Highlights
-
•
Ocrelizumab is not associated to increased severity in MS patients with COVID-19.
-
•
B-cell depleting treatment could impact on virus-specific antibody production.
-
•
B-cell depleting therapies can reduce IL-6 production, thus modulating inflammation.
Keywords: COVID-19, Disease modifying therapies, DMT, DMD, MS, IL-6
Abstract
Background
Recently SARS-CoV-2 has spread worldwide causing a pandemic. Little is known about disease severity in immunocompromised hosts and people receiving disease modifying therapies (DMTs). In the last decades DMTs have been widely employed, and ocrelizumab represents one of the newest therapies for the relapsing remitting and progressive forms of multiple sclerosis (MS).
Objectives
to describe SARS-CoV-2 related pneumonia in two MS patients under ocrelizumab treatment.
Methods
Case series.
Results
Patients showed a mild clinical course of SARS-CoV-2 related pneumonia without complications or sequelae.
Conclusion
Ocrelizumab treatment is not necessarily associated to increased severity in MS patients with SARS-CoV-2 infection.
1. Main text
1.1. Background
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV-2) is currently causing a pandemic. A few reports on multiple sclerosis (MS) patients receiving disease-modifying therapies (DMTs) are described, so far (Barzegar et al., 2020; Borriello and Ianniello, 2020; Foerch et al., 2020; Ghajarzadeh et al., 2020; Hughes et al., 2020; Meca-Lallana et al., 2020; Montero-Escribano et al., 2020; Novi et al., 2020; Suwanwongse and Shabarek, 2020). Here we analyze the cases of two patients affected by MS under ocrelizumab treatment, who experienced SARS-CoV-2 pneumonia, with a mild clinical course.
1.2. Methods
Demographic and clinical data were directly registered from patients’ clinical folders, while laboratory findings were extracted from the central laboratory electronic software. Concerning SARS-CoV-2 testing, real time reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2, targeting three distinct regions in the envelope (E), nucleoprotein (N) and RNA dependent RNA polymerase (RdRP) genes (AllplexTM 2019-nCoV Assay, Seegene) was performed on nasopharyngeal (NPh) swabs. SARS-CoV-2 serology was assessed either with a lateral flow immunoassay (detecting specific immunoglobulin [Ig]G and IgM), or with a quantitative chemiluminescent immunosorbent assay (CLIA) from DiaSorinTM (IgG only, cutoff: >15 AU/ml).
Case 1
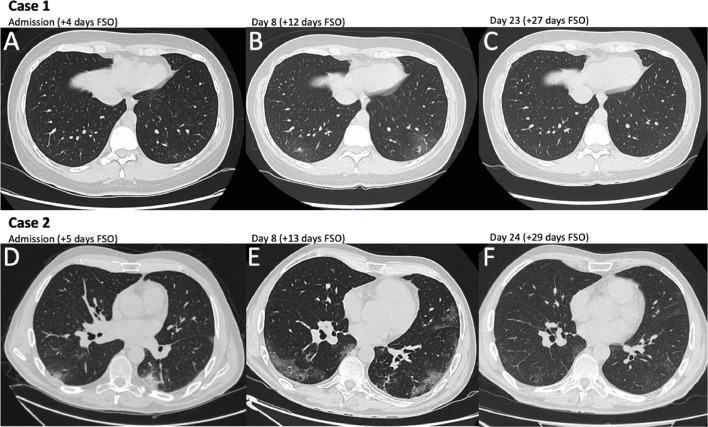
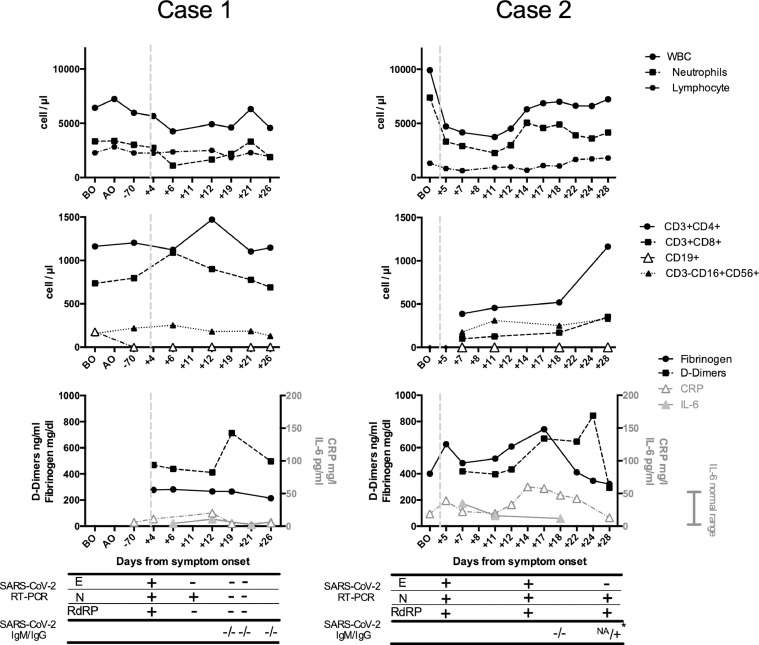
A 36-year-old Caucasian woman was admitted to Tor Vergata Hospital in Rome on March 29th, 2020, because of 4-day fever with dry cough and coryza. Her husband was previously diagnosed with SARS-CoV-2 infection. Her past medical history was notable for papillary thyroid carcinoma in 2014, HPV infection and diagnosis of highly active relapsing remitting multiple sclerosis (RRMS) in 2018. On March 2019 the patient was started on ocrelizumab (last infusion in September 2019). At hospital admission the Expanded Disability Status Scale (EDSS) was 5,5. SARS-CoV-2 infection was assessed with a RT-PCR on a NPh swab, with the detection of E, N and RdRP gene sequences. The chest CT-scan showed the presence of a single ground-glass area in the subpleural region of the inferior lobe of the left lung (Fig. 1 A). Other viral (Influenza, parainfluenza, syncytial respiratory virus, metapneumovirus, adenovirus, rhinovirus) and bacterial (Legionella pneumophila and Streptococcus pneumoniae) infections were excluded. Laboratory findings are represented in Fig. 2 . Plaquenil 200 mg twice daily for 10 days and lopinavir/ritonavir 400/100 mg twice daily for 12 days were administered. During hospitalization chest CT-scan was repeated after 8 days from hospitalization (+12 days from symptom onset), showing bilateral ground-glass opacities of the lungs (Fig. 1B). Notably, D-dimers peaked concomitantly with the worsening of lung infiltrates and tended to normalize with the resolution of pneumonia. No oxygen therapy was needed during hospitalization and the patient was discharged in good clinical condition and unremarkable arterial blood gases, on room air. Two negative NPh swabs for SARS-CoV-2 RT-PCR were obtained after 19 days from symptoms onset. IgG and IgM were undetectable up to 27 days from symptom onset. Follow-up chest CT-scan was performed 27 days after symptom onset and showed the complete resolution of lung ground glass opacities (Fig. 1C).
Fig. 1.
Chest Computed tomography scan imaging of the two MS patients. Computed tomography (CT) scan of the chest were performed in the two MS patients at three different timepoints. Case 1: CT scan at hospital admission (+4 days from symptom onset [FSO]) showed an isolated ground glass area in the subpleural region of the inferior lobe of the left lung (A). At 8 days after hospitalization (+12 days FSO) CT scan of the chest evidenced subpleural bilateral ground glass areas (B). At the follow up visit (+27 days FSO) CT scan of the chest showed the complete resolution of interstitial pneumonia (C). Case 2: CT scan at hospital admission (+5 days FSO) showed bilateral ground glass areas in the subpleural region of the inferior lobes (D). At 8 days after hospitalization (+13 days FSO), ground glass areas were increased in number and extension (E). At hospital discharge (+29 days FSO) CT scan of the chest showed almost complete resolution of interstitial pneumonia (F).
Fig. 2.
Laboratory findings in the two MS patients before and after SARS-CoV-2 infection.
White blood cell (WBC), neutrophil, total lymphocyte (upper panels) and subset (middle panels) absolute counts, fibrinogen (normal range 200-400 mg/dl), D-dimers (normal range 0-500 ng/ml), C-Reactive Protein (CRP, normal range 0-5 mg/l) and IL-6 levels (normal range 0-50 pg/ml) (lower panels) in the two MS patients (case 1 on the left, case 2 on the right), before ocrelizumab first administration (BO), during ocrelizumab treatment prior to SARS-CoV-2 infection (AO) and during hospitalization for SARS-CoV-2 infection are represented. Dashed vertical lines indicate the time of symptom onset. SARS-CoV-2 RT-PCR results on nasopharyngeal swabs and specific serology are reported in the grid, below the lower panels. SARS-CoV-2 IgG and IgM were detected with a lateral flow immunoassay in patient 1 and in patient 2 at +18 days from symptom onset, while a quantitative chemiluminescent immunosorbent assay (CLIA) from DiaSorinTM (asterisk) was employed in patient 2 at +28 days from symptom onset, detecting specific IgG at a very low concentration (17,9 AU/ml, cutoff: >15 AU/ml). IgM test was not available (NA). E: Envelope, N: nucleoprotein, RdRP: RNA dependent RNA polymerase.
Case 2
A 54-year-old Caucasian man was admitted to Tor Vergata Hospital in Rome on April 4th, 2020, because of 5-day fever. Before hospital admission, the patient was living in a nursing home, where other cases of COVID-19 have been diagnosed. His past medical history was notable for the diagnosis of secondary progressive multiple sclerosis (PPMS) in 2003. First line treatment was interferon beta 1a (2004-2011), followed by second line treatment with fingolimod (2011-2017). In 2018 the patient experienced a deep venous thrombosis treated with rivaroxaban and placement of an inferior vena cava filter for the prevention of pulmonary embolism. On November 2018 the patient was started on ocrelizumab (last infusion in November 2019). At hospital admission the EDSS was 7. SARS-CoV-2 infection was assessed with a RT-PCR assay on a NPh swab, with the positivity for E, N and RdRP genes of SARS-CoV-2. The chest CT-scan showed the presence of widespread bilateral ground-glass opacities (Fig. 1C). Other viral and bacterial infections were excluded. Laboratory findings are represented in Fig. 2. During hospitalization, interstitial pneumonia was monitored with chest CT-scans performed at 8 and 24 days after hospitalization, showing first the extension of bilateral ground-glass opacities of the lungs and then the complete resolution (Fig. 1D, E). Notably, leukocyte and CD4 absolute counts were reduced at hospital admission, while CRP, D-dimers and fibrinogen peaked concomitantly with the extension of lung infiltrates and normalized with the resolution of pneumonia. No oxygen therapy was necessary during hospitalization and the patient was discharged in good clinical condition and unremarkable peripheral oxygen saturation, on room air, while NPh swab for SARS-CoV-2 RT-PCR was still positive. IgG and IgM were undetectable up to 18 days from symptom onset, while IgG became slightly detectable after 28 days from symptom onset (17,9 AU/ml, cutoff: >15 AU/ml, CLIA DiaSorinTM)
2. Discussion
Ocrelizumab is an anti CD20 monoclonal antibody causing B-cell depletion, and is currently used for the treatment of MS (Hauser et al., 2017). Considering that the incidence of upper respiratory tract infection was increased in ocrelizumab registration studies (Hauser et al., 2017), there are some concerns about the risk of severe SARS-CoV-2 infection in MS patients treated with ocrelizumab. In our cases, besides the presence of comorbidities and high disability, representing additional risk factors for COVID-19 severity, clinical presentation was mild, IL-6 levels remained within normal ranges and patients did not require oxygen support. No changes in the EDSS or neurological symptoms and signs were observed during patients’ hospitalization. Our findings reflect the preliminary results of an Italian study in MS patients, in which only 5% of 232 cases of SARS-CoV-2 related infections were defined as severe or critical (Sormani, 2020). In a pharmacovigilance case series, reporting disease severity and outcomes of 100 suspected case (74 confirmed) of COVID-19, Hughes et al. concluded that there was no evidence of a more severe course of the disease in ocrelizumab-treated MS patients (Hughes et al., 2020). Furthermore, several authors have reported cases of SARS-CoV-2 related pneumonia in MS patients under ocrelizumab treatment, with a favorable outcome, in the absence of serious complications (Ghajarzadeh et al., 2020; Montero-Escribano et al., 2020; Suwanwongse and Shabarek, 2020). In a case series of 7 MS patients under anti CD20 therapies (1 rituximab and 6 ocrelizumab), only 2 patients showed a severe disease, while all patients recovered from SARS-CoV-2 infection (Meca-Lallana et al., 2020). Considering the documented CD19+ lymphocyte depletion observed in our two patients, B-cell mediated immunity seems to be not essential to recover from SARS-CoV-2 infection. However, B cell impairment could impact on virus-specific serum antibody production, as demonstrated in rituximab long-term treated patients, who can develop hypogammaglobulinemia and show an increased rate of upper respiratory tract infections (Tallantyre et al., 2018). Some reports have already demonstrated an attenuated antibody response to SARS-CoV-2 in patients under ocrelizumab treatment, characterized by the undetectability of specific IgG, several weeks after symptoms’ onset (Conte, 2020; Lucchini et al., 2020; Thornton and Harel, 2020). Very little is known about the effect of anti-CD20 treatment on secretory IgA production in the bronchial mucosa and its associated lymphoid tissue. One case report showed the presence of anti-SARS-CoV-2 specific IgA, despite the absence of IgG, indicating that the mucosal-associated lymphoid tissue (MALT) could be less influenced by ocrelizumab, thus sparing IgA response (Lucchini et al., 2020). Accordingly, in our two patients, serum specific IgG to SARS-CoV-2 were undetectable up to 27 days from symptom onset in case 1, and slightly detectable after 28 days from symptom onset, in case 2.
B-cell depleting therapies are able to reduce IL-6 production, thus modulating inflammation and T-cell activation (Barr et al., 2012). Consequently, we could speculate that ocrelizumab could have contributed to modulate IL-6 production in our two patients, despite the absence of specific anti-IL6 receptor treatment, such as tocilizumab and sarilumab, which have been administered in SARS-CoV-2 infected patients (Lu et al., 2020).
In conclusion, ocrelizumab is not necessarily associated to increased severity in MS patients with SARS-CoV-2 infection, even in patients with severe B-cell impairment. More studies and larger cohorts are needed to clarify the clinical management of MS patients under DMTs in the setting of SARS-CoV-2 epidemic and the impact of DMTs on antiviral antibody production.
Funding source
No funding declared.
CRediT authorship contribution statement
Marco Iannetta: Conceptualization, Writing - original draft, Writing - review & editing. Novella Cesta: Investigation, Data curation. Christof Stingone: Investigation, Data curation. Vincenzo Malagnino: Writing - review & editing. Elisabetta Teti: Writing - review & editing. Pietro Vitale: Investigation, Data curation. Giuseppe De Simone: Investigation, Data curation. Benedetta Rossi: Investigation, Data curation. Lorenzo Ansaldo: Investigation, Data curation. Mirko Compagno: Investigation, Data curation. Ilaria Spalliera: Investigation, Data curation. Andrea Di Lorenzo: Investigation, Data curation. Doriana Landi: Writing - review & editing. Carolina Gabri Nicoletti: Investigation, Data curation. Girolama Alessandra Marfia: Writing - review & editing. Massimo Andreoni: Supervision, Writing - review & editing. Loredana Sarmati: Supervision, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgments
We thank all the nurses and paramedics of the COVID Infectious Disease Unit of Policlinico Tor Vergata of Rome, Italy.
PTV-ID-COVID Group: Massimo Andreoni, Lorenzo Ansaldo, Filippo Barreca, Andrea Buoso, Federica Caldara, Laura Campogiani, Marcella Capozzi, Laura Ceccarelli, Novella Cesta, Davide Checchi, Mirko Compagno, Luigi Coppola, Angela Maria Antonia Crea, Giuseppe De Simone, Andrea Di Lorenzo, Luca Dori, Ludovica Ferrari, Luca Foroghi Biland, Adele Maria Gentile, Marco Iannetta, Alessandra Lodi, Vincenzo Malagnino, Giusella Moscato, Tiziana Mulas, Pier Giorgio Pace, Benedetta Rossi, Loredana Sarmati, Ilaria Spalliera, Christof Stingone, Simona Tedde, Elisabetta Teti, Pietro Vitale, Erika Zampieri, Marta Zordan
References
- Barr T.A., Shen P., Brown S., Lampropoulou V., Roch T., Lawrie S., Fan B., O'Connor R.A., Anderton S.M., Bar-Or A., Fillatreau S., Gray D. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J. Exp. Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Mirmosayyeb O., Nehzat N., Sarrafi R., Khorvash F., Maghzi A.-H., Shaygannejad V. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello G., Ianniello A. COVID-19 occurring during Natalizumab treatment: a case report in a patient with extended interval dosing approach. Mult. Scler. Relat. Disord. 2020;41 doi: 10.1016/j.msard.2020.102165. 10.1016/j.msard.2020.102165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W.L. Attenuation of antibody response to SARS-CoV-2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerch C., Friedauer L., Bauer B., Wolf T., Adam E.H. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajarzadeh M., Mirmosayyeb O., Barzegar M., Nehzat N., Vaheb S., Shaygannejad V., Maghzi A.-H. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Bar-Or A., Comi G., Giovannoni G., Hartung H.-P., Hemmer B., Lublin F., Montalban X., Rammohan K.W., Selmaj K., Traboulsee A., Wolinsky J.S., Arnold D.L., Klingelschmitt G., Masterman D., Fontoura P., Belachew S., Chin P., Mairon N., Garren H., Kappos L., OPERA I, and OPERA II Clinical Investigators Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- Hughes R., Pedotti R., Koendgen H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab – a pharmacovigilance case series. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.-C., Chen M.-Y., Lee W.-S., Chang Y.-L. Potential therapeutic agents against COVID-19: What we know so far. J. Chin. Med. Assoc. 2020;83:534–536. doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini M., Bianco A., Del Giacomo P., De Fino C., Nociti V., Mirabella M. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meca-Lallana V., Aguirre C., Río Beatrizdel, Cardeñoso L., Alarcon T., Vivancos J. COVID-19 in 7 multiple sclerosis patients in treatment with ANTI-CD20 therapies. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Escribano P., Matías-Guiu J., Gómez-Iglesias P., Porta-Etessam J., Pytel V., Matias-Guiu J.A. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novi G., Mikulska M., Briano F., Toscanini F., Tazza F., Uccelli A., Inglese M. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19:481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanwongse K., Shabarek N. Benign course of COVID-19 in a multiple sclerosis patient treated with Ocrelizumab. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Whittam D.H., Jolles S., Paling D., Constantinesecu C., Robertson N.P., Jacob A. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J. Neurol. 2018;265:1115–1122. doi: 10.1007/s00415-018-8812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]