Abstract
Background
Pulmonary infection with SARS-CoV-2 virus (severe acute respiratory syndrome coronavirus 2; COVID-19) has rapidly spread worldwide to become a global pandemic.
Objective
To collect paediatric COVID-19 cases worldwide and to summarize both clinical and imaging findings in children who tested positive on polymerase chain reaction testing for SARS-CoV-2.
Materials and methods
Data were collected by completion of a standardised case report form submitted to the office of the European Society of Paediatric Radiology from March 12 to April 8, 2020. Chest imaging findings in children younger than 18 years old who tested positive on polymerase chain reaction testing for SARS-CoV-2 were included. Representative imaging studies were evaluated by multiple senior paediatric radiologists from this group with expertise in paediatric chest imaging.
Results
Ninety-one children were included (49 males; median age: 6.1 years, interquartile range: 1.0 to 13.0 years, range: 9 days–17 years). Most had mild symptoms, mostly fever and cough, and one-third had coexisting medical conditions. Eleven percent of children presented with severe symptoms and required intensive unit care. Chest radiographs were available in 89% of patients and 10% of them were normal. Abnormal chest radiographs showed mainly perihilar bronchial wall thickening (58%) and/or airspace consolidation (35%). Computed tomography (CT) scans were available in 26% of cases, with the most common abnormality being ground glass opacities (88%) and/or airspace consolidation (58%). Tree in bud opacities were seen in 6 of 24 CTs (25%). Lung ultrasound and chest magnetic resonance imaging were rarely utilized.
Conclusion
It seems unnecessary to perform chest imaging in children to diagnose COVID-19. Chest radiography can be used in symptomatic children to assess airway infection or pneumonia. CT should be reserved for when there is clinical concern to assess for possible complications, especially in children with coexisting medical conditions.
Keywords: Children, Computed tomography, Coronavirus, COVID-19, Imaging, Lower respiratory tract infection, Pneumonitis, Radiography
Introduction
Since the outbreak in China in December 2019, pulmonary infection with the novel SARS-CoV-2 virus (severe acute respiratory syndrome coronavirus 2; causing COVID-19) has rapidly spread worldwide to become a global pandemic threatening the capacity of numerous national health care systems. Although COVID-19 predominantly affects adults, there have been reports of paediatric patients. The largest paediatric epidemiological report of COVID-19 in children by Dong et al. [1] found that children of all ages were susceptible to SARS-CoV-2 infection, but clinical manifestations were generally less severe than those in adults. Descriptions of imaging findings in children with COVID-19 are limited to small case series and reports, mostly written by non-radiologists with variable usage of standardised terminology.
Although chest radiography can be a useful tool in the investigation of lower respiratory tract infections, the extent to which this should be utilized in children with COVID-19 remains unclear [2]. Use of lung ultrasound (US) has been advocated to detect early pulmonary abnormalities in adults [3], however little published evidence exists regarding its clinical usefulness in children. Chest computed tomography (CT) has been proposed to identify adults with false-negative real-time reverse transcriptase polymerase chain reaction (PCR) results [4]. However, given that most children present with mild symptoms or are asymptomatic, the additional radiation dosage for screening CT or preoperative CT may not be justified. Furthermore, it is unclear whether the characteristic COVID-19 findings in adults are applicable to a paediatric population. The aim of this case series, therefore, was to describe the imaging appearances of a large cohort of children with COVID-19 and to provide some guidelines for paediatric imaging.
Materials and methods
Ethical approval for this multicentre retrospective observational study was obtained from Hospital Universitario Virgen del Rocío, Seville, Spain; Erasmus Medical Center, Rotterdam, The Netherlands, and from Amsterdam UMC, University of Amsterdam, The Netherlands. The European Society of Paediatric Radiology Cardiothoracic Taskforce conceived the study and members of multiple radiologic societies were invited to voluntarily submit anonymized demographic, clinical and imaging data for confirmed COVID-19 cases in children from their institutions. Parental/guardian approval was obtained for each child to share the anonymized data. Data were collected by completion of a standardised case report form submitted to the office of the European Society of Paediatric Radiology from March 12 to April 8, 2020. Chest radiographs and the five most representative CT images of each child were submitted by the physician collaborators on this study. The inclusion criteria were any patient ages 18 years or younger with a positive result from the PCR testing for SARS-CoV-2. All imaging modalities were included. Suspected cases, based on symptoms and/or clinical history, were not included without a confirmed PCR result. Chest radiographs were evaluated independently by three senior paediatric radiologists (M.N., M.G.M. and C.J.K., with 8, 11 and 25 years of experience in paediatric chest imaging, respectively). Chest CT images were analysed by a different group of three senior paediatric radiologists (A.S., M.B.D. and P.T., with 10, 15 and 43 years of experience in paediatric chest imaging, respectively) who reviewed the images independently before comparing their impressions and reaching a consensus decision on the abnormalities, without disagreements. Although the expert panel were aware that all children had tested positive for SARS-CoV-2 coronavirus, they were blinded to the demographic and clinical presentations, as well as the original radiologic reports. Descriptive analyses of the patient demographics, presenting complaints and imaging findings were performed and tabulated.
Results
Patients and clinical findings
Table 1 shows the demographic and clinical information for 91 children with confirmed cases of SARS-CoV-2 infection who underwent imaging and were included in this study (42 females/49 males). Median age was 6.1 years (interquartile range: 1.0 to 13.0 years, range: 9 days–17 years). Cases were submitted from Spain (n=30), Italy (n=28), France (n=8), Iran (n=7), United States of America (n=4), Switzerland (n=3), Germany (n=3), Sweden (n=2), Hong Kong (n=2), Netherlands (n=2), United Kingdom (n=1) and Mexico (n=1).
Table 1.
Summary of demographic and clinical findings in a series of 91 children with positive COVID-19 polymerase chain reaction tests who required imaging
| Age | |
| All cases median | 6.1 years |
| Interquartile range (range) | 1.0 to 13.0 years (9 days–17 years) |
| Age group n (%) | |
| <1 | 23 (25%) |
| 1–5 | 21 (23%) |
| 6–10 | 13 (14%) |
| 11–15 | 25 (28%) |
| >15 | 9 (10%) |
| Males n (%) | 49 (54%) |
| Symptoms n (%) | |
|
Fever Cough Dyspnoea Rhinorrhoea Sputum production Gastrointestinal (vomiting, abdominal pain) No symptoms |
66 (73%) 50 (55%) 33 (36%) 13 (14%) 8 (9%) 5 (6%) 5 (6%) |
|
Comorbidities n (%) Immunocompromised status Congenital heart disease Long-term respiratory conditions |
30 (33%) 16 (18%) 7 (8%) 7 (8%) |
Eighty-five children showed symptoms, and 6 children were asymptomatic (7%). The most common symptoms at presentation were fever, cough and dyspnoea; rhinorrhoea, sputum production, headaches, diarrhoea and chest pain were less frequent. The most frequent indications for imaging were clinically suspected lower tract respiratory infection, COVID-19 diagnosis and assessment of complications. One-third of the cases (33%) had significant comorbidities: 16 children were immunocompromised (post-transplant, immunosuppressive medication, malignancies), 7 had congenital heart diseases and 7 had long-term respiratory conditions (asthma, bronchial hyperreactivity, aspiration syndrome, preterm chronic lung disease).
Although a long-term clinical follow-up was not feasible, at the time of submission 85% of the reported children were discharged without complications. Two children had a pneumothorax, which improved after evacuation. A 16-year-old boy had deep venous thrombosis with pulmonary emboli and a 1-year-old girl had SARS-CoV-2 myocarditis. Ten children (11%) were admitted to the intensive care unit and nine of them required intubation and ventilation. A 3-year-old girl, whose polyarthritis was treated with methotrexate and prednisone, passed away due to SARS-CoV-2 infection despite aggressive resuscitation in the intensive care unit.
Chest radiography
Chest radiograph findings in our cohort are summarized in Table 2. In our study, 81 chest radiographs of children with COVID-19 (89%) were analysed. When chest radiography was used, 10% of cases did not have any radiographic findings despite being SARS-CoV-2 positive. All patients with normal chest radiographs had good clinical evolution without admission to the intensive care unit and those with multiple radiographs remained normal. One CT was performed in a child with a normal chest radiograph after an 8-day interval without significant findings.
Table 2.
Summary of findings on chest radiographs in 81 children with COVID-19, reviewed by three paediatric radiologists with 8, 11 and 25 years of experience, respectively
| Mean of the 3 readers Number of patients (percentage) | Reader 1 Number of patients |
Reader 2 Number of patients | Reader 3 Number of patients | |
|---|---|---|---|---|
| Perihilar peribronchial wall thickening | 47.0 (58%) | 47 | 51 | 43 |
| Consolidation | 28.3 (35%) | 23 | 31 | 31 |
| Ground glass opacities | 15.7 (19%) | 5 | 21 | 21 |
| Interstitial pattern | 12.7 (16%) | 13 | 14 | 11 |
| Normal chest radiograph | 10.0 (12%) | 8 | 11 | 11 |
| Pleural effusion | 6.0 (7%) | 6 | 6 | 6 |
| Pneumothorax | 2.0 (2%) | 2 | 2 | 2 |
| Atelectasis | 2.0 (2%) | 2 | 2 | 2 |
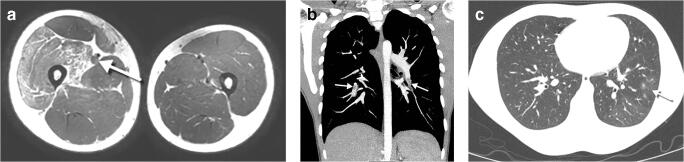
Increased central peribronchovascular markings (also called perihilar peribronchial wall thickening) in 58% of children (Figs. 1, 2) and consolidation in 35% (Figs. 2, 3, 4) were the most frequent abnormal findings. Ground glass opacities (19%) (Figs. 4, 5) and interstitial pattern (16%) (Figs. 4, 6, 7) were less frequent in our cohort. Pleural effusion (7%) (Fig. 2), pneumothorax (2%) (Figs. 8, 9) and atelectasis (2%) (Fig. 9) were uncommon in this group of children with COVID-19. Of note, our cohort was a mixture of patients with mild and severe disease, and discrimination of imaging findings based on the severity of disease was not possible with the existing data.
Fig. 1.
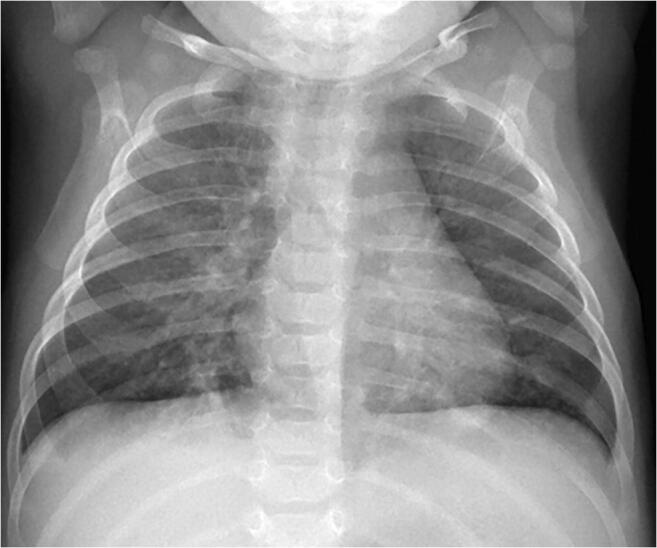
A 1-month-old boy presented with fever and cough. Anteroposterior chest radiograph shows bilateral perihilar peribronchial wall thickening along with subsegmental atelectasis in the lower lobes
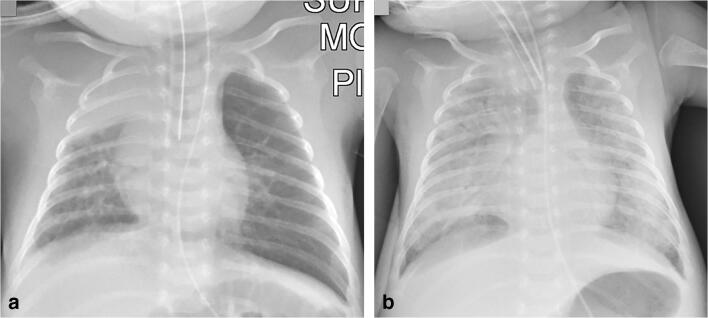
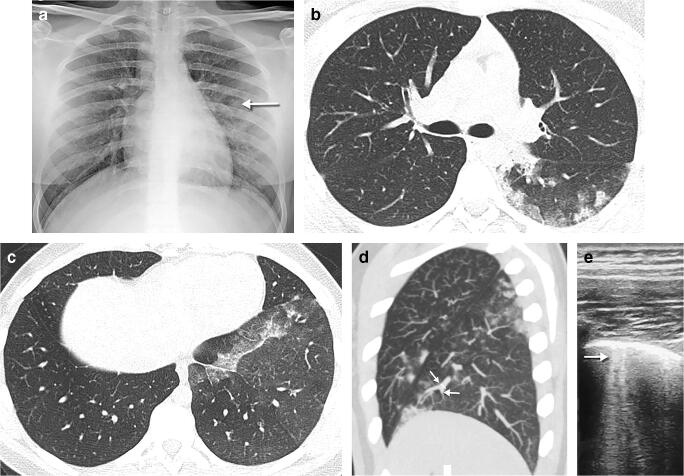
Fig. 2.
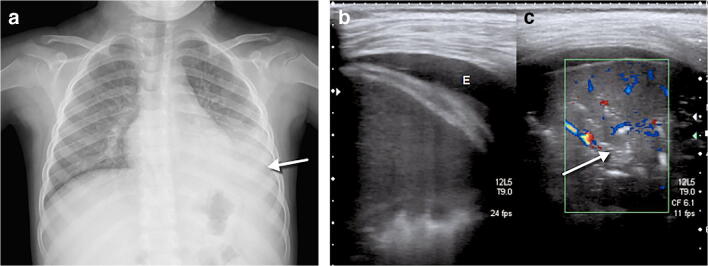
A 4-year-old girl presented after 2 days of fever and cough. a Anteroposterior chest radiograph shows bilateral perihilar peribronchial thickening along with left upper and lower lobe focal airspace consolidations and moderate left pleural effusion (arrow). b, c Coronal lung ultrasound image (b) and coronal colour Doppler image (c) show extensive subpleural consolidation within the posterobasal area of the left lung, as well as a simple pleural effusion (E). Within the subpleural consolidation there were air bronchograms (arrow) and normal flow on colour Doppler
Fig. 3.
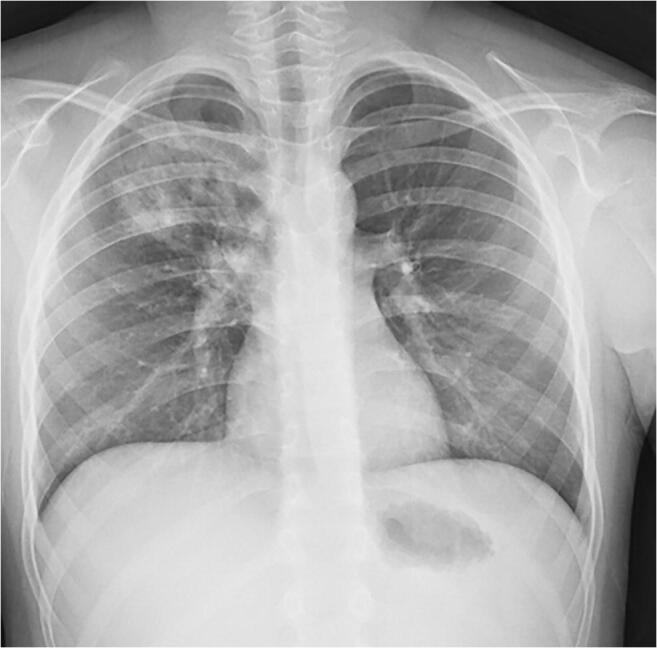
A 10-year-old boy presented with fever, dyspnoea and cough. Anteroposterior chest radiograph shows a focal airspace consolidation in the right upper lobe. This finding resolved on follow-up radiograph 3 days later (not shown)
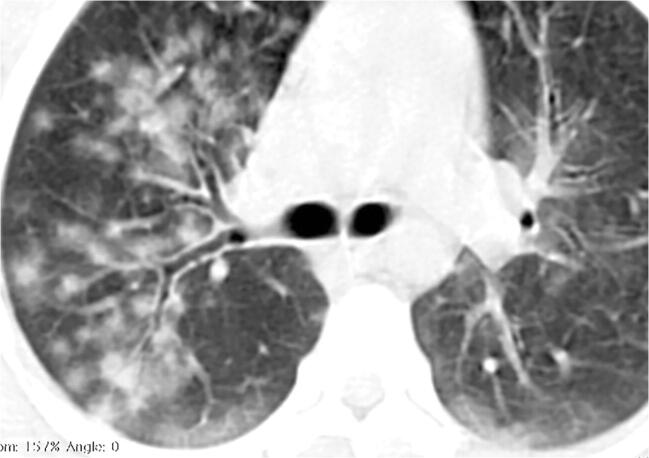
Fig. 4.
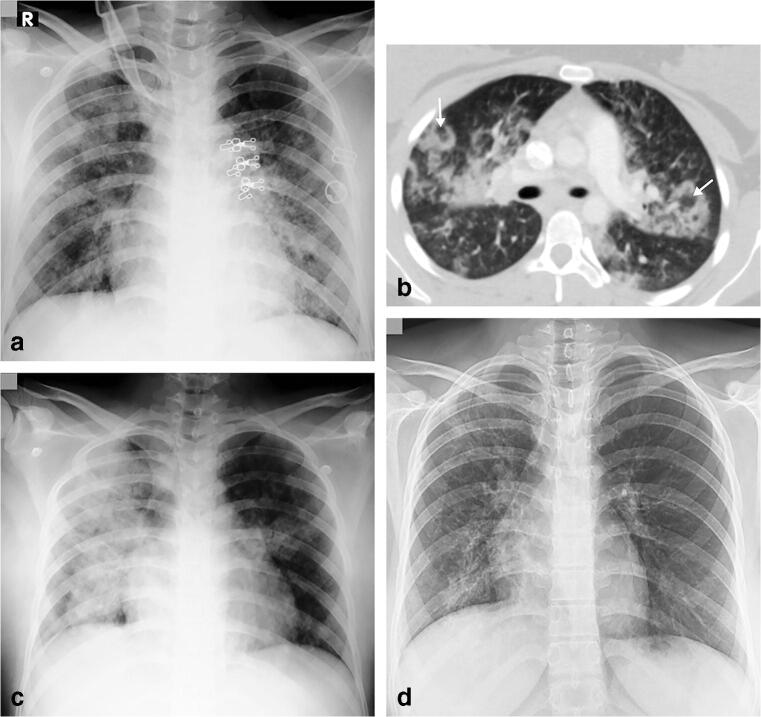
A 16-year-old girl presented with fever, cough, sputum, nasal discharge and dyspnoea. a Anteroposterior chest radiograph, on the day of admission, shows multifocal airspace consolidations, ground glass opacities and reticular opacities in both lungs. b Axial contrast-enhanced chest CT scan performed the same day demonstrates focal bilateral patchy rounded ground glass opacities (arrows) surrounded by a more or less complete ring-like consolidation (reverse halo sign) suggesting organizing pneumonia, which was a sign of severe extent of the disease. c Anteroposterior chest radiograph, 3 days later, showed increased right and improved left airspace opacities. d Posteroanterior chest radiograph, 17 days later, showed improvement in bilateral airspace opacities with residual reticular and ground glass opacities in the right upper, right lower and left lower lobes
Fig. 5.
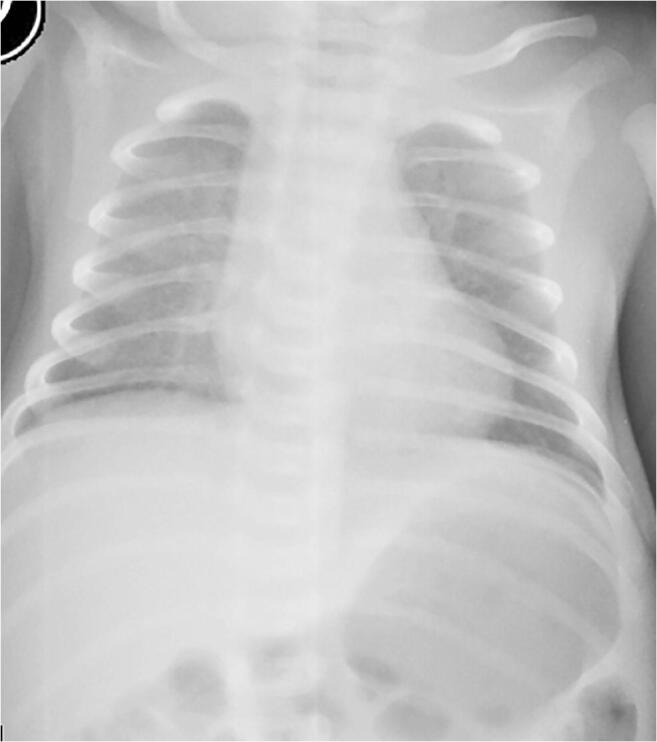
A 9-day-old girl presented with tachypnoea, after being asymptomatic following normal delivery at term. Anteroposterior chest radiograph shows bilateral diffuse ground glass opacity. The parents of the neonate were confirmed COVID-19 cases. This is the youngest PCR positive patient in our case series
Fig. 6.
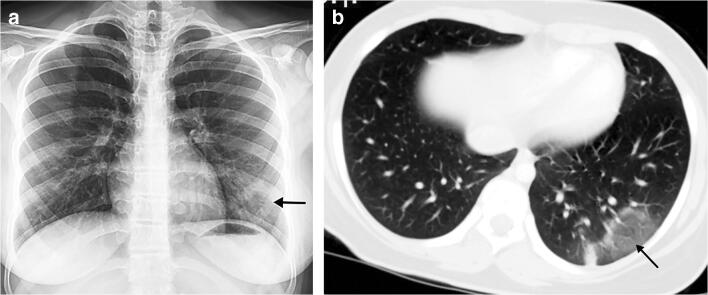
A 15-year-old girl presented with fever, dyspnoea, cough and sputum. a Posteroanterior chest radiograph shows reticular and focal ground glass opacities (arrow) in the left lower lobe. b Axial contrast-enhanced chest CT, performed 3 days later, confirms the left lower lobe focal ground glass opacity (arrow)
Fig. 7.
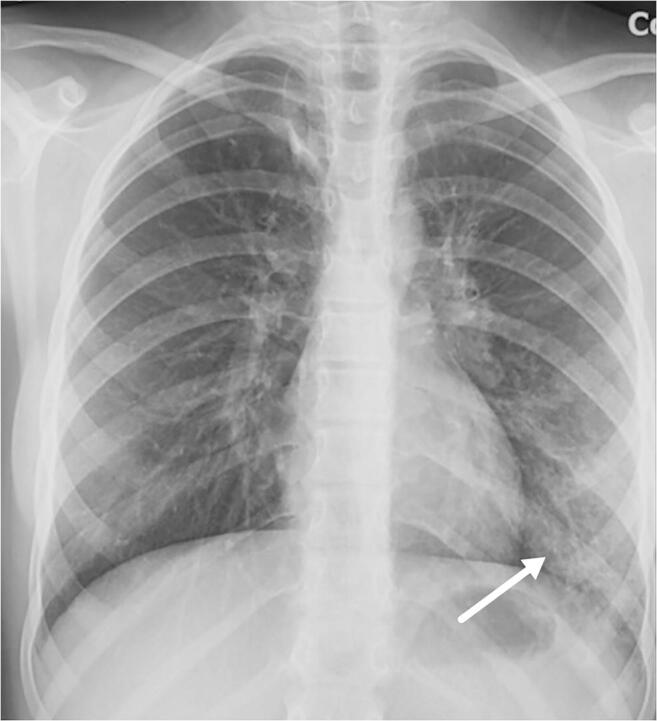
A 12-year-old girl presented with fever and cough. Anteroposterior chest radiograph shows subtle reticulonodular pattern and ground glass opacities (arrow) in the left lower lobe
Fig. 8.
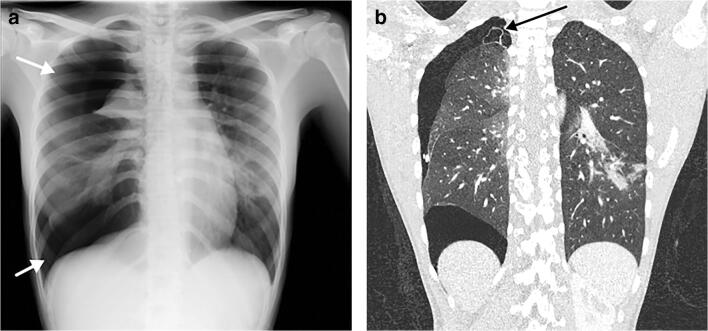
A 15-year-old girl presented with dyspnoea. a Posteroanterior chest radiograph shows a large right-side pneumothorax (arrows) with atelectasis of the right upper lobe and multifocal airspace opacities in the right lower and left lower lobes. There was a slight shift of the mediastinum to the left suggestive of a tension pnemothorax. b Coronal contrast-enhanced chest CT, performed 4 days later, demonstrates a small apical bullae (arrow) at the apex of the right upper lobe, along with persistence of the pneumothorax
Fig. 9.
A 5-week-old boy born at 32 weeks’ gestation with a history of intubation was discharged at 37 weeks’ corrected gestational age. On the following day, he presented with dyspnoea, apnoea, cough and nasal discharge and was admitted to the paediatric intensive care unit. a Anteroposterior chest radiograph demonstrates right upper lobe atelectasis and left lung hyperaeration (probably related to the low position of the endotracheal tube), multifocal airspace and ground glass opacities in the right middle and bilateral lower lobes and bilateral parahilar reticular opacities. b Anteroposterior chest radiograph, 6 days later, shows bibasilar pneumothoraces, which had increased in size compared to Day 3 (not shown) and increased bilateral airspace opacities with air bronchograms
Chest CT
Table 3 summarizes chest CT findings in 24 children with confirmed COVID-19. In our cohort of patients, a CT scan was performed after a few days of symptoms (mean: 4.8 days), mainly in adolescents (median: 13.5 years, interquartile range: 10.3 to 15.7 years) with a 50/50 male/female distribution.
Table 3.
Summary of chest CT findings in 24 children with COVID-2019, reviewed in consensus by three paediatric radiologists with 10, 15 and 43 years of experience
| Abnormal CT scans | n = 22 (92%) |
| Median age (interquartile range, range) | 13.5 years (10.3 to 15.7 years, 6 months–17.3 years) |
| Male/female distribution | 50/50 |
| Scan indications (percentage) |
10 assessment of lower respiratory tract infection 10 (42%) 10 assessment of complications (42%) 4 COVID-19 polymerase chain reaction test positive (17%) |
|
Interval between symptoms and CT Mean (median, range) |
4.8 days (3.5, 0–12) |
| Comorbidities (percentage) |
19 none (79%) 3 congenital heart disease (13%) 2 immunocompromised status (8%) |
| Ground glass opacities (percentage) |
21 (88%) 15 bilateral (63%) 17 left lower lobe (71%), 17 right lower lobe (71%) 9 segmental distribution (43%) 7 rounded morphology (29%) |
| Consolidations (percentage) |
14 (58%) 8/14 bilateral (57%) 10/14 left lower lobe (71%), 9/14 right lower lobe (64%) 8/14 segmental distribution (57%) |
| Linear opacities (percentage) |
8 (33%) 5/8 unilateral (63%) 6/8 right lower lobe (75%), 5/8 left lower lobe (63%) 6/8 vertical orientation (75%) |
| Nodules (percentage) |
6 (25%) 5/6 unilateral (83%) 3/6 right lower lobe (50%), 3/6 left lower lobe (50%), 3/6 right upper lobe (50%) 3/6 central distribution (50%) |
| Tree in bud appearance | 6/24 (25%) |
| Lymphadenopathy (percentage) |
4 (17%) 2/4 hilar (50%) 2/4 mediastinal (50%) |
| Vascular engorgement (percentage) | 3 (13%) |
| Crazy paving (percentage) |
2 (8%) 1/2 bilateral (50%) 2/2 right upper lobe and right lower lobe (100%) |
Out of 24 children, 2 (8%) had a normal CT scan, both with previous normal chest radiographs. CT appearances were a mixture of patterns such as rounded patchy ground glass opacities, consolidations and interlobular septal thickening. The most common abnormal CT finding was predominantly ground glass opacities (21/24), with 81% of abnormal cases, preferentially involving the lower lobes. Our series found patterns that mirror those in the adult: In the early stage of the disease, CT frequently showed ground glass opacities, mostly peripheral in distribution (Figs. 6, 10). These areas were relatively diffuse or multifocal, with a rounded shape (Figs. 10, 11). A pattern of ground glass opacities with airspace consolidation (Fig. 12) and interlobular septal thickening superimposed on the ground glass opacities, causing a crazy-paving configuration, was seen in a more severe stage of the infection (Figs. 13, 14). The reverse halo sign (Fig. 4) identified in one patient is a well-recognized CT pattern of organizing pneumonia [5], which is frequently seen in acute respiratory distress syndromes with diffuse alveolar damage.
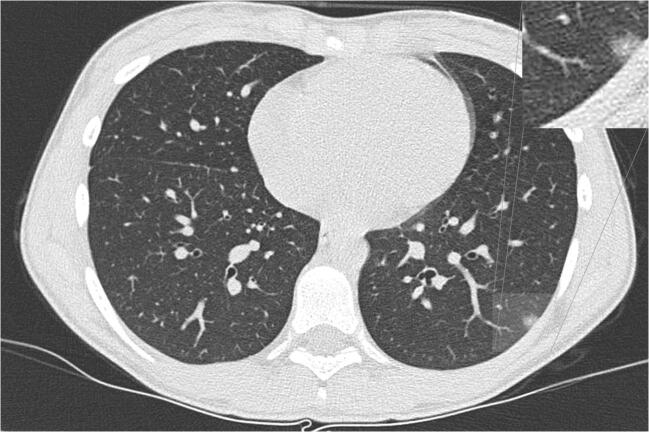
Fig. 10.
A 13-year-old girl with fever and cough. An axial non-contrast CT, 8 days after the onset of symptoms, shows a small rounded subpleural ground glass opacity in the left lower lobe. A magnification of the left lower lobe finding is inset
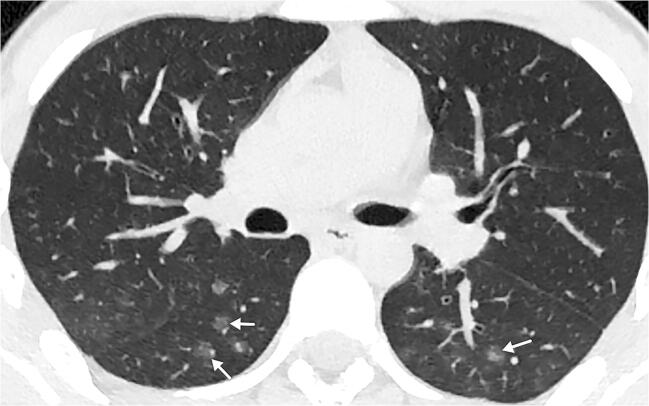
Fig. 11.
A 16-year-old boy following 1 day of fever. An axial non-enhanced CT shows small rounded multifocal ground glass opacities bilaterally in the lower lobes associated with small vessels (arrows)
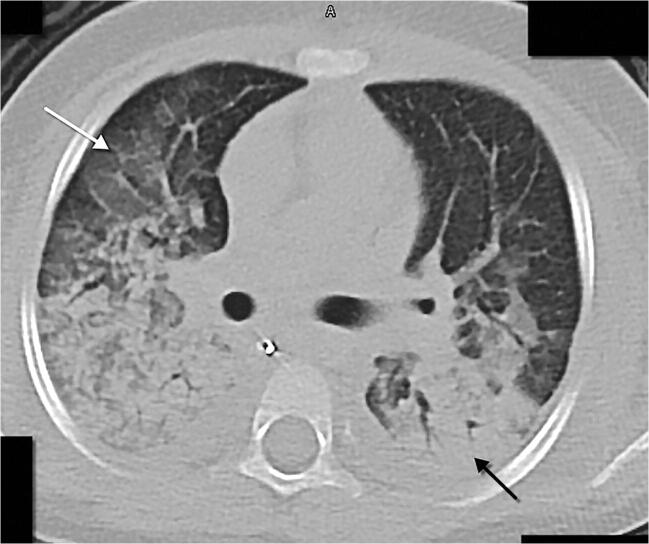
Fig. 12.
A 3-year-old girl with a previous history of polyartrhitis treated with methotrexate and prednisone who presented with fever and dyspnoea. An axial chest CT show areas of ground glass opacity in the upper lobes and middle lobe (white arrow), with confluent peribronchial consolidations (in keeping with the known COVID-19 histological association with diffuse alveolar damage), mostly in the lower lobes (black arrow), and mild bilateral pleural effusion. Despite resuscitation, this patient died
Fig. 13.
A 15-year-old girl in close contact with a relative with COVID-19 presented with 3 days of fever and dyspnoea. a A posteroanterior chest radiograph shows small opacities in the middle fields of the left lung (arrow). b An axial chest CT performed the same day demonstrates multifocal areas of rounded ground glass opacities, with a predominantly peripheral, subpleural location in the posterobasal segment of the left lower lobe. Intralobular reticulations can be seen superimposed on the ground glass opacities, resulting in a crazy paving pattern. c Axial images of the lung bases in the same CT show focal unilateral band of ground glass in the left lower lobe around the pleural reflection overlying the phrenic nerve. d Axial thin maximum intensity sagittal reconstruction in the same CT demonstrates focal vascular engorgement (arrows) in the anteromedial segment of the left lower lobe, compared with the upper lobe. e An axial lung ultrasound image obtained 2 days later as a follow-up diagnostic procedure shows B lines (arrow) within the lower posterior and lateral lung areas of the left lung, corresponding to the opacities seen on radiography, as well as pleural thickening
Fig. 14.
A 14-year-old boy unresponsive to wide-spectrum antibiotics. An axial non-contrast chest CT shows diffuse opacities with a rounded morphology and visible halo sign with both central and peripheral distribution and with relative subpleural sparing. Bilateral subpleural intralobular reticulations can be seen superimposed on the ground glass opacities, resulting in a crazy paving pattern of the posterobasal segments of the lower lobes
Tree in bud opacities (Fig. 15) were seen in 6 of 24 cases (25%), and when present, could be due to peripheral pulmonary vascular disease and not to bronchiolar disease. Focal vascular engorgement (Fig. 13) was seen in 3 patients (13%). A lobar pattern of consolidation without ground glass opacities, lymphadenopathy or significant pleural effusions was infrequently seen in our cohort.
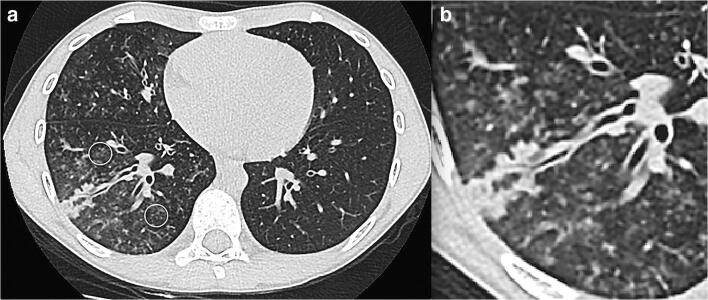
Fig. 15.
An 8-year-old boy with fever and dyspnoea. a An axial non-contrast chest CT shows peripheral tree in bud mostly in the right lower lobe (circles) and middle lobe either representing vasculitis from direct damage to the pulmonary vascular endothelium or exudative bronchiolitis due to hypersecretion/bacterial superimposed infection. b Magnification view of the right lower lobe findings
Other imaging modalities
Lung ultrasound (US) was used in three children -- as an initial diagnostic tool along with chest radiograph (Fig. 2), as a follow-up diagnostic procedure after CT (Fig. 13), and as a follow-up after chest radiograph in an 8-month-old boy with an extensive multiseptated pleural effusion and lung consolidation.
In one child, lung MRI abnormalities were identified (Fig. 16). This was an 11-year-old girl who was asymptomatic and underwent whole-body MRI for Ollier disease. MRI demonstrated hyperintense focal infiltration within the superior segment of the right lower lobe, as an incidental finding. Following the radiologist’s suggestion of COVID-19, she tested positive. A frontal chest radiograph performed 24 h after MRI did not show abnormalities.
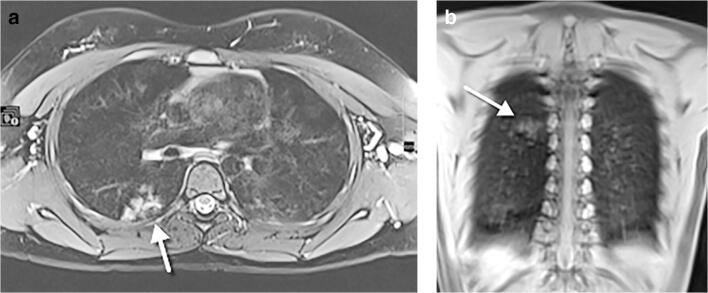
Fig. 16.
An 11-year-old girl, asymptomatic, who underwent whole-body MRI for Ollier disease. a An axial T2-weighted turbo spin echo fat-supressed image. b A coronal T1-weighted ultrashort echo time image. Both images demonstrate hyperintense focal infiltration (arrows) within the superior segment of the right lower lobe, as an incidental finding. Following a radiologist’s suggestion of COVID-19, the girl tested positive. A frontal chest radiograph performed 24 h after the MRI did not show abnormalities (not shown)
The cardiovascular system was affected in 2 patients within our cohort, a 1-year-old girl with myocarditis and a 16-year-old boy with deep venous thrombosis and bilateral pulmonary emboli (Fig. 17). COVID-19 seldom required imaging other than chest imaging, with only three patients needing abdominal US due to abdominal pain and two head CT due to headaches, without significant findings.
Fig. 17.
A 16-year-old boy with right leg pain due to deep vein thrombosis and pulmonary embolism, without respiratory symptoms, whose father had COVID-19. a An axial T2-weighted MR image shows a swollen right thigh, with oedema of the quadriceps muscle. Thrombus in the superficial femoral vein is seen as a distended vessel with absent flow void and increased signal intensity (arrow). b A coronal contrast-enhanced chest CT, performed due to tachycardia, demonstrates multiple emboli (arrows) within the segmental arteries of the lower lobes. c An axial contrast-enhanced CT in lung window shows small foci of ground glass opacity (arrow) with mosaic attenuation
Discussion
Chest radiography is the most commonly utilized diagnostic modality for evaluating lower respiratory tract infection and pneumonia in children. In previous case series as well as sporadic cases, most children had no findings on chest radiograph despite being SARS-CoV-2 positive [6, 7] and in most cases with mild presentation of COVID-19 the chest radiograph was normal [7, 8]. In our series, only 10% of patients had normal radiographs. This could be because the authors of previous reports might not have experience interpreting paediatric chest radiographs and misinterpreted increased central peribronchovascular markings, a sign of inflammatory lower airway disease related to viral infections, as normal exams. Interpretation of pneumonia on paediatric chest radiographs is known to have considerable intra-observer and interobserver variability [9]. Another factor that might have contributed to this difference is that in our cohort only 7% of children who had a radiograph were asymptomatic; this number of screening chest radiographs is much lower than that reported in the literature [8].
Among children with confirmed COVID-19 and initial normal chest radiographs, abnormalities could be seen on the same day or subsequent CT chest imaging [7]. In our cohort, only one child had a screening CT after a normal chest radiograph, without abnormalities. We advocate refraining from using CT for screening children with COVID-19.
The most frequent radiographic findings in confirmed COVID-19 patients who had an abnormal chest radiograph were focal or diffuse airspace consolidation or ground glass opacities [6, 10]. In children with severe clinical manifestations of SARS-CoV-2 infection who were admitted to the intensive care unit, the most common findings on chest radiograph were multifocal bilateral or unilateral airspace consolidation or multifocal ground glass opacities [8, 11, 12]. In addition, some of these children showed imaging features of complications, such as septic shock or multiple organ failure, and/or evidence of their underlying comorbidities [11]. Our case series confirmed that airspace consolidation is a frequent finding in paediatric COVID-19, while ground glass opacities are less frequently identified on chest radiographs than on chest CT. More importantly, we have described that increased central peribronchovascular markings were the most frequent abnormality in the chest radiographs of COVID-19 paediatric patients, similar to other lower airway inflammations and viral infections. After reviewing 81 chest radiographs of children with COVID-19, this group of paediatric chest imaging experts found that chest radiograph findings in COVID-19 are nonspecific and can be seen in any lower airway infection and pneumonia. Therefore, chest radiographs cannot differentiate between COVID-19 and any other childhood lung infection.
After the submission of this article, Foust et al. [13] published an International Expert Consensus Statement on Chest Imaging in Pediatric COVID-19 Patients. This article was written based on opinions of the experts, rather than imaging data. The references for the paper list mostly adult case series with very small case series in children (only 10 chest radiograph cases). The recommendations for imaging appear reasonable; however, the structured reporting is especially troublesome, after reviewing 81 chest radiographs where we could not reliably distinguish between COVID-19 and non-COVID-19 imaging findings. The structured reporting would not have been very helpful in our cases, especially the one for chest radiographs. Based on our findings, “bilateral, peripheral and subpleural ground glass opacities and/or consolidation” was not the typical finding of COVID-19 in children.
The CT findings in our 24 cases of COVID-19 in children confirm what has already been described in adults with COVID-19 [6, 14]. Ground glass opacities with a peripheral distribution in the lower lobes and airspace consolidation are the most frequent findings in this disease. In addition to the adult-type CT appearance, a more centrilobular or peribronchovascular tree in bud pattern is seen on CT in up to one-fourth of paediatric patients. It remains unclear if this is a different presentation of COVID-19, or coinfection with another infectious agent. This might overlap the appearance on CT. Similar to reports in the literature, a lobar pattern of consolidation without ground glass opacities, lymphadenopathy or significant pleural effusions was infrequently seen in our cohort and may suggest other diagnoses or complications such as superimposed bacterial infection. It is necessary to stress that one of the most frequent indications for CT imaging in this study was screening for COVID-19, as significant patient access issues including limited testing supplies have resulted in excessive waiting times to screen patients, leading some countries to utilize CT as a screening tool in adult hospitals [6].
Currently, lung US is not the first-line imaging tool in patients with respiratory symptoms caused by or suspected of SARS-CoV-2 infection. Pulmonary changes in COVID-19 are predominantly located peripherally in the lower lobes [15], and are particularly amenable to evaluation by lung US as a result of its ability to detect pulmonary pathology abutting the pleura. Several studies draw attention to the potential use of lung US in adults with COVID-19 [16, 17], especially in the intensive care unit. However, this has not been proposed in children even though the paediatric US image quality is superior compared to that in adults, due to a thinner thoracic wall.
At present, lung US in patients suspected to have COVID-19 is used mainly for triage (pneumonia/non-pneumonia) of symptomatic patients and to monitor therapeutic measures [18, 19]. The characteristic US features include: B lines (focal, multifocal and/or confluent); irregular, discontinuous, interrupted and/or thickened pleura (Fig. 13), sometimes with focal pleural effusion; subpleural consolidation of different sizes with occasionally detected air bronchograms (Fig. 3), and insufficient blood supply within the subpleural lesions [18–20]. A linear array high frequency transducer is essential to increase diagnostic accuracy. Operator dependence and the close contact between the person performing the lung US and the patient with COVID-19 are the main disadvantages of this technique, especially in countries where personal protective equipment is a limited resource. However, lung US might be of use in the clinical management of COVID-19 patients with respiratory involvement, due to its safety, availability, lack of radiation, low cost and point of care use, especially in the intensive care unit.
Lung MRI is not performed routinely for lung infections and, in a time of intense pressure on hospital resources, it is not an appropriate screening tool for COVID-19 pneumonia in children. Chest MRI is more expensive and time-consuming than CT, potentially requiring general anaesthesia in young children. However, developments in MRI make it a viable radiation-free alternative to chest CT in the appropriate clinical setting, such as in compliant children (older than 5 years) and in a tertiary centre with experience in chest MRI [21, 22]. Rapid MRI sequences are reported to have high sensitivity in detecting pneumonia [21, 23]. Lung parenchyma is proton poor and provides a black background for any infiltrative/infectious diseases [22]. Therefore, alveolar opacifications demonstrate high signal intensity on MRI, especially on T2-weighted sequences. However, some parenchymal findings might be overlooked, especially ground glass opacities and small nodules [22].
Recent studies in adults have shown that a severe complication of COVID-19 is the increased risk of deep venous thrombosis and have advocated for the use of anticoagulant therapy to improve prognosis [24]. We have identified one case of deep vein thrombosis of the lower limbs with pulmonary emboli. Thoracic contrast-enhanced CT angiography should be used in cases where this disease is suspected.
We did not encounter reports of any hyperinflammatory syndrome in our cohort of patients as recently described by Riphagen et al. [25]. None of the submitted cases was described to have any features similar to atypical Kawasaki disease, Kawasaki disease, hypotensive shock syndrome or toxic shock syndrome [25]. Coronary anomalies were not reported in any of the cases. A 1-year-old girl with lung involvement and poor clinical course showed signs of myocarditis on echocardiography, but no coronary aneurysms were identified.
This study has several limitations. It is a multicentre retrospective study, with heterogeneous data collection. CT protocols were heterogeneous in different centres and only the most relevant CT images submitted by collaborators were evaluated. The heterogeneous image quality of chest radiographs and CTs will impact the detection/interpretation of imaging findings. There is a lack of good clinical correlation because of the way the imaging was collected early in the pandemic. Children with proven COVID-19 might have concurrent bacterial or viral infections that could confound the imaging findings. Imaging findings may be influenced also by the availability of PCR testing and by the clinical protocols for access to PCR within each country [26]. Despite these limitations, we were able to describe the spectrum of chest findings in COVID-19.
Take-home messages
Based on the cases presented, the following take-home points can be made:
• Children of all ages are susceptible to COVID-19. However, clinical manifestations are less severe than in adults and probably as a consequence the radiologic findings are less marked.
• Imaging should not be considered as a screening tool for diagnosis in children.
• If imaging is needed, chest radiograph is the first imaging modality of choice.
• The most common findings on chest radiograph are increased central peribronchovascular markings and airspace consolidation.
• CT should be reserved for complex cases, suspected complications or possible differential diagnoses, particularly in children with associated medical conditions.
• The predominant pattern on CT is ground glass opacities, mainly in the lower lobes, and in contrast with adults, a more centrilobular or peribronchovascular/tree in bud pattern.
• Chest radiograph and CT findings in COVID-19 are nonspecific and resemble other lower respiratory tract infections.
• The role of lung US in children with suspected COVID-19 is not clear. More studies in the paediatric population are needed.
Conclusion
It seems unnecessary to screen children with imaging to diagnose COVID-19 because children seldom show severe symptoms and the imaging findings are neither specific nor sensitive. Chest radiography should be used as the first imaging obtained in symptomatic children to assess lower airway infection or pneumonia. CT should be reserved to assess for possible complications, especially in children with coexisting medical conditions.
Acknowledgements
We would like to thank Ms. Anca Cociuban from the European Society of Paediatric Radiology Office for her help in collecting the cases for this article and all collaborators who submitted the cases.
Collaborators of the European Society of Paediatric Radiology Cardiothoracic Task Force (listed in alphabetical surname order):
Owen Arthurs
Department of Radiology, Great Ormond Street Hospital, London, United Kingdom
Michiel Bannier
Maastricht University Medical Centre, Maastricht, The Netherlands
Francesco Bianco
Department of Cardiovascular Science, Cardiac Surgery, Paediatric and Congenital Cardiology Unit, Ospedali Riuniti, Ancona, Italy
Roham Borazjani
Medical Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
Mark Born
Paediatric Radiology, Bonn University Hospital, Bonn, Germany
Jasmin Buschl
Department of Diagnostic, Interventional and Pediatric Radiology, Inselspital, University of Bern, Bern, Switzerland
Marirosa Cristallo Lacalamita
Paediatric Radiology, EOC, IIMSI Istituto di Imaging della Svizzera Italiana, Switzerland
Francesca De Luca
ME Barnradiologi, ME Neuroradiologi, Karolinska Universitetssjukhuset, Stockholm, Sweden
Marco Di Maurizio
MD UOC Radiology Unit, AOU Meyer Children Hospital, Florence, Italy
Francesca Finazzo
UOC Radiologia Pediatrica, P.O. Di Cristina Arnas Civico Palermo, Palermo, Italy
Karsten Jablonka
Department of Pediatric Radiology, Bremen Childrens’ Hospital, Germany
Mark Jenkins
Department of Radiology, Great Ormond Street Hospital, London, United Kingdom
Karmella Kamali
Medical Imaging Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Letizia Macconi
MD UOC Radiology Unit, AOU Meyer Children Hospital, Florence, Italy
Carlos Marín
Radiología Pediátrica, Hospital General Universitario Gregorio Marañón, Madrid, Spain
María Martínez León
Radiología Pediátrica, Hospital Materno-Infantil, Hospital Regional Universitario, Málaga, Spain
Baptiste Morel
Pediatric Radiology Department, Clocheville Hospital, CHRU de Tours, Université de Tours, Tours, France
Inmaculada Mota Goitia
Radiología pediátrica, Hospital Universitario Ramón y Cajal, Madrid, Spain
Marcello Napolitano
Department of Paediatric Radiology and Neuroradiology, V. Buzzi Children’s Hospital, Milan, Italy
Nin-Yuan Pan
Department of Radiology, Princess Margaret Hospital, Hong Kong
Elham Pourbkhtyaran
Shahid Beheshti University of Medical Sciences, Tehran, Iran
Friederike Prüfer
Paediatric Radiology, University Children’s Hospital Basel, Basel, Switzerland
Enrica Rossi
MD UOC Radiology Unit, AOU Meyer Children Hospital, Florence, Italy
Carrie Ruzal-Shapiro
Chief Division of Pediatric Radiology Morgan Stanley Children’s Hospital of New York Presbyterian, New York, United States
Anahita Sanaei Dashti
Professor Alborzi Clinical Microbiology Research Centerm Shiraz University of Medical Sciences, Shiraz, Iran
Ana Gabriela Sangri Pinto
Star Medica Private Children’s Hospital, México City, Mexico
Charlotte Seiler
Radiopediatrics Department, Hôpital Timone Enfants à Marseille, Marseille, France
Maria Sole Prevedoni Gorone
Fondazione IRCCS Policlinico San Matteo, Pediatric Radiology, Pavia, Italy
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pablo Caro-Dominguez, Email: pablocaro82@hotmail.com.
Collaborators of the European Society of Paediatric Radiology Cardiothoracic Task Force:
Owen Arthurs, Michiel Bannier, Francesco Bianco, Roham Borazjani, Mark Born, Jasmin Buschl, Marirosa Cristallo Lacalamita, Francesca De Luca, Marco Di Maurizio, Francesca Finazzo, Karsten Jablonka, Mark Jenkins, Karmella Kamali, Letizia Macconi, Carlos Marín, María Martínez León, Baptiste Morel, Inmaculada Mota Goitia, Marcello Napolitano, Nin-Yuan Pan, Elham Pourbkhtyaran, Friederike Prüfer, Enrica Rossi, Carrie Ruzal-Shapiro, Anahita Sanaei Dashti, Ana Gabriela Sangri Pinto, Charlotte Seiler, and Maria Sole Prevedoni Gorone
References
- 1.Dong Y, Mo X, Hu Y et al (2020) Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 10.1542/peds.2020-0702
- 2.Zu ZY, Jiang MD, Xu PP et al (2020) Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 200490 [DOI] [PMC free article] [PubMed]
- 3.Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295:E6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Zhong Z, Zhao W et al (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 10.1148/radiol.2020200343:200343 [DOI] [PMC free article] [PubMed]
- 5.Maturu VN, Agarwal R. Reversed halo sign: a systematic review. Respir Care. 2014;59:1440–1449. doi: 10.4187/respcare.03020. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Xu J, Lin D et al (2020) A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 28:ciaa198 [DOI] [PMC free article] [PubMed]
- 7.Park JY, Han MS, Park KU, et al. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci. 2020;35:e124. doi: 10.3346/jkms.2020.35.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;S1473-3099(20):30198–30195. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson J, Kline JA. Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs. Emerg Radiol. 2010;17:285–290. doi: 10.1007/s10140-009-0854-2. [DOI] [PubMed] [Google Scholar]
- 10.Zeng LK, Tao XW, Yuan WH et al (2020) [First case of neonate infected with novel coronavirus pneumonia in China.] Zhonghua Er Ke Za Zhi 58:E009 [DOI] [PubMed]
- 11.Sun D, Li H, Lu X-X et al (2020) Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed]
- 12.Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:179–182. doi: 10.3760/cma.j.issn.0578-1310.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Foust AM, Philips GS, Chu WC, et al. International expert consensus statement on chest imaging in pediatric COVID-19 patient management: imaging findings, imaging study reporting and imaging study recommendations. Radiol: Cardiothoracic Imaging. 2020;2:2. doi: 10.1148/ryct.2020200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen A, Huang J, Liao Y, et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiol: Cardiothoracic Imaging. 2020;2:2. doi: 10.1148/ryct.2020200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua er ke za zhi. 2020;58:269–274. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 16.Buonsenso D, Pata D, Chiaretti A (2020) COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med. 10.1016/S2213-2600(20)30120-X [DOI] [PMC free article] [PubMed]
- 17.Buonsenso D, Piano A, Raffaelli F, et al. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 18.Soldati G, Smargiassi A, Inchingolo R et al (2020) Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 10.1002/jum.15284 [DOI] [PMC free article] [PubMed]
- 19.Peng Q-Y, Wang X-T, Zhang L-N, Chineses Critical Care Ultrasound Study Group (CCUSG) (2020) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med 46:849–850 [DOI] [PMC free article] [PubMed]
- 20.Huang Y, Wang S, Liu Y et al (2020) A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). SSRN. 10.2139/ssrn.3544750
- 21.Liszewski MC, Gorkem S, Sodhi KS, Lee EY. Lung magnetic resonance imaging for pneumonia in children. Pediatr Radiol. 2017;47:1420–1430. doi: 10.1007/s00247-017-3865-2. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch FW, Sorge I, Vogel-Claussen J, et al. The current status and further prospects for lung magnetic resonance imaging in pediatric radiology. Pediatr Radiol. 2020;50:734–749. doi: 10.1007/s00247-019-04594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorkem SB, Coskun A, Yikilmaz A, et al. Evaluation of pediatric thoracic disorders: comparison of unenhanced fast-imaging-sequence 1.5-T MRI and contrast-enhanced MDCT. AJR Am J Roentgenol. 2013;200:1352–1357. doi: 10.2214/AJR.12.9502. [DOI] [PubMed] [Google Scholar]
- 24.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riphagen S, Gomez X, Gonzalez-Martinez C et al (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395:1607–1608 [DOI] [PMC free article] [PubMed]
- 26.Tagarro A, Epalza C, Santos M et al (2020) Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid. Spain JAMA Pediatr. 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed]