Abstract
Aim:
To describe the epidemiologic, humanistic and economic burdens of hepatocellular carcinoma (HCC) in the USA.
Materials & methods:
Studies describing the epidemiology and economic burden from national cohorts, any economic models, or any humanistic burden studies published 2008–2018 were systematically searched.
Results:
HCC incidence was 9.5 per 100,000 person-years in most recent data, but was ∼100-times higher among patients with hepatitis/cirrhosis. Approximately a third of patients were diagnosed with advanced disease. Patients with HCC experienced poor quality of life. Direct costs were substantial and varied based on underlying demographics, disease stage and treatment received. Between 25–77% of patients did not receive surgical, locoregional or systemic treatment.
Conclusion:
Better treatments are needed to extend survival and improve quality of life for patients with HCC.
Keywords: : epidemiology, hepatocellular carcinoma, pharmacoeconomics, risk factors, staging
Liver cancer is the sixth most common cancer diagnosis and fourth most common cause of cancer deaths in the world [1]. In the USA, cancer of the liver and bile duct was estimated to incur over 42,000 new cases and over 31,000 deaths in 2019, with men experiencing approximately two- to three-times as many new cases and deaths as women [2]. Approximately 70% of liver cancer cases are thought to be associated with modifiable risk factors, such as viral hepatitis, alcohol, smoking and obesity [3]. Despite declines in overall incidence and deaths from all cancers in the USA, liver cancer incidence and mortality are rising [4].
Hepatocellular carcinoma (HCC) accounts for 75–85% of all liver cancers, making HCC a major cause of cancer and cancer-related deaths [5]. Similar to liver cancers overall, the risk of HCC increases dramatically in patients with pre-existing liver disease caused by hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, alcohol abuse, nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD) [6]. A study using data from the Surveillance, Epidemiology and End Results (SEER) Program suggests that there will be over 38,000 HCC cases in 2020 and over 56,000 HCC cases in 2030 in the USA [7].
The treatment decision for HCC depends upon disease stage and patient characteristics. Potentially curative options include liver transplant and surgical resection (cure fractions based on overall survival of 74 and 41%, respectively) [8]. However, these options are appropriate only for patients with early stage disease [9–11]. Additionally, in cases of liver-donor shortages or ineligibility for resection due to underlying liver dysfunction and/or portal hypertension, ablation may be a curative option. For patients with inoperable disease due to performance status or comorbidity, or for those with locoregional disease with or without minimal extrahepatic disease, guideline-recommended locoregional options include ablation (e.g., radiofrequency ablation, microwave ablation or cryoablation), radiation, or liver-directed treatment (e.g., conventional or drug-eluting bead [DEB] transarterial chemoembolization [TACE], transarterial radioembolization [TARE]). Systemic treatment (e.g., sorafenib, lenvatinib, atezolizumab + bevacizumab, regorafenib, cabozantinib, ramucirumab, nivolumab, pembrolizumab) is recommended by guidelines for patients with advanced HCC disease (i.e., metastatic disease or when patients experience extensive liver tumor burden) who have preserved liver function (i.e., Child-Pugh A) and good performance status (i.e., Eastern Cooperative Oncology Group [ECOG] 1–2) [9–11].
Approximately half of HCC patients in the USA do not undergo any treatment; factors commonly correlated with not receiving treatment include older age, African-American race, and being enrolled in Medicaid or having no insurance [12]. Left untreated, the median survival is 13.4 months for early stage patients, 9.5 months for intermediate stage patients, 3.4 months for advanced stage patients and 1.6 months for end stage patients [13]. Survival is associated with both clinical (e.g., tumor size and extent, evidence of metastasis, patient age, surgical treatment) and nonclinical (e.g., income, education) factors [14].
Since liver cancer incidence and mortality are rising, and HCC accounts for the majority of all liver cancers, we sought to gain a comprehensive understanding of the epidemiological, economic and humanistic burden among patients with any stage of HCC in the USA who are not eligible for liver transplant or resection.
Methods
Methods of the literature review were specified in advance and documented in an internal study protocol. The systematic literature review (SLR) was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15].
Search strategy
A systematic search was conducted to identify all available literature investigating the epidemiological, humanistic and economic burden associated with HCC patients in the USA (Supplementary Table 1). The population of interest was persons of any age in the USA with confirmed HCC; studies only describing patients with liver transplant or resection were excluded. Studies describing patient characteristics, epidemiology, or economic burden required national sampling for inclusion. Included citations for all outcomes were limited to observational studies (e.g., cross-sectional/surveys, retrospective or prospective cohort, case–control) and economic modeling studies, with SLRs/meta-analyses reviewed for study identification purposes only. The search was conducted in the MEDLINE® (1946-present) and Embase® (1947-present) databases for articles and conference abstracts, respectively, written in the English language and published between 1 January 2008 and 15 December 2018 (Supplementary Table 2). The publication limit of 2008 was intended to ensure that the treatment landscape was relatively consistent throughout the time periods in which data were collected in the included studies. The search for epidemiological studies was limited by an observational study design filter and a publication date after 1 January 2013, to ensure the more recent and relevant information was captured. Additional references were identified by searching the bibliographies of SLRs. Targeted keyword searches were performed on 5 August 2019, to identify citations published since the systematic search (15 December 2018) to ensure the most up-to-date information was captured. No study authors were contacted to identify additional studies or to confirm data.
Study selection & data extraction
Results of all searches were compiled into a common Excel database. Study inclusion and exclusion at each screening step were based on pre-defined criteria. Eligible records were screened first at the title and abstract level and then as full-texts by one reviewer, and quality control of records was performed by a second, senior reviewer. Conference abstracts with data that were subsequently published as manuscripts were excluded from further review as ‘duplicates.’ An Excel data extraction template was developed and piloted to ensure usability. The extraction sheet was then populated to capture study characteristics, study populations, HCC patient characteristics, epidemiology, humanistic burden, and economic burden data from each study (Supplementary Table 3). Full text review and extraction were performed by two independent reviewers, and any discrepancies were reconciled. A third independent reviewer was consulted as necessary and adjudicated where consensus could not be reached.
Outcomes evaluated
The main outcomes of interest were HCC patient characteristics, epidemiology, humanistic burden and economic burden. Comparable data points from each study were presented alone or as ranges; no summary measures combining studies were calculated. Epidemiology outcomes included incidence and prevalence. Patient-related characteristics included sex, ethnicity, age, comorbidities, insurance/payer, disease stage at diagnosis and survival. Humanistic outcomes included health-related quality of life (measured using generic or disease-specific instruments), symptom burden and treatment burden. Economic outcomes included direct costs (medical and nonmedical), indirect costs, inpatient length of stay (LOS), specialist visits and treatments, as well as a brief survey of economic modeling study inputs and results.
Risk of bias
Risk of bias was assessed at the outcome level by determining whether each study population represented a special, ‘selected’ cohort (i.e., a cohort selected based on receiving a specific intervention, having an underlying etiology, or having other special characteristics) or a ‘nonselected’ cohort. Within each outcome of interest, results are reported from citations describing nonselected cohorts and by payer when possible. No assessment of risk of bias affecting cumulative evidence was performed.
Results
Patient/study selection
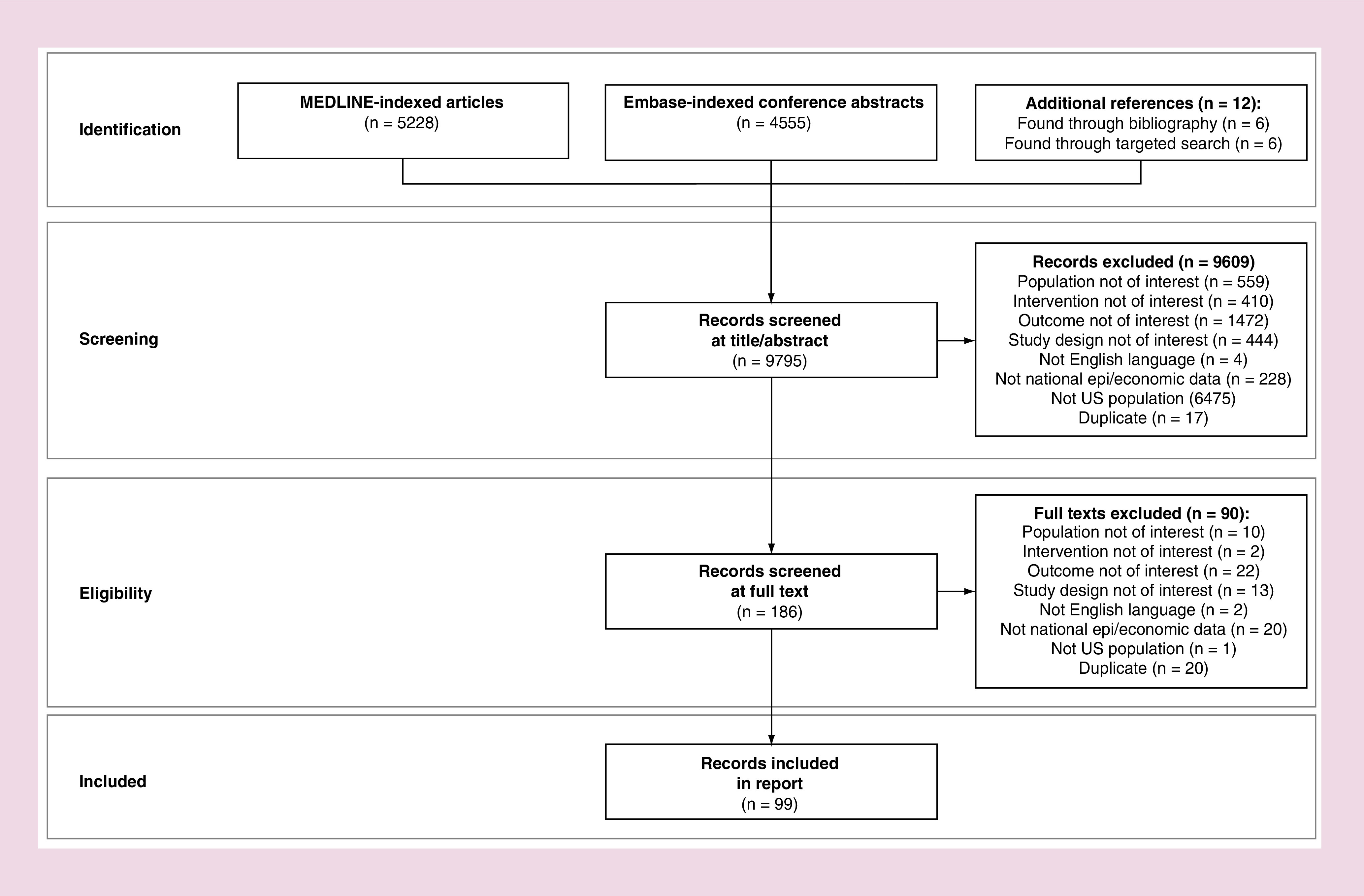
The searches identified 5228 journal articles from MEDLINE and 4555 conference abstracts from Embase for a total of 9795 citations for title and abstract review. From 9795 total citations screened, 99 citations were included (Figure 1).
Figure 1. . PRISMA flow diagram.
Epidemiology
Incidence
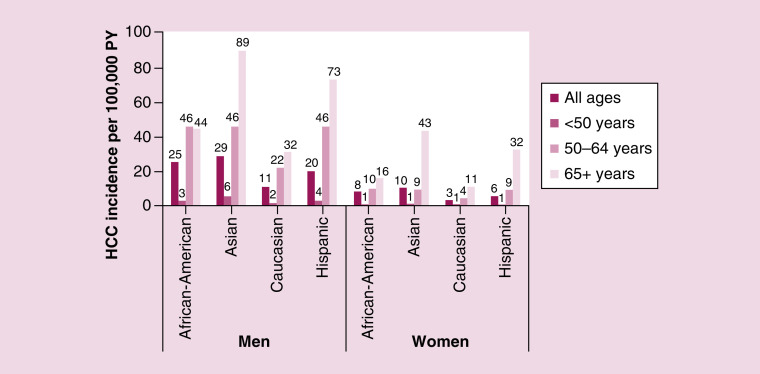
A total of nine studies presented incidence among nonselected cohorts (i.e., SEER and US Cancer Statistics registry, Medicare, and Veterans Affairs) [16–24], of which five studies presented incidence for individual years (Figure 2) [16,19,21,23,24]. Using the most recent data available (year 2015), the incidence of HCC in the USA among all payers was 9.5 per 100,000 person-years (PY) [19]. Based on the national population of 329 million people in 2019, an incidence of 9.5 per 100,000 PY equated to 31,255 annual HCC cases. The most recent incidence was higher in Medicare and Veterans Affairs patients (22.3 and 45 per 100,000 PY, respectively) [16,21]. In one study using data from SEER, incidence varied among groups by sex, race and age (Figure 3): across all racial groups, the incidence among men was approximately three-times higher than the incidence among women; by race, the incidence among Asians (18.6 per 100,000 PY), African-Americans (15.0 per 100,000 PY) and Hispanics (11.8 per 100,000 PY) was higher than Caucasians (7.0 per 100,000 PY); and persons aged 50 years and older typically had higher incidence than younger patients [24].
Figure 2. . Incidence.
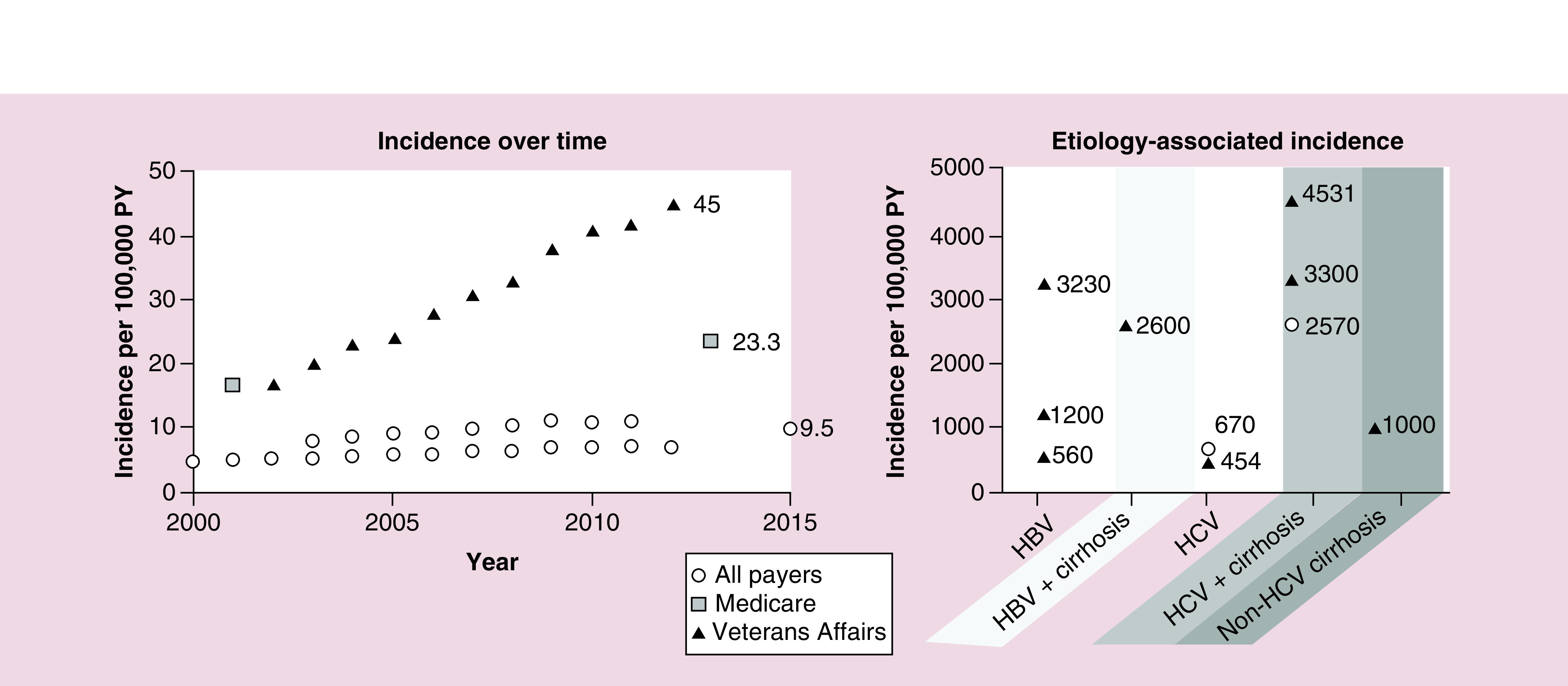
Each symbol represents an estimate from a given study.
HBV: Hepatitis B virus; HCV: Hepatitis C virus; PY: Person-years.
(Incidence over time) All payers sources: Ha, 2016 (SEER) [24]; Rich, 2019 (SEER) [19]; and White, 2017 (US Cancer Statistics registry) [23]; Medicare source: Shiels, 2019 [21]; Veterans Affairs source: Beste, 2015 [16]. (Etiology-associated incidence) Choi, 2015 [25]; El-Serag, 2016 [26]; Ioannou, 2018 [27]; Li, 2018 [28]; Mittal, 2014 [29]; Park, 2019 [30]; Su, 2018 [31].
Figure 3. . Incidence by patient demographics.
Data from SEER, 2003–2011; n = 50,723 patients with HCC included. Bars represent an annual estimate for 2003–2011.
HCC: Hepatocellular carcinoma; PY: Person-year.
Ha, 2016 [24].
Incidence was reported in 11 studies among etiology-related cohorts [25–35], of which 6 reported incidence irrespective of treatment with direct-acting antivirals (Figure 2) [25–30]. Estimates varied by study; however, the incidence of HCC in patients with HCV, HBV, or cirrhosis was approximately 100-times higher than incidence in the general population. Although HCC incidence among cohorts with heavy alcohol use was not found, 2 studies demonstrated an 87–92% increased risk of HCC with heavy alcohol consumption [36,37].
Prevalence
Six studies reported prevalence of HCC among nonselected cohorts [16,18,20,24,33,36]. In the most recent and generalizable study cohort, the prevalence of HCC in 2003–2011 was 0.0096%, with higher prevalence among Asian (0.0164%) populations than African-American (0.0109%), Caucasian (0.0082%) and Hispanic (0.0098%) populations [24]. The study authors did not have additional information on underlying disease etiologies and other risk factors and were unable to definitively state reasons for these racial discrepancies in prevalence. Furthermore, the similarity in incidence and prevalence rates was not explained by study authors, who noted that prevalence rates increased more from 2003 to 2011 than incidence rates, possibly due to improved treatment of underlying etiologies and better screening and surveillance programs. Prevalence of HCC was reported in 20 studies among etiology-related cohorts [16,26–33,38–49]. The prevalence of HCC among patients with HCV, HBV, NAFLD/NASH, cirrhosis or chronic liver disease was 1–10%.
Patient characteristics
Demographics & etiology
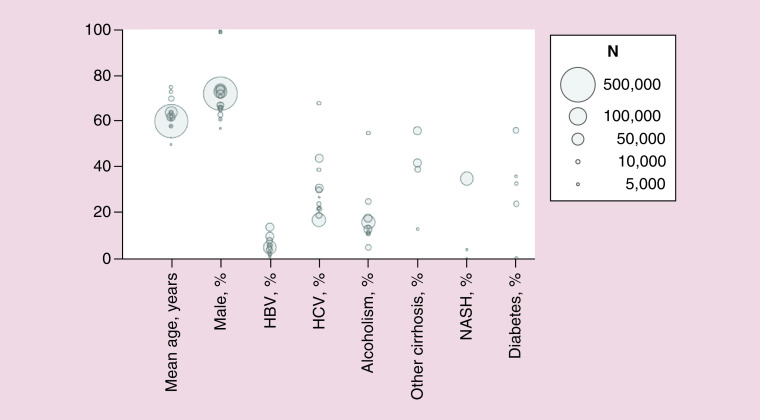
A total of 41 studies with national sampling presented demographic and etiology information [16,17,20,21,25,26,29,32,35,39,46,50–79], of which 21 studies presented data for nonselected cohorts (Figure 4) [16,17,20,21,33,50,54,55,58–60,62,65,66,68–70,72,74,75,77,79]. Reported values from the largest study including both inpatients and outpatients (n = 50,723) [77], with ranges provided from other studies, described patients with HCC as mean age 64 (50–75) years and 74% (57–100%) male (57–74% male if excluding studies from Veterans Affairs). Prevalence of HCC etiologies varied widely between cohorts, with ranges of 1–14% with HBV, 17–68% with HCV, 5–55% with alcoholism/alcoholic cirrhosis, 13–56% with other cirrhosis, 0–35% with NASH and 0–56% with diabetes (Figure 4).
Figure 4. . Patient characteristics.
HBV: Hepatitis B virus; HCV: Hepatitis C virus; NASH: Nonalcoholic steatohepatitis.
Aggarwal, 2018 [50]; Beste, 2015 [16]; Campbell, 2016 [54]; Cauble, 2013 [55]; Ha, 2016 [24]; Hester, 2019 [17]; Hyder, 2013 [58]; Jinjuvadia, 2017 [59]; Kaplan, 2018 [60]; Kasmari, 2017 [62]; Menzin, 2011 [65]; Mishra, 2013 [66]; Poklepovic, 2010 [68]; Sanoff, 2017 [69]; Sanyal, 2010 [20]; Serper, 2017 [79]; Shaya, 2014 [70]; Shiels, 2019 [21]; Sobotka, 2019 [72]; Tsong, 2010 [74]; Tsong, 2010 [75].
Each bubble represents a figure from a given study. Bubble size indicates total study sample size. Note that the circles indicating 99% male populations represent 3 studies using data from Veterans Affairs [16,61,79].
Payers
Cohorts from inpatient databases were used to identify the prevalence of different payers among patients with HCC, because all other data sources providing this information were based on databases from a single payer (e.g., SEER-Medicare, Veterans Affairs). Therefore, it is important to caveat that the only data available that described the proportions of payers for patients with HCC relates to those who were admitted as inpatients. A total of four studies presented payer data for inpatients (Figure 5) [55,59,66,72]. Among inpatients, payers included Medicare (37–45%), commercial insurance (27–33%), Medicaid (16–19%) and self-pay/other (8–12%) [55,59,66,72].
Figure 5. . Payers among inpatients with hepatocellular carcinoma. .
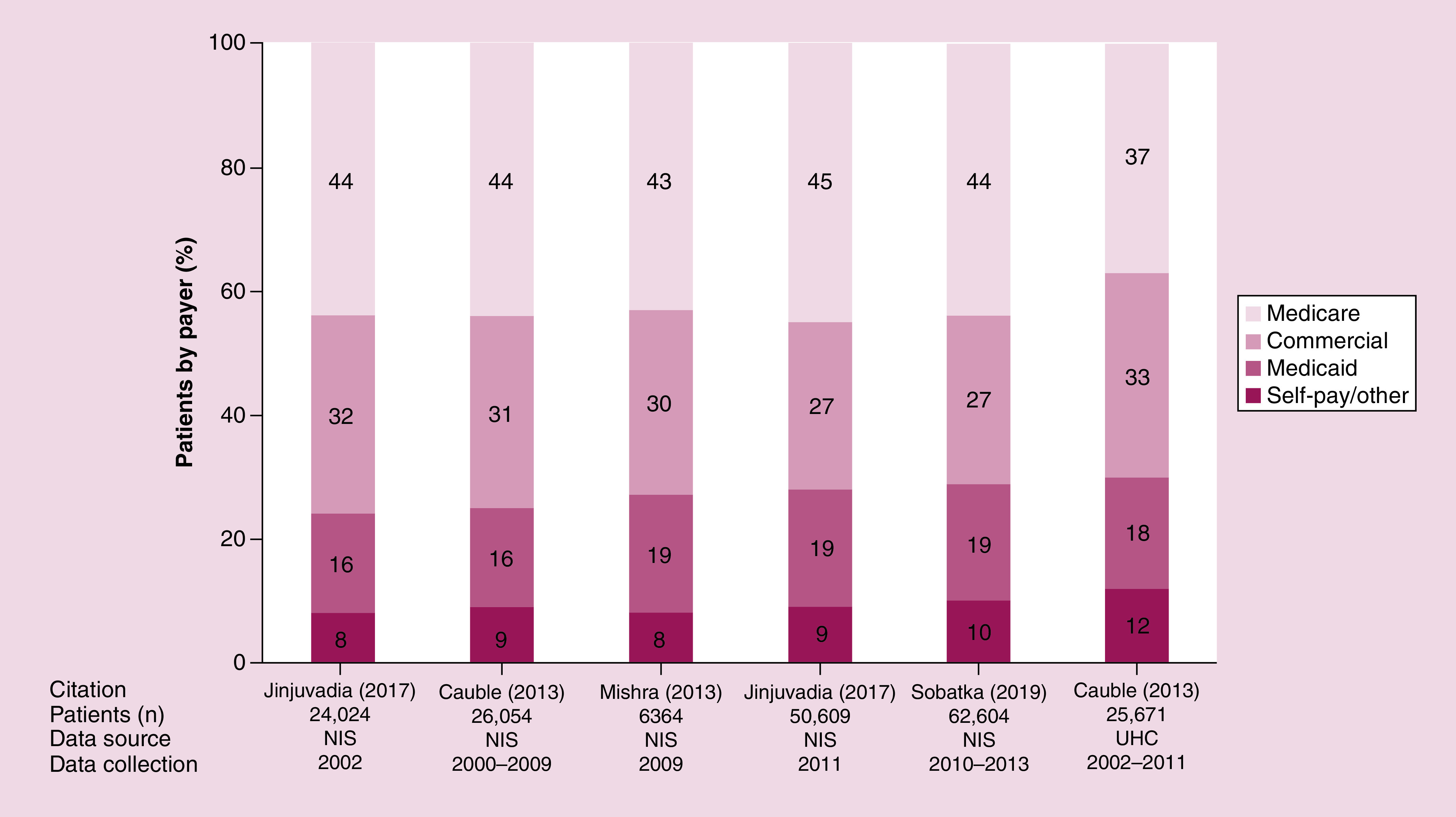
Each stacked bar represents a cohort from a given year or timeframe, as indicated.
HCC: Hepatocellular carcinoma; NIS: National Inpatient Sample; UHC: University Health Consortium.
Sources: Cauble, 2013 [55]; Jinjuvadia, 2017 [59]; Mishra, 2013 [66]; Sobotka, 2019 [72].
Disease stage
A total of ten studies described disease staging at diagnosis [53,56,60,64,65,69,70,77–79], of which six presented data for nonselected cohorts (Figure 6) [60,65,69,70,77,79]. Based on staging criteria from American Joint Committee on Cancer (AJCC) or Barcelona Clinic Liver Cancer (BCLC), approximately two-thirds of patients had early or intermediate stage disease at diagnosis, while a third had advanced stage disease [60,65,69,70,77,79].
Figure 6. . Disease stage.
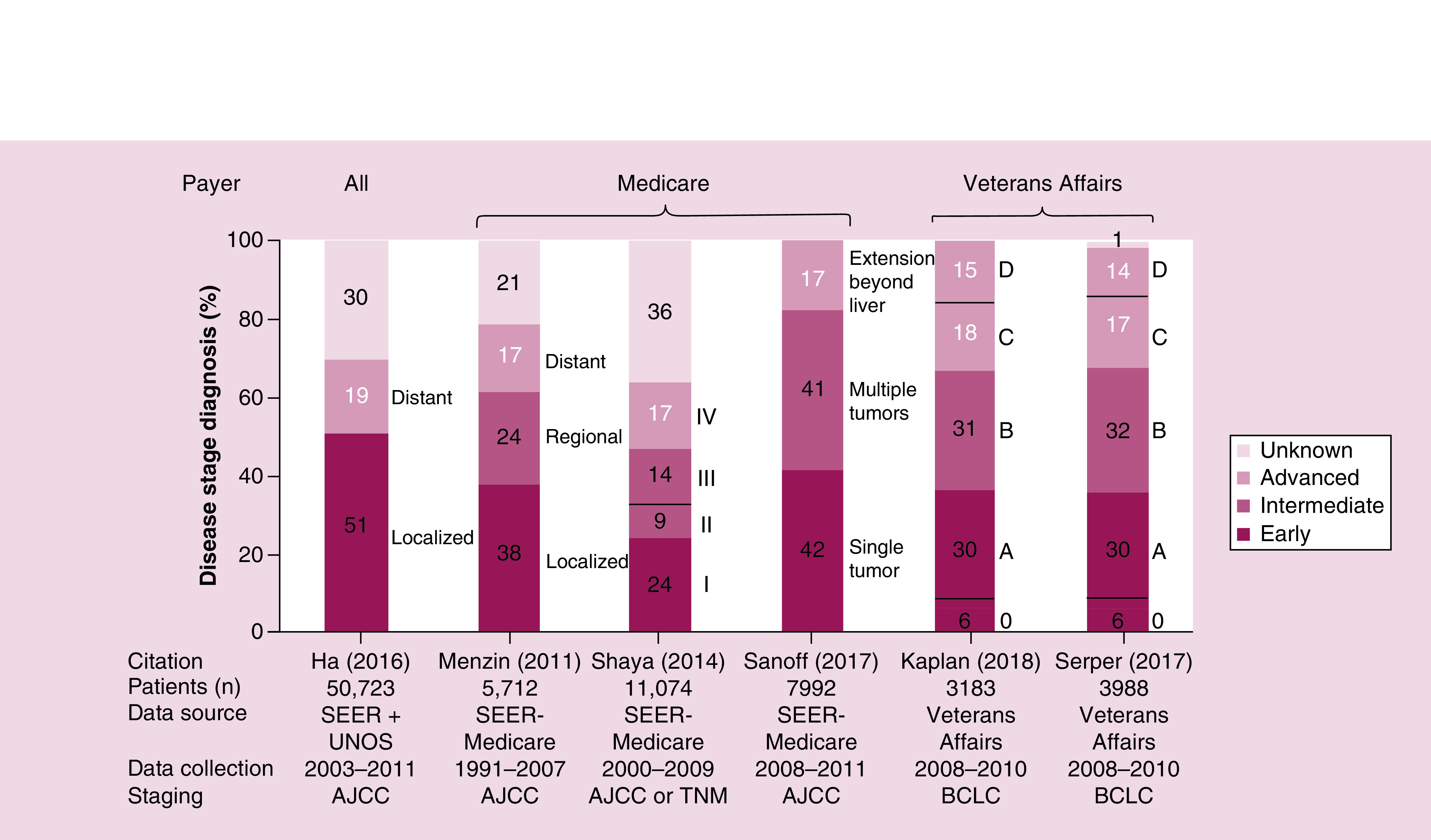
Each bar represents the staging information within a given study cohort. Text adjacent to each bar notes the disease stage as described by study authors.
AJCC: American Joint Committee on Cancer; BCLC: Barcelona Clinic Liver Cancer; SEER: Surveillance, Epidemiology, and End Results; UNOS: United Network on Organ Sharing.
Sources: Ha, 2016 [77]; Kaplan, 2018 [60]; Menzin, 2011 [65]; Sanoff, 2017 [69]; Serper, 2017 [79]; Shaya, 2014 [70].
Survival
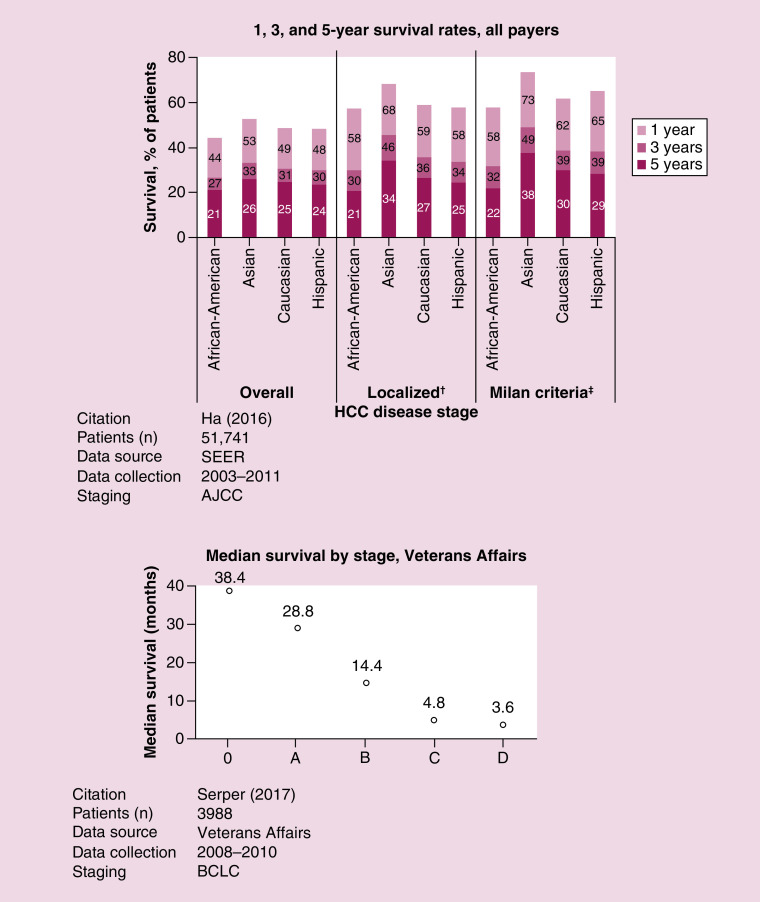
Fifteen studies reported survival (mean, median or proportion at timepoint) among patients with HCC [17,52,53,56,60,64,65,69,70,73,77,79–82]. Several of these studies described survival in a selected population, such as selection based on intervention (e.g., rounds of TACE; rounds of systemic treatment) or patient characteristics (e.g., inpatient vs outpatient; underlying HBV vs HCV). The largest study describing overall survival at specific timepoints in a nonselected population relied upon data from SEER [24]. The authors found that overall survival ranged 44–53% at 1 year, 27–33% at 3 years and 21–26% at 5 years among African-American, Asian, Caucasian and Hispanic patients with HCC; longer survival at 1 year was observed in the subset of patients with localized disease (i.e., tumor confined to 1 liver lobe) or disease within Milan Criteria [83] (i.e., a single lesion measuring <5 cm, or no more than 3 lesions measuring <3 cm each), which is typically used to determine eligibility for liver transplant (Figure 7). Although disparities were observed in survival data based only on race, the authors reported that multivariate analyses that adjusted for age, sex, race, disease stage, year of diagnosis and treatment received determined that Asian and African-American patients had higher 5-year survival rates than Caucasian patients [24].
Figure 7. . Survival.
†“Localized” refers to tumors involving a single lobe of the liver.
‡Milan Criteria refers to a single lesion measuring <5 cm, or no more than 3 lesions measuring <3 cm each [83].
AJCC: American Joint Committee on Cancer; BCLC: Barcelona Clinic Liver Cancer; SEER: Surveillance, Epidemiology and End Results.
Only 1 citation was identified that provided median overall survival based on BCLC disease stage at diagnosis (Figure 7) [79]. The authors reported that the survival of Veterans Affairs patients was 3.2 years for those diagnosed in BCLC 0, 2.4 years for BCLC A, 1.2 years for BCLC B, 0.4 years for BCLC C and 0.3 years for BCLC D [79].
Humanistic burden
A total of 15 studies described humanistic burden among patients with HCC (Supplementary Table 4) [84–98]. These studies tended to be small and performed in one to two centers. The most commonly used instruments were the Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) and 36-Item Short Form Survey (SF-36).
Symptom burden
Seven studies assessed symptom burden on patients with HCC or their caregivers [84–89,91]. The major symptom assessed was pain, although the evidence regarding pain prevalence varied across studies. Patients with HCC at end of life experienced lack of energy, pain, feeling drowsy, difficulty sleeping, problems with sexual interest, itching, lack of appetite, difficulty concentrating, worrying and feeling irritable [86]. Diarrhoea, fatigue, skin toxicities and appetite loss had the most impact on patients' quality of life [89].
Quality of life & survival
Two studies assessed the association between quality of life and survival in patients with HCC [90,93]. One found that overall health-related quality of life and some subscales on the FACT-Hep were significantly associated with survival. The second study found significant correlations between advanced BCLC stage and reduced global quality of life and individual components of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30).
Treatment on quality of life
Six studies assessed the impact of HCC treatment on quality of life [92,94–98]. Quality of life was higher at baseline than after 3 months of treatment. In one study describing a comparison of patients receiving TARE versus patients receiving TACE, TARE patients had better quality of life than TACE patients 4 weeks after treatment [92]. Other studies not comparing different locoregional modalities found mixed results of TARE, TACE and DEB-TACE on quality of life [94–98]. No studies were identified describing the impact of systemic treatment on quality of life.
Economic burden
Direct costs or charges per admission
A total of six studies reported admission-related payer costs [63,75,76,99–101]; however, all of these were in selected populations, including patients with malnutrition [100], patients born 1945–1965 [99], patients who underwent radiofrequency ablation [101], patients who received palliative care [63], or patients with HCC combined with patients with hepatobiliary diseases [75]. Admission-related hospital charges were described in five publications [41,59,66,67,102]; in the most recent data in a nonselected population (year 2011), mean charges per admission were US$59,686 [59].
Direct costs per month
A total of seven studies reported mean per month direct medical costs [52,57,65,68,71,74,103]; two of these studies described only patients receiving systemic treatment [52,57] and one compared costs among patients with HCV and cirrhosis, HCC or transplant [103]. The remaining four studies described monthly costs among nonselected cohorts (Supplementary Table 5) [65,68,71,74]. These studies described the difference in monthly payer costs before and after diagnosis [74], compared HCC and non-HCC cohorts [68], described costs based on treatment used [71], and described costs based on disease stage [65].
Long-term direct costs or charges
A total of 16 studies reported long-term direct medical costs [17,39,43,44,46,49,50,53,56,57,60,61,70,71,81,104]; three of these studies described charges [17,56,104], four described costs related to specific interventions [53,57,61,81] and five described costs among specific etiologies [39,43,44,46,49]. The remaining four studies described long-term costs among nonselected cohorts (Supplementary Table 6) [50,60,70,71]. One study described cumulative payer costs for patients with HCC that ranged US$87,556 for best supportive care to US$208,595 for liver-directed treatment + sorafenib (sorafenib alone, US$79,212; liver-directed treatment alone, US$203,502) [71]. A second study described inpatient costs of US$71,937, with different costs based on age and payer [50]. A third study described costs based on disease stage, with lowest costs for no treatment and highest cost for liver transplant [70]. Finally, a fourth study described 3-year Veterans Affairs payer costs of US$154,688 in cirrhosis and HCC versus US$69,010 in noncancer patients with cirrhosis; 3-year payer costs based on BCLC stage were the following: 0, US$221,046; A, US$193,859; B, US$149,177; C, US$96,838; D, 137,771 [60].
Indirect costs
One study provided costs due to sick leave, workers compensation and disability among employees with HCV and no cirrhosis, cirrhosis, HCC, liver transplant or non-HCV in the 6-month period following the most severe claim (Figure 8) [39]. The costs of work loss and numbers of days missed over a 6-month period were the following: no HCV/no HCC, US$652 (3.4 days); noncirrhotic HCV with no HCC, US$1003 (5.1 days); compensated cirrhosis HCV with no HCC, US$732 (3.1 days); decompensated cirrhosis HCV with no HCC, US$1354 (9.3 days); any HCV who have HCC, US$3594 (22.1 days); liver transplant, US$2800 (31.3 days) [39].
Figure 8. . Indirect costs.
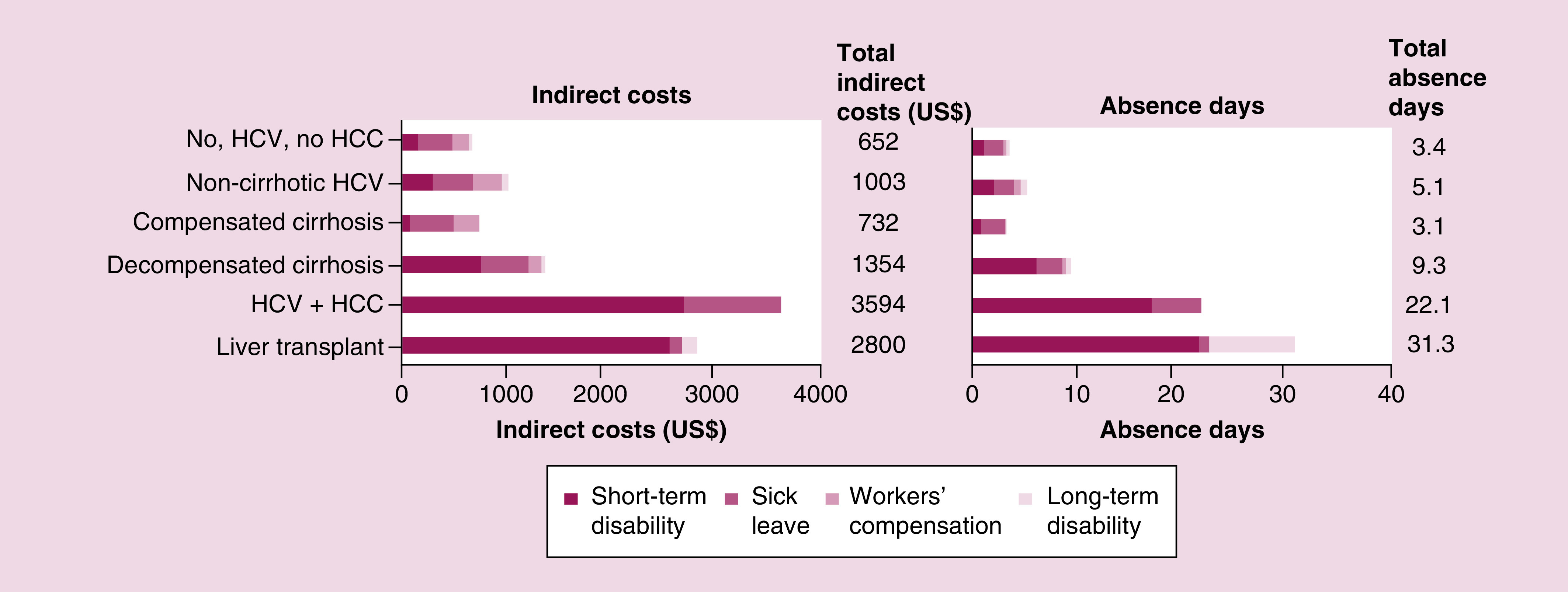
Data source: Human Capital Management Services integrated databases; 2001–2013.
Patients with HCV, n = 1,386; non-HCV patients, n = 727,588.
Each stacked bar represents data from one patient group, as indicated.
HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus.
Source: Baran, 2015 [39].
Healthcare resource use
A total of 13 studies reported inpatient length of stay [17,41,50,57,59,63,66,67,75,76,99,100,102], of which four studies described nonselected cohorts and reported trends over time [59,66], by etiology [102], and by age or payer [50]. The typical inpatient length of stay was approximately 6 days.
A total of six studies reported specialist visits [17,52,56,58,79,105]; four of these studies described nonselected cohorts (Supplementary Table 7) [17,58,79,105]. Nearly all patients in the Veterans Affairs cohort visited a specialist within 30 days of diagnosis; specialist visit rates were lower in the two Medicare cohorts, which suggests that the likelihood of visiting a specialist may be related to the payer involved.
A total of 15 studies reported treatments and procedures [20,52,53,55,57,64,69–72,77,79,106,107]. Of these, four studies described subpopulations based on intervention (i.e., systemic treatment [52,57] or liver-directed treatment [53,64]), two described likelihood of treatment (but not specific treatments received) based on insurance status [106] and race [107], and one did not report proportions receiving potentially curative treatments [71]. The remaining seven studies described nonselected populations (Figure 9) [20,55,69,70,72,77,79]. Data collection dates and treatment setting must be considered in interpreting these results, since some treatments (i.e., sorafenib) were not available within some studies' data collection dates, and treatments requiring hospital admission (i.e., transplant or resection) may be overrepresented in the inpatient study data. Across all studies, most patients did not undergo liver transplant or resection, and a large proportion of patients did not undergo any locoregional or systemic treatment [20,55,69,70,72,77,79]. Notably, in the three studies providing treatment based on disease stage, a substantial proportion did not receive treatment in even the earliest disease stages (patients not receiving treatment: 52–67% of patients within Milan criteria [range based on race] vs 77% overall [77], 43% of patients in AJCC stage I vs 68% overall [70], and 14% of patients in BCLC 0 vs 25% overall [79]).
Figure 9. . Treatment.
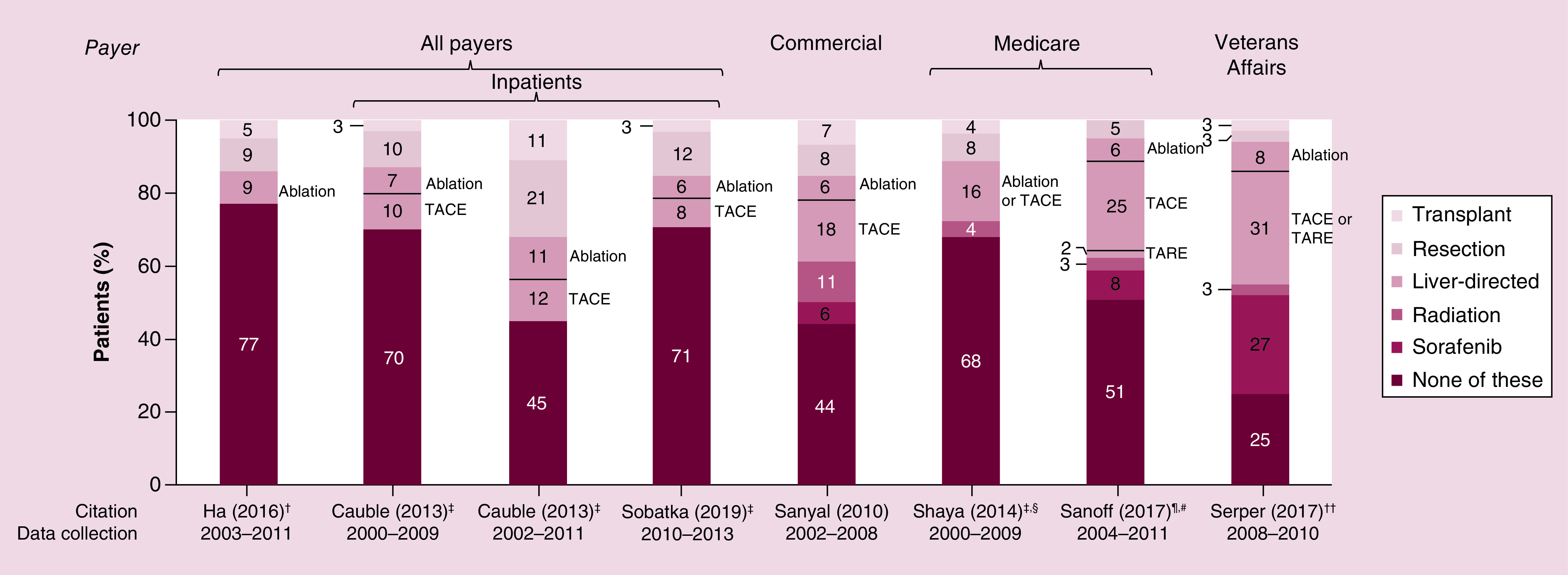
Each bar represents treatment utilization within a given study cohort. Text adjacent to each bar notes the specific liver-direct treatment used, as described by study authors.
†Treatment categories considered in analysis were transplant, resection, ablation, or none.
‡Most invasive treatment listed if patient underwent multiple procedures.
§Sorafenib was not considered in analysis.
¶Initial treatment.
#Data from subset of patients in which sorafenib and radiation were separated were used for this figure.
††Treatments not mutually exclusive.
NIS: National Inpatient Sample; SEER: Surveillance, Epidemiology and End Results; TACE: Transarterial chemoembolization; TARE: Transarterial radioembolization; UHC: University Health Consortium; UNOS: United Network on Organ Sharing.
Sources: Cauble, 2013 [55]; Ha, 2016 [77]; Sanoff, 2017 [69]; Sanyal, 2010 [20]; Serper, 2017 [79]; Shaya, 2014 [70]; Sobotka, 2019 [72].
Economic models
Eleven studies described economic models for various aspects of HCC (Supplementary Table 8) [60,70,78,108–115]. Two of these studies are described in the sections above [60,70]. Four involved a comparison against TACE [70,110,113,114], 4 involved a comparison against a systemic treatment (sorafenib, 2; cabozantinib, 1; regorafenib, 1) [78,108,111,115], 1 involved stereotactic body radiation therapy versus radiofrequency ablation [112], 1 involved independent factors affecting cost and survival [60] and 1 was treatment-agnostic and evaluated the cost to society [109].
Discussion
This review provides a comprehensive understanding of the epidemiology, patient characteristics, and economic burden (including healthcare resource use) of HCC in the USA. The data presented here indicate that many patients with HCC are diagnosed in intermediate or advanced stage disease; as such, it may not be surprising that few patients are eligible for liver transplant or resection. However, many patients also do not receive locoregional or systemic treatment. It is unclear from this research whether the high proportion of patients not receiving treatment is due to patient choice, ineligibility based on disease stage, or other factors (e.g., healthcare access, cost). It is unlikely that disease stage accounts entirely for the lack of treatment, as a substantial proportion of patients did not receive treatment in the earliest disease stages. All treatments for HCC, including supportive care, are associated with a substantial economic burden to payers and patients. Limited information suggests that patients with HCC experience a significant quality of life burden; however, little information was found comparing different locoregional modalities, and no information was found regarding quality of life or preferences associated with systemic treatment. In addition to the quality of life and economic burden associated with HCC, patients experience poor clinical outcomes, with approximately three-quarters of patients with HCC dying within 5 years of diagnosis.
This study provides a broad perspective on the epidemiology of HCC in the USA in recent years, but changing population demographics are anticipated to affect HCC incidence generally and within demographic subgroups in the near future. Using existing HCC incidence data from SEER and projected population data from the US Census Bureau, a study published in 2016 forecasted that HCC incidence will be 21.1 per 100,000 PY in 2030, but incidence will increase more quickly in men versus women and in Hispanic and Caucasian populations versus African-American and Asian populations due to shifts in projected populations in the USA [7]. This study also forecasted that HCC patients (both men and women) diagnosed in 2020–2030 will be older on average than patients diagnosed 2000–2013 [7]. However, this study does not take changes in prevalence of HCC risk factors into account, such as hepatitis infection, NASH, and NAFLD, and possible prevention of subsequent HCC. Both HBV and HCV prevalence may diminish in coming years due to HBV vaccination [116] and increased use of direct-acting antiviral therapies in HCV [117]. Conversely, prevalence of NASH and NAFLD in the USA are expected to rise 44 and 8%, respectively, by 2030 [118].
This study also describes the costs and healthcare resource use associated with HCC in recent years; these costs will likely rise in the near future. The US Centers for Medicare and Medicaid Services estimated that healthcare spending will grow 5.4% per year from 2019 to 2028 [119]. This projected increase in healthcare spending will likely be reflected in increased costs per HCC patient, while the anticipated rise in HCC incidence by 2030 will certainly drive total healthcare-related societal costs (currently estimated at US$405 million annually [109]) higher in the coming years.
The prevalence of HCC-related risk factors varies substantially globally; the average cohort of patients with HCC in the USA likely has a different risk factor profile than patients with HCC in other parts of the world. For example, cases attributable to HBV, smoking and obesity vary substantially across high-income regions globally [120]. The payer and healthcare system in the USA is different than other countries with high HCC incidence. Therefore, this study's emphasis on patients from USA provides a unique perspective, but further analyses and comparisons with global data are warranted [121–123].
Our review has some limitations. First, relative to the number of studies describing epidemiology, patient characteristics and economic burden, few studies describing humanistic burden were identified. More humanistic burden information may be available from randomized controlled trials; however, the scope of this review included only observational studies to capture a real-world understanding of patients with HCC. Second, this review does not fully capture emerging additions in the treatment landscape for advanced HCC. In addition to sorafenib, some options recently added to NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) [9] for the first-line setting include lenvatinib, nivolumab for patients ineligible for tyrosine kinase inhibitors or other anti-angiogenic agents, and atezolizumab + bevacizumab, which is undergoing review for an indication in HCC from the US Food and Drug Administration [124]. The changing treatment landscape in advanced HCC is likely to impact survival and costs, but real-world data describing systemic treatments other than sorafenib was not readily available for this review. Third, this review did not include citations describing epidemiology, patient characteristics, or economic burden based on data sources representing a single state/region. We also note that there are regional differences in the treatment pathways and clinical outcomes of HCC within the USA [125]. However, we believe that including studies requiring national sampling for these outcomes provides the most generalizable data.
Conclusion
The incidence and costs of HCC represent a major burden to patients, caregivers, and the healthcare system in the USA. Patients with intermediate or advanced HCC may elect to forgo treatment due to high costs and limited survival benefits of existing treatments. More efficacious, accessible treatment options are necessary to extend survival and improve patient quality of life.
Summary points.
Using the most recent data available, the incidence of hepatocellular carcinoma (HCC) in the USA among all payers was 9.5 per 100,000 person-years; the most recent incidence was higher in Medicare and Veterans Affairs patients.
The incidence of HCC in patients with HCV, HBV or cirrhosis was approximately 100-times higher than incidence of HCC in the overall population (i.e., populations not selected based on these etiologies).
HCC etiologies varied widely between studies, ranging 1–14% with HBV, 17–68% with HCV, 5–55% with alcoholism/alcoholic cirrhosis and 13–56% with other cirrhosis.
Using the most recent data available, payers of inpatients with HCC included Medicare (44%), commercial (27%), Medicaid (19%) and self-pay/other (10%).
Approximately two-thirds of patients had early or intermediate disease at diagnosis, while a third had advanced disease.
Patients with HCC at end of life experienced lack of energy, pain, feeling drowsy, difficulty sleeping, problems with sexual interest, itching, lack of appetite, difficulty concentrating, worrying and feeling irritable. Diarrhoea, fatigue, skin toxicities and appetite loss had the most impact on patients' quality of life.
Most patients with early stage HCC did not receive transplant or resection. A large proportion of patients with intermediate or advanced HCC did not undergo any locoregional or systemic treatment.
The incidence and costs of HCC represent a major burden to patients and to the healthcare system in the USA. Patients not eligible for transplant or resection may elect to forgo treatment due to high costs and limited survival benefits of existing therapies.
Supplementary Material
Acknowledgments
Manuscript editorial support was provided by Catherine Mirvis of Pharmerit - an OPEN Health Company.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/hep-2020-0024
Author contributions
A Aly, Y Doleh and F Benavente contributed to study conception, design and revisions to the manuscript; S Ronnebaum and D Patel contributed to study design, data analysis, drafting and revision of the manuscript.
Financial & competing interests disclosure
Funding for this study was provided by AstraZeneca. A Aly, Y Doleh and F Benavente are employees and stockholders of AstraZeneca. S Ronnebaum and D Patel are employees of Pharmerit - an OPEN Health Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Global Cancer Observatory (2018). https://gco.iarc.fr/today/home
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 69(1), 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Islami F, Goding Sauer A, Miller KD. et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 68(1), 31–54 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Ward EM, Sherman RL, Henley SJ. et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20–49 years. J. Natl Cancer Inst. 111(12), 1279–1297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 68(3), 526–549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrick JL, Kelly SP, Altekruse SF, Mcglynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J. Clin. Oncol. 34(15), 1787–1794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinna AD, Yang T, Mazzaferro V. et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann. Surg. 268(5), 868–875 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hepatobiliary Cancers V1.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed April 16, 2020. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way (2020). www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- 10.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69(1), 182–236 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Vogel A, Cervantes A, Chau I. et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29(Suppl. 4), iv238–iv255 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment. Pharmacol. Ther. 38(7), 703–712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalaf N, Ying J, Mittal S. et al. Natural history of untreated hepatocellular carcinoma in a US cohort and the role of cancer surveillance. Clin. Gastroenterol. Hepatol. 15(2), 273–281.e271 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Tian H, Cao S, Hu M. et al. Identification of predictive factors in hepatocellular carcinoma outcome: a longitudinal study. Oncol. Lett. 20(1), 765–773 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7), e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 149(6), 1471–1482.e1475 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Hester D, Golabi P, Paik J, Younossi I, Mishra A, Younossi ZM. Among Medicare patients with hepatocellular carcinoma, nonalcoholic fatty liver disease is the most common etiology and cause of mortality. J. Clin. Gastroenterol. 54(5), 459–467 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Simon TG, Tabung FK. et al. A prospective study of inflammatory diet potential and risk of hepatocellular carcinoma (HCC). Cancer Res. 78(13), 5256 (2018). [Google Scholar]
- 19.Rich NE, Yopp AC, Singal AG, Murphy CC. Hepatocellular carcinoma incidence Is decreasing among younger adults in the United States. Clin Gastroenterol Hepatol 18(1), 242–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports hepatocellular carcinoma (HCC) incidence within 10-year age groups by race/ethnicity and by sex from 1992–2015.
- 20.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 26(9), 2183–2191 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Shiels MS, Engels EA, Yanik EL, Mcglynn KA, Pfeiffer RM, O'brien TR. Incidence of hepatocellular carcinoma among older Americans attributable to hepatitis C and hepatitis B: 2001 through 2013. Cancer 125(15), 2621–2630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Lopez A, Liu B, Bhuket T, Wong RJ. Hepatocellular carcinoma (HCC) incidence in Hispanics overtakes Asians to become the ethnic group with the highest HCC incidence in the U.S.: an updated analysis of 2000–2014 Surveillance Epidemiology and End Results cancer registry. Hepatology 66, 744A–745A (2017). [Google Scholar]
- 23.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 152(4), 812–820.e815 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha J, Yan M, Aguilar M. et al. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 122(16), 2512–2523 (2016). [DOI] [PubMed] [Google Scholar]; •• Reports HCC incidence by race and sex, changes in incidence based on race/ethnicity and disease stage at diagnosis and survival based on race/ethnicity stratified by age groups and by disease stage at diagnosis.
- 25.Choi G, Serper M, Kaplan DE, Forde KA. Length of antiviral therapy and cirrhosis are key factors in the development of hepatocellular carcinoma among U.S. veterans with chronic hepatitis B. Hepatology 62, 271A (2015). [Google Scholar]
- 26.El-Serag HB, Kramer J, Duan Z, Kanwal F. Epidemiology and outcomes of hepatitis C infection in elderly US veterans. J. Viral Hepat. 23(9), 687–696 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Ioannou G, Green P, Lowy E, Mun E, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis: a cohort of 116,404 patients with cirrhosis including 10,042 who developed HCC. Hepatology 68, 168A–169A (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li DK, Ren Y, Fierer DS. et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: an ERCHIVES study. Hepatology 67(6), 2244–2253 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Mittal S, Kramer JR, Richardson P, El-Serag H, Kanwal F. Effect of race on the risk of hepatocellular cancer in U.S. veterans with chronic hepatitis B virus infection. Hepatology 60, 972A (2014). [Google Scholar]
- 30.Park H, Wang W, Henry L, Nelson DR. Impact of all-oral direct-acting antivirals on clinical and economic outcomes in patients with chronic hepatitis C in the United States. Hepatology 69(3), 1032–1045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su F, Green P, Berry K, Ioannou G. Estimating HCC risk in patients with chronic hepatitis B infection on antiviral therapy: a prediction model derived from a Veterans Affairs cohort. Hepatology 68, 1186A–1187A (2018). [Google Scholar]
- 32.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis C virus infection. Hepatology 64(1), 130–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer JR, Lin M, Feng H. et al. Absolute risk of hepatocellular carcinoma in a large, geographically and ethnically diverse cohort of patients with nonalcoholic fatty liver disease. Gastroenterology 152(5), S683 (2017). [Google Scholar]
- 34.Mun E, Green P, Berry K, Ioannou G. No difference between direct-acting antivirals for hepatitis C in hepatocellular carcinoma risk. Hepatology 68, 389A (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Lo Re V, Iii, Xiao H, Brown J, Yi G, Park H. Incidence of hepatocellular carcinoma and extrahepatic cancers among HCV-infected patients in the era of direct-acting antivirals. Value Health 21, S12–S13 (2018). [Google Scholar]
- 36.Persson EC, Schwartz LM, Park Y. et al. Alcohol consumption, folate intake, hepatocellular carcinoma, and liver disease mortality. Cancer Epidemiol. Biomarkers Prev. 22(3), 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrick JL, Campbell PT, Koshiol J. et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Brit. J. Cancer 118(7), 1005–1012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asfari MM, Rocio L, Zein NN, Mccullough AJ. The association of nonalcoholic steatohepatitis and hepatocellular carcinoma. Hepatology 66, 749A–750A (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baran RW, Samp JC, Walker DR. et al. Costs and absence of HCV-infected employees by disease stage. J. Med. Econ. 18(9), 691–703 (2015). [DOI] [PubMed] [Google Scholar]; • Describes medical, prescription and disability costs and absence days among employees with chronic hepatitis C, including those with HCC.
- 40.Butt AA, Yan P, Bonilla H. et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology 62(2), 365–374 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Devani K, Charilaou P, John F, Patel P, Young M, Reddy C. Hepatocellular carcinoma amongst cirrhosis: trends in hospitalization and inpatient mortality in United States. Hepatology 63(Suppl. 1), 712A–712A (2016).26646162 [Google Scholar]
- 42.Fukui N, Golabi P, Otgonsuren M, Mishra A, Venkatesan C, Younossi ZM. Demographics, resource utilization, and outcomes of elderly patients with chronic liver disease receiving hospice care in the United States. Am. J. Gastroenterol. 112(11), 1700–1708 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Gordon SC, Fraysse J, Li S, Wong RJ. Increasing healthcare resource utilization (HCRU) and costs associated with advanced liver disease - a multivariate analyses of real-world Medicare nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) patients. Hepatology 68, 573A (2018). [Google Scholar]
- 44.Harrison SA, Kachru N, Parker E, Korrer S, Loomba R. Longitudinal analyses of comorbidities and healthcare costs among nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) patients with advanced liver disease. Hepatology 68, 572A–573A (2018). [Google Scholar]
- 45.Matsuda T, Mccombs J, Lee MH. et al. External validation of the risk-prediction model for hepatocellular carcinoma HCC from the REVEAL-HCV study using data from the U.S. Veterans Affairs (VA) health system. J. Hepatol. 60(1), S16 (2014). [Google Scholar]
- 46.Mcadam-Marx C, Mcgarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J. Manag. Care Pharm. 17(7), 531–546 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scaife J, Kuti E, Acampa L. et al. Prevalence of hepatic outcomes among Medicare beneficiaries diagnosed with chronic hepatitis C virus. Value Health 16(3), A82 (2013). [Google Scholar]
- 48.Waghray A, Menon KVN. Preventive surveillance in patients that develop hepatocellular carcinoma: how are we doing? Gastroenterology 148(4), S-967 (2015). [Google Scholar]
- 49.Wong RJ, Kachru N, Meyer N, Gordon SC. Rising and higher healthcare resource utilization (HCRU) and costs of nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) patients with advanced liver disease of increasing severity – results of a US real-world analysis. Hepatology 68, 1283A–1284A (2018). [Google Scholar]
- 50.Aggarwal S, Kumar S, Topaloglu O. Trends in hospitalization length of stay and costs in patients with hepatocellular carcinoma (HCC): analysis of US national in-patient data for 2015. Value Health 21, S34 (2018). [Google Scholar]
- 51.Aslinia F, Tilton NA, Howell CD. Gender disparity and risk factors for hepatocellular carcinoma in hospitalized patients in the United States in 2006. Gastroenterology 138(5), S840 (2010). [Google Scholar]
- 52.Bonafede MM, Korytowsky B, Singh P. et al. Treatment patterns and economic burden by lines of therapy among patients with advanced hepatocellular carcinoma treated with systemic cancer therapy. J. Gastrointest. Cancer 51(1), 217–226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breunig IM, Shaya FT, Hanna N, Seal B, Chirikov VV, Daniel Mullins C. Transarterial chemoembolization treatment: association between multiple treatments, cumulative expenditures, and survival. Value Health 16(5), 760–768 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Campbell PT, Newton CC, Freedman ND. et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. adults. Cancer Res. 76(20), 6076–6083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cauble S, Abbas A, Balart L, Bazzano L, Medvedev S, Shores N. United States women receive more curative treatment for hepatocellular carcinoma than men. Dig. Dis. Sci. 58(10), 2817–2825 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golabi P, Jeffers T, Younoszai Z. et al. Independent predictors of mortality and resource utilization in viral hepatitis related hepatocellular carcinoma. Ann. Hepatol. 16(4), 555–564 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Hess L, Cui Z, Li X, Wu Y, Girvan A, Abada P. Treatment patterns and costs of care for patients diagnosed with hepatocellular carcinoma (HCC) in the United States (U.S.). Ann. Oncol. 29, v27 (2018). [Google Scholar]
- 58.Hyder O, Dodson RM, Nathan H. et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: a United States population-based study. J. Am. Coll. Surg. 217(5), 896–906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jinjuvadia R, Salami A, Lenhart A, Jinjuvadia K, Liangpunsakul S, Salgia R. Hepatocellular carcinoma: a decade of hospitalizations and financial burden in the United States. Am. J. Med. Sci. 354(4), 362–369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaplan DE, Chapko MK, Mehta R. et al. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin. Gastroenterol. Hepatol. 16(1), 106–114.e105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaplan DE, Yu S, Taddei TH. et al. Up-titration of sorafenib for hepatocellular carcinoma: impact on duration of exposure and cost. J. Clin. Oncol. 35(4), 385 (2017). [Google Scholar]
- 62.Kasmari AJ, Welch A, Liu G, Leslie D, Mcgarrity T, Riley T. Independent of cirrhosis, hepatocellular carcinoma risk is increased with diabetes and metabolic syndrome. Am. J. Med. 130(6), 746.e741–746.e747 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Liu A, Do A. Palliative care utilization is associated with reduced costs and reduced procedures in the setting of hepatocellular carcinoma with mortality: a study of the National Inpatient Sample. Hepatology 64(1), 719A–720A (2016). [Google Scholar]
- 64.Massarweh NN, Davila JA, El-Serag HB. et al. Transarterial bland versus chemoembolization for hepatocellular carcinoma: rethinking a gold standard. J. Surg. Res. 200(2), 552–559 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Menzin J, Korn J, Lang K. et al. Medical care costs and survival associated with hepatocellular carcinoma in a Medicare population. J. Clin. Oncol. 29(15), 547–554 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Mishra A, Otgonsuren M, Venkatesan C, Afendy M, Erario M, Younossi ZM. The inpatient economic and mortality impact of hepatocellular carcinoma from 2005 to 2009: analysis of the US nationwide inpatient sample. Liver Int 33(8), 1281–1286 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Mumtaz K, Patel N, Modi RM. et al. Trends and outcomes of transarterial chemoembolization in hepatocellular carcinoma: a national survey. Hepatobiliary Pancreat. Dis. Int. 16(6), 624–630 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Poklepovic AS, Sanyal A, Moyneur E, Meyers S, Barghout V. Retrospective health care claims database analysis of direct medical costs of newly diagnosed patients (pts) with hepatocellular carcinoma (HCC) in the United States. J. Clin. Oncol. 28(15), 6078 (2010). [Google Scholar]
- 69.Sanoff HK, Chang Y, Reimers M, Lund JL. Hospice utilization and its effect on acute care needs at the end of life in Medicare beneficiaries with hepatocellular carcinoma. J. Oncol. Pract. 13(3), e197–e206 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports detailed patient characteristics and healthcare resource use prior to hospice entry.
- 70.Shaya FT, Breunig IM, Seal B, Mullins CD, Chirikov VV, Hanna N. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-Medicare. Pharmacoeconomics 32(1), 63–74 (2014). [DOI] [PubMed] [Google Scholar]; • Provides survival and cumulative costs based on treatment and disease stage.
- 71.Singavi AK, Szabo A, Thomas JP. et al. Costs of care with liver directed therapy (LDT) and sorafenib (S) in patients (pts) with hepatocellular carcinoma (HCC). J. Clin. Oncol. 36(4), 383 (2018).28671856 [Google Scholar]
- 72.Sobotka LA, Hinton A, Conteh LF. Disparities in the treatment of hepatocellular carcinoma based on geographical region are decreasing. J. Gastroenterol. Hepatol. 34(3), 575–579 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Tohme S, Chidi A, Tsung A. Radioembolization for hepatocellular carcinoma: a nationwide 10-year experience of 1,222 cases. Ann. Surg. Oncol. 24(1), S121 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Tsong W, Ray S. First year insurer and patient costs associated with hepatocellular carcinoma diagnosis in the U.S. managed care population. Value Health 13(3), A31–A32 (2010). [Google Scholar]
- 75.Tsong W, Singer ME, Ray S. Burden of hospitalizations for hepatocellular carcinoma patients in a US population. Value Health 13(7), A260 (2010). [Google Scholar]
- 76.Cholankeril G, Perumpail RB, Aggarwal A. et al. Comparing the inpatient disease burden of HCV and HIV infections: trends in hospitalization, cost of care, and length of stay. Hepatology 64(1), 853A (2016).27014967 [Google Scholar]
- 77.Ha J, Yan M, Aguilar M. et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J. Clin. Gastroenterol. 50(5), 423–430 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Parikh ND, Marshall VD, Singal AG. et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: an analysis of the SEER-Medicare database. Hepatology 65(1), 122–133 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Serper M, Taddei TH, Mehta R. et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology 152(8), 1954–1964 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides treatment received and survival based on Barcelona Clinic Liver Cancer stage; also describes key disease, provider and geographic factors associated with treatment and with survival.
- 80.Davila JA, Sada YH, Kanwal F. et al. Outcomes associated with co-utilization of VA and Medicare healthcare benefits in patients with hepatocellular carcinoma. Gastroenterology 146(5), S-1003–S-1004 (2014). [Google Scholar]
- 81.Reiss KA, Yu S, Mamtani R. et al. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: a retrospective, multi-institutional study. J. Clin. Oncol. 35(31), 3575–3581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Liu B, Bhuket T, Wong R. Disparities in health insurance coverage do not fully explain race/ethnicity-specific disparities in hepatocellular carcinoma survival in the United States. Am. J. Gastroenterol. 111, S386–S387 (2016). [Google Scholar]
- 83.Mazzaferro V, Regalia E, Doci R. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 334(11), 693–700 (1996). [DOI] [PubMed] [Google Scholar]
- 84.Butt Z, Lai JS, Beaumont JL. et al. Psychometric properties of a brief, clinically relevant measure of pain in patients with hepatocellular carcinoma. Qual. Life Res. 23(9), 2447–2455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carr BI, Pujol L. Pain at presentation and survival in hepatocellular carcinoma. J. Pain 11(10), 988–993 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Hansen L, Dieckmann NF, Kolbeck KJ, Naugler WE, Chang MF. Symptom distress in patients with hepatocellular carcinoma toward the end of life. Oncol. Nurs. Forum 44(6), 665–673 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Hansen L, Rosenkranz SJ, Vaccaro GM, Chang MF. Patients with hepatocellular carcinoma near the end of life: a longitudinal qualitative study of their illness experiences. Cancer Nurs. 38(4), E19–27 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Hansen L, Rosenkranz SJ, Wherity K, Sasaki A. Living with hepatocellular carcinoma near the end of life: family caregivers' perspectives. Oncol. Nurs. Forum 44(5), 562–570 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Kaiser K, Mallick R, Butt Z, Mulcahy MF, Benson AB, Cella D. Important and relevant symptoms including pain concerns in hepatocellular carcinoma (HCC): a patient interview study. Support. Care Cancer 22(4), 919–926 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Meier A, Yopp A, Mok H, Kandunoori P, Tiro J, Singal AG. Role functioning is associated with survival in patients with hepatocellular carcinoma. Qual. Life Res. 24(7), 1669–1675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paris J, Du Q, Morrison R. Assessing symptom burden of patients with advanced hepatocellular carcinoma. J. Am. Geriatr. Soc. 59(S1), S10 (2011). [Google Scholar]
- 92.Salem R, Gilbertsen M, Butt Z. et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 11(10), 1358–1365.e1351 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Steel JL, Geller DA, Robinson TL. et al. Health-related quality of life as a prognostic factor in patients with advanced cancer. Cancer 120(23), 3717–3721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun V, Ferrell B, Juarez G, Wagman LD, Yen Y, Chung V. Symptom concerns and quality of life in hepatobiliary cancers. Oncol. Nurs. Forum 35(3), E45–52 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Webber G, Chen Z, Ei-Rayes B. et al. Update with longitudinal analysis of quality of life assessment after doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE) in patients with hepatocellular carcinoma (HCC). J. Vasc. Interv. Radiol. 24(4), S141 (2013). [Google Scholar]
- 96.Wible BC, Rilling WS, Drescher P. et al. Longitudinal quality of life assessment of patients with hepatocellular carcinoma after primary transarterial chemoembolization. J. Vasc. Interv. Radiol. 21(7), 1024–1030 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Xing M, Kokabi N, Camacho JC, Kim HS. Prospective longitudinal quality of life and survival outcomes in patients with advanced infiltrative hepatocellular carcinoma and portal vein thrombosis treated with Yttrium-90 radioembolization. BMC Cancer 18(1), 75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xing M, Webber G, Prajapati HJ. et al. Preservation of quality of life with doxorubicin drug-eluting bead transarterial chemoembolization for unresectable hepatocellular carcinoma: longitudinal prospective study. J. Gastroenterol. Hepatol. 30(7), 1167–1174 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Cholankeril G, Al Juboori A, Perumpail RB. et al. Rising inpatient healthcare resource utilization in baby boomers with hepatocellular carcinoma. Hepatology 64(1), 679A (2016).27123567 [Google Scholar]
- 100.Cholankeril R, Copelin EL, Yoo ER. et al. Malnourished HCC patients associated with highest inpatient mortality among GI cancers. Gastroenterology 152(5), S294 (2017). [Google Scholar]
- 101.Huo J, Chung TH, Aloia TA, Sheu T, Shih YT. Adoption of computed tomography image guidance among hepatocellular carcinoma patients with percutaneous radiofrequency ablation. Value Health 19(3), A136 (2016). [Google Scholar]
- 102.McNicoll C, Jadhav R, Louie A. et al. Inpatient mortality, total charges, and length of stay disparities exist for hepatocellular carcinoma in Nevada compared to the United States. HPB 19, S144 (2017). [Google Scholar]
- 103.Walker DR, Manthena SR, Juday TR. Healthcare costs by stage of liver disease in chronic hepatitis C patients in the United States. J. Hepatol. 62, S593–S594 (2015). [Google Scholar]
- 104.Shahab O, Hester D, Paik J. et al. In Medicare patients with hepatocellular carcinoma (HCC), nonalcoholic fatty liver disease (NAFLD) is among the top causes for mortality and resource utilization. Hepatology 68, 168A (2018). [Google Scholar]
- 105.Breunig IM, Shaya FT, Mullins CD, Chirikov V, Seal BS, Hanna N. Multispecialty care among hepatocellular carcinoma patients in SEER-Medicare. J. Clin. Oncol. 30(15_supp), e14638 (2012). [Google Scholar]
- 106.Wang J, Ha J, Tana M. et al. Insured patients with hepatocellular carcinoma in the United States are more likely to have hepatocellular carcinoma within Milan criteria, are more likely to receive treatment, and have higher survival compared to uninsured and Medicaid patients. J. Hepatol. 64(2), S328–S329 (2016). [Google Scholar]
- 107.Rajbhandari R, Chung RT, Ananthakrishnan AN. Racial disparities in in-hospital outcomes for hepatocellular carcinoma in the United States. Gastroenterology 148(4), S1069–S1070 (2015). [Google Scholar]
- 108.Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J. Gastroenterol. Hepatol. 25(11), 1739–1746 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J. Hepatol. 50(1), 89–99 (2009). [DOI] [PubMed] [Google Scholar]
- 110.Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 16(10), 1186–1194 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Parikh ND, Singal AG, Hutton DW. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer 123(19), 3725–3731 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Pollom EL, Lee K, Durkee BY. et al. Cost-effectiveness of stereotactic body radiation therapy versus radiofrequency ablation for hepatocellular carcinoma: a Markov modeling study. Radiology 283(2), 460–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ray CE, Jr, Battaglia C, Libby AM, Prochazka A, Xu S, Funaki B. Interventional radiologic treatment of hepatocellular carcinoma-a cost analysis from the payer perspective. J. Vasc. Interv. Radiol. 23(3), 306–314 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Rostambeigi N, Dekarske AS, Austin EE, Golzarian J, Cressman EN. Cost–effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J. Vasc. Interv. Radiol. 25(7), 1075–1084 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Soto-Perez-De-Celis E, Aguiar PN, Cordon ML, Chavarri-Guerra Y, Lopes GL. Cost-effectiveness of cabozantinib in the second-line treatment of advanced hepatocellular carcinoma. J. Natl Compr. Canc. Netw. 17(6), 669–675 (2019). [DOI] [PubMed] [Google Scholar]
- 116.United States of America: WHO and UNICEF estimates of immunization coverage: 2018 revision. www.who.int/immunization/monitoring_surveillance/data/usa.pdf
- 117.Burstow NJ, Mohamed Z, Gomaa AI. et al. Hepatitis C treatment: where are we now? Int. J. Gen. Med. 10, 39–52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Estes C, Anstee QM, Arias-Loste MT. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69(4), 896–904 (2018). [DOI] [PubMed] [Google Scholar]
- 119.National Health Expenditure Projections 2019–2028 (2019). www.cms.gov/files/document/nhe-projections-2019-2028-forecast-summary.pdf
- 120.Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur. J. Cancer Prev. 27(3), 205–212 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cai H, Zhang L, Li N, Zheng B, Liu M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: a cost–effectiveness analysis. J. Comp. Eff. Res. 9(8), 553–562 (2020). [DOI] [PubMed] [Google Scholar]
- 122.Kobayashi M, Kudo M, Izumi N. et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J. Gastroenterol. 54(6), 558–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pollock RF, Colaone F, Guardiola L, Shergill S, Brennan VK. A cost analysis of SIR-Spheres yttrium-90 resin microspheres versus tyrosine kinase inhibitors in the treatment of unresectable hepatocellular carcinoma in France, Italy, Spain and the UK. J. Med. Econ. 23(6), 593–602 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Roche submits supplemental Biologics License Application to the FDA for Tecentriq in combination with Avastin for the most common form of liver cancer (2020). www.roche.com/media/releases/med-cor-2020-01-27.htm
- 125.Cheng E, Hung P, Wang SY. Geographic variations of potentially curative treatments for hepatocellular carcinoma in the United States: a SEER-Medicare study. J. Natl Compr. Canc. Netw. 18(6), 729–736 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.