Abstract
Background
Gastric cancer (GC) is a malignancy with high morbidity/mortality, partly due to a lack of reliable biomarkers for early diagnosis. It is important to develop reliable biomarker(s) with specificity, sensitivity and convenience for early diagnosis. The role of tumour-associated macrophages (TAMs) and survival of GC patients are controversial. Macrophage colony stimulating factor (MCSF) regulates monocytes/macrophages. Elevated MCSF is correlated with invasion, metastasis and poor survival of tumour patients. IL-34, a ligand of the M-CSF receptor, acts as a “twin” to M-CSF, demonstrating overlapping and complimentary actions. IL-34 involvement in tumours is controversial, possibly due to the levels of M-CSF receptors. While the IL-34/M-CSF/M-CSFR axis is very important for regulating macrophage differentiation, the specific interplay between these cytokines, macrophages and tumour development is unclear.
Methods
A multi-factorial evaluation could provide more objective utility, particularly for either prediction and/or prognosis of gastric cancer. Precision medicine requires molecular diagnosis to determine the specifically mutant function of tumours, and is becoming popular in the treatment of malignancy. Therefore, elucidating specific molecular signalling pathways in specific cancers facilitates the success of a precision medicine approach. Gastric cancer tissue arrays were generated from stomach samples with TNM stage, invasion depth and the demography of these patients (n = 185). Using immunohistochemistry/histopathology, M-CSF, IL-34 and macrophages were determined.
Results
We found that IL-34 may serve as a predictive biomarker, but not as an independent, prognostic factor in GC; M-CSF inversely correlated with survival of GC in TNM III–IV subtypes. Increased CD68+ TAMs were a good prognostic factor in some cases and could be used as an independent prognostic factor in male T3 stage GC.
Conclusion
Our data support the potency of IL-34, M-CSF, TAMs and the combination of IL-34/TAMs as novel biological markers for GC, and may provide new insight for both diagnosis and cellular therapy of GC.
Background
Gastric cancer (GC) is an important disease with high morbidity and mortality. Due to a lack of relatively convenient and reliable biomarkers, large numbers of GC are diagnosed at an advanced stage, with poor prognosis [1]. It is fundamentally important to develop reliable biomarker(s) with enough specificity, sensitivity and convenience for early diagnosis. Whilst cell-mediated immunity may exhibit anti-tumour activity, epidemiological, preclinical and clinical studies demonstrate that chronic inflammation plays a vital role in the initiation and/or development of gastric cancer [2]. Chronic inflammation mediates tumourigenesis, including cellular survival, proliferation, migration, angiogenesis and metastasis via cytokine mediated signalling pathways.
The inflammatory microenvironment surrounding a tumour is a complex ecology of immune cells interconnected with tumour cells. Among the leucocytes present at the tumour site, macrophages are abundantly present at all stages of tumour progression [3]. Tumour-associated macrophages (TAMs) are correlated with poor survival of GC patients, as TAMs promote invasion and metastasis through enhancing angiogenesis [4]. However, others have reported a positive correlation between TAMs and 5 year survival rate of GC patients [5]. Multiple factors may contribute to this discrepancy, including tumour type and stage [6].
Macrophage colony stimulating factor (M-CSF) is a growth factor important in the regulation of differentiation, proliferation and survival of haematopoietic cell lineages [7]. Circulating M-CSF is increased in many tumours (e.g. breast, prostate and pancreatic cancers) and is positively correlated with invasion, metastasis and poor survival of tumour patients [8–10]. By contrast, monocytes/macrophages are able to kill cancerous cells by paraptosis, driven by over-expression of membrane M-CSF [11].
IL-34 was first identified by Lin et al. in 2008, as a protein that is able to bind to CD14+ monocytes in peripheral blood mononuclear cells. IL-34 stimulates the differentiation of monocytes into macrophages via the CSF-1 receptor [12]. Subsequently, IL-34, including mRNA and protein, can be detected in various tissues secreted by fibroblast-like cells. The order of the level of production in the tissues is: spleen, heart, brain, thymus, lung, kidney, liver, small intestine, colon, testes, ovary and prostate [13]. Using an IL-34 reporter gene, for IL-34 a high level of expression has been detected in the skin and in the brain compared to other non-lymphoid and lymphoid tissues [14].
IL-34 is also a ligand of the M-CSF receptor, and acts as a “twin” to the M-CSF cytokine, demonstrating overlapping and complimentary actions [15]. IL-34 acts similarly to MCSF in promoting osteoclastic differentiation of giant cell tumours [16], but IL-34 also displays singular function during brain development [17]. Furthermore, the role of IL-34 in tumours is controversial particularly in the development, metastasis and prognosis of cancers, although the response to MCSF is tumour-type dependant, possibly due to the levels of M-CSF receptors [18].
Studies have long sought specific biological markers that could characterize GC [19]. However, no existing marker(s) have proven to be sufficiently specific to GC. While the IL-34/M-CSF/MCSFR axis is very important for regulating macrophage differentiation [20], the specific interplay between these cytokines, macrophages and the development of tumours is unclear. Accordingly, a multi-factor evaluation could provide more objective utility, particularly for either prediction and/or prognosis of gastric cancer, compared to studies in the current literature.
Conventional chemotherapy kills cancers non-specifically, based on the high rate of cancer cell division. Precision medicine is becoming popular in the treatment of malignancy, which tailors intervention to the individual patient with customization of medical decisions and healthcare [21]. However, the success of a precision medicine approach replies on the identification of highly specific targets in each specific tumour. Therefore, elucidating specific molecular signalling pathways in specific cancers is necessary to facilitate the success of a precision medicine approach.
In our current study, we determined the production of M-CSF and IL-34, and the number of infiltrating CD68+-TAMs in GC. The relationship between M-CSF, IL-34 and CD68+-TAMs infiltration in GC was explored with a view to elucidate potential molecular targets.
Results
IL-34, M-CSF and CD68+-TAMs in GC
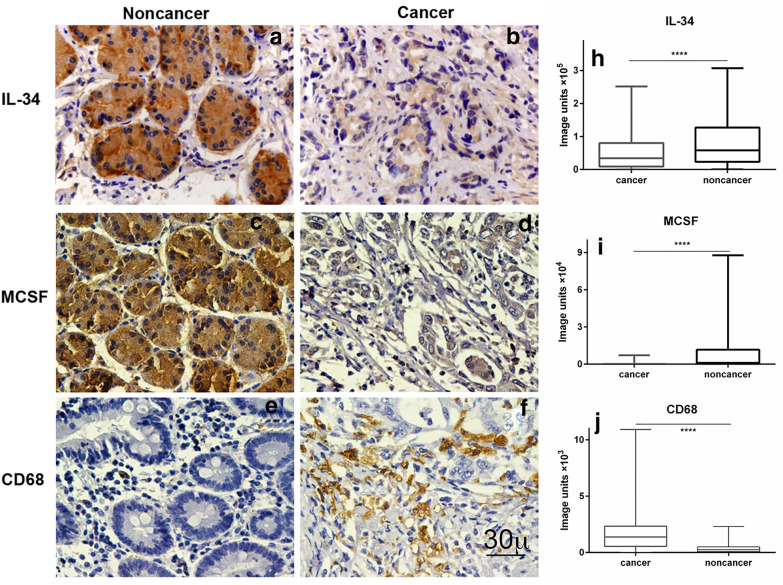
The expression levels of IL-34, M-CSF and CD68+-TAMs in GC were investigated. Following immunohistochemical staining (Fig. 1), the densities of IL-34, M-CSF and the number of CD68+-TAMs were determined and are presented as box plots, including medians and 25th and 75th percentiles. IL-34 and M-CSF were decreased > 40% and > 95%, respectively, in GC compared to tumour adjacent gastric tissues (p < 0.05), whereas CD68+-TAMs were increased 5.5 fold (p < 0.05) (Fig. 1).
Fig. 1.
Representative images of IL-34, MCSF and CD68+ TAMs immunohistochemistry staining and their densities in noncancerous and GC tissues. Positive IL-34, MCSF and CD68+ TAMs were stained in brown mainly expressed in the cytoplasm of gastric cancer and noncancerous tissues. The densities of IL-34 and MCSF were decreased in GC compared to tumour adjacent gastric tissues, whereas the density of CD68 was increased. Magnification, ×600
Correlation between IL-34, M-CSF and CD68+-TAMs in GC and clinicopathological parameters
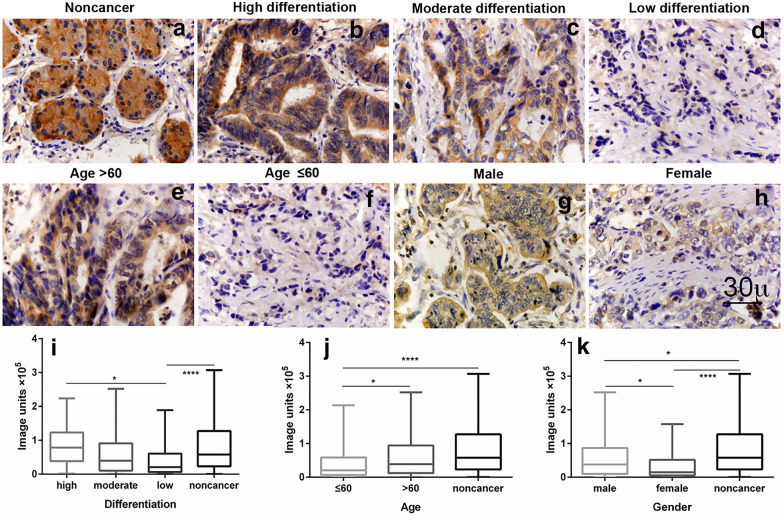
The median values obtained for IL-34, M-CSF and CD68+-TAMs expressions were found to significantly differ between subgroups defined by a range of clinicopathological parameters (Table 1, Fig. 2 and Additional file 1: Figure S1, Additional file 2: Figure S2). The median expression of IL-34 differed significantly with age, gender and tumour differentiation of GC patients (Fig. 2). There was less IL-34 (decreased 46.70%) in the group of patients aged ≤ 60 years compared to patients aged > 60 (p < 0.05). Lower IL-34 (decreased 61%) was also observed in female compared to male GC patients (p < 0.05). In addition, there was a significant correlation between IL-34 and differentiation of GC (low differentiation group of GC had 74% decreased IL-34 compared to the high differentiation group) (p < 0.05), suggesting that IL-34 correlates with the state of differentiation of GC. There was no correlation between IL-34 and other parameters, such as tumour size, lymph node metastasis, tumour invasion depth and TNM stage of GC. Additionally, there was no correlation between clinical parameters and M-CSF or CD68+-TAMs (Additional file 1: Figure S1, Additional file 2: Figure S2).
Table 1.
Correlations between IL-34, MCSF and CD68+ TAMs and clinical pathological features in patients with GC (n = 180)
| Characteristics | Patients | IL-34 | CD68 | MCSF | |||
|---|---|---|---|---|---|---|---|
| Median | p | Median | p | Median | p | ||
| All cancer | 180 | 33,827 | < 0.001 | 1366 | < 0.001 | 36.61 | < 0.001 |
| Noncancer (non) | 159 | 57,921 | 246.0 | 1007 | |||
| Gender | |||||||
| Male | 140 | 37,935 | 0.030 | 1325 | > 0.999 | 38.02 | > 0.999 |
| Female | 40 | 14,847 | 1535 | 27.25 | |||
| Age | |||||||
| ≤ 60 | 79 | 20,658 | 0.048 | 1066 | 0.083 | 25.53 | 0.742 |
| > 60 | 101 | 38,759 | 1702 | 48.79 | |||
| Tumour size (diameter) | |||||||
| < 5 cm | 87 | 40,818 | 0.358 | 1418 | > 0.999 | 45.35 | > 0.999 |
| ≥ 5 cm | 93 | 24,419 | 1321 | 32.54 | |||
| Lymph node metastasis | |||||||
| No | 75 | 43,296 | > 0.999 | 1715 | 0.169 | 48.20 | > 0.999 |
| Yes | 105 | 30,495 | 1185 | 28.30 | |||
| Differentiation | |||||||
| High | 14 | 78,613 |
H/L: 0.043 H/M: 0.499 M/L: 0.495 |
1250 | All > 0.999 | 223.8 |
H/L: 0.051 H/M: 0.471 M/L: 0.791 |
| Moderate | 78 | 39,376 | 1757 | 40.12 | |||
| Low | 88 | 20,788 | 1186 | 26.00 | |||
| Tumour invasion depth | |||||||
| T1 | 4 | 87,693 | All > 0.999 | 1762 | All > 0.999 | 20.82 | All > 0.999 |
| T2 | 27 | 51,850 | 1644 | 26.86 | |||
| T3 | 75 | 27,518 | 1593 | 40.12 | |||
| T4 | 74 | 32,160 | 1101 | 39.80 | |||
| TNM | |||||||
| I | 12 | 22,371 |
I/II, I/III I/IV, II/III > 0.999 II/IV: 0.278 III/IV: 0.643 |
1644 |
I/II, I/III I/IV, II/IV III/IV > 0.999 II/III: 0.636 |
27.25 |
I/II, I/III II/III > 0.999 I/IV: 0.942 II/IV: 0.277 III/IV: 0.183 |
| II | 70 | 42,580 | 1564 | 48.79 | |||
| III | 92 | 32,051 | 1147 | 42.15 | |||
| IV | 6 | 6544 | 936.6 | 3.390 | |||
Fig. 2.
Correlation of IL-34 expression with age, gender and differentiation subtypes. IL-34 decreased in the groups of GC patients aged less than or equal to 60 years old, female and low differentiation subtypes of GC
Correlation of decreased IL-34, M-CSF but increased CD68+-TAMs with overall survival of GC patients
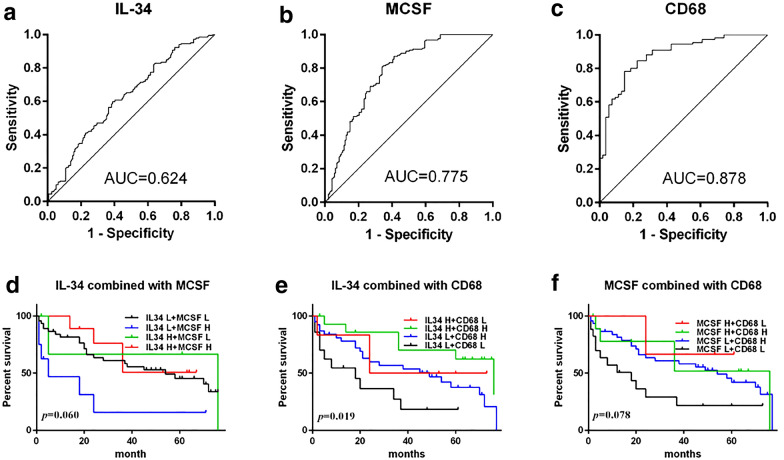
To evaluate whether decreased IL-34, M-CSF and increased CD68+-TAMs correlated with survival of GC patients, low and high cut-off points for IL-34, M-CSF and CD68 were defined by ROC curve analysis (Fig. 3).
Fig. 3.
ROC and combination analysis of IL-34, MCSF and CD68+ TAMs for prognosis of GC. ROC curves analysis displaying the diagnostic of GC by expression levels of IL-34, MCSF and CD68. Area under the curve, IL-34: 48,550, AUC = 0.624; MCSF: 233.7, AUC = 0.775; CD68: 768.7, AUC = 0.878. Kaplan–Meier survival analysis of combination of IL-34, MCSF and CD68+ TAMs for prognosis of GC, the combination of IL-34 and CD68+ TAMs had relationship with the prognosis of GC patients
The area under the curve (AUC) derived from the ROC curves showed that CD68+-TAM was the most sensitive marker for prognosis (AUC = 0.878), demonstrating a moderate to high accuracy, while IL-34 (AUC = 0.624) and M-CSF (AUC = 0.775) demonstrated only moderate accuracy [22].
Kaplan–Meier analysis was further applied to compare overall survival of GC patients according to combinations of IL-34, M-CSF and CD68+-TAMs (Fig. 3). Patients with high IL-34 plus high CD68+-TAMs had the longest survival of GC patients, while those with low IL-34 plus low CD68+-TAMs had the lowest survival. However, there was no significant difference in survival for the combination of IL-34 and M-CSF, or CD68+-TAMs and M-CSF.
Furthermore, to determine whether IL-34 was an independent prognostic marker for GC, we performed univariate and multivariate Cox regression analysis, including IL-34, age, gender, tumour differentiation, lymph node invasion, tumour size, the depth of tumour invasion and TNM stage. The effect of IL-34 on patient survival in GC was determined. Univariate analysis (Table 2) revealed that the expression of IL-34, advanced TNM stage, lymph node metastasis, the depth of tumour invasion and tumour diameter were correlated with the prognosis of GC patients. In multivariate analysis (Table 2), only advanced TNM stage remained a significant independent prognostic factor for the survival of patients.
Table 2.
Univariate and multivariate analysis of IL-34 and clinicopathological factors affecting survival of patients with GC
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| IL-34 (low/high) | 2.205 (1.072–4.537) | 0.032 | 1.457 (0.639–3.325) | 0.371 |
| Gender | ||||
| Female/male | 0.886 (0.443–1.775) | 0.733 | ||
| Age (≤ 60/> 60) | 1.058 (0.573–1.955) | 0.857 | ||
| Diameter (< 5/≥ 5, cm) | 0.363 (0.189–0.698) | 0.002 | 0.495 (0.217–1.129) | 0.095 |
| Lymph node metastasis | ||||
| No/yes | 0.267 (0.122–0.582) | 0.001 | 0.772 (0.274–2.173) | 0.624 |
| Tumour differentiation | ||||
| Low (reference) | 1 | 0.545 | ||
| High | 0.725 (0.218–2.411) | 0.600 | ||
| Moderate | 0.693 (0.349–1.375) | 0.294 | ||
| Invasion depth | ||||
| T4 | 1 | 0.142 | 1 | 0.955 |
| T1 | 0.000 (0.000–) | 0.977 | 0.900 (0.000–) | 1.000 |
| T2 | 0.261 (0.084–0.808) | 0.020 | 1.528 (0.326–7.165) | 0.591 |
| T3 | 0.750 (0.380–1.478) | 0.405 | 1.049 (0.463–2.378) | 0.909 |
| TNM | ||||
| IV (reference) | 1 | 0.000 | 1 | 0.001 |
| I | 0.000 (0.000–) | 0.966 | 0.000 (0.000–) | 0.969 |
| II | 0.085 (0.028–0.260) | 0.000 | 0.075 (0.021–0.270) | 0.000 |
| III | 0.195 (0.068–0.559) | 0.002 | 0.154 (0.047–0.503) | 0.002 |
Bold italic values indicate significance (p < 0.05)
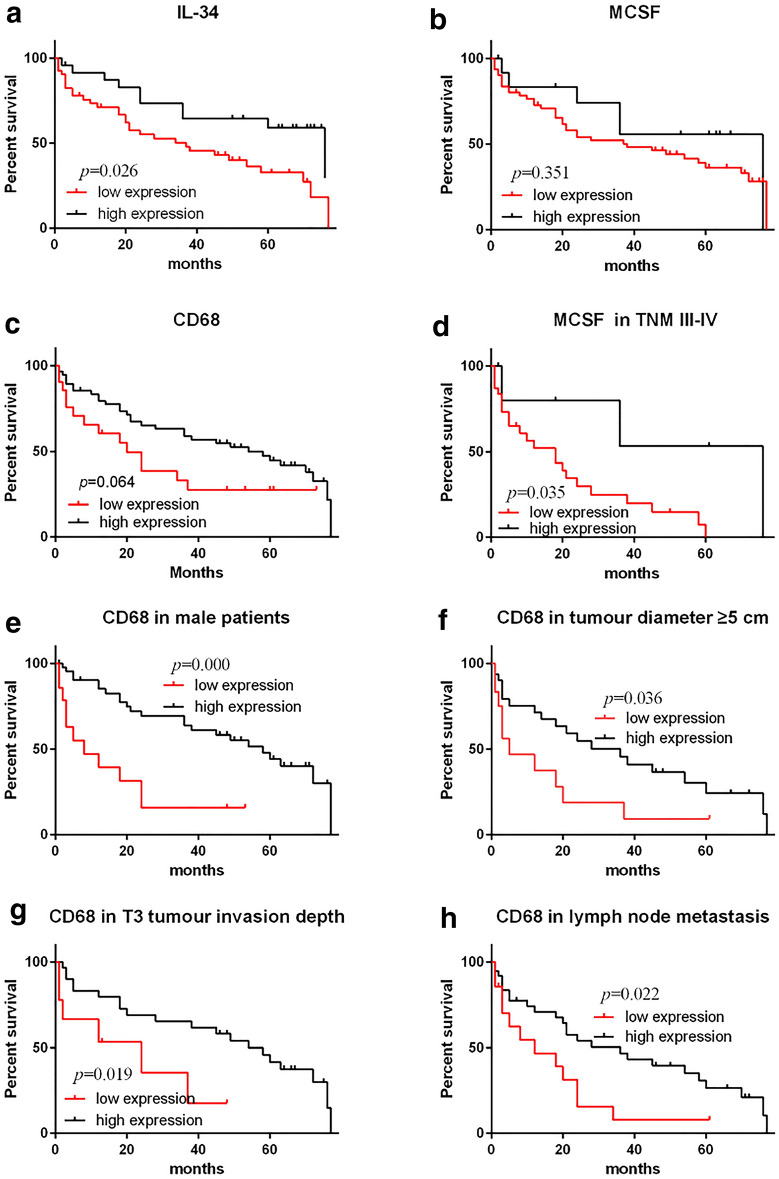
Further analysis of correlation of M-CSF and CD68+-TAMs with overall survival in subgroups of GC patients
Kaplan–Meier analysis was also applied to further compare overall survival according to M-CSF and CD68+-TAMs in different subgroups of GC. As results show in Fig. 4, M-CSF correlated significantly with the survival of patients in the TNM III-IV tumour stage. CD68+-TAMs correlated with survival significantly in male GC patients, larger tumour size (diameter ≥ 5 cm), lymph node metastasis and tumour invasion depth T3. There was no significance in other subgroups between survival and M-CSF and CD68+-TAMs (Additional file 3: Figure S3, Additional file 4: Figure S4, Additional file 5: Figure S5, Additional file 6: Figure S6).
Fig. 4.
Survival analysis of IL-34, MCSF and CD68+ TAMs for prognosis of GC. Kaplan–Meier survival analysis of GC patients: decreased IL-34 expression correlates with a poor survival for GC patients. Decreased MCSF expression correlates with poor survival of GC patients in TNM III-IV stage. Increased CD68+ TAMs expression correlates with good survival of GC patients in male, Diameter ≥ 5 cm, lymph node metastasis and T3 stage
Furthermore, to examine whether M-CSF and CD68+-TAMs were independent prognostic markers for subgroups in GC, we again performed univariate and multivariate Cox regression analysis, including M-CSF and CD68+-TAMs, age, gender, tumour differentiation, lymph node invasion, tumour size, the depth of tumour invasion and TNM stage to study the effects of M-CSF and CD68+-TAMs on patient survival in GC subgroups.
Using univariate analysis a correlation was observed between survival of GC patients and CD68+ TAMs, TNM stage or lymph node metastasis in the T3 stage subgroup of GC patients, respectively (Table 3). Using multivariate analysis, it was demonstrated that CD68+ TAMs and TNM stage remained as significant independent prognostic factors for survival of GC patients within these subgroups.
Table 3.
Univariate and multivariate analysis of CD68+ TAMs and clinicopathological factors affecting survival of patients in T3 stage of GC
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD68 (low/high) | 3.080 (1.135–8.360) | 0.027 | 5.471 (1.825–16.397 | 0.002 |
| Gender | ||||
| Female/male | 0.638 (0.216–1.886) | 0.416 | ||
| Age (≤ 60/> 60) | 1.052 (0.479–2.310) | 0.900 | ||
| Diameter(< 5/≥ 5, cm) | 0.464 (0.196–1.100) | 0.081 | ||
| Lymph node metastasis | ||||
| No/yes | 0.407 (0.167–0.993) | 0.048 | 0.363 (0.123–1.075) | 0.067 |
| Tumour differentiation | ||||
| Low (reference) | 1 | 0.436 | ||
| High | 0.269 (0.035–2.059) | 0.206 | ||
| Moderate | 0.825 (0.348–1.955) | 0.663 | ||
| TNM | ||||
| IV (reference) | 1 | 0.019 | 1 | 0.007 |
| II | 0.140 (0.029–0.683) | 0.015 | 0.061 (0.011–0.351) | 0.002 |
| III | 0.337 (0.070–1.619) | 0.174 | 0.101 (0.016–0.634) | 0.014 |
Bold italic values indicate significance (p < 0.05)
Using univariate analysis, it was demonstrated that there is a correlation between survival of GC patients and CD68+ TAMs, tumour diameter, advanced TNM stage and lymph node metastasis in the male GC patients’ subgroup (Table 4). Multivariate analysis demonstrated that CD68+ TAMs, tumour diameter ≥ 5 cm and advanced TNM stage remained as significant independent prognostic factors of survival of male GC patients.
Table 4.
Univariate and multivariate analysis of CD68+ TAMs and clinicopathological factors affecting survival of male patients of GC
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD68 (low/high) | 3.843 (1.773–8.329) | 0.001 | 3.905 (1.599–9.538) | 0.003 |
| Age (≤ 60/> 60) | 0.876 (0.441–1.740) | 0.706 | ||
| Diameter(< 5 vs ≥ 5, cm) | 0.308 (0.150–0.636) | 0.001 | 0.393 (0.172–0.901) | 0.027 |
| Lymph node metastasis | ||||
| No/yes | 0.300 (0.133–0.677) | 0.004 | 0.847 (0.328–2.186) | 0.731 |
| Tumour differentiation | ||||
| Low (reference) | 1 | 0.737 | ||
| High | 0.633 (0.186–2.153) | 0.464 | ||
| Moderate | 0.848 (0.402–1.790) | 0.666 | ||
| Invasion depth | ||||
| T4 | 1 | 0.366 | ||
| T1 | 0.000 (0.000–) | 0.975 | ||
| T2 | 0.300 (0.080–1.129) | 0.075 | ||
| T3 | 0.740 (0.332–1.652) | 0.462 | ||
| TNM | ||||
| IV (reference) | 1 | 0.002 | 1 | 0.040 |
| I | 0.000 (0.000–) | 0.978 | 0.000 (0.000–) | 0.977 |
| II | 0.104 (0.030–0.356) | 0.000 | 0.137 (0.035–0.529) | 0.004 |
| III | 0.299 (0.094–0.948) | 0.299 | 0.232 (0.066–0.816) | 0.023 |
Bold italic values indicate significance (p < 0.05)
Using univariate analysis for survival in the subgroup of tumour diameter ≥ 5 cm (Table 5) and lymph node metastasis subgroup (Table 6), CD68+-TAMs and TNM stage were correlated with the prognosis of GC patients within these two subgroups. However, only TNM stage remained a significant independent prognostic factor of survival of GC patients in multivariate analysis in both subgroups. In the TNM III–IV subgroup of GC patients there was no significant outcome in M-CSF using univariate analysis (Additional file 7: Table S1).
Table 5.
Univariate and multivariate analysis of CD68+ TAMs and clinicopathological factors affecting survival of GC in subtype of diameter ≥ 5 cm
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD68 (low/high) | 2.222 (1.014–4.872) | 0.046 | 1.781 (0.776–4.087) | 0.173 |
| Gender | ||||
| Female/male | 0.778 (0.349–1.733) | 0.539 | ||
| Age (≤ 60/> 60) | 0.924 (0.442–1.928) | 0.832 | ||
| Lymph node metastasis | ||||
| No/yes | 0.820 (0.311–2.165) | 0.689 | ||
| Tumour differentiation | ||||
| Low (reference) | 1 | 0.533 | ||
| High | 1.100 (0.144–8.377) | 0.927 | ||
| Moderate | 0.636 (0.284–1.426) | 0.272 | ||
| Invasion depth | ||||
| T4 | 1 | 0.548 | ||
| T2 | 0.610 (0.131–2.836) | 0.529 | ||
| T3 | 0.645 (0.286–1.453) | 0.290 | ||
| TNM | ||||
| IV (reference) | 1 | 0.007 | 1 | 0.025 |
| II | 0.095 (0.022–0.412) | 0.002 | 0.125 (0.027–0.568) | 0.007 |
| III | 0.157 (0.040–0.616) | 0.008 | 0.184 (0.047–0.724) | 0.015 |
Bold italic values indicate significance (p < 0.05)
Table 6.
Univariate and multivariate analysis of clinicopathological factors affecting survival of GC in subtype of lymph node metastasis
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD68 (low/high) | 2.220 (1.083–4.553) | 0.029 | 1.559 (0.706–3.443) | 0.272 |
| Gender | ||||
| Female/male | 0.968 (0.472–1.988) | 0.930 | ||
| Age (≤ 60/> 60) | 0.957 (0.497–1.843) | 0.896 | ||
| Diameter (< 5/≥ 5 cm) | 0.773 (0.384–1.557) | 0.472 | ||
| Tumour differentiation | ||||
| Low (reference) | 1 | 0.360 | ||
| High | 0.772 (0.230–2.598) | 0.676 | ||
| Moderate | 0.570 (0.263–1.237) | 0.155 | ||
| Invasion depth | ||||
| T4 | 0.529 | |||
| T2 | 0.538 (0.174–1.660) | 0.281 | ||
| T3 | 0.774 (0.378–1.584) | 0.483 | ||
| TNM | ||||
| IV (reference) | 1 | 0.001 | 1 | 0.011 |
| II | 0.083 (0.022–0.314) | 0.000 | 0.113 (0.027–0.476) | 0.003 |
| III | 0.152 (0.046–0.498) | 0.002 | 0.188 (0.055–0.647) | 0.008 |
Bold italic values indicate significance (p < 0.05)
Discussion
We have demonstrated that decreased IL-34 in GC is inversely correlated with tumour differentiation, age and female gender of GC patients, which is consistent with others showing that younger, particularly female GC patients had more severe malignancy than older, male patients [23, 24]. Decreased IL-34 was closely related to the poor survival rate of GC patients, but not as an independent prognostic factor. Liu et al. demonstrate that > 60% of GC patients had low M-CSF, using positive expression rate only [25]. This is in line with our current study revealed that M-CSF was decreased in GC compared to the control. However, there was no correlation between M-CSF and clinical–pathological parameters, as well as, prognosis of GC in the current study. Interestingly, in the TNM III–IV subtype of GC, decreased M-CSF was inversely correlated with prognosis of GC, but M-CSF still cannot be considered an independent prognostic factor.
IL-34 induces differentiation of leukaemia cells into monocyte-like, macrophage-like cells and mature macrophages through the JAK/STAT and PI3K/Akt signalling pathways [26, 27], suggesting that IL-34 enhances differentiation of other cancers, and supporting our current finding that IL-34 was correlated with the differentiation of GC. Our data may provide an explanation for the possible role of IL-34 in the development of GC, i.e. IL-34 also regulates GC differentiation, which would have potential clinical relevance regarding IL-34 as a therapeutic target for malignancy.
IL-34 and M-CSF can induce macrophage polarization, mainly into the M2 phenotype, which subsequently leads to M2 macrophage mediated immunosuppression [28], promoting tumour progression and metastasis [26]. However, a controversial report showed no significant correlation between the serum level of M-CSF and the stage and prognosis in GC patients [29]. Anti-M-CSF antibody doesn’t induce cytotoxic effects on breast cancer in vitro, which may be either due to a differential effect in these different cancer models and/or variance between in vivo and in vitro [30]. Additionally, IL-34- and MCSF-induced macrophages can switch memory T cells into Th17 cells [18] to support anti-tumour immunity in established ovarian cancers [31].
Macrophage mediated host cellular immunity is important in tumour oncogenesis [32]. Monocytes differentially polarize into M1 and M2 macrophages [33], but in TAMs M1 and M2 polarization is rarely observed [34]. CD68 is frequently used as a marker of infiltrated TAMs, regardless of their polarization state in many studies. Our data show there was no correlation between CD68+-TAMs and any clinicopathological parameters, as well as, prognosis of GC, except for increased number of CD68+-TAMs in GC. We found there was better prognosis in GC patients with high CD68+-TAMs in males, with GC size ≥ 5 cm, lymph node metastasis and T3 subtypes. This is consistent with findings in colon and colorectal cancers [35, 36], but not in other cancer [37, 38].
TAMs are mixed phenotype, expressing M1 or M2 markers [39], and may be influenced by different microenvironments in different regions and/or in different individuals. The functions of TAMs can be modified by cancer and cancer cell secretions, which in turn could affect tumour growth and differentiation [40]. However, our current observation invites speculation that the increased infiltrating CD68+-TAMs may be M1 dominant, contributing to anti-tumour activity, which will be determined in our future experiments. The possible relationship between M1 vs M2 and IL-34 has been recently reviewed [41].
A significant correlation was observed between the combination of IL-34/CD68+-TAMs and the prognosis of GC. Tumours with high IL-34 plus high CD68+-TAMs had the best prognosis, tumours with high or low IL-34 plus low or high CD68+-TAMs had mid-level prognosis and tumours with low IL-34 plus low CD68+-TAMs had the worst prognosis in GC patients. Our results verify a significant correlation between the combination of IL-34 and CD68+-TAMs and prognosis of GC patients. The precise underlying mechanism of IL-34/M-CSF/M-CSFR axis in tumorigenesis, particular in GC, will be determined in future work. Finally, our data may also provide useful information in personalised decision making for precision medicine, which may substantially reduce adverse effects of chemotherapy, and improve the outcome.
At this stage, our data demonstrate that IL-34 and its related markers M-CSF and TAM correlated well with prognosis of gastric cancer patients, particularly in the male gastric cancer patients. Our observation suggests IL-34 might be a potential biomarker for predicting the prognosis of gastric cancer patients. However, there is still a long way to go before IL-34 can be used for this purpose. This practical issue is currently being investigated using IL-34 transgenic and IL-34 gene knockout mice, as well as further investigation ex vivo in human tissues. Clinical application is also being examined in gastric biopsy samples.
Although our data suggest that IL-34 and CD68+ TAM might be useful biomarkers in gastric cancer, other factors, for example, Epstein–Barr virus infection, are well known to be linked with gastric cancer [42]. Such linkage will be determined in our future studies.
In our study we have compared cancer tissue with adjacent normal gastric mucosa from the same patients as a control. Ideally, we should use normal gastric tissue from non-cancer patients, however, due to ethics issues we are not able to obtain normal gastric tissue from non-cancer patients for our research purposes. Perhaps in the future we may collect normal gastric tissue from organ donors, e.g. heart, lung, kidney donors. Our current observation is based on immunohistochemistry exclusively. However, it remains to be clarified whether mRNA for IL-34, M-CSF and macrophages markers are also expressed in the same way, which will be determined in our future experiments. A tissue array methodology was used in the current study for immunohistochemical staining. Thus, due to limited size of the tumour sample within the array, we are not able to demonstrate the expression of CD68+-TAMs and the loss of IL-34/M-CSF at the interface of tumour invasion region in the existing immunohistochemically stained slides. We also are unable to undertake more fresh immunostaining at this stage, due to COVID-19 limitations for undertaking any wet laboratory experiments in the University of Sydney. This excellent suggestion will be performed in our future experiments. Finally, as stated above, there is overlapping functions between IL-34 and M-CSF, which may explain why IL-34 is not an independent prognostic factor in GC.
Conclusion
Reduced IL-34 was associated with poor differentiation, poor survival rate, relatively young patients and female GC patients. IL-34 may serve as a predictive biomarker, but not as an independent, prognostic factor in GC. M-CSF inversely correlated with survival of GC in TNM III–IV subtype, but was also not an independent prognostic factor. Increased CD68+-TAMs were a good prognostic factor of GC in male, tumour diameter ≥ 5 cm, lymph node metastasis and T3 subgroups. CD68+-TAMs could therefore be used as an independent prognostic factor in male T3 stage GC. Furthermore, the combination of IL-34 and CD68+-TAMs might serve as a useful prognostic marker in GC. These results collectively support the potency of IL-34, M-CSF, CD68+-TAMs and the combination of IL-34 and CD68+-TAMs as novel biological markers for GC, thus may provide new insight for both diagnosis and cellular therapy of GC.
Methods
Patients and samples
GC tissue and adjacent histologically normal gastric tissue (control) was obtained from 180 GC patients undergoing gastrectomy without prior chemotherapy in Xuzhou Medical University, China (2008–2010). These GC patients were 140 males and 40 females (aged 23–85 years) (Table 1) with complete clinical information. Among them, 77 had follow-up until their death or until their most recent contact (May, 2015). At the time of the most recent contact, 14 of 77 were still alive, whereas 42 were dead and the other 21 were lost contacts during the following-up. Among the 14 surviving patients, the longest survival period was 76 months. The tissues within the pathology blocks were obtained from the patents at surgery. The consent for surgery included consent for the tissues to be used for diagnostic and research purpose in an unidentified manner. A written explanation of the surgical procedures and the potential research use of the tissues was provided to the patient prior to surgery. All of the patients were adults who were older than 16 years. This study was approved by the Human Ethical Committee, Xuzhou Medical University (xyfylw2012002).
Immunohistochemistry
Sections (5 µm) from tissue microarray blocks were labelled with three antibodies, as described previously [43]. The antibodies were: rabbit anti-IL-34 polyclonal antibody (bs-18170R, Beijing Biosynthesis Biotechnology, China), rabbit anti-M-CSF (Abcam, Cambridge, UK) and mouse monoclonal anti-CD68 (Dako, Copenhagen, Denmark). HRP-conjugated secondary antibody (Beijing Sequoia Jinqiao Biological Technology) was used. The specific target(s) was visualized with 3, 3′-diaminobenzidine (DAB) detection kit and counterstained with hematoxylin. IL-34, M-CSF and CD68 production was quantified.
Briefly, all the images were taken by an Olympus BX51 microscope with fixed exposure time and light sources to avoid any additional unwanted errors. The quantitative analysis was conducted using ImagePro Plus 7.1 software (Media Cybernetics, Silver Spring, MD) as described by Liu et al. [43–45]. The positive staining threshold was defined by an independent pathologist in a double-blind fashion. The defined threshold was applied to analyse all of the images, using a pre-programmed macro in ImagePro Plus 7.1 software to obtain the objective positive value (pixels) of the staining. The positive pixels were expressed as image units. The mean of these values represents the amount of staining per treatment group used for subsequent statistical comparison, as described below.
Statistical analysis
The SPSS 16.0 statistical software package was used for the statistical analysis. Comparison between two groups was performed via Mann–Whitney U test, as described [44, 45]. Comparisons among multi-groups were performed via Kruskal–Wallis test. Low and high cutoff values for cytokine expression was defined by ROC curve analysis [46]. Survival curves were plotted by the Kaplan–Meier method and compared by the log-rank test. Cox’s proportional hazards model was used to identify the prognostic factors that influenced survival. p < 0.05 was considered statistically significant.
Supplementary information
Additional file 1: Figure S1. Correlation of IL-34, MCSF and CD68+ TAMs expression with clinicopathological parameters of tumour size, lymph node metastasis, tumour invasion depth and TNM subtypes of GC. IL-34, MCSF and CD68+ TAMs all have no correlations with any clinicopathological parameters of tumour size, lymph node metastasis, tumour invasion depth and TNM subtypes of GC.
Additional file 2: Figure S2. Correlation of MCSF and CD68+ TAMs with clinicopathological parameters of age, gender and differentiation subtypes of GC. MCSF and CD68+ TAMs both have no correlations with any clinicopathological parameters of age, gender and differentiation subtypes of GC.
Additional file 3: Figure S3. Survival analysis of MCSF for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of GC patients: there were no correlations of MCSF with survival of gender, age, diameter and lymph node metastasis subtypes of GC patients.
Additional file 4: Figure S4. Survival analysis of MCSF for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of MCSF for prognosis of GC in differentiation, tumour invasion depth and TNM stage subtypes.
Additional file 5: Figure S5. Survival analysis of CD68+ TAMs for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of CD68+ TAMs for prognosis of GC in female, tumour diameter < 5 cm, no lymph node metastasis and T4 stage subtypes.
Additional file 6: Figure S6. Survival analysis of CD68+ TAMs for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of CD68+ TAMs for prognosis of GC in age, tumour differentiation and TNM stage subtypes.
Additional file 7: Table S1. Univariate analysis of MCSF and clinicopathological factors affecting survival of patients with GC in TNM III–IV.
Acknowledgements
We appreciate the assistance from the Department of Pathology, Xuzhou Medical University, China.
Abbreviations
- CRC
Gastric cancer
- IL
Interleukin
- TAM
Tumour associate macrophages
- M-CSF
Macrophage colony stimulating factor
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- TNM
Tumour, node and metastasis
- NFkb
Nuclear factor kappa B
- MAPKs
Mitogen-activated protein kinase
- DCs
Dendritic cells
Authors’ contributions
QL: performed the experiment, analysed the data, and wrote manuscript. YZ: immunohistochemistry. JZ, KT, BH and SB: designed the experiment and critically reviewed the manuscript. SB and QL: provided financial support for the experiment. All authors read and approved the final manuscript.
Funding
This study was supported by Grants from National Natural Science Foundation of China (NO. 81502030) for the reagents and histopathology and immunohistochemistry, and The Bosch small equipment grands SB), the University of Sydney, Australia for image analysis. Liu was a recipient of a Travel fellowship, Minster of Education, Jiangsu Province, China (QL) sponsored Dr. Liu’s stipend during her stay in Australia.
Availability of data and materials
Yes.
Ethics approval and consent to participate
The consent for surgery included consent for the tissues to be used for diagnostic and research purpose in an unidentified manner. The informed consent obtained from study participants was written. A written explanation of the surgical procedures and the potential research use of the tissues was provided to the patient prior to surgery. All of the patients were adults who were older than 16 years. This study was approved by the Human Ethical Committee, Xuzhou Medical University (xyfylw2012002).
Consent for publication
NA.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qinghua Liu, Email: liuqinghua1@126.com.
Ying Zhang, Email: 807411916@qq.com.
Jiwei Zhang, Email: 398164963@qq.com.
Kun Tao, Email: taokun@shtrhospital.com.
Brett D. Hambly, Email: brett.hambly@sydney.edu.au
Shisan Bao, Email: bob.bao@sydney.edu.au.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13578-020-00454-8.
References
- 1.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9(1):5–17. doi: 10.5009/gnl14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai EZ, Siveen KS, Shanmugam MK, Arfuso F, Sethi G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015;468(1):1–15. doi: 10.1042/BJ20141337. [DOI] [PubMed] [Google Scholar]
- 3.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Yan Y, Yang Y, Wang L, Li M, Wang J, Liu X, Duan X, Wang J. High infiltration of tumor-associated macrophages influences poor prognosis in human gastric cancer patients, associates with the phenomenon of EMT. Medicine (Baltimore) 2016;95(6):e2636. doi: 10.1097/MD.0000000000002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, Zheng L, Li L. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol. 2011;18(9):2585–2593. doi: 10.1245/s10434-011-1609-3. [DOI] [PubMed] [Google Scholar]
- 6.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99(9):1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 7.Stanley ER, Berg KL, Einstein DB, Lee PS, Pixley FJ, Wang Y, Yeung YG. Biology and action of colony–stimulating factor-1. Mol Reprod Dev. 1997;46(1):4–10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Lawicki S, Omyla J, Mroczko B, Szmitkowski M, Czygier M. Plasma level of macrophage-colony stimulating factor (M-CSF) in the course of breast cancer treatment. Pol Arch Med Wewn. 2004;112(4):1181–1187. [PubMed] [Google Scholar]
- 9.Ide H, Hatake K, Terado Y, Tsukino H, Okegawa T, Nutahara K, Higashihara E, Horie S. Serum level of macrophage colony-stimulating factor is increased in prostate cancer patients with bone metastasis. Hum Cell. 2008;21(1):1–6. doi: 10.1111/j.1749-0774.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 10.Groblewska M, Mroczko B, Wereszczynska-Siemiatkowska U, Mysliwiec P, Kedra B, Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin Chem Lab Med. 2007;45(1):30–34. doi: 10.1515/CCLM.2007.025. [DOI] [PubMed] [Google Scholar]
- 11.Jadus MR, Chen Y, Boldaji MT, Delgado C, Sanchez R, Douglass T, Al-Atar U, Schulz W, Lloyd C, Wepsic HT. Human U251MG glioma cells expressing the membrane form of macrophage colony-stimulating factor (mM-CSF) are killed by human monocytes in vitro and are rejected within immunodeficient mice via paraptosis that is associated with increased expression of three different heat shock proteins. Cancer Gene Ther. 2003;10(5):411–420. doi: 10.1038/sj.cgt.7700583. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320(5877):807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 13.Dembic Z. Cytokines of the immune system: interleukins. In: Dembic Z, editor. The cytokines of the immune system: the role of cytokines in disease related to immune response. 1. Amsterdam: Elsevier; 2015. pp. 143–239. [Google Scholar]
- 14.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37(6):1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6(6):a021857. doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baud’huin M, Renault R, Charrier C, Riet A, Moreau A, Brion R, Gouin F, Duplomb L, Heymann D. Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. J Pathol. 2010;221(1):77–86. doi: 10.1002/path.2684. [DOI] [PubMed] [Google Scholar]
- 17.Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367(2):100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foucher ED, Blanchard S, Preisser L, Descamps P, Ifrah N, Delneste Y, Jeannin P. IL-34- and M-CSF-induced macrophages switch memory T cells into Th17 cells via membrane IL-1alpha. Eur J Immunol. 2015;45(4):1092–1102. doi: 10.1002/eji.201444606. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzon L, Cippitelli C, Avantifiori R, Uccini S, French D, Torrisi MR, Ranieri D, Mercantini P, Canu V, Blandino G, et al. Down-regulated miRs specifically correlate with non-cardial gastric cancers and Lauren’s classification system. J Surg Oncol. 2017;116:184–194. doi: 10.1002/jso.24648. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto D, Chow A, Greter M, Saenger Y, Kwan WH, Leboeuf M, Ginhoux F, Ochando JC, Kunisaki Y, van Rooijen N, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208(5):1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma M. Personalized medicine and cancer. J Pers Med. 2012;2(1):1–14. doi: 10.3390/jpm2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 23.Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn HS, et al. A risk prediction model based on lymph-node metastasis in poorly differentiated-type intramucosal gastric cancer. PLoS ONE. 2016;11(5):e0156207. doi: 10.1371/journal.pone.0156207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Yao T, Niho Y, Tsuneyoshi M. A clinicopathological study in young patients with gastric carcinoma. J Surg Oncol. 1999;71(4):214–219. doi: 10.1002/(sici)1096-9098(199908)71:4<214::aid-jso2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Zhang H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Increased expression of CSF-1 associates with poor prognosis of patients with gastric cancer undergoing gastrectomy. Medicine (Baltimore) 2016;95(9):e2675. doi: 10.1097/MD.0000000000002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segaliny AI, Mohamadi A, Dizier B, Lokajczyk A, Brion R, Lanel R, Amiaud J, Charrier C, Boisson-Vidal C, Heymann D. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer. 2015;137(1):73–85. doi: 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]
- 27.Booker BE, Clark RS, Pellom ST, Adunyah SE. Interleukin-34 induces monocytic-like differentiation in leukemia cell lines. Int J Biochem Mol Biol. 2015;6(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 28.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. Antagonistic effects of GM-CSF and IFNgamma. PLoS ONE. 2013;8(2):e56045. doi: 10.1371/journal.pone.0056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou R, Zhou Y, Chen Z. Exploration of macrophage colony-stimulating factor as a new type of tumor marker. Biomed Rep. 2013;1(6):845–849. doi: 10.3892/br.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rietkotter E, Bleckmann A, Bayerlova M, Menck K, Chuang HN, Wenske B, Schwartz H, Erez N, Binder C, Hanisch UK, et al. Anti-CSF-1 treatment is effective to prevent carcinoma invasion induced by monocyte-derived cells but scarcely by microglia. Oncotarget. 2015;6(17):15482–15493. doi: 10.18632/oncotarget.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, Thomas G, Zhou S, Wang Q, Elakkad A, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016;1(2):e85841. doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35(5):585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 34.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4(2):141–154. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13(5):1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 37.Kumagai S, Marumo S, Shoji T, Sakuramoto M, Hirai T, Nishimura T, Arima N, Fukui M, Huang CL. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer. 2014;85(3):457–464. doi: 10.1016/j.lungcan.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schworer AM, Wagner U, Muller-Brusselbach S, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134(1):32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 40.Nabeshima A, Matsumoto Y, Fukushi J, Iura K, Matsunobu T, Endo M, Fujiwara T, Iida K, Fujiwara Y, Hatano M, et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br J Cancer. 2015;112(3):547–555. doi: 10.1038/bjc.2014.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao S, Hu R, Hambly BD. IL-34, IL-36 and IL-38 in colorectal cancer-key immunoregulators of carcinogenesis. Biophys Rev. 2020 doi: 10.1007/s12551-020-00726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa H, Pinto-Correia AL, Medeiros R, Dinis-Ribeiro M. Epstein–Barr virus is associated with gastric carcinoma: the question is what is the significance? World J Gastroenterol. 2008;14(27):4347–4351. doi: 10.3748/wjg.14.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puranik R, Fox OJ, Sullivan DS, Duflou J, Bao S. Inflammatory characteristics of premature coronary artery disease. Int J Cardiol. 2010;145(2):288–290. doi: 10.1016/j.ijcard.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Chen F, Qu M, Zhang F, Tan Z, Xia Q, Hambly BD, Bao S, Tao K. IL-36 s in the colorectal cancer: is interleukin 36 good or bad for the development of colorectal cancer? BMC Cancer. 2020;20(1):92. doi: 10.1186/s12885-020-6587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F, Zhang F, Tan Z, Hambly BD, Bao S, Tao K. Interleukin-38 in colorectal cancer: a potential role in precision medicine. Cancer Immunol Immunother. 2020;69(1):69–79. doi: 10.1007/s00262-019-02440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Z, Li Z, Wang H, Liu Y, Xu Y, Mo R, Ren P, Chen L, Lu J, Li H, et al. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int. 2017;37(11):1612–1621. doi: 10.1111/liv.13536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Correlation of IL-34, MCSF and CD68+ TAMs expression with clinicopathological parameters of tumour size, lymph node metastasis, tumour invasion depth and TNM subtypes of GC. IL-34, MCSF and CD68+ TAMs all have no correlations with any clinicopathological parameters of tumour size, lymph node metastasis, tumour invasion depth and TNM subtypes of GC.
Additional file 2: Figure S2. Correlation of MCSF and CD68+ TAMs with clinicopathological parameters of age, gender and differentiation subtypes of GC. MCSF and CD68+ TAMs both have no correlations with any clinicopathological parameters of age, gender and differentiation subtypes of GC.
Additional file 3: Figure S3. Survival analysis of MCSF for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of GC patients: there were no correlations of MCSF with survival of gender, age, diameter and lymph node metastasis subtypes of GC patients.
Additional file 4: Figure S4. Survival analysis of MCSF for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of MCSF for prognosis of GC in differentiation, tumour invasion depth and TNM stage subtypes.
Additional file 5: Figure S5. Survival analysis of CD68+ TAMs for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of CD68+ TAMs for prognosis of GC in female, tumour diameter < 5 cm, no lymph node metastasis and T4 stage subtypes.
Additional file 6: Figure S6. Survival analysis of CD68+ TAMs for prognosis of subtypes of GC patients. Kaplan-Meier survival analysis of CD68+ TAMs for prognosis of GC in age, tumour differentiation and TNM stage subtypes.
Additional file 7: Table S1. Univariate analysis of MCSF and clinicopathological factors affecting survival of patients with GC in TNM III–IV.
Data Availability Statement
Yes.