Dear Editor:
Patients with coronavirus disease 2019 (COVID-19) can develop acute respiratory distress syndrome (ARDS), which has been linked to poor prognosis and is a major contributor to patient death [1]. A better understanding of the pathophysiology of COVID-19-related ARDS would benefit early, precise treatment.
Cell death plays a major role in ARDS pathogenesis. While apoptosis in acute lung injury is well studied, newly identified cell death signaling has drawn attention as a potential mediator of ARDS [2]. Necroptosis, a caspase-independent form of necrosis involving receptor-interacting kinase 3 (RIPK-3), has been implicated in ARDS development with sepsis and trauma [3]. Since this highly regulated cell death signaling leads to rupture of the plasma membrane and release of damage-associated molecular patterns [4], necroptosis may be a therapeutic target for ARDS. However, the relationship between necroptosis and COVID-19-induced ARDS remains unclear.
Here, we describe serum RIPK-3 levels in COVID-19 patients measured on the first day of hospitalization. Patients were recruited from March 1 to May 30, 2020, and diagnosed as “severe” if any of the following conditions were met [5]: (1) respiratory rate > 30 breaths/min, (2) saturation of peripheral oxygen < 93% in ambient air, (3) ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen < 300 mmHg, or (4) lung infiltrates > 50% within 24–48 h. Blood samples were centrifuged within 30 min and refrigerated at 4 °C, and plasma aliquots were frozen within 12 h. RIPK-3 levels were measured using an enzyme-linked immunosorbent assay (Wuhan Huamei Biotech, Wuhan, China).
This observational study enrolled 16 COVID-19 patients (11 males, 68.8%) (Table 1). Confirmation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was by real-time reverse transcription polymerase chain reaction of nasopharyngeal swabs. Patients’ median age was 55 years (interquartile range [IQR] 40.5–71.5 years), and the median duration from symptom onset to hospitalization was 7 days (3.25–9 days). On admission, 14 patients (87.5%) were confirmed to have COVID-19 pneumonia by chest computed tomography, 10 patients were diagnosed with severe COVID-19 and ARDS, and 6 patients were diagnosed as mild. While hospitalized, the antiviral drug favipiravir was administrated to 11 patients (68.8%) in the context of a clinical trial, whereas azithromycin (n = 11, 68.8%), nafamostat (n = 12, 75%), and tocilizumab (n = 7, 43.7%) were commenced as off-label use. The median levels of serum RIPK-3 were significantly higher in severe COVID-19 cases than in mild cases (483.5 pg/mL, IQR 329.6–867.7 pg/mL vs. 139.9 pg/mL, IQR 95.37–286.8 pg/mL, p = 0.0075) (Fig. 1). Fifteen patients recovered and were discharged, whereas three patients in the severe group were intubated due to severe acute respiratory failure and one of these patients died.
Table 1.
Clinical characteristics of patients (n = 16)
| Male | 11 (68.8%) |
| Median age, years (IQR) | 55 (40.5–71.5) |
| Median duration from onset of symptoms to hospitalization, days (IQR) | 7 (3.25–9) |
| Underlying disease | |
| Diabetes mellitus | 4 (25%) |
| Hypertension | 2 (12.5%) |
| Heart disease | 2 (12.5%) |
| Treatment | |
| Azithromycin | 11 (68.8%) |
| Favipiravir | 11 (68.8%) |
| Nafamostat | 12 (75%) |
| Tocilizumab | 7 (43.7%) |
| Disease severity | |
| Mild | 6 (37.5%) |
| Severe | 10 (62.5%) |
| PaO2/FiO2 ratio | |
| > 350 | 6 (37.5%) |
| 200–300 | 5 (31.25%) |
| 150–200 | 3 (18.75%) |
| < 150 | 2 (12.5%) |
IQR interquartile range, PaO2/FiO2ratio ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen
Fig. 1.
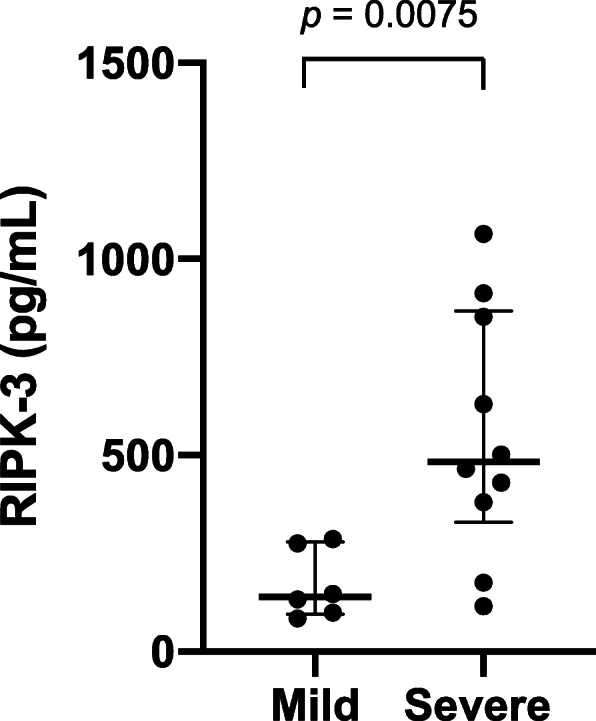
Serum levels of receptor-interacting kinase 3 (RIPK-3) in 16 patients with COVID-19. Serum RIPK-3 levels were measured by the enzyme-linked immunosorbent assay in patients with mild (n = 6) or severe (n = 10) COVID-19. For each dataset, the horizontal bars represent the median and interquartile range. Statistics were analyzed using Prism (GraphPad Software, CA, USA); p < 0.05 was considered significant
This is the first study to analyze RIPK-3 in COVID-19 patients. The higher serum RIPK-3 levels in severe patients suggest that RIPK-3-mediated signaling, such as necroptosis, might be involved in the development of acute lung injury associated with COVID-19 pneumonia. Siempos et al. reported plasma RIPK-3 levels were significantly higher in ARDS patients compared to those of non-ARDS patients [6]. Shashaty et al. demonstrated that among patients with sepsis or trauma, the change in plasma RIPK-3 levels 48 h after admission was independently associated with ARDS [3]. Because RIPK-3 mediates not only necroptosis but also other inflammatory pathways [2], the elevation of RIPK-3 does not directly indicate the execution of necroptosis. To confirm the role of RIPK-3 in COVID-19-ARDS patients, further studies are needed including a larger number of participants and histological evaluation of lung tissues, especially since RIPK-3-mediated necroptosis could be a potential therapeutic target for COVID-19-related ARDS.
Acknowledgements
Collaborating author names: Mariko Otsuki, Yuri Higure, Naoya Nishiyama, Masashi Nakamatsu, and Shusaku Haranaga. Department of Infectious, Respiratory and Digestive Medicine, Graduate School of Medicine, University of the Ryukyus, Okinawa, Japan.
Abbreviations
- COVID-19
Coronavirus disease 2019
- ARDS
Acute respiratory distress syndrome
- RIPK1/3
Receptor-interacting kinase 1 and 3 (RIPK1/3)
- IQR
Interquartile range
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Authors’ contributions
HN designed the study and had full access to all data in the study. TK and WA contributed to the laboratory work. MT and JF supervised the study and contributed to the writing of the manuscript. All authors contributed to data acquisition, data analysis, or data interpretation and reviewed and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
Full de-identified data of the analyses are available upon request sent to the corresponding author.
Ethics approval and consent to participate
This study was approved by the ethics committee of the University of the Ryukyus for Medical and Health Research Involving Human Subjects (approval number: 1616).
Consent for publication
Written informed consent was obtained from all patients.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao Y, Liu X, Xiong L, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 2020;3:10.1002/jmv.25822. [DOI] [PMC free article] [PubMed]
- 2.Faust H, Mangalmurti NS. Collateral damage: necroptosis in the development of lung injury. Am J Physiol Lung Cell Mol Physiol. 2020;318(2):L215–L225. doi: 10.1152/ajplung.00065.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shashaty MGS, Reilly JP, Faust HE, Forker CM, Glttner CA, Zhang PX, et al. Plasma receptor interacting protein kinase-3 levels are associated with acute respiratory distress syndrome in sepsis and trauma: a cohort study. Crit Care. 2019;23(1):235. doi: 10.1186/s13054-019-2482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;30(5):455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;24. 10.1001/jama.2020.2648. [DOI] [PubMed]
- 6.Siempos II, Ma KC, Imamura M, Baron RM, Fredenburgh LE, Huh JW, et al. RIPK3 mediates pathogenesis of experimental ventilator-induced lung injury. JCI Insight. 2018;3(9):e97102. doi: 10.1172/jci.insight.97102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full de-identified data of the analyses are available upon request sent to the corresponding author.


