Abstract
Cardioversion is widely used in patients with atrial fibrillation (AF) and atrial flutter when a rhythm control strategy is pursued. We sought to summarize the current evidence on this important area of clinical management of patients with AF including electrical and pharmacological cardioversion, peri-procedural anticoagulation and thromboembolic complications, success rate, and risk factors for recurrence to give practical guidance.
Keywords: Atrial fibrillation, Atrial flutter, Electrical cardioversion, Pharmacological cardioversion, Anticoagulation, Thromboembolism
Introduction
Cardioversion, either by a synchronized direct current (DC) electrical shock (electrical cardioversion, ECV) or by the application of antiarrhythmic drugs (AADs; pharmacological cardioversion, PCV), is an integral part of the management of atrial fibrillation (AF) and atrial flutter (AFL) in symptomatic patients who require a rhythm control strategy.1 Electrical cardioversion may also be appropriate as a one-time diagnostic shock in supposedly asymptomatic patients with persistent AF to evaluate, whether they nevertheless show improved exercise tolerance during sinus rhythm.2 The first reports on PCV of AF using quinidine were published in the late 1940s, while ECV of AF by synchronized DC shock was introduced in the early 1960s.3,4 These procedures are readily available and easy to perform with a high overall success rate. Nevertheless, several important points must be considered before embarking on this treatment, among others the need for cardioversion,5 the mode (ECV or PCV) and timing of cardioversion, assessment of the individual peri-procedural thromboembolic risk of the patient, anticoagulant therapy, and peri-procedural or subsequent long-term therapy with AADs.
This review summarizes the current scientific evidence for undertaking ECV and PCV, the occurrence of thromboembolic events with cardioversion, image-guiding of cardioversion, and antithrombotic therapy when performing cardioversion. We also give some practical advice for this widely used therapy.
Electrical and pharmacological cardioversion
Electrical cardioversion terminates AF in over 90% of cases and is the treatment of choice in severely haemodynamically compromised patients with new-onset AF or AFL.1 Pharmacological cardioversion mainly converts recent-onset or paroxysmal (i.e. in principle self-terminating) AF to sinus rhythm in 50–70% of cases within a few hours, when sodium channel blockers (mainly propafenone or flecainide) or vernakalant are used, while these drugs rarely convert AF of longer duration.1,6 Compared to AF, ECV is more effective in AFL, also requiring less energy.7,8
Electrical cardioversion can be performed safely under short sedation with i.v. midazolam and/or propofol and continuous blood pressure monitoring and oximetry during the procedure.9 Electrical cardioversion is more effective when using a biphasic defibrillator, and around 40% of patients are pre-treated with an AAD at their ECV.10 An antero-posterior electrode position (Figure 1) restores sinus rhythm better compared to antero-apical.11 Starting with the maximum shock energy available seems more effective than escalating shock energies.12 In patients with an implanted pacemaker or implantable cardioverter-defibrillator (ICD), damage to the system can be avoided by biphasic ECV in the antero-posterior paddle position.13 Even in patients with implanted defibrillators, ECV seems preferable to internal cardioversion performed with the ICD.14
Figure 1.
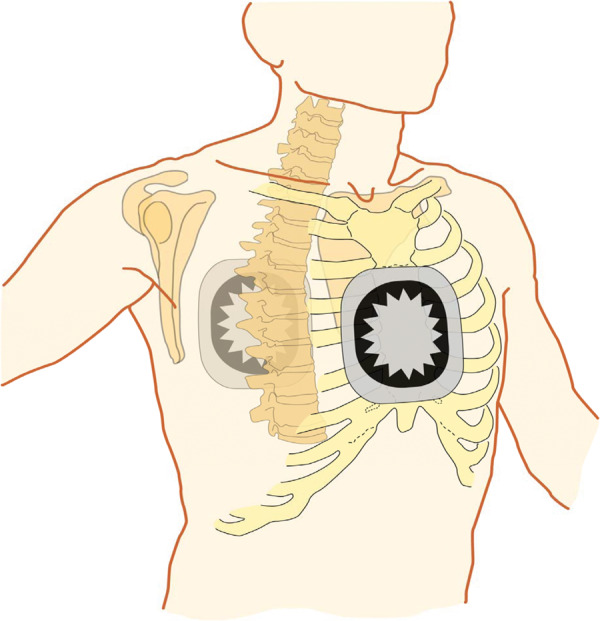
Antero-posterior electrode position for ECV. ECV, electrical cardioversion. Modified after Kirchhof et al.,15 with permission.
Complications of rhythm control with ECV include sedation-related complications, hypotension, ventricular fibrillation due to inappropriate shock synchronization, bradycardias (frequently diagnostic, i.e. unmasking sick sinus or sick atrioventricular node syndrome), tachycardias, such as AFL with 1:1 conduction or torsade de pointes. Cardiac biomarker release and transient ST-segment elevation seen after ECV are self-limiting and may relate to previous cardiac surgery.16 Real-world data from a contemporary cohort of 1801 patients undergoing ECV or PCV show that complications, in general, are rare (Table 1).17
Table 1.
Major complications of PCV and ECV in 1801 real-world patients from the Euro Heart Survey
| PCV (n = 1089), N (%) | ECV (n = 712), N (%) | |
|---|---|---|
| Non-sudden cardiac death | 1 (0.1) | 2 (0.3) |
| Sick sinus syndrome | 5 (0.5) | 5 (0.7) |
| Ventricular tachycardia | 2 (0.2) | 6 (0.8) |
| Torsades de pointes | 3 (0.3) | 1 (0.1) |
| Ventricular fibrillation | 0 | 3 (0.4) |
| Asystole | 7 (0.7) | 2 (0.3) |
| Cardiac syncope | 8 (0.8) | 1 (0.1) |
| Pulmonary embolism | 1 (0.1) | 0 |
| Myocardial infarction | 4 (0.4) | 0 |
| Transient ischaemic attack | 13 (1.3) | 2 (0.3) |
| Non-haemorrhagic stroke | 1 (0.1) | 2 (0.3) |
| Heart failure | 9 (1.0) | 7 (1.1) |
| Major bleeding | 10 (1.0) | 9 (1.3) |
Modified after Pisters et al.,17 undefined with permission.
ECV, electrical cardioversion; PCV, Pharmacological cardioversion.
Key points
Electrical cardioversion terminates AF in over 90% of cases and is the treatment of choice in haemodynamically compromised patients.
Pharmacological cardioversion mainly converts recent-onset AF of <48 h duration.
Electrical cardioversion with an antero-posterior electrode position restores sinus rhythm better than with an antero-apical position.
Complications of ECV and PCV are generally rare.
Timing of electrical cardioversion
The RACE 7 ACWAS trial (Rate Control vs. Electrical Cardioversion Trial 7–Acute Cardioversion vs. Wait and See) showed that a wait-and-see approach (with initial rate control and delayed cardioversion only if needed) allowed almost 70% of patients with recent-onset AF reporting at the emergency department to regain sinus rhythm spontaneously vs. only 16% under immediate cardioversion. At 48 h and 4 weeks after index AF, the number of patients in sinus rhythm was similar, i.e. over 90% in both groups. Atrial fibrillation found at 30 days was incidental recurrences or persistent AF. Notably, up to 30% had a recurrence of self-terminating paroxysmal AF after the index conversion, which was also similar between groups.5
Key points
In most patients with recent-onset AF, immediate cardioversion may be replaced by a wait-and-see approach as the default approach with delayed cardioversion as needed.
Timing of recurrences after cardioversion of persistent atrial fibrillation
The response to ECV of persistent (i.e. clinically non-self-terminating) AF is represented by the 1-1-1-1-1 rule (Figure 2). Immediately after the shock, it may be apparent that no single sinus beat was seen (no conversion and shock failure), which may be due to failure of complete capture of the atria by the DC shock. In the subsequent minute, immediate recurrence of AF (IRAF) may occur, which may relate to instantaneous post-shock hyper-vulnerability.18,19 Thereafter, 1 day of uninterrupted sinus rhythm occurs, during which the atria are incapable of fibrillating (the so-called relapse gap), which presumably relates to atrial stunning.19 Thereafter, sub-acute recurrences are common over a period of 1–2 weeks due to spatially non-uniform electrical reverse remodelling that enhances electrical instability of the atria.20 Once reversed electrical remodelling is complete, the rate of recurrences decreases, which is represented by the subsequent phase of late re-occurrences during which AF recurrences appear at a much lower rate (Figure 2).
Figure 2.
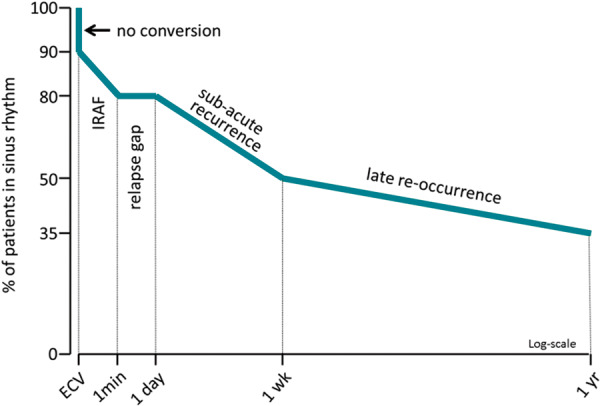
The 1-1-1-1-1 pattern of recurrence after ECV of persistent AF. Modified after Van Gelder et al.,2 with permission. AF, atrial fibrillation; ECV, electrical cardioversion; IRAF, immediate recurrence of atrial fibrillation.
Predictors of successful ECV of persistent AF include AF duration, age of the patient, better functional class of the patients, and whether or not pre-treatment with AAD is applied.1,21 Drugs affect the various stages differently. First, acute failure of cardioversion can be prevented by pre-treatment with AADs (Table 2).1 Next, the influence of AADs on recurrences depends on stage-related arrhythmogenic mechanisms and their subsidence during reversed electrical remodelling induced by persistent sinus rhythm after conversion. Immediate recurrences of AF can be prevented by ibutilide but also by sodium channel blockers and probably by sotalol and amiodarone.1,22,23 Immediate recurrences of AF may be further reduced by adding verapamil to Class I or III AADs.24 Immediate recurrence of AF-related cardioversion failure can also be ameliorated by reapplying a shock immediately after it appears, since the longer the sinus rhythm episodes last between the consecutive IRAFs, the higher the chance the relapse gap can be reached. The relapse gap may be lengthened by all AADs but notably also by intracellular calcium-lowering drugs including verapamil and beta-blockers as well as angiotensin receptor blockers.19 Later (sub-acute) recurrences are prevented by all AADs but even better if combined with an angiotensin receptor blocker or verapamil.25,26 Beta-blockers alone may also reduce sub-acute recurrences.27 Presumably, these (add-on) agents control intracellular calcium overflow in atrial cells not used to large calcium transients after having been in AF for months. Late re-occurrences (Figure 2) respond well to AADs and may be managed by season-ticket-ECV, i.e. repeat single ECVs, in patients with low risk of recurrence.23,28 However, if recurrence risk is high, catheter ablation is the preferred option.1,29
Table 2.
Drugs affecting cardioversion by lowering ECV threshold or suppressing IRAF
| Decrease threshold for cardioversion or suppress IRAF | Suppress sub-acute recurrences |
|---|---|
| Quinidine | Quinidine |
| Propafenone | Propafenone |
| Flecainide | Flecainide |
| Amiodarone | Amiodarone |
| Sotalol | Amiodarone + ARBs |
| Ibutilide | Beta-blockers |
| Verapamil on top of other AADs | Verapammil on top of other AADs |
| Uncertain effect | Uncertain effect |
| Procainamide | Verapamil |
| Disopyramide | Diltiazem |
| Dofetilide | Dofetilide |
| Beta-blockers | |
| Verapamil | |
| Diltiazem |
Also, drugs that suppress sub-acute (see Figure 2) recurrences are shown.
AAD, antiarrhythmic drug; ARB, angiotensin receptor blocker; ECV, electrical cardioversion; IRAF, immediate recurrence of atrial fibrillation.
‘Diagnostic electrical cardioversion’—a possible new indication
In some patients with persistent AF, e.g. those with heart failure both with reduced and with preserved ejection fraction but also others, the relationship between symptoms and arrhythmia may be unclear. In those patients, a ‘diagnostic ECV’ may be performed to show improvement of symptoms (or not) when in stable sinus rhythm. To enhance such assessment, the period in sinus rhythm may be lengthened by using temporary amiodarone or flecainide.23,30 Studies are needed in this area to show usefulness of such an approach.
Key points
Predictors of successful ECV of persistent AF are AF duration, patient age, better function class, and pre-treatment with AADs.
Termination of recent-onset or paroxysmal atrial fibrillation
Successful drug conversion of paroxysmal or recent-onset AF depends on pre-defined time to conversion, previous AF duration, type of AF (persistent AF does not usually respond), type of drug (Class Ic and vernakalant vs. all other AADs), intravenous vs. oral route of administration, and extend of underlying heart disease. Electro-echocardiography may show electrophysiological effects of AADs and predict drug conversion, although the value of these tools needs further assessment.31,32
For immediate restoration of sinus rhythm with PCV, intravenous flecainide, propafenone, or vernakalant are most effective in patients with recent-onset AF. These agents are very safe in patients without significant structural heart disease.1,10,23 Flecainide or propafenone may also be given orally using specific dosing schemes, including pill-in-the-pocket.33,34 Atrial flutter responds to Class III AAD, while Class Ic agents are not useful since they almost always fail and can actually create a substrate for flutter.35,36 If applied for the first time, it is reasonable to perform PCV in-hospital to observe potential adverse effects.1,37,38 Also, other agents are used for immediate cardioversion, among them even the typical rhythm control agents amiodarone and sotalol (Figure 3), which mainly control rate rather than rhythm in the initial hours of treatment and, therefore, are ineffective conversion drugs.10 In persistent AF, marinating the atria in amiodarone or sotalol administered orally for 1 month is associated with a modest conversion rate around 25%.28 Also, chronic administration of flecainide and propafenone may convert persistent AF but—as a side effect—may cause fast ventricular rates during ongoing AF.39,40 Verapamil may enhance chronic drug conversion with amiodarone.41 Although this pharmacological effect seems modest, it could be useful when temporary AAD therapy prior to ECV is considered.23,30
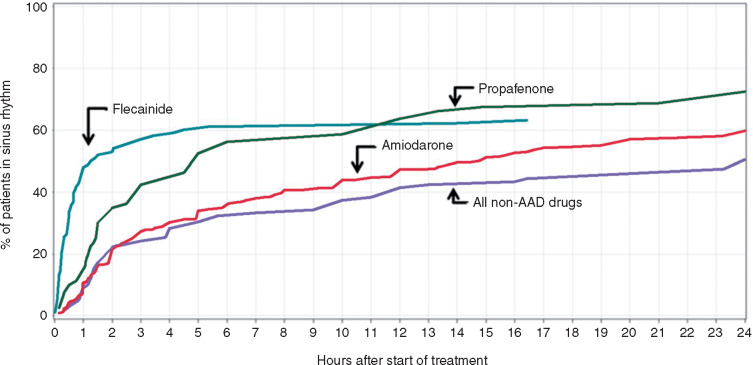
Figure 3.
Conversion to sinus rhythm over time after start of drug therapy for recent-onset AF. Class Ic AADs foreshorten time to sinus rhythm significantly. Amiodarone and non-AAD (rate control drugs) are associated with spontaneous conversion, with minimal conversion action of amiodarone discernable from 6 h on. At the end of the day around 60–70% of patients reached sinus rhythm. Modified after Crijns et al.,10 with permission. AAD, antiarrhythmic drug; AF, atrial fibrillation.
Notwithstanding the above, recent-onset or paroxysmal AF may terminate spontaneously (Figure 3). Antiarrhythmic drugs foreshorten time to sinus rhythm but at the end of the day, numbers of patients in sinus rhythm are the same.5,10
A risk-based approach to choosing long-term rhythm control therapy after cardioversion (no therapy, AADs, catheter ablation, or combination of these two treatments) is desirable. Prediction models for recurrent AF are being developed and will need to be based on representative, combined data sets.42
Key points
Intravenous Class Ic AADs or vernakalant are most effective in restoring recent-onset AF.
When Class Ic AADs are intended to be used as pill-in-the-pocket approach, the very first administration should be performed in-hospital to observe potential adverse effects.
Atrial flutter is restored by Class III AADs but not Class Ic AADs.
Thromboembolic events with cardioversion
Although cardioversion of AF or AFL is considered safe in general, cardioversion is associated with an increased risk of thromboembolic events.1,43 There is no apparent difference in the risk of thromboembolic events of PCV or ECV and no difference between AF and AFL.44 Thromboemboli after cardioversion are considered due to embolization of already existing thrombi present in the atrium at the time of cardioversion or to the formation and subsequent embolization of de novo thrombi in the atrium that form while atrial function is still depressed in the weeks after cardioversion.45,46
Key points
Electrical cardioversion and PCV carry the same thromboembolic risk.
Cardioversion of AF and AFL carry the same thromboembolic risk.
Thromboembolic events in atrial fibrillation patients without anticoagulant therapy
Historical data showed an incidence of thromboembolic events of 2% in AF patients without anticoagulation and 0.33% in those receiving vitamin K antagonists (VKAs),47 while a more recent large retrospective Danish study of 16 274 patients discharged from hospital after a first-time ECV for AF reported a lower thromboembolic event rate during the first 30 days after cardioversion of 1.1% without and 0.28% with VKA treatment.48 The different event rates reported may also be due to study design and definitions of thromboembolic events collected. An incidence rate of ∼1% was also observed in a retrospective study by Gallagher et al.,44 who looked at 1950 case records of patients who underwent 2639 ECV. Cardioversion was preceded by VKA treatment for at least 3 weeks in 73% of all cardioversions. Of 756 cases with an international normalized ratio (INR) <2.5 or no measurement before conversion, a thromboembolic event occurred in 1.2%, while in those with an INR >2.5 no thromboembolic events were reported. Patients without anticoagulation and those with inadequate anticoagulation seem to have a comparable thromboembolic event rate of around 1%.
A specific population are patients with AF lasting <48 h. These patients are considered to have a low risk of thromboembolic events post-cardioversion.49 The retrospective Finnish CardioVersion (FinCV) study included 7660 cardioversions in 3143 patients.50 The 30-day thromboembolic event rate was 0.7%, which was in concordance with six prior small retrospective studies.50,51 The FinCV study also demonstrated that in cardioversions of AF <48 h without anticoagulation, the risk of thromboembolic events increased with an increasing CHA2DS2-VASc score (from 0.4% in those with a score of 0–2.3% in those with score of ≥5). The incidence of thromboembolic events was significantly lower in cardioversions performed on anticoagulation, and the preventive effect of anticoagulation was significantly greater in patients with a CHA2DS2-VASc score of ≥2.52 A large retrospective Swedish study in more than 22 000 patients, who were cardioverted with or without oral anticoagulant (OAC) pre-treatment, found similar results.53
Thromboembolic events in atrial fibrillation patients receiving anticoagulant therapy
In anticoagulated patients as included in the European RHYTHM-AF registry, a thromboembolic event rate of 0.51% (15 embolic events in 2940 patients) was reported with no differences in thromboembolic risk between AF of >48 h or unknown duration compared to AF of <48 h (0.4% vs. 0.3%).54 Similar event rates in anticoagulated patients were reported from the Flec-SL (Flecainide Short-Long) trial in 508 patients after ECV and 127 patients after PCV. In total, six patients developed a thromboembolic event (event rate 0.8%) independent of the type of cardioversion, of which three occurred in the first 5 days after cardioversion.55 Lastly, a meta-analysis of four randomized controlled trials comparing non-vitamin K antagonist oral anticoagulant (NOAC) therapy with VKAs, including 4517 cardioversions in 3635 patients, found a thromboembolic event rate of 0.41% on NOAC therapy and 0.61% on VKAs.56
Key points
Reported peri-cardioversion thromboembolic event rates are between 1.1% and 2% in patients not or insufficiently anticoagulated and between 0.28% and 0.8% in patients sufficiently anticoagulated.
In patients with AF lasting <48 h the thromboembolic event rate without anticoagulation is around 0.7% and increases with CHA2DS2-VASc score. It is significantly reduced with anticoagulation.
Temporal incidence of thromboembolic events after cardioversion
The timing of thromboembolic events after ECV of AF or AFL was analysed by Berger and Schweitzer,57 based on data from 32 studies (published up to 1997) and a total of 4621 patients, including 92 patients with a thromboembolic event post-cardioversion. The interval between cardioversion and thromboembolic episodes ranged from <1 to 18 days (Figure 4). Of the 92 embolic events, 75 (82%) occurred within 3 days, 88 (96%) within 1 week, and 90 (98%) within 10 days of ECV. Current guidelines, therefore, recommend using anticoagulation up to 4 weeks after cardioversion.1,43 Factors contributing to peri-procedural thromboembolism include the presence of pre-existing thrombi, transient atrial stunning after cardioversion, changes in mechanical atrial systolic function, left atrial size, and a prothrombotic state.58
Figure 4.
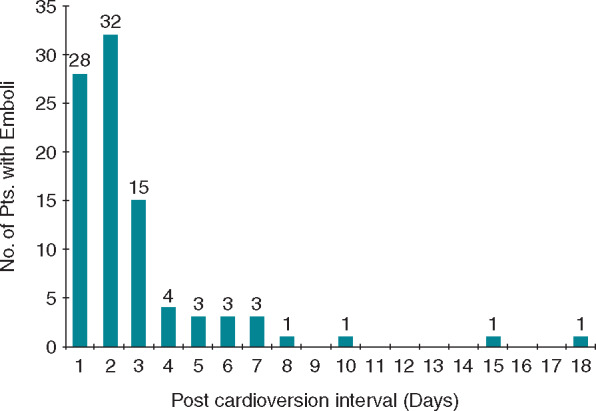
Interval between cardioversion and thromboembolic events in 92 patients. From Berger and Schweitzer57 with permission.
Key points
Almost all thromboembolic events with cardioversion occur within 10 days after the procedure.
Therefore, anticoagulation up to 4 weeks after cardioversion is recommended.
Image-guided cardioversion
Risk factors for thromboembolism: clinical factors and information from transoesophageal echocardiography
A recent meta-analysis showed that left atrial (LA) thrombus is observed in about 10% of non-valvular AF patients with increased risk in patients with higher age, hypertension, female gender, diabetes, and heart failure.59 Patients with LA thrombus have a higher CHADS2 score (mean difference 0.88, 95% confidence interval: 0.68–1.07) and a 3.5-fold increased risk of stroke/systemic embolism.59 A recent post hoc analysis from the ENSURE-AF (edoxaban vs. warfarin in subjects undergoing cardioversion of AF) study demonstrated that only age and heart failure were independent risk factors for the detection of LA thrombi.60 Thrombus formation is most frequently observed in the left atrial appendage (LAA) but may also occur in the LA cavity,61 although this is more often associated with mitral valve disease rather than AF or AFL.62
Transoesophageal echocardiography (TOE) enables evaluation of LAA morphology and flow patterns within it, and TOE is the gold standard to rule out thrombus formation,1,63 whereas transthoracic echocardiography has limited ability to evaluate the LAA. TOE has a sensitivity of about 92% and a specificity of 98% (with negative and positive predictive values of 100 and 86%) for the identification of LAA thrombosis in patients with AF when compared to intraoperative findings.64,65 The sensitivity of TOE can be improved by ultrasound contrast agents that opacify the appendage and facilitate identification of filling defects.66 Standard TOE may also be complemented by the use of three-dimensional TOE and tissue-Doppler imaging, including speckle tracking.67,68 Three-dimensional TOE allows a more comprehensive LAA assessment by overcoming inadequate imaging planes and other limitations of two-dimensional imaging.63
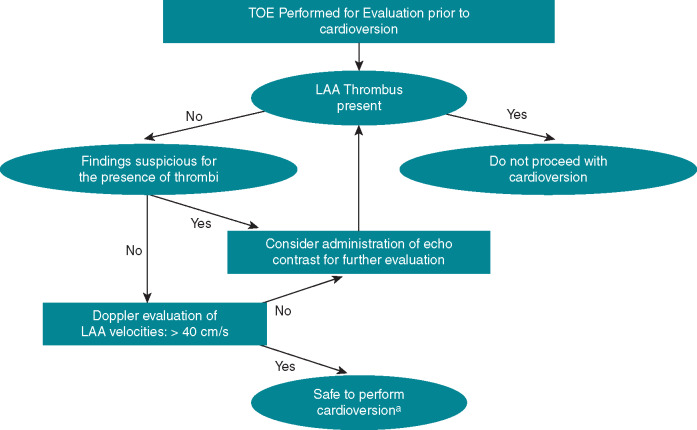
Because of the multilobed and complex anatomy of the LAA, visualizing the entire LAA to exclude small thrombi is often challenging. Absence of colour flow in the distal part of the LAA or side lobes may indicate a filling defect caused by thrombus formation. Functional evaluation of LAA and the risk of thromboembolism by Doppler echocardiography is recommended.63 Left atrial appendage flow can be assessed upon alignment of the pulsed-wave Doppler signal using colour flow imaging with sampling in the proximal third of the LAA, where maximal flow velocities are obtained. Velocities <40 cm/s are associated with presence of spontaneous echocardiographic contrast (SEC) and, in particular, velocities below 20 cm/s, associate with identification of LAA thrombi and increased risk of thromboembolic events.69–72 In fact, SEC, which is promoted by reduced LAA flow velocities, is the cardiac factor most strongly associated with LAA thrombus and embolic events.70 Accordingly, the presence of low flow velocities and/or SEC requires meticulous evaluation of the LAA before cardioversion. In addition to SEC and low LAA flow velocities, TOE may also help identifying other predictors of thromboembolism, e.g. complex aortic plaques. An algorithm detailing echocardiographic evaluation of LAA prior to cardioversion has previously been provided (Figure 5).63
Figure 5.
Schematic approach for the TOE evaluation of the LAA before cardioversion. From Beigel et al.,63 with permission. aSafe if no other contraindications exist for the patient. TOE refers to 2D TOE, but 3D TOE should be used, if available, to increase sensitivity and specificity of findings. 2D, 2-dimensional; 3D, 3-dimensional; LAA, left atrial appendage; TOE, transoesophageal echocardiography.
Key points
A left atrial thrombus is observed in about 10% of non-valvular AF.
Thrombus formation is most frequently observed in the LAA.
Transoesophageal echocardiography is the gold standard to rule out left-atrial thrombus formation.
Low flow in the LAA is associated with SEC, LAA thrombi, and thromboembolic events.
Imaging of the left atrial appendage: new modalities
Excluding in situ thrombosis is crucial before cardioversion of AF and AFL, and although TOE is the gold standard,1,63multidetector computed tomography (MDCT) and cardiac magnetic resonance (CMR), are emerging as new valuable modalities for imaging and assessment of LAA anatomy and function.63 Also, as described above, the number of opportunities for increasing the information gained from echocardiography is growing. Although transthoracic echocardiography currently has a limited role in the evaluation on the LAA, the use of harmonic imaging and ultrasound contrast agents have enhanced the sensitivity for detection of LAA thrombi.73 During planned interventional cardiac procedures, intracardiac echocardiography (ICE) provides an alternative imaging method, when TOE is not feasible. Intracardiac echocardiography reliably diagnoses LAA thrombi74 but is less sensitive than TOE.75
Multidetector computed tomography provides three-dimensional volumetric data of the entire heart, including the LAA, with high spatial (0.24–0.4 mm) and temporal (66–100 ms) resolution enabling identification of LAA thrombi (Figure 6) and spontaneous contrast formation similar to the SEC observed by TOE.76–78 The sensitivity of MDCT to detect LAA thrombus is up to 100% compared to TOE,79 whereas the positive predictive value is between 41% and 92% depending on the type of data acquisition.80 The performance of MDCT is enhanced when delayed imaging is used to differentiate between reduced LAA filling and SEC or thrombus.80 Cardiac magnetic resonance has high temporal resolution (30–50 ms) and visualizes LAA size and function, and CMR may be used for detection of thrombus in patients with AF. Also, LAA blood flow can be measured by velocity-encoded techniques.81 The lack of need for radiation is an advantage, whereas limitations of CMR include a lower spatial resolution (1–2 mm), lengthy scanning procedures and inability to be performed in the majority of patients with implanted cardiac devices.
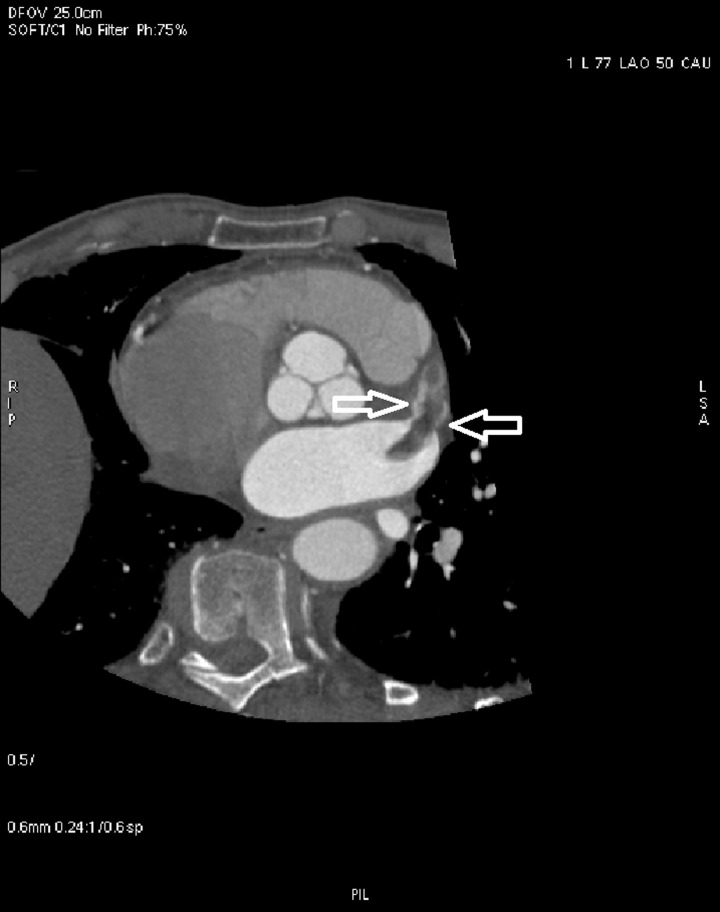
Figure 6.
LAA thrombus (arrows) on cardiac CT. CT, computed tomography; LAA, left atrial appendage.
A recent systematic review and meta-analysis of 22 MDCT and 4 CMR studies compared the diagnostic performance of MDCT and CMR with TOE for identification of LAA thrombi.82 Multidetector computed tomography demonstrated sensitivity and specificity of 0.99 and 0.94 vs. TOE with significantly improved specificity of the delayed imaging protocols. Cardiac magnetic resonance demonstrated sensitivity and specificity of 0.80 and 0.98 when compared with TOE. There was no significant difference in the sensitivity or specificity between MDCT and CMR.
Transoesophageal echocardiography-guided cardioversion
Current guidelines recommend TOE to exclude intra-cardiac thrombi, if a strategy of early cardioversion without being therapeutically anticoagulated the preceding 3 weeks is pursued in patients who have been in AF for >48 h.1,83 Oral anticoagulation treatment should be initiated immediately in all patients scheduled for cardioversion and maintained for at least 4 weeks. Long-term OAC treatment is based on the thromboembolic risk profile of the individual patient and should be assessed using the CHA2DS2-VASc score.1,83 Cardioversion can be performed safely, if no LA thrombus is identified, provided that sufficient anticoagulation is achieved before TOE.83 Thus, timing of cardioversion in relation to initiation of anticoagulant therapy is crucial and should depend on the pharmacokinetics of the drug chosen, as the procedure should be performed under therapeutic anticoagulation. If a thrombus is identified on TOE, appropriate anticoagulation is recommended for at least 3 weeks, before a repeat TOE is done to ensure thrombus resolution.84
Several randomized and observational studies investigating both low-molecular-weight heparin (LMWH)/warfarin and different NOACs have demonstrated that TOE-guided cardioversion is as safe as a conventional cardioversion strategy with at least 3 weeks of OAC pre-treatment in terms of thromboembolic events and bleeding.85–88 In the ACUTE (Assessment of Cardioversion Using Transesophageal Echocardiography) Study, the rate of haemorrhagic events was even significantly lower in the TOE-guided cardioversion group. On the other hand, the TOE-group showed a numerical trend towards more deaths.85 While some studies show greater rates of successful restoration of sinus rhythm with TOE-guided cardioversion,85,86 inconsistent results exist as to long-term maintenance of sinus rhythm.85,89
Key points
When no LA thrombus is identified on TOE, cardioversion can be performed safely, provided that sufficient anticoagulation is achieved before TOE.
Studies comparing cardioversion on non-vitamin K oral anticoagulant vs. vitamin K antagonist and special subgroups
Since the first NOAC became available for stroke prevention in AF in Europe in 2011, their use has been rapidly increasing.90–92 A similar trend has been observed in patients undergoing cardioversion.93–95 Nevertheless, registry data from 2015 still showed a relatively high proportion of cardioversions performed on VKAs.93
There is growing evidence that NOACs can safely be used for stroke prevention in patients undergoing cardioversion of AF. The first data came from post hoc analyses of the pivotal Phase III studies comparing NOACs with warfarin. In the RE-LY trial, 1983 cardioversions were performed in 7% of the 18 113 patients included, equally distributed across the three treatment arms (dabigatran 150 mg b.i.d., dabigatran 110 mg b.i.d., and dose-adjusted warfarin). Most patients were treated with the study drug for ≥3 weeks before cardioversion. Rates of thromboembolic events and major bleeding were low across all treatment arms (Table 3) suggesting that treatment with both doses dabigatran was as safe and effective as with warfarin.96 Three minor post hoc analyses from the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation), and ENGAGE AF-TIMI 48 [Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction 48 (edoxaban)] showed comparable results.97–99
Table 3.
Large studies investigating NOACs vs. VKA in the cardioversion setting
| Study (year) | RE-LY (2011)96 undefined | X-VeRT (2014)87 undefined | ENSURE-AF (2016)88 undefined | EMANATE (2018)100 undefined |
|---|---|---|---|---|
| Study type | Multicentre, international, post hoc analysis | Multinational, randomized, open-label, parallel-group Phase IIIb study | Multicentre, prospective, randomized, open-label, parallel-group with blinded endpoint | Multinational, prospective, randomized, open-label with blinded endpoint adjudication |
| NOAC | Dabigatran | Rivaroxaban | Edoxaban | Apixaban |
| Total number of patients (NOAC/warfarin) (N) | 1270 (1319/664)a | 1504 (1002/502) | 2199 (1095/1104) | 1500 (753/747) |
| Follow-up | 30 days | 30 days | 58 days | 30 days (90 days in patients not converted) |
| NOAC dosing | 110 mg b.i.d. and 150 mg b.i.d. | 20 mg o.d.b | 60 mg o.d.c | 5 mg b.i.d.d |
| Outcomes | Primary: stroke, systemic embolism and major bleeding |
|
|
|
| Age (years) | 71.5 ± 8.8 (dabigatran 150 mg), 71.4 ± 8.6 (dabigatran 110 mg), 71.6 ± 8.6 (warfarin)e | 64.9 ± 10.6 (rivaroxaban), 64.7 ± 10.5 (VKA) | 64.3 ± 10.3 (edoxa), 64.2 ± 10.8 (enoxaparin + warfarin) | 64.7 ± 12.2 (apixaban), 64.5 ± 12.8 (heparin/warfarin) |
| CHA2DS2-VASc score ≥2 | N/R | 959/1504 (63.76%) | 1707/2199 (77.63%) | mean 2.4 ± 1.7 |
| TTR in warfarin-treated patients (%) | N/R | N/R | 70.8 ± 27.4 | 65% (beyond first 2 weeks of treatment) |
| TOE-guided cardioversion, n (%) |
|
|
|
|
| Patients with primary efficacy outcome, n (%) |
|
|
|
|
| Patients with primary safety outcome, n (%) |
|
|
|
|
b.i.d., twice a day; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65–74 years, Sex category (female); CRNM bleeds, clinically relevant non-major bleeds; EMANATE, Eliquis evaluated in acute cardioversion compared to usual treatments for anticoagulation in subjects with atrial fibrillation; ENSURE-AF, edoxaban vs. warfarin in subjects undergoing cardioversion of atrial fibrillation; MI, myocardial infarction; N/R, not reported; NOAC, non-vitamin K oral anticoagulant; o.d., once daily; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; TIA, transient ischaemic attack; TOE, transoesophageal echocardiography; TTR, time in therapeutic range; VKA, vitamin K antagonist; X-VeRT, explore the efficacy and safety of once-daily oral rivaroxaban for the prevention of cardiovascular events in patients with non-valvular atrial fibrillation scheduled for cardioversion.
Total number of cardioversions.
15 mg o.d. in patients with CrCL of 30–49 mL/min.
30 mg o.d., if CrCl 15–50 mL/min, body weight ≤60 kg or use of P-gp inhibitors.
2.5 mg b.i.d., if at least two of the following: age ≥80 years, weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (≥133 µmol/L). Cardioversion could be performed 2 h after a loading dose of 10 mg (5 mg, if at least two of the following: age ≥80 years, weight ≤60 kg, or serum creatinine ≥1.5 mg/dL [≥133 µmol/L]).
From main study.
Prospective trials with the factor-Xa inhibitors rivaroxaban (X-VeRT, Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Subjects with Non-Valvular Atrial Fibrillation Scheduled for Cardioversion), edoxaban (Edoxaban vs. Enoxaparin–Warfarin in Patients Undergoing Cardioversion of Atrial Fibrillation, ENSURE-AF), and apixaban (Eliquis Evaluated in Acute Cardioversion Compared to Usual Treatments for Anticoagulation in Subjects with Atrial Fibrillation, EMANATE), which markedly differed in design, confirmed the generally low peri-cardioversion rates of stroke, systemic embolism, death, and serious bleeding events during treatment with NOACs compared with heparin/VKA (Table 3), regardless of whether a standard care approach with ≥3 weeks OAC pre-treatment or an early TOE-guided approach was pursued, although these trials were not adequately powered statistically to demonstrate non-inferiority of NOAC treatment.87,88,100
While patients in X-VeRT and ENSURE-AF only initiated OAC treatment with standard doses of rivaroxaban and edoxaban, respectively, before early cardioversion,87,88 early cardioversion in EMANATE was performed either after initiating treatment with standard doses apixaban or after a single loading dose [10 mg or 5 mg, if patients fulfilled two of the following criteria: age ≥80 years, weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 µmol/L)] given at least 2 h before the procedure, the latter mainly in patients with new-onset AF.100
The comparable efficacy and safety of NOACs and VKA in patients undergoing cardioversion of AF were also confirmed in several recent meta-analyses.101–103 Similar to the results of the prospective studies and meta-analyses, several cohort studies with different post-procedural follow-up times and settings also demonstrated very low rates of thromboembolic events (0.15–1.62%) and major bleeding (0.4–1.7%) in patients with AF undergoing cardioversion during NOAC treatment suggesting that NOACs are associated with an acceptable benefit-risk profile in this setting.94,95,104–108 Moreover, a pre-specified post hoc analysis of the ENSURE-AF study found that patients receiving edoxaban were more satisfied with their treatment than patients on warfarin.109 Of note, the use of NOACs also lead to faster cardioversion compared to warfarin use when pursuing a standard care approach.87,95,100,107,110 Finally, a meta-analysis of the warfarin vs. NOAC cardioversion trials found a lower stroke rate in patients randomized to NOAC therapy.111
A major concern when performing cardioversion on NOAC treatment is how to ensure compliance, because unlike VKAs there is currently no test to monitor the quality of peri-procedural NOAC treatment, and observational data in patients on VKA treatment have also shown more frequent complications in patients with suboptimal anticoagulation intensity at the time of cardioversion.44 Therefore, patient education on importance of a strict intake schedule, information about adherence aids, and the utilization of telemonitoring systems are crucial and can improve treatment adherence.112 Verbal confirmation of NOAC intake and retrospective pill counting can help to ensure that anticoagulation was taken.
Key points
The peri-cardioversion rates of stroke, systemic embolism, and bleeding are low with NOACs.
Non-vitamin K oral anticoagulants can safely be used for stroke prevention in patients undergoing cardioversion of AF.
Measures to ensure treatment adherence are crucial.
Cardioversion in patients with atrial fibrillation after acute coronary syndrome and/or percutaneous coronary intervention
Atrial fibrillation and coronary artery disease commonly coexist,113 and ∼5–10% of patients undergoing percutaneous coronary intervention (PCI) also have AF.114 Therefore, the vast majority of these patients require combination therapy with OACs and antiplatelet drugs for a limited period of time after the procedure.115 Acute cardioversion can be justified in patients who are haemodynamically unstable, but usually cardioversion can be deferred. If a cardioversion is planned during the chronic phase after PCI, while patients are on combination therapy with a NOAC and an antiplatelet drug, attention must be paid to the appropriate NOAC dosage as used in the pivotal cardioversion trials.
Cardioversion in patients with atrial fibrillation after left atrial appendage occlusion
Left atrial appendage occlusion (LAAO) is an alternative to OAC treatment if the bleeding risk is high or if OAC treatment is contraindicated. There are currently no data suggesting the optimal management of these patients, if they require a cardioversion, because LAAO and contraindications to OAC were exclusion criteria in the randomized trials.116–118 A pre-procedural TOE should be performed in these patients and a short duration of OAC should be considered in patients with concomitant antiplatelet therapy.
In summary, cardioversion under peri-procedural treatment with NOACs appears as safe and effective as under treatment with heparin/VKA regardless of whether pursuing a standard care approach with ≥3 weeks pre-treatment or an early approach with a TOE performed immediately before the procedure. The latter is more convenient for patients and healthcare professionals. Immediate cardioversion at least 2 h after a single loading dose of apixaban is feasible and, therefore, might become a more convenient alternative to heparin pre-treatment. Ensuring adherence to NOAC is important, as patients undergoing cardioversion are at increased risk of stroke.
A personalized cardioversion approach to patients after PCI or LAAO is preferred.
Conclusion
Cardioversion is widely used as part of a rhythm control strategy in patients with AF. Nevertheless, a wait-and-see approach is reasonable in patients with recent-onset AF, as the majority will convert spontaneously within 48 h. Recurrences after ECV of persistent AF show a specific pattern which may help guide rhythm control. Although complications of cardioversion overall are rare, it is of utmost importance to assess thromboembolic risk before the procedure, initiate timely OAC and continue life-long in patients with increased stroke risk. The advent of NOACs facilitates the streamlining of the peri-procedural anticoagulation management and, thus, performing cardioversion without major delays, provided that patients have been adequately counselled about the necessity for compliance to NOAC treatment. After the procedure, a close structured clinical follow-up is mandatory to recognize AF recurrences, to ensure appropriate and effective rhythm control therapy, to evaluate symptoms, and to optimize treatment of underlying cardiovascular conditions.
Conflict of interest: A.B. has received lecture fees from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, MSD; and research support from Gilead, the Region of Southern Denmark, and the Zealand Region. E.L.G. has received speaker honoraria or consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, MSD, Portola Pharmaceuticals, and Roche. He is an investigator in the THEMIS, SATELLITE, and FLAVOUR studies (AstraZeneca) and has received research grants from Boehringer Ingelheim. P.K. receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past. P.K. is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). All other authors have no conflict of interest to declare.
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 2. Van Gelder IC, Tuinenburg AE, Schoonderwoerd BS, Tieleman RG, Crijns HJ.. Pharmacologic versus direct-current electrical cardioversion of atrial flutter and fibrillation. Am J Cardiol 1999;84:147–51R. [DOI] [PubMed] [Google Scholar]
- 3. Phillips E, Levine SA.. Auricular fibrillation without other evidence of heart disease; a cause of reversible heart failure. Am J Med 1949;7:478–89. [DOI] [PubMed] [Google Scholar]
- 4. Lown B, Amarasingham R, Neuman J.. New method for terminating cardiac arrhythmias. Use of synchronized capacitor discharge. JAMA 1962;182:548–55. [PubMed] [Google Scholar]
- 5. Pluymaekers N, Dudink E, Luermans J, Meeder JG, Lenderink T, Widdershoven J. et al. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med 2019;380:1499–508. [DOI] [PubMed] [Google Scholar]
- 6. Hernandez-Madrid A, Svendsen JH, Lip GY, Van Gelder IC, Dobreanu D, Blomstrom-Lundqvist C; conducted by the Scientific Initiatives Committee, European Heart Rhythm Association (EHRA). Cardioversion for atrial fibrillation in current European practice: results of the European Heart Rhythm Association survey. Europace 2013;15:915–8. [DOI] [PubMed] [Google Scholar]
- 7. Van Gelder IC, Crijns HJ, Van Gilst WH, Verwer R, Lie KI.. Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct-current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol 1991;68:41–6. [DOI] [PubMed] [Google Scholar]
- 8. Gallagher MM, Guo XH, Poloniecki JD, Guan Yap Y, Ward D, Camm AJ.. Initial energy setting, outcome and efficiency in direct current cardioversion of atrial fibrillation and flutter. J Am Coll Cardiol 2001;38:1498–504. [DOI] [PubMed] [Google Scholar]
- 9. Furniss SS, Sneyd JR.. Safe sedation in modern cardiological practice. Heart 2015;101:1526–30. [DOI] [PubMed] [Google Scholar]
- 10. Crijns HJ, Weijs B, Fairley AM, Lewalter T, Maggioni AP, Martin A. et al. Contemporary real life cardioversion of atrial fibrillation: results from the multinational RHYTHM-AF study. Int J Cardiol 2014;172:588–94. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Eckardt L, Loh P, Weber K, Fischer RJ, Seidl KH. et al. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: a randomised trial. Lancet 2002;360:1275–9. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt AS, Lauridsen KG, Torp P, Bach LF, Rickers H, Lofgren B.. Maximum-fixed energy shocks for cardioverting atrial fibrillation. Eur Heart J 2020;41:626–631. [DOI] [PubMed] [Google Scholar]
- 13. Manegold JC, Israel CW, Ehrlich JR, Duray G, Pajitnev D, Wegener FT. et al. External cardioversion of atrial fibrillation in patients with implanted pacemaker or cardioverter-defibrillator systems: a randomized comparison of monophasic and biphasic shock energy application. Eur Heart J 2007;28:1731–8. [DOI] [PubMed] [Google Scholar]
- 14. Pluymaekers N, Dudink E, Boersma L, Erkuner O, Gelissen M, van Dijk V. et al. External electrical cardioversion in patients with cardiac implantable electronic devices: is it safe and is immediate device interrogation necessary? Pacing Clin Electrophysiol 2018;41:1336–40. [DOI] [PubMed] [Google Scholar]
- 15. Kirchhof P, Mönnig G, Wasmer K, Heinecke A, Breithardt G, Eckardt L. et al. A trial of self-adhesive patch electrodes and hand-held paddle electrodes for external cardioversion of atrial fibrillation (MOBIPAPA). European Heart Journal 2005;26:1292–7. [DOI] [PubMed] [Google Scholar]
- 16. Van Gelder IC, Crijns HJ, Van der Laarse A, Van Gilst WH, Lie KI.. Incidence and clinical significance of ST segment elevation after electrical cardioversion of atrial fibrillation and atrial flutter. Am Heart J 1991;121:51–6. [DOI] [PubMed] [Google Scholar]
- 17. Pisters R, Nieuwlaat R, Prins MH, Le Heuzey JY, Maggioni AP, Camm AJ. et al. ; Euro Heart Survey Investigators. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace 2012;14:666–74. [DOI] [PubMed] [Google Scholar]
- 18. Rossi M, Lown B.. The use of quinidine in cardioversion. Am J Cardiol 1967;19:234–8. [DOI] [PubMed] [Google Scholar]
- 19. Weijs B, Limantoro I, Delhaas T, de Vos CB, Blaauw Y, Houben RPM. et al. Cardioversion of persistent atrial fibrillation is associated with a 24-hour relapse gap: observations from prolonged postcardioversion rhythm monitoring. Clin Cardiol 2018;41:366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tieleman RG, Van Gelder IC, Crijns HJ, De Kam PJ, Van Den Berg MP, Haaksma J. et al. Early recurrences of atrial fibrillation after electrical cardioversion: a result of fibrillation-induced electrical remodeling of the atria? J Am Coll Cardiol 1998;31:167–73. [DOI] [PubMed] [Google Scholar]
- 21. Van Gelder IC, Crijns HJ, Tieleman RG, Brugemann J, De Kam PJ, Gosselink AT. et al. Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med 1996;156:2585–92. [DOI] [PubMed] [Google Scholar]
- 22. Van Noord T, Van Gelder IC, Schoonderwoerd BA, Crijns HJ.. Immediate reinitiation of atrial fibrillation after electrical cardioversion predicts subsequent pharmacologic and electrical conversion to sinus rhythm on amiodarone. Am J Cardiol 2000;86:1384–5, A5. [DOI] [PubMed] [Google Scholar]
- 23. Kirchhof P, Andresen D, Bosch R, Borggrefe M, Meinertz T, Parade U. et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet 2012;380:238–46. [DOI] [PubMed] [Google Scholar]
- 24. Daoud EG, Hummel JD, Augostini R, Williams S, Kalbfleisch SJ.. Effect of verapamil on immediate recurrence of atrial fibrillation. J Cardiovasc Electrophysiol 2000;11:1231–7. [DOI] [PubMed] [Google Scholar]
- 25. Madrid AH, Bueno MG, Rebollo JMG, Marín I, Penña G, Bernal E. et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation 2002;106:331–6. [DOI] [PubMed] [Google Scholar]
- 26. De Simone A, Stabile G, Vitale DF, Turco P, Di Stasio M, Petrazzuoli F. et al. Pretreatment with verapamil in patients with persistent or chronic atrial fibrillation who underwent electrical cardioversion. J Am Coll Cardiol 1999;34:810–4. [DOI] [PubMed] [Google Scholar]
- 27. Kuhlkamp V, Schirdewan A, Stangl K, Homberg M, Ploch M, Beck OA.. Use of metoprolol CR/XL to maintain sinus rhythm after conversion from persistent atrial fibrillation: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 2000;36:139–46. [DOI] [PubMed] [Google Scholar]
- 28. Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL. et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005;352:1861–72. [DOI] [PubMed] [Google Scholar]
- 29. Mont L, Bisbal F, Hernandez-Madrid A, Perez-Castellano N, Vinolas X, Arenal A. et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmed S, Rienstra M, Crijns HJ, Links TP, Wiesfeld AC, Hillege HL. et al. Continuous vs episodic prophylactic treatment with amiodarone for the prevention of atrial fibrillation: a randomized trial. JAMA 2008;300:1784–92. [DOI] [PubMed] [Google Scholar]
- 31. Duytschaever M, Heyse A, de Sutter J, Crijns H, Gillebert T, Tavernier R. et al. Transthoracic tissue Doppler imaging of the atria: a novel method to determine the atrial fibrillation cycle length. J Cardiovasc Electrophysiol 2006;17:1202–9. [DOI] [PubMed] [Google Scholar]
- 32. Limantoro I, De Vos CB, Delhaas T, Marcos E, Blaauw Y, Weijs B. et al. Tissue velocity imaging of the left atrium predicts response to flecainide in patients with acute atrial fibrillation. Heart Rhythm 2014;11:478–84. [DOI] [PubMed] [Google Scholar]
- 33. Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L. et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med 2004;351:2384–91. [DOI] [PubMed] [Google Scholar]
- 34. Crijns HJ, van Wijk LM, van Gilst WH, Kingma JH, van Gelder IC, Lie KI.. Acute conversion of atrial fibrillation to sinus rhythm: clinical efficacy of flecainide acetate. Comparison of two regimens. Eur Heart J 1988;9:634–8. [DOI] [PubMed] [Google Scholar]
- 35. Crijns HJ, Van Gelder IC, Kingma JH, Dunselman PH, Gosselink AT, Lie KI.. Atrial flutter can be terminated by a class III antiarrhythmic drug but not by a class IC drug. Eur Heart J 1994;15:1403–8. [DOI] [PubMed] [Google Scholar]
- 36. Crijns HJ, van Gelder IC, Lie KI.. Supraventricular tachycardia mimicking ventricular tachycardia during flecainide treatment. Am J Cardiol 1988;62:1303–6. [DOI] [PubMed] [Google Scholar]
- 37. Pappone C, Radinovic A, Manguso F, Vicedomini G, Sala S, Sacco FM. et al. New-onset atrial fibrillation as first clinical manifestation of latent Brugada syndrome: prevalence and clinical significance. Eur Heart J 2009;30:2985–92. [DOI] [PubMed] [Google Scholar]
- 38. van Opstal JM, Volders PG, Crijns HJ.. Provocation of silence. Europace 2009;11:385–7. [DOI] [PubMed] [Google Scholar]
- 39. Borgeat A, Goy JJ, Maendly R, Kaufmann U, Grbic M, Sigwart U.. Flecainide versus quinidine for conversion of atrial fibrillation to sinus rhythm. Am J Cardiol 1986;58:496–8. [DOI] [PubMed] [Google Scholar]
- 40. Kochiadakis GE, Igoumenidis NE, Parthenakis FI, Chlouverakis GI, Vardas PE.. Amiodarone versus propafenone for conversion of chronic atrial fibrillation: results of a randomized, controlled study. J Am Coll Cardiol 1999;33:966–71. [DOI] [PubMed] [Google Scholar]
- 41. Tieleman RG, Gosselink AT, Crijns HJ, van Gelder IC, van den Berg MP, de Kam PJ. et al. Efficacy, safety, and determinants of conversion of atrial fibrillation and flutter with oral amiodarone. Am J Cardiol 1997;79:53–7. [DOI] [PubMed] [Google Scholar]
- 42. Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR. et al. Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 2016;13:230–7. [DOI] [PubMed] [Google Scholar]
- 43. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr. et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gallagher MM, Hennessy BJ, Edvardsson N, Hart CM, Shannon MS, Obel OA. et al. Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol 2002;40:926–33. [DOI] [PubMed] [Google Scholar]
- 45. Fatkin D, Kuchar DL, Thorburn CW, Feneley MP.. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol 1994;23:307–16. [DOI] [PubMed] [Google Scholar]
- 46. Ito T, Suwa M, Otake Y, Kobashi A, Hirota Y, Ando H. et al. Assessment of left atrial appendage function after cardioversion of atrial fibrillation: relation to left atrial mechanical function. Am Heart J 1998;135:1020–6. [DOI] [PubMed] [Google Scholar]
- 47. Moreyra E, Finkelhor RS, Cebul RD.. Limitations of transesophageal echocardiography in the risk assessment of patients before nonanticoagulated cardioversion from atrial fibrillation and flutter: an analysis of pooled trials. Am Heart J 1995;129:71–5. [DOI] [PubMed] [Google Scholar]
- 48. Hansen ML, Jepsen RM, Olesen JB, Ruwald MH, Karasoy D, Gislason GH. et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace 2015;17:18–23. [DOI] [PubMed] [Google Scholar]
- 49. Nuotio I, Hartikainen JE, Gronberg T, Biancari F, Airaksinen KE.. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA 2014;312:647–9. [DOI] [PubMed] [Google Scholar]
- 50. Airaksinen KE, Gronberg T, Nuotio I, Nikkinen M, Ylitalo A, Biancari F. et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol 2013;62:1187–92. [DOI] [PubMed] [Google Scholar]
- 51. Weigner MJ, Caulfield TA, Danias PG, Silverman DI, Manning WJ.. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med 1997;126:615–20. [DOI] [PubMed] [Google Scholar]
- 52. Gronberg T, Hartikainen JE, Nuotio I, Biancari F, Ylitalo A, Airaksinen KE.. Anticoagulation, CHA2DS2VASc score, and thromboembolic risk of cardioversion of acute atrial fibrillation (from the FinCV Study). Am J Cardiol 2016;117:1294–8. [DOI] [PubMed] [Google Scholar]
- 53. Sjalander S, Svensson PJ, Friberg L.. Atrial fibrillation patients with CHA2DS2-VASc >1 benefit from oral anticoagulation prior to cardioversion. Int J Cardiol 2016;215:360–3. [DOI] [PubMed] [Google Scholar]
- 54. Lip GY, Gitt AK, Le Heuzey JY, Bash LD, Morabito CJ, Bernhardt AA. et al. Overtreatment and undertreatment with anticoagulation in relation to cardioversion of atrial fibrillation (the RHYTHM-AF study). Am J Cardiol 2014;113:480–4. [DOI] [PubMed] [Google Scholar]
- 55. Apostolakis S, Haeusler KG, Oeff M, Treszl A, Andresen D, Borggrefe M. et al. Low stroke risk after elective cardioversion of atrial fibrillation: an analysis of the Flec-SL trial. Int J Cardiol 2013;168:3977–81. [DOI] [PubMed] [Google Scholar]
- 56. Dentali F, Botto GL, Gianni M, Ambrosino P, Di Minno MN.. Efficacy and safety of direct oral anticoagulants in patients undergoing cardioversion for atrial fibrillation: a systematic review and meta-analysis of the literature. Int J Cardiol 2015;185:72–7. [DOI] [PubMed] [Google Scholar]
- 57. Berger M, Schweitzer P.. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol 1998;82:1545–7. A8. [DOI] [PubMed] [Google Scholar]
- 58. Lip GY. Cardioversion of atrial fibrillation. Postgrad Med J 1995;71:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Di Minno MN, Ambrosino P, Dello Russo A, Casella M, Tremoli E, Tondo C.. Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb Haemost 2016;115:663–77. [DOI] [PubMed] [Google Scholar]
- 60. Merino JL, Lip GYH, Heidbuchel H, Cohen AA, De Caterina R, de Groot JR. et al. Determinants of left atrium thrombi in scheduled cardioversion: an ENSURE-AF study analysis. Europace 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Blackshear JL, Odell JA.. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–9. [DOI] [PubMed] [Google Scholar]
- 62. Jaber WA, Prior DL, Thamilarasan M, Grimm RA, Thomas JD, Klein AL. et al. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: a transesophageal echocardiographic study. Am Heart J 2000;140:150–6. [DOI] [PubMed] [Google Scholar]
- 63. Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ.. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging 2014;7:1251–65. [DOI] [PubMed] [Google Scholar]
- 64. Acar J, Cormier B, Grimberg D, Kawthekar G, Iung B, Scheuer B. et al. Diagnosis of left atrial thrombi in mitral stenosis–usefulness of ultrasound techniques compared with other methods. Eur Heart J 1991;12 Suppl B:70–6. [DOI] [PubMed] [Google Scholar]
- 65. Manning WJ, Weintraub RM, Waksmonski CA, Haering JM, Rooney PS, Maslow AD. et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med 1995;123:817–22. [DOI] [PubMed] [Google Scholar]
- 66. von der Recke G, Schmidt H, Illien S, Luderitz B, Omran H.. Use of transesophageal contrast echocardiography for excluding left atrial appendage thrombi in patients with atrial fibrillation before cardioversion. J Am Soc Echocardiogr 2002;15:1256–61. [DOI] [PubMed] [Google Scholar]
- 67. Parvathaneni L, Mahenthiran J, Jacob S, Foltz J, Gill WJ, Ghumman W. et al. Comparison of tissue Doppler dynamics to Doppler flow in evaluating left atrial appendage function by transesophageal echocardiography. Am J Cardiol 2005;95:1011–4. [DOI] [PubMed] [Google Scholar]
- 68. Tenekecioglu E, Karabulut A, Yilmaz M.. Comparison of tissue Doppler dynamics with Doppler flow in evaluating left atrial appendage function by transesophageal echocardiography in prehypertensive and hypertensive patients. Echocardiography 2010;27:677–86. [DOI] [PubMed] [Google Scholar]
- 69. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med 1998;128:639–47. [DOI] [PubMed] [Google Scholar]
- 70. Fatkin D, Kelly RP, Feneley MP.. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol 1994;23:961–9. [DOI] [PubMed] [Google Scholar]
- 71. Mugge A, Kuhn H, Nikutta P, Grote J, Lopez JA, Daniel WG.. Assessment of left atrial appendage function by biplane transesophageal echocardiography in patients with nonrheumatic atrial fibrillation: identification of a subgroup of patients at increased embolic risk. J Am Coll Cardiol 1994;23:599–607. [DOI] [PubMed] [Google Scholar]
- 72. Li YH, Lai LP, Shyu KG, Hwang JJ, Kuan P, Lien WP.. Clinical implications of left atrial appendage flow patterns in nonrheumatic atrial fibrillation. Chest 1994;105:748–52. [DOI] [PubMed] [Google Scholar]
- 73. Sallach JA, Puwanant S, Drinko JK, Jaffer S, Donal E, Thambidorai SK. et al. Comprehensive left atrial appendage optimization of thrombus using surface echocardiography: the CLOTS multicenter pilot trial. J Am Soc Echocardiogr 2009;22:1165–72. [DOI] [PubMed] [Google Scholar]
- 74. Ren JF, Marchlinski FE, Supple GE, Hutchinson MD, Garcia FC, Riley MP. et al. Intracardiac echocardiographic diagnosis of thrombus formation in the left atrial appendage: a complementary role to transesophageal echocardiography. Echocardiography 2013;30:72–80. [DOI] [PubMed] [Google Scholar]
- 75. Saksena S, Sra J, Jordaens L, Kusumoto F, Knight B, Natale A. et al. ; ICE-CHIP Investigator Study Group. A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: the intracardiac echocardiography guided cardioversion helps interventional procedures study. Circ Arrhythm Electrophysiol 2010;3:571–7. [DOI] [PubMed] [Google Scholar]
- 76. Kim YY, Klein AL, Halliburton SS, Popovic ZB, Kuzmiak SA, Sola S. et al. Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J 2007;154:1199–205. [DOI] [PubMed] [Google Scholar]
- 77. Patel A, Au E, Donegan K, Kim RJ, Lin FY, Stein KM. et al. Multidetector row computed tomography for identification of left atrial appendage filling defects in patients undergoing pulmonary vein isolation for treatment of atrial fibrillation: comparison with transesophageal echocardiography. Heart Rhythm 2008;5:253–60. [DOI] [PubMed] [Google Scholar]
- 78. Hur J, Kim YJ, Lee HJ, Nam JE, Ha JW, Heo JH. et al. Dual-enhanced cardiac CT for detection of left atrial appendage thrombus in patients with stroke: a prospective comparison study with transesophageal echocardiography. Stroke 2011;42:2471–7. [DOI] [PubMed] [Google Scholar]
- 79. Martinez MW, Kirsch J, Williamson EE, Syed IS, Feng D, Ommen S. et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging 2009;2:69–76. [DOI] [PubMed] [Google Scholar]
- 80. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ.. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 2013;6:185–94. [DOI] [PubMed] [Google Scholar]
- 81. Muellerleile K, Sultan A, Groth M, Steven D, Hoffmann B, Adam G. et al. Velocity encoded cardiovascular magnetic resonance to assess left atrial appendage emptying. J Cardiovasc Magn Reson 2012;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vira T, Pechlivanoglou P, Connelly K, Wijeysundera HC, Roifman I.. Cardiac computed tomography and magnetic resonance imaging vs. transoesophageal echocardiography for diagnosing left atrial appendage thrombi. Europace 2018. [DOI] [PubMed] [Google Scholar]
- 83. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 84. Lip GY, Hammerstingl C, Marin F, Cappato R, Meng IL, Kirsch B. et al. Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J 2016;178:126–34. [DOI] [PubMed] [Google Scholar]
- 85. Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW. et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411–20. [DOI] [PubMed] [Google Scholar]
- 86. Seidl K, Rameken M, Drogemuller A, Vater M, Brandt A, Schwacke H. et al. Embolic events in patients with atrial fibrillation and effective anticoagulation: value of transesophageal echocardiography to guide direct-current cardioversion. Final results of the Ludwigshafen Observational Cardioversion Study. J Am Coll Cardiol 2002;39:1436–42. [DOI] [PubMed] [Google Scholar]
- 87. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY. et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J 2014;35:3346–55. [DOI] [PubMed] [Google Scholar]
- 88. Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J. et al. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet 2016;388:1995–2003. [DOI] [PubMed] [Google Scholar]
- 89. Osmanagic A, Moller S, Osmanagic A, Sheta HM, Vinther KH, Egstrup K.. Effect of early direct current cardioversion on the recurrence of atrial fibrillation in patients with persistent atrial fibrillation. Am J Cardiol 2015;116:225–9. [DOI] [PubMed] [Google Scholar]
- 90. Staerk L, Fosbol EL, Gadsboll K, Sindet-Pedersen C, Pallisgaard JL, Lamberts M. et al. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: temporal trends 2011-2015 in Denmark. Sci Rep 2016;6:31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Apenteng PN, Gao H, Hobbs FR, Fitzmaurice DA; UK GARFIELD-AF Investigators and GARFIELD-AF Steering Committee. Temporal trends in antithrombotic treatment of real-world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD-AF registry. BMJ Open 2018;8:e018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Haastrup SB, Hellfritzsch M, Rasmussen L, Pottegard A, Grove EL.. Use of non-vitamin K antagonist oral anticoagulants 2008-2016: a Danish Nationwide Cohort Study. Basic Clin Pharmacol Toxicol 2018;123:452–63. [DOI] [PubMed] [Google Scholar]
- 93. Papp J, Zima E, Bover R, Karaliute R, Rossi A, Szymanski C. et al. Changes in oral anticoagulation for elective cardioversion: results from a European cardioversion registry. Eur Heart J Cardiovasc Pharmacother 2017;3:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Femia G, Fetahovic T, Shetty P, Lee A.. Novel oral anticoagulants in direct current cardioversion for atrial fibrillation. Heart Lung Circ 2018;27:798–803. [DOI] [PubMed] [Google Scholar]
- 95. Frederiksen AS, Albertsen AE, Christesen AMS, Vinter N, Frost L, Moller DS.. Cardioversion of atrial fibrillation in a real-world setting: non-vitamin K antagonist oral anticoagulants ensure a fast and safe strategy compared to warfarin. Europace 2018;20:1078–85. [DOI] [PubMed] [Google Scholar]
- 96. Nagarakanti R, Ezekowitz MD, Oldgren J, Yang S, Chernick M, Aikens TH. et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011;123:131–6. [DOI] [PubMed] [Google Scholar]
- 97. Piccini JP, Stevens SR, Lokhnygina Y, Patel MR, Halperin JL, Singer DE. et al. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol 2013;61:1998–2006. [DOI] [PubMed] [Google Scholar]
- 98. Flaker G, Lopes RD, Al-Khatib SM, Hermosillo AG, Hohnloser SH, Tinga B. et al. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol 2014;63:1082–7. [DOI] [PubMed] [Google Scholar]
- 99. Plitt A, Ezekowitz MD, De Caterina R, Nordio F, Peterson N, Giugliano RP; ENGAGE AF-TIMI 48 Investigators. Cardioversion of atrial fibrillation in ENGAGE AF-TIMI 48. Clin Cardiol 2016;39:345–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ezekowitz MD, Pollack CV Jr, Halperin JL, England RD, VanPelt Nguyen S, Spahr J. et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J 2018;39:2959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Renda G, Ricci F, De Caterina R.. Non-vitamin K antagonist oral anticoagulants for cardioversion in atrial fibrillation: an updated meta-analysis. Am J Med 2017;130:457–61. [DOI] [PubMed] [Google Scholar]
- 102. Telles-Garcia N, Dahal K, Kocherla C, Lip GYH, Reddy P, Dominic P.. Non-vitamin K antagonists oral anticoagulants are as safe and effective as warfarin for cardioversion of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol 2018;268:143–8. [DOI] [PubMed] [Google Scholar]
- 103. Mincu RI, Mahabadi AA, Totzeck M, Rassaf T.. Novel anticoagulants versus vitamin K antagonists for cardioversion of non- valvular atrial fibrillation - a meta-analysis of more than 17000 patients. Sci Rep 2019;9:3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Camm AJ, Turpie AGG, Hess S, Amarenco P, Lambelet M, Haas S. et al. ; XANTUS Investigators. Outcomes after catheter ablation and cardioversion in patients with non-valvular atrial fibrillation: results from the prospective, observational XANTUS study. Europace 2018;20:e87–95. [DOI] [PubMed] [Google Scholar]
- 105. Itainen S, Lehto M, Vasankari T, Mustonen P, Kotamaki M, Numminen A. et al. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients undergoing elective cardioversion. Europace 2018;20:565–8. [DOI] [PubMed] [Google Scholar]
- 106. Kochhauser S, Khaykin Y, Beardsall J, Juta R, Hache P, Trought K. et al. Comparison of outcomes after cardioversion or atrial fibrillation ablation in patients with differing periprocedural anticoagulation regimens. Can J Cardiol 2014;30:1541–6. [DOI] [PubMed] [Google Scholar]
- 107. Pallisgaard JL, Lindhardt TB, Hansen ML, Schjerning AM, Olesen JB, Staerk L. et al. Cardioversion and risk of adverse events with dabigatran versus warfarin—a nationwide cohort study. PLoS One 2015;10:e0141377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Coleman CM, Khalaf S, Mould S, Wazni O, Kanj M, Saliba W. et al. Novel oral anticoagulants for DC cardioversion procedures: utilization and clinical outcomes compared with warfarin. Pacing Clin Electrophysiol 2015;38:731–7. [DOI] [PubMed] [Google Scholar]
- 109. Goette A, Kwong WJ, Ezekowitz MD, Banach M, Hjortshoj SP, Zamoryakhin D. et al. Edoxaban therapy increases treatment satisfaction and reduces utilization of healthcare resources: an analysis from the EdoxabaN vs. warfarin in subjectS UndeRgoing cardiovErsion of atrial fibrillation (ENSURE-AF) study. Europace 2018;20:1936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wall C, Jankowski T, Naruka V, Mota P.. Comparing the delay with different anticoagulants before elective electrical cardioversion for atrial fibrillation/flutter. PLoS One 2019;14:e0210170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kotecha D, Pollack CV Jr, De Caterina R, Renda G, Kirchhof P.. Direct oral anticoagulants halve thromboembolic events after cardioversion of AF compared with warfarin. J Am Coll Cardiol 2018;72:1984–6. [DOI] [PubMed] [Google Scholar]
- 112. Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR. et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 2017;390:1737–46. [DOI] [PubMed] [Google Scholar]
- 113. Weijs B, Pisters R, Haest RJ, Kragten JA, Joosen IA, Versteylen M. et al. Patients originally diagnosed with idiopathic atrial fibrillation more often suffer from insidious coronary artery disease compared to healthy sinus rhythm controls. Heart Rhythm 2012;9:1923–9. [DOI] [PubMed] [Google Scholar]
- 114. Secemsky EA, Butala NM, Kartoun U, Mahmood S, Wasfy JH, Kennedy KF. et al. Use of chronic oral anticoagulation and associated outcomes among patients undergoing percutaneous coronary intervention. J Am Heart Assoc 2016;5:e004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L. et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019;21:192–3. [DOI] [PubMed] [Google Scholar]
- 116. Lip GY, Merino J, Ezekowitz M, Ellenbogen K, Zamoryakhin D, Lanz H. et al. A prospective evaluation of edoxaban compared to warfarin in subjects undergoing cardioversion of atrial fibrillation: the EdoxabaN vs. warfarin in subjectS UndeRgoing cardiovErsion of Atrial Fibrillation (ENSURE-AF) study. Am Heart J 2015;169:597–604.e5. [DOI] [PubMed] [Google Scholar]
- 117. Ezekowitz MD, Cappato R, Klein AL, Camm AJ, Ma CS, Le Heuzey JY. et al. Rationale and design of the eXplore the efficacy and safety of once-daily oral riVaroxaban for the prEvention of caRdiovascular events in patients with nonvalvular aTrial fibrillation scheduled for cardioversion trial: a comparison of oral rivaroxaban once daily with dose-adjusted vitamin K antagonists in patients with nonvalvular atrial fibrillation undergoing elective cardioversion. Am Heart J 2014;167:646–52. [DOI] [PubMed] [Google Scholar]
- 118. Ezekowitz MD, Pollack CV, Sanders P, Halperin JL, Spahr J, Cater N. et al. Apixaban compared with parenteral heparin and/or vitamin K antagonist in patients with nonvalvular atrial fibrillation undergoing cardioversion: rationale and design of the EMANATE trial. Am Heart J 2016;179:59–68. [DOI] [PubMed] [Google Scholar]