Patients undergoing solid-organ transplantation (SOT) or allogeneic hematopoietic cell transplantation (HCT) are at increased risk for infectious complications. Community-acquired respiratory viruses (CARVs) pose a particular challenge due to the frequent exposure pre-, peri-, and posttransplantation.
KEYWORDS: bone marrow transplantation, diagnosis, respiratory viruses, solid-organ transplantation, treatment, vaccination
SUMMARY
Patients undergoing solid-organ transplantation (SOT) or allogeneic hematopoietic cell transplantation (HCT) are at increased risk for infectious complications. Community-acquired respiratory viruses (CARVs) pose a particular challenge due to the frequent exposure pre-, peri-, and posttransplantation. Although influenza A and B viruses have a top priority regarding prevention and treatment, recent molecular diagnostic tests detecting an array of other CARVs in real time have dramatically expanded our knowledge about the epidemiology, diversity, and impact of CARV infections in the general population and in allogeneic HCT and SOT patients. These data have demonstrated that non-influenza CARVs independently contribute to morbidity and mortality of transplant patients. However, effective vaccination and antiviral treatment is only emerging for non-influenza CARVs, placing emphasis on infection control and supportive measures. Here, we review the current knowledge about CARVs in SOT and allogeneic HCT patients to better define the magnitude of this unmet clinical need and to discuss some of the lessons learned from human influenza virus, respiratory syncytial virus, parainfluenzavirus, rhinovirus, coronavirus, adenovirus, and bocavirus regarding diagnosis, prevention, and treatment.
INTRODUCTION
Patients undergoing allogeneic hematopoietic cell transplantation (HCT) or solid-organ transplantation (SOT) are at increased risk for infectious complications (1), among which community-acquired respiratory viruses (CARVs) are unique due to the frequent exposure pre-, peri-, and posttransplantation (2–5). Since, following successful transplantation, return to a normal life situation is among the key goals, the avoidance of CARV exposure becomes a challenging task.
Only a decade ago, the seasonal increase in patients presenting with symptoms and signs of respiratory tract infectious disease (RTID) was the main parameter defining “influenza-like illness” (ILI). The epidemiology of ILI was complemented by parallel increases of influenza virus A and B (IV-A and IV-B) detection in the population using classic techniques, such as direct antigen detection (DAD) or virus isolation in cell culture (VIC), for subtyping and vaccine prediction of IV-A and IV-B strains (6). Even though such sentinel testing was also available for human respiratory syncytial virus (HRSV) and human parainfluenzaviruses (HPIV) (7, 8), CARV-specific diagnostics in individual patients presenting with RTID were largely limited to academic medical centers (9) to serve patients at high risk for complications, such as neonates, patients with chronic heart and lung diseases, and transplant patients (10–13). The general landscape has fundamentally changed following the IV-A/H1N1/pdm09 pandemic in 2009 (14), rendering molecular detection of influenza and other CARVs by nucleic acid testing (NAT) widely available in general health care in resource-rich parts of the world (15). Moreover, multiplex NAT and (semi)quantitative NAT (QNAT) now provide comprehensive information about the diversity, dynamics, and impact of CARVs in the general pediatric and adult population (15–18). Apart from classical ILI, CARVs, including IV-A/B, HRSV, HPIV, human rhinovirus (HRV), human metapneumovirus (HMPV), human coronaviruses (HCoV), human bocavirus (HBoV), and human adenovirus (HAdV), are now known to cause overlapping manifestations of other RTIDs, including exacerbations of wheezing/asthma, croup, bronchiolitis, and pneumonitis/pneumonia (18). For example, a large study of 6,073 children analyzed CARV-attributed symptoms and signs and compared the results with data obtained from a literature meta-analysis covering 49,858 patients (19). The authors concluded that although there were significant associations of, for instance, IV-A/B with fever or headache or of HRSV with cough, dyspnea, and wheezing, none of the RTID presentations were pathognomonic or even unique for a specific CARV. As no clinical algorithm exists that reliably predicts the etiology of CARV-RTID (19), laboratory testing has become essential for both timely diagnosis and intervention. A practical guidance document has recently been published outlining preanalytical and analytical considerations with recommendations regarding specimen collection and preferred laboratory testing for diagnosis of CARV causing acute RTID (20). The intelligent use of clinical and laboratory resources in real time will undoubtedly improve the management of CARV, which includes restricting empirical antibiotic administration, improving infection control and patient cohorting, as well as delivering specific antiviral treatment for CARVs, if available. Moreover, careful review of the data will serve to define patient populations that potentially benefit from future antiviral drug and vaccine development.
While the global impact of CARVs among lower RTIDs has been estimated to have increased in recent years (21) and the clinical impact of specific CARVs in the routine care of RTID is being addressed in ongoing investigations (22–24), the widespread availability of multiplex NAT results already raises questions about CARV prevention and treatment in transplant patients.
It has become clear that classic definitions of ILI and severe acute respiratory infection (SARI) (25) are not helpful for CARV-mediated RTID in transplant patients (26–30). This is not only due to altered, often mitigated and delayed clinical presentation requiring more appropriately adapted case definitions (Table 1) but also reflects the overlapping epidemiology of CARVs, as summarized elsewhere (5). Conventionally, upper RTID is defined as CARVs affecting anatomic sites above and including the larynx (and virus is detectable in samples from nose, pharynx, larynx, conjunctivae, or sinuses), whereas lower RTID includes trachea, bronchus, and bronchoalveolar sites. A frequently used definition of lower RTID uses a combination of detecting CARVs in respiratory fluids obtained from the lower respiratory tract by bronchoalveolar lavage (BAL) fluid (presumptive) or biopsy specimens (proven) together with a lowered oxygen saturation and new pulmonary infiltrates (5). Recently, detailed definitions for lower RTID by HPIV in allogeneic HCT patients have been proposed (31), which could be adapted for other CARVs, except that proven (histological evidence) and presumptive (laboratory detection in BAL fluid) lower RTID have been combined. Although this approach seems reasonable for retrospective studies and cohorts, it is clear that the level of evidence is not equivalent in the two situations. Moreover, highly sensitive NAT detection is faced with the potential for contamination of respiratory fluids from the lower respiratory tract by viruses replicating at high level in the upper respiratory tract, which require a thorough understanding of semiquantitative levels and relevant diagnostic thresholds (32, 33). Here, we review the current knowledge on diversity and impact of CARVs in SOT and allogeneic HCT patients to better define the magnitude of this unmet clinical need and to discuss some of the lessons learned from IV-A/B, HRSV, HPIV, and other CARVs regarding prevention and antiviral treatment.
TABLE 1.
Case definitions of CARV-RTIDa
| Case definition |
|---|
| Case classification |
|
| Clinical criteria |
|
| AND |
|
| AND |
|
| Epidemiological criteria |
|
| Laboratory criteria |
|
| AND |
|
Modified from reference 5.
EPIDEMIOLOGY AND DIVERSITY OF CARVs IN THE GENERAL POPULATION
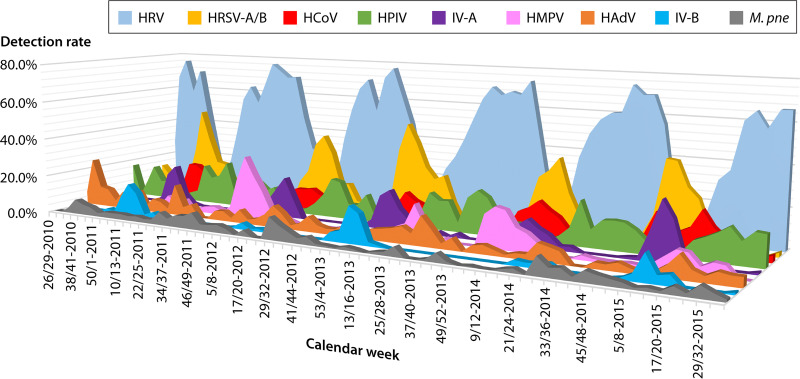
Large comprehensive studies using multiplex NAT for symptomatic children and adults have linked a multitude of CARVs to partly overlapping clinical presentations of RTID (18, 19). In a cross-sectional study including nasopharyngeal swabs (71%) and BAL fluid (27%) from 116 symptomatic children and 155 adults attending tertiary care centers, the most frequently detected pathogen was HRV, followed by HRSV, IV-A/B, and HPIV (34). In a comprehensive longitudinal Swiss study of a total of 11,446 samples from 5,135 symptomatic children (N = 5,135) and adults (N = 6,316) tested by multiplex NAT from 2010 to 2015, HRVs (38%) predominated in both populations during the summer months (Fig. 1) and were seasonally replaced during autumn and winter by HRSV (17%), IV-A/B (11%), HCoVs (11%), HPIVs (9%), HPMV (5%), and, rarely, HAdV (4%) (35). However, the detection rates in children were around 70%, significantly higher than the 30% found in adults, despite the higher rates of underlying morbidities, including being immunocompromised, and the use of BAL fluid in the latter (35). Interestingly, similar CARV rates have been recently reported among mostly very young pediatric SARI cases in a resource-limited setting in Uganda, where the initial molecular IV-A/B testing of 8% was complemented by metagenomic deep sequencing (36, 37). Notably, little new information is available for human polyomaviruses, such as WU and KI, originally discovered in CARV-negative respiratory fluids (for a review, see reference 38), which may be due to the fact that they are not part of current multiplex NAT panels.
FIG 1.
CARV epidemiology over 5 years in symptomatic children and adults attending a tertiary care center, adapted from reference 35. HAdV, human adenoviruses; HCoV, human coronavirus (-229E, -OC43, -NL63, and -HKU1); IV-A, influenza virus A; IV-B, influenzavirus B; HMPV, human metapneumovirus; HPIV, human parainfluenzavirus (1 to 4); HRSV, human respiratory syncytial virus (A/B); HRV, human rhinovirus; M.pne, Mycoplasma pneumoniae (for comparison).
A study of adult patients admitted for ILI during the 2009–2011 season in Hong Kong indicated that HRSV was the cause of severe lower RTID in older adults, resulting in respiratory failure, prolonged hospitalization, and high mortality similar to that of seasonal influenza (22). The clinical impact of HPIV and the respective subtypes 1 to 4 was recently described in a 7-year retrospective U.S. study from Chicago covering 550 adults with a mean age of 60.4 years (23). Admission to intensive care units (ICUs) and death were seen in 129 and 28 patients, respectively, and there was no significant difference between patients above or below 65 years of age or those who were or were not immunocompromised (23). In a multicenter Italian study of 414 patients with community-acquired pneumonia requiring admission to intensive care, 226 (55%) had evidence of CARV-RTID, where IV-A was the most common virus in 140 patients (62%), followed by HRV (15%), HRSV (6%), IV-B (4%), HCoV (4%), cytomegalovirus (CMV; 4%), and HMPV (0.4%) (39). The underlying risk factors were similar to those known to contribute to excess mortality during influenza season, namely, older age and chronic organ failures, especially heart and lung diseases (22). In a Dutch study of patients older than 65 years, IV-A and HRSV were associated with excess mortality, whereas IV-B and HPIV particularly affected those aged 75 years and older (40). In hospital-acquired pneumonia of nonventilated patients, CARVs were detected in 22.4%, a rate similar to that for bacterial pathogens, where the most common viruses were HRV, IV-A/B, and HPIV. A French and Belgian collaborative study reported that detection of CARVs by multiplex NAT was associated with higher ICU mortality, and this association was strongest for IV-A/B, HPIV, and HRSV (41). CARV detection independently predicted ICU mortality in patients with acute respiratory failure (41), an observation shared by other studies (42, 43). Together, the currently available evidence suggests that influenza and non-influenza CARVS represent recurrent, often seasonally exacerbating causes of RTID morbidity in the general population around the globe, requiring significant health care resources and causing excess mortality in the very young, in the very old, and in patients with chronic medical conditions of heart, lung, kidney, diabetes mellitus, and transplantation (21, 44, 45). Indeed, among 747 critically ill hematology patients, CARV-RTID was identified by multiplex NAT in 21.3%, occurring particularly in patients with malignancy and HCT and increasing the risk of ICU mortality by an odds ratio (OR) of 2.07 (95% confidence interval [CI], 1.22 to 3.50) (41).
GENERAL ASPECTS OF CARVs IN THE TRANSPLANT POPULATION
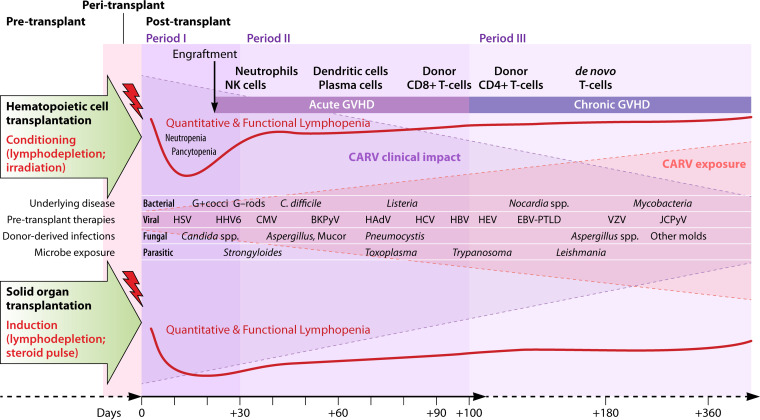
The impact of CARVs in transplant recipients depends on diverse factors, including the susceptibility of the host, the overall number (quantity) and effector functions (quality) of immune responses, and also the inoculum size and intrinsic pathogenicity of the specific infectious agent. Obviously, an allogeneic constellation between virus-infected cells and immune effectors contributes to impaired immune control while at the same time enhancing inflammatory (allo-)immune responses necessitating prophylactic and therapeutic immunosuppressive treatment (46). Not unexpectedly, CARVs have their highest impact in lung transplantation and allogeneic HCT (46). In addition, CARV epidemiology and transplantation intersect, seasonally exposing transplant patients to different CARVs in different periods of vulnerability (Fig. 2). For both SOT and allogeneic HCT, the underlying disease and other preexisting medical conditions and their treatments prior to transplantation may already cause significant impairment of the innate and adaptive immune defense, thereby increasing their vulnerability pre- and peritransplant, an aspect that is often underestimated (Fig. 2). Importantly, deferral of transplantation remains an option to be considered in patients with RTID pretransplant, particularly for those patients who are considered to be at higher risk of significant morbidity and mortality, e.g., after allogeneic HCT, if the underlying disease permits (5, 47). These considerations deserve explicit attention for pretransplant infections with IV-A/B, HRSV, HPIV, and HMPV in patients scheduled for allogeneic HCT. For SOT recipients, especially for lung transplant recipients, these issues are equally challenging and need to be balanced against the risk of transplant deferral, particularly in view of the organ donor shortage, the transplanted organ, and the availability of antiviral treatment (48, 49).
FIG 2.
Timetable of infectious disease events after allogeneic hematopoietic cell transplantation and solid-organ transplantation. BKPyV, BK polyomavirus; C. difficile, Clostridium difficile; CARV, community-acquired respiratory viruses; CMV, cytomegalovirus; EBV, Epstein-Barr virus; G+, Gram-positive; G−, Gram negative; HAdV, human adenovirus; HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; HHV-6, human herpesvirus-6; HSV, herpes simplex virus; JCPyV, JC polyomavirus; PTLD, posttransplant lymphoproliferative disorder.
After allogeneic HCT, infectious disease (ID) events unfold their impact in three conceptual periods of vulnerability, defined first by conditioning and neutropenia (period I), followed by engraftment and immune reconstitution, e.g., from day +30 to day +100 (period II), and period III, beyond day +100 (Fig. 2). In clinical practice, these periods represent a continuum whereby the second and third period frequently overlap, and this needs to be integrated in the individual patient assessment. Key variables influencing the net state of immunodeficiency are the intensity and toxicity of conditioning (myeloablative versus nonmyeloablative; total body irradiation), the graft source (bone marrow, peripheral stem cells, and cord blood), the quantity and quality (CD34 count, related versus unrelated donor, and HLA mismatching), the transplanted donor immunity, and the desired and undesired immunologic consequences of graft versus malignancy and graft versus host, where the latter extends the vulnerable period due to immunosuppressive prophylaxis or therapy. However, neutropenia may be short or missing in some protocols of nonmyeloablative conditioning, reduced intensity, and reduced toxicity conditioning (50–52).
After SOT, ID events can also be attributed to 3 periods (53), where nosocomial, surgical, donor-derived, and early herpesvirus infections predominate in the first 4 weeks posttransplant (period I), followed by reactivating latent, opportunistic, and environmental infectious agents during the subsequent periods II and III of 3 to 6 and up to 12 months posttransplant, respectively (Fig. 2). During the first 3 months after SOT, immunosuppression is generally highest and may be even more intense if lymphocyte-depleting induction, higher maintenance immunosuppression, and antirejection treatments are necessary for countering pronounced antidonor/graft immunity, e.g., as defined by panel-reactive antibodies and/or acute cell- or antibody-mediated rejection episodes. Therefore, antimicrobial immune responses may be significantly compromised in proliferation and effector functions that may extend far beyond the first 3 to 12 months posttransplant.
In SOT, the epidemiology of CARVs follows the local seasonal pattern seen in the general population, for example, in the Northern Hemisphere (Fig. 1), but differs in tropical and subtropical countries (36, 54). However, most SOT data are available from lung transplant patients due to the increased surveillance and clinical impact (33, 55–58). IV-A/B and HRSV occur predominantly in the winter months, HRV and HCoV predominate in spring and fall together with peaks of HPIV, while HAdV is detected year-round (55). Clinical disease is generally mild and self-limited in non-lung transplant recipients, although severe and progressive illness can be seen in any transplant patient. Young children, because of limited preexisting immunity and heavy exposure, are at greater risk for severe RTID and their complications (57). This also applies to patients that are more profoundly immunosuppressed due to the type of transplant and/or the receipt of lymphocyte-depleting agents within the past 3 to 6 months. Lastly, patients with severe hypogammaglobulinemia of <4.5 g/liter or lymphopenia of <300/μl appear to be at heightened risk of severe and progressive CARV-RTID. However, initial signs and symptoms are less prevalent and are not specific to one particular CARV (59). Among IV-A/B-infected SOT recipients, for instance, only 64% had fever, 35% had sore throat, and 40% had myalgias (60). Notably, CARVs are associated with long-term consequences, particularly in lung transplant recipients (55, 61).
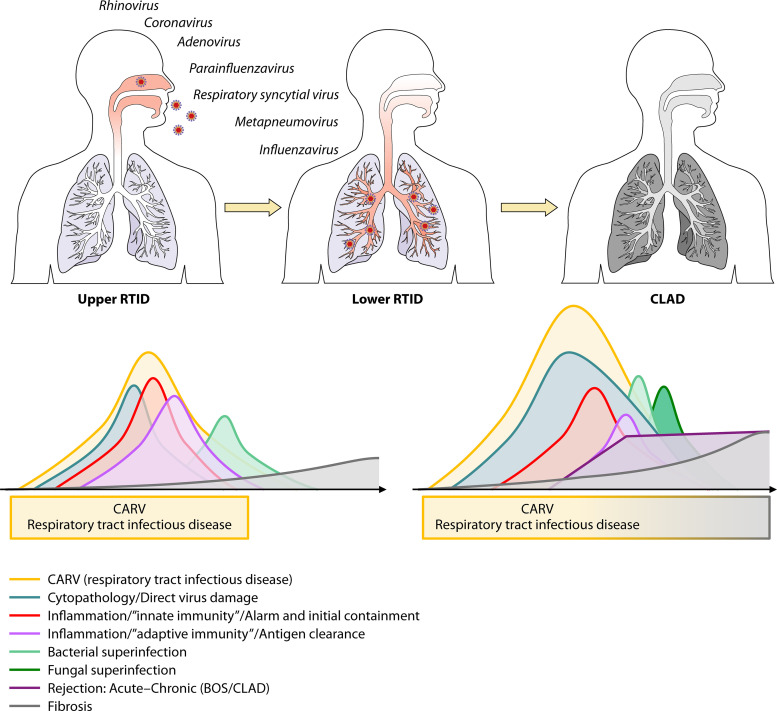
While there is concern that CARVs can trigger local alloimmune responses, i.e., acute rejection (62), other recent studies suggest that the inflammatory infiltrates noted on biopsy specimens during acute rejection are mostly antiviral responses helping to clear the infection (63). Following the viral cytopathic insult and the resulting impairment of the mucociliary clearance (Fig. 3), an increased risk of bacterial superinfection ensues, which extends to fungal complications in immunocompromised patients, mandating an increased index of suspicion in clinical care (22, 64). Furthermore, through the elicited innate and adaptive immune responses, CARVs have been discussed as a trigger of acute and chronic rejection, bronchiolitis obliterans syndrome (BOS), and chronic lung allograft dysfunction (CLAD) (Fig. 3). The historic and current definitions of CLAD have been recently reviewed (65). Thus, a reduced forced expiratory volume in the first second of expiration (FEV1) or forced vital capacity (FVC) of less than or equal to 90% for 3 weeks or more raises the suspicion of CLAD. Subsequently, restrictive CLAD (restrictive allograft syndrome) is diagnosed when FVC is ≤80% for >3 weeks and can be distinguished from obstructive CLAD-BOS (FEV1 of ≤80%) for >3 weeks and mixed forms. These different terms are still used in the relevant CARV literature and will be presented as used in the original works.
FIG 3.
Schematic illustration of community-acquired respiratory virus infection and pathogenesis in immunocompetent and immunocompromised patients posttransplant in the allogeneic constellation of lung transplantation and hematopoietic cell transplantation. (Top) Infections with CARVs causing mostly upper- and lower-respiratory-tract infectious disease (RTID) and following lower RTID progression to chronic allograft disease/dysfunction (CLAD) in the allogeneic setting of lung or hematopoietic cell transplantation. (Bottom, left) Relative level and intensity of direct viral cytopathology in the respiratory tract, of the inflammation caused by the innate and adaptive immune response, and of the bacterial superinfection in a patient with no or moderate immunodeficiency. (Bottom, right) The same parameters for allogeneic HCT or lung transplant patients with severe immunodeficiency but including an alloimmune response (rejection), an increased risk of bacterial and fungal superinfection, and progression to bronchiolitis obliterans syndrome (BOS) and CLAD.
With respect to the pathogenesis, it remains to be demonstrated by randomized specific antiviral, anti-inflammatory, and/or antifibrogenic interventions whether or not CARVs are causal or just a cytopathic inducer of an aberrant immune response and how these responses are aggravated in transplant patients across allogeneic immune barriers in lung and allogeneic HCT patients (Fig. 3). Most likely due to small sample sizes and study heterogeneities, a review and meta-analysis did not identify a significant association of CARVs with acute rejection but possibly with CLAD in lung transplant recipients (63). However, in a more recent large single-center retrospective study of 250 lung transplantations performed from 2007 to 2012, 50 (20%) developed CLAD during the median follow-up of 143 weeks, consisting of BOS in 33 (66%) and restrictive CLAD in 17 (34%). No surveillance testing was done, but 117 episodes of upper and lower CARV-RTID were identified by multiplex NAT in 79 (31.6%) patients, where the first episode occurred at a median of 133 days posttransplant (interquartile range [IQR], 63 to 441 days). In multivariate models, CLAD was independently associated with prior acute rejection (adjusted hazard ratio [HR], 2.16; 95% CI, 1.18 to 3.93; P = 0.01) and CARV-RTID (adjusted HR, 1.92; 95% CI, 1.07 to 3.45; P = 0.03). In a prospective cohort study from 2009 to 2014 enrolling 98 lung transplant recipients, CLAD was diagnosed in 38% at a median time of 20.4 months (IQR, 12 to 30.4) (61). In the time-controlled multivariate analysis, CLAD was associated with CARV-LRTID (HR, 3.00; 95% CI, 1.52 to 5.91; P = 0.002), acute rejection (HR, 2.97; 95% CI, 1.51 to 5.83; P = 0.002), and CMV pneumonitis (HR, 3.76; 95% CI, 1.23 to 11.49; P = 0.02) (61).
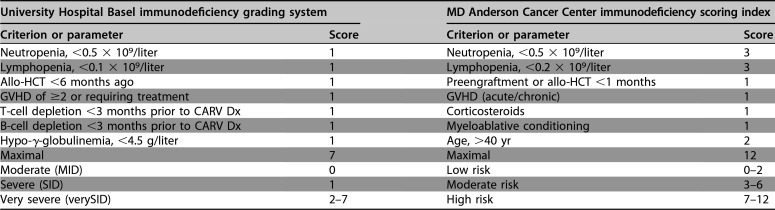
In allogeneic HCT, CARV exposure also follows the local epidemiology, placing significant emphasis on infection control measures inside and outside the hospital, particularly for neutropenic and lymphopenic patients during the winter months. In a seminal study of 122 HCT patients using the complete set of clinical and laboratory assessments, including DAD, VIC, and NAT, 30% developed CARV infections up to day +100 (26). HPIV were detected in approximately two-thirds of patients, followed by IV-A/B, HRSV, and HMPV in less than 10% each. Cough, rhinorrhea, or nasal congestion were leading clinical presentations and somewhat unspecific across all the different CARVs, but they were significantly more frequent among virus-positive than virus-negative patients. Fever above 38°C and myalgia only occurred in a third of HCT patients with IV-A/B or HRSV, emphasizing that standard ILI definitions cannot be applied (5). Interestingly, CARV loads were lower in patients without or with only one symptom/sign, suggesting that (semi)quantitative viral loads are of interest for clinical and virological follow-up (27, 66). Conversely, prolonged asymptomatic shedding of HRSV and HPIV has been shown to give rise to nosocomial outbreaks among in- and outpatients (26, 67–71). In a Spanish report, 36% of CARVs in lower RTID occurred between day +100 and 12 months posttransplant, and the mean time from HCT to HRSV infection was +466 days (IQR, 163 to 1,028) in a recent U.S. study (72). In a Swiss study of symptomatic allogeneic HCT recipients between 2010 and 2014 using comprehensive multiplex NAT, 419 (39%) CARV ID events were detected in 1,069 tests, where the leading pathogens were HRV in 21%, occurring year-round but peaking during summer, followed by Paramyxo- and Pneumoviridae (14% HPIV, HRSV, and HMPV) and IV-A/B (3%) (73). Notably, RTID caused by HRSV, HMPV, and HPIV occurred during the cold seasons at a median time of days +382, +504, and +628 after allogeneic HCT (73), respectively. A large retrospective multicenter study of 1,560 cases of pediatric HCT identified at least one CARV infection in 259 patients (16.6%) occurring until 1 year posttransplant and typically affected younger children (4.8 versus 7.1 years; P < 0.001) (74). Importantly, 48% required some respiratory support, 14% suffered significant pulmonary sequelae, and all-cause and attributable case fatality rates within 3 months were 11% and 5.4%, respectively. Independent risk factors for morbidity and mortality were CARV infection within 60 days of HCT, need for respiratory support, and steroid administration within 1 week of RTID (74). Together, various factors reflecting impaired immune control were linked to progression to lower RTID and mortality, in line with the notion that vulnerability to CARVs extends beyond day +100 and may be approximated by clinical and laboratory parameters attempting to capture the degree of vulnerability as either immunodeficiency grading (moderate, severe, and very severe) or as immunodeficiency score index (27, 66, 75–78) (Table 2). A few studies have compared both tools (73, 77, 78) and found a significant overlap in predicting progression to HRSV lower RTID and mortality despite the fact that only portions of the parameters were identical. Similar grading tools have not been proposed for lung transplant recipients.
TABLE 2.
Clinical criteria proposed to identify patients undergoing allo-HCT at risk for complicated CARV lower RTID caused by HRSV, HPIV, or IV-A/Ba
Allo-HCT, allogeneic hematopoietic cell transplantation; CARV, community-acquired respiratory virus; Dx, diagnosis; GVHD, graft-versus-host disease; HRSV, human respiratory syncytial virus; HPIV, human parainfluenzavirus; IV-A/B, influenza virus A or B; RTID, respiratory tract infectious disease.
CARV-SPECIFIC ASPECTS IN TRANSPLANT PATIENTS
Influenza Viruses
According to the current taxonomy, the Orthomyxoviridae family contains the genus Influenzavirus and four species, named A, B, C, and D, with the proposed acronyms IV-A, -B, -C, and -D (https://talk.ictvonline.org/taxonomy/). IVs are enveloped virions in helical capsids of approximately 100-nm diameter that contain 7 to 8 single-stranded RNA segments of negative polarity in a nucleoprotein complex, each carrying an RNA-dependent RNA polymerase. IV-A and -B cause the bulk of the seasonal RTID outbreaks during the respiratory virus seasons, typically from November to May in the Northern Hemisphere (Fig. 1) and May to October in the Southern Hemisphere (25). Rates of infection among transplant patients vary depending on the specific population, type of immunosuppression used, and the circulating influenza strain. Likewise, seasonal variability in vaccine efficacy contributes to the respective rates in the transplant population. Influenza can be complicated by progressive disease, including viral pneumonia, secondary bacterial and fungal pneumonia, and extrapulmonary complications. Such severe and progressive complications are more common among allogeneic HCT and lung transplant recipients. To prevent IV-A/B infections, current guidelines recommend respiratory hygiene, influenza vaccination of recipients and their close contacts, and contact and droplet precautions in the health care setting for potentially infected patients (79–81). The vaccine immunogenicity is reduced in SOT and HCT patients, particularly when administered within periods I and II posttransplant (60, 82–85). Early antiviral therapy with one of the neuraminidase inhibitors (oseltamivir, peramivir, or zanamivir) has been associated with reduced risk of hospitalization, reduced progression to pneumonia, reduced need for ICU-level care, and reduced mortality (60) (Fig. 4). In a phase 3 trial, the recently FDA-approved IV-A/B polymerase PB2 inhibitor baloxavir caused faster reductions in IV-A/B loads and alleviated symptoms 1 day earlier than placebo in uncomplicated influenza (86), but data on high-risk and transplant patients are not available so far. Seasonal antiviral prophylaxis can be considered for those patients who are expected not to respond to influenza vaccine or where vaccine is not available (87). While postexposure prophylaxis is discussed for those at high risk of complications, the risk of resistance emergence during the incubation phase and during presymptomatic replication typically favors the use of the higher treatment dose or monitoring the patient for the onset of symptoms to start early therapy (88).
FIG 4.
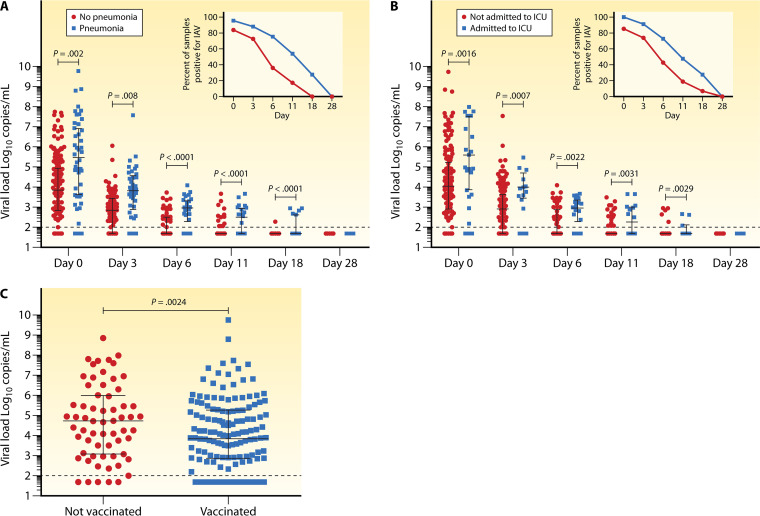
Influenza A viral loads and disease outcomes. (A) Viral loads were significantly higher in patients with lower RTID (pneumonia) than in patients with upper RTID both at presentation (day 0) and over the time of follow-up. (B) Viral loads were significantly higher in patients admitted to intensive care units (ICU) versus patients not admitted to ICU at diagnosis (day 0) and over the time of follow-up. (C) Viral loads were significantly lower at presentation in patients who had received influenza vaccine in the same season that infection occurred. (Reproduced from reference 60 with permission of the Infectious Diseases Society of America.)
Specific aspects of influenza in SOT.
Presentation of influenza in the SOT population in those coming to clinical attention in a recent study is associated with reduced incidence of classic symptoms: 64% had fever, 35% had a sore throat, and 40% had myalgias, while about 85% had cough (60). Radiologic evidence of pneumonia is present in 22%, and bacterial coinfections were rare at this early stage. Most will require hospital admission within a median of 6 days, suggesting that hospitalization is generally needed for more than just observation early in the clinical course. Very severe infection, requiring ICU-level care (11%) or mechanical ventilation (8%), can occur in the minority of patients, but mortality in SOT recipients remains sizable (2% to 4%) (29, 60).
Prevention and management of influenza in SOT patients.
The primary approach to prevention of influenza is through the use of inactivated vaccines covering IV-A/H1N1 or -A/H3N2 combined with one IV-B strain (trivalent) or with two IV-B strains (quadrivalent) of the circulating IV-A/H1N1, -A/H3N2, -B/Victoria, and -B/Yamagata viruses. The impact of the quadrivalent IV-A/B vaccine became evident during the 2017/2018 season, when the dominant subtype in Europe was -B/Yamagata, which was not present in the trivalent vaccine. Hence, the quadrivalent IV-A/B vaccine should be preferred, although there are no head-to-head comparisons in the transplant setting. Adjuvanted IV-A/B vaccines appear to be safe in transplant patients, and some studies report a similar response to unadjuvanted vaccines (89, 90). However, compared to results in healthy controls, influenza vaccines result in lower antibody responses (geometric mean) in transplant recipients (91). Since the peak antibody titer is assumed to correlate with the longevity of IV-A/B protection, transplant recipients may have waning protection later in the season, particularly if the vaccine is given early (51, 52). As such, optimal vaccine timing would be as late as possible before the start of the season, for example, in late October to early November in the Northern Hemisphere. Recent clinical data suggest that IV-A/B vaccines not only reduced the frequency of infection and the respiratory viral loads but also protected against progression to more severe disease (60). Intradermal IV-A/B vaccine does not appear to result in improved protection responses in lung transplant recipients and is therefore not preferred over standard injectable vaccine (92). Use of high-dose vaccines has been associated with improved seroconversion and higher postimmunization geometric mean titers (93, 94). Whether or not high-dose vaccines should indeed be preferred requires larger, preferably multicenter studies. Alternatively, a secondary booster dose of IV-A/B vaccine was shown in some studies to improve vaccine responses (51). While head-to-head studies between high-dose and booster vaccines have not been conducted, interpretation of the limited data would favor the high-dose approach.
For patients who would be predicted to have a poor IV-A/B vaccine response (i.e., recent transplant, intense immunosuppression, and use of lymphocyte-depleting therapy), seasonal antiviral prophylaxis can be considered. This approach was associated with reduced incidence of NAT (1.7% versus 8.4%)- and VIC (<1% versus 3.8%)-defined viral breakthrough infections yet without evidence for dominant NAI resistance mutations (95). While generally well tolerated, clinical experience demonstrates challenges in getting the prescription approved by health insurance and a low incidence of discontinuation because of intolerance. Postexposure prophylaxis using the lower prophylactic dose is not recommended, since transmission may have already occurred and the risk of clinical and virological influenza breakthrough should be clearly avoided, especially in immunocompromised patients. Instead, empirical treatment using the therapeutic dosing (i.e., 10 days of twice daily [BID] oseltamivir) should be considered for high-risk patients (i.e., lung transplant or recent transplant or rejection treatment) and close observation of low-risk patients with early treatment when they develop symptoms.
Most of the currently available experience for the treatment of influenza in transplant patients stems from NAIs, since all circulating IV-A/B strains show adamantane (M2-inhibitor) resistance. Prospective studies have not been performed in the transplant setting to define the optimal dose, route, or duration of antiviral NAI therapy or the role of combination therapies. NAI therapy is associated with reduced mortality, reduced progression to pneumonia, reduced duration of shedding, and, among lung transplant recipients, reduced risk of development of chronic lung allograft dysfunction (60, 96). Early therapy, defined as initiation in less than 24 to 48 h after symptom onset, is associated with the best outcomes in uncomplicated influenza infection of healthy nonimmunosuppressed patients as well as transplant patients (29, 97, 98). Nonetheless, therapy appears to be beneficial in this population beyond 48 h, most likely due to the higher and more prolonged viral replication load (Fig. 2), and all symptomatic transplant patients should be treated (28, 29, 97, 98). Given the atypical and mild degree of symptoms, IV-A/B should remain in the differential diagnosis for any patient with fever or any respiratory or systemic symptoms consistent with influenza. Antivirals should be initiated as soon as IV-A/B is considered in the differential diagnosis (Table 1, possible or probable case) and should not wait for confirmation of infection with diagnostic testing, especially when there is documented local activity in the community (Table 1, probable or presumptive case). In this setting, molecular point-of-care testing with high sensitivity and specificity and turn-around times of less than 20 min become of interest (99–101). If testing is negative by sensitive validated NAT, antivirals can be discontinued; in lung transplant recipients or patients with evidence of lower RTID or outright pneumonia, testing of lower airway specimens should be performed before stopping therapy, since discordant viral and clinical activity in the upper and lower airways has been documented (32). Similar to HCT recipients (27), influenza replication in SOT recipients tends to be prolonged compared to that in healthy adults, particularly among those with severe illness requiring ICU-level care (60), which requires appropriate testing and infection control measures. Given prolonged shedding, most experts recommend at least 10 days of therapy. Higher-than-usual doses (i.e., oseltamivir 150 mg per os BID) may be associated with reduced risk of resistance emergence and should also be considered in immunocompromised patients with influenza B due to the higher inhibitory concentration of IV-B and slower viral load decline (28, 102, 103).
Donor-derived influenza is a rare complication in SOT. Generally, IV-A/B replication is restricted to the respiratory tract except in rare cases where viral RNA is detected in blood, as viremia is present in patients with very severe seasonal influenza or possibly in patients infected with novel or avian viral strains (104). Viremia typically declines by several orders of magnitude to undetectable levels with the use of antiviral therapy. If the donor has detectable IV-A/B, current guidelines recommend that the lungs should be not be used until completion of antiviral therapy. For donors infected with novel or avian influenza, intestinal transplants should be avoided (104). Other organs generally can be used from IV-A/B-infected donors, conferring practically zero risk of transmission. If non-lung recipients experience donor-derived influenza, atypical presentation may be more common, since systemic symptoms may predate respiratory symptoms; hence, testing of blood and respiratory secretions should be considered (56).
Specific aspects of influenza in allogeneic HCT.
Clinical presentation of influenza in allogeneic HCT and SOT patients is similar to that demonstrated in a recent prospective study, in which cough was the leading symptom in 90% of patients, whereas only 70% had fever, 55% rhinorrhea, 40% sore throat and gastrointestinal symptoms, and 30% myalgia or headache (60). As pointed out earlier, the onset of symptoms and signs appears to be significantly less overt in HCT patients shortly after transplantation (26, 27, 105). Most patients typically will be symptomatic for 10 days, followed by a period of prolonged asymptomatic shedding. Absolute lymphocyte count (ALC) at diagnosis correlated inversely with viral shedding (P < 0.001) (27). Similar to observations in children (106), consecutive infections of IV-A followed by IV-B have been observed in approximately 10% of patients, and one-third of cases occurred despite prior vaccination (27). However, data from two prospective observational studies indicate that vaccination was associated with lower viral loads and lower risk of progression to lower RTID (60, 107). About a quarter of high-risk HCT (i.e., those with infection early posttransplant, profound lymphopenia, and/or therapy for acute or chronic graft-versus-host disease [GVHD]) progress to pneumonia typically after a week of symptomatic upper RTID (Fig. 3). Mortality rates of up to 25% have been reported in HCT patients with lower RTID (108). Retrospective comparative studies suggest more severe outcomes of the pandemic influenza A/H1N1/pdm09 than previous seasonal IV-A, with more patients suffering from lower RTID, hypoxemia, and mortality, where these outcomes were predicted by profound lymphopenia and delayed antiviral therapy (109, 110). However, another large retrospective analysis of influenza A/H1N1/pdm09 diagnosed in 286 HCT patients (222 allogeneic, 64 autologous) at 18 months posttransplant reported cough, fever, and rhinorrhea in 85%, 81%, and 49%, respectively (30). Lower RTID was seen in 33% of patients and correlated with lymphopenia, defined as <200 per μl (OR, 2.5; P < 0.001), and older age (OR, 1.025; P = 0.002). A death rate of 6.3% was noted, which was associated with neutropenia (P = 0.03) and older age (P = 0.04) (30). Clinical criteria have been proposed to identify allogeneic HCT patients at increased risk of IV-A/B progression to lower RTID and mortality using the Basel immunodeficiency grading (27, 73) or the MD Anderson immunodeficiency score index (ISI) (76, 77, 107, 111) (Table 2).
Prevention and management of influenza in allogeneic HCT patients.
The primary approach to prevention of influenza is through the use of inactivated vaccines. Patients in the first 6 months following myeloablative stem cell transplants or within 7 days of receipt of conventional chemotherapy are unlikely to respond to current influenza vaccines. However, earlier vaccination is still considered if allogeneic HCT takes place in an outbreak situation or within the ongoing season (85). Independently, vaccination of close contacts should be strongly considered, and careful infection prevention through the use of contact and droplet precautions in the health care setting should be utilized (81). High-dose influenza vaccine appears to result in improved vaccine responses in pediatric HCT patients (112) and can be considered in adults (113). A study is currently ongoing to assess the efficacy of high-dose vaccine in adult HCT recipients (ClinicalTrials registration number NCT03179761) and in pediatric HCT recipients (ClinicalTrials registration number NCT02860039). As described above, seasonal antiviral prophylaxis can be considered for patients who would be predicted to have a poor vaccine response, whereas postexposure prophylaxis should use therapeutic doses (95).
Prospective studies have not been performed in the transplant setting to define the optimal duration of antiviral therapy, the optimal dose or route of therapy, or the role of combination therapies. Influenza replication tends to be prolonged compared to that in healthy adults, particularly among those with pneumonia or severe illness requiring ICU-level care (27). Antiviral therapy is associated with reduced mortality, reduced progression to pneumonia, and reduced duration of shedding (108, 109). Among allogeneic HCT patients, especially those with poor T-cell reconstitution having received alternative donor transplants, T-cell depletion, and GVHD, antivirals should be initiated as soon as influenza is considered in the differential diagnosis and should not wait for diagnostic IV-A/B testing, but a rapid test of sufficient sensitivity and specificity should be sought (20). If positive, antiviral therapy should be continued for at least 10 days or longer if patients have prolonged viral shedding along with clinical symptoms.
Although neuraminidase inhibitor therapy begun <48 h of symptom start was shown to be clinically effective during a short course of 5 days in immunocompetent adults, drug resistance variants can be detected as minority species by the time the immune system is able to start clearing the virus. Recently, the Committee for Medicinal Products for Human Use of the European Medicnes Agency has recommended the marketing of intravenous zanamivir for patients with severe influenza for compassionate use (114). Further studies of IV-A enumerated oseltamivir resistance on day +1 of treatment in 3.6% of patients, which reached 11.8% in 1- to 5-year-old children (115, 116). Resistant IV-A/B showed delayed clearance of 8 versus 11 days but had little effect on symptom resolution. The expected synergy of NAI and immune control or vaccination in immunocompetent patients (Fig. 3 and 4) was experimentally confirmed in the well-established influenza ferret model, an issue also relevant in the care of transplant patients (117). Similarly, experimental ferret models supported a therapeutic effect of ribavirin when administered early with respect to viral loads and clinical symptomatology (118). Although the effect of ribavirin for prophylaxis and treatment of IV-A/B RTID is not well documented, combination therapy with amantadine, NAI, and ribavirin has demonstrated a more rapid decline in viral titers, which has not translated into clinical benefit in immunocompetent patients (119, 120), most likely because of innate and adaptive inflammatory components. This may be different in the HCT setting showing prolonged viral shedding (27), where anecdotal success of combination therapy has been reported for NAI-resistant cases (121). Thus, combinations of NAI and ribavirin cannot be generally recommended but would be considered in otherwise desperate single cases. Reliable data regarding the combination therapy of NAI with the novel cap-snatching inhibitor baloxavir are pending, but preliminary data suggest that this is a viable and potent combination (122, 123). Monotherapy with baloxavir in immunocompromised patients may not be preferred at the present time of limited clinical evidence because of the potential risk of resistance emergence.
Approved therapies that are currently recommended for the treatment of influenza A and B include the neuraminidase inhibitors zanamivir (inhalation approved globally; intravenous administration approved only in Europe), oseltamivir (globally approved), peramivir (widely approved), and lanimavir (approved in Japan and South Korea). Novel antiviral therapies for the treatment of influenza include three polymerase-targeted inhibitors, favipiravir (approved with limited indication in Japan), pimodivir (not approved), and baloxavir (approved in Japan and the United States, pending in Europe) (Table 3). Some of the new drugs appear to have the potential for greater reduction in viral titers and show synergy when combined with neuraminidase inhibitors, which may not translate into clinical benefit in immunocompetent patients (124), as discussed above, but may do so in SOT or HCT populations, though study results are currently lacking. Presumably, they may provide benefit in combination with neuraminidase inhibitors, in preventing resistance emergence or reducing viral shedding. Further, they may provide an alternative for the treatment of resistant variants. However, the rate of resistance emergence of the newer drugs in otherwise healthy adults and children may also challenge their use in immunocompromised populations (86). A number of monoclonal antibodies are in early stages of clinical development and may also prove to be beneficial if they advance further with regulatory approval. Plasma and immunoglobulin products with high influenza titers have been studied in patients with severe influenza pneumonia and are associated with reduced mortality and the need for ventilators (125). As such, these approaches may be considered adjunctive therapy in critically ill transplant patients.
TABLE 3.
Influenza polymerase inhibitors in advanced clinical development or recently approveda
| Feature | Favipiravir (T-705) | Pimodivir (JNJ-63623872) | Baloxavir (S-033188) |
|---|---|---|---|
| Viral target | PB1 | PB2 | PA |
| Spectrum | IV-A, IV-B, IV-C | IV-A only | IV-A and IV-B (better than OTV against IV-B) |
| Synergy with NAIs | Yes | Yes | Yes |
| Activity against M2-resistant strains, activity against NAI-resistant strains | Yes | Yes | Yes |
| Agent-resistant variants | None to date | Yes | Yes |
| Administration | Oral (i.v. in development) | Oral (i.v. in development) | Oral |
| Reduction in i.v. viral loads | Modest | Good (better than NAI) | Strong (3.5-log10 reduction) |
| Development status | Approval in Japan, investigational in rest of world | Phase 2 ambulatory done, hospitalized study ongoing | Approval in Japan and in USA, pending in Europe |
IV-A, influenza virus A; IV-B, influenza virus B; i.v., intravenous; NAI, neuraminidase inhibitors; OTV, oseltamivir.
Human Pneumo- and Paramyxoviridae
Although previously grouped together as Paramyxoviridae, an increasing number of viral genome sequences have led to the taxonomic separation of the Pneumoviridae and Paramyxoviridae families (https://viralzone.expasy.org/). Whereas the former includes the genus of human Pneumovirus with the species HRSV and HMPV, the latter includes the respiroviruses HPIV-1, -2, -3, and -4. The virions of both families are enveloped helical particles of 150-nm diameter containing a single-stranded 15-kb-long RNA genome of negative polarity in a nucleoprotein-polymerase complex. The different families are morphologically similar and cannot be distinguished by transmission electron microscopy (https://viralzone.expasy.org/). Measles and mumps virus are not considered CARVs, despite similar virology and transmission modalities, including severe lower RTID/pneumonia as a feared short-term complication, especially among adults. However, measles and mumps are examples of the protective potential of vaccination, and vaccines are under current development for HRSV and HPIV. At the same time, they illustrate the principle limitation of live-attenuated vaccines being contraindicated in transplant patients. Modeling studies suggest that the epidemiologic dynamics of HRSVs, HMPV, and HPIV involve limited cross-immunity (126), and human-derived monoclonal antibodies directed against the prefusion conformation of the F-protein cross-neutralizing HRSV and HMPV have been characterized molecularly, in cell culture, and in murine models (127).
Human respiratory syncytial virus.
There are two antigenic types, called HRSV-A and -B, which cocirculate in the community but may differ locally in their relative abundance. Children <5 years of age are key drivers of transmission and disease, putting immunocompromised contacts in families and institutions at high risk for infection (5, 45). In SOT, HRSV infection has the highest impact in the first year posttransplant, except in lung transplant recipients, where the detection rates remain similar over time posttransplant (55, 128). Among HCT recipients, between 2% and 17% of recipients are diagnosed with HRSV infections (12, 26, 73, 129, 130). Progression to lower-tract infection occurs relatively quickly after onset of upper-tract symptoms and is more common among lung transplant recipients and allogeneic or cord blood transplant recipients, and increased risk of progression has been noted for infections occurring early posttransplant subsequent to lymphocyte-depleting antibody therapy or in patients with pronounced lymphocytopenia (5). Mortality is greatest among HCT recipients, with lower RTID showing average rates of about 25%, although lower mortality rates have been reported more recently (66, 73, 129).
Currently, there are no vaccines available for the prevention of HRSV, but several candidate vaccines are in advanced clinical development. Prevention is based on rigorous infection control and contact precautions. Several reports have described the devastating impact of nosocomial HRSV transmission, particularly in hematology and HCT centers (69). Palivizumab is a monoclonal antibody directed at the abundant postfusion F-protein of HRSV. Palivizumab should be considered for infants that are born at ≤28 weeks 6 days gestational age and <12 months at the start of HRSV season, who have chronic lung disease of prematurity, and who have hemodynamically significant chronic heart or premature lung disease necessitating medical therapy within 6 months prior to the beginning of the HRSV season (131). Palivizumab prophylaxis can be considered in children <24 months who are immunocompromised or have undergone cardiac transplantation for the HRSV season (131). Earlier studies suggested that many transplant centers were using palivizumab for the prevention of HRSV in children at risk, but more recent data suggest that there is little increase in disease incidence when palivizumab use is more restricted (132).
(i) Specific aspects of HRSV in SOT.
No specific antivirals are licensed for prevention or treatment of HRSV infection. In the lung transplant setting, ribavirin has become the standard of care, typically in addition to steroids, despite the lack of randomized trials demonstrating efficacy (133). Early studies administered systemic ribavirin intravenously (33 mg/kg of body weight on day 1 and then 20 mg/kg/day in 3 divided doses until resolution of symptoms) (134). As the intravenous formulation of ribavirin has been difficult to obtain and aerosolized ribavirin is associated with high costs and safety issues for pregnant health care staff, many centers have begun using systemic ribavirin perorally for treatment of HRSV in SOT. A retrospective analysis compared the outcomes of oral ribavirin versus the best supportive care for paramyxovirus infections in lung/heart-lung transplant recipients. Oral ribavirin treatment (15 to 20 mg/kg/day in 2 divided doses for 14 days) was associated with better recovery of graft function at 30 days postinfection (84% versus 59%; P = 0.02) and reduced development of new-onset BOS within 6 months (5% versus 24%; P = 0.02) (135). Thus, oral ribavirin was adopted as a presumed standard of care, often combined with intravenous immunoglobulin (IVIG), as it was found to be a well-tolerated alternative to intravenous or inhaled ribavirin (136, 137). However, appropriately sized randomized controlled trial data are needed to document safety and efficacy of systemic ribavirin (oral and/or intravenous) in SOT and in lung transplant recipients in particular.
(ii) Specific aspects of HRSV in allogeneic HCT.
To date, there have not been large, prospective, randomized trials establishing a specific HRSV therapy in HCT patients. Nonetheless, there is a large body of case reports, case series, and retrospective analyses investigating aerosolized or systemic (oral or intravenous) ribavirin, antibody-based therapy (standard IVIG, high-titer HRSV Ig, or palivizumab), or combinations thereof. A summary compilation of outcome data suggested that any treatment was associated with less progression to lower RTID (P < 0.001) and less mortality (P < 0.001) (129). However, there was no significant difference between individual approaches of aerosolized ribavirin, aerosolized ribavirin plus immunoglobulins, or systemic ribavirin plus immunoglobulins with respect to progression to lower RTID, whereas mortality rates were significantly higher for aerosolized ribavirin alone or systemic ribavirin than for aerosolized ribavirin plus an antibody-based therapy (129). This analysis is faced with uncertainties regarding patient bias, time point of diagnosis, late decisions regarding treatment initiation, and the underlying medical conditions, e.g., preferential treatment of sicker cases with more advanced HRSV lower RTID, whose outcome cannot be significantly modified (Fig. 3). Given the absence of better evidence, the principle approach of administering oral ribavirin plus immunoglobulins at an early stage of HRSV upper RTID in severely immunodeficient (SID) patients was introduced in some centers more than 10 years ago (138, 139) in order to reduce HRSV-associated mortality compared to rates seen in historic controls and earlier reports (66). Compared to those of HCT patients with moderate immunodeficiency (MID), patients with SID and very severe immunodeficiency (verySID) (Table 2) showed more prolonged symptoms and HRSV shedding (66). Subsequently, some limited but dramatic evidence was obtained during an HRSV outbreak in the winter season of 2010/2011 in a hematology and transplant unit affecting, in a short time, 56 patients, including 16 allogeneic HCT patients, of whom 40 developed HRSV lower RTID and 14 died. In line with the SID criteria, fatal outcome was associated with hypogammaglobulinemia (OR, 11.8; P = 0.007), whereas rapid delivery of systemic ribavirin was protective (OR, 0.14; P = 0.02) (69). In a large retrospective analysis of 280 allogeneic HCT patients in a single center over 2 decades (140), aerosolized ribavirin (91 with and 66 without adjuvant immunoglobulin) administered to 157 patients at the stage of upper RTID was associated with reduced progression to lower RTID (<0.001) and all-cause mortality (P = 0.013) compared to levels for 123 patients not receiving this treatment. Based on the risk factor profile in a subgroup of 237 patients, a weighted ISI was developed to identify patients at high risk for progression to lower RTID and all-cause mortality and to potentially guide treatment (Table 2 and Fig. 5). An advantage of the SID grading is the simplicity of use, where the critical parameters are equally weighted and, if coexisting, can readily shift patients into the verySID group. Advantages of the ISI are a fine gradation over several rank scores and independent validation data for HRSV, but its use is complicated by the different weights, some of which cannot be generalized, e.g., the threshold of 40 years of age.
FIG 5.
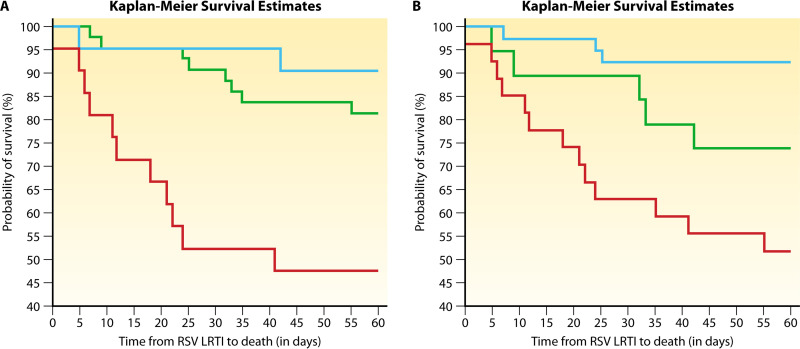
Immunodeficiency and probability of survival in allogeneic HCT patients presenting with lower RTID caused by human respiratory syncytial virus. Immunodeficiency scoring index (left) and immunodeficiency grading (right) were calculated as indicated in Table 2 for 85 allogeneic HCT recipients diagnosed with HRSV lower RTID and compared for mortality. High-risk individuals, as identified by a high immunodeficiency scoring index (left, score of 7 to 12; red line), had a higher risk for mortality than those having a moderate index (left, score of 3 to 6; green line) or low index (left, score of 0 to 2; blue line) (OR, 10.5; 95% CI, 1.9 to 56.6; P = 0.01). Similarly, individuals with very severe immunodeficiency (right, 2 to 7 criteria; red line) had a higher risk for mortality than those with moderate immunodeficiency (right, 1 criterion, green line) or moderate risk (right, 0 criteria; blue line) (OR, 11.1; 95% CI, 2.8 to 45.1; P < 0.001). Both grading approaches predicted time to mortality, as demonstrated by Kaplan-Meier survival curves. (Reproduced from reference 78 with permission of John Wiley and Sons.)
Importantly, a number of studies have now investigated the association of Basel immunodeficiency grades and MD Anderson ISI scores in HCT and hematologic malignancy patients not only for HRSV but also for IV-A/B, HPIV, and other CARV infections (27, 73, 76, 77, 107, 111). Some of these studies include side-by-side comparisons (77, 78). Indeed, many of the parameters present in the SID grades and ISI overlap and permit separation of patients into groups of similar clinical outcomes regarding progression from upper to lower RTID or mortality (Fig. 5), despite an apparently moderate Cohen’s kappa coefficient (78).
Because of the high costs and required logistics for long daily administration and teratogenicity concerns when administering aerosolized ribavirin, many centers have adopted the use of oral ribavirin plus an antibody-based therapy (141). A recent retrospective study of 124 adult HCT patients comparing aerosolized (n = 70) and systemic (oral) ribavirin (n = 54) reported that all-cause mortality was not significantly different between both groups at day 30 (10% versus 9%; P = 1.0) and day 90 (23% versus 11%; P = 0.10) postdiagnosis (72). The optimal dose and duration of therapy has been poorly studied, but therapy is generally continued until symptoms have markedly improved or resolved. Dosing of oral ribavirin should be aggressive, as the 50% inhibitory concentration for ribavirin is relatively high and may be challenging to achieve in the alveolar fluid. As discussed previously (66, 140), optimal therapy should be initiated early, before the development of lower RTID, as this preemptive approach is associated with the best outcomes. Such early therapy should be considered for any HCT recipient within 90 days of transplant, those requiring steroids chronically, those undergoing treatment for GVHD, or those who are severely lymphopenic (ALC of <100 cells per microliter). Although different dosing schemes of systemic ribavirin have been reported, the guidelines of the fourth European Conference on Infections in Leukaemia (ECIL-4) (5) recommend starting with a loading dose of 600 mg followed by 400 mg every 8 h in order to reach 10 mg/kg bodyweight every 8 h by day 3 (Table 4).
TABLE 4.
Use of systemic ribavirin for RTID with HRSV, HPIV, and HMPVa
| Treatment course | Description |
|---|---|
| Oral or intravenous ribavirin maximal dosing at 10 mg/kg body weight every 8 h for adults | Day 1: start with 600 mg loading dose, then 200 mg every 8 h |
| Day 2: 400 mg every 8 h | |
| Day 3: Increase the dose to a maximum of 10 mg/kg body weight every 8 h | |
| In case of adverse events, decrease dose or discontinue ribavirin | |
| Oral or intravenous administration according renal function | Dose for a creatinine clearance of 30 to 50 ml/min: a maximum of 200 mg every 8 h |
| For a creatinine clearance of 10 to 30 ml/min, no dose recommendation can be givenb |
(iii) New options in the prevention and management of HRSV.
There are currently four drugs undergoing development for the treatment of HRSV. ALN-RSV01 is a small interfering RNA (siRNA) that was studied in the treatment of HRSV in lung transplant recipients (142, 143). The drug was studied in two phase 2 studies and demonstrated a reduced risk of development of BOS (13.6% versus 30.3%) in treated patients compared to placebo. Treatment effect was enhanced when ALN-RSV01 was started <5 days from symptom onset and was observed even without ribavirin treatment (142). Despite these promising results, the drug is no longer undergoing clinical development. MEDI8897, a recombinant human IgG1 kappa monoclonal antibody that targets the prefusion conformation of the HRSV F-protein, shows excellent safety and pharmacokinetics in adults and infants (144, 145). Currently, no studies are planned with this compound in immunocompromised patients, but initial studies in otherwise healthy patients are ongoing.
Two direct-acting antivirals are undergoing clinical development for HRSV (presatovir [GS-5806] and lumicitabine [ALS-8176]). Presatovir is a novel oral small molecule that inhibits HRSV entry at low-nanomolar concentrations by blocking viral envelope fusion with the host cell membrane. In experimental infection studies in healthy adults, presatovir was associated with reduction in viral load and severity of clinical disease (146). The drug was studied in four phase 2 studies: one in hospitalized adults with HRSV, one in lung transplant recipients with HRSV, and one in HCT recipients with upper RTID and one with lower RTID, and it was well tolerated in all of these studies. In the lung transplant study, 40 patients were given presatovir and 20 were given placebo. Despite achieving plasma trough levels that were 4-fold higher than the protein binding-adjusted 95% effective concentration of HRSV for at least 21 days, the drug had no effect on clinical symptoms, FEV1, or the viral load kinetics in upper respiratory secretions (147). In the HCT trials, 96 patients after allogeneic or autologous transplantation with upper HRSV RTID received presatovir and 93 received placebo. The coprimary endpoints were not met regarding a faster average decline in upper HRSV load from day 1 to day 9 or progression to lower RTID relative to placebo (148). In a post hoc analysis, patients on presatovir were less likely to progress to lower RTID when having lymphopenia of <200/μl (P = 0.02), when being treated in fewer than 4 days from symptom onset (P = 0.07), or when being in the hospital at the first dose (P = 0.02). In the HCT lower RTID study, 31 patients received presatovir and 29 received placebo, and no difference in virologic or clinical endpoints was observed. Although presatovir was well tolerated, there was no difference in the median number of supplemental oxygen-free days, proportion of patients developing respiratory failure, or all-cause mortality compared to levels for the placebo (149). However, the exploratory small sample size did not permit a meaningful interpretation of the results.
Lumicitabine is an orally bioavailable prodrug of the novel HRSV replication inhibitor ALS-008112, a cytidine nucleoside analogue. The nucleoside triphosphate analogue inhibits HRSV replication by means of chain termination. In experimental infection studies in healthy adults, lumicitabine was associated with more rapid HRSV clearance, a greater reduction of viral load, and more rapid improvements in the severity of clinical disease than placebo (150). A planned clinical trial in hospitalized adults (ClinicalTrials registration number NCT02935673) was recently discontinued, and no studies in transplant populations are currently planned.
Human metapneumovirus.
HMPV has been detected in SOT and HCT recipients, and rates of 4% (range, 1% to 10%) have been reported when using comprehensive CARV-multiplex NAT in the high-risk population of lung transplantation or allogeneic HCT (32, 55, 56, 73). Like HRSV and HPIV, HMPV has a higher propensity for lower RTID than HRV and for acute and chronic lung dysfunction (Fig. 3), and it has been associated with increased mortality.
Only limited data are available for SOT, which stem largely from lung transplant recipients and are usually of small size. In a 5-year prospective study of 98 patients, HMPV was detected by multiplex-NAT in 7.7%, half of which were symptomatic, and two-thirds presented with lower RTID with tracheobronchitis (55). Given the intrinsic propensity for lower RTID, a systematic literature search reviewed all available reports on HMPV infection and attempted to exclude other confounding infections and lung transplant rejection within 6 months. Among a total of 1,007 patients, sole HMPV infection was identified in 57 cases, of which 35% were associated with acute rejection within 3 months, followed by CLAD/BOS (151). However, in other studies, reactivation of cytomegalovirus has been identified as an important cofactor that is not exclusive to HMPV (61).
In allogeneic HCT, a 1-year single-center study of 93 patients tested with CARV-multiplex NAT reported eight (8.6%) patients, seven of which had lower RTID and ground-glass patterns on computer tomography imaging of the chest, six received IVIG combined with oral ribavirin, and one patient died (12.5%) (152). In a systematic review of the literature, the overall incidence of HPMV was 7% in HCT patients (103 of 1,504) and was not different from that for patients with hematological malignancies (153). Progression to lower RTID occurred in approximately 15% and HMPV-associated mortality was 6%, corresponding to 27% among those with lower RTID (153). In a retrospective study of 118 HCT, 30 had lower RTID, which was associated with steroid use (>1 mg/kg), low lymphocyte counts, and diagnosis less than day +30 posttransplant (154). No clear benefit from antiviral intervention using aerosolized or systemic ribavirin has been documented, and even the role of IVIG is controversial (154). The uncertainty of antiviral treatments may have multiple confounding reasons besides low or limited efficacy and may include patient characteristics, with undefined rates of spontaneous recovery being best documented among patients with upper RTID, severity of immunodeficiency, the underlying disease, and the rates of advanced lower RTID, the course of which can no longer be modified by treatment, as well as the potential role of coexisting cytomegalovirus reactivation. However, some centers use systemic ribavirin, as summarized in Table 4. Clearly, large prospective multicenter studies are needed to define risk factors and clinical impact of HMPV.
Human parainfluenzaviruses.
HPIVs cause significant morbidity and mortality in HCT recipients, in patients with leukemia, and in SOT, particularly among lung transplant recipients (155, 156). The incidence of HPIV is about 2 to 7% in HCT recipients and about 5% in lung transplant recipients. Asymptomatic shedding has been documented in SOT and HCT recipients and can give rise to nosocomial outbreaks and a high fatality rate (157–159). Prospective data from Spain from 98 lung transplant recipients identified HPIV in 7.7%, only half of which are symptomatic with mostly lower RTID (55). A systematic review of the literature of 1,196 HPIV infections in 31,730 patients identified an incidence of 5% and 3% HPIV detection rates among allogeneic and autologous HCT patients, which was approximately 2-fold higher than the 2% reported in patients with hematological malignancies (156). Traditional diagnostics covered only HPIV-1, -2, and -3, whereas consistent HPIV-4 data were obtained in the clinical stage only following the use of CARV-multiplex NAT. Indeed, in a comprehensive study of 1,069 CARV-multiplex NAT of allogeneic HCT patients, HPIV-1 and HPIV-2 were detected in 1%, HPIV-4 in 2%, and HPIV-3 in 4%, together amounting to 18% of the CARV-positive samples (73). Most cases of severe RTID have been linked to HPIV-3 (5, 160). Literature data suggest a progression rate to lower RTID in HCT patients of 30% and to HPIV-associated mortality of 12% overall and 27% of those with HPIV lower RTID (156). Similar outcomes were noted in a study of 200 patients, including 120 HCT recipients, where relevant risk factors were neutropenia, steroid use, and refractory underlying malignancy (161). In a recent comparative study, progression to lower RTID and death was only 6% and not different from that for HRSV or HPMV, but it was clearly linked to verySID grading (73). Similarly, in a large single-center study of more than 500 HCT patients, the mean progression rate from upper to lower RTID was 7 days and was associated with monocyte counts of <200/μl, high-dose steroids (1 mg/kg), and HPIV-3 detection (162). An earlier study from the same group reported the 90-day mortality of HPIV lower RTID as being 37%, whereby risk factors were low monocyte counts, being <1 years after HCT, use of high-dose steroids (>2 mg/kg), and oxygen requirement at diagnosis, where no impact of ribavirin and/or IVIG was noted (31).
In addition to the immediate direct effects of the virus, HPIV has been associated with longer-term pulmonary dysfunction in HCT and lung transplant patients. Lung transplant recipients with lower-tract HPIV infections are associated with a high rate of development of BOS or chronic rejection (163). Among HCT recipients, there is an 18-fold increased risk of developing severe late-onset airflow obstruction (164). Unfortunately, this appears to be associated with a frequently progressive and potentially fatal course.
There are no vaccines currently available for the prevention of parainfluenza, so prevention is focused on pulmonary hygiene and contact precautions in the health care setting. There are no approved drugs that have been demonstrated to be active against HPIV clinically. While most studies have failed to demonstrate a clinical benefit, especially for advanced stages (31, 162), many clinicians will administer IVIG and, less frequently, oral or aerosolized ribavirin for high-risk patients (5, 73) (Table 4) despite lack of evidence for efficacy, especially for lower RTID.
(i) Prevention and new option in management of HPIV.
DAS181 is a novel host-directed antiviral with enzymatic activity that is delivered by inhalation. DAS181 is a recombinant protein composed of a sialidase catalytic domain derived from Actinomyces viscosus and is linked to a glycosaminoglycan-binding domain derived from human amphiregulin (Table 5). Similar to other virally encoded neuraminidases, DAS181 destroys sialic acid receptors on epithelial cells of the respiratory tract, which are used by IV-A/B and HPIV for attaching to the host cell. Due to the large size of this novel protein, immunogenicity must be postulated upon repeat administration, but there is currently little evidence of this in immunocompromised patients. In a number of case series, DAS181 inhalation was followed by improved pulmonary function and reduced viral loads in immunosuppressed patients (165, 166). In those that failed to respond, most had clinically significant coinfections. A randomized, placebo-controlled study of DAS181 in immunocompromised patients with HPIV has been completed (ClinicalTrials registration number NCT01644877), and preliminary results were presented at a recent meeting (tct.confex.com/tct/2019/meetingapp.cgi/Paper/13497).
TABLE 5.
DAS181 treatment of HPIV infection in immunocompromised patientsa
| Series | Age (yr) | Serotype | Posttreatment viral load | Pulmonary function |
Mortality | |
|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment | |||||
| Chalkias et al. (221) | 64 | HPIV-1 | 1.91-log decrease | Intubated | Extubated | N |
| 69 | HPIV-3 | 1.81-log decrease | Intubated | Extubated | N | |
| Waghmare et al. (165) | 12 | HPIV-3 | Negative (D+48) | N | ||
| 7 mo | HPIV-2 | Negative (D+69) | N | |||
| 4 | HPIV-3 | Negative (D+4) | N | |||
| 3 | HPIV-3 | Negative (D+45) | N | |||
| Chen et al. (166) | 63 | HPIV-3 | Negative (D+8) | FEV1 = 0.76 liters, DLCO = 36% | FEV1 = 0.91 liters, DLCO = 56% | Y (D+14) |
| Guzman-Suarez et al. (222) | 55 | HPIV-3 | 1-log decrease (D+2) | FEV1 = 0.93 liters, FVC = 1.12 liters | FEV1 = 1.18 liters, FVC = 1.31 liters | N |
| 59 | HPIV-3 | Unchanged (D+2) | FEV1 = 1.52 liters, FVC = 1.74 liters | FEV1 = 1.69 liters, FVC = 1.98 liters | N | |
| Dhakal et al. (223) | 74 | HPIV-3 | Intubated | Intubated w/ improved oxygen requirements | Y | |
| 35 | HPIV-3 | Intubated | Intubated w/ improved oxygen requirements | N | ||
| Drozd et al. (224) | 64 | HPIV-3 | Negative (D+6) | High flow mask | Nasal cannula | N |
| Salvatore et al. (225) | 53 | HPIV-1 | 3.94-log10 decrease (D+10) | 90% on 90% FIO2 (vent) | 100% on 55% FIO2 (vent) | Y (D+21) |
| 69 | HPIV-3 | 96% on 50% FIO2 (facemask) | 95% on 4-liter NC | N | ||
| 64 | HPIV-3 | 1.14-log10 decrease (D+5) | 96% on 35% FIO2 (vent) | 97% on 50% FIO2 (vent) | N | |
| 66 | HPIV-4 | 1.69-log10 decrease (D+7) | 96% on 3L NC | 96% on 2-liter NC | N | |
| 67 | HPIV-3 | 97% on 50% FIO2 (facemask) | 98% on 50% FIO2 (vent) | Y (D+10) | ||
| 58 | HPIV-4 | 94% on 3-liter (NC) | 99% on 50% FIO2 (vent) | N | ||
| 65 | HPIV-3/4 | 95% on RA | 99% on RA | N | ||
| 29 | HPIV-1 | 3.51-log10 decrease (D+7) | 98% on 3-liter NC | 99% on 3-liter NC (off oxygen D+10) | N | |
| 50 | HPIV-1 | 0.19-log10 increase (D+7) | 96% on 3-liter NC | 95% on 3-liter NC | N | |
| 65 | HPIV-3 | 94% on 5-liter NC | 96% on 2-liter NC | N | ||
| 72 | HPIV-3 | 90% on RA | 94% on RA | N | ||
| 65 | HPIV-3 | 97% on RA | 97% on RA | N | ||
| 60 | HPIV-3 | 1.60-log10 increase (D+5) | 96% on 6-liter NC | 97% on 4-liter NC (off oxygen D+6) | Y (D+21) | |
| 66 | HPIV-4 | 98% on RA | 99% on RA | N | ||
| 59 | HPIV-4 | 100% on RA | 100% on RA | N | ||
| 71 | HPIV-4 | 2.60-log10 decrease (D+7) | 94% on RA | 95% on RA | N | |
Literature search was conducted using PubMed. Studies were included if DAS181 was used therapeutically to treat HPIV in immunocompromised patients. Viral load, pulmonary function, and mortality were assessed from data available in the study text. No studies were excluded. D+#, days after end of treatment; NC, nasal cannula; RA, room air; HPIV, human parainfluenzavirus; DLCO, diffusion capacity of lung for carbon monoxide.
Other CARVs in SOT and Allogeneic HCT
Numerous other viruses have been detected in respiratory samples, including annello-, polyoma-, and picornavirus genera, such as parechovirus, Aichi virus, and Saffold virus. Some of these agents have been shown to change in abundance according to time and immunosuppression posttransplant (38, 167–171) but will not be reviewed here. Other regularly detected CARVs in SOT and HCT include HRV, HCoV, HAdV, and HBoV, yet information on clinical presentation and impact is limited to selective case series in SOT and retrospective studies in allogeneic HCT recipients. A significant component of the uncertainty regarding these agents derives from the fact that (i) prolonged asymptomatic shedding has been observed (172); (ii) there are numerous serotypes/genotypes of HRVs and other enteroviruses, HCoVs, and HAdVs that are not readily differentiated or quantified by multiplex NAT diagnostics; (iii) the serotype/genotype may confer only limited cross-immunity; and (iv) the respective immune status with respect to antibody titers or cellular immunity has not been consistently assessed (173–177). Thus, persistent and prolonged shedding versus re-/superinfection with another subtype is not readily identified. Clearly, dedicated comprehensive clinical and diagnostic studies are needed to shed more light on the role of these CARVs in RTID and the associated risk factors.
Human rhinoviruses.
HRVs belong to the Picornaviridae and have been merged into the genus Enterovirus, which share nonenveloped icosahedral virions of 30-nm diameter surrounding a single-stranded 8-kb-long RNA of positive polarity (https://viralzone.expasy.org/). HRVs have been little investigated in SOT, but a retrospective study on 116 lung transplant recipients identified HRV in 11% of patients, whereby higher HRV loads correlated with the presence of RTID (174). Recent prospective data in 98 lung transplant recipients from Spain identified HRV in approximately half of the upper RTID episodes (47 of 97, or 48.5%) and a third of lower RTIDs (23 of 65, or 35.3%), including in 2 fatal cases (55). Similarly, HRVs were retrospectively identified in 33.9% of 116 mostly lower RTID episodes among 250 lung transplant patients. In both studies, lower RTID was independently associated with acute rejection and CLAD, but even though HRV represented the largest CARV genus, a specific attribution was not possible.
In allogeneic HCT, HRVs are the most frequently diagnosed entities among children according to a large retrospective multicenter study, contributing 162 (62%) of 259 CARV events in the first year posttransplant (74). A quarter of these children had a diagnosis of lower RTID at onset and approximately one-half required some form of respiratory support, but ICU admission remained rare, 6 of 162 (4%). A diagnostic surveillance study reported a cumulative HRV incidence until day +100 of 22.3% (45 of 215) adult HCT recipients. The median duration of HRV shedding was for 3 weeks but lasted more than 3 months in 6 patients, and lower RTID was seen in 2 patients (178). In a study of 195 adult patients from Hong Kong from December 2010 to March 2015, HRVs were detected in 38 episodes (19.9%) as the sole CARV, of which 9 (23.7%) presented with lower RTID, 4 (10.4%) required ventilatory support, and 2 (5.3%) died (111). The immunodeficiency score was not predictive of lower RTID or death, but the numbers were small (111). A recent retrospective study of 382 allogeneic HCT patients from Australia identified 65 episodes in 56 (14.7%) patients with CARV-RTID until day +100, which involved HRV in 69.2%. Lower RTID occurred in 18 cases (40%), requiring ICU transfer and mechanical ventilation in 7 (15.6%) (179). Similarly, HRV was detected in 8% of 77 bronchoalveolar lavage samples obtained from HCT patients with new-onset lower RTID and possibly contributed, together with other coinfections, to a high fatality rate of 83% (3). A large retrospective study from the United States identified 697 HRV-positive HCT patients transplanted between 1993 and 2015 (180). Patients with HRV upper RTID had a 90-day mortality of 6%, whereas the mortality rate was 41% for those with lower RTID. The survival rate was not significantly affected by copathogen detection, but in multivariate analysis, the overall mortality was associated with a degree of respiratory failure, captured as oxygen use (P = 0.015); immunodeficiency, captured as a low monocyte count of <300/μl (P = 0.027); or steroid dose greater than 1 mg/kg/day (P = 0.003) before diagnosis. In univariate analysis, a low lymphocyte, but not monocyte, count of <300/μl was significantly associated with respiratory failure. This raises questions about the different functional effector or surrogate roles of lymphocytes versus monocytes, as well as the significance versus convenience of various thresholds of <300, <200, and <100 (13, 66). However, one may conclude that HRVs, which typically have a propensity for the upper respiratory tract, with its colder body temperature, have a clinical impact similar to that of HRSV, HPIV, or IV-A/B once established as lower RTID (180). Indeed, a comparative study of 47 HRV cases versus controls matched for time of transplant, stem cell source, and malignancy identified a 2-fold increased rate of hospital readmissions (P < 0.007) (181). HRV infection pretransplant was noted in 13 patients, but the authors could not identify an increase in poor outcome (181). In view of the prolonged shedding, defining predictors of HRV lower RTID and mortality, such as grading SID or ISI, are warranted to guide decisions regarding transplant deferral and/or selective treatment of allogeneic HCT patients at highest risk (5). In a recent retrospective single-center study of 588 HRV upper RTID events in 150 autologous and 438 allogeneic HCT recipients, 16 and 84 were found to progress to lower RTID, respectively (182). Following multivariate analysis, a novel weighted risk score was derived consisting of a total of 99 points, which were given at upper RTID diagnosis, including for steroid use (20 for ≥2 mg/kg bodyweight; 12 for ≥1 to <2 mg/kg), 16 for lymphocyte counts of <100/μl, 14 for allogeneic/unrelated donor, and 12 for a positive CMV recipient status. Other less obvious scoring factors were 11 for low serum albumin of <30 mg/liter, 14 for recipient statin use, and 12 for previous HRV events. In the total population, four risk strata of scores of <16, 16 to <27, 27 to <46, and >46 were associated with progression to lower HRV RTID within 90 days of 9%, 12%, 22%, and 42%, respectively. Using a binary model for HRV events in allogeneic HCT patients only, a score of ≥27 was associated with a cumulative incidence of 28% versus 11%. Further studies will be needed to evaluate the clinical utility of this HRV progression score as well as the comparison with other risk scores. In addition, more data on the role of HRV types, viral loads in respiratory fluids and in blood, and possibly variants detected by next-generation sequencing are needed (173, 174).
Human coronaviruses.
HCoVs belong to the Coronaviridae family, and the species circulating in humans, HCoV-229E, -NL63, -OC43, and -HKU1, belong to the Alphacoronavirus (HCoV-229E and -NL63) and Betacoronavirus (HCoV-OC43 and –HKU1) genera, respectively. Animal CoVs are known for respiratory zoonosis, such as the past severe acute respiratory syndrome (SARS) outbreak and the ongoing Middle East respiratory syndrome (MERS)-CoV transmissions, with severe RTIDs justifying the name (183). The virions are enveloped particles of 120 nm surrounding a helical capsid and a very large single-stranded RNA genome of approximately 30 kb (https://viralzone.expasy.org/). HCoVs have been linked to severe pneumonia in case studies in HCT and lung transplant programs (184), but only recently, more comprehensive data have become available due to NAT-based diagnostics specifically detecting HCoV-229E, -NL63, -OC43, and -HKU1 (185). A prospective study of 279, mostly adult, transplant patients from Switzerland identified all 4 HCoVs in 29 (5.4%) of 540 BAL fluid samples. Of these, 18 (62%) had a new infiltrate on chest X-rays, and 9 (31%) were admitted to ICU (185). A large retrospective U.S. study compared HCoV detection in 85 immunocompromised and 1,152 nonimmunocompromised children with median ages of 6.3 and 1.6 years, respectively (186). The overall rates of HCoV lower RTID were not different between the groups, but severe lower RTID defined clinically by oxygen requirement was more frequently seen in immunocompromised children (adjusted OR, 2.5; 95% CI, 1.2 to 4.9; P = 0.01) and was not attributable to one or another HCoV species. Underlying respiratory disorders, HRSV infection, other copathogens, and younger age were significant in the univariate analysis.
In adult allogeneic HCT recipients, HCoVs were the second most frequently detected CARVs after HRV and were followed by IV-A/B (178, 187). Prolonged shedding of more than 21 days was common and not different between the types but rather reflected the immunodeficiency of the host- and treatment-related factors (178, 188) associated with high HCoV loads, steroid dosing of >1 mg/kg, and myeloablative conditioning (188). No association between lymphocyte or monocyte count and the rate of progression was observed. HCoV lower RTID ranged from 0% to 10% of allogeneic HCT patients. Focusing on 35 patients (allogeneic HCT, N = 28; hematologic malignancy, N = 7) having 37 episodes with HCoV lower RTID, 21 (60%) required oxygen therapy at diagnosis, and the overall mortality was 54% (n = 19) (189). Other pathogens were detected in 21 (57%) episodes, including viruses in 12, fungi in 10, and bacteria in 8, but were not significantly associated with mortality. In fact, the 90-day mortality rate of HCoV lower RTID was not statistically different from those observed in patients with IV-A/B (n = 36), HRSV (n = 113), or HPIV (n = 119) in the same center (189). The key factors in multivariate models were bone marrow as stem cell source (adjusted HR, 1.64; 95% CI, 1.13 to 2.40), oxygen use at diagnosis (adjusted HR, 3.00; 95% CI, 1.98 to 4.53), monocyte counts of <300/μl (adjusted HR, 1.87; 95% CI, 1.12 to 3.13), and neutrophil count of <500/μl (adjusted HR, 1.61; 95% CI, 1.00 to 2.58) (189).
Human adenoviruses.
HAdVs belong to the Adenoviridae family, characterized by nonenveloped virions of 60- to 90-nm diameter containing a linear double-stranded DNA of approximately 37 kb. HAdVs are known to persist for prolonged times, typically in lymphoid-rich tissues, and shedding into mucosal sites has been reported. HAdVs include more than 80 different types, which are grouped into 7 species (A to G), some having a higher propensity to cause RTID, such as HAdV-1, -3, -7, -16, -21, and -50 (subtype B1); HAdV-11, -14, -34, and -35 (subtype B2); HAdV-1, -2, -5, and -6 (subtype C); and HAdV-4 (subtype E) (190). These HAdVs cause approximately 1% to 10% of febrile RTIDs in adults and children. Immunocompromised young children with no or limited preexisting HAdV-specific immunity are at the highest risk for organ-invasive disease, particularly those after allogeneic HCT and SOT (191). HAdVs can disseminate to other organs with corresponding local disease manifestations, including parapneumonic effusion, hepatitis, gastrointestinal disease, and, more rarely, central nervous system disease. Although respiratory HAdVs are detectable year round (Fig. 1), the impressive HAdV disease potential is demonstrated in regional and institutional outbreaks of certain subtypes (192–197).
In SOT, comprehensive data on HAdV RTID are mostly available from lung transplantation, indicating rates of 1% to 10% among adult and pediatric patients (198–200). In a small prospective study of 16 mostly pediatric lung transplant patients, HAdV RTID was identified in 8 patients and was significantly associated with BOS. However, HAdV RTID was rare in a recent prospective study of adult lung transplant recipients and not associated with CLAD (55). Although there may be a predilection for HAdV involvement of the respective allograft, such as lung, heart, liver, and kidney, the manifestations in non-lung allografts may result from secondary spread from a mucosal entry site to these internal organs, with the possible exception of intestinal transplant recipients. In a kidney transplant recipient, HAdV multiorgan involvement was described as presenting as a febrile illness with sinusitis and conjunctivitis, and the patient was eventually diagnosed with renal allograft nephritis (201). In two other cases, pulmonary infiltrates and hepatitis occurred (202). This emphasizes the role of systemic humoral and cellular immunity and may explain the susceptibility of the pediatric population. However, in a small prospective study of 75 pediatric kidney transplant recipients, HAdV DNAemia was detected in 11 (14.7%) patients at a median of 173 days (interquartile range, 109 to 310 days) posttransplant, of whom 5 (6.7%) were symptomatic, including showing hematuria in 2 cases and acute febrile RTID in 3 cases (198).
In allogeneic HCT, HAdV replication is more frequently detected, which is, however, mostly due to the widespread screening of blood and gastrointestinal specimens from a- or presymptomatic patients considered to be at highest risk, such as children and those receiving alemtuzumab therapies (203). Conversely, HAdV is less frequently reported in respiratory specimens from allogeneic HCT patients with RTID (204, 205). Accordingly, the levels and dynamics of HAdV in different anatomic sites is not well understood, with the exception of blood and stool samples, and no generalized thresholds using international calibrators have been established (203, 206, 207). A large retrospective study of 687 allogeneic HCT patients identified HAdV in 64 (9.3%) cases presenting mostly as gastrointestinal disease or hemorrhagic cystitis but in 3 (0.5%) cases as HAdV pneumonitis, which eventually had a lethal outcome (204). A retrospective study of 572 pediatric HCT patients of a median age of 6.2 years identified HAdV by NAT in 76 (13.3%) patients, consisting of 8 (11%) autologous and 68 (89%) allogeneic HCT patients. In only a quarter of the latter, RTID attributable to HAdV was diagnosed using nasopharyngeal and bronchoalveolar lavage fluids (205). Thus, HAdV contributes to significant posttransplant RTID complications in less than 10% of allogeneic, preferentially pediatric HCT recipients.
Treatment of HAdV-RTID is limited to reducing immunosuppressive treatment and the off-label administration of intravenous cidofovir (199, 204, 208), systemic ribavirin, ganciclovir, and acyclovir (209, 210). Frequently, these antiviral agents have been combined with IVIG, but the overall response was either disappointing, moderate, or confounded by immune recovery of overall lymphocyte counts, including HAdV-specific T cells, best demonstrated in allogeneic HCT recipients (211). Thus, newer agents with more potent antiviral activity, such as oral or intravenous brincidofovir (formerly called CMX001) and adoptive T-cell transfer from an HCT donor or third party are currently being investigated as potentially more effective therapies (210, 212–214).
Human bocaviruses.
HBoVs belong to the Parvoviridae family, characterized by small nonenveloped virions of 25-nm diameter containing a 5-kbp single-stranded DNA carrying at either end telomeric hairpin loops of approximately 150 bp to facilitate genome replication (https://viralzone.expasy.org/). HBoV1 was first detected in respiratory secretions of children with lower RTIDs involving none of the hitherto known respiratory agents, but at least three additional types have been described, of which HBoV2 has been associated with gastroenteritis in infants (167). Similar to human parvovirus B19, detection of HBoV genomes may persist for prolonged periods, which, together with the frequent codetection with other CARVs, has complicated the attribution to disease and to RTID in particular as persistence, reactivation, or super-/reinfection (215, 216). This problem has been partly addressed by quantitative NAT in a large retrospective cohort of 426 patients contributing 685 respiratory specimens (217). The HBoV detection rate was 14.1% in 283 children and 1.4% in 143 adults, peaking in winter-early spring and in infants less than 1 year old. However, two-thirds represented coinfections. The single HBoV infections almost exclusively occurred in children less than 1 year old, having HBoV DNA loads above 5 log10 c/ml, but was found in none of the adults. Although these data suggest a cutoff, confirmatory studies of by-stander versus copathogen have not been rigorously conducted, and most reports on HBoV detection in transplant recipients by multiplex NAT are only qualitative. Another approach to identify significant HBoV exposure has been taken by examining the HBoV1 IgG and IgM seroresponse. Recent data were gathered from 759 otherwise healthy children from Brazil (mean age, 2.2 years; range, 1.2 to 3.4 years) presenting with community-acquired lower RTID (218). Acute or recent HBoV1 exposure was serologically identified in 38 (5%) children, including 4 of 8 cases in which HBoV1 was the only viral pathogen detected (218). Although serological responses may not be reliable in immunosuppressed transplant patients, this topic has not been well studied in SOT or allogeneic HCT.
Since immunocompromised and transplant patients are more likely to have coinfections, the role of HBoV1 (co)detection in RTID has been difficult to understand. A prospective study focusing on 522 bronchoalveolar lavage specimens from 299 adults, including HCT and lung transplant recipients, identified HBoV in 3 (1%) cases (32). A recent study from Brazil detected HBoV in 6 (3%) of 192 viral RTID episodes after allogeneic HCT, five of which occurred as coinfection and only one in a case of lower RTID (218). Unlike the well-known parvovirus B19, HBoV1-DNAemia was not detectable in a study of 53 children from Finland after allogeneic HCT (219). Thus, low rates (220), especially in adults, frequent coinfections, together with extended low-level persistence (215) more commonly seen for Parvoviridae render a simple attribution of HBoV1 to disease and necessity of treatment by, e.g., IVIG questionable.
CLOSING REMARKS AND OUTLOOK
CARVs are a common cause of morbidity and mortality among transplant patients and reflect the local activity in the community. Increasing use of modern molecular diagnostics in real time has expanded our understanding of the epidemiology of these viruses. Importantly, viruses once thought to be rare and clinically unimportant, notably rhinovirus and coronaviruses, are now being recognized as significant and common. Prediction of clinical impact remains important in order to optimize vaccine/prophylaxis strategies and to target antiviral interventions. The vulnerability of the patient and the pathogenicity of the respective CARVs, as well as the dynamics of progression to lower RTID, need to be considered. Clinical and laboratory scores, such as the ISI or SID grading, may help to guide early and extended antiviral (combination) treatment if further developed and validated. Currently approved therapies are only available for influenza and HRSV. Notably, newer classes of antivirals and antibodies are undergoing advanced development for the treatment of influenza, HRSV, HPIV, and adenovirus. Massive parallel sequencing techniques providing comprehensive and (semi)quantitative information on the respiratory virome as part of the microbiome and the respective host transcriptome response will generate new insights into pathogenesis of CARVs in transplant patients. Integrating this information into the real-time risk assessment and corresponding treatment approaches is likely to permit reducing the impact of respiratory viruses in the transplant population.
ACKNOWLEDGMENTS
We are very thankful to Patrick Lane of ScEYEnce Studios for working with us to prepare the graphically enhanced figures. We also thank Erika Hofmann, Basel, for excellent assistance with the references and the bibliography.
Biographies

Michael G. Ison completed his Internal Medicine Residency and General Internal Medicine Fellowship at Oregon Health Sciences University in Portland, Oregon. He then obtained his Master of Science in Health Evaluation Sciences and did his Infectious Diseases Fellowship at the University of Virginia. Dr. Ison undertook additional training in Transplant Infectious Diseases at the Massachusetts General Hospital and Harvard Medical School. He then joined the faculty of the Divisions of Infectious Diseases and Organ Transplantation at Northwestern University Feinberg School of Medicine in 2005. Dr. Ison is currently the Medical Director of the Transplant & Immunocompromised Host Infectious Diseases Service, Northwestern University Comprehensive Transplant Center, and Professor of Medicine and Surgery. His research focuses on respiratory viral infections in transplant recipients, and he has been engaged in studies in this population since 2001.

Hans H. Hirsch (FMH Infectious Diseases, FMH Internal Medicine, FAMH Medical Microbiology) is a professor at the Medical Faculty of the University of Basel, senior clinical consultant in Infectious Diseases & Hospital Epidemiology, and head of Clinical Virology at the University Hospital Basel in Basel, Switzerland. He has a special clinical and diagnostic expertise regarding HIV and other viral diseases, including those of relevance in solid-organ and hematopoietic cell transplantation. Dr. Hirsch has (co)authored more than 200 peer-reviewed papers and has given invited lectures at internationally relevant conferences in virology, infectious diseases, and transplantation. He has contributed to clinical guidelines and to key textbooks. Dr. Hirsch is co-senior editor of Transplant Infectious Disease, a consulting editor of the American Journal of Transplantation, and an editorial board member of the Journal of Clinical Microbiology and Journal of Virology. His research interests focus on translational aspects of viral infections in immunocompromised patients to improve clinical management.
REFERENCES
- 1.Rubin RH, Hirsch HH. 2008. Transplant infectious disease: a moving target. Transpl Infect Dis 10:1–2. doi: 10.1111/j.1399-3062.2008.00297.x. [DOI] [PubMed] [Google Scholar]
- 2.Englund JA, Sullivan CJ, Jordan MC, Dehner LP, Vercellotti GM, Balfour HH Jr.. 1988. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med 109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 3.Ison MG, Hayden FG, Kaiser L, Corey L, Boeckh M. 2003. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis 36:1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ison MG. 2007. Respiratory viral infections in transplant recipients. Antivir Ther 12:627–638. [PubMed] [Google Scholar]
- 5.Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. 2013. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. 2000. The impact of influenza epidemics on hospitalizations. J Infect Dis 181:831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 7.Laurichesse H, Dedman D, Watson JM, Zambon MC. 1999. Epidemiological features of parainfluenza virus infections: laboratory surveillance in England and Wales, 1975–1997. Eur J Epidemiol 15:475–484. doi: 10.1023/A:1007511018330. [DOI] [PubMed] [Google Scholar]
- 8.Treanor J, Falsey A. 1999. Respiratory viral infections in the elderly. Antiviral Res 44:79–102. doi: 10.1016/S0166-3542(99)00062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambon MC, Stockton JD, Clewley JP, Fleming DM. 2001. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet 358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 10.Hayden FG. 1997. Prevention and treatment of influenza in immunocompromised patients. Am J Med 102:55–60. doi: 10.1016/s0002-9343(97)80013-7. [DOI] [PubMed] [Google Scholar]
- 11.Apalsch AM, Green M, Ledesma-Medina J, Nour B, Wald ER. 1995. Parainfluenza and influenza virus infections in pediatric organ transplant recipients. Clin Infect Dis 20:394–399. doi: 10.1093/clinids/20.2.394. [DOI] [PubMed] [Google Scholar]
- 12.Whimbey E, Champlin RE, Couch RB, Englund JA, Goodrich JM, Raad I, Przepiorka D, Lewis VA, Mirza N, Yousuf H, Tarrand JJ, Bodey GP. 1996. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis 22:778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 13.Nichols WG, Gooley T, Boeckh M. 2001. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant 7(Suppl):11S–15S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 14.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, Feikin DR, Fowler KB, Gordon A, Hien NT, Horby P, Huang QS, Katz MA, Krishnan A, Lal R, Montgomery JM, Molbak K, Pebody R, Presanis AM, Razuri H, Steens A, Tinoco YO, Wallinga J, Yu H, Vong S, Bresee J, Widdowson MA. 2012. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 15.Dumoulin A, Widmer AF, Hirsch HH. 2009. Comprehensive diagnostics for respiratory virus infections after transplantation or after potential exposure to swine flu A/H1N1: what else is out there? Transpl Infect Dis 11:287–289. doi: 10.1111/j.1399-3062.2009.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickel CH, Stephan FP, Dangel M, Blume K, Gehrisch R, Dumoulin A, Tschudin S, Keller DI, Hirsch HH, Widmer AF, Bingisser R. 2009. First wave of the influenza A/H1N1v pandemic in Switzerland. Swiss Med Wkly 139:731–737. https://smw.ch/en/article/doi/smw.2009.12952/. [DOI] [PubMed] [Google Scholar]
- 17.Webb SA, Pettila V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW, Hart GK, Howe B, Iredell JR, McArthur C, Mitchell I, Morrison S, Nichol AD, Paterson DL, Peake S, Richards B, Stephens D, Turner A, Yung M. 2009. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 18.Pavia AT. 2011. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 52(Suppl 4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. 2011. Viral pneumonia. Lancet 377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton CL, Babady E, Ginocchio CC, Hatchette TF, Jerris RC, Li Y, Loeffelholz M, McCarter YS, Miller MB, Novak-Weekley S, Schuetz AN, Tang YW, Widen R, Drews SJ. 2019. Practical guidance for clinical microbiology laboratories: viruses causing acute respiratory tract infections. Clin Microbiol Rev 32:e00042-18. doi: 10.1128/CMR.00042-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, Fullman N, Mosser J, Thompson RL, Reiner RC, Abajobir A, Alam N, Alemayohu MA, Amare AT, Antonio CA, Asayesh H, Avokpaho E, Barac A, Beshir MA, Boneya DJ, Brauer M, Dandona L, Dandona R, Fitchett JRA, Gebrehiwot TT, Hailu GB, Hotez PJ, Kasaeian A, Khoja T, Kissoon N, Knibbs L, Kumar GA, Rai RK, El Razek HMA, Mohammed MSK, Nielson K, Oren E, Osman A, Patton G, Qorbani M, Roba HS, Sartorius B, Savic M, Shigematsu M, Sykes B, Swaminathan S, Topor-Madry R, Ukwaja K, Werdecker A, Yonemoto N, El Sayed Zaki M, Lim SS, Naghavi M, Vos T, Hay SI, Murray CJL, Mokdad AH. 2017. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee N, Lui GC, Wong KT, Li TC, Tse EC, Chan JY, Yu J, Wong SS, Choi KW, Wong RY, Ngai KL, Hui DS, Chan PK. 2013. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 57:1069–1077. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- 23.Russell E, Yang A, Tardrew S, Ison MG. 2019. Parainfluenza virus in hospitalized adults: a 7-year retrospective study. Clin Infect Dis 68:298–305. doi: 10.1093/cid/ciy451. [DOI] [PubMed] [Google Scholar]
- 24.Stolz D, Hirsch HH, Schilter D, Louis R, Rakic J, Boeck L, Papakonstantinou E, Schindler C, Grize L, Tamm M. 2018. Intensified therapy with inhaled corticosteroids and long-acting beta2-agonists at the onset of upper respiratory tract infection to prevent chronic obstructive pulmonary disease exacerbations. A multicenter, randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 197:1136–1146. doi: 10.1164/rccm.201709-1807OC. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2014. Global epidemiological surveillance standards for influenza. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/resources/documents/influenza_surveillance_manual/en/. [Google Scholar]
- 26.Peck AJ, Englund JA, Kuypers J, Guthrie KA, Corey L, Morrow R, Hackman RC, Cent A, Boeckh M. 2007. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood 110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna N, Steffen I, Studt JD, Schreiber A, Lehmann T, Weisser M, Fluckiger U, Gratwohl A, Halter J, Hirsch HH. 2009. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 11:100–105. doi: 10.1111/j.1399-3062.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 28.Ison MG, Hirsch HH. 2010. Influenza: a recurrent challenge to transplantation. Transpl Infect Dis 12:95–97. doi: 10.1111/j.1399-3062.2010.00501.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, Danziger-Isakov L, Stosor V, Estabrook M, Gantt S, Marr KA, Martin S, Silveira FP, Razonable RR, Allen UD, Levi ME, Lyon GM, Bell LE, Huprikar S, Patel G, Gregg KS, Pursell K, Helmersen D, Julian KG, Shiley K, Bono B, Dharnidharka VR, Alavi G, Kalpoe JS, Shoham S, Reid GE, Humar A, American Society of Transplantation H1N1 Collaborative Study Group. 2010. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis 10:521–526. doi: 10.1016/S1473-3099(10)70133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljungman P, de la Camara R, Perez-Bercoff L, Abecasis M, Nieto Campuzano JB, Cannata-Ortiz MJ, Cordonnier C, Einsele H, Gonzalez-Vicent M, Espigado I, Halter J, Martino R, Mohty B, Sucak G, Ullmann AJ, Vázquez L, Ward KN, Engelhard D. 2011. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica 96:1231–1235. doi: 10.3324/haematol.2011.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo S, Xie H, Campbell AP, Kuypers JM, Leisenring WM, Englund JA, Boeckh M. 2014. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 58:1357–1368. doi: 10.1093/cid/ciu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garbino J, Soccal PM, Aubert JD, Rochat T, Meylan P, Thomas Y, Tapparel C, Bridevaux PO, Kaiser L. 2009. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax 64:399–404. doi: 10.1136/thx.2008.105155. [DOI] [PubMed] [Google Scholar]
- 33.Soccal PM, Aubert JD, Bridevaux PO, Garbino J, Thomas Y, Rochat T, Rochat TS, Meylan P, Tapparel C, Kaiser L. 2010. Upper and lower respiratory tract viral infections and acute graft rejection in lung transplant recipients. Clin Infect Dis 51:163–170. doi: 10.1086/653529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckmann C, Hirsch HH. 2016. Comparing Luminex NxTAG-Respiratory Pathogen Panel and RespiFinder-22 for multiplex detection of respiratory pathogens. J Med Virol 88:1319–1324. doi: 10.1002/jmv.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Compagno F, Braunacker-Mayer L, Khanna N, Stolz D, Battegay M, Frey U, Tamm M, Kouyos RD, Hirsch HH. 2017. Comparing viral respiratory tract infections in symptomatic children and adults: multiplex NAT from 2010–2015 (EP0641). Abstr ECCMID 2017 ESCMID, Basel, Switzerland https://www.escmid.org/escmid_publications/escmid_elibrary/?q=Compagno±Hirsch+ePoster+2016&id=2173&L=0&x=0&y=0. [Google Scholar]
- 36.Cummings MJ, Bakamutumaho B, Kayiwa J, Byaruhanga T, Owor N, Namagambo B, Wolf A, Wamala JF, Morse SS, Lutwama JJ, O’Donnell MR. 2016. Epidemiologic and spatiotemporal characterization of influenza and severe acute respiratory infection in Uganda, 2010-2015. Annals ATS 13:2159–2168. doi: 10.1513/AnnalsATS.201607-561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch HH. 2019. Spatio-temporal virus surveillance for severe acute respiratory infections in resource-limited settings: how deep need we go? Clin Infect Dis 68:1126–1128. doi: 10.1093/cid/ciy663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch HH, Babel N, Comoli P, Friman V, Ginevri F, Jardine A, Lautenschlager I, Legendre C, Midtvedt K, Munoz P, Randhawa P, Rinaldo CH, Wieszek A, Hosts E. 2014. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect 20(Suppl 7):74–88. doi: 10.1111/1469-0691.12538. [DOI] [PubMed] [Google Scholar]
- 39.Piralla A, Mariani B, Rovida F, Baldanti F. 2017. Frequency of respiratory viruses among patients admitted to 26 intensive care units in seven consecutive winter-spring seasons (2009–2016) in Northern Italy. J Clin Virol 92:48–51. doi: 10.1016/j.jcv.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Asten L, van den Wijngaard C, van Pelt W, van de Kassteele J, Meijer A, van der Hoek W, Kretzschmar M, Koopmans M. 2012. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis 206:628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 41.Legoff J, Zucman N, Lemiale V, Mokart D, Pene F, Lambert J, Kouatchet A, Demoule A, Vincent F, Nyunga M, Bruneel F, Contejean A, Mercier-Delarue S, Rabbat A, Lebert C, Perez P, Meert AP, Benoit D, Schwebel C, Jourdain M, Darmon M, Resche-Rigon M, Azoulay E. 2018. Clinical significance of upper airway virus detection in critically ill hematology patients. Am J Respir Crit Care Med 199:518–528. doi: 10.1164/rccm.201804-0681OC. [DOI] [PubMed] [Google Scholar]
- 42.Shorr AF, Fisher K, Micek ST, Kollef MH. 2018. The burden of viruses in pneumonia associated with acute respiratory failure: an underappreciated issue. Chest 154:84–90. doi: 10.1016/j.chest.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. 2017. Viruses are prevalent in non-ventilated hospital-acquired pneumonia. Respir Med 122:76–80. doi: 10.1016/j.rmed.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 45.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirsch HH. 2005. Virus infections post transplant: risk and immunity. Transpl Infect Dis 7:97–98. doi: 10.1111/j.1399-3062.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 47.Campbell AP, Guthrie KA, Englund JA, Farney RM, Minerich EL, Kuypers J, Corey L, Boeckh M. 2015. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis 61:192–202. doi: 10.1093/cid/civ272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.L’Huillier AG, Green M, Danziker-Isakov L, Chaudhuri A, Höcker B, VanderLinden D, Goddard L, Ardura MI, Stephens D, Verma A, Evans HM, McCulloch M, Michaels MG, Posfay-Barbe KM. 2019. Infections among pediatric transplant candidates: an approach to decision‐making. Pediatr Transplant 23:1–13. doi: 10.1111/petr.13375. [DOI] [PubMed] [Google Scholar]
- 49.Malinis M, Boucher HW, AST Infectious Diseases Community of Practice. 2019. Screening of donor and candidate prior to solid organ transplantation–guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019:e13548. doi: 10.1111/ctr.13548. [DOI] [PubMed] [Google Scholar]
- 50.Nagler A, Shimoni A. 2019. Conditioning, p 99–107. In Carreras E, Dufour C, Mohty M, Kröger N (ed), The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. Springer, Cham, Switzerland. doi: 10.1007/978-3-030-02278-5_13. [DOI] [PubMed] [Google Scholar]
- 51.Cordero E, Roca-Oporto C, Bulnes-Ramos A, Aydillo T, Gavaldà J, Moreno A, Torre-Cisneros J, Montejo JM, Fortun J, Muñoz P, Sabé N, Fariñas MC, Blanes-Julia M, López-Medrano F, Suárez-Benjumea A, Martinez-Atienza J, Rosso-Fernández C, Pérez-Romero P, TRANSGRIPE 1–2 Study Group. 2017. Two doses of inactivated influenza vaccine improve immune response in solid organ transplant recipients: results of TRANSGRIPE 1-2, a randomized controlled clinical trial. Clin Infect Dis 64:829–838. doi: 10.1093/cid/ciw855. [DOI] [PubMed] [Google Scholar]
- 52.Branagan AR, Duffy E, Albrecht RA, Cooper DL, Seropian S, Parker TL, Gan G, Li F, Zelterman D, Boddupalli CS, Zhang L, Verma R, Ferencz TM, Dhodapkar MV. 2017. Clinical and serologic responses after a two-dose series of high-dose influenza vaccine in plasma cell disorders: a prospective, single-arm trial. Clin Lymphoma Myeloma Leuk 17:296–304. doi: 10.1016/j.clml.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fishman JA. 2017. Infection in organ transplantation. Am J Transplant 17:856–879. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 54.Huang G, Yu D, Mao N, Zhu Z, Zhang H, Jiang Z, Li H, Zhang Y, Shi J, Zhang S, Wang X, Xu W. 2013. Viral etiology of acute respiratory infection in Gansu province, China, 2011. PLoS One 8:e64254. doi: 10.1371/journal.pone.0064254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peghin M, Hirsch HH, Len Ó, Codina G, Berastegui C, Sáez B, Solé J, Cabral E, Solé A, Zurbano F, López-Medrano F, Román A, Gavaldá J. 2017. Epidemiology and immediate indirect effects of respiratory viruses in lung transplant recipients: a 5-year prospective study. Am J Transplant 17:1304–1312. doi: 10.1111/ajt.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar D, Husain S, Chen MH, Moussa G, Himsworth D, Manuel O, Studer S, Pakstis D, McCurry K, Doucette K, Pilewski J, Janeczko R, Humar A. 2010. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation 89:1028–1033. doi: 10.1097/TP.0b013e3181d05a71. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Worley S, Arrigain S, Aurora P, Ballmann M, Boyer D, Conrad C, Eichler I, Elidemir O, Goldfarb S, Mallory GB, Mogayzel PJ, Parakininkas D, Visner G, Sweet S, Faro A, Michaels M, Danziger-Isakov LA. 2009. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis 11:304–312. doi: 10.1111/j.1399-3062.2009.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottlieb J, Schulz TF, Welte T, Fuehner T, Dierich M, Simon AR, Engelmann I. 2009. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation 87:1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 59.Paulsen GC, Danziger-Isakov L. 2017. Respiratory viral infections in solid organ and hematopoietic stem cell transplantation. Clin Chest Med 38:707–726. doi: 10.1016/j.ccm.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P, Aydillo T, Danziger-Isakov L, Limaye AP, Carratala J, Munoz P, Montejo M, Lopez-Medrano F, Farinas MC, Gavalda J, Moreno A, Levi M, Fortun J, Torre-Cisneros J, Englund JA, Natori Y, Husain S, Reid G, Sharma TS, Humar A. 2018. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis 67:1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 61.Peghin M, Los-Arcos I, Hirsch HH, Codina G, Monforte V, Bravo C, Berastegui C, Jauregui A, Romero L, Cabral E, Ferrer R, Sacanell J, Roman A, Len O, Gavalda J. 2018. Community-acquired respiratory viruses are a risk factor for chronic lung allograft dysfunction. Clin Infect Dis 2018:ciy1047. doi: 10.1093/cid/ciy1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cainelli F, Vento S. 2002. Infections and solid organ transplant rejection: a cause-and-effect relationship? Lancet Infect Dis 2:539–549. doi: 10.1016/S1473-3099(02)00370-5. [DOI] [PubMed] [Google Scholar]
- 63.Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. 2011. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am J Transplant 11:1071–1078. doi: 10.1111/j.1600-6143.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCullers JA. 2014. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 65.Shah RJ, Diamond JM. 2017. Update in chronic lung allograft dysfunction. Clin Chest Med 38:677–692. doi: 10.1016/j.ccm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Khanna N, Widmer AF, Decker M, Steffen I, Halter J, Heim D, Weisser M, Gratwohl A, Fluckiger U, Hirsch HH. 2008. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis 46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 67.Harrington RD, Hooton TM, Hackman RC, Storch GA, Osborne B, Gleaves CA, Benson A, Meyers JD. 1992. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis 165:987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 68.Cortez KJ, Erdman DD, Peret TC, Gill VJ, Childs R, Barrett AJ, Bennett JE. 2001. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis 184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 69.Lehners N, Schnitzler P, Geis S, Puthenparambil J, Benz MA, Alber B, Luft T, Dreger P, Eisenbach C, Kunz C, Benner A, Buchholz U, Aichinger E, Frank U, Heeg K, Ho AD, Egerer G. 2013. Risk factors and containment of respiratory syncytial virus outbreak in a hematology and transplant unit. Bone Marrow Transplant 48:1548–1553. doi: 10.1038/bmt.2013.94. [DOI] [PubMed] [Google Scholar]
- 70.Lehners N, Tabatabai J, Prifert C, Wedde M, Puthenparambil J, Weissbrich B, Biere B, Schweiger B, Egerer G, Schnitzler P. 2016. Long-term shedding of influenza virus, parainfluenza virus, respiratory syncytial virus and nosocomial epidemiology in patients with hematological disorders. PLoS One 11:e0148258. doi: 10.1371/journal.pone.0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikulska M, Del Bono V, Gandolfo N, Dini S, Dominietto A, Di Grazia C, Bregante S, Varaldo R, Orsi A, Ansaldi F, Bacigalupo A, Viscoli C. 2014. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol 93:669–676. doi: 10.1007/s00277-013-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foolad F, Aitken SL, Shigle TL, Prayag A, Ghantoji S, Ariza-Heredia E, Chemaly RF. 2018. Oral versus aerosolized ribavirin for the treatment of respiratory syncytial virus infections in hematopoietic cell transplantation recipients. Clin Infect Dis 2018:ciy760. doi: 10.1093/cid/ciy760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spahr Y, Tschudin-Sutter S, Baettig V, Compagno F, Tamm M, Halter J, Gerull S, Passweg J, Hirsch HH, Khanna N. 2018. Community-acquired respiratory paramyxovirus infection after allogeneic hematopoietic cell transplantation: a single-center experience. Open Forum Infect Dis 5:ofy077. doi: 10.1093/ofid/ofy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher BT, Danziger-Isakov L, Sweet LR, Munoz FM, Maron G, Tuomanen E, Murray A, Englund JA, Dulek D, Halasa N, Green M, Michaels MG, Madan RP, Herold BC, Steinbach WJ. 2018. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatric Infect Dis Soc 7:275–282. doi: 10.1093/jpids/pix051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim YJ, Guthrie KA, Waghmare A, Walsh EE, Falsey AR, Kuypers J, Cent A, Englund JA, Boeckh M. 2014. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 209:1195–1204. doi: 10.1093/infdis/jit832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kmeid J, Vanichanan J, Shah DP, El Chaer F, Azzi J, Ariza-Heredia EJ, Hosing C, Mulanovich V, Chemaly RF. 2016. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant 22:542–548. doi: 10.1016/j.bbmt.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinana JL, Gomez MD, Perez A, Madrid S, Balaguer-Rosello A, Gimenez E, Montoro J, Gonzalez EM, Vinuesa V, Moles P, Hernandez-Boluda JC, Salavert M, Calabuig M, Sanz G, Solano C, Sanz J, Navarro D. 2018. Community-acquired respiratory virus lower respiratory tract disease in allogeneic stem cell transplantation recipient: risk factors and mortality from pulmonary virus-bacterial mixed infections. Transpl Infect Dis 20:e12926. doi: 10.1111/tid.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vakil E, Sheshadri A, Faiz SA, Shah DP, Zhu Y, Li L, Kmeid J, Azzi J, Balagani A, Bashoura L, Ariza-Heredia E, Chemaly RF. 2018. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl Infect Dis 20:e12994. doi: 10.1111/tid.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaia J, Baden L, Boeckh MJ, Chakrabarti S, Einsele H, Ljungman P, McDonald GB, Hirsch H. 2009. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant 44:471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ariza-Heredia EJ, Gulbis AM, Stolar KR, Kebriaei P, Shah DP, McConn KK, Champlin RE, Chemaly RF. 2014. Vaccination guidelines after hematopoietic stem cell transplantation: practitioners’ knowledge, attitudes, and gap between guidelines and clinical practice. Transpl Infect Dis 16:878–886. doi: 10.1111/tid.12312. [DOI] [PubMed] [Google Scholar]
- 81.Engelhard D, Mohty B, de la Camara R, Cordonnier C, Ljungman P. 2013. European guidelines for prevention and management of influenza in hematopoietic stem cell transplantation and leukemia patients: summary of ECIL-4 (2011), on behalf of ECIL, a joint venture of EBMT, EORTC, ICHS, and ELN. Transpl Infect Dis 15:219–232. doi: 10.1111/tid.12054. [DOI] [PubMed] [Google Scholar]
- 82.Engelhard D, Nagler A, Hardan I, Morag A, Aker M, Baciu H, Strauss N, Parag G, Naparstek E, Ravid Z. 1993. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant 11:1–5. [PubMed] [Google Scholar]
- 83.Smith KG, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. 1998. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transplant 13:160–164. doi: 10.1093/ndt/13.1.160. [DOI] [PubMed] [Google Scholar]
- 84.Danziger-Isakov L, Kumar D, AST ID Community of Practice. 2019. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019:e13563. doi: 10.1111/ctr.13563. [DOI] [PubMed] [Google Scholar]
- 85.Cordonnier C, Einarsdottir S, Cesaro S, Di Blasi R, Mikulska M, Rieger C, de Lavallade H, Gallo G, Lehrnbecher T, Engelhard D, Ljungman P, European Conference on Infections in Leukaemia. 2019. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19:e200–e212. doi: 10.1016/S1473-3099(18)30600-5. [DOI] [PubMed] [Google Scholar]
- 86.Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A. 2018. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 87.Ison MG, Szakaly P, Shapira MY, Krivan G, Nist A, Dutkowski R. 2012. Efficacy and safety of oral oseltamivir for influenza prophylaxis in transplant recipients. Antivir Ther 17:955–964. doi: 10.3851/IMP2192. [DOI] [PubMed] [Google Scholar]
- 88.Canini L, Conway JM, Perelson AS, Carrat F. 2014. Impact of different oseltamivir regimens on treating influenza a virus infection and resistance emergence: insights from a modelling study. PLoS Comput Biol 10:e1003568. doi: 10.1371/journal.pcbi.1003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kimball P, Verbeke S, Flattery M, Rhodes C, Tolman D. 2000. Influenza vaccination does not promote cellular or humoral activation among heart transplant recipients. Transplantation 69:2449–2451. doi: 10.1097/00007890-200006150-00042. [DOI] [PubMed] [Google Scholar]
- 90.Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. 2017. Heterologous Immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 17:281–286. doi: 10.1111/ajt.13960. [DOI] [PubMed] [Google Scholar]
- 91.Birdwell KA, Ikizler MR, Sannella EC, Wang L, Byrne DW, Ikizler TA, Wright PF. 2009. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis 54:112–121. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manuel O, Humar A, Berutto C, Ely L, Giulieri S, Lien D, Meylan PR, Weinkauf J, Pascual M, Nador R, Aubert JD, Kumar D. 2011. Low-dose intradermal versus intramuscular trivalent inactivated seasonal influenza vaccine in lung transplant recipients. J Heart Lung Transplant 30:679–684. doi: 10.1016/j.healun.2011.01.705. [DOI] [PubMed] [Google Scholar]
- 93.Baluch A, Humar A, Eurich D, Egli A, Liacini A, Hoschler K, Campbell P, Berka N, Urschel S, Wilson L, Kumar D. 2013. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant 13:1026–1033. doi: 10.1111/ajt.12149. [DOI] [PubMed] [Google Scholar]
- 94.Natori Y, Shiotsuka M, Slomovic J, Hoschler K, Ferreira V, Ashton P, Rotstein C, Lilly L, Schiff J, Singer L, Humar A, Kumar D. 2018. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 66:1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 95.Ison MG. 2012. Antiviral therapies for respiratory viral infections in lung transplant patients. Antivir Ther 17:193–200. doi: 10.3851/IMP2058. [DOI] [PubMed] [Google Scholar]
- 96.Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. 2008. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 27:282–288. doi: 10.1016/j.healun.2007.11.575. [DOI] [PubMed] [Google Scholar]
- 97.Manuel O, Estabrook M, American Society of Transplantation Infectious Diseases Community of Practice. 2019. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 28:e13511. doi: 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, Gravenstein S, Hayden FG, Harper SA, Hirshon JM, Ison MG, Johnston BL, Knight SL, McGeer A, Riley LE, Wolfe CR, Alexander PE, Pavia AT. 2019. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 68:895–902. doi: 10.1093/cid/ciy874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beckmann C, Hirsch HH. 2015. Diagnostic performance of near-patient testing for influenza. J Clin Virol 67:43–46. doi: 10.1016/j.jcv.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bell J, Bonner A, Cohen DM, Birkhahn R, Yogev R, Triner W, Cohen J, Palavecino E, Selvarangan R. 2014. Multicenter clinical evaluation of the novel Alere i influenza A&B isothermal nucleic acid amplification test. J Clin Virol 61:81–86. doi: 10.1016/j.jcv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Brendish NJ, Schiff HF, Clark TW. 2015. Point-of-care testing for respiratory viruses in adults: the current landscape and future potential. J Infect 71:501–510. doi: 10.1016/j.jinf.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee N, Hui DS, Zuo Z, Ngai KL, Lui GC, Wo SK, Tam WW, Chan MC, Wong BC, Wong RY, Choi KW, Sin WW, Lee EL, Tomlinson B, Hayden FG, Chan PK. 2013. A prospective intervention study on higher-dose oseltamivir treatment in adults hospitalized with influenza a and B infections. Clin Infect Dis 57:1511–1519. doi: 10.1093/cid/cit597. [DOI] [PubMed] [Google Scholar]
- 103.South East Asia Infectious Disease Clinical Research Network. 2013. Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ 346:f3039. doi: 10.1136/bmj.f3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar D, Morris MI, Kotton CN, Fischer SA, Michaels MG, Allen U, Blumberg EA, Green M, Humar A, Ison MG, AST Infectious Diseases Community of Practice and Transplant Infectious Diseases Section of TTS. 2010. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant 10:18–25. doi: 10.1111/j.1600-6143.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 105.Nichols WG, Guthrie KA, Corey L, Boeckh M. 2004. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 106.Most J, Weiss G. 2016. Consecutive infections with influenza A and B virus in children during the 2014-2015 seasonal influenza epidemic. J Infect Dis 214:1139–1141. doi: 10.1093/infdis/jiw104. [DOI] [PubMed] [Google Scholar]
- 107.Pinana JL, Perez A, Montoro J, Gimenez E, Dolores Gomez M, Lorenzo I, Madrid S, Gonzalez EM, Vinuesa V, Hernandez-Boluda JC, Salavert M, Sanz G, Solano C, Sanz J, Navarro D. 2018. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional prospective observational study. Clin Infect Dis 2018:ciy792. doi: 10.1093/cid/ciy792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Casper C, Englund J, Boeckh M. 2010. How I treat influenza in patients with hematologic malignancies. Blood 115:1331–1342. doi: 10.1182/blood-2009-11-255455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reid G, Huprikar S, Patel G, Razonable RR, Mossad S, Levi M, Gregg K, Shoham S, Humar A, Adams W, Kumar D. 2013. A multicenter evaluation of pandemic influenza A/H1N1 in hematopoietic stem cell transplant recipients. Transpl Infect Dis 15:487–492. doi: 10.1111/tid.12116. [DOI] [PubMed] [Google Scholar]
- 110.Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. 2011. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood 117:5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang L, Allen J, Diong C, Goh YT, Gopalakrishnan S, Ho A, Hwang W, Lim F, Oon L, Tan TT, Linn YC, Tan BH. 2017. Respiratory virus infection after allogeneic hematopoietic stem cell transplant in a tropical center: predictive value of the immunodeficiency scoring index. Transpl Infect Dis 19:e12693. doi: 10.1111/tid.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.GiaQuinta S, Michaels MG, McCullers JA, Wang L, Fonnesbeck C, O'Shea A, Green M, Halasa NB. 2015. Randomized, double-blind comparison of standard-dose vs. high-dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatr Transplant 19:219–228. doi: 10.1111/petr.12419. [DOI] [PubMed] [Google Scholar]
- 113.Halasa NB, Savani BN, Asokan I, Kassim A, Simons R, Summers C, Bourgeois J, Clifton C, Vaughan LA, Lucid C, Wang L, Fonnesbeck C, Jagasia M. 2016. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 22:528–535. doi: 10.1016/j.bbmt.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 114.European Medicines Agency. 2011. Summary on compassionate use for IV zanamivir. Procedure no. EMA/H/K/2287/CU. 114 European Medicines Agency, Amsterdam, the Netherlands: https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-iv-zanamivir-rev-1_en.pdf. [Google Scholar]
- 115.Whitley RJ, Boucher CA, Lina B, Nguyen-Van-Tam JS, Osterhaus A, Schutten M, Monto AS. 2013. Global assessment of resistance to neuraminidase inhibitors, 2008-2011: the Influenza Resistance Information Study (IRIS). Clin Infect Dis 56:1197–1205. doi: 10.1093/cid/cis1220. [DOI] [PubMed] [Google Scholar]
- 116.Lina B, Boucher C, Osterhaus A, Monto AS, Schutten M, Whitley RJ, Nguyen-Van-Tam JS. 2018. Five years of monitoring for the emergence of oseltamivir resistance in patients with influenza A infections in the Influenza Resistance Information Study. Influenza Other Respir Viruses 12:267–278. doi: 10.1111/irv.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van der Vries E, Stittelaar KJ, van Amerongen G, Veldhuis Kroeze EJ, de Waal L, Fraaij PL, Meesters RJ, Luider TM, van der Nagel B, Koch B, Vulto AG, Schutten M, Osterhaus AD. 2013. Prolonged influenza virus shedding and emergence of antiviral resistance in immunocompromised patients and ferrets. PLoS Pathog 9:e1003343. doi: 10.1371/journal.ppat.1003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schofield KP, Potter CW, Edey D, Jennings R, Oxford JS. 1975. Antiviral activity of ribavirin on influenza infection in ferrets. J Antimicrob Chemother 1:63–69. doi: 10.1093/jac/1.suppl_4.63. [DOI] [PubMed] [Google Scholar]
- 119.Beigel JH, Bao Y, Beeler J, Manosuthi W, Slandzicki A, Dar SM, Panuto J, Beasley RL, Perez-Patrigeon S, Suwanpimolkul G, Losso MH, McClure N, Bozzolo DR, Myers C, Holley HP, Hoopes J, Lane HC, Hughes MD, Davey RT, Winnie M, Dinh DV, Seethala R, Garcia H, Pouzar J, Seep M, Riffer E, Bart B, Dar S, Hoppers M, Panuto J, Rowe H, Slandzicki A, Wolfe C, Desantis D, Baynton B, Beasley RL, Markowitz N, Stearns ZA, Cho J, Goisse M, Wolf TA, Kay J, Dharan N, Fitzgibbons W, Woodruff M, Bell T, Lenzmeier T, Schooley R, Elie M-C, Winokur P, Finberg R, Hurt C, Tebas P, Sattler FR, Ampajwala M, Batts D, Bloch M, Moore R, Dwyer D, Romo-Garcia J, Patrigeon SP, Zulueta APR, Manosuthi W, Chetchotisakd P, Ruxrungtham K, Avihingsanon A, Suwanpimolkul G, Ratanasuwan W, Lupo S, Trape L, Losso MH, Macias LM, Lopardo G, Barcelona L, Mykietuk A, Alzogaray MF. 2017. Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: a multicentre, double-blind, randomised phase 2 trial. Lancet Infect Dis 17:1255–1265. doi: 10.1016/S1473-3099(17)30476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoopes JD, Driebe EM, Kelley E, Engelthaler DM, Keim PS, Perelson AS, Rong L, Went GT, Nguyen JT. 2011. Triple combination antiviral drug (TCAD) composed of amantadine, oseltamivir, and ribavirin impedes the selection of drug-resistant influenza A virus. PLoS One 6:e29778. doi: 10.1371/journal.pone.0029778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seo S, Englund JA, Nguyen JT, Pukrittayakamee S, Lindegardh N, Tarning J, Tambyah PA, Renaud C, Went GT, de Jong MD, Boeckh MJ. 2013. Combination therapy with amantadine, oseltamivir and ribavirin for influenza A infection: safety and pharmacokinetics. Antivir Ther 18:377–386. doi: 10.3851/IMP2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fukao K, Noshi T, Yamamoto A, Kitano M, Ando Y, Noda T, Baba K, Matsumoto K, Higuchi N, Ikeda M, Shishido T, Naito A. 2019. Combination treatment with the cap-dependent endonuclease inhibitor baloxavir marboxil and a neuraminidase inhibitor in a mouse model of influenza A virus infection. J Antimicrob Chemother 74:654–662. doi: 10.1093/jac/dky462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kawaguchi N, Koshimichi H, Ishibashi T, Wajima T. 2018. Evaluation of drug-drug interaction potential between baloxavir marboxil and oseltamivir in healthy subjects. Clin Drug Investig 38:1053–1060. doi: 10.1007/s40261-018-0697-2. [DOI] [PubMed] [Google Scholar]
- 124.Finberg RW, Lanno R, Anderson D, Fleischhackl R, van Duijnhoven W, Kauffman RS, Kosoglou T, Vingerhoets J, Leopold L. 2019. Phase 2b study of pimodivir (JNJ-63623872) as monotherapy or in combination with oseltamivir for treatment of acute uncomplicated seasonal influenza A: TOPAZ Trial. J Infect Dis 219:1026–1034. doi: 10.1093/infdis/jiy547. [DOI] [PubMed] [Google Scholar]
- 125.Beigel JH, Tebas P, Elie-Turenne MC, Bajwa E, Bell TE, Cairns CB, Shoham S, Deville JG, Feucht E, Feinberg J, Luke T, Raviprakash K, Danko J, O’Neil D, Metcalf JA, King K, Burgess TH, Aga E, Lane HC, Hughes MD, Davey RT, IRC002 Study Team. 2017. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med 5:500–511. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bhattacharyya S, Gesteland PH, Korgenski K, Bjornstad ON, Adler FR. 2015. Cross-immunity between strains explains the dynamical pattern of paramyxoviruses. Proc Natl Acad Sci U S A 112:13396–13400. doi: 10.1073/pnas.1516698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Guarino B, Silacci C, Marcandalli J, Marsland BJ, Piralla A, Percivalle E, Sallusto F, Baldanti F, Lanzavecchia A. 2013. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 501:439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 128.Hynicka LM, Ensor CR. 2012. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 46:558–566. doi: 10.1345/aph.1Q553. [DOI] [PubMed] [Google Scholar]
- 129.Shah JN, Chemaly RF. 2011. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 117:2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 130.Rowan CM, Gertz SJ, Zinter MS, Moffet J, Bajwa RPS, Barnum JL, Kong M. 2018. A multicenter investigation of respiratory syncytial viral infection in children with hematopoietic cell transplantation. Transpl Infect Dis 20:e12882. doi: 10.1111/tid.12882. [DOI] [PubMed] [Google Scholar]
- 131.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. 2014. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 132.Teusink-Cross A, Davies SM, Danziger-Isakov L, El-Bietar J, Grimley MS. 2016. Restrictive palivizumab use does not lead to increased morbidity and mortality in pediatric hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 22:1904–1906. doi: 10.1016/j.bbmt.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 133.Uckay I, Gasche-Soccal PM, Kaiser L, Stern R, Mazza-Stalder J, Aubert JD, van Delden C. 2010. Low incidence of severe respiratory syncytial virus infections in lung transplant recipients despite the absence of specific therapy. J Heart Lung Transplant 29:299–305. doi: 10.1016/j.healun.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Glanville AR, Scott AI, Morton JM, Aboyoun CL, Plit ML, Carter IW, Malouf MA. 2005. Intravenous ribavirin is a safe and cost-effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant 24:2114–2119. doi: 10.1016/j.healun.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 135.Fuehner T, Dierich M, Duesberg C, DeWall C, Welte T, Haverich A, Warnecke G, Simon AR, Gottlieb J. 2011. Single-centre experience with oral ribavirin in lung transplant recipients with paramyxovirus infections. Antivir Ther 16:733–740. doi: 10.3851/IMP1811. [DOI] [PubMed] [Google Scholar]
- 136.Burrows FS, Carlos LM, Benzimra M, Marriott DJ, Havryk AP, Plit ML, Malouf MA, Glanville AR. 2015. Oral ribavirin for respiratory syncytial virus infection after lung transplantation: efficacy and cost-efficiency. J Heart Lung Transplant 34:958–962. doi: 10.1016/j.healun.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 137.Chemaly RF, Aitken SL, Wolfe CR, Jain R, Boeckh MJ. 2016. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis 18:634–636. doi: 10.1111/tid.12551. [DOI] [PubMed] [Google Scholar]
- 138.Chakrabarti S, Collingham KE, Holder K, Fegan CD, Osman H, Milligan DW. 2001. Pre-emptive oral ribavirin therapy of paramyxovirus infections after haematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant 28:759–763. doi: 10.1038/sj.bmt.1703216. [DOI] [PubMed] [Google Scholar]
- 139.Hirsch HH, Widmer AF, Francioli P. 2004. Respiratorisches syncytial-virus (RSV) infektion: massnahmen beim immunsupprimierten patienten. Praxis 95:61–66. doi: 10.1024/0369-8394.95.3.61. [DOI] [PubMed] [Google Scholar]
- 140.Shah DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U, Hosing C, Rondon G, Tarrand JJ, Champlin RE, Chemaly RF. 2013. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 68:1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beaird OE, Freifeld A, Ison MG, Lawrence SJ, Theodoropoulos N, Clark NM, Razonable RR, Alangaden G, Miller R, Smith J, Young JA, Hawkinson D, Pursell K, Kaul DR. 2016. Current practices for treatment of respiratory syncytial virus and other non-influenza respiratory viruses in high-risk patient populations: a survey of institutions in the Midwestern Respiratory Virus Collaborative. Transpl Infect Dis 18:210–215. doi: 10.1111/tid.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gottlieb J, Zamora MR, Hodges T, Musk AW, Sommerwerk U, Dilling D, Arcasoy S, DeVincenzo J, Karsten V, Shah S, Bettencourt BR, Cehelsky J, Nochur S, Gollob J, Vaishnaw A, Simon AR, Glanville AR. 2016. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant 35:213–221. doi: 10.1016/j.healun.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 143.DeVincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I, Nechev L, Murugaiah V, Van Vliet A, Vaishnaw AK, Meyers R. 2008. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV). Antiviral Res 77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 144.Domachowske JB, Khan AA, Esser MT, Jensen K, Takas T, Villafana T, Dubovsky F, Griffin MP. 2018. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J 37:886–892. doi: 10.1097/INF.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Griffin MP, Khan AA, Esser MT, Jensen K, Takas T, Kankam MK, Villafana T, Dubovsky F. 2017. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother 61:e01714-16. doi: 10.1128/AAC.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, McBride S, Lambkin-Williams R, Jordan R, Xin Y, Ramanathan S, O’Riordan T, Lewis SA, Li X, Toback SL, Lin SL, Chien JW. 2014. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 147.Gottlieb J, Torres F, Hadddad T, Dhillon G, Dilling DF, Knoop C, Rampolla R, Walia R, Ahya V, Kessler R, Mason DP, Budev M, Neurorh C, Glanville AR, Jordan R, Porter D, McKevitt MT, German P, Guo Y, Chien JW, Watkins TR, Zamora MR. 2018. A phase 2b randomized controlled trial of presatovir, an oral RSV fusion inhibitor, for the treatment of respiratory syncytial virus (RSV) in lung transplant (LT) recipients, abstr 372. J Heart Lung Transplant 37:S155 https://www.jhltonline.org/article/S1053-2498(18)30376-0/abstract. [DOI] [PubMed] [Google Scholar]
- 148.Chemaly RF, Dadwal SS, Bergeron A, Ljungman PT, Kim Y-J, Cheng GS, Pipavath S, Limaye AP, Blanchard E, Winston DJ, Stiff PJ, Marty FM, Zuckerman T, Lachance S, Rahav G, Small CB, Mullane KM, Patron R, Lee D-G, Hirsch HH, Waghmare A, McKevitt MT, Jordan R, Guo Y, German P, Porter D, Gossage D, Watkins TR, Chien JW, Boeckh MJ. 2018. A phase 2b, randomized, double-blind, placebo-controlled trial of presatovir (GS-5806), an oral fusion inhibitor for the treatment of RSV upper tract respiratory tract infection in hematopoietic-cell transplant recipients. Abstr BMT Tandem Meet, abstr LBA7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marty FM, Chemaly RF, Bergeron A, ljungman PT, Kim Y-J, Boeckh MJ, Waghmare A, Mullane KM, Lee D-G, Small CB, Hirsch HH, Blanchard E, McKevitt MT, Porter D, Jordan R, Guo Y, Watkins TR, Chien JW, Dadwal SS. 2018. A phase 2b, randomized, double-blind, placebo-controlled trial of presatovir (GS-5806), an oral fusion inhibitor for the treatment of RSV lower tract respiratory tract infection in hematopoietic-cell transplant recipients. Abstr BMT Tandem, abstr LBA6. [Google Scholar]
- 150.DeVincenzo JP, McClure MW, Symons JA, Fathi H, Westland C, Chanda S, Lambkin-Williams R, Smith P, Zhang Q, Beigelman L, Blatt LM, Fry J. 2015. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med 373:2048–2058. doi: 10.1056/NEJMoa1413275. [DOI] [PubMed] [Google Scholar]
- 151.Dosanjh A. 2015. Respiratory metapneumoviral infection without co-infection in association with acute and chronic lung allograft dysfunction. J Inflamm Res 8:79–82. doi: 10.2147/JIR.S78259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Egli A, Bucher C, Dumoulin A, Stern M, Buser A, Bubendorf L, Gregor M, Servida P, Sommer G, Bremerich J, Gratwohl A, Khanna N, Widmer AF, Battegay M, Tamm M, Hirsch HH, Halter JP. 2012. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection 40:677–684. doi: 10.1007/s15010-012-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shah DP, Shah PK, Azzi JM, El Chaer F, Chemaly RF. 2016. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett 379:100–106. doi: 10.1016/j.canlet.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seo S, Gooley TA, Kuypers JM, Stednick Z, Jerome KR, Englund JA, Boeckh M. 2016. Human metapneumovirus infections following hematopoietic cell transplantation: factors associated with disease progression. Clin Infect Dis 63:178–185. doi: 10.1093/cid/ciw284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Russell E, Ison MG. 2017. Parainfluenza virus in the hospitalized adult. Clin Infect Dis 65:1570–1576. doi: 10.1093/cid/cix528. [DOI] [PubMed] [Google Scholar]
- 156.Shah DP, Shah PK, Azzi JM, Chemaly RF. 2016. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett 370:358–364. doi: 10.1016/j.canlet.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Piralla A, Percivalle E, Di Cesare-Merlone A, Locatelli F, Gerna G. 2009. Multicluster nosocomial outbreak of parainfluenza virus type 3 infection in a pediatric oncohematology unit: a phylogenetic study. Haematologica 94:833–839. doi: 10.3324/haematol.2008.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hodson A, Kasliwal M, Streetly M, Macmahon E, Raj K. 2011. A parainfluenza-3 outbreak in a SCT unit: sepsis with multi-organ failure and multiple co-pathogens are associated with increased mortality. Bone Marrow Transplant 46:1545–1550. doi: 10.1038/bmt.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Berrueco R, Anton A, Rives S, Catala A, Toll T, Ruiz A, Camos M, Torrebadell M, Estella J, Munoz-Almagro C. 2013. Multiplex real-time PCR for prompt diagnosis of an outbreak of human parainfluenza 3 virus in children with acute leukemia. Infection 41:1171–1175. doi: 10.1007/s15010-013-0498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ustun C, Slaby J, Shanley RM, Vydra J, Smith AR, Wagner JE, Weisdorf DJ, Young JA. 2012. Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biol Blood Marrow Transplant 18:1580–1588. doi: 10.1016/j.bbmt.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chemaly RF, Hanmod SS, Rathod DB, Ghantoji SS, Jiang Y, Doshi A, Vigil K, Adachi JA, Khoury AM, Tarrand J, Hosing C, Champlin R. 2012. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood 119:2738–2745. doi: 10.1182/blood-2011-08-371112. [DOI] [PubMed] [Google Scholar]
- 162.Seo S, Xie H, Leisenring WM, Kuypers JM, Sahoo FT, Goyal S, Kimball LE, Campbell AP, Jerome KR, Englund JA, Boeckh M. 2019. Risk factors for parainfluenza virus lower respiratory tract disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 25:163–171. doi: 10.1016/j.bbmt.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Billings JL, Hertz MI, Savik K, Wendt CH. 2002. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant 21:559–566. doi: 10.1016/S1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 164.Erard V, Chien JW, Kim HW, Nichols WG, Flowers ME, Martin PJ, Corey L, Boeckh M. 2006. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis 193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Waghmare A, Wagner T, Andrews R, Smith S, Kuypers J, Boeckh M, Moss R, Englund JA. 2015. Successful treatment of parainfluenza virus respiratory tract infection with DAS181 in 4 immunocompromised children. J Pediatric Infect Dis Soc 4:114–118. doi: 10.1093/jpids/piu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen YB, Driscoll JP, McAfee SL, Spitzer TR, Rosenberg ES, Sanders R, Moss RB, Fang F, Marty FM. 2011. Treatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantation. Clin Infect Dis 53:e77–e80. doi: 10.1093/cid/cir501. [DOI] [PubMed] [Google Scholar]
- 167.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tapparel C, Cordey S, Junier T, Farinelli L, Van Belle S, Soccal PM, Aubert JD, Zdobnov E, Kaiser L. 2011. Rhinovirus genome variation during chronic upper and lower respiratory tract infections. PLoS One 6:e21163. doi: 10.1371/journal.pone.0021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Young JC, Chehoud C, Bittinger K, Bailey A, Diamond JM, Cantu E, Haas AR, Abbas A, Frye L, Christie JD, Bushman FD, Collman RG. 2015. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant 15:200–209. doi: 10.1111/ajt.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, Okamoto J, Snyder TM, Cornfield DN, Nicolls MR, Weill D, Bernstein D, Valantine HA, Quake SR. 2013. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hirsch HH, Pergam SA. 2016. Human adenovirus, polyomavirus, and parvovirus infections in patients undergoing hematopoietic stem-cell transplantation, p 1090–1104. In Forman SJ, Negrin RS, Antin JH, Appelbaum FR (ed), Thomas’ hematopoietic cell transplantation, 5th ed John Wiley & Sons Ltd., Chichester, United Kingdom. [Google Scholar]
- 172.Piralla A, Zecca M, Comoli P, Girello A, Maccario R, Baldanti F. 2015. Persistent rhinovirus infection in pediatric hematopoietic stem cell transplant recipients with impaired cellular immunity. J Clin Virol 67:38–42. doi: 10.1016/j.jcv.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Esposito S, Daleno C, Scala A, Castellazzi L, Terranova L, Sferrazza Papa S, Longo MR, Pelucchi C, Principi N. 2014. Impact of rhinovirus nasopharyngeal viral load and viremia on severity of respiratory infections in children. Eur J Clin Microbiol Infect Dis 33:41–48. doi: 10.1007/s10096-013-1926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ambrosioni J, Bridevaux PO, Aubert JD, Soccal P, Wagner G, Kaiser L. 2015. Role of rhinovirus load in the upper respiratory tract and severity of symptoms in lung transplant recipients. J Clin Virol 64:1–5. doi: 10.1016/j.jcv.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 175.Megremis S, Niespodziana K, Cabauatan C, Xepapadaki P, Kowalski ML, Jartti T, Bachert C, Finotto S, West P, Stamataki S, Lewandowska-Polak A, Lukkarinen H, Zhang N, Zimmermann T, Stolz F, Neubauer A, Akdis M, Andreakos E, Valenta R, Papadopoulos NG. 2018. Rhinovirus species-specific antibodies differentially reflect clinical outcomes in health and asthma. Am J Respir Crit Care Med 1490–1499. doi: 10.1164/rccm.201803-0575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sester M, Leboeuf C, Schmidt T, Hirsch HH. 2016. The “ABC” of virus-specific T cell immunity in solid organ transplantation. Am J Transplant 16:1697–1706. doi: 10.1111/ajt.13684. [DOI] [PubMed] [Google Scholar]
- 177.Sester M, Sester U, Alarcon Salvador S, Heine G, Lipfert S, Girndt M, Gartner B, Kohler H. 2002. Age-related decrease in adenovirus-specific T cell responses. J Infect Dis 185:1379–1387. doi: 10.1086/340502. [DOI] [PubMed] [Google Scholar]
- 178.Milano F, Campbell AP, Guthrie KA, Kuypers J, Englund JA, Corey L, Boeckh M. 2010. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sim SA, Leung VKY, Ritchie D, Slavin MA, Sullivan SG, Teh BW. 2018. Viral respiratory tract infections in allogeneic hematopoietic stem cell transplantation recipients in the era of molecular testing. Biol Blood Marrow Transplant 24:1490–1496. doi: 10.1016/j.bbmt.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Seo S, Waghmare A, Scott EM, Xie H, Kuypers JM, Hackman RC, Campbell AP, Choi SM, Leisenring WM, Jerome KR, Englund JA, Boeckh M. 2017. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica 102:1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abandeh FI, Lustberg M, Devine S, Elder P, Andritsos L, Martin SI. 2013. Outcomes of hematopoietic SCT recipients with rhinovirus infection: a matched, case-control study. Bone Marrow Transplant 48:1554–1557. doi: 10.1038/bmt.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Waghmare A, Xie H, Kuypers J, Sorror ML, Jerome KR, Englund JA, Boeckh M, Leisenring WM. 2019. Human rhinovirus infections in hematopoietic cell transplant recipients: risk score for progression to lower respiratory tract infection. Biol Blood Marrow Transplant 25:1011–1021. doi: 10.1016/j.bbmt.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim SH, Chang SY, Sung M, Park JH, Bin Kim H, Lee H, Choi JP, Choi WS, Min JY. 2016. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis 63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Folz RJ, Elkordy MA. 1999. Coronavirus pneumonia following autologous bone marrow transplantation for breast cancer. Chest 115:901–905. doi: 10.1378/chest.115.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Garbino J, Crespo S, Aubert JD, Rochat T, Ninet B, Deffernez C, Wunderli W, Pache JC, Soccal PM, Kaiser L. 2006. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (non-SARS)-related human coronavirus infection. Clin Infect Dis 43:1009–1015. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. 2018. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc 8:21–28. doi: 10.1093/jpids/pix093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wolfromm A, Porcher R, Legoff J, Peffault de Latour R, Xhaard A, de Fontbrune FS, Ribaud P, Bergeron A, Socie G, Robin M. 2014. Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long-term incidence and outcome. Biol Blood Marrow Transplant 20:1238–1241. doi: 10.1016/j.bbmt.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ogimi C, Greninger AL, Waghmare AA, Kuypers JM, Shean RC, Xie H, Leisenring WM, Stevens-Ayers TL, Jerome KR, Englund JA, Boeckh M. 2017. Prolonged shedding of human coronavirus in hematopoietic cell transplant recipients: risk factors and viral genome evolution. J Infect Dis 216:203–209. doi: 10.1093/infdis/jix264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ogimi C, Waghmare AA, Kuypers JM, Xie H, Yeung CC, Leisenring WM, Seo S, Choi SM, Jerome KR, Englund JA, Boeckh M. 2017. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 64:1532–1539. doi: 10.1093/cid/cix160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lion T. 2014. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Michaels MG, Green M, Wald ER, Starzl TE. 1992. Adenovirus infection in pediatric liver transplant recipients. J Infect Dis 165:170–174. doi: 10.1093/infdis/165.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Centers for Disease Control and Prevention. 2007. Acute respiratory disease associated with adenovirus serotype 14–four states, 2006–2007. MMWR Morb Mortal Wkly Rep 56:1181–1184. [PubMed] [Google Scholar]
- 193.Kandel R, Srinivasan A, D’Agata EM, Lu X, Erdman D, Jhung M. 2010. Outbreak of adenovirus type 4 infection in a long-term care facility for the elderly. Infect Control Hosp Epidemiol 31:755–757. doi: 10.1086/653612. [DOI] [PubMed] [Google Scholar]
- 194.Barnadas C, Schmidt DJ, Fischer TK, Fonager J. 2018. Molecular epidemiology of human adenovirus infections in Denmark, 2011–2016. J Clin Virol 104:16–22. doi: 10.1016/j.jcv.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu CY, Huang LM, Fan TY, Cheng AL, Chang LY. 2018. Incidence of respiratory viral infections and associated factors among children attending a public kindergarten in Taipei City. J Formos Med Assoc 117:132–140. doi: 10.1016/j.jfma.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 196.Shen CF, Wang SM, Ho TS, Liu CC. 2017. Clinical features of community acquired adenovirus pneumonia during the 2011 community outbreak in southern Taiwan: role of host immune response. BMC Infect Dis 17:196. doi: 10.1186/s12879-017-2272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swartling L, Allard A, Torlen J, Ljungman P, Mattsson J, Sparrelid E. 2015. Prolonged outbreak of adenovirus A31 in allogeneic stem cell transplant recipients. Transpl Infect Dis 17:785–794. doi: 10.1111/tid.12443. [DOI] [PubMed] [Google Scholar]
- 198.Engen RM, Huang ML, Park GE, Smith JM, Limaye AP. 2018. Prospective assessment of adenovirus infection in pediatric kidney transplant recipients. Transplantation 102:1165–1171. doi: 10.1097/TP.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Doan ML, Mallory GB, Kaplan SL, Dishop MK, Schecter MG, McKenzie ED, Heinle JS, Elidemir O. 2007. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant 26:883–889. doi: 10.1016/j.healun.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 200.Ohori NP, Michaels MG, Jaffe R, Williams P, Yousem SA. 1995. Adenovirus pneumonia in lung transplant recipients. Hum Pathol 26:1073–1079. doi: 10.1016/0046-8177(95)90268-6. [DOI] [PubMed] [Google Scholar]
- 201.Lachiewicz AM, Cianciolo R, Miller MB, Derebail VK. 2014. Adenovirus causing fever, upper respiratory infection, and allograft nephritis complicated by persistent asymptomatic viremia. Transpl Infect Dis 16:648–652. doi: 10.1111/tid.12248. [DOI] [PubMed] [Google Scholar]
- 202.Saquib R, Melton LB, Chandrakantan A, Rice KM, Spak CW, Saad RD, Fenves AZ, Barri YM. 2009. Disseminated adenovirus infection in renal transplant recipients: the role of cidofovir and intravenous immunoglobulin. Transpl Infect Dis 12:77–83. doi: 10.1111/j.1399-3062.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 203.Hiwarkar P, Kosulin K, Cesaro S, Mikulska M, Styczynski J, Wynn R, Lion T. 2018. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: State-of-the-art and real-life current approach: a position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev Med Virol 28:e1980. doi: 10.1002/rmv.1980. [DOI] [PubMed] [Google Scholar]
- 204.Symeonidis N, Jakubowski A, Pierre-Louis S, Jaffe D, Pamer E, Sepkowitz K, O’Reilly RJ, Papanicolaou GA. 2007. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transpl Infect Dis 9:108–113. doi: 10.1111/j.1399-3062.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 205.Fisher BT, Boge CLK, Petersen H, Seif AE, Bryan M, Hodinka RL, Cardenas AM, Purdy DR, Loudon B, Kajon AE. 2018. Outcomes of human adenovirus infection and disease in a retrospective cohort of pediatric hematopoietic cell transplant recipients. J Pediatr Infect Dis Soc 2018:piy049. doi: 10.1093/jpids/piy049. [DOI] [PubMed] [Google Scholar]
- 206.Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, Milano F, Delaney C, Jerome KR, Zerr DM, Nichols G, Boeckh M, Schiffer JT. 2018. Kinetics of double-stranded DNA viremia after allogeneic hematopoietic cell transplantation. Clin Infect Dis 66:368–375. doi: 10.1093/cid/cix804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Funk GF, Gosert R, Hirsch HH. 2007. Viral dynamics in transplant patients: implication for disease. Lancet Infect Dis 7:460–472. doi: 10.1016/S1473-3099(07)70159-7. [DOI] [PubMed] [Google Scholar]
- 208.Neofytos D, Ojha A, Mookerjee B, Wagner J, Filicko J, Ferber A, Dessain S, Grosso D, Brunner J, Flomenberg N, Flomenberg P. 2007. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant 13:74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 209.Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C, Ljungman P. 2012. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 14:555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 210.Lee YJ, Prockop SE, Papanicolaou GA. 2017. Approach to adenovirus infections in the setting of hematopoietic cell transplantation. Curr Opin Infect Dis 30:377–387. doi: 10.1097/QCO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 211.Lugthart G, Oomen MA, Jol-van der Zijde CM, Ball LM, Bresters D, Kollen WJ, Smiers FJ, Vermont CL, Bredius RG, Schilham MW, van Tol MJ, Lankester AC. 2015. The effect of cidofovir on adenovirus plasma DNA levels in stem cell transplantation recipients without T cell reconstitution. Biol Blood Marrow Transplant 21:293–299. doi: 10.1016/j.bbmt.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 212.Baugh KA, Tzannou I, Leen AM. 2018. Infusion of cytotoxic T lymphocytes for the treatment of viral infections in hematopoetic stem cell transplant patients. Curr Opin Infect Dis 31:292–300. doi: 10.1097/QCO.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, Kuvalekar M, Gee AP, Wu MF, Liu H, Grilley BJ, Krance RA, Gottschalk S, Brenner MK, Rooney CM, Heslop HE, Leen AM, Omer B. 2017. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 35:3547–3557. doi: 10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, Dey BR, Grilley BJ, Gee AP, Brenner MK, Rooney CM, Heslop HE. 2013. Multicenter study of banked third party virus-specific T-cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schenk T, Maier B, Hufnagel M, Strahm B, Kontny U, Neumann-Haefelin D, Falcone V. 2010. Persistence of human bocavirus DNA in immunocompromised children. Pediatr Infect Dis J 30:82–84. doi: 10.1097/INF.0b013e3181f12fcf. [DOI] [PubMed] [Google Scholar]
- 216.Jula A, Waris M, Kantola K, Peltola V, Soderlund-Venermo M, Hedman K, Ruuskanen O. 2013. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis 19:1328–1331. doi: 10.3201/eid1908.130074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gerna G, Piralla A, Campanini G, Marchi A, Stronati M, Rovida F. 2007. The human bocavirus role in acute respiratory tract infections of pediatric patients as defined by viral load quantification. New Microbiol 30:383–392. [PubMed] [Google Scholar]
- 218.Pinana JL, Madrid S, Perez A, Hernandez-Boluda JC, Gimenez E, Terol MJ, Calabuig M, Navarro D, Solano C. 2018. Epidemiologic and clinical characteristics of coronavirus and bocavirus respiratory infections after allogeneic stem cell transplantation: a prospective single-center study. Biol Blood Marrow Transplant 24:563–570. doi: 10.1016/j.bbmt.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rahiala J, Koskenvuo M, Norja P, Meriluoto M, Toppinen M, Lahtinen A, Vaisanen E, Waris M, Vuorinen T, Saarinen-Pihkala U, Lappalainen M, Allander T, Ruuskanen O, Hedman K, Soderlund-Venermo M, Vettenranta K. 2013. Human parvoviruses B19, PARV4 and bocavirus in pediatric patients with allogeneic hematopoietic SCT. Bone Marrow Transplant 48:1308–1312. doi: 10.1038/bmt.2013.63. [DOI] [PubMed] [Google Scholar]
- 220.Miyakis S, van Hal SJ, Barratt J, Stark D, Marriott D, Harkness J. 2009. Absence of human bocavirus in bronchoalveolar lavage fluid of lung transplant patients. J Clin Virol 44:179–180. doi: 10.1016/j.jcv.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 221.Chalkias S, Mackenzie MR, Gay C, Dooley C, Marty FM, Moss RB, Li T, Routh RL, Walsh SR, Tan CS. 2014. DAS181 treatment of hematopoietic stem cell transplant patients with parainfluenza virus lung disease requiring mechanical ventilation. Transpl Infect Dis 16:141–144. doi: 10.1111/tid.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guzman-Suarez BB, Buckley MW, Gilmore ET, Vocca E, Moss R, Marty FM, Sanders R, Baden LR, Wurtman D, Issa NC, Fang F, Koo S. 2012. Clinical potential of DAS181 for treatment of parainfluenza-3 infections in transplant recipients. Transpl Infect Dis 14:427–433. doi: 10.1111/j.1399-3062.2012.00718.x. [DOI] [PubMed] [Google Scholar]
- 223.Dhakal B, D’Souza A, Pasquini M, Saber W, Fenske TS, Moss RB, Drobyski WR, Hari P, Abidi MZ. 2016. DAS181 treatment of severe parainfluenza virus 3 pneumonia in allogeneic hematopoietic stem cell transplant recipients requiring mechanical ventilation. Case Rep Med 2016:8503275. doi: 10.1155/2016/8503275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Drozd DR, Limaye AP, Moss RB, Sanders RL, Hansen C, Edelman JD, Raghu G, Boeckh M, Rakita RM. 2013. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transpl Infect Dis 15:E28–E32. doi: 10.1111/tid.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Salvatore M, Satlin MJ, Jacobs SE, Jenkins SG, Schuetz AN, Moss RB, Van Besien K, Shore T, Soave R. 2016. DAS181 for the treatment of parainfluenza virus infections in 16 hematopoietic stem cell transplant recipients at a single center. Biol Blood Marrow Transplant 22:965–970. doi: 10.1016/j.bbmt.2016.02.011. [DOI] [PubMed] [Google Scholar]