Abstract
In myotonic dystrophy type 1 (DM1), the CUG expansion (CUGexp) in the 3′ untranslated region of the dystrophia myotonica protein kinase messenger ribonucleic acid affects the homeostasis of ribonucleic acid-binding proteins, causing the multiple symptoms of DM1. We have previously reported that Staufen1 is increased in skeletal muscles from DM1 mice and patients and that sustained Staufen1 expression in mature mouse muscle causes a progressive myopathy. Here, we hypothesized that the elevated levels of Staufen1 contributes to the myopathic features of the disease. Interestingly, the classic DM1 mouse model human skeletal actin long repeat (HSALR) lacks overt atrophy while expressing CUGexp transcripts and elevated levels of endogenous Staufen1, suggesting a lower sensitivity to atrophic signaling in this model. We report that further overexpression of Staufen1 in the DM1 mouse model HSALR causes a myopathy via inhibition of protein kinase B signaling through an increase in phosphatase tensin homolog, leading to the expression of atrogenes. Interestingly, we also show that Staufen1 regulates the expression of muscleblind-like splicing regulator 1 and CUG-binding protein elav-like family member 1 in wild-type and DM1 skeletal muscle. Together, data obtained from these new DM1 mouse models provide evidence for the role of Staufen1 as an atrophy-associated gene that impacts progressive muscle wasting in DM1. Accordingly, our findings highlight the potential of Staufen1 as a therapeutic target and biomarker.
Introduction
Myotonic dystrophy type 1 (DM1) is the most common muscular dystrophy observed in adults. DM1 is caused by an expansion of Cytosine Thymine Guanine (CTG) repeats in the 3′ untranslated region of the dystrophia myotonica protein kinase (DMPK) gene (1–3), which form Cytosine Uracil Guanine expansion (CUGexp) in messenger ribonucleic acids (mRNAs) that aggregate in the nucleus as ribonucleic acid (RNA) foci (4, 5). As a result, various RNA-binding proteins (RBPs) are sequestered and/or misregulated causing a toxic cellular effect, which directly affects the expression, metabolism and splicing of target mRNAs (6). DM1 is a complex multi-systemic disorder affecting skeletal and cardiac muscles as well as the neurological, endocrine, gastrointestinal and reproductive systems.
The most studied RBPs in DM1 are muscleblind-like splicing regulator 1 (MBNL1) and CUG-binding protein elav-like family member 1 (CELF1; also referred to as CUGBP1) (7). Their misregulation/mislocalization in DM1 cells are known to cause mis-splicing of several key pre-mRNAs (8–16). More recently, several studies have demonstrated that members of the heterogeneous nuclear ribonucleoproteins and the DEAD-box helicase families are also misregulated in DM1 (17–22). We have previously reported that the RBP Staufen1 is elevated in DM1 skeletal muscle from human patients and mouse models (23). The double-stranded RBP Staufen is a multi-functional protein that regulates several post-transcriptional events involved in RNA metabolism (24–27). The increased expression of Staufen1 that we observed in DM1 cells appeared initially to be beneficial for DM1 because further overexpression of Staufen1 rescued key aberrant splicing events in DM1 skeletal muscle, namely, exon 11 exclusion of the insulin receptor (INSR) mRNA and intron 2 inclusion of the voltage-gated chloride channel 1 (CLCN1) mRNA (23). Recently, we determined that Staufen1 regulates numerous alternative splicing events (ASEs) in DM1 myoblasts confirming our initial observations of Staufen1’s role in splicing regulation (23, 28). In this latter work, we showed that Staufen1 is capable of rescuing several ASEs in DM1 but that it can also affect other splicing events in a detrimental fashion for DM1 (28). Thus, Staufen1’s splicing role in DM1 is consistent with that of a disease modifier.
In separate studies, our laboratory has shown that Staufen1 is also highly regulated during skeletal muscle differentiation and that its expression is elevated in denervated skeletal muscle (29). The developmental regulation of Staufen1 in skeletal muscle has since been confirmed in several other studies (30–33) including our own, establishing that Staufen1 progressively decreases during myogenesis and in early postnatal muscle for normal muscle development (34). To gain insights into the functional role of Staufen1 in skeletal muscle, we recently generated and characterized a transgenic mouse model that specifically overexpresses Staufen1 in skeletal muscle. We reported that sustained expression of Staufen1 in mature skeletal muscles causes a progressive myopathy characterized by profound morphological and functional deficits (35). These deficits were accompanied by an increase in the levels of several atrogenes and a Staufen1-induced negative regulation of phoisphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling through the modulation of phosphatase tensin homolog (PTEN) expression (35). Collectively, our findings using this mouse model indicate that Staufen1 is a novel atrophy-associated gene and may represent a biomarker and therapeutic target for neuromuscular disorders and conditions such as DM1 and muscle atrophy.
DM1 is characterized by a progressive muscle weakness, atrophy and myotonia (36). However, the mechanisms leading to muscle atrophy in DM1 are not well understood (37, 38). Over the years, several mouse models have been developed for DM1 (39). The DM300 mouse model (40, 41), the inducible EpA960/HSA-Cre-ERT2 (42) and TREDT960I (or CUG960) (38) mouse models, expressing a portion of the human DMPK locus, all recapitulate muscle wasting. However, the most commonly used human skeletal actin long repeat (HSALR) mouse model (43), which carry the CUGexp in the human skeletal actin (HSA) transgene, develop many symptoms associated with DM1 including myotonia, central nucleation, variable muscle fiber size and aberrant alternative splicing (43, 44) but without overt and consistent muscle atrophy (43, 44).
Given the fact that Staufen1 levels are increased in muscles from DM1 patients in a CUGexp length-dependent manner (23) and that Staufen1 is a novel atrophy-associated gene promoting muscle atrophy (35), we hypothesized that a Staufen1 increase promotes muscle atrophy in DM1. Although Staufen1 is increased in muscles from HSALR (23), we postulated that this increase is below the sensitivity threshold necessary to promote atrophic features and that a further increase in Staufen1 levels would promote the atrophy lacking in the HSALR DM1 mouse model. Therefore, to gain additional insight into the mechanisms by which Staufen1 drives DM1 symptoms, we have designed a series of complementary experiments to investigate the implication of Staufen1 upregulation in the muscle-specific HSALR DM1 mouse model. Together, our data provide strong evidence for the role of Staufen1 as an atrophy-associated gene, impacting progressive muscle wasting in DM1.
Results
Sustained Staufen1 expression alters muscle histology, regulates CELF1 and MBNL1 levels and alternative splicing in skeletal muscle
In our recent work, we generated muscle-specific Staufen1 transgenic mice (MCK-Staufen1-HA, here called MCK-Stau1) and discovered that sustained expression of Staufen1 causes a progressive myopathy with profound morphological changes characterized by smaller fibers with increased central nucleation (Fig. 1A and B) (35). This is accompanied by a progressive muscle weakness and functional deficits (35). Given the known role of Staufen1 in alternative pre-mRNA splicing (23, 28, 45), we wondered whether muscles from MCK-Stau1 transgenic mice display aberrant ASEs similar to those seen in DM1. We initially focused on sarco/endoplasmic reticulum calcium-ATPase (Serca1) and ryanodine receptor 1 (RyR1), two well-characterized aberrant ASEs in DM1. In DM1 muscles, the neonatal variant of the SERCA1b is expressed with exon 22 exclusion (46), whereas the expression of the RyR1 fetal isoform that is generated by exon 70 (ASI) exclusion is increased (46). The analyses of these splicing events in MCK-Stau1 mouse muscles from lines Tg-551 and Tg-6898 showed no change in Serca1 exon 22 splicing between wild-type controls (CTL) and MCK-Stau1 with Serca1a being the predominant isoform (Fig. 1C and D). However, we observed an increase in the expression of the RyR1 ASI fetal isoform in MCK-Stau1 compared with CTL muscles (90% ± 1.8 versus 76% ± 1.1, P < 0.001 for Tg-551 and 94% ± 0.4 versus 68% ± 2.7, P < 0.001 for Tg-6898; Fig. 1C and D).
Figure 1.
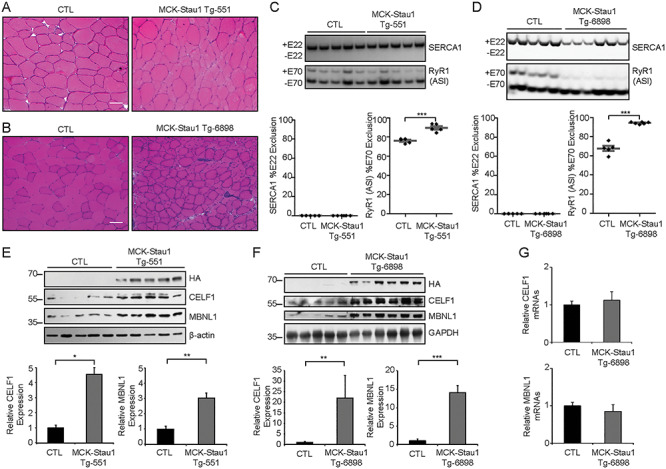
Sustained Staufen1 expression regulates CELF1 and MBNL1 levels and alternative splicing in wild-type skeletal muscle. (A and B) H&E staining of TA muscle cross-section from 16-week-old wild-type FVB/N (CTL) and MCK-Stau1 mice (Tg-551 and Tg-6898). Scale bars, 50 μm. (C and D) Alternative splicing profiles from 16-week-old wild-type FVB/N (CTL) and MCK-Stau1 mice were analyzed by RT-PCR. The quantification of Serca1 exon 22 exclusion and RyR1 ASI exon 70 exclusion. (E and F) Western blot and quantification from TA muscles of 16-week-old wild-type FVB/N (CTL) and MCK-Stau1 mice for CELF1, MBNL1 and β-actin and GAPDH as the loading control. CTL (N = 5) and MCK-Stau1 (N = 6). (G) The quantification of CELF1 and MBNL1 mRNA expression by RT-qPCR. CTL (N = 5) and MCK-Stau1 (N = 6). Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t-test, two-tailed, equal variance.
In parallel, we examined the expression of CELF1 and MBNL1 in muscles from MCK-Stau1 mice (35). First, we confirmed transgene expression in tibialis anterior (TA) muscles from 16-week-old MCK-Stau1 transgenic mice via western blot using anti-HA antibodies (Fig. 1E and F) and anti-Staufen1 antibodies (Supplementary Material, Fig. S1A). Next, we determined CELF1 and MBNL1 protein levels in these mice. Interestingly, results showed a marked increase in the expression of CELF1 (~4 and ~ 22-fold, P < 0.05 and 0.01, for Tg-551 and Tg-6898, respectively) and MBNL1 (~3 and ~ 14-fold, P < 0.01 and 0.001, for Tg-551 and Tg-6898, respectively) in MCK-Stau1 TA muscles (Fig. 1E and F). The increase in CELF1 and MBNL1 was greater in the Tg-6898 line compared with the Tg-551 line, consistent with the fact that Tg-6898 mice display a more severe muscle phenotype than Tg-551 (35). In contrast, the level of CELF1 and MBNL1 mRNAs remained unchanged by real-time quantitative polymerase chain reaction (RT-qPCR), indicating a post-transcriptional regulation in MCK-Stau1 muscles (P > 0.05) (Fig. 1G). Together, these data demonstrate that Staufen1 overexpression in skeletal muscle induces CELF1 and MBNL1 expression and consistent with our previous findings (23, 28), causes only modest changes in alternative splicing of key DM1 pre-mRNAs.
Sustained Staufen1 expression causes a myopathy in ‘mild’ and ‘severe’ DM1 skeletal muscle
To evaluate the impact of Staufen1 on DM1 muscles, we overexpressed Staufen1 in DM1 mouse models using complementary strategies. First, we made a hemizygous transgenic cross between the more severe hemizygous MCK-Stau1 mouse line Tg-6898 (35) and homozygous HSALR mice (43), denoted as HSALR Tg/0/MCK-Stau1 (Fig. 2A). These mice represent ‘mild’ DM1 as a result of the hemizygous HSALR transgene expression. Positive mice were identified via genotyping by polymerase chain reaction (PCR) using primers specific for the MCK-Staufen1-HA and HSALR transgenes as previously described (35, 43). Western blot analysis of TA muscles from HSALR Tg/0/MCK-Stau1 mice confirmed Staufen1-HA expression (Fig. 2B and Supplementary Material, Fig. S1A). Hematoxylin and Eosin (H&E) staining was next performed on TA muscle cross-sections obtained from these transgenic mice (Fig. 2C). Histological analyses revealed a marked increase in the percentage of muscle fibers containing central nuclei in HSALR Tg/0/MCK-Stau1 mice compared with CTL (P < 0.05) and hemizygous HSALR littermates (HSALR Tg/0) (P < 0.001; Fig. 2D). The variance coefficient (VC) was also calculated to assess fiber size variability. For this parameter, we observed no change (P > 0.05) in HSALR Tg/0/MCK-Stau1 mice compared with CTL or HSALR Tg/0 mice (Fig. 2E). Cross-sectional area (CSA) of TA muscle fibers was measured. As shown in Figure 2F, we noted an ~ 40% decrease in the mean CSA (P < 0.001) of TA fibers in HSALR Tg/0/MCK-Stau1 mice. In agreement with these data, the frequency distribution of CSA showed an increased frequency of smaller fibers concomitant with a decreased frequency of larger fibers as compared with CTL and HSALR Tg/0 littermates (Fig. 2G).
Figure 2.
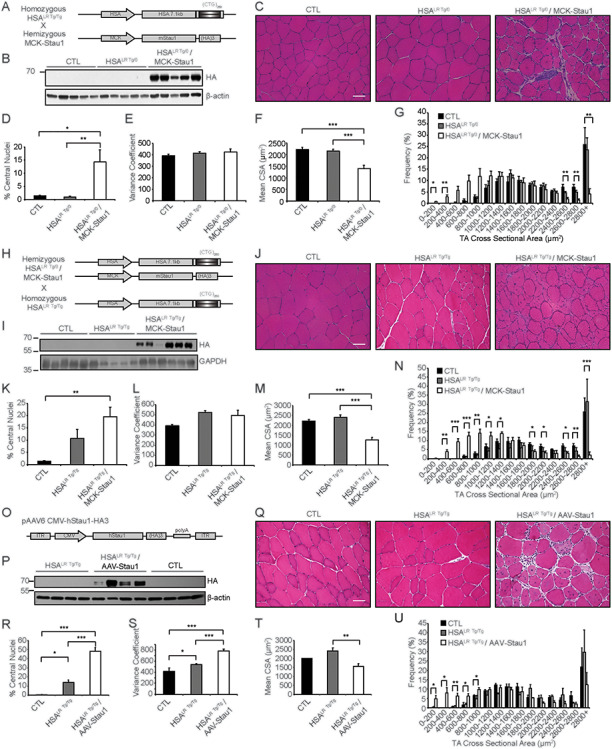
Sustained Staufen1 expression causes morphological changes in DM1 skeletal muscle. (A) Schematic diagram of transgenic cross between MCK-Stau1 (line Tg-6898) with HSALR mice. (B) Western blotting to confirm transgene expression using HA antibodies with β-actin as a loading control. (C) H&E staining of TA muscle cross-sections from wild-type FVB/N (CTL), hemizygous HSALR Tg/0 and HSALR Tg/0/MCK-Stau1 15-week-old mice. (D) % central nuclei. (E) VC of TA fiber CSA. (F) Mean CSA (μm2). (G) TA fiber CSA (μm2) displayed as a frequency distribution (% of total) of 15-week-old mice. CTL (N = 5), HSALR Tg/0 (N = 7) and HSALR Tg/0/MCK-Stau1 (N = 6). (H) Schematic diagram of transgenic cross between HSALR Tg/0/MCK-Stau1 and HSALR Tg/Tg mice. (I) Western blotting to confirm transgene expression using HA antibodies with GAPDH as a loading control. (J) H&E staining of TA muscle cross-sections from wild-type FVB/N (CTL), homozygous HSALR Tg/Tg and HSALR Tg/Tg/MCK-Stau1 15–18-week-old mice. (K) % central nuclei. (L) VC of TA fiber CSA. (M) Mean CSA (μm2). (N) TA fiber CSA (μm2) displayed as a frequency distribution (% of total) of 15-week-old mice. CTL (N = 5), HSALR Tg/Tg (N = 5) and HSALR Tg/Tg/MCK-Stau1 (N = 6). (O) Schematic diagram of pAAV6-CMV-hStaufen1-HA3 vector used to produce recombinant AAV viral particles injected into TA muscle of homozygous HSALR Tg/Tg mice. (P) Western blotting to confirm transgene expression using HA antibodies with β-actin as a loading control. (Q) H&E staining of TA muscle cross-sections from wild-type FVB/N (CTL), homozygous HSALR Tg/Tg and AAV-Staufen1 injected (AAV-Stau1) mice. (R) % central nuclei. (S) VC of TA fiber CSA. (T) Mean CSA (μm2). (U) TA fiber CSA (μm2) displayed as a frequency distribution (% of total) of 18 week-old mice. CTL (N = 4), HSALR Tg/Tg (N = 4) and HSALR Tg/Tg/MCK-Stau1 (N = 4). Scale bars, 50 μm. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA, with Bonferroni post hoc test.
To determine the impact of Staufen1 overexpression in ‘severe’ DM1 skeletal muscle, we took two approaches. In the first approach, we crossed the above hemizygous transgenic mouse model, HSALR Tg/0/MCK-Stau1, with homozygous HSALR mice (43) to generate HSALR Tg/Tg/MCK-Stau1 (Fig. 2H). Positive mice were identified via genotyping by PCR using primers specific for the MCK-Staufen1-HA and HSALR transgenes as previously described (35, 43). Western blot analysis of TA muscles confirmed Staufen1-HA expression (Fig. 2I and Supplementary Material, Fig. S1A). To assess muscle morphology, H&E staining was performed on TA muscle cross-sections (Fig. 2J). As seen with HSALR Tg/0/MCK-Stau1 mice above, there is a marked increase in the percentage of muscle fibers containing central nuclei in HSALR Tg/Tg/MCK-Stau1 mice compared with CTL (P < 0.01) as well as a trend toward an increase in comparison to HSALR Tg/Tg littermates (P > 0.05; Fig. 2K). As also observed with HSALR Tg/Tg/MCK-Stau1 mice, there was no change in the VC (P > 0.05; Fig. 2L). However, in agreement with results from hemizygous HSALR Tg/0/MCK-Stau1 mice, we observed an ~ 40% decrease in the mean CSA in HSALR Tg/Tg/MCK-Stau1 mice compared with CTL and HSALR Tg/Tg mice (P < 0.001; Fig. 2M). Consistent with this, the frequency distribution of CSA showed an increased frequency of smaller fibers concomitant with a decreased frequency of larger fibers as compared with CTL and HSALR Tg/Tg littermates (Fig. 2N).
In a second approach, and to circumvent any effect of Staufen1 overexpression on the embryonic development of skeletal muscle, we also generated recombinant adeno-associated virus (AAV) particles encoding Staufen1-HA (Fig. 2O). The adeno-associated viral serotype 6 (AAV6) pseudotype was selected to ensure a wide and sustained expression of the transgene in skeletal muscle after intramuscular injections (47–50). We thus injected AAV-Staufen1-HA into TA muscles of homozygous HSALR adult mice and collected samples 4 weeks post-infection. Western blot analysis of TA muscles confirmed Staufen1-HA expression in infected muscle fibers (Fig. 2P and Supplementary Material, Fig. S1B). H&E staining was initially performed on muscle cross-sections. In excellent agreement with the data presented above using transgenic mouse models, the overexpression of Staufen1 in adult DM1 skeletal muscle appears to negatively impact muscle histology (Fig. 2Q). Indeed, TA muscles from these mice displayed a higher incidence of central nucleation (~90- and ~ 3.5-fold; P < 0.001 compared with CTL and HSALR tg/tg, respectively; Fig. 2R) as well as an increase in muscle fiber size variability as determined by the VC (P < 0.001; Fig. 2S). In addition, we observed an ~ 20% decrease in mean CSA in AAV-Staufen1 injected DM1 mice (P < 0.01; Fig. 2T). The overall decrease in CSA was characterized by a marked increase in the frequency of smaller muscle fibers compared with CTL and HSALR Tg/Tg mice (Fig. 2U).
Conversely, we attempted to downregulate Staufen1 in HSALR mice using AAV-mediated depletion to test whether the decrease in Staufen1 levels would improve the DM1 skeletal muscle phenotype. However, we were unable to efficiently down-regulate Staufen1 levels in DM1 mice with an AAV-mediated shRNA delivery strategy, either due to technical limitations or elimination of myofibers depleted in Staufen1.
Overexpression of Staufen1 modestly impacts alternative splicing in DM1 muscle
Staufen1 is known to regulate alternative pre-mRNA splicing in DM1 (23, 28). As observed in MCK-Stau1 mice (Fig. 1C and D), Serca1 exon 22 does not appear to be alternatively spliced in muscles from HSALR Tg/0/MCK-Stau1 mice (Fig. 3A and B). Surprisingly, this was also the case in HSALR Tg/0 littermates as compared to homozygous HSALR Tg/Tg mice, indicating that the hemizygous nature of the HSALR transgene is not sufficient to induce aberrant alternative splicing of Serca1 (Fig. 3A and B). The analysis of the RyR1 ASI exon 70 showed ~ 97% ± 3.1 skipping in HSALR Tg/0/MCK-Stau1, thereby mimicking patterns observed in homozygous HSALR Tg/Tg mice, as compared with ~ 77% ± 2.9 skipping in HSALR Tg/0 and ~ 68% ± 2.7 in CTL littermates (P < 0.001; Fig. 3A and B). As controls, we also examined the splicing patterns of Serca2b and RyR1 ASII, splicing events not affected in DM1. As expected, our data show that these events are unaffected in HSALR Tg/0/MCK-Stau1 skeletal muscles (Fig. 3A and B).
Figure 3.
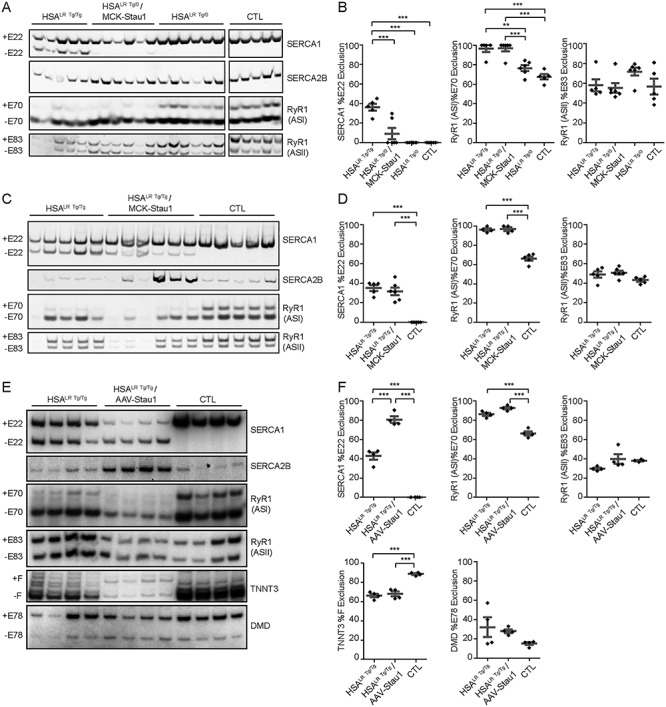
Overexpression of Staufen1 modulates alternative splicing in DM1 muscle. (A) Alternative splicing profiles from TA muscles of 15–18 week-old homozygous HSALR Tg/Tg, hemizygous HSALR Tg/0, wild-type FVB/N (CTL) and HSALR Tg/0/MCK-Stau1 mice were analyzed by RT-PCR. The same CTL for Serca1 and RyR1 ASI were used in Figure 1B. (B) The quantification of Serca1 exon 22 exclusion, RyR1 ASI Exon 70 exclusion and RyR1 ASII Exon 83 exclusion. CTL (N = 5), HSALR Tg/Tg (N = 5), HSALR Tg/0 (N = 6) and HSALR Tg/0/MCK-Stau1 (N = 6). (C) Alternative splicing profiles from TA muscles of 15–18 week-old homozygous HSALR Tg/Tg, wild-type FVB/N (CTL) and HSALR Tg/Tg/MCK-Stau1 mice were analyzed by RT-PCR. (D) The quantification of Serca1 exon 22 exclusion, RyR1 ASI Exon 70 Exclusion and RyR1 ASII Exon 83 ASII exclusion. CTL (N = 5), HSALR Tg/Tg (N = 5) and HSALR Tg/Tg/MCK-Stau1 (N = 6). (E) Alternative splicing profiles from TA muscles of 10 week-old homozygous HSALR Tg/Tg, Control (CTL) and AAV-Staufen1 injected homozygous HSALR mice (AAV-Stau1) were analyzed by radioactive RT-PCR. (F) The quantification of Serca1 exon 22 exclusion, RyR1 ASI Exon 70 Exclusion, RyR1 ASII Exon 83 exclusion, Tnnt3 Exon F exclusion and Dmd Exon 78 exclusion. CTL (N = 4), HSALR Tg/Tg (N = 4), AAV-Stau1 (N = 4). Data are means ± SEM. **P < 0.01, ***P < 0.001; one-way ANOVA, with Bonferroni post hoc test.
In contrast to the overexpression of Staufen1 in mild DM1 skeletal muscle, HSALR Tg/Tg/MCK-Stau1 mice showed a significant increase in Serca1 exon 22 skipping (32% ± 3.5) as compared with CTL mice (0%), consistent with the alternative splicing patterns observed in HSALR Tg/Tg mice (P < 0.001; Fig. 3C and D). The analysis of the RyR1 ASI showed increased exon 70 skipping in HSALR Tg/Tg/MCK-Stau1 (97% ± 1.8) as compared with CTL mice (66% ± 2.3; P < 0.001), consistent with ratios observed in HSALR Tg/Tg mice (96% ± 1.4) (Fig. 3C and D). As observed above, splicing patterns of Serca2b and RyR1 ASII, used as controls, were unchanged (Fig. 3C and D).
In agreement with our findings with HSALR Tg/Tg/MCK-Stau1 mice, AAV-Staufen1-injected TA muscles displayed a significant increase in Serca1 exon 22 skipping (80.6% ± 3.4) as compared with HSALR Tg/Tg (43% ± 3.9) and CTL mice (0%) (P < 0.001; Fig. 3E and F). Moreover, we observed an increase in the RyR1 ASI fetal isoform as compared with CTL skeletal muscle (P < 0.001; 93% ± 1.3 versus 66% ± 2.1; Fig. 3E and F). These splicing patterns are consistent with those observed in HSALR Tg/Tg mice (Fig. 3C and D). As controls, and as seen above, splicing patterns of Serca2b and RyR1 ASII were unaffected by AAV-mediated overexpression of Staufen1 (Fig. 3E and F). In this most severe model, we evaluated the impact of Staufen1 overpression on two additional well-characterized aberrant splicing events. In DM1 muscles, the inclusion of the fetal F exon of fast skeletal muscle troponin T3 (Tnnt3) is increased (11), whereas the exclusion of the exon 78 of the dystrophin (Dmd) transcript is increased (51). Our results show that AAV-Staufen1-injection had no significant effect on the alternative splicing of these splicing events compared with HSALR Tg/Tg mice (P > 0.05; Fig. 3E and F). Altogether, our results show that Staufen1 overexpression only has mild effects on alternative splicing in DM1 mice.
Overexpression of Staufen1 increases CELF1 and MBNL1 levels in DM1 muscle
Next, we investigated the expression of CELF1 and MBNL1 in skeletal muscles from these mice. Our data show an ~ 5-fold increase in CELF1 levels in HSALR Tg/0/MCK-Stau1 mice as compared with CTL (P < 0.01), as well as a trend toward an increase in comparison to HSALR Tg/0, whereas MBNL1 expression remained unchanged in HSALR Tg/0/MCK-Stau1 mice as compared with CTL and HSALR Tg/0 littermates (Fig. 4A and B). Next, we investigated expression of CELF1 and MBNL1 in TA muscles from the ‘severe’ DM1 mouse models. Our data show an ~ 13- and ~ 6-fold increase in CELF1 (P < 0.001) and MBNL1 (P < 0.05), respectively, in HSALR Tg/Tg/MCK-Stau1 mice as compared with CTL mice (Fig. 4C and D). CELF1 levels were also increased by ~ 3.4-fold (P < 0.01) compared with HSALR Tg/Tg littermates (Fig. 4C and D). Similar analyses of AAV-Staufen1-injected TA muscles showed increases in the expression of CELF1 (~8-fold; P < 0.01) and MBNL1 (~2-fold; P < 0.05; Fig. 4E and F) as compared with CTL and HSALR Tg/Tg mice.
Figure 4.
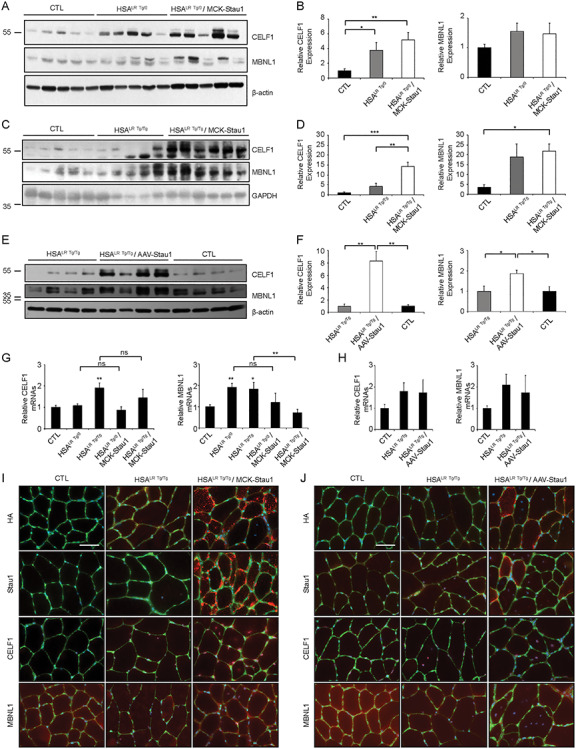
Sustained transgenic Staufen1 expression increases CELF1 and MBNL1 levels. (A and B) Western blot from TA muscles of 15 week-old hemizygous HSALR Tg/0, wild-type FVB/N (CTL) and HSALR Tg/0/MCK-Stau1 mice analyzed for CELF1, MBNL1 and β-actin as the loading control. (C and D) Western blot from TA muscles of 15–18 week-old homozygous HSALR Tg/Tg, wild-type FVB/N (CTL) and HSALR Tg/Tg/MCK-Stau1 mice analyzed for CELF1, MBNL1 and GAPHD as the loading control. (E and F) Western blot from TA muscles of 14–17 week-old homozygous HSALR Tg/Tg, Control (CTL) and AAV-Staufen1 injected homozygous HSA LR mice (AAV-Stau1) were analyzed for CELF1, MBNL1 and β-actin as the loading control. The same β-actin control was used in Figure 2P. (G and H) The quantification of CELF1 and MBNL1 mRNA expression by RT-qPCR. (N = 4–6). (I and J) Representative immunofluorescence showing Staufen1-HA, Staufen1, CELF1 and MBNL1 expression and localization in TA muscles (red). Laminin (green) and DAPI (blue) were used to delimit muscle fibers and nuclei, respectively. Scale bars, 50 μm. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA, with Bonferroni post hoc test or Student’s t-test, two-tailed, equal variance.
In addition, we performed RT-qPCR in HSALR Tg/0/MCK-Stau1, HSALR Tg/Tg/MCK-Stau1 and AAV-Staufen1-injected HSALR mice along with their respective controls. Interestingly, no significant increase in the expression of CELF1 and MBNL1 transcript levels were observed across all our mouse models overexpressing Staufen1 and their respective controls (P > 0.05) (Fig. 4G and H), indicating that the observed increase in CELF1 and MBNL1 are a result of post-transcriptional regulation.
To complement these findings, we investigated the level and subcellular localization of Staufen1-HA, CELF1 and MBNL1 by immunofluorescence. As expected with anti-HA antibodies, Staufen1-HA expression is restricted to HSALR Tg/Tg/MCK-Stau1 and AAV-Staufen1 injected HSALR mice (Fig. 4I and J). Staufen1 localization is observed in the cytoplasm, sarcolemma, and to a lesser extent nuclei, as previously described with MCK-Stau1 mice, line 6898 (35). In addition, we observed a marked increase of Staufen1 levels and similar localization using anti-Staufen1 antibodies in HSALR Tg/Tg/MCK-Stau1 and AAV-Staufen1 injected HSALR mice compared with CTL and HSALR Tg/Tg mice (Fig. 4I and J). Consistent with results obtained by western blot, we also observed an increase in CELF1 and MBNL1 nuclear and cytoplasmic staining in HSALR Tg/Tg/MCK-Stau1 and AAV-Staufen1 injected HSALR Tg/Tg mice compared with CTL and HSALR Tg/Tg mice (Fig. 4I and J). Altogether, these data show that the overexpression of Staufen1 increases CELF1 and MBNL1 levels in DM1 muscle.
As an additional control for the AAV-Staufen1 experiments, we also injected AAV-GFP into TA muscles of HSALR mice and collected tissues 4 weeks post-infection as performed above. The analysis of the AAV-GFP expressing fibers (Supplementary Material, Fig. S2A) shows that the AAV infection procedure does not alter Staufen1, CELF1 and MBNL1 protein levels (P > 0.05) (Supplementary Material, Fig. S2B and C), CELF1 and MBNL1 transcripts levels (P > 0.05) (Supplementary Material, Fig. S2D), or alternative splicing of Serca1 exon 22 and RyR1 ASI exon 70 in TA muscles from HSALR mice (P > 0.05) (Supplementary Material, Fig. S2E and F). These data confirm that the results obtained after the injection of TA muscles with AAV-Staufen1 are a direct consequence of Staufen1 upregulation.
Staufen1 expression promotes PTEN expression and inhibition of Akt signaling
We have previously shown that Staufen1 negatively regulates PI3K/Akt signaling through the activation of PTEN expression in skeletal muscle (35). We thus investigated whether increased expression of Staufen1 in DM1 mice also affects Akt signaling via PTEN regulation in skeletal muscles. To this end, we focused on the most severe of our models, namely, the AAV-Staufen1 mice. First, we compared the levels of Akt signaling and PTEN, a known inhibitor of Akt, in CTL, HSALR and AAV-Staufen1 injected-HSALR mice. The expression of PTEN, phosphorylated Akt (Ser473) and total Akt was determined by western blot and a ratio of phosphorylated Akt to total Akt (p-Akt/Akt) was calculated. Although PTEN and total Akt levels remained unchanged, we observed an increase in p-Akt/Akt ratio (P < 0.01) in HSALR Tg/Tg mice compared with CTL (Fig. 5A and B). Furthermore, in AAV-Staufen1 injected-HSALR mice, we observed a marked increase in PTEN levels (~3-fold, P < 0.001) compared with HSALR Tg/Tg mice (Fig. 5A and B). Accordingly, although total levels of Akt were increased by Staufen1 (~7-fold, P < 0.001), we observed a decrease in p-Akt/Akt ratio (P < 0.01) compared with HSALR Tg/Tg mice (Fig. 5A and B). In agreement with our previous work (35), these data show that Staufen1 represses Akt signaling by upregulating PTEN in DM1 mice.
Figure 5.
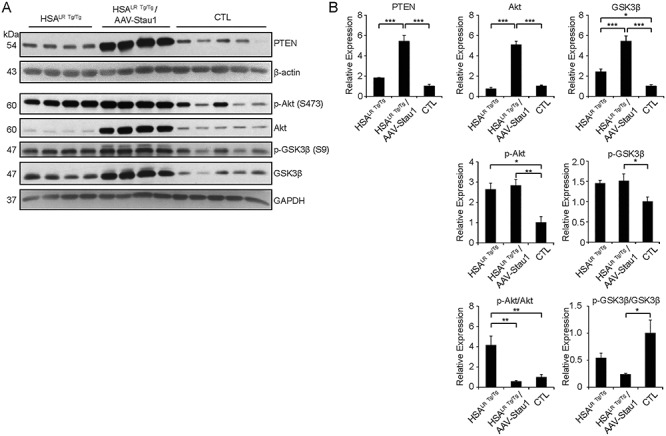
Overexpression of Staufen1 increases PTEN levels and inhibits Akt signaling in DM1 muscle. (A and B) Western blot and quantification from TA muscles of 14–17 week-old homozygous HSALR Tg/Tg, Control (CTL) and AAV-Staufen1 injected homozygous HSALR mice (AAV-Stau1) analyzed for PTEN, p-Akt, Akt, p-GSK3β, GSK3β, along with GAPDH and β-actin as the loading controls. (N = 4–5). Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA, with Bonferroni post hoc test.
In addition, we assessed the level of glycogen synthase kinase-3β (GSK3β) signaling by measuring the expression of the total, and the inactive phosphorylated (Ser9) form of GSK3β. As expected (52), we observed an activation of GSK3β signaling in HSALR Tg/Tg mice, as shown by the increase in total GSK3β (P < 0.05) and a trend toward a decrease in the ratio of inactive p-GSK3β/GSK3β (P > 0.05) compared with CTL mice (Fig. 5A and B). Interestingly, in AAV-Staufen1 injected-HSALR mice, while the total levels of GSK3β were increased by Staufen1 (P < 0.001), we observed a trend towards a decrease in the ratio of p-GSK3β/GSK3β compared with HSALR Tg/Tg mice (P > 0.05) and CTL (P < 0.05) (Fig. 5A and B), suggesting that Staufen1 expression further activates GSK3β signaling in DM1 mice. This augmentation in active GSK3β is consistent with the increase in CELF1 levels observed after Staufen1 upregulation in Figure 4.
Staufen1 expression increases atrogene expression in DM1 mice
Skeletal muscle atrophy is linked to the upregulation of several E3 ubiquitin ligases, such as muscle RING-finger protein-1 (MuRF1) and muscle atrophy F-box (MAFbx)/Atrogin-1 (53). We thus examined the expression of these atrogenes by RT-qPCR in TA muscles from HSALR Tg/0/MCK-Stau1 mice, HSALR Tg/Tg/MCK-Stau1 mice and CTL, HSALR Tg/0 and HSALR Tg/Tg mice. No difference in MuRF1 and MAFbx expression were observed in HSALR Tg/0 and HSALR Tg/Tg mice compared with CTL mice P > 0.05 (Fig. 6A and B). Our findings show a trend toward an increase in MuRF1 (~3.2-fold) and MAFbx (~2.5-fold) in muscles from HSALR Tg/0/MCK-Stau1 compared with hemizygous HSALR Tg/0 mice (Fig. 6A and B). A similar trend was obtained when compared to CTL mice (Fig. 6A and B). In addition, we observed a marked increase (P < 0.05) and a clear trend toward an increase in MAFbx mRNA expression in HSALR Tg/Tg/MCK-Stau1 mice compared with CTL and homozygous HSALR Tg/Tg mice, respectively (Fig. 6C and D). In contrast, the expression of MuRF1 was unchanged. Such an apparent discrepancy may be related to the known transient pattern of atrogene expression during muscle atrophy (53–55). Finally, the expression of MAFbx was significantly increased in AAV-Staufen1-HA-injected TA muscles compared with HSALR (P < 0.05), with no increase in MuRF1 mRNA levels (Fig. 6E and F). Collectively, our results show that Staufen1 overexpression increases atrogene expression.
Figure 6.
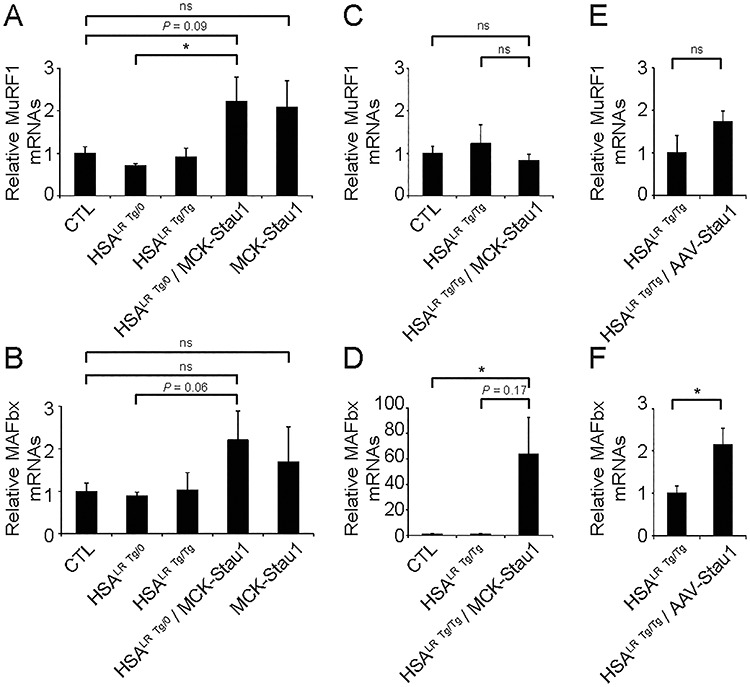
Overexpression of Staufen1 increases atrogene expression in DM1 muscle. The quantification of MuRF1 and MAFbx expression by RT-qPCR in TA muscles from: (A and B) 15–18 week-old wild-type FVB/N (CTL), hemizygous HSALR Tg/0, homozygous HSALR Tg/Tg, HSALR Tg/0/MCK-Stau1 mice and MCK-Stau1 (Tg-6898); (C and D) 15–18 week-old wild-type FVB/N (CTL), homozygous HSALR Tg/Tg and HSALR Tg/Tg/MCK-Stau1 mice and (E and F) 14–17 week-old homozygous HSALR Tg/Tg and AAV-Staufen1 injected (AAV-Stau1) mice. (N = 3–7). Data are means ± SEM. *P < 0.05; Student’s t-test, two-tailed, equal variance.
Discussion
In our previous work, we found that Staufen1 is highly expressed during early stages of muscle development, in denervated muscle, rhabdomyosarcoma as well as in skeletal muscles from DM1 patients and mice (23, 28, 29, 34, 56, 57). Moreover, we uncovered a novel role for Staufen1 in the regulation of alternative splicing in muscle (23, 28), and showed that it acts as a disease modifier in DM1 (28). Finally, we discovered that sustained expression of Staufen1 promotes a progressive myopathy characterized by morphological and functional deficits (35).
To build upon these findings and to gain insights into the functional impact of Staufen1 in DM1 muscle, we overexpressed Staufen1 in ‘mild’ and ‘severe’ DM1 mouse models to better define the impact that Staufen1 has on DM1 muscle. Overall, we report that the overexpression of Staufen1 in DM1 skeletal muscles exacerbates dystrophic and atrophic features via the inhibition of PI3K/Akt signaling by PTEN and atrogene expression, thereby recapitulating several morphological abnormalities observed in the MCK-Staufen1-HA mice (MCK-Stau1) (35). In addition, our data are consistent with the notion that Staufen1 fine-tunes ASEs rather than drastically modulating splicing patterns of key pre-mRNAs in DM1 muscles (23, 28). Finally, our findings reveal for the first time that Staufen1 regulates key RBPs known to significantly impact the DM1 pathology namely, CELF1 and MBNL1, further supporting the notion that Staufen1 is a disease modifier in DM1.
In the present study, we demonstrate using complementary approaches that Staufen1 expression promotes muscle atrophy in both mild hemizygous HSALR Tg/0 and severe homozygous HSALR Tg/Tg DM1 mouse models. Our results show that sustained overexpression of Staufen1 in DM1 muscle induces profound histological modifications in muscle, characterized by smaller myofibers and increased central nucleation. Mechanistically, this is accompanied by an increase in the levels of PTEN, which negatively regulates PI3K/Akt signaling and promotes the expression of MuRF1 and MAFbx atrogenes. These data are consistent with our previous findings that Staufen1 regulates PTEN expression and in turn PI3K/Akt signaling in MCK-Staufen1-HA mice causing muscle atrophy (35). Collectively, our results show that Staufen1 expression in HSALR mice promotes muscle atrophy in DM1 muscle and further support the concept that Staufen1 participates in the DM1 pathomechanism.
It is well established that MBNL1 sequestration by CUGexp mRNAs plays a central role in DM1 disease mechanisms by compromising alternative splicing regulation (9, 11, 12). As expected, the upregulation of MBNL1 in HSALR mice via localized AAV-mediated delivery, reverses multiple ASEs characteristic of DM1 including for example CLCN1, thus rescuing myotonia in these mice (8). This work established the safety and efficacy of MBNL1 overexpression for the treatment of DM1 (8). Dual hemizygous transgenic mice were later generated by crossing MBNL1 transgenic with HSALR mice, and showed a complete rescue of the myopathy together with a significant decrease in central nucleation (13). However, in light of our current findings showing that hemizygous HSALR Tg/0 mice do not display significant splicing abnormalities compared with control mice, it would appear necessary to examine the impact of transgenic MBNL1 overexpression in a homozygous HSALR Tg/Tg background. Here, our data show that Staufen1 induces a strong induction of MBNL1 expression, but failed to rescue the DM1 pathology. While surprising, a recent study also showed that the overexpression of MBNL1 in a DM1 mouse model is not necessarily sufficient to rescue alternative splicing in DM1 (58).
In addition to MBNL1 sequestration, another key player in the DM1 pathomechanism is elevated expression of CELF1 (59, 60). CELF1 transgenic mice display morphological impairments including muscle atrophy and increased muscle regeneration with the most severe cases (~6 to 8-fold increase of CEFL1) showing a phenotype consistent with congenital DM1 (15, 61). Our results show that Staufen1 overexpression increases the nuclear and cytoplasmic levels of CELF1. CELF1 is regulated by several signaling pathways in skeletal muscle, including PKC (62), PI3K/Akt (62, 63), cyclin D3-cdk4/6 and GSK3β (52, 64), which phosphorylate CELF1 at various sites resulting in its stabilization and/or changes in affinity for targets. In the current study, our data show that Staufen1 promotes an increase in total GSK3β levels in parallel to a decrease in the inactive phosphorylated (Ser9) form of GSK3β compared with HSALR mice. These findings are consistent with muscle biopsy samples from DM1 patients (52). Given the regulatory role of GSK3β on CELF1 and the fact that CELF1 also participates in muscle atrophy (16, 37), our observed phenotype may be partially attributed to a Staufen1-dependent increase of CELF1.
In cell culture, the overexpression of Staufen1 in C2C12, HeLa and HEK293 cells does not affect CELF1 and MBNL1 levels (23, 28). Here, our data show that sustained expression of Staufen1 in muscle tissues resulted in a marked increase in CELF1 and MBNL1 levels, indicating in vivo-specific regulations. Since CELF1 and MBNL1 protein expression is increased during muscle regeneration (65), it is possible that the increase in our mouse models is restricted to the skeletal muscle or our data could be impacted by infiltrating immune cells. We observed by immunofluorescence an increase in both nuclear and cytoplasmic staining of MBNL1 and CELF1 inside myofibers. However, we also observed signal outside of the sarcolemma. It is therefore likely that the increase in CELF1 and MBNL1 proteins can be attributed to both a Staufen1-dependent increase through the activation of atrophic pathways and their upregulation in response to regenerating fibers and infiltrating immune cells.
It is well documented that the misregulation of RBPs in DM1, in particular MBNL1 and CELF1, causes aberrant alternative pre-mRNA splicing and contributes to various key hallmarks of the disease (8, 11, 15, 16, 66). In an attempt to identify additional RBPs involved in DM1, we previously showed that Staufen1 is increased in DM1 skeletal muscle and that it regulates alternative splicing of the INSR and the CLCN1 pre-mRNAs (23). More recently, we expanded on this work and characterized Staufen1 as a disease modifier because it fine-tunes multiple ASEs and regulates stress granule formation in DM1 skeletal muscle (28, 57). Here, we demonstrate that Staufen1 regulates the splicing of RyR1 ASI by promoting the expression of the fetal isoform in all three mouse models compared with wild-type, whereas it regulates Serca1 splicing in the AAV-Staufen1 model. However, Staufen1 did not further regulate Troponin T3 (Tnnt3) or Dmd in the AAV-Staufen1 injected HSALR mice. Therefore, further increasing Staufen1 in DM1 mice only modestly impact missplicing patterns, indicating that Staufen1 exacerbates dystrophic and atrophic features independently of alternative splicing.
In the present study, we demonstrate that the overexpression of Staufen1 in the HSALR mouse model causes a myopathy, which, in contrast to DM1 patients, is lacking in the traditional HSALR model. This may appear contradictory as skeletal muscles from both DM1 patients and mice show elevated levels of endogenous Staufen1 as we previously demonstrated (23). A plausible explanation to reconcile this apparent paradox is that the ability of Staufen1 to induce myopathic and atrophic changes in DM1 muscles is related to muscle types, the number of repeats, the level of endogenous Staufen1 overexpression and/or the sensitivity of species to Staufen1-mediated signaling causing muscle morphological defects. Thus, in HSALR mice, the endogenous increase in Staufen1 is insufficient to detrimentally impact the myofiber architecture, and further overexpression is necessary to induce morphological abnormalities. In addition, a certain degree of variability in Staufen1 overexpression can be observed in muscle tissues such that TA muscles showed less of an increase (Supplementary Material, Fig. S1A). We also observed variability in CELF1 and MBNL1 levels in HSALR mice (Fig. 4). It would therefore appear timely to then carry out a detailed study on the levels of misregulated RBPs (MBNL1, CELF1 and Staufen1) in several affected muscles from patients with varying repeat numbers and phenotypes. Overall, these data are in agreement with our recent findings showing that the muscle-specific expression of Staufen1 causes a progressive myopathy in wild-type mouse skeletal muscle (35). Accordingly, the various DM1 mouse models generated in the present study may prove important to gain insight into the basic mechanisms causing muscle abnormalities in DM1, while also being useful for designing therapeutic interventions that simultaneously target splicing defects and morphological features.
Materials and Methods
Generation of transgenic animals
All animal experimental protocols were approved by the University of Ottawa Institutional Animal Care Committee and were in accordance with the Canadian Council of Animal Care guidelines. MCK-Staufen1-HA transgenic mice (here called MCK-Stau1) lines Tg-551 and Tg-6898, were previously described (35). MCK-Stau1 line Tg-6898 was crossed with homozygous HSALR20b (called HSALR Tg/Tg) mice (43) to generate HSALR Tg/0/MCK-Stau1, yielding mice hemizygous for both the MCK-Staufen1-HA and HSALR transgenes. Hemizygous HSALR20b (called HSALR Tg/0) and FvB/N (CTL) mice were used for comparison. The HSALR Tg/Tg/MCK-Stau1 transgenic mouse line was generated by breeding homozygous HSALR Tg/Tg mice with HSALR Tg/0/MCK-Stau1 to generate mice hemizygous for the MCK-Staufen1-HA transgene and homozygous for HSALR.
Genotyping of transgenic animals
Genomic DNA was isolated from mice (Macherey-Nagel Inc., PA, USA) and screened by PCR (EZ BioResearch PCR Ready Mix) using transgene-specific primers. For MCK-Staufen1-HA mice (fwd 5′-CACTTAGTTTAGGAACCAGTG-3′, rev 5′-CGACCAGAGGAGGGAAGAG-3′), primers were used to detect a single band migrating at 345 bp. Additional primers were designed to amplify both endogenous (870 bp) and exogenous MCK-Staufen1-HA (137 bp) (fwd 5′-GCCAGAGTACATGCTCCTTAC-3′, rev 5′-TGTTCTCAGCAGCATTACGC -3′) to confirm genotyping.
For HSALR Tg/0/MCK-Stau1 and HSALR Tg/Tg/MCK-Stau1 mice, primers specific for MCK-Staufen1-HA as described above and primers specific for the HSALR transgene were used (fwd 5′- GCCATCGTAAACTGACACAGTG-3′, rev 5′- GTTCCACAGGGCTTTGTTTCG-3′) detecting a single band at ~ 750 bp. Interindividual length of the CTG250 expansion was monitored using these primers. Additional primers were used as a positive PCR control for mouse skeletal actin primers (fwd 5′- TCCTCAGGACGACAATCGAC-3′, rev 5′- CCTAAGGAGTTCACCCAGTCTG-3′).
Production and injection of AAV6-hStaufen1-HA3
The hStaufen155-HA3 cDNA (67) was subcloned into the pAAV-MCS Helper Free System (generous gift from Dr David Park, uOttawa). Restriction digest using SmaI was performed to ensure proper insertion between the inverted terminal repeat sequences. AAV6 particles were generated via the University of North Carolina Vector Core Facility with a viral titer of 3.6 × 1012 vector genomes/ml. As a control, AAV6-CMV-eGFP viral particles were purchased from the University of North Carolina Vector Core Facility (#4334523) with a viral titer of 3 × 1012 vector genomes/ml. The right TA muscle of 10–13 week-old HSALR20b mice was injected with 1 × 1011 vector genomes/ml in 30 μl of saline. Muscles were collected 4 weeks post-infection for analysis.
H&E staining and analysis
Dissected muscles were embedded in Tissue-Tek OCT compound (VWR, Mississauga, Ontario, Canada) and subsequently frozen in melting isopentane pre-cooled with liquid nitrogen. Samples were stored at −80°C until use. Cross sections of muscles (10 μm thick) were stained with H&E, dehydrated via a series of ethanol washes (70, 95 and 100%), cleared with toluene, mounted using Permount (Fisher Scientific, Ottawa, Canada) and visualized via light microscopy at 20X magnification. The analysis of 5 fields of view was performed using Northern Eclipse Software (NES, Expix Imaging, Mississauga, Ontario, Canada). The central nuclei percentage was calculated by [(number centrally nucleated fibers/total fibers) × 100%]. The CSA of each fiber was measured using NES, and the VC was calculated by (VC Z = 1000 × standard deviation of muscle fiber CSA/mean muscle fiber CSA).
Western blotting
Frozen muscle samples were crushed in liquid nitrogen and the powder was resuspended in radioimmunoprecipitation assay buffer (50 mm Tris–HCl, pH 8.0, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1X protease inhibitor cockail, 1X PhospoStop [Roche, Laval, Quebec]) and centrifuged at 13 000 rpm, 10 min. Protein concentration was measured with the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Ottawa, Canada). In total, 10–30 μg of protein was separated by SDS-PAGE. An equal amount of proteins were loaded in each well to ensure even loading within individual western blots. Proteins were transferred onto 0.2 μm nitrocellulose membranes (Bio-Rad, Mississauga, Ontario, Canada). Non-specific binding was blocked with 5% skim milk in 1X PBS + 0.05% Tween 20. Primary antibodies were diluted in 1% skim milk and incubated with Membranes for 1 h at room temperature or overnight at 4°C. The antibodies used were HA.11 clone 16B12 (1:1000; BioLegend, California, USA), anti-β-actin (1:20 000–40:000; #47778, Santa Cruz Biotechnology, Santa Cruz, CA), anti-GAPDH-HRP (1:10 000; #47724, Santa Cruz Biotechnology, Santa Cruz, CA), anti-MBNL1 (1:200; #47740 Santa Cruz Biotechnology, Santa Cruz, CA), and anti-CELF1 (1:500; #20003, Santa Cruz Biotechnology, Santa Cruz, CA), anti-Staufen1 (1:2000; ab73478, Abcam, Toronto, Ontario, Canada), anti-PTEN (1:2000; #9188, Cell Signaling Technology, Massachusetts, USA), anti-Akt (pan) (1:2000; #4691, Cell Signaling Technology, Massachusetts, USA), anti-p-Akt (Ser473) (1:2000; #4060, Cell Signaling Technology, Massachusetts, USA), anti-GSK3 (1:1000; #12456, Cell Signaling Technology, Massachusetts, USA) and anti-p-GSK3 (Ser9) (1:1000; #5558, Cell Signaling Technology, Massachusetts, USA). Membranes were washed 3 × 10 min with 1X PBS + 0.05% Tween 20 and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. For HA, CELF1 and MBNL1 detection in mouse models, anti-mouse light chain-specific secondary antibodies were used (111-035-174, Jackson ImmunoResearch Laboratories Inc., PA, USA). Membranes were then washed 3 × 10 min with 1X PBS + 0.05% Tween 20 and signal detection was performed using Pierce ECL western blotting substrate and autoradiographed with x-ray film (Thermo Fisher Scientific, Ottawa, Canada). Quantification was performed using Image Lab Software (Bio-Rad, Mississauga, Ontario, Canada).
Immunofluorescence
Immunofluorescent staining was performed on 10 μm thick muscle cross-sections using the M.O.M Immunodetection Kit (#BM-2202, Vector Laboratories, Ontario, Canada) as per manufacturer instructions. Sections were incubated with primary antibodies anti-HA.11 clone 16B12 (1:100; BioLegend, California, USA), anti-Staufen1 (1:100; ab73478, Abcam, Toronto, Ontario, Canada), anti-CELF1-A594 (3B1) (1:100; sc-20 003 AF594, Santa Cruz Biotechnology, Santa Cruz, CA), anti-MBNL1-A594 (3A4) (1:50; sc-47 740 AF594, Santa Cruz Biotechnology, Santa Cruz, CA), for 30 min at room temperature. Sections were also incubated with Rabbit anti-Laminin (1:200; L9393, Sigma-Aldrich, Oakville, Ontario, Canada) or Mouse anti-Laminin clone LAM-89 (1:200; L8271, Sigma-Aldrich, Oakville, Ontario, Canada) for 30 min at room temperature. A Texas Red conjugated streptavidin detection system was applied (1:500) for fluorescent detection. Secondary antibodies with Alexa Fluor 488 antibodies (1:500) (Invitrogen Life Technologies, Burlington, Canada) were also used. The slides were mounted with Vectasheild mounting medium containing DAPI (Vector Laboratories, Ontario, Canada) and visualized using a Zeiss AxioImager.M2 microscope. A no-primary control was performed and acquisition parameters setup to ensure no signal in no-primary control condition.
RNA extraction, reverse transcription, and real-time quantitative PCR
Total RNA was extracted using TRIpure Isolation Reagent (Sigma-Aldrich, Oakville, Ontario, Canada). Extracted RNA was treated for 1 h at 37°C with DNAse I (Ambion Life Technologies, Burlington, Ontario, Canada). Reverse transcription (RT) was performed on DNAse I treated samples in a reaction mixture (5 mm MgCl2, 1× PCR buffer, 1 mm dNTP, 1 U/ml RNase inhibitor, 5 U/ml Moloney murine leukemia virus reverse transcriptase and 2.5 mm random hexamers) (Applied Biosystems, CA, USA) and incubated at 42°C, 45 min; 95°C, 5 min. mRNA expression was analyzed by RT-qPCR using QuantiTect SYBR Green PCR kit (QIAGEN, Toronto, Canada) and the MX3005p real-time PCR system (Stratagene, La Jolla, CA, USA) according to manufacturer’s instructions. Primer sequences were as follows: CELF1 (fwd 5′-CATCCCCAATGGTAAAG-3′, rev 5′-GTGTTGAGGTTCCCAGAG GA-3′), MBNL1 (fwd 5′-TGACTGTCGGTTTGCTCATC-3′, rev 5′-CAGCCTGGTTGACCTGGTAT-3′), MuRF1 (fwd 5′-TGTCTGGAGGTCGTTTCCG −3′, rev 5′-ATGCCGGTCCATGATCACTT-3′), MAFbx (fwd 5′- AGCGACCTCAGCAGTTACTGC-3′, rev 5′-CTTCTGGAATCCAGGATGGC-3′) and normalized to 18S (fwd 5′-CGCCGCTAGAGGTGAAATC-3′, rev 5′-CCAGTCGGCATCGTTTATGG-3′), or GAPDH (fwd 5′-GGGTGTGAACCACGAGAAAT-3′, rev 5′-CCTTCCACAATGCCAAAGTT-3′).
RNA splicing
Total RNA was extracted, and RT was performed as described above. Alternative splicing PCR was then carried out by either labeling 1.5 ng of the forward primer with 0.7 μCi of [γ32P]-dATP using 5U of T4 Polynucleotide Kinase and 1X Buffer A (Thermo Fisher Scientific, Ottawa, Canada) in a 10 μl reaction mixture incubated for 30 min at 37°C. The radiolabelled forward primer was then added to the PCR amplification mixture (1.75 mm MgCl2, 1X GoTaq® Buffer, 0.2 mm dNTP, 0.62 μm Forward Primer, 0.62 μm Reverse Primer, 1U GoTaq® DNA polymerase and Nuclease Free H2O ~50 μl) (Promega, Madison, WI, USA). Subsequent PCR was performed [95°C, 2 min; (95°C, 30 s; 55°C, 30 s; 72°C, 30 s) × 25 cycles; 72°C, 2 min]. Or by non-radiolabelled PCR for SERCA1 exon 22 (fwd exon 21 5′-ATCTTCAAGCTCCGGGCCCT-3′ rev; exon 23 5′-CAGCTTTGGCTGAAGATGCA-3′) (46), SERCA2b (fwd exon 19 5′-CTCCATCTGCTTGTCCAT-3′; rev exon 20 5′-GCGGTTACTCCAGTATTG-3′) (46), RyR1 ASI exon 70 (fwd exon 69 5′-GACAATAAGAGCAAAATGGC-3′; rev exon 71 5′-CTTGGTGCGTTCCTGATCTG-3′) (46), RyR1 ASII exon 83 (fwd exon 82 5′-CGAGAGGCAGAACAAGGCAG-3′; rev exon 84 5′-GGTCCTGTGTGAACTCGTCA-3′) (46), SERCA2b exon 19 (fwd 5′-CTCCATCTGCTTGTCCAT-3′; rev 5′-GCGGTTACTCCAGTATTG-3′) (46), TNNT3 exon F (fwd 5′-TCTGACGAGGAAACTGAACAAG-3′; rev 5′-TGTCAATGAGGGCTTGGAG-3′) (11), and DMD exon 78 (fwd 5′-TGGATCTGACATCTCATCAAGGAC-3′; rev 5′-CCATGCTAGCTACCCTGAGAC-3) (51). PCR products were resolved on 5% non-denaturing polyacrylamide gels, radiolabelled gels were dried using the gel drying system model 583 (Bio-Rad, Mississauga, Ontario, Canada) followed by autoradiography using GE Healthcare Amersham Hyperfilm (Thermo Fisher Scientific, Ottawa, Canada) and non-radiolabelled PCR gels were subsequently stained with GelRed™ Nucleic Acid Stain (Biotium Inc., Fremont, California, USA) and imaged using the Molecular Imager Gel Doc™ XR+ (Bio-Rad, Mississauga, Ontario, Canada). All alternative splicing ratios were quantified using Image Lab Software (Bio-Rad, Mississauga, Ontario, Canada).
Statistical analysis
The data were analyzed using Student’s t-test (two-tailed, equal variance) or One-way analysis of variance (ANOVA) with Bonferroni post hoc test. The level of significance was set at P ≤ 0.05. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. Error bars represent standard error of the mean (SEM).
Supplementary Material
Acknowledgements
The authors would like to acknowledge John Lunde, Amanda Tran and Dr Guy Bélanger for expert technical assistance.
Conflicts of Interest Statement: None declared.
Author Contributions
T.E.C.P., K.A.M., C.P. and A.R.C. performed the experiments. T.E.C.P., A.R.C. and B.J.J. conceived the project, designed the experiments and wrote the manuscript.
Funding
This work was supported by grants from the Canadian Institutes of Health Research, the Rachel Fund (Canadian Institutes of Health Research/Institute of Musculoskeletal Health and Arthritis, and Muscular Dystrophy Canada), the Association Française contre les Myopathies and the Muscular Dystrophy Association (USA). T.E.C.P. was a recipient of the Queen Elizabeth II Graduate Scholarships in Science and Technology.
References
- 1. Brook J.D., McCurrach M.E., Harley H.G., Buckler A.J., Church D., Aburatani H., Hunter K., Stanton V.P., Thirion J.P. and Hudson T. (1992) Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell, 68, 799–808. [DOI] [PubMed] [Google Scholar]
- 2. Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O’Hoy K. et al. (1992) Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science, 255, 1253–1255. [DOI] [PubMed] [Google Scholar]
- 3. Fu Y., Pizzuti A., Fenwick R.J., King J., Rajnarayan S., Dunne P., Dubel J., Nasser G., Ashizawa T., De Jong P. et al. (1992) An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science, 255, 1256–1258. [DOI] [PubMed] [Google Scholar]
- 4. Taneja K.L., McCurrach M., Schalling M., Housman D. and Singer R.H. (1995) Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol., 128, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis B.M., McCurrach M.E., Taneja K.L., Singer R.H. and Housman D.E. (1997) Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results. Proc. Natl. Acad. Sci. U. S. A., 94, 7388–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Rourke J.R. and Swanson M.S. (2009) Mechanisms of RNA-mediated disease. J. Biol. Chem., 284, 7419–7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J. and Cooper T. (2009) Pathogenic mechanisms of myotonic dystrophy. Biochem. Soc. Trans., 37, 1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanadia R.N., Shin J., Yuan Y., Beattie S.G., Wheeler T.M., Thornton C.A. and Swanson M.S. (2006) Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc. Natl. Acad. Sci. U. S. A., 103, 11748–11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller J.W., Urbinati C.R., Teng-Umnuay P., Stenberg M.G., Byrne B.J., Thornton C.A. and Swanson M.S. (2000) Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J., 19, 4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Philips A.V., Timchenko L.T. and Cooper T.A. (1998) Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science, 280, 737–741. [DOI] [PubMed] [Google Scholar]
- 11. Kanadia R.N., Johnstone K.A., Mankodi A., Lungu C., Thornton C.A., Esson D., Timmers A.M., Hauswirth W.W. and Swanson M.S. (2003) A muscleblind knockout model for myotonic dystrophy. Science, 302, 1978–1980. [DOI] [PubMed] [Google Scholar]
- 12. Ho T.H., Charlet-B N., Poulos M.G., Singh G., Swanson M.S. and Cooper T.A. (2004) Muscleblind proteins regulate alternative splicing. EMBO J., 23, 3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamberlain C.M. and Ranum L.P.W. (2012) Mouse model of muscleblind-like 1 overexpression: skeletal muscle effects and therapeutic promise. Hum. Mol. Genet., 21, 4645–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanger J.W., Kang S., Siebrands C.C., Freeman N., Du A., Wang J., Stout A.L. and Sanger J.M. (2005) How to build a myofibril. J. Muscle Res. Cell Motil., 26, 343–354. [DOI] [PubMed] [Google Scholar]
- 15. Ho T.H., Bundman D., Armstrong D.L. and Cooper T.A. (2005) Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet., 14, 1539–1547. [DOI] [PubMed] [Google Scholar]
- 16. Ward A.J., Rimer M., Killian J.M., Dowling J.J. and Cooper T.A. (2010) CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum. Mol. Genet., 19, 3614–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paul S., Dansithong W., Kim D., Rossi J., Webster N.J.G., Comai L. and Reddy S. (2006) Interaction of muscleblind, CUG-BP1 and hnRNP H proteins in DM1-associated aberrant IR splicing. EMBO J., 25, 4271–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D.H., Langlois M.A., Lee K.B., Riggs A.D., Puymirat J. and Rossi J.J. (2005) HnRNP H inhibits nuclear export of mRNA containing expanded CUG repeats and a distal branch point sequence. Nucleic Acids Res., 33, 3866–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paul S., Dansithong W., Jog S.P., Holt I., Mittal S., Brook J.D., Morris G.E., Comai L. and Reddy S. (2011) Expanded CUG repeats dysregulate RNA splicing by altering the stoichiometry of the muscleblind 1 complex. J. Biol. Chem., 286, 38427–38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pettersson O.J., Aagaard L., Andrejeva D., Thomsen R., Jensen T.G. and Damgaard C.K. (2014) DDX6 regulates sequestered nuclear CUG-expanded DMPK-mRNA in dystrophia myotonica type 1. Nucleic Acids Res., 42, 7186–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laurent F.X., Sureau A., Klein A.F., Trouslard F., Gasnier E., Furling D. and Marie J. (2012) New function for the RNA helicase p68/DDX5 as a modifier of MBNL1 activity on expanded CUG repeats. Nucleic Acids Res., 40, 3159–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones K., Wei C., Schoser B., Meola G., Timchenko N. and Timchenko L. (2015) Reduction of toxic RNAs in myotonic dystrophies type 1 and type 2 by the RNA helicase p68/DDX5. Proc. Natl. Acad. Sci. U. S. A., 112, 8041–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravel-Chapuis A., Bélanger G., Yadava R.S., Mahadevan M.S., DesGroseillers L., Côté J. and Jasmin B.J. (2012) The RNA-binding protein Staufen1 is increased in DM1 skeletal muscle and promotes alternative pre-mRNA splicing. J. Cell Biol., 196, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. St Johnston D., Beuchle D. and Nüsslein-Volhard C. (1991) Staufen, a gene required to localize maternal RNAs in the drosophila egg. Cell, 66, 51–63. [DOI] [PubMed] [Google Scholar]
- 25. Li P., Yang X., Wasser M., Cai Y., Chia W. and Chiat W. (1997) Inscuteable and staufen mediate asymmetric localization and segregation of prospero RNA during drosophila neuroblast cell divisions. Cell, 90, 437–447. [DOI] [PubMed] [Google Scholar]
- 26. Schuldt A.J., Adams J.H.J., Davidson C.M., Micklem D.R., Haseloff J., St. Johnston D. and Brand A.H. (1998) Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev., 12, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breitwieser W., Markussen F.H., Horstmann H. and Ephrussi A. (1996) Oskar protein interaction with vasa represents an essential step in polar granule assembly. Genes Dev., 10, 2179–2188. [DOI] [PubMed] [Google Scholar]
- 28. Bondy-Chorney E., Crawford Parks T.E., Ravel-Chapuis A., Klinck R., Rocheleau L., Pelchat M., Chabot B., Jasmin B.J. and Côté J. (2016) Staufen1 regulates multiple alternative splicing events either positively or negatively in DM1 indicating its role as a disease modifier. PLoS Genet., 12, e1005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bélanger G., Stocksley M.A., Vandromme M., Schaeffer L., Furic L., DesGroseillers L. and Jasmin B.J. (2003) Localization of the RNA-binding proteins Staufen1 and Staufen2 at the mammalian neuromuscular junction. J. Neurochem., 86, 669–677. [DOI] [PubMed] [Google Scholar]
- 30. Gong C., Kim Y.K., Woeller C.F., Tang Y. and Maquat L.E. (2009) SMD and NMD are competitive pathways that contribute to myogenesis: effects on PAX3 and myogenin mRNAs. Genes Dev., 23, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang Y., Chou H., Yen Y., Chang H., Hong Y., Huang H. and Tseng C. (2012) Agrin induces association of Chrna1 mRNA and nicotinic acetylcholine receptor in C2C12 myotubes. FEBS Lett., 586, 3111–3116. [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi Y., Oohinata R., Naiki T. and Irie K. (2008) Stau1 negatively regulates myogenic differentiation in C2C12 cells. Genes Cells, 13, 583–592. [DOI] [PubMed] [Google Scholar]
- 33. Yamaguchi Y., Naiki T. and Irie K. (2012) Stau1 regulates Dvl2 expression during myoblast differentiation. Biochem. Biophys. Res. Commun., 417, 427–432. [DOI] [PubMed] [Google Scholar]
- 34. Ravel-Chapuis A., Crawford T.E., Blais-Crépeau M.-L., Bélanger G., Richer C.T. and Jasmin B.J. (2014) The RNA-binding protein Staufen1 impairs myogenic differentiation via a c-myc-dependent mechanism. Mol. Biol. Cell, 25, 3765–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crawford Parks T.E., Ravel-chapuis A., Bondy-chorney E., Renaud J., Côté J. and Jasmin B.J. (2017) Muscle-specific expression of the RNA-binding protein Staufen1 induces progressive skeletal muscle atrophy via regulation of phosphatase tensin homolog. Hum. Mol. Genet., 26, 1821–1838. [DOI] [PubMed] [Google Scholar]
- 36. Mateos-Aierdi A.J., Goicoechea M., Aiastui A., Fernández-Torrón R., Garcia-Puga M., Matheu A., de Munain A.L. and López de Munain A. (2015) Muscle wasting in myotonic dystrophies: a model of premature aging. Front. Aging Neurosci., 7, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Timchenko L. (2013) Molecular mechanisms of muscle atrophy in myotonic dystrophies. Int. J. Biochem. Cell Biol., 45, 2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morriss G.R., Rajapakshe K., Huang S., Coarfa C. and Cooper T.A. (2018) Mechanisms of skeletal muscle wasting in a mouse model for myotonic dystrophy type 1. Hum. Mol. Genet., 27, 2789–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gomes-Pereira M., Foiry L., Nicole A., Huguet A., Junien C., Munnich A. and Gourdon G. (2007) CTG trinucleotide repeat “big jumps”: large expansions, small mice. PLoS Genet., 3, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vignaud A., Ferry A., Huguet A., Baraibar M., Trollet C., Hyzewicz J., Butler-Browne G., Puymirat J., Gourdon G. and Furling D. (2010) Progressive skeletal muscle weakness in transgenic mice expressing CTG expansions is associated with the activation of the ubiquitin-proteasome pathway. Neuromuscul. Disord., 20, 319–325. [DOI] [PubMed] [Google Scholar]
- 41. Seznec H., Agbulut O., Sergeant N., Savouret C., Ghestem A, Tabti N., Willer J. C., Ourth L., Duros C., Brisson E.. et al (2001) Mice transgenic for the human myotonic dystrophy region with expanded CTG repeats display muscular and brain abnormalities. Hum. Mol. Genet., 10, 2717–2726. [DOI] [PubMed] [Google Scholar]
- 42. Orengo J.P., Chambon P., Metzger D., Mosier D.R., Snipes G.J. and Cooper T.A. (2008) Expanded CTG repeats within the DMPK 3’ UTR causes severe skeletal muscle wasting in an inducible mouse model for myotonic dystrophy. Proc. Natl. Acad. Sci., 105, 2646–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mankodi A., Logigian E., Callahan L., McClain C., White R., Henderson D., Krym M. and Thornton C. (2000) Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science, 289, 1769–1772. [DOI] [PubMed] [Google Scholar]
- 44. Mankodi A., Takahashi M., Jiang H., Beck C., Bowers W., Moxley R., Cannon S. and Thornton C. (2002) Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell, 10, 35–44. [DOI] [PubMed] [Google Scholar]
- 45. Sugimoto Y., Vigilante A., Darbo E., Zirra A., Militti C., Ambrogio A.D., Luscombe N.M. and Ule J. (2015) hiCLIP reveals the in vivo atlas of mRNA secondary structures recognized by Staufen 1. Nature, 519, 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kimura T., Nakamori M., Lueck J.D., Pouliquin P., Aoike F., Fujimura H., Dirksen R.T., Takahashi M.P., Dulhunty A.F. and Sakoda S. (2005) Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum. Mol. Genet., 14, 2189–2200. [DOI] [PubMed] [Google Scholar]
- 47. Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Meuse L., Miller D.G., Russell D.W. and Chamberlain J.S. (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med., 10, 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blankinship M.J., Gregorevic P., Allen J.M., Harper S.Q., Harper H., Halbert C.L., Miller A.D. and Chamberlain J.S. (2004) Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol. Ther., 10, 671–678. [DOI] [PubMed] [Google Scholar]
- 49. Wang Z., Tapscott S.J., Chamberlain J.S. and Storb R. (2011) Immunity and AAV-mediated gene therapy for muscular dystrophies in large animal models and human trials. Front. Microbiol., 2, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naso M.F., Tomkowicz B., Perry W.L. and Strohl W.R. (2017) Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs, 31, 315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rau F., Lainé J., Ramanoudjame L., Ferry A., Arandel L., Delalande O., Jollet A., Dingli F., Lee K.Y., Peccate C. et al. (2015) Abnormal splicing switch of DMD’s penultimate exon compromises muscle fibre maintenance in myotonic dystrophy. Nat. Commun., 6, 7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jones K., Wei C., Iakova P., Bugiardini E., Schneider-gold C., Meola G., Woodgett J., Killian J., Timchenko N.A. and Timchenko L.T. (2012) GSK3 β mediates muscle pathology in myotonic dystrophy. J. Clin. Invest., 122, 4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K. et al. (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science, 294, 1704–1708. [DOI] [PubMed] [Google Scholar]
- 54. Ratti F., Ramond F., Moncollin V., Simonet T., Milan G., Méjat A., Thomas J.L., Streichenberger N., Gilquin B., Matthias P. et al. (2015) Histone deacetylase 6 is a FoxO transcription factor dependent effector in skeletal muscle atrophy. J. Biol. Chem., 290, 4215–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bodine S.C. and Baehr L.M. (2014) Skeletal muscle atrophy and the E3 ubiquitin ligases, MuRF1 and MAFbx/Atrogin-1. Am. J. Physiol. Endocrinol. Metab., 307, E469–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crawford Parks T.E., Marcellus K.A., Langill J., Ravel-chapuis A., Michaud J., Cowan K.N., Côté J. and Jasmin B.J. (2017) Novel roles for Staufen1 in embryonal and alveolar rhabdomyosarcoma via c-myc- dependent and -independent events. Sci. Rep., 17, 42342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ravel-Chapuis A., Gunnewiek A.K., Belanger G., Crawford Parks T.E., Cote J. and Jasmin B.J. (2016) Staufen1 impairs stress granule formation in skeletal muscle cells from Myotonic dystrophy type 1 patients. Mol. Biol. Cell, 27, 1728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yadava R.S., Kim Y.K., Mandal M., Mahadevan K., Gladman J.T., Yu Q. and Mahadevan M.S. (2019) MBNL1 overexpression is not sufficient to rescue the phenotypes in a mouse model of RNA toxicity. Hum. Mol. Genet., 28, 2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Savkur R.S., Philips A.V. and Cooper T.A. (2001) Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet., 29, 40–47. [DOI] [PubMed] [Google Scholar]
- 60. Timchenko N.A., Cai Z.J., Welm A.L., Reddy S., Ashizawa T. and Timchenko L.T. (2001) RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J. Biol. Chem., 276, 7820–7826. [DOI] [PubMed] [Google Scholar]
- 61. Timchenko N.A., Patel R., Iakova P., Cai Z., Quan L. and Timchenko L.T. (2004) Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. J. Biol. Chem., 279, 13129–13139. [DOI] [PubMed] [Google Scholar]
- 62. Kuyumcu-Martinez N.M., Wang G.-S. and Cooper T.A. (2007) Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol. Cell, 28, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salisbury E., Sakai K., Schoser B., Huichalaf C., Schneider-Gold C., Nguyen H., Wang G.-L., Albrecht J.H. and Timchenko L.T. (2008) Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein. CUGBP1. Exp. Cell Res., 314, 2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huichalaf C., Sakai K., Jin B., Jones K., Wang G.L., Schoser B., Schneider-Gold C., Sarkar P., Pereira-Smith O.M., Timchenko N. et al. (2010) Expansion of CUG RNA repeats causes stress and inhibition of translation in myotonic dystrophy 1 (DM1) cells. FASEB J., 24, 3706–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Orengo J.P., Ward A.J. and Cooper T.A. (2011) Alternative splicing dysregulation secondary to skeletal muscle regeneration. Ann. Neurol., 69, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Charizanis K., Lee K.-Y., Batra R., Goodwin M., Zhang C., Yuan Y., Shiue L., Cline M., Scotti M.M., Xia G. et al. (2012) Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron, 75, 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wickham L., Duchaîne T., Luo M., Nabi I.R. and DesGroseillers L. (1999) Mammalian Staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol. Cell. Biol., 19, 2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


