counterbalance the reduced repolarization reserve and prevent excessive APD prolongation. In addition, reduced Ca2+ transient amplitudes in heart failure would decrease Ca2+-dependent blocking of ISK and decrease the possibility of LTCC reactivation in phases 2 and 3 of the AP, leading to a lower probability of EAD generation.
Despite several studies indicating a role of SK channels in cardiac arrhythmias, there is still no clear consensus on whether SK channels are anti- or proarrhythmic. In VCMs, ISK or its effects on APs are barely detectable in several animal models and humans and only become prominent in disease or with β-adrenergic stimulation (5,19,20,25,38,42,43,64). Under pathological conditions of prolonged APD or dysregulated Ca2+, there are several studies supporting an antiarrhythmic role of SK channels. For instance, enhancing ISK is protective in human and dog heart failure VCMs because apamin blocking in these cells promotes proarrhythmic behavior in VCMs (5). There is an upregulation of apamin-sensitive potassium currents in patients with heart failure (42). SK channels are also expressed in mitochondria to modulate mitochondrial Vm and ROS production and enhancing mitochondrial SK channels in a rat hypertrophic model of heart failure reduced Ca2+-dependent arrhythmias (8). In addition, blocking SK channels with ondansetron increased EADs and vulnerability to ventricular fibrillation in the rabbit model of pacing-induced heart failure (65). In the same model, inhibiting SK channels with apamin in ventricles was shown to generate EADs and polymorphic ventricular tachycardia because of secondary rises in Ca2+ (18). These results demonstrate that the presence of ISK is protective in chronic long QT conditions as a compensatory mechanism to counterbalance prolonged QT interval or proarrhythmic Ca2+-dependent depolarizing currents. However, in pathological conditions with shortened APD, SK channels may exacerbate APD shortening and can be proarrhythmic. During acute induction of arrhythmias, blocking SK channels has been shown to be antiarrhythmic. Blocking SK currents with apamin or UCL-1684 reduced spontaneous sustained ventricular tachycardia in rats with acute myocardial infarction (22). Apamin reduced postshock APD shortening-induced recurrent spontaneous ventricular fibrillation in rabbits (19) and reduced pacing-induced ventricular fibrillation under ISO conditions in female rabbit ventricles (6). Finally, pacing-induced ventricular fibrillation in guinea pigs was partially alleviated by negative modulation with NS8395 or blockade with ICA (21).
Our results indicate that SK channels’ rectification properties may contribute to their multifaceted roles in arrhythmias. Fig. 6 shows that SK channels can suppress EADs and repolarization failures (Fig. 6 A, inset), suggesting that the presence of SK channels can be protective in many diseases associated with reduced repolarization reserve and prolonged APD, including long QT syndrome and heart failure in line with the studies just mentioned. The protective effect of SK channels through normalization of prolonged APD and suppression of triggered activity was demonstrated experimentally in rabbit ventricles (18). However, our results also show that increased SK channel conductance may cause excessive APD shortening, especially when both types of rectification are removed, which can increase risks for reentry formation. Enhancing ISK in rabbit hearts with the SK enhancer CyPPA shortens ventricular APD and leads to phase 2 reentry (66), in line with the prediction from excessive APD shortening by ISK with reduced rectification as in our simulation. In addition, transgenic mice overexpressing SK3 had an increased rate of sudden cardiac death associated with nearly a threefold greater ventricular APD80 dispersion in the homozygous transgenic mice (25). Interestingly, our simulation predicts that when intrinsic rectification is absent, a sudden APD shortening may occur in a small range of SK conductances (Fig. 6, red line), which may increase risks of APD dispersion. Modulation of the inward rectification properties of SK channels is, therefore, an important factor in determining whether ISK is anti- or proarrhythmic in normal and pathological conditions.
In addition to inward rectification of SK channels, the modulation of SK channel sensitivity to activation by Ca2+ is another important factor that may alter SK channel kinetics and its contribution to AP repolarization, as shown in a modeling study (67). Experimental results have shown that protein kinase CK2 interacts with SK channels and phosphorylates SK-bound calmodulin to reduce the Ca2+ sensitivity of SK channels (68,69). In our recordings of rat cardiomyocytes, we do not see a shift in Ca2+ sensitivity with the application of ISO (38); however, it is possible certain pathological conditions do result in a shift in Ca2+ sensitivity. Further studies are needed to delineate the effect of modulating rectification versus Ca2+ sensitivity in arrhythmogenesis.
This study has several limitations that should be noted. First, ISK was estimated by measuring an apamin-sensitive current between multiple APs under ISO and ISK here may have been underestimated because of the ISO rundown effect during the recording. Second, the [Ca2+]SM was estimated based on the parameter sets from data from the rabbit VCMs (70,71) because of lack of the parameter sets from the rat VCMs. Third, our SK channel model parameters were optimized to fit the overexpressed SK2 data with assumption that the overexpressed SK2 senses cytosolic Ca2+ concentration, and further studies are needed to better understand a relative contribution of SK1, SK2, and SK3 in SK currents in native myocytes. Fourth, the computer model of rat AP used in this study (50) has three Ca2+ compartments, which is suitable for studying SK channels and AP dynamics, but this model cannot replicate spontaneous Ca2+ release and Ca2+ wave propagation, which is often observed in pathological conditions. Further studies using a more realistic stochastic Ca2+ release model may reveal a detailed relationship during pathophysiological conditions, such as early or delayed afterdepolarizations.
In conclusion, the evidence in this study points to the requirement for a precisely tuned level of SK channel conductance via rectification properties to prevent cardiac arrhythmias. More studies will need to be done to determine which type of rectification or sites of phosphorylation make the best targets to maintain this tuning under pathological conditions. Dynamic voltage clamp experiments will also be part of future plans to further validate the importance of SK channel rectification by showing the AP dynamics can be modulated with model-based SK currents.
Author Contributions
Experimental design and interpretation were performed by P.B., T.Y.K., D.T., and B.-R.C. AP clamp experiments and analysis were performed by P.B. and D.T. rSK2 overexpression experiments and analysis were performed by I.P., D.T., P.B., S.H., R.T., and K.R. Modeling was performed by T.Y.K. and B.-R.C. Writing and editing the manuscript were performed by P.B., B.-R.C., T.Y.K., D.T., G.K., S.H., R.T., and K.R.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute, National Institutes of Health grants R01HL121796 and R01HL142588 to D.T. and R01HL139467 and R01HL110791 to G.K.
Editor: Andrew Plested
Footnotes
Peter Bronk and Tae Yun Kim contributed equally to this work.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.06.022.
Supporting Material
References
- 1.Xia X.M., Fakler B., Adelman J.P. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y., Tuteja D., Chiamvimonvat N. Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J. Biol. Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 3.Köhler M., Hirschberg B., Adelman J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 4.Stocker M., Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell. Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla I.M., Long V.P., III, Carnes C.A. Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PLoS One. 2014;9:e108824. doi: 10.1371/journal.pone.0108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M., Yin D., Chen P.S. Sex-specific activation of SK current by isoproterenol facilitates action potential triangulation and arrhythmogenesis in rabbit ventricles. J. Physiol. 2018;596:4299–4322. doi: 10.1113/JP275681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haugaard M.M., Hesselkilde E.Z., Jespersen T. Pharmacologic inhibition of small-conductance calcium-activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart Rhythm. 2015;12:825–835. doi: 10.1016/j.hrthm.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Kim T.Y., Terentyeva R., Terentyev D. SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR. Cardiovasc. Res. 2017;113:343–353. doi: 10.1093/cvr/cvx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skibsbye L., Poulet C., Jespersen T. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc. Res. 2014;103:156–167. doi: 10.1093/cvr/cvu121. [DOI] [PubMed] [Google Scholar]
- 10.Tuteja D., Rafizadeh S., Chiamvimonvat N. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ. Res. 2010;107:851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuteja D., Xu D., Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 12.Yang D., Wang T., Ma A. Apamin-sensitive K+ current upregulation in volume-overload heart failure is associated with the decreased interaction of CK2 with SK2. J. Membr. Biol. 2015;248:1181–1189. doi: 10.1007/s00232-015-9839-0. [DOI] [PubMed] [Google Scholar]
- 13.Qi X.Y., Diness J.G., Nattel S. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129:430–440. doi: 10.1161/CIRCULATIONAHA.113.003019. [DOI] [PubMed] [Google Scholar]
- 14.Chang P.C., Chen P.S. SK channels and ventricular arrhythmias in heart failure. Trends Cardiovasc. Med. 2015;25:508–514. doi: 10.1016/j.tcm.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diness J.G., Bentzen B.H., Grunnet M. Role of calcium-activated potassium channels in atrial fibrillation pathophysiology and therapy. J. Cardiovasc. Pharmacol. 2015;66:441–448. doi: 10.1097/FJC.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahida S. Expanding role of SK channels in cardiac electrophysiology. Heart Rhythm. 2014;11:1233–1238. doi: 10.1016/j.hrthm.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X.D., Lieu D.K., Chiamvimonvat N. Small-conductance Ca2+ -activated K+ channels and cardiac arrhythmias. Heart Rhythm. 2015;12:1845–1851. doi: 10.1016/j.hrthm.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang P.C., Hsieh Y.C., Chen P.S. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm. 2013;10:1516–1524. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua S.K., Chang P.C., Chen P.S. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ. Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements R.T., Terentyev D., Sellke F.W. Ca(2+)-activated K(+) channels as therapeutic targets for myocardial and vascular protection. Circ. J. 2015;79:455–462. doi: 10.1253/circj.CJ-15-0015. [DOI] [PubMed] [Google Scholar]
- 21.Diness J.G., Kirchhoff J.E., Grunnet M. Antiarrhythmic effect of either negative modulation or blockade of small conductance Ca2+-activated K+ channels on ventricular fibrillation in Guinea pig langendorff-perfused heart. J. Cardiovasc. Pharmacol. 2015;66:294–299. doi: 10.1097/FJC.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 22.Gui L., Bao Z., Chen Q.H. Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K+ channels. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H118–H130. doi: 10.1152/ajpheart.00820.2011. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy M., Bers D.M., Sato D. Dynamical effects of calcium-sensitive potassium currents on voltage and calcium alternans. J. Physiol. 2017;595:2285–2297. doi: 10.1113/JP273626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N., Timofeyev V., Chiamvimonvat N. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J. Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahida S., Mills R.W., Ellinor P.T. Overexpression of KCNN3 results in sudden cardiac death. Cardiovasc. Res. 2014;101:326–334. doi: 10.1093/cvr/cvt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skibsbye L., Bengaard A.K., Jespersen T. Inhibition of small conductance calcium-activated potassium (SK) channels prevents arrhythmias in rat atria during β-Adrenergic and muscarinic receptor activation. Front. Physiol. 2018;9:510. doi: 10.3389/fphys.2018.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X.D., Timofeyev V., Chiamvimonvat N. Critical roles of a small conductance Ca2+-activated K+ channel (SK3) in the repolarization process of atrial myocytes. Cardiovasc. Res. 2014;101:317–325. doi: 10.1093/cvr/cvt262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adelman J.P., Maylie J., Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu. Rev. Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 29.Hirschberg B., Maylie J., Marrion N.V. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J. Gen. Physiol. 1998;111:565–581. doi: 10.1085/jgp.111.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W., Aldrich R.W. Electrostatic influences of charged inner pore residues on the conductance and gating of small conductance Ca2+ activated K+ channels. Proc. Natl. Acad. Sci. USA. 2011;108:5946–5953. doi: 10.1073/pnas.1103090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soh H., Park C.S. Inwardly rectifying current-voltage relationship of small-conductance Ca2+-activated K+ channels rendered by intracellular divalent cation blockade. Biophys. J. 2001;80:2207–2215. doi: 10.1016/S0006-3495(01)76193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soh H., Park C.S. Localization of divalent cation-binding site in the pore of a small conductance Ca(2+)-activated K(+) channel and its role in determining current-voltage relationship. Biophys. J. 2002;83:2528–2538. doi: 10.1016/S0006-3495(02)75264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altschul S.F., Gish W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 34.Gish W., States D.J. Identification of protein coding regions by database similarity search. Nat. Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 35.Higham J., Sahu G., Marrion N.V. Preferred formation of heteromeric channels between coexpressed SK1 and IKCa channel subunits provides a unique pharmacological profile of Ca2+-activated potassium channels. Mol. Pharmacol. 2019;96:115–126. doi: 10.1124/mol.118.115634. [DOI] [PubMed] [Google Scholar]
- 36.Hancock J.M., Weatherall K.L., Marrion N.V. Selective activation of heteromeric SK channels contributes to action potential repolarization in mouse atrial myocytes. Heart Rhythm. 2015;12:1003–1015. doi: 10.1016/j.hrthm.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Hougaard C., Eriksen B.L., Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2007;151:655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton S., Polina I., Terentyev D. PKA phosphorylation underlies functional recruitment of sarcolemmal SK2 channels in ventricular myocytes from hypertrophic hearts. J. Physiol. 2019 doi: 10.1113/JP277618. Published online February 16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X.D., Coulibaly Z.A., Chiamvimonvat N. Coupling of SK channels, L-type Ca2+ channels, and ryanodine receptors in cardiomyocytes. Sci. Rep. 2018;8:4670. doi: 10.1038/s41598-018-22843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannell M.B., Kong C.H., Laver D.R. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys. J. 2013;104:2149–2159. doi: 10.1016/j.bpj.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shannon T.R., Wang F., Bers D.M. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys. J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang P.C., Turker I., Ai T. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. J. Am. Heart Assoc. 2013;2:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni Y., Wang T., Ma A. Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Mol. Cell. Biochem. 2013;384:95–103. doi: 10.1007/s11010-013-1785-5. [DOI] [PubMed] [Google Scholar]
- 44.Terentyev D., Rochira J.A., Li W. Sarcoplasmic reticulum Ca2+ release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H738–H746. doi: 10.1152/ajpheart.00621.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bers D.M., Patton C.W., Nuccitelli R. A practical guide to the preparation of Ca(2+) buffers. Methods Cell Biol. 2010;99:1–26. doi: 10.1016/B978-0-12-374841-6.00001-3. [DOI] [PubMed] [Google Scholar]
- 46.Cheng H., Lederer W.J., Cannell M.B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 47.Escobar A.L., Velez P., Vergara J.L. Kinetic properties of DM-nitrophen and calcium indicators: rapid transient response to flash photolysis. Pflugers Arch. 1997;434:615–631. doi: 10.1007/s004240050444. [DOI] [PubMed] [Google Scholar]
- 48.Trafford A.W., Díaz M.E., Eisner D.A. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J. Physiol. 1995;488:577–586. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber C.R., Piacentino V., III, Bers D.M. Na(+)-Ca(2+) exchange current and submembrane [Ca(2+)] during the cardiac action potential. Circ. Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 50.Pandit S.V., Clark R.B., Demir S.S. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys. J. 2001;81:3029–3051. doi: 10.1016/S0006-3495(01)75943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Hara T., Virág L., Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput. Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blatz A.L., Magleby K.L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- 53.Ohmori H., Yoshida S., Hagiwara S. Single K+ channel currents of anomalous rectification in cultured rat myotubes. Proc. Natl. Acad. Sci. USA. 1981;78:4960–4964. doi: 10.1073/pnas.78.8.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai M., Hussain M., Orchard C.H. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am. J. Physiol. 1999;277:H603–H609. doi: 10.1152/ajpheart.1999.277.2.H603. [DOI] [PubMed] [Google Scholar]
- 55.Guia A., Stern M.D., Josephson I.R. Ion concentration-dependence of rat cardiac unitary L-type calcium channel conductance. Biophys. J. 2001;80:2742–2750. doi: 10.1016/S0006-3495(01)76242-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ledoux J., Bonev A.D., Nelson M.T. Ca2+-activated K+ channels in murine endothelial cells: block by intracellular calcium and magnesium. J. Gen. Physiol. 2008;131:125–135. doi: 10.1085/jgp.200709875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravens U. Atrial-selective K+ channel blockers: potential antiarrhythmic drugs in atrial fibrillation? Can. J. Physiol. Pharmacol. 2017;95:1313–1318. doi: 10.1139/cjpp-2017-0024. [DOI] [PubMed] [Google Scholar]
- 58.Félétou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honrath B., Krabbendam I.E., Dolga A.M. Small conductance Ca2+-activated K+ channels in the plasma membrane, mitochondria and the ER: pharmacology and implications in neuronal diseases. Neurochem. Int. 2017;109:13–23. doi: 10.1016/j.neuint.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Lancaster B., Nicoll R.A., Perkel D.J. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J. Neurosci. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizukami K., Yokoshiki H., Tsutsui H. Small-conductance Ca2+-activated K+ current is upregulated via the phosphorylation of CaMKII in cardiac hypertrophy from spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1066–H1074. doi: 10.1152/ajpheart.00825.2014. [DOI] [PubMed] [Google Scholar]
- 62.Acsai K., Antoons G., Sipido K.R. Microdomain [Ca2+] near ryanodine receptors as reported by L-type Ca2+ and Na+/Ca2+ exchange currents. J. Physiol. 2011;589:2569–2583. doi: 10.1113/jphysiol.2010.202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shamsaldeen Y.A., Culliford L., Marrion N.V. Role of SK channel activation in determining the action potential configuration in freshly isolated human atrial myocytes from the SKArF study. Biochem. Biophys. Res. Commun. 2019;512:684–690. doi: 10.1016/j.bbrc.2019.03.074. [DOI] [PubMed] [Google Scholar]
- 64.Lee Y.S., Chang P.C., Chen P.S. Apamin-sensitive calcium-activated potassium currents in rabbit ventricles with chronic myocardial infarction. J. Cardiovasc. Electrophysiol. 2013;24:1144–1153. doi: 10.1111/jce.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin D., Yang N., Chen P.S. Effects of ondansetron on apamin-sensitive small conductance calcium-activated potassium currents in pacing-induced failing rabbit hearts. Heart Rhythm. 2020;17:332–340. doi: 10.1016/j.hrthm.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen M., Xu D.Z., Chen P.S. Concomitant SK current activation and sodium current inhibition cause J wave syndrome. JCI Insight. 2018;3:122329. doi: 10.1172/jci.insight.122329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Landaw J., Zhang Z., Qu Z. Small-conductance Ca2+-activated K+ channels promote J-wave syndrome and phase 2 reentry. Heart Rhythm. 2020;S1547-5271:30355–30356. doi: 10.1016/j.hrthm.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen D., Fakler B., Adelman J.P. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J. Neurosci. 2007;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bildl W., Strassmaier T., Fakler B. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 70.Shiferaw Y., Karma A. Turing instability mediated by voltage and calcium diffusion in paced cardiac cells. Proc. Natl. Acad. Sci. USA. 2006;103:5670–5675. doi: 10.1073/pnas.0511061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiferaw Y., Watanabe M.A., Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys. J. 2003;85:3666–3686. doi: 10.1016/S0006-3495(03)74784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.