Highlights
-
•
Patient pathways are complex interventions and have become central to systematic and effective trauma care in high income countries.
-
•
Effective pathway evaluation processes are key to developing robust pathways with a demonstrable patient benefit.
-
•
There are unclear benefits to using patient pathways in the management of blunt thoracic injury where empirical evaluation has been conducted.
-
•
The importance of effective implementation methods is identified, there is a paucity of data indicating effective implementation clinically.
-
•
More robust experimental research is required to understand the impact of patient pathways on patient outcomes and experience.
Keywords: Rib fractures, Chest trauma, Patient pathway, Hospitalisation, Analgesia, Implementation science, Pathway development, Pathway evaluation, Injury, Trauma
Abstract
Background
Blunt thoracic injury is present in around 15% of all major trauma presentations. To ensure a standardised approach to the management of physical injury, patient pathway-based interventions have been established in many healthcare settings. It currently remains unclear how these complex interventions are implemented and evaluated in the literature. This systematic review aims to identify pathway effectiveness literature and implementation studies in relation to patient pathway-based interventions in blunt thoracic injury care.
Methods
The databases Medline, Embase, Web of Science, CINAHL, WHO Clinical Trials Register and both the GreyLit & OpenGrey databases were searched without restrictions on date or study type. A search strategy was developed including keywords and MeSH terms relating to blunt thoracic injury, patient pathway-based interventions, evaluation and implementation. Due to heterogeneity of intervention pathways, meta-analysis was not possible; analysis was undertaken using an iterative narrative approach.
Results
A total of 16 studies met the inclusion criteria and were included in analysis. Pathways were identified covering analgesic management, respiratory care, surgical decision making and reducing risk of complications. Studies evaluating pathways are generally limited by their observational and retrospective design, but results highlight the potential benefits of pathway driven care provision in blunt thoracic injury.
Conclusions
The results demonstrate the complexity of evaluating patient pathway-based interventions in blunt thoracic injury management. It is important that pathways undergo rigorous evaluation, refinement and validation to ensure quality and patient safety. Strong recommendations are precluded as the quality of the pathway evaluation studies are low.
Background
The incidence of blunt thoracic injury is estimated to be around 15% with variable mortality rates reported between 9–60% [1], [2], [3]. There is complexity and variability in injury severity for these patients which is associated with a need for effective and reliable treatment for both the injury and subsequent complications [4,5]. For this review, Blunt Thoracic Injury is defined as injury to the bony structure of the thorax or underlying soft tissues and organ systems characterised by injury that does not involve opening of the chest wall and is associated with a blunt mechanism of injury [6].
Whilst the implementation of clinical pathways in major trauma care continues to grow, they are frequently not fully evaluated [2,5]. It is therefore important to consider how these pathways are evaluated to measure their clinical impact. There is evidence that patient pathway based interventions may have a positive effect on health outcomes in non-trauma populations [7], [8], [9] but these benefits have not been consistently identified across studies.
For this review, patient pathway based interventions have been defined as: “…a complex intervention for the mutual decision-making and organisation of care processes for a well-defined group of patients over a well-defined timeframe.” [10]. Further to this there is agreement that these interventions must have a clear focus on multidisciplinary teamworking and the delivery of care is focused on the local context to enable effective implementation and delivery [10].
From both clinical and academic perspectives, it is essential to understand the potential benefits and challenges associated with implementing a clinically effective pathway for patients with blunt thoracic injuries [3]. This systematic review will identify pathway effectiveness literature and implementation studies relating to patient pathway-based interventions in blunt thoracic injury care.
Methods
This study aims to answer the review question: How does the implementation of a patient pathway-based intervention influence the outcomes of major trauma patients with blunt thoracic injuries?
The review objectives are:
-
1.
Investigate the impact of patient pathway-based interventions on patient outcomes.
-
2.
Examine factors associated with successful implementation of patient pathway-based interventions for patients with blunt thoracic injuries.
Search strategies
This systematic review was conducted in accordance with the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) guidelines for reporting systematic reviews [11]. Search strategies were developed by combining free-text and index term searching. Two reviewers (EB, AW) independently performed a structured literature search (revised search: 05/01/2019). The databases Medline, Embase, Web of Science, CINAHL, WHO Clinical Trials Register and both the GreyLit and OpenGrey databases were searched without date restriction. To extend this search further, a hand search of reference lists of eligible publications was evaluated for inclusion. Search outputs were combined, and duplicates removed by the reference management software EndNote [12].
The following search strategy was used for all electronic databases with * denoting specific MeSH terms used in the review search strategy:
(Thoracic Injuries[Title/Abstract]*) OR (Rib Fractures [Title/Abstract]*) OR (Trauma [Title/Abstract]) OR (Flail Chest[Title/Abstract]*) OR (Costal Fracture[[Title/Abstract]) AND (Pathway[Title/Abstract]) OR (Guideline[Title/Abstract]*) OR (Care Bundle[Title/Abstract]*) OR (Integrated Care[Title/Abstract]) AND (Complications[Title/Abstract]) OR (Length of Stay[Title/Abstract]*) OR (Analgesia[Title/Abstract]*) OR (Mortality[Title/Abstract]*) OR (Intensive/Critical Care[Title/Abstract]*) OR (Ventilatory/Respiratory Support[Title/Abstract]) OR (Implementation [Title/Abstract]). Search terms were coded, and truncation/wildcards integrated using specified methodology for each database. Search strategies and article counts for both reviewers are included for Medline, Embase and CINHAL in the supplementary file.
Inclusion and exclusion criteria
This review included: (i) all empirical study designs; (ii) written in English; (iii) no limit on date of publication; (iv) Studies that included participants with a blunt thoracic injury component to their major trauma injury profile who received treatment at an acute hospital designated for the treatment of major trauma. Initial ‘a priori’ outcomes were set including hospital and critical care length of stay, mortality, complication rates and ventilatory support requirements. Studies not reporting these outcomes were not excluded automatically, rather where other outcomes were identified in the review process, these were discussed and where appropriate added for data analysis.
Data screening and extraction
The first reviewer (EB) screened all titles and abstracts and excluded duplicates. After this selection process, two reviewers (EB and AW) independently screened the title/abstract/full text of the remaining manuscripts. Possible differences in opinion were discussed and consensus was reached through a third reviewer (GL). Data extraction was undertaken using a pre-determined tool individually by two reviewers (EB and AW). The data extraction tool was piloted with a selection of studies prior to the main data extraction process to check for inter-reviewer reliability.
Quality assessment
Included studies were critically appraised independently and in duplicate by EB and AW for quality using the Cochrane Risk of Bias tool (with additional questions for observational studies). No studies were excluded due to issues identified in the quality appraisal process, but quality was considered in the data analysis and reporting process using Grading of Recommendations, Assessment, Development and Evaluations (GRADE) [13,14].
Data analysis
To identify whether there are variations in findings based on context, all studies were categorised according to the type and focus of the patient pathway-based intervention. Due to the heterogeneity identified between different interventions, a meta-analysis was not possible in this study. Synthesis was undertaken using a narrative approach.
Review registration
A protocol for this review has been published through the PROSPERO database maintained by York University (reference number: CRD42018100893) (available: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=100893].
Results
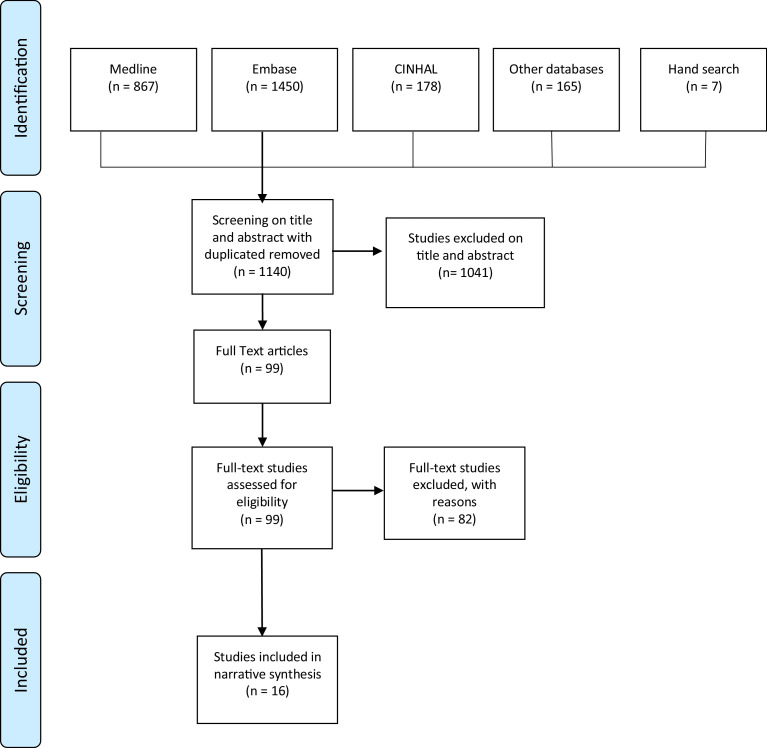
A total of 16 papers were included in the final systematic review, including a total of 9,212 participants and involving four countries (USA (n = 9); Australia (n = 5); Italy (n = 1); and France (n = 1). Included studies were published between 2001 and 2017. Eighty-two studies were excluded after full text review. The primary reason for exclusion was due to content being unrelated to the blunt thoracic injury population (n = 44). Other studies were excluded if they did not focus on a guideline or pathway-based process (n = 27) or focused on pathway development through an evidence-based review process but did not present empirical outcomes (n = 11), and one was a study protocol without results.Fig. 1 presents the PRISMA flow diagram representing the search and screening process for included articles.
Fig. 1.
PRISMA flow diagram demonstrating study selection.
Critical Appraisal summaries are included for all empirical studies and further critique was integrated into the presentation of the study results. Table 1presents the quality assessment of studies included in this review using the Cochrane Risk of bias tool with additional questions for the inclusion of observational studies [15].
Table 1.
Quality assessment summary table.
| Author | Random sequence generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of participants and personnel (Performance Bias) | Blinding or outcome assessment (Detection bias) | Incomplete Outcome data (Attrition bias) | Selective reporting (Reporting bias) | Other Bias | Reporting Bias (Observational Study) | External Validity (Observational Study) | Internal Validity (Observational Study) | Internal Validity-Confounding/ Selection Bias (Observational Study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flarity et al. (2017)[16] | – | – | – | – | – | – | ? | + | – | ? | ? |
| Curtis et al. (2017) [31] | – | – | – | – | – | – | – | ? | – | – | – |
| Carrie et al. (2017) [18] | – | – | – | – | – | ? | ? | + | + | + | ? |
| Curtis et al. (2016) [17] | – | – | – | – | – | ? | ? | + | + | + | ? |
| Dennis et al. (2017) [24] | – | – | – | – | ? | ? | ? | + | ? | – | – |
| Nyland et al. (2016) [22] | – | – | – | – | ? | ? | ? | + | + | + | ? |
| Anderson et al. (2015) [25] | – | – | – | – | – | – | – | ? | – | – | – |
| Gonzalez et al. (2015)[29] | – | – | – | – | – | – | – | + | + | + | + |
| Sahr et al. (2013) [23] | – | – | – | – | – | – | ? | + | + | ? | – |
| Menditto et al.(2012) [26] | – | – | – | – | – | ? | – | – | ? | ? | – |
| Frederickson et al. (2011)[30] | – | – | – | – | – | ? | ? | + | – | ? | ? |
| Morrison et al. (2009)[27] | – | – | – | – | – | – | ? | – | + | + | – |
| Todd et al. (2006) [19] | – | – | – | – | + | ? | ? | + | + | ? | ? |
| Adrales et al. (2002)[28] | – | – | – | – | – | – | ? | ? | – | – | – |
| Wilson et al. (2001) [20] | – | – | – | – | – | – | – | – | ? | ? | – |
| Sesperez et al. (2001) [21] | – | – | – | – | – | – | – | – | ? | ? | – |
| Key: |
Quality criterion met + |
Partially met ? |
Not met - |
||||||||
Table 2 presents the participant characteristics and injury demographic data from these included studies. Table 3 presents an overview of the 16 studies included in this review. There was distinct variability in both the overall focus and individual components of the patient pathway-based interventions included in this study. The main focus areas of blunt thoracic injury care were identified as: analgesic management [16], [17], [18], [19], [20], [21]; respiratory care [16], [17], [18], [19], [20], [21], [22], [23]; surgical decision making (including chest drain management) [18,[24], [25], [26], [27], [28]; and reducing the risk of in-patient complications [[16], [17], [18], [19], [20], [21], 29,30]. Outcome measures analysed in these studies included: Hospital Length of Stay; Intensive Care Unit Length of Stay; Rates of Pneumonia; Ventilatory support/respiratory function; Mortality; Thoracic Surgical Interventions; Analgesia; and Financial savings.
Table 2.
(continued)
| Author / Sample size | Age | Sex% male | Injury Characteristics` | No. of thoracic fractures | Injury Severity Score |
|---|---|---|---|---|---|
| Menditto et al. (2009)[26] | Pre-protocol vs. Protocol group (Mean in years) 51.2 (±22) vs. 57.2 (±20.4); p = 0.40 |
Pre-protocol vs. Protocol group 67%vs. 64%; p = 0.68 |
Pre-protocol vs. Protocol group Sternal fractures: 25%vs. 18%; p = 0.14 Pulmonary Contusions: 23%vs. 16%; p = 0.03 Pneumothorax: 10%vs. 5%; p = 0.17 Pleural effusion: 9%vs. 15%; p = 0.14 |
Pre-protocol vs. Protocol group (Mean no. of #) 2.1 (±1.9) vs. 2.7 (±1.9); p = 0.10 |
Not Reported. |
| Morrison et al. (2009)[27] | Protocol group vs. Control group (Mean in years) 30.7 (±2) vs. 35.2 (±2.9); p = 0.205 |
Protocol group vs. Control group 89.7%vs. 84%; p = 0.692 |
Protocol group vs. Control group Blunt Thoracic Injury: 10.3%vs. 20%; p = 0.449 Motor Vehicle Collision: 10.3% (n = 3) vs. 20% (n = 5) Other blunt Mechanism: 3.4% (n = 1) vs. 0% (n = 0) |
Not Reported | Protocol group vs. Control group 11.6 (±0.8) vs. 15.6 (±2.5); p = 0.140 |
| Todd et al. (2006)[19] | Protocol group vs. control group (Median in years) 56 (IQR 51–65) vs. 60.5 (52-72); p = 0.02 |
Protocol group vs. control group 63% (n = 94) vs 65% (n = 97); p = 0.72 |
Protocol group vs. control group Sternal Fracture: 10% (n = 15) vs. 5% (n = 8); p = 0.13 Pulmonary Contusion: 33% (n = 50) vs. 37% (n = 55); p = 0.55 Pneumothorax: 53% (n = 79) vs. 39% (n = 58); p = 0.01 Haemothorax: 43% (n = 65) vs. 15% (n = 22); p<0.0001 |
Protocol group vs. control group (Median no. of #) 6 (IQR 5–7) vs. 7 (IQR 6–9); p<0.0001 |
Protocol group vs. control group ISS: 21 (IQR 17–29) vs. 21 ([17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]); p = 0.67 AIS Chest: 4 (IQR 3–4) vs. 4 ([3], [4]), p = 0.17 |
| Adrales et al.(2002)[28] | Pre-protocol vs. Protocol group (Mean in years) 38.0 (±3.7) vs. 31.6 (±3.9); p = 0.08 |
Pre-protocol vs. Protocol group 71% (n = 10/14) vs. 62% (n = 29/47) |
Pre-protocol vs. Protocol group Blunt Thoracic Injury: 71% (n = 10/14) vs. 57% (n = 27/47) Motor Vehicle Collision: 50% (n = 7/14) vs. 47% (n = 22/47) Fall: 14% (n = 2/14) vs. 9% (n = 4/47) Assault: 7% (n = 1/14) vs. 2% (n = 1/47) |
Not Reported | Pre-protocol vs. Protocol group 20.5 (±2.4) vs. 25.7 (±3.3); p = 0.33 |
| Sesperez et al. (2001)[21] | (Mean in years) Pre-implementation: 41.6 (±20.5) Evaluation: 43.4 (±20.9) Re-evaluation: 39.9 (±20.7) p = 0.59 |
Pre-implementation: 69.7% Evaluation: 62.4% Re-evaluation: 70.5% p = 0.48 |
Allocation to Rib Fracture Pathway: Total: n = 87/265 Pre-implementation: n = 28 Evaluation: n = 38 Re-evaluation: n = 21 (p = 0.07) |
Not Reported | Pre-implementation: 12.7 (±11.5) Evaluation: 11.1 (±11.3) Re-evaluation: 11.1 (±9.9) p = 0.57 |
| Wilson et al. (2001)[20] | (Mean in years) 41.9 (±20.7) |
65.8% (n = 96) |
Allocation to Rib Fracture Pathway: Total: n = 59/146 |
Not Reported | (Mean) 11.1 (±10.7) ISS BTI: 8.0 (±7.7) |
Table 3.
(continued)
| Reference: Single/multi: Country: | Design/Setting: | Description of Intervention: | Sample Size: | Outcome Measures: | Key Results: |
|---|---|---|---|---|---|
| Anderson et al. (2015)[25] Single Australia |
Retrospective Observational Study, (Before-After) Emergency Department |
Procedural checklist for trauma ICD insertion |
n = 3069 (Pre-Protocol: 2009) (Protocol: 1060) |
Rates of empyema post chest drain insertion | Incidence of empyema pre-protocol was 1.44% (n = 29/2009) Incidence of empyema post protocol was 0.57 (n = 6/1060) p = 0.038 |
| Gonzalez et al. (2015)[29] Single USA |
Retrospective Observational Study Trauma Ward |
Predictive model and BTI Pathway Development for Identification for high risk groups requiring ICU in BTI care | n = 400 | ICU LoS Healthcare Costs analysis |
Mean ICU length of study was 1.7 days (associated with increased cost of $2200 per patient. Variables predicting need for ICU included: COPD, low albumin, ICD, ISS and no. of Rib Fractures. These variables were used to create a risk score with a sensitivity (78.5%) and specificity (78.9%). |
| Sahr et al. (2013)[23] Single USA |
Retrospective Observational Study (Before-After) Trauma ICU |
BTI admissions algorithm (Clinical Pathway) |
n = 148 (Pre-intervention: 81) (Post Intervention: 67) |
Hospital LoS ICU LoS Mortality |
There was a reduction in both hospital LoS and ICU LoS in the post-intervention period. |
| Frederickson et al. (2013)[30] Single USA |
Retrospective Observational study (Before-After) Trauma ICU |
4 Clinical Pathways developed and tested for elderly trauma pts:
|
n = 2058 (Pre-intervention total: 902) (Post intervention total: 1156) (Pre-intervention BTI: 84) (Post intervention BTI: 114) |
Hospital LoS ICU LoS Reduced Ventilation needs Mortality, Healthcare Costs Analysis |
Overall, the implementation of 4 protocols resulted in a 32% reduction in Hospital LoS, 88.89% compliance in the post-intervention BTI protocol, ISS and admission systolic blood pressure were identified as significant predictors of LoS in the regression analysis (β-coefficient: 0.219, p = 0.000 and −0.020, p = 0.001, respectively). |
| Menditto et al. (2012)[26] Single Italy |
Retrospective Observational Study (Before-After) Emergency Department Observation Unit |
Clinical Pathway and decision-making protocol |
n = 240 (Pre-Intervention: 110) (Post- Intervention: 130) |
Mortality, Rate of ICD for delayed PTX or haemothorax, Readmission within 30 days, Rate of hospitalisation, Hospital LoS |
Reduced re-admission rates (12%vs.4%, p = 0.03) Rates of late ICD increased (1.9%vs. 12.5%, p<0.05) Rate of hospitalisation decreased (49%vs. 24%, p<0.005) Hospital LoS decreased (94.7hr (±79.6) vs. 65.7 (±60.6), p<0.02) Cost analysis identified not significant change in cost effectiveness |
| Morrison et al. (2009)[27] Single USA |
Retrospective Observational Study (Before-After) Trauma Ward |
Clinical decision pathway for management of retained Haemothorax |
n = 54 (Study group: 29) (Control: 25) |
Time to theatre, Hospital LoS, No. of VATS performed, Post-operative complications, Total Hospital Costs |
Study Group had reduced time to theatre (3.0 (±0.33) days vs. 9.9 (±2.0) days, p = 0.002), Shorter Hospital LoS: (10.8 (±0.8) days vs. 30.5 (±5.5) days, p = 0.003). 100% of the study group had VATs vs. 56% in the control group (p = 0.0003), No difference in the rates of post-operative complications and need for repeat surgery. Total hospital charges lower in study group: ($46471 vs. $126221, p = 0.03) |
| Todd et al. (2006)[19] Single USA |
Prospective Cohort Study (Before-After) Trauma Ward |
Clinical Pathway and decision-making protocol |
n = 300 (150 prospectively recruited) (150 historical control group) |
ICU LoS, Hospital LoS, Mortality. |
Decreased ICU LoS by 2.4 days (95%CI: −4.3 - −0.52, p = 0.01), Decreased Hospital LoS by 3.7 days (95%CI: −7.1 - −0.42, p = 0.02), Reduced odds of pneumonia (OR: 0.12 [95%CI: 0.04–0.34] p<0.001) Reduced odds of mortality (OR: 0.37 [95%CI: 0.13–1.03] p = 0.06) |
| Adrales et al. (2002)[28] Single USA |
Prospective Before and After Study (Quasi-experimental) Trauma Ward |
Algorithmic Practice Guideline for ICD management in BTI. |
n = 61 (Pre-intervention: 14) (Post-intervention: 47) |
Prophylactic antibiotic use, Duration of ICD insertion Pre-removal radiography Incidence of empyema Recurrent pneumothorax and retained haemothorax |
Duration of ICD insertion was 3 days less than preintervention in the post intervention period Complication rates were not different between pre-and post-intervention phases. In the post implementation phase, there was a positive reduction in radiology fees ($3000) due to reduced need for pre-ICD removal X-ray |
| (continued on next page) | |||||
Table 3.
(continued)
| Reference: Single/multi: Country: | Design/Setting: | Description of Intervention: | Sample Size: | Outcome Measures: | Key Results: |
|---|---|---|---|---|---|
| Wilson et al. (2001)[20] Single Australia |
Prospective Observational Study Trauma Ward |
5 Clinical Pathways developed:
|
n = 146 (n = 59 with primary BTI) |
Integrated Care pathway variance analysis (i.e. expected progression through care pathway) | 32027 potential variances recorded in BTI group 2100 non-applicable variances recorded in BTI group Applicability Index = 93.4% Patient Assessment, Pain management, Skin integrity and patient education were the most appropriate key elements of care. Discharge Planning, Patient Satisfaction, Treatment and Activity were the least applicable areas of care. |
| Sesperez et al. (2001) [21] Single Australia |
Prospective Observational Study (Before and After) Stage 1: Pre-intervention Phase Stage 2: Initial Post Intervention Phase Stage 3: Post Intervention Phase Trauma Ward |
5 Clinical Pathways developed:
|
n = 265 (Stage 1: 89) (Stage 2: 85) (Stage 3: 61) (Withdrew: 30) |
Integrated Care pathway variance analysis |
Stage 1: 19555 potential variances recorded in BTI group 1312 observed variance recorded in BTI group 93.3% (95%CI: 92.9–93.6) outcomes achieved Stage 2: 20468 potential variances recorded in BTI group 1477 observed variance recorded in BTI group 92.8% (95%CI: 92.4–93.1) outcomes achieved Stage 3: 9279 potential variances recorded in BTI group 347 observed variances recorded in BTI group 96.3% (95%CI: 95.9–96.6) outcomes achieved |
Abbreviations: BTI = Blunt Thoracic Injury; ChIP = Chest Injury Protocol; HFNP = High Flow Nasal Prongs; ICD = Intercostal Drain; LoS = Length of Stay; PCA = Patient Controlled Analgesics.
Table 2.
Characteristics and demographic data from pathway evaluation studies.
| Author / Sample size | Age | Sex% male | Injury Characteristics` | No. of thoracic fractures | Injury Severity Score |
|---|---|---|---|---|---|
| Carrie et al. (2017) [18] | Protocol group vs. Control group (Mean in years) 58 (±16) vs. 58 (±15); p = 0.85 |
Protocol group vs. Control group 78% (n = 54) vs. 78% (54); p = 1.0 |
Protocol group vs. Control group Bilateral Rib Fractures: 32% (n = 22) vs. 23% (n = 16); p = 0.25 Flail Segment: 22% (n = 15) vs. 17% (n = 12); p = 0.52 Pulmonary Contusion: 59% (n = 41) vs. 52% (n = 36); p = 0.39 Pneumothorax: 48% (n = 33) vs. 62% (n = 43); p = 0.09 Haemothorax: 39% (n = 27) vs. 57% (n = 39); p = 0.05 |
Protocol group vs. Control group (mean no. of #) 6 (±3) vs. 6 (±2); p = 0.56 |
Protocol group vs. Control group 17 (±7) vs. 17 (±7); p = 0.97 Thoracic Trauma Severity Score: 8 (±3) vs. 8 (±3); p = 0.97 |
| Curtis et al. (2017) [31] | No Pathway Group (median): 81.0 (IQR 66–88) Pathway Group (median): 79.5 (IQR 69–87) |
No Pathway Group: 41.8% (n = 56) Pathway Group: 46.2 (n = 134) |
Not reported |
No Pathway Group (median): (IQR 0–3) Pathway Group (Median): 0 (IQR 0–2) |
No Pathway Group (median): 4.0 (IQR 2–9) Pathway Group (median): 5.0 (IQR 2–9) |
| Dennis et al.(2017) [24] | Pre-protocol vs. Protocol group (Median in years) 46.32 (IQR 30.7–61.94) vs. 48.33 (IQR 34.51–64.16); p = 0.722 |
Pre-protocol vs. Protocol group 77.9% (n = 254) vs. 77.8% (n = 246); p = 0.984 |
Pre-protocol vs. Protocol group Blunt Thoracic Injury: 80.1% (n = 261) vs. 77.5% (n = 245); p = 0.484 |
Not Reported. | Pre-protocol vs. Protocol group 26 (IQR 18–34) vs. 25 (IQR 17–33); p = 0.364 |
| Curtis et al. (2016) [17] | Pre-protocol vs. Protocol group (Median in years) 82 (IQR 71–88) vs. 81 (IQR 70–87); p = 0.73 |
Pre-protocol vs. Protocol group 43.6% (n = 119) vs. 47.3% (n = 129); p = 0.39. |
Pre-protocol vs. Protocol group Haemothorax: 9.9% (n = 27) vs. 10.6% (n = 29); p = 0.78 Pneumothorax: 5.9% (n = 16) vs. 3.7% (n = 10); p = 0.23 Pulmonary Contusion: 1.5% (n = 4) vs. 0.7% (n = 2); p = 0.69 Mechanism: <1 m Fall: 82.1% (n = 224) vs. 85.7 (n = 234) Motor Vehicle Collision: 6.2% (n = 17) vs. 2.9% (n = 8) |
Pre-protocol vs. Protocol group (Median no. of #) 0 (IQR 0–2) vs. 0 (IQR 0–3); p = 0.42 |
Pre-protocol vs. Protocol group ISS: 3 (IQR 2–9) vs. 5 (IQR 2–9); p<0.001 AIS Chest: 1 (IQR 1–2) vs. 1 (IQR 1–3); p = 0.39 |
| Nyland et al. (2016) [22] | Pre-Protocol vs. Phase I vs. Phase II (Median in years) 60 (IQR 46–75) vs. 63 (54–73) vs. 59 (36-78); p = 0.47 |
Pre-Protocol vs. Phase I vs. Phase II 70% (n = 35), 62% (n = 31), 70% (n = 35); p = 0.62 |
Pre-Protocol vs. Phase I vs. Phase II Rib Fractures: 58% (n = 29) vs. 74% (n = 37) vs. 58% (n = 29); p = 0.16 Pulmonary Contusion: 44% (n = 22) vs. 24% (n = 12) vs. 18% (n = 9); p = 0.01 Pneumothorax: 8% (n = 4) vs. 10% (n = 5) vs. 20% (n = 10); p = 0.15 Flail Chest: 4% (n = 2) vs. 4% (n = 2) vs. 0%; p = 0.36 |
Not reported |
Pre-Protocol vs. Phase I vs. Phase II Median score 19 (IQR 10–25) vs. 13 ([9], [10], [11], [12], [13], [14], [15], [16], [17]) vs. 10 ([9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]); p = 0.002 |
| Anderson et al. (2015) [25] |
Not reported | Not reported | Not reported | Not reported | Not reported |
| Gonzalez et al. (2015) [29] | 57% (n = 229) aged 65 years or older | Not reported | Fall: 39.0% (n = 156); RTC: 35.25% (n = 141); Motorcycle collision: 8.25% (n = 33); Pedestrian vs. vehicle: 2.25% (n = 9); Other: 15.25% (n = 61); |
Not reported | Not reported |
| Frederickson et al. (2013)[30] | Not Reported |
Pre-protocol vs. Protocol group 36.25 (n = 327) vs. 36.25 (n = 419); NS |
Pre-protocol vs. Protocol group Treated on Rib # Protocol: 84 vs. 114 |
Pre-protocol vs. Protocol group <3 #: 28.57 (n = 24) vs. 36.84 (n = 42) 3–8 #: 28.57 (n = 24) vs. 505 (n = 57) ≥9 #: 42.86 (n = 36) vs. 13.16 (n = 15) |
Pre-protocol vs. Protocol group (Mean Score) 9.93 (±7.65) vs. 10.25 (±7.24); NS |
| Sahr et al. (2013)[23] | Pre-protocol vs. Protocol group (Mean in years) <3 Rib Fractures: 79.70 (±7.88) vs. 79.64 (±9.37) ≥3 Rib Fractures: 79.06 (±7.88) vs. 79.54 (±8.11) |
Pre-protocol vs. Protocol group 56.79% (n = 46) vs. 57.69% (n = 30) Total: 51.35% (n = 76) |
Pre-protocol vs. Protocol group Mechanism: Motor Vehicle Collision: 27.04% (n = 30) vs. 17.91% (n = 12) Mechanism: Falls: 54.32% (n = 44) vs. 58.21% (n = 39) Mechanism: Other: 8.65% (n = 7) vs. 8.96% (n = 6) |
Pre-protocol vs. Protocol group <3 Rib Fractures: 37.04% (n = 30) vs. 41.79% (n = 28) ≥3 Rib Fractures: 62.96% (n = 51) vs. 58.21% (n = 39) |
Pre-protocol vs. Protocol group <3 Rib Fractures: 11.50 (±7.20) vs. 10.68 (±9.25) ≥3 Rib Fractures: 18.63 (±12.44) vs. 17.13 (±9.67) |
| (continued on next page) | |||||
Table 3.
Included studies summary table: pathway evaluation studies.
| Reference: Single/multi: Country: | Design/Setting: | Description of Intervention: | Sample Size: | Outcome Measures: | Key Results: |
|---|---|---|---|---|---|
| Flarity et al. (2017)[16] Single USA |
Retrospective Observational Study (Before-After) Trauma Ward |
Clinical Practice Guideline for BTI care including: Pulmonary function, Analgesia, Identification of deterioration |
n = 571 | Hospital LoS, ICU LoS, Narcotic Usage, Pulmonary function Mortality |
Multivariable regression identified a significant decrease in LOS for those patients admitted in the intervention period (B = −2.29; P = 0.019). Despite being significantly older with more rib fractures in the ICU cohort, patients admitted after implementation of the intervention had a significantly reduced LOS on multivariable analysis, reducing LOS by over two days. Overall,it demonstrated reduced narcotic usage, improved pulmonary function and reduced hospital LoS after the implementation of a BTI clinical practice guideline. |
| Dennis et al. (2017)[24] Single USA |
Retrospective Observational Study (Before-After) Trauma Ward |
Clinical Pathway for management of retained haemothorax post chest trauma |
n = 642 (Pre: n = 326) (Post: n = 316) |
No. of patients requiring surgical interventions beyond ICD. Hospital LoS, ICU LoS, Ventilatory requirement, VATS, Open Thoracotomy, Image Guided Catheter, Intrapleural thrombolysis, Empyema |
The number of patients needing more than 1 surgical intervention decreased (49 vs. 28; p = 0.02). Number of patients requiring VATS decreased (27 vs. 10; p<0.01) Number of radiology guided catheters placed increased (2 vs. 10; p = 0.02) Intrapleural tPA, open thoracotomy, empyema and 6-month readmission rates were unchanged. ICU and hospital LoS were unchanged. |
| Curtis et al. (2017)[31] Single Australia |
Retrospective Observational Study & staff survey Emergency Department/Trauma Ward |
Evaluation of Chest Injury Protocol (ChIP) (Curtis et al. 2016)[31] |
n = 424 patient participants n = 99 staff participants |
No. of patient using ChIP, Barriers and Facilitators to implementation of ChIP protocol. |
Only 68.4% of eligible patients received the ChIP protocol at baseline. Key facilitators and barriers to implementation were identified through staff surveys and mapped to the theoretical domains framework. Revisions incorporating behavioural change techniques resulted in an improvement in use of the ChIP intervention to 91%. |
| Carrie et al. (2017)[18] Single France |
Retrospective Case Control Study (Before-After) Emergency Department |
Care bundle & Clinical Pathway including: Pulmonary function, Analgesia, Identification of deterioration, MDT working, Chest drain management, Oxygenation and ventilation support |
n = 138 (69 pre & 69 post) |
Rate of uncontrolled pain during initial 24 h of admission, Rate of Pulmonary, Complications, ICU LoS, Hospital LoS, Pain at rest, Opioid Usage. |
Reduction in rate of uncontrolled analgesia (55 vs. 17%, p<0.001). Increased rate of ICU admission (23 vs. 52%, p<0.001). No difference in rates of pulmonary complications, ICU LoS or Hospital LoS. Use of NSAID may results in reduced rates of respiratory complications. |
| Curtis et al. (2016)[17] Single Australia |
Retrospective Observational Study (Before-After) Emergency Department/Trauma Ward |
Chest Injury Protocol (ChIP) |
n = 546 (Pre-Intervention: 273) (Post-Intervention: 273) |
Rate of Pneumonia, Mortality, DVT and/or PE, Ventilatory Support, Hospital LoS, Time to Pain Team review, Time to Physiotherapy, Time to Trauma review, Use of PCA, Use of HFNP. |
Pneumonia rates reduced by 4.8% (95%CI: 0.5–9.2, p = 0.03), Increased access to pain team review (32%vs. 13%, p<0.001), physiotherapy (93%vs. 86%, p = 0.005) and trauma team review (95%vs. 39%, p<0.001). There was no reduction in Hospital LoS (p = 0.50). |
| Nyland et al. (2016)[22] Single USA |
Retrospective Observational Study (Before-After) Trauma Ward |
Decision making algorithm for the Volume Expansion Protocol (VEP) & Protocol |
n = 150 (Pre-Protocol: 50) (Phase 1 (post implementation): 50) (Phase 2 (re-evaluation): 50) |
Hospital LoS, ICU LoS. Unplanned admissions to ICU Bronchodilator usage Mortality |
Unplanned admissions to ICU were eliminated post implementation Hospital LoS was decreased by 1.5 days (p = 0.001) Ward LoS and ICU LoS was reduced (p = 0.001&p = 0.01 respectively) More patients were admitted directly to ward level care Bronchodilator use decreased (not statically significant). |
| (continued on next page) | |||||
Pathway evaluation
Hospital length of stay & intensive care length of stay
Seven studies identified a reduction in hospital LoS for patients treated using a patient pathway based intervention [18,19,22,23,26,27,30]. Conversely, three studies identified no difference in hospital LoS for patients treated using patient pathway based interventions (4 days (IQR 2–8) vs. 4 days (IQR 2–8); p = 0.5 and 6.76 (IQR 3.89–12.18) vs. 6.00 (IQR 3.86–11.00); p = 0.368, respectively) [17,21,24]. For patients with less than 3 fractured ribs, hospital LoS was longer (4.77 days (±3.93) vs. 4.93 (±3.33); p = 0.042) but it was unlikely that this is a clinically significant difference [23]. In the same study, the hospital LoS was less (10.24 days (±13.59) vs. 8.74 days (±9.83); p = 0.006) for patients with 3 or more fractured ribs treated with a patient pathway based intervention [23].
Two studies identified a reduction in the ICU LoS after the protocol was introduced (2.0-day and 2.4-day reduction respectively) [19,22]. Nyland et al. identified the elimination of unplanned admissions to the ICU for patients treated using a respiratory care patient pathway based intervention (pre-protocol: 12% (n = 6) vs. post-protocol 0%; p = 0.038) [22]. Conversely, two studies reported an increase in ICU LoS after implementation of a patient pathway based intervention for the management of blunt thoracic injuries [18,23]. Sahr et al. identified an increased ICU LoS for both patients with less than three fractured ribs (Pre-vs. Post implementation: 0.54 days (±1.24) vs. 1.90 (±2.33)) and for those with three or more fractures (Pre-vs. Post implementation: 3.67 days (±7.30) vs. 4.72 (±6.97)). When analysis of variance was performed, this was significant (F1,105=4.595; p = 0.028) [23]. Furthermore, two studies did not identify any change in ICU LoS after the implementation of the patient pathway based interventions [24,30].
Pneumonia
There was a decrease in the rate of pneumonia after the implementation of a patient pathway based intervention two studies (9.2%vs. 4.4%; p = 0.003 and 18%vs. 5%; p = 0.0003 respectively) [17,19]. Conversely, Carrie et al.reported no significant difference in pneumonia infections (17% (n = 12) vs. 19% (n = 13); p = 0.87) but an increased ICU LoS for blunt thoracic injury patients treated with a patient pathway based intervention, and increased primary placement of pathway patients from Emergency Department (ED) to ICU (23% (n = 16) vs. 52% (n = 36); p = 0.0004) [18].
Ventilatory support requirements
Two studies identified a non-significant reduction in ventilatory support requirements (2.2% (n = 6) (IQR 0.3–4.0) vs. 1.1% (n = 3 (IQR −0.2 – 2.4); p = 0.50& 2.6 (±6.7) vs. 1.8 (±8.1); p = 0.28, respectively) [17,19]. Furthermore, Carrie et al. did not identify any change in ventilatory needs of participants after the implementation of a patient pathway based intervention (12% (n = 8) vs. 12% (n = 8); p = 1.0) [18]. Conversely, one study measured a one-day increase in ventilatory-free days amongst a sample of patients treated using a patient pathway based intervention (27 (IQR 19.75–28) vs. 28 (IQR 25–28); p = 0.028) [24].
Mortality rates
Although a reduction in mortality was reported in five studies [[17], [18], [19], 22,23], this was significant in one study [19] (protocol vs. intervention group: 13%vs. 4%, p = 0.004). Conversely, one study did not find any difference in mortality rates after implementation of a clinical pathway for blunt thoracic injury management [30].
Chest drain management
Six studies evaluated the use of thoracic surgical procedures including chest drain use and operative drainage of retained haemothorax using Video Assisted Thoracic Surgery (VATS) [17], [18], [19],[26], [27], [28]. Two studies identified a reduction in the requirement for chest drain placement for patients treated using a patient pathway based intervention (29% and 14% reduction, respectively) [18,19]. Interestingly, despite the decrease in chest drain use, Todd et al. reported higher incidence of diagnosed pneumothorax (39% (n = 58) vs. 53% (n = 79); p = 0.01), haemothorax (15% (n = 22) vs. 43% (n = 65); p<0.0001) and haemo-pneumothorax (14% (n = 21) vs. 29% (n = 43); p<0.002) in the study group on the clinical pathway [19]. This suggests a change in both the diagnostic process and the clinical decision making around chest drain management. One study reported a 2.8 day reduction in the duration of chest drain insertion for patients treated with a patient pathway based intervention (7.0 days (±1.3) vs. 4.2 days (±0.4); p = 0.04) [28]. Conversely, Menditto et al. reported a 10% increase in the usage of chest drains using a patient pathway based interventions for the management of blunt thoracic injuries in an Emergency Department observation ward [26] whilst Curtis et al. reported no change in chest drain usage after the implementation of the inpatient Chest Injury Protocol (ChIP) [17].
Two studies investigated the impact of patient pathway-based interventions on the treatment of retained haemothorax after chest trauma [24,27]. Dennis et al. identified no reduction in the number of patients requiring interventions beyond chest drainage (69.9% (n = 228) vs. 64.9% (n = 205); 0.179). There was a reduction in patients requiring more than one surgical intervention (15.0% (n = 49) vs. 8.9% (n = 28); p = 0.021) [24]. These studies also identified a decreased requirement for VATS (Protocol vs. Control group: 8.3% (n = 27) vs. 3.2% (n = 10); p = 0.0006) [24], reduced time to theatre for VATS (Protocol vs. Control group: 3 days (±0.3) vs. 9.9 days (±2); p = 0.002) [27], and increased use of minimally invasive surgical techniques (Protocol vs. Control group: 100%vs. 56%; p = 0.0003) [27]. Furthermore, whilst Morrison et al. identified a reduced conversion to open surgical procedures (3.4%vs. 16%; p = 0.017), Dennis et al. did not find any change in the incidence of open thoracotomy (2.5% (n = 8) vs. 2.2% (n = 7); p = 1.0) [24,27].
Analgesia and pain assessment/management
Analgesic usage associated with a patient pathway-based intervention was investigated in three studies [17], [18], [19]. Two studies identified increased use of Patient Controlled Analgesia (PCA) (10.3% (n = 28) vs. 16.1 (n = 44); p = 0.04& 92% (n = 138) vs 31% (n = 47); p<0.0001, respectively) post clinical pathway implementation [17,19]. Similarly, Todd et al. and Carrie et al. identified significantly increased use of epidural analgesia (42% (n = 63) vs. 11% (n = 17); p<0.0001 and 1% (n = 1) vs. 33% (n = 23); p<0.0001, respectively) [18,19] and paravertebral block (4% (n = 3) vs. 30% (n = 21); p<0.0001) [18]. Todd et al. reported the number of failed or refused epidural attempts which demonstrates an improved risk governance associated with the use of a patient pathway based intervention [19]. Curtis et al. highlighted improved access to specialist pain services for patient treated using the Chest Injury Protocol (ChIP) [17] and Carrie et al. reported a two-point reduction in pain score on day one of admission, reduced total opioid usage and reduced episodes of uncontrolled pain when assessed using a visual analogue pain Likert scale [18].
Financial savings and gains
Changes in the cost of patient care was an outcome measure in three studies [26], [27], [28]. Two studies identified important treatment cost reductions associated with the use of patient pathway-based interventions [26,28]. Furthermore, Adrales et al. identified specific resource use savings through the limitation of prophylactic antibiotic usage to 24-hours only (50% (n = 7/14) vs.74% (n = 35/47)) and reduction in X-ray use prior to chest drain removal (before vs. after: 93%vs. 55%; p = 0.02) [28]. Conversely, Menditto et al. did not identify any improvement in cost despite improvement in patient outcomes when treated using a patient pathways based intervention [26].
Implementation analysis
Variance analysis in integrated care pathways
Two studies analysed the provision of care through integrated care pathways which included a rib fracture management pathway [20,21]. In these studies, the pathway implementation was evaluated through measurement of the number of episodes of variance from the expected progress of patient recovery after injury. Sesperez et al. observed substantial number of variances in stage one (Pre-implementation) with decreased numbers of variance from the integrated pathway in stage two and three (both post implementation) (51.7 (±43.5), 42.3 (±32.9) & 23.2 (±21.7); p = 0.0001) [21]. In the analysis of variances from the integrated care pathways, 0.2% related to system errors, 25% related to patient factors, and 75.8% to staff related factors. In stage 3, staff related variances were substantially reduced [21].
Evaluating process and implementation science
One mixed-methods study evaluated the intervention implementation process and used the outcomes to refine the clinical pathway [31]. Curtis et al. identified that only 68.4% of eligible patients were receiving care through the patient pathway-based intervention. Furthermore, using a qualitative staff survey, 25 themes were identified which influenced the implementation and uptake of the intervention (15 facilitators and 10 barriers to effective implementation) [31]. These themes included knowledge of the pathway, positive feedback, beliefs about the benefits of the pathway and environmental context. After refinement and a relaunch programme, uptake of the intervention increased from 68.4% to 91.0% of eligible patients [31]. This demonstrates how implementation science underpinning the introduction of a patient pathway-based intervention can positively impact on uptake in clinical practice.
Discussion
Most pathway-based interventions reported in the literature have been developed through a process of evidence review and/or expert opinion. Although there appears to be no consensus in the literature on the most appropriate approach for pathway development, there is evidence that pathways, like other complex interventions, need to be developed and evaluated using a systematic theoretical framework. Although not explicitly reported in any publication included, the Medical Research Council framework for development and evaluation of complex interventions is one commonly used approach [32], [33], [34]. Although this systematic review did not focus on the pathway development process, it is important to recognise the importance of robust development methods to precede and underpin effective evaluation processes.
This systematic review identified several patient pathway-based interventions which were associated with a reduced Hospital LoS and reduced ICU LoS. Although this was not a consistent finding amongst all included studies, it is consistent with the finding of clinical pathway evaluations in other complex health care settings [7,9,[35], [36], [37]. There is evidence that extending a hospital LoS for any reason is associated with altered patient outcomes, particularly in older adults [38]. Similarly, the impact of extended ICU LoS has been well documented in the literature [39], [40], [41] and the variables that impact on ICU LoS is multi-faceted and complex [42], [43], [44]. Despite these data, it is important to remember that ICU LoS is confounded by delayed discharge, and this is often due to lack of acute care beds where patients should be discharged within four hours of a decision to discharge [45,46]. Similarly, in the US, this measure is confounded by long-term care facility bed provision [47]. Whilst these patients remain on ICU, they continue to receive critical care and it is not clear how this has impacted on these reported data. More robust methods are needed when evaluating any patient pathway-based intervention for the management of blunt thoracic injuries to allow for this potential artefact.
It is also important to note that the impact of patient pathway-based interventions are context dependent [48,49]. Although not measured in the included evaluation studies, there is potential for contrary outcomes when patient pathway-based interventions are introduced at different centres. Furthermore, pathways in trauma care have been criticised for not providing enough flexibility to account for the diversity in factors such as injury severity and individual patient characteristics. It is important that any evaluation process considers how well a pathway fits the local population and ensures there are sufficient mechanisms in place to allow flexibility and targeted patient care [10,50,51]. These factors highlight the need for site evaluation as part of any evaluation of a patient pathway-based intervention, particularly in relation to interventions with higher risk or skills requirements.
Curtis et al. identify the importance of effectively embedding pathways in clinical practice using recognised implementation science methods [31]. Several factors have been reported as facilitators to effectively embed a pathway in clinical practice. These include but are not limited to staff engagement at design, implementation and evaluation phases and ensuring the pathway is context specific [50]. Interestingly, no other authors report considering the implementation process when presenting or evaluating a blunt thoracic injury pathway in clinical practice. As these factors also form a substantial threat to successfully embedding a pathway in practice, further work is needed to understand how local barriers and facilitators can be built into pathway design, implementation and evaluation in the trauma sphere. Interestingly, as the pathway evaluation studies have used cross-sectional approaches rather than longitudinal methods, we have been unable to identify if the intervention was successfully embedded in long-term clinical practice.
It is clear from the literature that evaluation is a key construct in interventional development and although this has been explicitly stated by a small number of studies, it appears that further work is needed to promote the evaluation and refinement of pathway-based interventions in this area. For the studies that have evaluated an intervention in relation to patient outcomes, this is often limited by methodology and design with most of these studies using retrospective observational designs with small sample sizes or quasi-experimental design at risk of bias [52]. There is currently a paucity of experimental research. When assessing the effectiveness of a complex intervention with multiple components, there is a substantial risk of heterogeneity in the interaction between components of the interventions [53]. Although measuring this was beyond the scope of this review, it remains an important consideration in the future evaluations of patient pathway-based intervention in blunt thoracic injury.
Strengths and limitations
The lack of time limit for publication within the search strategy may be considered a potential limitation due to the changes in clinical practice and management strategies. However, the focus of this systematic review is on the impact of patient pathway-based interventions on clinical outcomes rather than the specific pathway content. It was clear in the development of the protocol for this systematic review that there would be high levels of heterogeneity in the studies included due to the broad inclusion of outcomes and the diversity in the patient pathway-based interventions that have been included. For this reason, meta-analysis was not possible, and a process of narrative synthesis was used for data analysis. The included studies were undertaken in several different high-income countries making it difficult to assess the generalisability and application of these results locally. Despite these potential limitations, to our knowledge, this study remains the first systematic review to be undertaken synthesising evidence on patient pathway-based interventions for blunt thoracic injuries.
Conclusion
Despite some reported improvements in patient outcomes after the implementation of a patient pathway-based intervention in blunt thoracic injury care, the quality of the available evidence is low precluding strong recommendations.
Funding
Edward Baker is funded by a National Insitute for Health Research (NIHR), Clincial Doctoral Research Fellowship for this research project. The publication presents independent research funded by the National Insitute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Author contribution
EB, AXE, CN, PH& GL conceived this study, designed the tool and protocol. GL, AXE, CN &PH are EB's academic supervisor at King's College London. Data collection was undertaken by EB & AW. Initial analysis and data integration were undertaken by EB. EB undertook the initial draft of the manuscript, and AW, AXE, CN, PH, GL contributed substantially to subsequent drafts and revisions. All authors approved the final version of the manuscript. EB takes responsibility for the paper.
Declaration of Competing Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.injury.2020.06.002.
Contributor Information
Edward Baker, Email: edward.e.baker@kcl.ac.uk.
Alison Woolley, Email: awooley@nhs.net.
Andreas Xyrichis, Email: andreas.xyrichis@kcl.ac.uk.
Christine Norton, Email: christine.norton@kcl.ac.uk.
Philip Hopkins, Email: p.hopkins@nhs.net.
Geraldine Lee, Email: gerry.lee@kcl.ac.uk.
Appendix. Supplementary materials
References
- 1.Dennis B.M., Bellister S.A., Guillamondegui O.D. Thoracic Trauma. Surg Clin North Am. 2017;97(5):1047–1064. doi: 10.1016/j.suc.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Unsworth A., Curtis K., Asha S.E. Treatments for blunt chest trauma and their impact on patient outcomes and health service delivery. Scand J Trauma Resusc Emerg Med. 2015;23:17. doi: 10.1186/s13049-015-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker E.J., Lee G.A. A Retrospective Observational Study Examining the Effect of Thoracic Epidural and Patient Controlled Analgesia on Short-term Outcomes in Blunt Thoracic Trauma Injuries. Medicine (Baltimore) 2016;95(2) doi: 10.1097/MD.0000000000002374. e2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battle C., Hutchings H., Lovett S., Bouamra O., Jones S., Sen A. Predicting outcomes after blunt chest wall trauma: development and external validation of a new prognostic model. Crit Care. 2014;18(3):R98. doi: 10.1186/cc13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kourouche S., Buckley T., Munroe B., Curtis K. Development of a blunt chest injury care bundle: an integrative review. Injury. 2018;49(6):1008–1023. doi: 10.1016/j.injury.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Baker E., Xyrichis A., Norton C., Hopkins P., Lee G. The long-term outcomes and health-related quality of life of patients following blunt thoracic injury: a narrative literature review. Scand J Trauma Resusc Emerg Med. 2018;26(1):67. doi: 10.1186/s13049-018-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts H.C., Pickering R.M., Onslow E., Clancy M., Powell J., Roberts A. The effectiveness of implementing a care pathway for femoral neck fracture in older people: a prospective controlled before and after study. Age Ageing. 2004;33(2):178–184. doi: 10.1093/ageing/afh063. [DOI] [PubMed] [Google Scholar]
- 8.Devapriam J., Alexander R., Gumber R., Pither J., Gangadharan S. Impact of care pathway-based approach on outcomes in a specialist intellectual disability inpatient unit. J Intellect Disabil. 2014;18(3):211–220. doi: 10.1177/1744629514532453. [DOI] [PubMed] [Google Scholar]
- 9.Kwan J., Hand P., Dennis M., Sandercock P. Effects of introducing an integrated care pathway in an acute stroke unit. Age Ageing. 2004;33(4):362–367. doi: 10.1093/ageing/afh104. [DOI] [PubMed] [Google Scholar]
- 10.Vanhaecht K., De Witte K., Panella M., Sermeus W. Do pathways lead to better organized care processes? J Eval Clin Pract. 2009;15(5):782–788. doi: 10.1111/j.1365-2753.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 b2535. [PMC free article] [PubMed] [Google Scholar]
- 12.Bramer W., Glustini D., de Jonge G., Holland L., Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):4. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Kerwin A.J., Haut E.R., Burns J.B., Como J.J., Haider A., Stassen N. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287. doi: 10.1097/TA.0b013e31827013e9. [DOI] [PubMed] [Google Scholar]
- 15.Galvagno S.M., Smith C.E., Varon A.J., Hasenboehler E.A., Sultan S., Shaefer G. Pain management for blunt thoracic trauma: a joint practice management guideline from the Eastern Association for the Surgery of Trauma and Trauma Anesthesiology Society. J Trauma Acute Care Surg. 2016;81(5):936–951. doi: 10.1097/TA.0000000000001209. [DOI] [PubMed] [Google Scholar]
- 16.Flarity K., Rhodes W.C., Berson A.J., Leininger B.E., Reckard P.E., Riley K.D. Guideline-Driven Care Improves Outcomes in Patients with Traumatic Rib Fractures. Am Surg. 2017;83(9):1012–1017. [PubMed] [Google Scholar]
- 17.Curtis K., Asha S.E., Unsworth A., Lam M., Goldsmith H., Langcake M. ChIP: an early activation protocol for isolated blunt chest injury improves outcomes, a retrospective cohort study. Australas Emerg Nurs J. 2016;19(3):127–132. doi: 10.1016/j.aenj.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Carrie C., Stecken L., Cayrol E., Cottenceau V., Petit L., Revel P. Bundle of care for blunt chest trauma patients improves analgesia but increases rates of intensive care unit admission: a retrospective case-control study. Anaesth Crit Care Pain Med. 2018;37(3):211–215. doi: 10.1016/j.accpm.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Todd S.R., McNally M.M., Holcomb J.B., Kozar R.A., Kao L.S., Gonzalez E.A. A multidisciplinary clinical pathway decreases rib fracture-associated infectious morbidity and mortality in high-risk trauma patients. Am J Surg. 2006;192(6):806–811. doi: 10.1016/j.amjsurg.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 20.Wilson S., Bin J., Sesperez J., Seger M., Sugrue M. Clinical pathways–can they be used in trauma care. An analysis of their ability to fit the patient. Injury. 2001;32(7):525–532. doi: 10.1016/s0020-1383(00)00199-6. [DOI] [PubMed] [Google Scholar]
- 21.Sesperez J., Wilson S., Jalaludin B., Seger M., Sugrue M. Trauma case management and clinical pathways: prospective evaluation of their effect on selected patient outcomes in five key trauma conditions. J Trauma. 2001;50(4):643–649. doi: 10.1097/00005373-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Nyland B.A., Spilman S.K., Halub M.E., Lamb K.D., Jackson J.A., Oetting T.W. A Preventative Respiratory Protocol to Identify Trauma Subjects at Risk for Respiratory Compromise on a General In-Patient Ward. Respir Care. 2016;61(12):1580–1587. doi: 10.4187/respcare.04729. [DOI] [PubMed] [Google Scholar]
- 23.Sahr S.M., Webb M.L., Renner C.H., Sokol R.K., Swegle J.R. Implementation of a rib fracture triage protocol in elderly trauma patients. J Trauma Nurs. 2013;20(4):172–175. doi: 10.1097/JTN.0000000000000008. quiz 6-7. [DOI] [PubMed] [Google Scholar]
- 24.Dennis B.M., Gondek S.P., Guyer R.A., Hamblin S.E., Gunter O.L., Guillamondegui O.D. Use of an evidence-based algorithm for patients with traumatic hemothorax reduces need for additional interventions. J Trauma Acute Care Surg. 2017;82(4):728–732. doi: 10.1097/TA.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 25.Anderson M., Fitzgerald M., Martin K., Santamaria M., Arendse S., O’Reilly G. A procedural check list for pleural decompression and intercostal catheter insertion for adult major trauma. Injury. 2015;46(1):42–44. doi: 10.1016/j.injury.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Menditto V.G., Gabrielli B., Marcosignori M., Screpante F., Pupita G., Polonara S. A management of blunt thoracic trauma in an emergency department observation unit: pre-post observational study. J Trauma Acute Care Surg. 2012;72(1):222–228. doi: 10.1097/TA.0b013e3182140cad. [DOI] [PubMed] [Google Scholar]
- 27.Morrison C.A., Lee T.C., Wall M.J., Carrick M.M. Use of a trauma service clinical pathway to improve patient outcomes for retained traumatic hemothorax. World J Surg. 2009;33(9):1851–1856. doi: 10.1007/s00268-009-0141-0. [DOI] [PubMed] [Google Scholar]
- 28.Adrales G., Huynh T., Broering B., Sing R.F., Miles W., Thomason M.H. A thoracostomy tube guideline improves management efficiency in trauma patients. J Trauma. 2002;52(2):210–214. doi: 10.1097/00005373-200202000-00002. discussion 4-6. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez K.W., Ghneim M.H., Kang F., Jupiter D.C., Davis M.L., Regner J.L. A pilot single-institution predictive model to guide rib fracture management in elderly patients. J Trauma Acute Care Surg. 2015;78(5):970–975. doi: 10.1097/TA.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 30.Frederickson T.A., Renner C.H., Swegle J.R., Sahr S.M. The cumulative effect of multiple critical care protocols on length of stay in a geriatric trauma population. J Intensive Care Med. 2013;28(1):58–66. doi: 10.1177/0885066611432420. [DOI] [PubMed] [Google Scholar]
- 31.Curtis K., Van C., Lam M., Asha S., Unsworth A., Clements A. Implementation evaluation and refinement of an intervention to improve blunt chest injury management-A mixed-methods study. J Clin Nurs. 2017 doi: 10.1111/jocn.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore G.F., Audrey S., Barker M., Bond L., Bonell C., Hardeman W. Process evaluation of complex interventions: medical Research Council guidance. BMJ. 2015;350 doi: 10.1136/bmj.h1258. h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013;50(5):587–592. doi: 10.1016/j.ijnurstu.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 34.O’Cathain A., Croot L., Duncan E., Rousseau N., Sworn K., Turner K.M. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DuBose J.J., Inaba K., Shiflett A., Trankiem C., Teixeira P.G., Salim A. Measurable outcomes of quality improvement in the trauma intensive care unit: the impact of a daily quality rounding checklist. J Trauma. 2008;64(1):22–27. doi: 10.1097/TA.0b013e318161b0c8. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhu L., Li J., Li X.K., Feng J.Q., Gao J.M. Impact of a clinical pathway on hospital costs, length of stay and early outcomes after hepatectomy for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2014;15(13):5389–5393. doi: 10.7314/apjcp.2014.15.13.5389. [DOI] [PubMed] [Google Scholar]
- 37.Fujino Y., Kubo T., Muramatsu K., Murata A., Hayashida K., Tomioka S. Impact of regional clinical pathways on the length of stay in hospital among stroke patients in Japan. Med Care. 2014;52(7):634–640. doi: 10.1097/MLR.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 38.van Vliet M., Huisman M., Deeg D.J.H. Decreasing Hospital Length of Stay: effects on Daily Functioning in Older Adults. J Am Geriatr Soc. 2017;65(6):1214–1221. doi: 10.1111/jgs.14767. [DOI] [PubMed] [Google Scholar]
- 39.Moitra V.K., Guerra C., Linde-Zwirble W.T., Wunsch H. Relationship Between ICU Length of Stay and Long-Term Mortality for Elderly ICU Survivors. Crit Care Med. 2016;44(4):655–662. doi: 10.1097/CCM.0000000000001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan A., Anderson C., Kypson A., Kindell L., Ferguson T.B., Chitwood W.R. Clinical outcomes in patients with prolonged intensive care unit length of stay after cardiac surgical procedures. Ann Thorac Surg. 2012;93(2):565–569. doi: 10.1016/j.athoracsur.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 41.Evans J., Kobewka D., Thavorn K., D'Egidio G., Rosenberg E., Kyeremanteng K. The impact of reducing intensive care unit length of stay on hospital costs: evidence from a tertiary care hospital in Canada. Can J Anaesth. 2018;65(6):627–635. doi: 10.1007/s12630-018-1087-1. [DOI] [PubMed] [Google Scholar]
- 42.Böhmer A.B., Just K.S., Lefering R., Paffrath T., Bouillon B., Joppich R. Factors influencing lengths of stay in the intensive care unit for surviving trauma patients: a retrospective analysis of 30,157 cases. Crit Care. 2014;18(4):R143. doi: 10.1186/cc13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Yu J., Huang W., Huang Q., Yan J., Liang H. Risk factors analysis of acute respiratory distress syndrome in intensive care unit traumatic patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30(10):978–982. doi: 10.3760/cma.j.issn.2095-4352.2018.010.015. [DOI] [PubMed] [Google Scholar]
- 44.Almashrafi A., Elmontsri M., Aylin P. Systematic review of factors influencing length of stay in ICU after adult cardiac surgery. BMC Health Serv Res. 2016;16:318. doi: 10.1186/s12913-016-1591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilligan S. Critical care delayed discharge: good or bad? J Intens Care Soc. 2017;18(2):146–148. doi: 10.1177/1751143716678637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiruvoipati R., Botha J., Fletcher J., Gangopadhyay H., Majumdar M., Vij S. Intensive care discharge delay is associated with increased hospital length of stay: a multicentre prospective observational study. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0181827. e0181827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson D.W., Schmidt U.H., Bittner E.A., Christensen B., Levi R., Pino R.M. Delay of transfer from the intensive care unit: a prospective observational study of incidence, causes, and financial impact. Crit Care. 2013;17(4):R128. doi: 10.1186/cc12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panella M., Marchisio S., Di Stanislao F. Reducing clinical variations with clinical pathways: do pathways work? Int J Qual Health Care. 2003;15(6):509–521. doi: 10.1093/intqhc/mzg057. [DOI] [PubMed] [Google Scholar]
- 49.Partington A., Chew D.P., Ben-Tovim D., Horsfall M., Hakendorf P., Karnon J. Screening for important unwarranted variation in clinical practice: a triple-test of processes of care, costs and patient outcomes. Aust Health Rev. 2017;41(1):104–110. doi: 10.1071/AH15101. [DOI] [PubMed] [Google Scholar]
- 50.Evans-Lacko S., Jarrett M., McCrone P., Thornicroft G. Facilitators and barriers to implementing clinical care pathways. BMC Health Serv Res. 2010;10:182. doi: 10.1186/1472-6963-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hipp R., Abel E., Weber R.J. A Primer on Clinical Pathways. Hosp Pharm. 2016;51(5):416–421. doi: 10.1310/hpj5105-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyko E.J. Observational research–opportunities and limitations. J Diab Complicat. 2013;27(6):642–648. doi: 10.1016/j.jdiacomp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribeiro D.C., Abbott J.H., Sharma S., Lamb S.E. Process evaluation of complex interventions tested in randomised controlled trials in musculoskeletal disorders: a systematic review protocol. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-028160. e028160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.